Highlights
-
•
Hormonal receptors positive breast tumor and prostate cancer are managed with endocrine therapies.
-
•
Endocrine therapies designed for breast and prostate cancer are often associated to serious adverse skeletal related events, such fractures.
-
•
Denosumab is a monoclonal anti-body binding RANKL which acts as inhibitor of osteoclasts activity, thus increasing bone mass.
-
•
Denosumab was showed to strongly prevent hormonal therapies-related skeletal issues.
-
•
Denosumab administration results safe in bone mass increase and reduction of fractures risk.
Keywords: Breast, Prostate, Cancer, Hormone, Denosumab, Fracture
Abbreviations: ADT, androgen deprivation therapy; BMD, bone mass density; CI, confidence interval; HR, hazard ratio; MD, mean difference; RANKL, receptor activator of nuclear factor-kB ligand; RCTs, randomized clinical trials; SAEs, serious adverse events
Abstract
Hormonal therapies for receptor positive-breast and prostate cancer patients have shown clinical efficacy but also several side effects including osteoporosis, loss of bone mass and increased fracture risk. Denosumab represents an anti RANKL (receptor activator of nuclear factor-kB ligand) monoclonal anti-body acting as inhibitor of osteoclasts formation, function, and survival, then increasing bone mass. Herein, we performed a systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate the role of Denosumab in saving bone health in prostate and breast cancer patients receiving respectively androgen deprivation therapy and adjuvant endocrine therapy. Moreover, selected patients have to be treated with Denosumab at the dose of 60 mg every six month or placebo. Outcomes studied included the bone mass density (BMD) increase at 24 and 36 months, BMD loss, reduction of fractures risk (in particular vertebral) at 24 and 36 months and safety (overall, serious adverse events – SAEs and discontinuation rate). Our results showed a reduction of the BMD loss up to 36 months both at the lumbar and femoral level and a BMD increase both at 24 and 36 months. It was also found a reduction in the number of new vertebral and femoral fractures at 24 and 36 months. Finally, our pooled analysis showed that Denosumab did not affect both the SAEs and therapy discontinuation risk. In conclusion, Denosumab administration can be considered effective and safe in the prevention and management of the above mentioned adverse events related to hormonal therapies designed for breast and prostate tumors.
1. Introduction
Prostate and breast cancer are among the most commonly diagnosed cancers worldwide, with 0.9 million diagnoses of prostate cancer and 1.4 million diagnoses of breast cancer every year [1]. Early stages diagnosis and the development of effective therapies have reduced cancer mortality [1]; 10-year recurrence-free survival is estimated at up to 80% in women with breast cancer and 68%–97% for men with prostate cancer [2], [3]. These patients are treated with hormone therapy to reduce the risk of recurrence or progression. In prostate cancer, androgen deprivation therapy (ADT) is widely used for men with hormone-sensitive cancer [4], [5], [6], [7], [8]. ADT includes orchiectomy, gonadotropin-releasing hormone (GnRH) agonists (e.g., leuprolide, goserelin) and antagonists (e.g., degarelix), given either alone or in combination with androgen receptor antagonists (e.g., flutamide, bicalutamide, nilutamide) [9]. A recent meta analysis provided a significant correlation between ADT and BMD reduction in prostate cancer patients suggesting a strong role for medical therapy, lifestyle intervention, and nutritional support for BMD loss. In breast cancer, up to 75% of cancers expressing either estrogen or progesterone receptors, would be expected to benefit from endocrine therapy [8], [10], [11], [12], [13], [14], [15], [16]. It includes estrogen receptor modulators (SERMs; e.g., tamoxifen), aromatase inhibitors (e.g., letrozole, anastrozole, exemestane), and luteinizing hormone-releasing hormone (LHRH) agonists (e.g., goserelin, leuprolide). In post-menopausal women, aromatase inhibitors are the standard of care for hormone receptor-positive (HR+) early-stage cancer, but its use is associated with several side effects including osteoporosis, loss of bone mass and increased fracture risk. Indeed aromatase inhibitors suppress the conversion of androgens to estrogens, resulting in estrogen depletion, which in turn leads to lower bone mineral density and increased fracture risk [17]. Bone loss is mediated by osteoclasts, whose formation, function, and survival depend on the receptor activator of nuclear factor-kB ligand (RANKL). RANKL binds to its receptor RANK on preosteoclasts and mature osteoclasts and activates and maintains osteoclast-mediated bone resorption. Denosumab is a drug able to inhibit this process. It is a fully human monoclonal anti- body that specifically binds to the receptor activator of nuclear factor-κB ligand, a key mediator of osteoclast formation, function, and survival, increasing bone mass in patients undergoing hormone ablation therapy [18]. In postmenopausal women with osteoporosis who do not have cancer, Denosumab reduces the risk of vertebral, non-vertebral, and hip fractures. In previous trials, the subcutaneous administration of 60 mg of Denosumab every 6 months reduced bone turnover and increased bone mineral density [19]. The purpose of this meta-analysis is to compare studies of men with prostate cancer receiving ADT and women with breast cancer following adjuvant endocrine therapy in order to evaluate the effects of Denosumab in terms of BMD increase at 24 and 36 months, the BMD loss, the reduction of fractures risk (in particular vertebral fractures) at 24 and 36 months and safety (overall, serious adverse events – SAEs and discontinuation rate).
