Abstract
Cancer cells utilize vitamin folate to fulfill their excessive demand for nucleotides and amino acids. Dihydrofolate reductase (DHFR), an enzyme involved in folate metabolism converts dihydrofolate into tetrahydrofolate, which is required for the de novo synthesis of purines, and certain amino acids. DHFR inhibitors are used as a chemotherapeutic agent. Cancer sequencing analysis has identified additional enzymes in folate metabolism that are dysregulated in cancer. Methylenetetrahydrofolate dehydrogenase 1 like (MTHFD1L), one such enzyme is overexpressed in bladder cancer. MTHFD1L is a mitochondrial enzyme involved in the folate cycle by catalyzing the reaction of formyl-tetrahydrofolate to formate and tetrahydrofolate (THF). THF is crucial for de novo purine and thymidylate synthesis and is also involved in the regeneration of methionine. Cancer cells rely on purines derived from the de novo pathway for the nucleotides whereas normal cells favor the salvage pathway. In this study we examined MTHFD1L expression in bladder cancer. By using publicly available cancer transcriptome data analysis web-portal UALCAN, we found overexpression of MTHFD1L in bladder cancer and expression was associated with overall survival. RT-PCR and immunoblot analysis confirmed the overexpression of MTHFD1L in muscle invasive bladder cancer tissues compared to normal urothelium. Furthermore, our investigations suggested a critical role for MTHFD1L in bladder cancer cell proliferation, colony formation and invasion. Thus, in this study, we show the significance of the folate metabolic enzyme MTHFD1L in aggressive bladder cancers and suggest that being an enzyme, MTHFD1L serves as a valuable therapeutic target.
Introduction
Bladder cancer is the most common malignancy of the urinary tract with estimated 80,470 new cancer cases in 2019 in the USA [1]. Bladder cancer most commonly manifests as superficial disease with high recurrence rates. However about 10–15% of tumors will progress to muscle invasive bladder cancer (MIBC) [2]. For these tumors surgical removal with or without neoadjuvant chemotherapy remains the standard treatment option [3], [4]. Once metastatic, urothelial carcinoma of the bladder is generally incurable by current chemotherapy and leads to early mortality [5]. Immune checkpoint inhibitors (e.g. atezolizumab, nivolumab, pembrolizumab) have recently provided durable benefits to a minority (∼20%) of post-platinum or selected untreated PD-L1 low cisplatin-ineligible patients and platinum-ineligible patients [6], [7], [8], [9]. Erdafitinib was recently approved for selected post-platinum patients with somatic FGFR2/3 alterations [10]. However, these agents are not curative and benefits are modest, suggesting a major role for new tolerable agents and combinations for this elderly population.
Multiple molecular alterations play a role in the progression of this aggressive disease. Recent studies have identified molecular subtypes in MIBC with different sensitivities to frontline therapy suggesting the heterogeneity in these tumors and the importance of molecular characterization of MIBC to provide effective treatment [11], [12], [13].
Cancer cells harbor specific metabolic requirements due to their enhanced proliferation rate [14], [15], [16]. One of the common dysregulated pathways in this setting is folate metabolism. Inhibitors of this pathway predominantly target dihydrofolate reductase (DHFR), an enzyme that reduces folate to dihydrofolate and converts it to tetrahydrofolate (THF). Methotrexate, a component of one of the standard regimens used to treat metastatic urothelial carcinoma, i.e. MVAC (methotrexate, vinblastine, doxorubicin, cisplatin), is a DHFR inhibitor [17], [18], [19], [20]. In the present study, we have identified and characterized another folate metabolic enzyme methylenetetrahydrofolate dehydrogenase 1 like (MTHFD1L) in bladder cancer. MTHFD1L is a mitochondrial enzyme that catalyzes the reaction of formyl-tetrahydrofolate to formate and THF [21], [22], [23]. THF is crucial for de novo purine and thymidylate synthesis and is also involved in the regeneration of methionine [24]. Cancer cells rely on purines derived from the de novo pathway for nucleotides whereas normal cells favor the salvage pathway [25], [26]. Our previous studies have shown that PAICS, an enzyme involved in de novo purine biosynthesis is overexpressed in bladder cancer and plays a critical role in bladder cancer growth [27]. Similarly, targeting enzymes involved in the associated folate pathway, like SHMT2, MTHFD2, and MTHFD1L, may harbor a therapeutic potential [28], [29]. MTHFD1L, being a potential therapeutic target overexpressed in bladder cancer, warrants further investigation.
In this study we characterized the expression and investigated the role of MTHFD1L in bladder cancer. Our studies show significant overexpression of MTHFD1L in primary bladder cancer. Furthermore, knockdown of MTHFD1L in multiple bladder cancer cells showed a decrease in cancer cell proliferation. In addition, our studies also revealed a reduced colony forming and invasion ability of bladder cancer cells upon MTHFD1L knockdown indicating the therapeutic potential of targeting this gene. Further studies are needed to target MTHFD1L with small molecule inhibitors.
