Abstract
Background
Apathy is the neuropsychiatric syndrome that correlates most highly with Huntington's disease progression, and, like early patterns of neurodegeneration, is associated with lesions to cortico-striatal connections. However, due to its multidimensional nature and elusive etiology, treatment options are limited.
Objectives
To disentangle underlying white matter microstructural correlates across the apathy spectrum in Huntington's disease.
Methods
Forty-six Huntington's disease individuals (premanifest (N = 22) and manifest (N = 24)) and 35 healthy controls were scanned at 3-tesla and underwent apathy evaluation using the short-Problem Behavior Assessment and short-Lille Apathy Rating Scale, with the latter being characterized into three apathy domains, namely emotional, cognitive, and auto-activation deficit. Diffusion tensor imaging was used to study whether individual differences in specific cortico-striatal tracts predicted global apathy and its subdomains.
Results
We elucidate that apathy profiles may develop along differential timelines, with the auto-activation deficit domain manifesting prior to motor onset. Furthermore, diffusion tensor imaging revealed that inter-individual variability in the disruption of discrete cortico-striatal tracts might explain the heterogeneous severity of apathy profiles. Specifically, higher levels of auto-activation deficit symptoms significantly correlated with increased mean diffusivity in the right uncinate fasciculus. Conversely, those with severe cognitive apathy demonstrated increased mean diffusivity in the right frontostriatal tract and left dorsolateral prefrontal cortex to caudate nucleus tract.
Conclusions
The current study provides evidence that white matter correlates associated with emotional, cognitive, and auto-activation subtypes may elucidate the heterogeneous nature of apathy in Huntington's disease, as such opening a door for individualized pharmacological management of apathy as a multidimensional syndrome in other neurodegenerative disorders.
Keywords: Apathy, Diffusion MRI, Huntington's disease, Individual differences, Neurodegeneration, White matter microstructure
Graphical abstract
Highlights
-
•
Global Apathy is a biomarker of Huntington's disease progression.
-
•
Symptoms in a specific apathy domain appear prior to motor onset.
-
•
Global apathy is related to specific dorsal and ventral cortico-striatal tracts.
-
•
Cortico-striatal tracts selectively predict specific apathy domains.
1. Introduction
Huntington's disease (HD) is an autosomal dominant, neurodegenerative disorder caused by a cytosine-adenine-guanine polyglutamine expansion in the HTT gene (MacDonald et al., 1993). Typically manifesting in mid-adulthood, the disease is characterized by progressive motor and cognitive deficits as well as neuropsychiatric symptoms such as apathy.
Apathy represents one of the most common psychiatric symptoms in HD. Occurring at a prevalence of 52–75% in HD individuals (Paoli et al., 2017), apathy constitutes a significant burden on the quality of life of patients and caregivers. Additionally, apathy have been shown to act as a biomarker of disease progression (Craufurd et al., 2001; Kingma et al., 2008; Thompson et al., 2012; Tabrizi et al., 2013; van Duijn et al., 2014; Fritz et al., 2018). However, other studies using comprehensive psychiatric scales not specific to HD have revealed that many indices increase with disease severity, including depression, anxiety, and obsessive-compulsiveness (Duff et al., 2007) as well as ‘frontal’ behaviors such as disinhibition and executive dysfunction in addition to apathy (Duff et al., 2010). Nonetheless, an understanding of the expression patterns and potential underlying mechanisms of apathy in HD is essential to clinicians providing care to those impacted by apathy across neurodegenerative disorders.
Apathy is a multidimensional, transdiagnostic syndrome characterized by decreased motivation with a quantitative reduction in goal-directed behaviors. Traditionally, apathy has been associated with cortico-striatal connections, specifically ventral, limbic loops (Tekin and Cummings, 2002; Thompson et al., 2002; Bonelli and Cummings, 2007; Delmaire et al., 2013). This has also been posited in HD populations, where early degeneration has been targeted to cortico-basal ganglia networks (Camacho et al., 2018). However, recent reviews have associated apathy to a wider range of neural substrates specific to three domains: cognitive, emotional, and auto-activation deficit (Levy and Dubois, 2006; Pagonabarraga et al., 2015). Cognitive apathy, described as inertia of executive functioning needed to elaborate an objective-oriented behavioral plan (e.g., “I find it difficult to organize future goals”), has been posited to involve the dorsolateral prefrontal cortex and caudate nucleus. On the other hand, emotional apathy is defined as a blunting in affect not attributable to depression (e.g., “I feel indifference for many issues that I was previously interested in”) and relates more closely with lesions to the orbitomedial prefrontal cortex, anterior cingulate cortex, amygdala, and ventral striatum. The third domain refers to auto-activation deficit, which translates to difficulties in self-activating thoughts or behavior (e.g., “I need a push to get started on things”) (Levy and Dubois, 2006; Pagonabarraga et al., 2015). Auto-activation deficit may represent the most severe form of apathy, and is also seen in form of akinetic mutism, abulia, and Laplane syndrome (Lhermitte et al., 1986; Bonelli and Cummings, 2007). Neurologically, it is encountered in individuals with large frontal and basal ganglia lesions (Levy and Dubois, 2006).
Neuroimaging studies using diffusion tensor imaging (DTI) have demonstrated potential for parsing out white matter correlates of apathy in HD. Measures such as mean diffusivity (MD) and fractional anisotropy (FA) function as proxies to study white matter microstructural properties that include not only demyelination accompanying neurodegeneration, but also incipient tissue swelling and redistribution of fluid that occurs at earlier stages of the disease (Sen and Basser, 2005). However, such studies have yielded inconsistent results (Delmaire et al., 2013; Gregory et al., 2015; McColgan et al., 2017), perhaps due to the great variability among HD individuals in the degree and evolution of apathy symptoms. One possible source of individual differences in apathy symptomology could be explained by variability in the degree of neurodegeneration of different neural circuits. In this regard, neuroimaging studies can contribute to the understanding of the neurobiological basis of phenotypic heterogeneity in terms of tract-specific white matter microstructure. To date, however, there has been no study investigating the relationship between apathy subtypes and white matter connectivity in HD. Disentangling the white matter correlates associated with emotional, cognitive, and auto-activation subtypes in HD may elucidate the picture further, clarifying potential profiles of the disease across the apathy spectrum and as such facilitating the diagnosis and treatment of apathy.
Aligning reviews by Levy and Dubois (2006), Bonelli and Cummings (2007), Pagonabarraga et al. (2015), we consider apathy as a multidimensional transdiagnostic syndrome that may be represented by white matter dysfunction in specific cortico-striatal tracts. First, the frontostriatal tract (FST) and the dorsolateral prefrontal cortex to caudate nucleus tract (dlPFC-cn) are hypothesized to be more involved in cognitive apathy in addition to general apathy. The FST connects the dorsal caudate to the presupplementary motor area, a region anatomically and functionally associated with motor preparation as well as non-motor, cognitive tasks (Nachev et al., 2008; Morris et al., 2016). In regard to the dlPFC-cn, the cortical dlPFC projection is involved predominantly in executive function (Thompson et al., 2002) and emotional regulation (Davidson et al., 2000).