2. Materials and methods
We have searched for randomized controlled trials (RCTs) including histological or cytological proven prostate cancer patients receiving ADT or breast cancer patients following adjuvant endocrine therapy. In particular, we include in our analysis trials in which prostate cancer patients were treated with ADT including, gonadotropin-releasing hormone (GnRH) agonists (e.g., leuprolide, goserelin) and antagonists (e.g., degarelix), given either alone or in combination with androgen receptor antagonists (e.g., flutamide, bicalutamide, nilutamide). Breast cancer patients were treated with receptor modulators (SERMs; e.g., tamoxifen), aromatase inhibitors (e.g., letrozole, anastrozole, exemestane), and luteinizing hormone-releasing hormone (LHRH) agonists (e.g., goserelin, leuprolide), according to the pre- or post-menopausal hormonal status. Both prostate and breast cancer patients have to be treated with Denosumab at the dose of 60 mg every six month or placebo. The outcomes are the BMD increase at 24 and 36 months, the BMD loss, the reduction of fractures risk (in particular vertebral fractures) at 24 and 36 month and safety (overall, serious adverse events – SAEs and discontinuation rate). We excluded trials in which data were unavailable, ongoing studies and studies with small sample size (less than 10 patients for arm). To minimize the risk of bias, we excluded observational or retrospective trials. For the articles with multiple follow-up over time, we decided to choose the most updated and methodically valid. Data extraction and assessment were made independently by two different authors (A.G. and D.S.) and disagreement were solved by discussion with another author (A.R.). We searched for RCTs using Medline (PubMed), Embase-databases and Cochrane-Library up to May 2019, with no language restrictions. We included also relevant abstracts from the American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), AIOM (Italian Association of Medical Oncology) and ISPRM (The International Society of Physical and Rehabilitation Medicine). Other unpublished data were explored through the ClinicalTrials.gov site (www.clinicaltrials.gov), the reference lists of selected RCTs. We made a quality analysis of selected trials following the criteria reported in the Cochrane Handbook for Systematic Reviews of Intervention [20] including: allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; sequence generation; elective outcome reporting; other sources of bias. For each study we defined “Yes” as at low risk of bias and as “No” at high risk of bias. We define also “unclear” if there were insufficient data for a precise judgement. The risk of selective outcome reporting bias was also evaluated by two independent reviewers (A.G. and D.S.) and disagreement were solved by consensus [21].
Outcomes (BMD loss, reduction of fractures risk and safety) were analyzed using Risk ratio (RR), with a 95% of confidence interval (CI). For each study we first collected the number of patients with an event and the total number of patients to perform meta-analysis. BMD increase was analyzed using the mean difference (MD) and for each study we retrieved the mean and its standard deviation (SD). Heterogeneity between studies was explored using I-square and Chi-square tests. If I-square value was higher than 75% it was considered as at high risk of heterogeneity and meta-analysis was performed using random effect-based model of Der Simonian and Laird. If not, we used the fixed effect-based Mantel-Haenszel model [22]. As regards the risk of bias across studies, we performed a publication bias analysis using Egger's test and a Funnel Plot (Fig. 2). The meta-analysis was performed according to the PRISMA – guidelines for reporting of systematic review [23], using Cochrane RevMan ver. 5.3 statistical software and Comprehensive Meta – Analysis ver. 2.0 to assess the risk of publication bias (Egger's Test).When data were not reported in the text, we used specific software (GetData Graph Digitizer – free version) to extract data from figure the more accurate as possible. All the p-values were considered as statistically significant if p<0.05.