Materials and Methods
Gene Expression from The Cancer Genome Atlas (TCGA)
MTHFD1L gene expression levels in bladder cancer and normal bladder were interrogated utilizing UALCAN [30], a web portal that provides boxplots depicting each gene's expression based on 3 RNA-sequencing data from the TCGA transcriptome sequencing datasets.
Benign and Bladder Cancer Tissue Samples for PCR and Immunoblot Blot Analysis
Fresh frozen MIBC (≥pT2 stage) tissue samples (T) with adjacent normal tissue (N), were obtained from the Cooperative Human Tissue Network (CHTN) based at the University of Alabama at Birmingham (UAB). CHTN complies with federal human subjects regulations (The “Common Rule;” 45 CFR part 46) to collect and distribute biospecimens. Tumor samples were obtained from patients undergoing radical cystectomy surgery without preceding neoadjuvant systemic therapy, snap frozen and stored in liquid N02 tanks (23). Specimens underwent central pathological assessment for confirmation of diagnosis. Then, macrodissection of tissue was conducted after histologic demarcation of tumor and normal bladder epithelial tissue. The study was IRB approved (IRB-120917005) at UAB.
Tissue Immunohistochemistry (IHC)
To evaluate MTHFD1L expression in bladder cancer and benign urothelium formalin-fixed, paraffin-embedded tissue sections were stained with rabbit polyclonal antibody against MTHFD1L (#16113–1-AP, PTG Labs, Rosemont, IL). For antigen retrieval tissue sections were boiled for 10 minutes in citrate buffer (#C9999-1000ML, Sigma Aldrich, MO). Immunostaining was performed using Vector Laboratories staining kit following the manufacturer's protocol. Primary antibody was added in a 1:250 dilution for 1 h at room temperature according to dilution protocol optimized in our laboratory. After washing with PBS, secondary antibody (anti-rabbit, #MP-7401 Vector Laboratories, Burlingame, CA) was added for 45 min at room temperature. Antibody signals were detected using ImmPACT DAB (#SK-4105, Vector Laboratories, Burlingame, CA). Hematoxylin QS (# H-3404, Vector Laboratories, Burlingame, CA) was used as a counterstain.
Cell Culture
RT-112 and VMCUB-1 cells were obtained from Leibniz Institut DSMZ, Germany. HT-1376, 5637, HT-1197 and T24 were purchased from ATCC. RT-112, 5637, VMCUB-1 cells were cultivated at 37 °C in a humidified environment with 5% CO2 in RPMI medium (Gibco™ RPMI 1640 Medium, Life Technologies™), while HT-1376 and HT-1197 were cultivated in MEM medium (Gibco MEM (1x), Life Technologies™) and T24 in McCoy's 5a modified medium (Gibco™ McCoy's 5A (Modified) Medium, Life Technologies™). All media was supplemented with 10% fetal bovine serum and 100 U/ml penicillin G and 100 μg/ml streptomycin.
Immunoblot Analyses
For immunoblot analysis, protein samples were separated on SDS–PAGE (NuPAGE 4–12%, # WG1402BOX, Thermo Fischer Scientific, Waltham, MA). Equal amounts of proteins were loaded and transferred for 2 h at 0.35 A onto an Immobilon1-P PVDF membrane (EMD Millipore, Billerica, MA). To block non-specific binding, the membrane was incubated for 1 h in blocking buffer (Tris-buffered saline, 0.1% Tween 20 [TBS-T], 5% nonfat dry milk) followed by incubation overnight at 4 °C with the primary antibody. After two washes with TBS-T for 5 min, the blot was incubated with horseradish peroxidase-conjugated secondary antibody (1:5000) for 1 h at room temperature. The membrane was again washed with TBS-T and TBS twice for 5 min each and signals were visualized by LuminataTM Forte Western HRP Substrate (#WBLUF0500, EMD Millipore, Billerica, MA) as per manufacturer's protocol. β-actin served as a loading control. Antibodies were implemented as follows: anti-MTHFD1L: #16113–1-AP (1:1000; PTG Labs, Rosemont, IL), anti–HRP-β-actin: # HRP-60008 (1:200000; PTG Labs, Rosemont, IL), and anti-rabbit IgG HRP: # SA00001–2 (1:5000; PTG Labs, Rosemont, IL). All antibodies were employed at dilutions optimized in our laboratory.
MTHFD1L Knockdown in Bladder Cancer Cells
MTHFD1L (siGenome SMARTpool, # M-009949-01-0005) and non-targeting small interfering RNA (siRNA) were obtained from Dharmacon (Lafayette, CO) and transfection experiments were performed following the manufacture's protocol. For transfection Lipofectamine RNAiMAX reagent (#13778150, Thermo Fisher, Waltham, MA) was applied. Cells were seeded 1 x 105 in a 6-well plate and simultaneously transfected with MTHFD1L siRNA and control non-targeting siRNA. Cells were harvested 72 h after transfection for RNA isolations and immunoblot experiments.