Conversely, the uncinate fasciculus (UF) is a ventral associative bundle, putatively involved in emotional processing, that connects the anterior temporal lobe with the amygdala and orbitofrontal cortex (Catani et al., 2002; Von Der Heide et al., 2013). Indeed, it has recently been shown that changes in gray matter volume in the amygdala and temporal lobe, as well as decreased glucose metabolism in the anterior cingulate and ventromedial PFC, play a critical role in apathy severity in HD (Martinez-Horta et al., 2018). All such regions constitute the medial orbitofrontal circuit, a cortico-striatal loop that has been directly associated with general apathy (Tekin and Cummings, 2002; Thompson et al., 2002) and which may be involved in emotional apathy.
The last subtype, auto-activation deficit, is associated with more widespread neural correlates. These include terminations of both the FST and UF. With regards to the FST, both the medial superior frontal gyrus and caudate nucleus are cited as anatomical involvements of auto-activation deficit (Pagonabarraga et al., 2015), and the caudate nucleus is associated with planning and execution of self-generated novel action (Monchi et al., 2006). Likewise, the orbitofrontal cortex, to which the UF projects, is associated with deficits in auto-activation (Levy and Dubois, 2006). Given this evidence, we hypothesize that disturbances in FST or UF white matter may be related with auto-activation deficit.
With regards to global apathy, we utilize an exploratory approach in all three fronto-cortico-striatal tracts, namely the cognitively-oriented FST and dlPFC-cn as well as the limbic UF.
The present exploratory study aims to investigate the neural bases underlying individual differences in both global apathy and its three subtypes in HD. As such, we explored the relationship between apathy and white matter microstructure of cortico-striatal connections. To do so, we virtually dissected specific cortico-striatal tracts in vivo in order to characterize the FST, dlPFC-cn, and UF. We hypothesized that these discrete cortico-striatal tracts are associated with both global apathy and distinct apathy domains. Specifically, we predicted a positive relationship between white matter microstructure disturbance and cognitive apathy in the dorsal, cognitively-associated FST and dlPFC-cn and, comparatively, a positive relationship between emotional apathy and disturbance of the ventrally located UF. Finally, we predicted that a more severe disruption of the FST and UF tracts would evince a positive association with increased levels of auto-activation deficit.
2. Methods
2.1. Participants
Participants' demographics are detailed in Table 1. Forty-six HD gene-expansion carriers and 35 healthy control participants who matched for age (t(79) = 0.05, P = .959), sex (t(69.8) = 1.65, P = .103), and years of education (t(78) = −1.31, P = .194) participated in this study. Control participant data was utilized in two stages of the analysis: 1) to compare apathy levels and 2) to explore white matter microstructure disturbance.
Table 1.
Sociodemographic and clinical characteristics of study participants.
| Control | Manifest | Premanifest | P (Cohen's d) | |
|---|---|---|---|---|
| Na | 35 | 24 | 22 | - |
| Sex (f/m) | 18/17 | 14/10 | 18/4 | .103 |
| Age (years) | 44.00 ± 11 | 51.00 ± 9.5 | 36.64 ± 8.7 | .959 |
| Education (years) | 12.74 ± 2.7 | 10.71 ± 2.6 | 13.29 ± 2.6, N = 21 | .194 |
| UHDRS-TMS | - | 22.13 ± 12 | 1.524 ± 3.0, N = 21 | <.001⁎ (2.362) |
| UHDRS-cogscore | - | 183.1 ± 58, N = 21 | 299.1 ± 65, N = 19 | <.001⁎ (1.939) |
| CAP | - | 113.86 ± 18 | 80.78 ± 16, N = 21 | <.001⁎ (1.956) |
| TFC | - | 11.17 ± 2.0 | 12.76 ± 0.70, N = 21 | .001⁎ (1.066) |
Data presented as mean ± standard deviation. P-values refer to independent two-tailed t-tests between all Huntington's disease gene-expansion carriers combined vs. controls (gray background) and premanifest vs. manifest Huntington's disease gene-expansion carriers (white background). Cohen's d is displayed for significant results.
N = number of participants; f = females; m = males; UHDRS-cogscore = Unified Huntington's Disease Rating Scale total cognitive score; UHDRS-TMS = Unified Huntington's Disease Rating Scale total motor score (Huntington Study Group, 1996); CAP = standardized age-CAG product (Ross et al., 2014); TFC = Total Functional Capacity.
P-values retained significance after false discovery rate correction (q = 0.05).
Number of participants listed in individual cells when differing from this number for each group.
HD individuals were grouped into premanifest (N = 22) and manifest (N = 24) stages based on their Unified Huntington's Disease Rating Scale (UHDRS) diagnostic confidence score for motor abnormalities (Huntington Study Group, 1996).
Despite the fact that HD is clinically diagnosed based on motor onset, pathological changes are often present long before motor symptoms (Thompson et al., 2012; Martinez-Horta et al., 2016). As such, when examining the association between white matter microstructure and apathy subtypes, we studied the disease as a continuum.
Antidepressants are widely used to treat mood disturbances in HD, and tetrabenazine and benzodiazapines are prescribed to manage motor symptoms. However, such medications may worsen apathy severity (Frank, 2014). As such, medication use (SSRIs, SARIs, NASSAs, NSRIs, NDRIs, benzodiazapines, tetrabenazines, antipsychotics, and anti-epileptics) was recorded in order to create a binary code delineating the presence or absence of medication at the visit that may affect mood or apathy scores.
Due to time constrains, not all tests were administered to all participants. The specific N is detailed for each test. One control and two HD participants did not receive a diffusion-weighted image scan due to claustrophobia. Furthermore, outliers whose Z-scores were greater than |3.5| were excluded. No participants reported previous history of traumatic brain injury or neurological disorder other than HD. The study was approved by the ethics committee of Bellvitge Hospital in accordance with the Helsinki Declaration of 1975 and all participants provided written informed consent.
2.2. Clinical evaluation
All HD participants underwent the UHDRS evaluation (Huntington Study Group, 1996), which comprises subscales for motor function (UHDRS-TMS) and cognition (UHDRS-cogscore).
In order to further describe the HD sample, we defined the standardized CAG-Age Product (CAP) score for each group, computed as CAP = 100 × age × (CAG – 35.5) / 627 (Ross et al., 2014). In addition, total functional capacity (TFC) was employed as a measure of independence in daily activities, ranging from thirteen (full capacity) to zero (total incapacity) (Huntington Study Group, 1996). TFC has been shown to be reliable based on radiographic measures of HD progression (Young et al., 1986). All evaluations were carried out by neuropsychologists and psychiatrists specializing in movement disorders.
2.3. Neuropsychiatric assessment
Previous literature has recommended concurrent implementation of multiple apathy questionnaires in order to promote a more consistent evaluation of apathy across studies (Passamonti et al., 2018). As such, we implemented both the short forms of the Problem Behavior Assessment (PBA-s) and the Lille Apathy Rating Scale (LARS-s). The PBA-s was specifically designed for neuropsychiatric evaluation in HD individuals; however, it cannot be decomposed to measure multiple aspects of apathy, which has been recommended in recent studies (Fritz et al., 2018; Misiura et al., 2019). The comprehensive nature of the LARS-s, in contrast, allows a more in-depth assessment of apathy than that which is available in the PBA-s or other commonly used neuropsychiatric assessments such as the Frontal System Behavior Scale and Neuropsychiatric Inventory (Duff et al., 2010; Reijnders et al., 2010; Clarke et al., 2011), while maintaining applicability for practical and reliable use in everyday clinical practice (Dujardin et al., 2013).