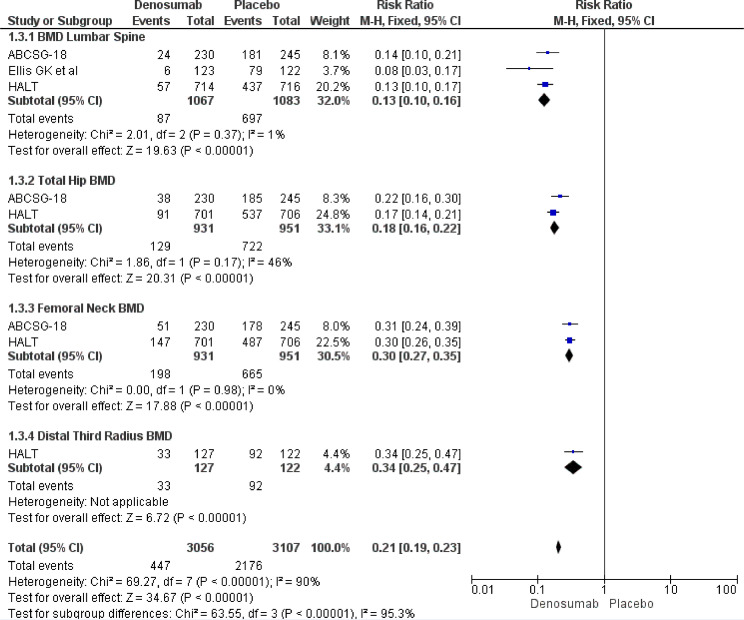
Fig. 2.
BMD loss at 36 months according to different bone sites.
3. Results
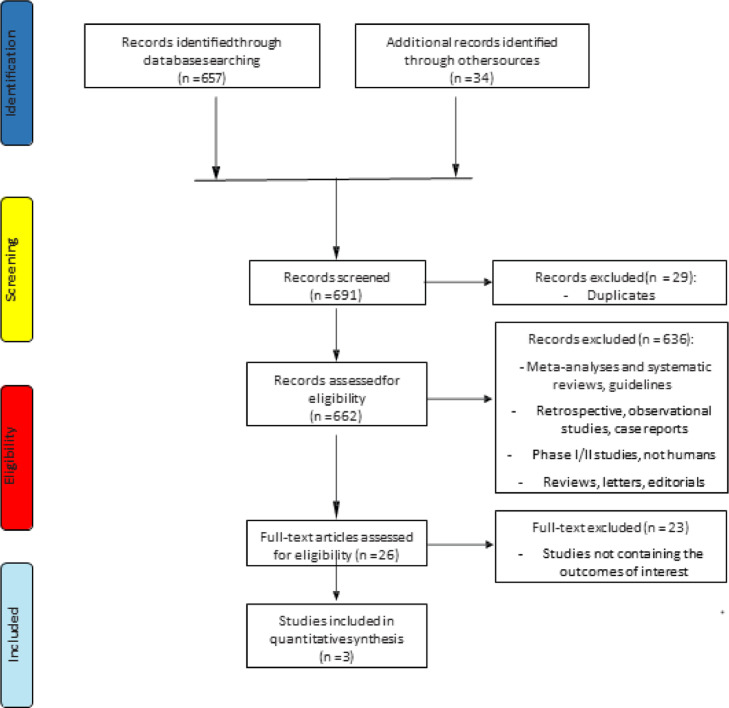
The search for literature identified in a total of 691 records, of which 29 were excluded because duplicates; 636 records were excluded because systematic reviews, meta-analyses, retrospective, observational or phase I/II studies, not in humans studies, letters, commentaries or guidelines. A total of 26 trials were assessed for eligibility and 23 were excluded because no data about the principal outcomes of our meta-analysis (BMD loss, BMD increase, reduction of fractures incidence and safety) were reported. Finally, 3 studies [24], [25], [26] for a total of 5140 patients met all the inclusion/exclusion criteria and were included in the meta-analysis (Fig. 1) (Table 1 – Supplementary files). The clinical outcomes of the included trials are reported in Table 2 (Supplementary files).
Fig. 1.
Flow chart of trials selection process.