RNA Extraction and qRT-PCR
Total RNA from bladder cancer cells was extracted by using the Direct-zol RNA MiniPrep Plus kit (#R2071, Zymo Research, Irvine, CA) according to manufacturer's protocol. For bladder cancer tissue RNA was harvested by employing the Qiagen RNAeasy kit. Each sample was transcribed into complementary DNA by using Superscript III Reverse Transcriptase (#18080093, Thermo Fischer Scientific, Waltham, MA). For each qRT-PCR amplification, 2 μl of the cDNA product (200 ng/μl), 5 μl SYBR green PCR Master Mix (Applied Biosystems, Waltham, MA), 1 μl primer solution, and 2 μl of DNAse/RNAse free water was added for a final volume of 10 μl. Thermocycling conditions were as suggested by the manufacturer: 95° for 10 min to activate the polymerase followed by 40 cycles of 95° for 15 sec and 60° for 1 min. SYBR green was used to determine the mRNA expression level of a gene of interest. Levels were normalized to beta-Actin and analyzed using the ΔΔCT method. All primers for SYBR green were synthesized by Integrated DNA Technologies (Coralville, IA). Primer sequences are: MTHFD1L: forward primer, TGTGCCAAGGGACTTCATCT and reverse primer, AGTCCTGGCATGGTGCTC, ACTB: forward primer, GCACAGAGCCTCGCCTT, and reverse primer, GTTGTCGACGACGAGCG. All PCR reactions were performed in triplicates.
Cell Proliferation Assays
Cell proliferation was measured by cell counting. For this, transient MTHFD1L knockdowns cells were used. Non-targeting siRNA and untreated cells served as control. After 48 hours of transfection using pooled siRNA, the cells were trypsinized and seeded at a density of 1500 cells/well in 12-well plates. Then, the cells were trypsinized and counted at specified time points by Z2 Coulter particle counter (Beckman Coulter, Brea, CA). Each experiment was performed with three replicates.
Matrigel Invasion Assay
For Matrigel Invasion assay cells in medium without fetal bovine serum were seeded onto Corning BioCoat Matrigel matrix (# 354480, Corning, New York, NY) in the upper chamber of a 24-well culture plate. The lower chamber containing adequate medium was supplemented with 10% fetal bovine serum as a chemoattractant. After 48 hours, the non-invading cells and Matrigel matrix were gently removed with a cotton swab. Invasive cells located on the lower side of the chamber were fixed with 10% (v/v) glutaraldehyde (#BP25471, Fisher Scientific, Pittsburg, PA) for 15 minutes and stained with crystal violet (# HT901-8FOZ, Sigma-Aldrich, St. Louis, MO) for 15 minutes and photographed using an inverted microscope (4×).
Colony Formation Assay
After 48 hours of transfection, the various test cells were counted and seeded at a density of 1000 cells per 1 well of 6-well plates (triplicates) and incubated at 37 °C, 5% CO 2 for 6 days. Colonies were fixed with 10% (v/v) glutaraldehyde (#BP25471, Fisher scientific, Pittsburg, PA) for 15 minutes and stained with crystal violet (#HT901-8FOZ, Sigma-Aldrich, St. Louis, MO) for 15 minutes. Then, the photographs of the colonies were taken using Amersham Imager 600RGB (GE Healthcare Life Sciences, Pittsburgh, PA).
Statistical Analysis
To determine significant differences between two groups, the Wilcoxon rank sum test was applied for continuous variables using JMP® 13.1.0. For paired samples the pairwise t-test was utilized. P values <.05 were considered significant.
Results
The Mitochondrial Folate Enzyme MTHFD1L is Overexpressed in Bladder Cancer and Its Expression Predicts Poor Patient Survival
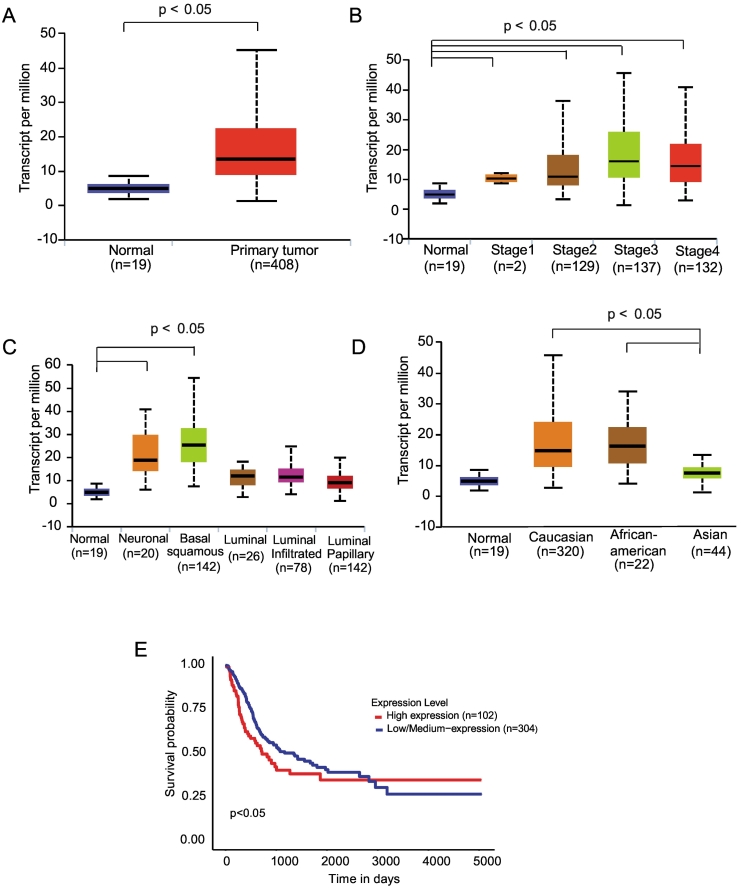
Transcriptome sequence analysis of the TCGA bladder cancer dataset revealed an overexpression of MTHFD1L in bladder cancer (Figure 1A). This overexpression was observed in all stages (Figure 1B). Looking at the molecular TCGA subtypes described by Robertson et al. [13], demonstrated higher MTHFD1L levels in the basal/squamous and neuronal subtypes (Figure 1C) and in patients with African American and Caucasian race compared to patients of Asian race (Figure 1D). Furthermore, Kaplan–Meier survival analysis indicated decreased overall survival in patients whose tumors expressed higher MTHFD1L levels (Figure 1E). The clinical and demographic characteristics of the bladder cancer samples from TCGA are given in Table 1.