2.3.1. Problem behavior assessment, short-form
The short form of the Problem Behavior Assessment (PBA-s) was designed for the evaluation of neuropsychiatric symptoms in HD (Craufurd et al., 2001; Kingma et al., 2008). The PBA-s consists of eleven items, from which three main components measuring apathy, irritability, and affective symptoms have been identified (Callaghan et al., 2015). Following this delineation, we calculated apathy and affective components by summing the corresponding PBA-scores (frequency × severity) of the corresponding items, where ‘apathy’ is the sum of lack of initiative, perseverative thinking or behavior, and disoriented behavior, and ‘affective behavior’ is the sum of depressed mood, suicidal ideation, and anxiety. Higher scores indicate more severe/frequent symptoms.
2.3.2. Lille apathy rating scale, short-form
Global apathy is measured as the total score of the Lille Apathy Rating Scale, short-form (LARS-s) (Sockeel et al., 2006; Dujardin et al., 2013). The LARS-s global score ranges from −15 to +15, in which higher scores represent a greater degree of apathy. The cut-off for clinically relevant global apathetic syndromes is defined as a score greater than −7 (Dujardin et al., 2013). We measured apathy domains by combining items into cognitive apathy (everyday productivity, interests), emotional apathy (novelty seeking, emotional responses), and auto-activation deficit (initiative, motivation). These domains of apathy were categorized based on previous apathy literature (Levy and Dubois, 2006; Starkstein and Leentjens, 2008; Robert et al., 2009; Pagonabarraga et al., 2015).
2.4. MRI data acquisition
MRI data were acquired through a 3T whole-body MRI scanner (Siemens Magnetom Trio; Hospital Clínic, Barcelona), using a 32-channel phased array head coil. Structural images were comprised of conventional high-resolution 3D T1 image [magnetization-prepared rapid-acquisition gradient echo sequence, 208 sagittal slices, repetition time 1970 ms, echo time 2.34 ms, inversion time 1050 ms, flip angle 9°, field of view 25.6 cm, 1 mm isotropic voxel with no gap between slices].
Diffusion-weighted MRI data were acquired using a dual spin-echo DTI sequence with GRAPPA (reduction factor of 4) cardiac gating, with echo time 92 ms. Images were measured using 2 mm isotropic voxels, no gap, 60 axial slices, field of view 23.6 cm. In order to obtain the diffusion tensors, diffusion was measured along 64 non-collinear directions, using a single b-value of 1500 s/mm2 interleaved with 9 non-diffusion (b = 0) images. To avoid chemical shift artifacts, frequency-selective fat saturation was used to suppress fat signal.
2.5. DW-MRI tractography analysis
2.5.1. Preprocessing of DTI data
Brain extraction was performed using the FSL Brain Extractor Tool (Smith, 2002). Head motion and eddy-current correction were then performed using the FMRIB's Diffusion Toolbox (FDT) in FMRIB's Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl/fdt) and the gradient matrix was rotated (Leemans and Jones, 2009). The diffusion tensor was then reconstructed using Diffusion Toolkit's least-squares estimation algorithm for each voxel provided in Diffusion Toolkit (http://www.trackvis.org/dtk) and its corresponding eigenvalues and eigenvectors were extracted to calculate FA and MD maps.
Fiber orientation distributions were reconstructed using a spherical deconvolution approach based on the damped version of the Richardson-Lucy algorithm (Dell'Acqua et al., 2010) implemented in StarTrack software (http://www.natbrainlab.co.uk). Fiber orientation distribution fields in selected a priori fiber crossing regions (splenium of the corpus callosum and corona radiata) were first visualized. Then, a combination of spherical deconvolution parameters was selected to resolve crossing and avoid spurious peaks in gray matter or cerebral spinal fluid (fixed fiber response corresponding to a shape factor of α = 2 × 10–3 mm2/s; 200 algorithm iterations, regularization threshold ƞ = 0.04 and regularization geometric parameter v = 8). (See Dell'Acqua et al. (2010), for further details.)
Whole-brain tractography was then performed using a b-spline interpolation of the diffusion tensor field and Euler integration to propagate streamlines following the directions of the principal eigenvector with a step size of 0.5 mm (Basser et al., 2000). Tractography was started in the different regions of interest and was stopped when FA < 0.2 or when the angle between two consecutive tractography steps was larger than 35°. Finally, tractography data and diffusion tensor maps were exported into TrackVis (http://www.trackvis.org) for manual dissection of the tracts.
2.5.2. Tractography dissections
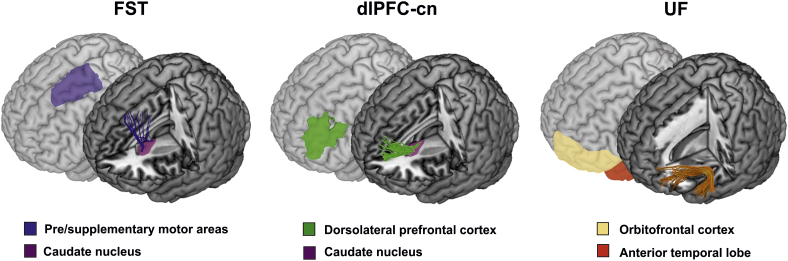
Virtual in vivo DTI dissections of the three tracts of interest were carried out bilaterally in the native space FA maps and a two-region of interest (ROI) approach (Catani et al., 2002; Catani and Thiebaut de Schotten, 2008; Craig et al., 2009). These include two cortico-striatal tracts, namely the FST and dlPFC-cn, and the cortico-striato-cortical UF (Fig. 1).
Fig. 1.
Three cortico-striatal tracts of interest.
Virtual in vivo dissections of frontal cortico-striatal projections for one participant, rendered onto the Montreal Neurological Institute template. Where applicable, tracts are shown projecting to the subcortical region of interest (caudate nucleus). Cortical regions of interest are delineated in shadow images, behind. Relevant region of interest are labeled below each respective tract. From left to right: FST (frontostriatal tract; pre/supplementary motor areas to caudate nucleus tract), dlPFC-cn (dorsolateral prefrontal cortex to caudate nucleus), and UF (uncinate fasciculus; anterior temporal lobe to orbitofrontal cortex).
To dissect the FST, fibers projecting from the ROI containing the presupplementary and supplementary motor areas, as defined on the axial plane anterior to the hand knob region following anatomical guidelines described by Catani et al. (2012), were restricted to terminate within the caudate nucleus ROI. The caudate nucleus ROIs were segmented by using the FSL FIRST toolbox (Nugent et al., 2013) and then registered to the individual native diffusion space using the FSL FLIRT (Jenkinson and Smith, 2001) and FNIRT (Andersson et al., 2007) modules after normalizing both the structural T1 images and FA maps.
To dissect the dlPFC-cn, fibers projecting from the dlPFC ROI were restricted to terminate within the caudate nucleus area. The dlPFC ROIs were defined based on (Yi et al., 2016) using the Sallet Dorsal Frontal Connectivity Based Parcellation Atlas Clusters 5, 6, and 7 (Brodmann areas 9/46 dorsal, 9/46 ventral, and 46) in FSL and subsequent transformation to the native FA space for each participant.