3.1. Outcomes
3.1.1. BMD loss at 36 months
Pooled results showed a strong statistically significant result in terms of overall BMD loss risk reduction favoring Denosumab (RR 0.21, 95% CI 0.19–0.23). The same risk reduction was recorded at the lumbar spine (RR 0.13, 95% CI 0.10–0.16), total hip (RR 0.18, 95% CI 0.16–0.22), femoral neck (RR 0.30, 95% CI 0.27–0.35) and distal third radius (RR 0.34, 95% CI 0.25–0.47) (Fig. 2).
3.1.2. BMD increase at 24 and 36 months
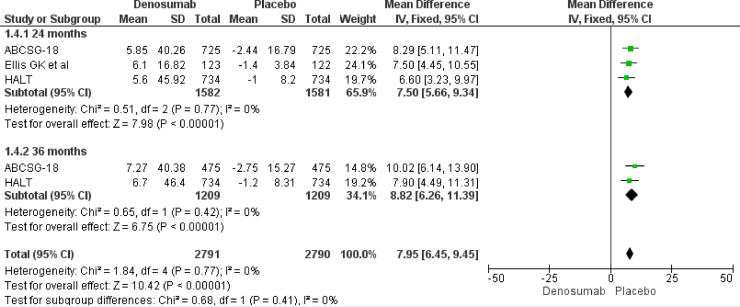
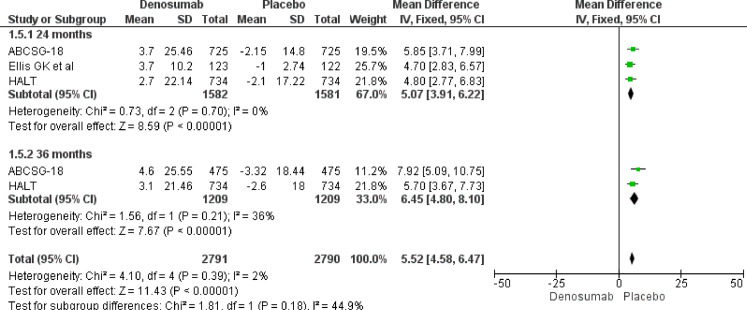
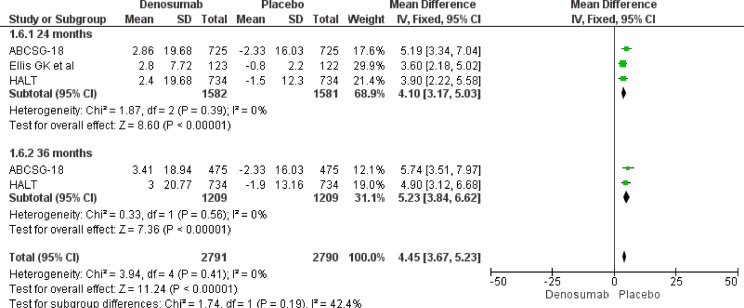
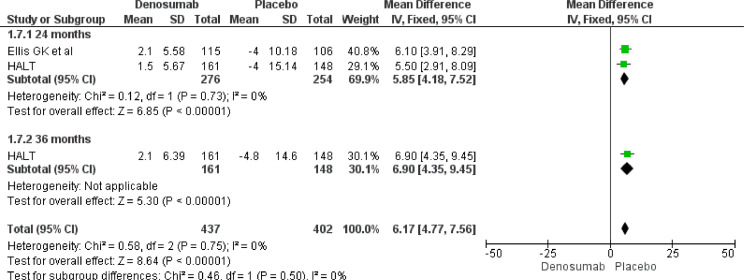
Our meta-analysis results underline a role of Denosumab in BMD increase both at 24 and 36 months. At 24 months, Denosumab showed a robust BMD increase risk at lumbar spine (MD 7.50, 95% CI 5.66–9.34), total hip (MD 5.07, 95% CI 3.91–6.22), femoral neck (MD 4.10, 95% CI 3.17–5.03) and distal third radium (MD 5.85, 95% CI 4.18–7.52). The same magnitude of effect was maintained also at 36 months at lumbar spine (MD 8.82, 95% CI 6.26–9.45), total hip (MD 6.45, 95% CI 4.80–8.10), femoral neck (MD 5.23, 95% CI 3.84–6.62) and distal third radius (MD 6.90, 95% CI 4.35–9.45) (Fig. 3, Fig. 4, Fig. 5, Fig. 6).