Figure 1.
The folate metabolic pathway enzyme MTHFD1L is overexpressed in bladder urothelial carcinoma and predicts poor patient survival. (A) Expression of MTHFD1L in normal bladder and primary tumor samples using TCGA data analyzed by the UALCAN web portal. (B) Boxplots of MTHFD1L expression analyzed by stage. (C) Boxplots represent MTHFD1L expression level across normal bladder and molecular bladder cancer subtypes. (D) Analysis of MTHFD1L expression by race. (E) Overall survival analysis of patients with high vs low/medium MTHFD1L expression.
Table 1.
Clinical and demographic characteristics of TCGA Bladder urothelial carcinoma (BLCA) samples categorized based on MTHFD1L expression level (Clinical and demographic information are not available for few samples)
| Clinical/demographic parameters | TCGA BLCA samples with high MTHFD1L expression (n = 102) | TCGA BLCA samples with low/medium MTHFD1L expression (n = 304) | |
|---|---|---|---|
| Gender | Male | 76 (74.51%) | 219 (72.04%) |
| Female | 26 (25.49%) | 79 (25.99%) | |
| Race | Caucasian | 89 (87.25%) | 230 (75.66%) |
| African American | 5 (4.9%) | 17 (5.59%) | |
| Asian | 4 (3.92%) | 39 (12.83%) | |
| Pathologic N | N0 | 61 (59.8%) | 175 (57.57%) |
| N1 | 11 (10.78%) | 25 (8.22%) | |
| N2 | 14 (13.73%) | 32 (10.53%) | |
| N3 | 13 (12.75%) | 62 (20.39%) | |
| NX | 1 (0.98%) | 6 (1.97%) | |
| Pathologic T | T0 | 0 | 1 (0.33%) |
| T1 | 1 (0.98%) | 2 (0.66%) | |
| T2 | 5 (4.9%) | 32 (10.53%) | |
| T2a | 3 (2.94%) | 22 (7.24%) | |
| T2b | 16 (15.69%) | 40 (13.16%) | |
| T3 | 14 (13.73%) | 29 (9.54%) | |
| T3a | 17 (16.67%) | 52 (17.11%) | |
| T3b | 29 (28.43%) | 52 (17.11%) | |
| T4 | 4 (3.92%) | 6 (1.97%) | |
| T4a | 6 (5.88%) | 37 (12.17%) | |
| T4b | 0 | 5 (1.64%) | |
| TX | 0 | 1 (0.33%) | |
| Stage | Stage I | 0 | 2 (0.66%) |
| Stage II | 26 (25.49%) | 102 (33.55%) | |
| Stage III | 45 (44.12%) | 92 (30.26%) | |
| Stage IV | 31 (30.39%) | 100 (32.89%) | |
| Histology | Papillary | 21 (20.59%) | 109 (35.86%) |
| Non-papillary | 78 (76.47%) | 193 (63.49%) | |
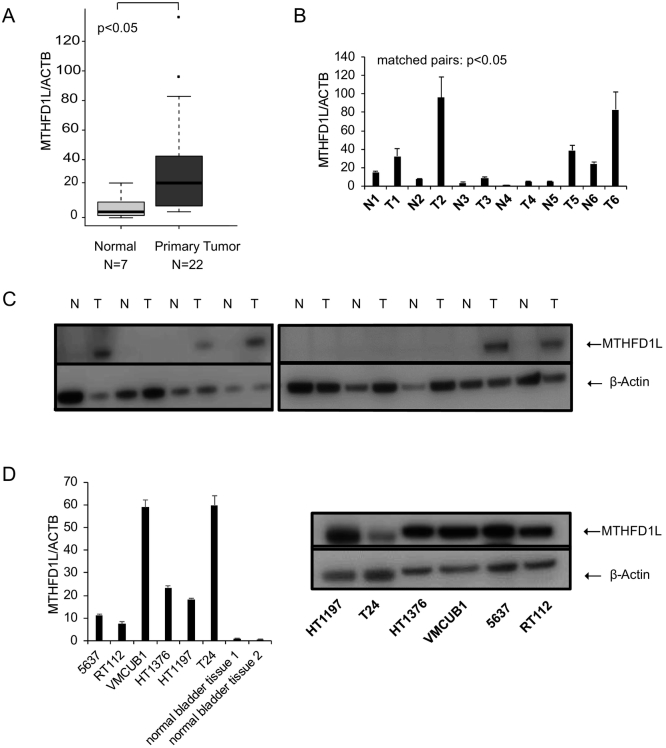
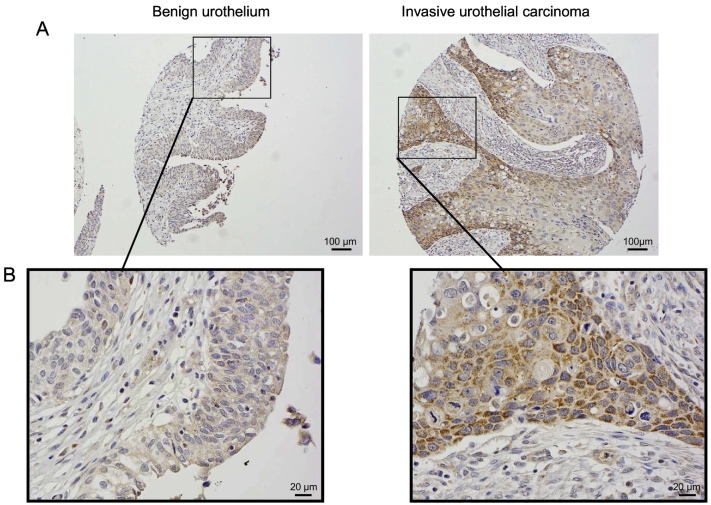