To dissect the UF, the ROIs were manually defined on the color FA images of each participant to include white matter of the anterior temporal lobe and external capsule, based on previous in-house tractography guidelines (Sierpowska et al., 2015; François et al., 2016).
To blind dissectors to participant identity, controls and patients were randomized. Importantly, due to the presence of marked atrophy in prefrontal regions, the dorsal tracts were unable to be segmented in all patients. Specifically, the FST was not dissected in twelve (four right, four left, four bilaterally) and the dlPFC-cn in ten (one right, seven left, two bilaterally) out of 45 HD individuals with diffusion data. For those participants in which segmentation was possible, FA and MD values were extracted for analysis of white matter microstructure.
2.6. Statistical analyses
Statistical analyses were performed in SPSS v.24 (SPSS Inc., Chicago, USA). Independent two-tailed t-tests and Cohen's d were used to describe clinical and sociodemographic differences between groups (Cohen, 1977; Lakens, 2013), each surveyed for homogeneity of variance. Pearson's correlations were used to assess associations between apathy levels, clinical markers, or white matter microstructure. In order to study differences in apathy between premanifest, manifest, and control groups, we employed one-way ANOVA with post-hoc comparisons.
Prior to investigating the relationship between structural connectivity and apathy, we examined the level of white matter disturbance within each dissected tract of manifest HD patients compared with controls (independent two-tailed t-tests). Specifically, we examined extracted FA and MD values of the FST, UF, and dlPFC-cn bilaterally. Only those tracts that were affected were further investigated. Lastly, we studied the association between structural connectivity and apathy using Pearson's correlations. When studying global apathy via the PBA-s and LARS-s, we utilized the PBA-s ‘affective behavior’ component as a covariate of no interest in order to examine apathy as an independent psychiatric syndrome from depression. (For a similar approach, see Reyes et al. (2009), Martinez-Horta et al. (2016), and Misiura et al. (2019).) Similarly, when studying cognitive apathy, UHDRS-cogscore and TFC effects were also controlled.
The false discovery rate (FDR) approach was used to correct all t-tests and correlations for multiple comparisons based on the number of tracts tested (q = 0.05). The number of comparisons is specified in each analysis. Both raw P-values (P) and the P-adjusted FDR values (P-adj) are reported. Differences were considered statistically significant when P-adj ≤ 0.05.
3. Results
3.1. Behavioral results
Up to 45% of HD gene-expansion carriers demonstrated clinical apathy (PBA-s apathy >2). HD participants also presented PBA-s scores of >2 for perseverative phenomena (33%), depressed mood (29%), anxiety (22%), irritability (22%), angry or aggressive behavior (11%), obsessive-compulsive behaviors (11%), delusions/paranoid thinking (6.7%), hallucinations (4.4%), suicidal ideation (4.4%), and disoriented behavior (2.2%). The overall apathy scores of the LARS-s and the PBA-s significantly correlated (r = 0.33, P = .038, N = 39), supporting the use of the LARS-s to measure apathy in HD. Furthermore, the cognitive apathy domain demonstrated a significant negative correlation with both TFC (r = −0.35, P = .036, P-adj = 0.049, N = 36) and UHDRS-cogscore (r = −0.46, P = .008, P-adj = 0.032, N = 32), while the TFC also presented a significant relationship with the LARS-s (r = −0.48, P = .003, P-adj = 0.012, N = 36) and auto-activation deficit (r = −0.35, P = .037, P-adj = 0.049, N = 36) (four comparisons; four apathy scales × one clinical measure). As a control analysis, the PBA-s affective behavior sub-score did not show such a relationship with TFC nor UHDRS-cogscore. UHDRS-TMS did not demonstrate a significant correlation with any behavioral markers.
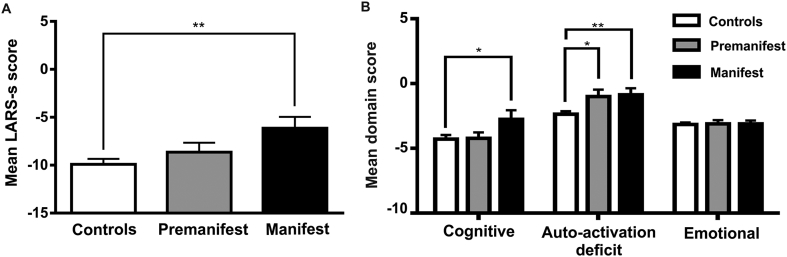
For each scale and subscale, both manifest and premanifest participants presented more severe apathy on average than control participants, with manifest patients demonstrating the most severe apathy. One-way ANOVA demonstrated that these group differences were significant after correction for four comparisons for the global LARS-s (F(2,66) = 5.12, P = .009, P-adj = 0.018, ηp2 = 0.134) and auto-activation deficit (F(2,66) = 5.25, P = .008, P-adj = 0.018, ηp2 = 0.137), whereas differences in cognitive apathy did not remain significant (F(2,66) = 3.13, P = .050, P-adj = 0.067 ηp2 = 0.087) (Table 2; Fig. 2). Post-hoc comparisons revealed a significant difference between controls and manifest patients for the global LARS-s (P = .002), auto-activation deficit (P = .005), and cognitive apathy (P = .022). Premanifest participants, however, only significantly differed from controls in the auto-activation deficit domain (P = .017) (Table 2; Fig. 2), while manifest patients exemplified a tendency toward more severe global apathy and cognitive apathy than premanifest participants (P = .070 and P = .055, respectively).
Table 2.
Behavioral results for three participant groups.
| Control | Premanifest | Manifest | P | |
|---|---|---|---|---|
| LARS-s % clinically relevanta | −9.90 ± 3.1, N = 31 12.9% | −8.65 ± 4.1, N = 17 29.4% | −6.14 ± 5.4, N = 21 52.2% | .009⁎ |
| Cognitive apathyb | −4.29 ± 1.8 | −4.24 ± 1.9 | −2.76 ± 3.2 | .050 |
| Auto-activation deficitb | −2.36 ± 1.2 | −1.00 ± 2.2 | −0.86 ± 2.3 | .008⁎ |
| Emotional apathyb | −3.16 ± 0.90 | −3.12 ± 1.2 | −2.95 ± 1.3 | .279 |
| PBA-s, apathyc | - | 4.76 ± 8.6, N = 21 | 8.08 ± 7.6, N = 24 | - |
| PBA-s, affectivec | - | 4.91 ± 5.9 | 2.63 ± 3.29 | - |
Data presented as mean ± standard deviation. Greater or more positive numbers indicate more severe behavioral symptoms. P-values refer to one-way ANOVA between controls, premanifest, and manifest groups.
N = number of participants; LARS-s = Lille Apathy Rating Scale, short-from; PBA-s = Problem Behavior Assessment, short-form.
P-values retained significance after false discovery rate correction (q = 0.05).
Cut-off for clinically relevant global apathetic syndromes is defined as a total LARS-s score > −7 (Dujardin et al., 2013).
Subscale computed from the LARS-s.
Measured based on ‘apathy’ and ‘affective behavior’ components of factor analysis in Callaghan et al. (2015).
Fig. 2.
Levels of apathy across participant groups.
Differences in global apathy (A) and three apathy domains (B) between controls, premanifest, and manifest Huntington's disease gene-expansion carriers, shown as mean ± standard error of the mean. Global apathy is measured by the short-form Lille Apathy Rating Scale (LARS-s; range -15 to +15), where larger and more positive scores indicate more severe apathy. *P-value < .05, **P-value < .01 after controlling for multiple comparisons.