Fig. 3.
BMD increase at 24 and 36 months. Lumbar Spine.
Fig. 4.
BMD increase at 24 and 36 months. Total hip.
Fig. 5.
BMD increase at 24 and 36 months. Femoral neck.
Fig. 6.
BMD increase at 24 and 36 months. Distal third radius.
3.2. New fractures
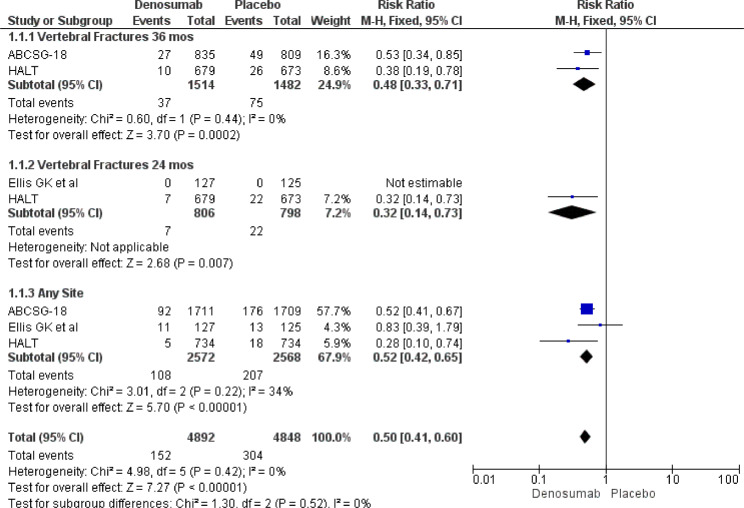
Meta-analysis pooled results defined an effective role for Denosumab in reducing new fractures risk. In particular Denosumab showed its efficacy both in any site (RR 0.52, 95% CI 0.42–0.65) and in different times (vertebral fractures at 24 months – RR 0.32, 95% CI 0.14–0.73 and vertebral fractures at 36 months RR 0.48, 95% CI 0.33–0.71) (Fig. 7).
Fig. 7.
New vertebral and any bone site fractures at 24 or 36 months.
3.2.1. Safety
Regards safety endpoints, the addiction of Denosumab did not produce any clinically relevant and statistically significant modification if compared to the placebo group. In particular, our pooled results showed that Denosumab did not affect both the SAEs risk (RR 1.06, 95% CI 0.98–1.15) and the risk of Denosumab discontinuation (RR 0.90, 95% CI 0.69–1.18) (Fig.8 – Supplementary files).
3.2.2. Risk of bias assessment
Publication bias test is required in a meta-analysis including at least 3 studies. In our analysis, Egger's test was calculated only for the Denosumab vs. placebo comparisons showing no statistical significance (p>0.05 for all outcomes) (Fig. 9 – Supplementary Files). The overall quality of included trials was also investigated following the CONSORT checklist statement. We reported an average good quality of all trials, without relevant considerations for high risk of bias (Fig.10 – Supplementary files).