Next, we validated these in silico findings by performing additional analysis. We used bladder cancer tissue and adjacent normal bladder tissue for further analysis. qRT-PCR and immunoblot analysis of bladder tissue using a specific antibody showed overexpression of MTHFD1L in bladder cancer compared to normal bladder tissue (Figure 2, A and C). We found that in all cases with available corresponding uninvolved tissue, MTHFD1L mRNA expression and protein levels to be higher in the malignant tissue compared to normal tissue (Figure 2, B and C). Similarly, elevated levels of MTHFD1L were observed in bladder cancer cell lines compared to normal bladder tissue (Figure 2D). To visualize the localization of MTHFD1L in bladder cancer and paired benign tissue, we performed IHC showing a granular cytoplasmic staining pattern (Figure 3).
Figure 2.
MTHFD1L expression in bladder cancer tissue and cell lines.
(A) QRT-PCR analysis in normal bladder and primary bladder urothelial carcinoma. (B) MTHFD1L mRNA levels in cases with available matched normal bladder and tumor tissues. (C) Immunoblot analysis showing MTHFD1L expression in matched normal bladder and tumor tissues. β-actin served as a loading control. (D) MTHFD1L mRNA and protein levels in various bladder cancer cell lines by qRT-PCR and immunoblot analysis.
Figure 3.
Immunohistochemical analysis of MTHFD1L in urothelial carcinoma of bladder. Overview (A) and detailed photo micrographic (B) depiction of MTHFD1L imunnohistochemical staining in invasive urothelial carcinoma and paired benign urothelium.
Knockdown of MTHFD1L Inhibits Bladder Cancer Cell Proliferation
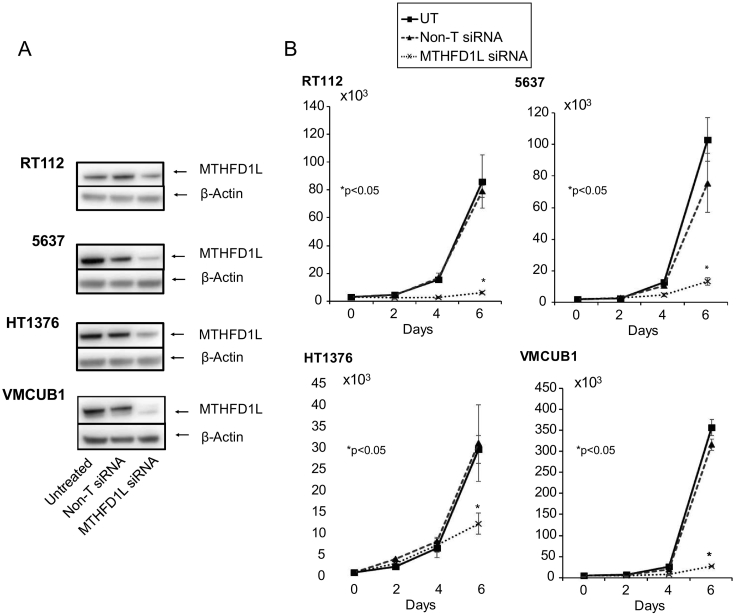
Since folate metabolism is a critical requirement for the cancer cells, we went on to evaluate the role of MTHFD1L using multiple bladder cancer cell lines. To investigate the role of MTHFD1L in bladder cancer cell proliferation, we performed RNA interference using highly specific pooled siRNA in the bladder cancer cell lines 5637, RT112, HT1376, and VMCUB-1. The knockdown efficiency of MTHFD1L was evaluated by performing immunoblot analysis (Figure 4A). Proliferation experiments were performed over a period of 6 days. Our results indicated a dramatic decrease in cancer cell growth upon knockdown in all four bladder cancer cell lines (Figure 4B). This experiment clearly shows the requirement of MTHFD1L for cancer cell proliferation.