Similarly, when manifest and premanifest participants were pooled together, the group maintained significant differences compared to the control group in the global LARS (t(63.2) = 2.69, P = .009, P-adj = 0.018, Cohen's d = 0.63) and auto-activation deficit (t(59.2) = 3.44, P = .001, P-adj = 0.004, Cohen's d = 0.80). It should be noted that while the premanifest and manifest groups differed in age, this factor could not explain apathy levels across these groups (Supplementary Table 1). There were no significant differences in global apathy measures (LARS-s t(30) = 0.63, P = .990, PBA-apathy (t(34) = 1.25, P = .261), mood scores (PBA-dep (t(34) = 0.43, P = .956), and apathy subtypes (cognitive apathy(t(30) = −0.90, P = .263, activation deficit t(30) = 0.218, P = .193) when comparing HD individuals on or off the specified medications. However, emotional apathy showed a trend that was not significant after multiple comparison correction (t(78) = 1,82, P = .015, P-adj = 0.09, six comparisons; six behavioral measures). Seven HD participants and five controls did not complete the apathy scales. One HD patient did not submit demographic data for years of education.
3.2. Differences in structural connectivity between Huntington's disease patients and controls
Manifest HD patients overall showed lower mean FA values and higher mean MD values in the majority of tracts when compared with controls, demonstrating disturbed white matter microstructure (Supplementary Table 2). Specifically, there was a significant increase in MD values in the right UF (t(54) = 3.32, P = .002, P-adj = 0.006, Cohen's d = 0.92), the right FST (t(47) = 2.77, P = .008, P-adj = 0.016, Cohen's d = 0.82), and the dlPFC-cn in both the left (t(38) = 2.21, P = .033, P-adj = 0.050, Cohen's d = 0.72) and right hemispheres (t(50) = 5.18, P < .001, Cohen's d = 1.50) (six comparisons; three tracts × two hemispheres). Post-hoc one-tailed analyses examining differences in structural connectivity between all HD participants and controls replicated these results in three of the four tracts, again in MD values: right dlPFC-cn (t(68.7) = 3.02, P = .002), right UF (t(74) = 2.27, P = .013), and right FST (t(62) = 1.81, P = .038), with the left dlPFC-cn showing a trend (t(55) = 1.39, P = .086).
3.3. Association between structural connectivity and apathy domains
When examining the association with global apathy, we first employed the PBA-s as an apathy measure in order to more closely parallel previous literature (Delmaire et al., 2013; Gregory et al., 2015). Significant correlations were found between structural connectivity and global apathy in that patients with higher PBA-s apathy values exemplified increased MD in the right FST (r = 0.46, P = .005, P-adj = 0.020, N = 36) (four comparisons; four tracts × one apathy scale).
Post-hoc comparisons controlling for CAP, age, and sex maintained significance in the right FST (r = 0.43, P = .014). Further controlling for affective behavior strengthened the relationship in the right FST (r = 0.67, P < .001) and revealed a similar relationship between apathy levels and structural connectivity in the right UF (r = 0.36, P = .026). No significant correlations were found between global apathy scores and white matter connectivity in the dlPFC-cn.
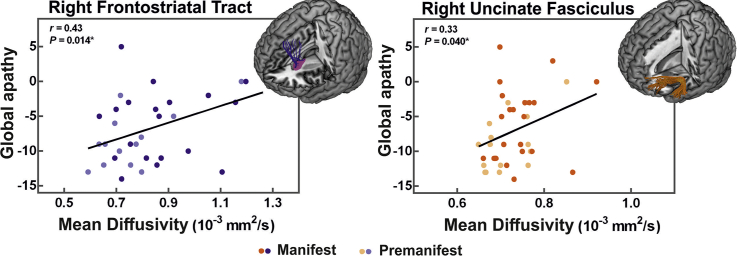
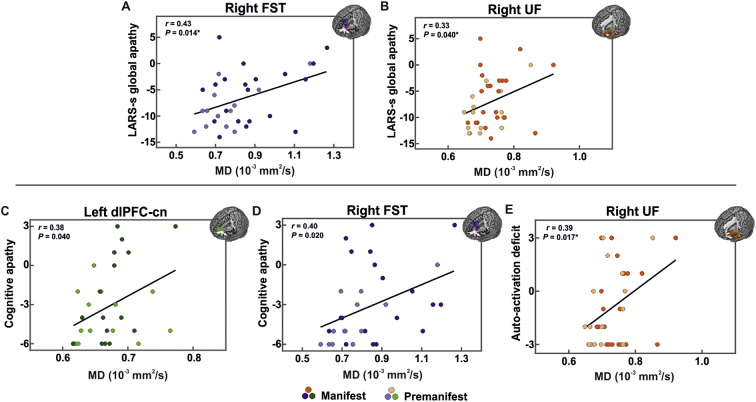
Following these findings, we reproduced these relationships in the right FST and right UF in HD participants using the LARS-s. Specifically, HD individuals with higher LARS-s apathy values exemplified increased MD in both the right FST (r = 0.43, P = .014, P-adj = 0.028, N = 33) and the right UF (r = 0.33, P = .040, P-adj = 0.040, N = 38) (two comparisons; two tracts × one apathy scale; Fig. 3A,B). We again controlled for CAP, age, and sex, revealing a trend toward significance in the right FST (r = 0.32, P = .095). This trend was maintained when additionally controlling for affective behavior in the right FST (r = 0.31, P = .106).
Fig. 3.
Positive relationship between apathy levels and white matter disturbance in the three tracts of interest.
Bivariate plot displaying the significant association between global apathy and mean diffusivity (MD) in the (A) right frontostriatal tract (FST) and (B) right uncinate fasciculus (UF). Apathy is measured with the short-form Lille Apathy Rating Scale (LARS-s), ranging from −15 to +15, where larger and more positive scores indicate more severe apathy. Larger MD values indicate more severe damage to white matter microstructure. Bivariate plots displaying the associations between apathy domains and the MD of the dorsolateral prefrontal cortex to caudate nucleus tract (dlPFC-cn), FST, and UF, respectively. Cognitive apathy demonstrated a strong association with both the left dlPFC-cn (C) and right FST (D), while auto-activation deficit exemplified a significant relationship with the right UF (E). Correlation coefficients (r) and raw P-values (P) for each hemisphere are shown in upper left. Linear regression line is fit for each scatterplot to aid interpretation. *P-value survives false discovery rate correction at q = 0.05.
In relation to apathy domains, higher cognitive apathy was associated with increases in MD in the left dlPFC-cn (r = 0.38, P = .040, P-adj = 0.060, N = 30) and right FST (r = 0.40, P = .020, P-adj = 0.060, N = 33) (three comparisons; three cognitive tracts × one apathy scale; Fig. 3C,D). When controlling or CAP, age, and sex, we found that this trend was maintained in both the left dlPFC-cn (r = 0.36, P = .071) and right FST (r = 0.34, P = .071). Additionally controlling for UHDRS-cogscore and TFC strengthened the relationship in the right FST (r = 0.51, P = .010), while removing the association in the left dlPFC-cn.