4. Discussion
It is well known that adjuvant treatment with aromatase inhibitors is the first-line treatment in postmenopausal women with hormone receptor-positive breast cancer, although it represents a risk factor for the onset of osteoporosis and therefore of fragility fractures. The same occurs in men on ADT with prostate cancer. The drugs used to counteract the osteoporosis are Denosumab, Alendronate, Risedronate and Zoledronate. Denosumab is a human IgG2 monoclonal antibody produced in a mammalian cell line (CHO) by recombinant DNA technology. Denosumab binds with high affinity and specificity RANK ligand, preventing the activation of its receptor, RANK, present on the surface of osteoclasts and their precursors. The blockage of the interaction between RANKL and RANK inhibits the formation, functionality and survival of osteoclasts, reducing bone resorption both at cortical and trabecular level. It is, therefore, an anti-restorative [27]. The bisphosphonates are derivatives of the pyrophosphate to which the P–O–P bridge has been replaced with a non-hydrolysable P–C–P bridge. In particular the non-aminobiphosphonates have the nitrogen atom in an amino group. They are able to increase bone density by inhibiting the action of osteoclasts, the main target of these drugs. The activation of the osteoclast and the consequent dissolution of the hydroxyapatite triggers the liberation of the bisphosphonates previously "buried" in the bone matrix and bound to the calcium salts of the bone. Once released from the bone matrix, the drug comes into contact with the osteoclasts of which it inhibits the action. These can also be classified as antiresorptive drugs [28]. The bibliographic research carried out for our meta-analysis has identified 691 studies, this has certainly demonstrated a high interest of the scientific world regarding the effectiveness of Denosumab in the prevention of osteoporosis fragility fractures secondary to adjuvant endocrine therapy in the breast cancer and androgen deprivation in the prostate cancer. However, only 3 studies [24], [25], [26] met our inclusion criteria for a total of 5140 patients. The small number of studies represents a limitation of our meta-analysis, but at the same time the sample size of each individual study represents a strength. Three meta analyses investigated the role of bone targeted therapies and nonmetastatic cancers. O’ Carrigan et al. [29] and Hayes et al. [30] did not account endpoints directly linked to bone health such as BMD loss and BMD increase. while Alibhai SMH et al. [31] provided limited results only on 12-month BMD increase for bisphosphonates and Denosumab (only one study, only in prostate cancer setting). The results obtained suggest that Denosumab at a dose of 60 mg twice a year is able to counter the risk of osteoporosis during endocrine therapy at 24 and 36 months. It reduces the loss of BMD up to 36 months both at the lumbar and femoral level and increases the BMD both at 24 and at 36 months maintaining the same amount over time. This statement is further reinforced by another extremely important fact: the reduction in the number of new fractures both at the vertebral and femoral level at 24 and 36 months with the confirmation of a high level of safety.
5. Conclusions
Therefore therapy with Denosumab is an effective and safe treatment for the prevention of the main complications represented by vertebral and femoral fragility fractures. Studies on the anti-fracture efficacy of Denosumab for the duration of endocrine therapy would be desirable.
Acknowledgments
Acknowledgments
All authors thank to Dr. Chiara Drago for the English revision.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
All Authors declare no potential conflict of interest to disclose.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2019.100252.
Contributor Information
Antonio Galvano, Email: antoniogalvano@hotmail.it.
Dalila Scaturro, Email: dalila.scaturro@unipa.it.
Giuseppe Badalamenti, Email: giuseppe.badalamenti@unipa.it.
Lorena Incorvaia, Email: lorena.incorvaia@unipa.it.
Sergio Rizzo, Email: sergiorizzo77@gmail.com.
Luisa Castellana, Email: luisa.castellana22@gmail.com.
Stefania Cusenza, Email: stefaniacusenza1@gmail.com.
Sofia Cutaia, Email: sofia.cutaia@gmail.com.
Daniele Santini, Email: d.santini@unicampus.it.
Fiorella Guadagni, Email: fiorella.guadagni@sanraffaele.it.
Mario Roselli, Email: mario.roselli@uniroma2.it.
Stefania Gori, Email: stefania.gori@sacrocuore.it.
Mario Adelfio Latteri, Email: mario.latteri@unipa.it.
Viviana Bazan, Email: viviana.bazan@usa.net.
Letizia Mauro Giulia, Email: giulialetizia@unipa.it, giulia.letiziamauro@unipa.it.
Antonio Russo, Email: antonio.russo@usa.net.