Figure 4.
MTHFD1L regulates bladder cancer cell proliferation.
(A) Immunoblot analysis showing MTHFD1L protein in RT112, 5637, HT1376, and VMCUB1 cells transfected with a MTHFD1L siRNA or control non-targeting siRNA (non-T siRNA) and untreated cells. (B) Transient knockdown of MTHFD1L reduces cell proliferation of bladder cancer cell lines RT1122, 5637, HT1376, and VMCUB1.
MTHFD1L Regulates Bladder Cancer Cell Colony Formation and Invasion
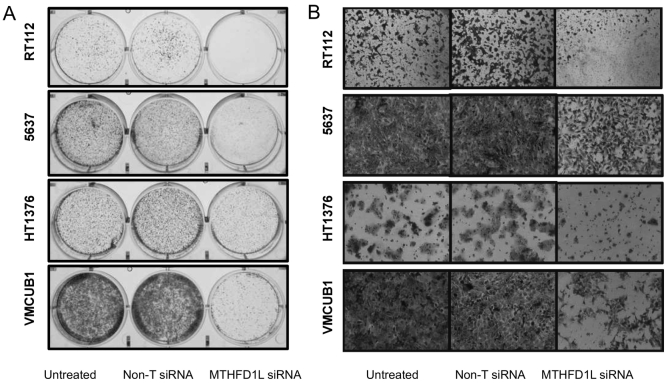
Next, in order to further investigate the role of MTHFD1L in other oncogenic phenotypes, we performed cancer cell colony formation and invasion assays. MTHFD1L knockdown resulted in formation of fewer colonies compared to non-targeting and untreated cells (Figure 5A). Boyden Chamber Matrigel invasion assay (Figure 5B) showed reduced cancer cell invasion upon MTHFD1L knockdown suggesting potential role of MTHFD1L in bladder cancer invasion and metastasis.
Figure 5.
MTHFD1L regulates bladder cancer cell colony formation and invasion.
(A) Photomicrographs of colony formation assay using untreated, non-targeting siRNA (non-T siRNA) and MTHFD1L siRNA bladder cancer lines RT1122, 5637, HT1376, and VMCUB-1 (B) MTHFD1L siRNA-treated bladder cancer cells showed reduced invasion in Boyden chamber Matrigel invasion assay. Invaded cells were stained with crystal violet.
Discussion
Alterations in cancer cell energetics and metabolism is one of the emerging hallmarks in cancer [14]. It is a field of active investigations in cancer and serves as an avenue to establish new treatment options. Cancer cells have a higher metabolic rate than benign cells [14], [15]. Due to increased growth rates and proliferation, tumor cells show an increased demand for nucleotides and amino acids. One of the main pathways that provide these nutrients is the folate cycle (also known C1 metabolism or one carbon metabolism), which helps to supply cells with one-carbon (C1) groups needed for amino acids and nucleotide synthesis [29], [31]. In the initial step of folate metabolism folate is converted to dihydrofolate (DHF) and further to tetrahydrofolate (THF) by the enzyme dihydrofolate reductase (DHFR) [32]. THF can then be used for DNA synthesis. Many chemotherapeutics, such as methotrexate act by compromising the folate metabolism through inhibition of DHFR. However, given that normal cells also require THF, these dugs cause non-specific toxicity in both cancer cells and in non-cancerous cells. As C1 metabolism has a mitochondrial and cytoplasmic component, studies have hypothesized that targeting only the mitochondrial pathways could potentially cause less side effects, as a parallel pathway in the cytoplasm exists [32]. The mitochondrial enzymes involved in the folate cycle are MTHFD1L, ALDH1L2, SHMT2, AMT and MTHFD2 [24], [33].