We investigated the relationship between auto-activation deficit and the structural connectivity of both the right FST and right UF. While the FST showed only a trend toward significance in increased right MD (r = 0.33, P = .058, N = 33), the UF manifested a strong positive relationship between right MD and the auto-activation deficit score (r = 0.39, P = .017, P-adj = 0.034, N = 38), which survived correction for two comparisons (two tracts × one apathy scale; Fig. 3E). Controlling for CAP, age, and sex maintained this association in the right UF (r = 0.36, P = .038), while decreasing the association in the right FST (r = 0.26, P = .170). The UF was not significantly associated with emotional apathy.
Lastly, in order to verify that these correlations were specific to our hypothesized apathy subtypes, we performed control correlations of the dlPFC-cn with auto-activation deficit, both the dlPFC-cn and FST with emotional apathy, and the UF with cognitive apathy. No significant correlations were found.
4. Discussion
The present exploratory study aimed to disentangle the different dimensions of apathy in HD individuals in relation to discrete white matter tracts and potential apathy profiles, namely emotional, cognitive, and auto-activation deficit subtypes. In particular, manifest HD patients showed significantly higher levels of global apathy and auto-activation deficit compared with controls, whereas premanifest individuals presented higher levels of auto-activation deficit only. We next hypothesized that inter-individual variability in the disruption of discrete cortico-striatal tracts could explain the differential severity of each apathy profile. Indeed, higher levels of global apathy correlated with a lateralized increased MD in the right FST and right UF. We further revealed that domain-specific apathy correlated with an increase in MD in the dlPFC-cn and FST for the cognitive domain of apathy and in the UF for auto-activation deficit, also predominantly on the right side.
Overall, we found that increases in apathy were significantly associated with HD progression, as measured by TFC. This finding corroborates the relationship that has consistently been established between increasing apathy and lower TFC (Thompson et al., 2002; Hamilton et al., 2003; Naarding et al., 2009; van Duijn et al., 2014). Such a relationship indicates that apathy may be a suitable biomarker for potentially capturing the spectrum of neurodegeneration.
Affective behavior, however, was not found to correlate with TFC. This supports previous studies showing that, in contrast with apathy, neuropsychiatric symptoms such as anxiety, irritability, and depression do not exhibit a consistent relationship with disease progression or duration, instead manifesting at any stage of the disease progression (Craufurd et al., 2001; Thompson et al., 2002; Naarding et al., 2009) or even showing a reduction over time (Thompson et al., 2012). Secondly, cognitive function, as measured by UHDRS-cogscore, bore a specific relationship with levels of cognitive apathy, but not global apathy.
Compared to controls, both premanifest and manifest groups presented more severe apathy on average. However, it is important to point out that premanifest individuals presented significantly higher levels of auto-activation deficit than controls. This emphasizes the gradual nature of disease onset, which affects neuropsychiatric functioning years or even decades prior to formal disease diagnosis by motor onset (Ross et al., 2014; Martinez-Horta et al., 2016).
Moreover, our findings suggest that specific apathy domains may develop along differential timelines in HD, with auto-activation deficit emerging prior to formal diagnosis. Thus, early in the disease process, difficulty in self-activating thoughts or behavior can signal disease progression. Additionally, levels of cognitive apathy and global apathy were significantly increased in manifest patients when compared with the control group. The fact that significantly higher levels of apathy domains were specific to auto-activation deficit and cognitive apathy supports findings that apathy in HD is particularly associated with disease evolution in cognitive and functional modalities (Hamilton et al., 2003; Naarding et al., 2009), but not necessarily in motor symptomology.
Regarding the association between MD and apathy subtypes, our findings highlight that damage to white matter microstructure in specific tracts may directly contribute to the severity of precise apathy domains in HD. First, those with elevated MD in left dlPFC-cn white matter presented higher levels of cognitive apathy. In support of this finding, the gray matter regions of the dlPFC circuit have been specifically associated with cognitive apathy previously, including cognitive regions of the basal ganglia such as the dorsal caudate in addition to the dlPFC itself (Levy and Dubois, 2006; Pagonabarraga et al., 2015).
Second, the right FST also exhibited increased MD in association with higher cognitive apathy levels. To the best of our knowledge and unlike the dlPFC-cn, the cortical terminations of the FST have not yet been cited as being involved in cognitive apathy. Nonetheless, the presupplementary motor area shares connections with the dlPFC and, furthermore, is also functionally associated with non-motor, cognitive processing (Nachev et al., 2008; Morris et al., 2016). In addition, preparatory motor regions involved in action anticipation have been found to be associated with the behavioral apathy domain using the LARS (Bonnelle et al., 2016). This proposes the FST as a suitable candidate for involvement in the cognitive form of apathy. Anatomically, cognitive or dorsal territories of the caudate nucleus, to which the FST projects (Riley et al., 2011; Robinson et al., 2012), are described as being involved in this apathy subtype (Levy and Dubois, 2006), and the supplementary motor area is specifically referenced as involved in auto-activation deficit (Pagonabarraga et al., 2015) and apathy (Bonnelle et al., 2016). In addition, the presupplementary motor area shares a key role in voluntary action (Nachev et al., 2008). Imaging studies report greater (Jenkins et al., 2000) and earlier (Cunnington et al., 2002) activation in this cortical region when participants perform internally initiated movements in contrast to those generated by external cues and instruction.
Third, heightened MD in the UF also portrayed higher levels of auto-activation deficit, also in the right hemisphere. Gray matter targets of the UF have explicitly been cited as being involved in auto-activation deficit, specifically the ventral anterior cingulate cortex, anterior prefrontal cortex, and limbic territories of the basal ganglia including the ventral caudate nucleus (Levy and Dubois, 2006). Although its function is rather poorly understood (Catani and Thiebaut de Schotten, 2008), the UF is often considered a limbic tract involved in emotional processing (Catani et al., 2002; Von Der Heide et al., 2013). However, we did not find a relationship between UF white matter and emotional apathy. This suggests that another neural correlate may be more associated with emotional apathy in HD. Alternatively, it is also possible that participants in our sample simply did not present sufficiently high or variable degrees of emotional apathy, as this subtype may affect more advanced stages of the disease.
The fact that only MD was related to apathy severity and disease progression markers suggests that the results may be attributed to atrophy (Steventon et al., 2016) or white matter pathology that may be restricted to damage to tissue microstructures, representing different pathophysiological processes, whether due to fiber reorganization, increased membrane permeability, destruction of intracellular compartments, or glial alterations (Beaulieu, 2002; Acosta-Cabronero et al., 2010). Another intriguing result is the rightward bias observed in those tracts that show a significant association with apathy. Of the four associations found between white matter and apathy subtypes, three involved the right hemisphere, with only the dlPFC-cn tract showing a left-localized relationship with cognitive apathy. Interestingly, the relationship between apathy and right rather than left hemisphere structural damage has been demonstrated in a number of patient populations, such as in Parkinson's disease (Aarsland et al., 1999; Bogdanova and Cronin-Golomb, 2012), frontotemporal dementia (Peters et al., 2006; Mendez et al., 2008; Zamboni et al., 2008), Alzheimer's disease (Lanctôt et al., 2007), and brain damage patients (Andersson et al., 1999) (but see Joseph, 1999; Wager et al., 2003; Roth et al., 2004; Bruen et al., 2008). Tying these results with the rightward trend observed in our paper (both in microstructure effect and its association with apathy), we propose that this rightward bias in white matter microstructural abnormalities is an underlying contributor to the prevalent and progressive levels of apathy throughout HD.