Appendix. Supplementary materials
References
- 1.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Brewster A.M., Hortobagyi G.N., Broglio K.R., Kau S.W., Santa-Maria C.A., Arun B. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J. Natl. Cancer Inst. 2008;100(16):1179–1183. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roehl K.A., Han M., Ramos C.G., Antenor J.A., Catalona W.J. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3478 consecutive patients: long-term results. J. Urol. 2004;172(3):910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich A., Bellmunt J., Bolla M., Joniau S., Mason M., Matveev V. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur. Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Parker C., Gillessen S., Heidenreich A., Horwich A., Committee E.G. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26(Suppl 5):v69–v77. doi: 10.1093/annonc/mdv222. [DOI] [PubMed] [Google Scholar]
- 6.Loblaw D.A., Virgo K.S., Nam R., Somerfield M.R., Ben-Josef E., Mendelson D.S. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American society of clinical oncology practice guideline. J. Clin. Oncol. 2007;25(12):1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 7.Mottet N., Bellmunt J., Bolla M., Joniau S., Mason M., Matveev V. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Actas Urol. Esp. 2011;35(10):565–579. doi: 10.1016/j.acuro.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Wood D.E. National comprehensive cancer network (NCCN) clinical practice guidelines for lung cancer screening. Thorac. Surg. Clin. 2015;25(2):185–197. doi: 10.1016/j.thorsurg.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Sharifi N., Gulley J.L., Dahut W.L. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 10.Anderson W.F., Chatterjee N., Ershler W.B., Brawley O.W. Estrogen receptor breast cancer phenotypes in the surveillance, epidemiology, and end results database. Breast Cancer Res. Treat. 2002;76(1):27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 11.Aebi S., Davidson T., Gruber G., Cardoso F., Group E.G.W. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2011;22(Suppl 6):vi12–vi24. doi: 10.1093/annonc/mdr371. [DOI] [PubMed] [Google Scholar]
- 12.Burstein H.J., Prestrud A.A., Seidenfeld J., Anderson H., Buchholz T.A., Davidson N.E. American society of clinical oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J. Clin. Oncol. 2010;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillner B.E., Ingle J.N., Chlebowski R.T., Gralow J., Yee G.C., Janjan N.A. American society of clinical oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J. Clin. Oncol. 2003;21(21):4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Limburg C.E. Screening, prevention, detection, and treatment of cancer therapy-induced bone loss in patients with breast cancer. Oncol. Nurs. Forum. 2007;34(1):55–63. doi: 10.1188/07.ONF.55-36. [DOI] [PubMed] [Google Scholar]
- 15.Trémollieres F.A., Ceausu I., Depypere H., Lambrinoudaki I., Mueck A., Pérez-López F.R. Osteoporosis management in patients with breast cancer: EMAS position statement. Maturitas. 2017;95:65–71. doi: 10.1016/j.maturitas.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso F., Fallowfield L., Costa A., Castiglione M., Senkus E., Group E.G.W. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2011;22(Suppl 6):vi25–vi30. doi: 10.1093/annonc/mdr372. [DOI] [PubMed] [Google Scholar]
- 17.Forbes J.F., Cuzick J., Buzdar A., Howell A., Tobias J.S., Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 18.Wolters R., Regierer A.C., Schwentner L., Geyer V., Possinger K., Kreienberg R. A comparison of international breast cancer guidelines – do the national guidelines differ in treatment recommendations? Eur. J. Cancer. 2012;48(1):1–11. doi: 10.1016/j.ejca.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Cummings S.R., San Martin J., McClung M.R., Siris E.S., Eastell R., Reid I.R. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011. [Google Scholar]; The Cochrane Collaboration. Available from http://handbook.cochrane.org.
- 21.Jüni P., Altman D.G., Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323(7303):42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 23.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis G.K., Bone H.G., Chlebowski R., Paul D., Spadafora S., Smith J. Randomized trial of Denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J. Clin. Oncol. 2008;26(30):4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 25.Gnant M., Pfeiler G., Dubsky P.C., Hubalek M., Greil R., Jakesz R. Adjuvant Denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–443. doi: 10.1016/S0140-6736(15)60995-3. [DOI] [PubMed] [Google Scholar]
- 26.Smith M.R., Egerdie B., Hernández Toriz N., Feldman R., Tammela T.L., Saad F. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2009;361(8):745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller P.D. Denosumab: anti-RANKL antibody. Curr. Osteoporos. Rep. 2009;7(1):18–22. doi: 10.1007/s11914-009-0004-5. [DOI] [PubMed] [Google Scholar]
- 28.Martin T.J., Grill V. Bisphosphonates – mechanisms of action. Exp. Clin. Pharmacol. 2000:130–132. [Google Scholar]
- 29.O'Carrigan B., Wong M.H., Willson M.L., Stockler M.R., Pavlakis N., Goodwin A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2017;10 doi: 10.1002/14651858.CD003474.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes A.R., Brungs D., Pavlakis N. Osteoclast inhibitors to prevent bone metastases in men with high-risk, non-metastatic prostate cancer: a systematic review and meta-analysis. PLoS ONE. 2018;13(1) doi: 10.1371/journal.pone.0191455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alibhai S.M.H., Zukotynski K., Walker-Dilks C., Emmenegger U., Finelli A., Morgan S.C. Bone health and bone-targeted therapies for nonmetastatic prostate Cancer: a systematic review and meta-analysis. Ann. Intern. Med. 2017;167(5):341–350. doi: 10.7326/M16-2577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.