In our study, by performing in silico analysis, we show a significant upregulation of MTHFD1L in bladder cancer. We validated these findings by performing RT-PCR, immunoblot and immunohistochemistry using bladder cancer tissue mRNA and protein lysates. The TCGA cancer RNA sequencing data using the UALCAN web portal indicated decreased overall survival in bladder cancer patients with high MTHFD1L expression [30]. To investigate the biological significance of MTHFD1L we conducted proliferation, colony formation and invasion assays by knocking down MTHHFD1L in bladder cancer cell lines. We showed that knockdown of MTHFD1L resulted in decreased proliferation, reduced colony forming and invasion, all of which suggest a critical role of this enzyme in regulating bladder cancer growth and invasion. Earlier studies have shown that MTHFD1L is upregulated in esophageal squamous cell carcinoma [34]. Investigations in hepatocellular carcinoma (HCC) indicated that MTHFD1L is transcriptionally upregulated through nuclear factor (erythroid-derived2)-like 2 (NRF2), which is a key player in the activation of antioxidant genes under oxidative stress. This suggests that metabolic reprogramming in cancer cells produce antioxidants for neutralizing high levels of reactive oxygen species (ROS) [24], [35]. Furthermore, the folate cycle was shown to be a major supplier of nicotinamide adenine dinucleotide phosphate (NADPH) which is an important antioxidant [36], [37]. Thus inhibition of the folate cycle resulted in reduced ROS anti-oxidant production, which induces intracellular oxidative stress and was shown to inhibit HCC growth [36].
Through its involvement in the folate cycle MTHFD1L is not only involved in purine and thymidylate synthesis but also helps cells in the methionine cycle [24]. S-Adenosylmethionine (SAM) is an important methyl donor utilized by histone and DNA methyltransferases [38]. In prostate cancer the availability of SAM was shown to lead to aggressive prostate cancer [39]. Thus, reduced THF levels through MTHFD1L inhibition potentially impair cells not only by reducing de novo purine and thymidine synthesis, but also through its involvement in the methionine cycle.
In summary we found that overexpression of MTHFD1L in bladder cancer portends to decreased overall survival. Furthermore, our studies show that MTHFD1L plays a critical role in bladder cancer growth and invasion, which suggests its potential as a valuable therapeutic target. Future studies will focus on rationally developing small molecule inhibitor to target MTHFD1L, as single agents and in combination with chemotherapy and checkpoint inhibitors, in biomarker selected patients with high tumor MTHFD1L expression.
Acknowledgements
This study was supported by institutional funds (Department of Pathology and School of Medicine of the University of Alabama at Birmingham) awarded to SV.
Footnotes
Competing interests: Guru Sonpavde: Consultant for BMS, Exelixis, Bayer, Sanofi, Pfizer, Novartis, Eisai, Janssen, Amgen, Astrazeneca, Merck, Genentech, EMD Serono, Astellas/Agensys; Research support to institution from Astrazeneca, Bayer, Amgen, Boehringer-Ingelheim, Janssen, Merck, Sanofi, Pfizer; Author for Uptodate; Steering committee for Astrazeneca, BMS, Bavarian Nordic; Speaker for Onclive; Research to Practice; Physician Education Resource (PER). The other authors declare that they have no conflict of interest.
Funding/support: UAB O'Neal Comprehensive Cancer Center Developmental fund
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Hedegaard J, Lamy P, Nordentoft I, Algaba F, Hoyer S, Ulhoi BP, Vang S, Reinert T, Hermann GG, Mogensen K. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell. 2016;30:27–42. doi: 10.1016/j.ccell.2016.05.004. doi. [DOI] [PubMed] [Google Scholar]
- 3.Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, Lotan Y, Meeks JJ, Michalski JM, Morgan TM. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. 2017;198:552–559. doi: 10.1016/j.juro.2017.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, Manley B, Spiess PE, Poch MA, Sexton WJ. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 2018;4:1535–1542. doi: 10.1001/jamaoncol.2018.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. 23/21/4602 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 8.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017 doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonpavde G. PD-1 and PD-L1 inhibitors as salvage therapy for urothelial carcinoma. N Engl J Med. 2017 doi: 10.1056/NEJMe1701182. [DOI] [PubMed] [Google Scholar]
- 10.Siefker-Radtke AO, Necchi A, Park SH, GarcÃa-Donas Js, Huddart RA, Burgess EF, Fleming MT, Rezazadeh A, Mellado B, Varlamov S. First results from the primary analysis population of the phase 2 study of erdafitinib (ERDA; JNJ-42756493) in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRalt) J Clin Oncol. 2018;36:4503. [Google Scholar]