Distinct from larger HD networks, our study can take advantage of a tailored methodology, employing specific apathy scales and an optimized DTI protocol in order to study the syndrome precisely and consider the underlying heterogeneity of HD at the individual level. This methodology is currently not available in other studies with HD individuals. However, it should be noted that, while the current analysis aims to elucidate apathy as a syndrome, the PBA-s affective behavior covariate is not capable of teasing out the full range of depressive symptoms that may confound the evaluation of apathy. Future studies should focus on utilizing more comprehensive neuropsychiatric measures to control for depressive features, and may also consider controlling for obsessive-compulsive behaviors in addition to depressed mood, suicidal ideation, and anxiety. On the other hand, future research may also consider addressing clinically relevant comorbid mental disorders as defined by current diagnostic manuals rather than simply controlling for neuropsychiatric symptoms, a limitation of the present study. Furthermore, while we have attempted to account for the presence or absence of specific medications (including psychotropic drugs), the heterogeneous nature of drug classes and variability in dosage and duration make it difficult to fully explain medication effects that may enhance or engender apathetic symptoms. Lastly, given the small group size, the present study is limited as an exploratory analysis, and replication in a larger independent sample is needed. This is especially important given that DTI data is inherently noisy, potentially resulting in reduced power when detecting abnormalities related to atrophy in white matter microstructure. Therefore, further studies need to be carried out in the future using large-scale HD cohorts (TRACK-HD, PREDICT-HD, IMAGE-HD) in order to better understand the neural correlates involved in the spectrum of apathy.
The present findings are highly relevant in corroborating the progressive nature of apathy as a biomarker in HD while also pioneering research on the disentanglement of apathy profiles in HD. As such, these results bear implications for a differential diagnosis of apathy subtypes and, subsequently, more individualized pharmacological management. For example, cholinesterase inhibitors (which increase acetylcholine levels) or cholinergic precursors has been shown to improve cognitive performance and thus may be preferred in the treatment of cognitive apathy, while methylphenidate (which increases levels of catecholamines) or dopaminergic agonists may be reserved for behavioral aspects of apathy in HD (Nobis and Husain, 2018), such as auto-activation deficit. As a whole, this research contributes to the elucidation of potential profiles of HD, which is currently diagnosed solely as a progressive movement disorder (Rosas et al., 2008), yet whose patients would benefit from profile development due to the heterogeneous nature of symptom presentation and patterns of degeneration (Georgiou et al., 1999; Friedman et al., 2005; Gómez-Esteban et al., 2007; Tippett et al., 2007; Thu et al., 2010).
Funding
This work was supported by the Instituto de Salud Carlos III, which is an agency of the MINECO, co-funded by FEDER funds/European Regional Development Fund (ERDF) – a way to Build Europe (CP13/00225 and PI14/00834, both to EC). This study has been also funded by Generalitat de Catalunya, Awarded Research Group 2017 SGR 1573. We thank CERCA Programme/Generalitat de Catalunya for institutional support.
Declaration of Competing Interests
None.
Acknowledgments
The authors are grateful to the patients and their families for their participation in this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101965.
Appendix A. Supplementary data
Supplementary material
References
- Aarsland D., Larsen J.P., Lim N.G., Janvin C., Karlsen K., Tandberg E. Range of neuropsychiatric disturbances in patients with Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 1999;67:492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Cabronero J., Williams G.B., Pengas G., Nestor P.J. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain. 2010;133:529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- Andersson S., Krogstad J.M., Finset A. Apathy and depressed mood in acquired brain damage: relationship to lesion localization and psychophysiological reactivity. Psychol. Med. 1999;29:447–456. doi: 10.1017/s0033291798008046. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S., Andersson J. FMRIB Technial Report TR07JA2. 2007. Non-linear registration aka spatial normalisation. [Google Scholar]
- Basser P.J., Pajevic S., Pierpaoli C., Duda J., Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bogdanova Y., Cronin-Golomb A. Neurocognitive correlates of apathy and anxiety in Parkinson's disease. Park Dis. 2012;2012:1–9. doi: 10.1155/2012/793076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli R.M., Cummings J.L. Frontal-subcortical circuitry and behavior. Dialogues Clin. Neurosci. 2007;9:141. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V., Manohar S., Behrens T., Husain M. Individual differences in premotor brain systems underlie behavioral apathy. Cereb. Cortex. 2016;26(2):807–819. doi: 10.1093/cercor/bhv247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruen P.D., McGeown W.J., Shanks M.F., Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain J. Neurol. 2008;131:2455–2463. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- Callaghan J., Stopford C., Arran N., Boisse M.-F., Coleman A., Santos R.D. Reliability and factor structure of the short problem behaviors assessment for Huntington's disease (PBA-s) in the TRACK-HD and REGISTRY studies. J. Neuropsychiatr. Clin. Neurosci. 2015;27:59–64. doi: 10.1176/appi.neuropsych.13070169. [DOI] [PubMed] [Google Scholar]
- Camacho M., Barker R.A., Mason S.L. Apathy in Huntington's disease: a review of the current conceptualization [internet] J. Alzheimer's Dis. Park. 2018:08. https://www.omicsonline.org/open-access/apathy-in-huntington8217s-disease-a-review-of-the-current-conceptualization-2161-0460-1000431-100935.html [cited 2018 Oct 26] Available from. [Google Scholar]
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo interactive dissection of white matter fasiculi in the human brain. NeuroImage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M., Dell'Acqua F., Vergani F., Malik F., Hodge H., Roy P. Short frontal lobe connections of the human brain. Cortex. 2012;48:273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Clarke D.E., Ko J.Y., Kuhl E.A., van Reekum R., Salvador R., Marin R.S. Are the available apathy measures reliable and valid? A review of the psychometric evidence. J. Psychosom. Res. 2011;70:73–97. doi: 10.1016/j.jpsychores.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Academic Press; 1977. CHAPTER 2 - The t test for means; pp. 19–74. Revised Edition. [Google Scholar]
- Craig M.C., Catani M., Deeley Q., Latham R., Daly E., Kanaan R. Altered connections on the road to psychopathy. Mol. Psychiatry. 2009;14:946–953. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Craufurd D., Thompson J.C., Snowden J.S. Behavioral changes in Huntington disease. Neuropsychiatry Neuropsychol. Behav. Neurol. 2001;14:219–226. [PubMed] [Google Scholar]