- 11.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConkey DJ, Choi W, Ochoa A, Siefker-Radtke A, Czerniak B, Dinney CP. Therapeutic opportunities in the intrinsic subtypes of muscle-invasive bladder cancer. Hematol Oncol Clin North Am. 2015;29:377–394, x-xi. doi: 10.1016/j.hoc.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA, Akbani R. Comprehensive molecular characterization of muscle-invasive bladder. Cancer Cell. 2017;171:540–556 e525. doi: 10.1016/j.cell.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Locasale JW, Cantley LC. Metabolic flux and the regulation of mammalian cell growth. Cell Metab. 2011;14:443–451. doi: 10.1016/j.cmet.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberti MV, Locasale JW. The Warburg Effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, Yu EY, Powles T, Moshier EL, Ladoire S. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121:2586–2593. doi: 10.1002/cncr.29387. [DOI] [PubMed] [Google Scholar]
- 18.Holmboe L, Andersen AM, Morkrid L, Slordal L, Hall KS. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br J Clin Pharmacol. 2012;73:106–114. doi: 10.1111/j.1365-2125.2011.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis JL., Jr. Choriocarcinoma: a success story for chemotherapy. Int J Radiat Oncol Biol Phys. 1980;6:897–898. doi: 10.1016/0360-3016(80)90333-8. [DOI] [PubMed] [Google Scholar]
- 20.Hertz R, Li MC, Spencer DB. Effect of methotrexate therapy upon choriocarcinoma and chorioadenoma. Proc Soc Exp Biol Med. 1956;93:361–366. doi: 10.3181/00379727-93-22757. [DOI] [PubMed] [Google Scholar]
- 21.Pike ST, Rajendra R, Artzt K, Appling DR. Mitochondrial C1-tetrahydrofolate synthase (MTHFD1L) supports the flow of mitochondrial one-carbon units into the methyl cycle in embryos. J Biol Chem. 2010;285:4612–4620. doi: 10.1074/jbc.M109.079855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walkup AS, Appling DR. Enzymatic characterization of human mitochondrial C1-tetrahydrofolate synthase. Arch Biochem Biophys. 2005;442:196–205. doi: 10.1016/j.abb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 24.Lee D, Xu IM, Chiu DK, Lai RK, Tse AP, Lan Li L, Law CT, Tsang FH, Wei LL, Chan CY. Folate cycle enzyme MTHFD1L confers metabolic advantages in hepatocellular carcinoma. J Clin Invest. 2017;127:1856–1872. doi: 10.1172/JCI90253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natsumeda Y, Prajda N, Donohue JP, Glover JL, Weber G. Enzymic capacities of purine de novo and salvage pathways for nucleotide synthesis in normal and neoplastic tissues. Cancer Res. 1984;44:2475–2479. [PubMed] [Google Scholar]
- 26.Yamaoka T, Kondo M, Honda S, Iwahana H, Moritani M, Ii S, Yoshimoto K, Itakura M. Amidophosphoribosyltransferase limits the rate of cell growth-linked de novo purine biosynthesis in the presence of constant capacity of salvage purine biosynthesis. J Biol Chem. 1997;272:17719–17725. doi: 10.1074/jbc.272.28.17719. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarthi B, Rodriguez Pena MDC, Agarwal S, Chandrashekar DS, Hodigere Balasubramanya SA, Jabboure FJ, Matoso A, Bivalacqua TJ, Rezaei K, Chaux A. A role for de novo purine metabolic enzyme PAICS in bladder cancer progression. Neoplasia. 2018;20:894–904. doi: 10.1016/j.neo.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pikman Y, Puissant A, Alexe G, Furman A, Chen LM, Frumm SM, Ross L, Fenouille N, Bassil CF, Lewis CA. Targeting MTHFD2 in acute myeloid leukemia. J Exp Med. 2016;213:1285–1306. doi: 10.1084/jem.20151574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, Huang J, Asplund A, Mootha VK. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun. 2014;5:3128. doi: 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia (New York, NY) 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Momb J, Lewandowski JP, Bryant JD, Fitch R, Surman DR, Vokes SA, Appling DR. Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice. Proc Natl Acad Sci U S A. 2013;110:549–554. doi: 10.1073/pnas.1211199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koseki J, Konno M, Asai A, Colvin H, Kawamoto K, Nishida N, Sakai D, Kudo T, Satoh T, Doki Y. Enzymes of the one-carbon folate metabolism as anticancer targets predicted by survival rate analysis. Sci Rep. 2018;8:303. doi: 10.1038/s41598-017-18456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kory N, Wyant GA, Prakash G, Uit de Bos J, Bottanelli F, Pacold ME, Chan SH, Lewis CA, Wang T, Keys HR. SFXN1 is a mitochondrial serine transporter required for one-carbon metabolism. Science. 2018;362 doi: 10.1126/science.aat9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang YS, Yuan Y, Hu WP, Shang QX, Chen LQ. The role of mitochondrial folate enzyme MTHFD1L in esophageal squamous cell carcinoma. Scand J Gastroenterol. 2018;53:533–540. doi: 10.1080/00365521.2017.1407440. [DOI] [PubMed] [Google Scholar]
- 35.Xu IM, Lai RK, Lin SH, Tse AP, Chiu DK, Koh HY, Law CT, Wong CM, Cai Z, Wong CC. Transketolase counteracts oxidative stress to drive cancer development. Proc Natl Acad Sci U S A. 2016;113:E725–E734. doi: 10.1073/pnas.1508779113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee D, Wong CC. The folate cycle is a new metabolic weakness of cancer. Mol Cell Oncol. 2017;4 doi: 10.1080/23723556.2017.1327004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X, Locasale JW, Reid MA. Serine and methionine metabolism: vulnerabilities in lethal prostate cancer. Cancer Cell. 2019;35:339–341. doi: 10.1016/j.ccell.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reina-Campos M, Linares JF, Duran A, Cordes T, L'Hermitte A, Badur MG, Bhangoo MS, Thorson PK, Richards A, Rooslid T. Increased serine and one-carbon pathway metabolism by PKClambda/iota deficiency promotes neuroendocrine prostate cancer. Cancer Cell. 2019;35:385–400 e389. doi: 10.1016/j.ccell.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]