- Cunnington R., Windischberger C., Deecke L., Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. NeuroImage. 2002;15:373–385. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Putnam K.M., Larson C.L. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua F., Scifo P., Rizzo G., Catani M., Simmons A., Scotti G. A modified damped Richardson–Lucy algorithm to reduce isotropic background effects in spherical deconvolution. NeuroImage. 2010;49:1446–1458. doi: 10.1016/j.neuroimage.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Delmaire C., Dumas E.M., Sharman M.A., van den Bogaard S.J.A., Valabregue R., Jauffret C. The structural correlates of functional deficits in early huntington's disease: structural correlates of functional deficits in HD. Hum. Brain Mapp. 2013;34:2141–2153. doi: 10.1002/hbm.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K., Paulsen J.S., Beglinger L.J. Predict-HD Investigators ofthe Huntington Study Group: Psychiatric symptoms in Hunting-ton’s disease before diagnosis: the predict-HD study. Biol. Psychiatry. 2007;62:1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- Duff K., Paulsen J.S., Beglinger L.J., Langbehn D.R., Wang C., Stout J.C. ‘Frontal’ behaviors before the diagnosis of Huntington's disease and their relationship to markers of disease progression: evidence of early lack of awareness. J. Neuro-Oncol. 2010;22:196–207. doi: 10.1176/appi.neuropsych.22.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin K., Sockeel P., Carette A.-S., Delliaux M., Defebvre L. Assessing apathy in everyday clinical practice with the short-form Lille apathy rating scale. Mov. Disord. 2013;28 doi: 10.1002/mds.25584. [DOI] [PubMed] [Google Scholar]
- François C., Ripollés P., Bosch L., Garcia-Alix A., Muchart J., Sierpowska J. Language learning and brain reorganization in a 3.5-year-old child with left perinatal stroke revealed using structural and functional connectivity. Cortex. 2016;77:95–118. doi: 10.1016/j.cortex.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Frank S. Treatment of Huntington's disease. Neurotherapeutics. 2014;11:153–160. doi: 10.1007/s13311-013-0244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J.H., Trieschmann M.E., Myers R.H., Fernandez H.H. Monozygotic twins discordant for Huntington disease after 7 years. Arch. Neurol. 2005;62:995–997. doi: 10.1001/archneur.62.6.995. [DOI] [PubMed] [Google Scholar]
- Fritz N.E., Boileau N.R., Stout J.C., Ready R., Perlmutter J.S., Paulsen J.S. Relationships among apathy, health-related quality of life, and function in Huntington's disease. J. Neuropsychiatr. Clin. Neurosci. 2018;30:194–201. doi: 10.1176/appi.neuropsych.17080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou N., Bradshaw J.L., Chiu E., Tudor A., O'Gorman L., Phillips J.G. Differential clinical and motor control function in a pair of monozygotic twins with Huntington's disease. Mov. Disord. Off. J. Mov. Disord. Soc. 1999;14:320–325. doi: 10.1002/1531-8257(199903)14:2<320::aid-mds1018>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Gómez-Esteban J.C., Lezcano E., Zarranz J.J., Velasco F., Garamendi I., Pérez T. Monozygotic twins suffering from Huntington's disease show different cognitive and behavioural symptoms. Eur. Neurol. 2007;57:26–30. doi: 10.1159/000097006. [DOI] [PubMed] [Google Scholar]
- Gregory S., Seunarine K.K., Stopford C., Zhang H., Zhang J., Orth M. Neuropsychiatry and white matter microstructure in Huntington's disease. J. Huntingt Dis. 2015;4:239–249. doi: 10.3233/JHD-150160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.M., Salmon D.P., Corey-Bloom J., Gamst A., Paulsen J.S., Jerkins S. Behavioural abnormalities contribute to functional decline in Huntington's disease. J. Neurol. Neurosurg. Psychiatry. 2003;74:120–122. doi: 10.1136/jnnp.74.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington Study Group Unified Huntington's disease rating scale: reliability and consistency. Mov. Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Jenkins I.H., Jahanshahi M., Jueptner M., Passingham R.E., Brooks D.J. Self-initiated versus externally triggered movements: II. The effet of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Joseph R. Frontal lobe psychopathology: mania, depression, confabulation, catatonia, perseveration, obsessive compulsions, and schizophrenia. Psychiatry. 1999;62:138–172. doi: 10.1080/00332747.1999.11024862. [DOI] [PubMed] [Google Scholar]
- Kingma E.M., van Duijn E., Timman R., van der Mast R.C., Roos R.A.C. Behavioural problems in Huntington's disease using the problem behaviours assessment. Gen. Hosp. Psychiatry. 2008;30:155–161. doi: 10.1016/j.genhosppsych.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs [internet] Front. Psychol. 2013:4. doi: 10.3389/fpsyg.2013.00863. https://www.frontiersin.org/articles/10.3389/fpsyg.2013.00863/full [cited 2018 Apr 19] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctôt K.L., Moosa S., Herrmann N., Leibovitch F.S., Rothenburg L., Cotter A. A SPECT study of apathy in Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2007;24:65–72. doi: 10.1159/000103633. [DOI] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Levy R., Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb. Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Lhermitte F., Pillon B., Serdaru M. Human autonomy and the frontal lobes. Part I: imitation and utilization behavior: a neuropsychological study of 75 patients. Ann. Neurol. 1986;19:326–334. doi: 10.1002/ana.410190404. [DOI] [PubMed] [Google Scholar]
- MacDonald M.E., Ambrose C.M., Duyao M.P., Myers R.H., Lin C., Srinidhi L. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Martinez-Horta S., Perez-Perez J., van Duijn E., Fernandez-Bobadilla R., Carceller M., Pagonabarraga J. Neuropsychiatric symptoms are very common in premanifest and early stage Huntington's disease. Parkinsonism Relat. Disord. 2016;30:58–64. doi: 10.1016/j.parkreldis.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Martinez-Horta S., Perez-Perez J., Sampedro F., Pagonabarraga J., Horta-Barba A., Carceller-Sindreu M. Structural and metabolic brain correlates of apathy in Huntington's disease: apathy in Huntington's disease. Mov. Disord. 2018;33:1151–1159. doi: 10.1002/mds.27395. [DOI] [PubMed] [Google Scholar]
- McColgan P., Razi A., Gregory S., Seunarine K.K., Durr A. A.C. Roos R, et al. structural and functional brain network correlates of depressive symptoms in premanifest Huntington's disease: depressive symptoms in preHD. Hum. Brain Mapp. 2017;38:2819–2829. doi: 10.1002/hbm.23527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M.F., Lauterbach E.C., Sampson S.M. ANPA committee on research. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA committee on research. J. Neuropsychiatr. Clin. Neurosci. 2008;20:130–149. doi: 10.1176/jnp.2008.20.2.130. [DOI] [PubMed] [Google Scholar]
- Misiura M.B., Ciarochi J., Vaidya J., Bockholt J., Johnson H.J., Calhoun V.D. Apathy is related to cognitive control and striatum volumes in prodromal Huntington's disease. J. Int. Neuropsychol. Soc. 2019:1–8. doi: 10.1017/S1355617719000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O., Petrides M., Strafella A.P., Worsley K.J., Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann. Neurol. 2006;59:257–264. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Morris L.S., Kundu P., Dowell N., Mechelmans D.J., Favre P., Irvine M.A. Fronto-striatal organization: defining functional and microstructural substrates of behavioural flexibility. Cortex J. Devoted Study Nerv. Syst. Behav. 2016;74:118–133. doi: 10.1016/j.cortex.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naarding P., Janzing J.G., Eling P., van der Werf S., Kremer B. Apathy is not depression in Huntington's disease. J. Neuropsychiatr. Clin. Neurosci. 2009;21:266–270. doi: 10.1176/jnp.2009.21.3.266. [DOI] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9 doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nobis L., Husain M. Apathy in Alzheimer's disease. Curr. Opin. Behav. Sci. 2018;22:7–13. doi: 10.1016/j.cobeha.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent A.C., Luckenbaugh D.A., Wood S.E., Bogers W., Zarate C.A., Drevets W.C. Automated subcortical segmentation using FIRST: test-retest reliability, inter-scanner reliability, and comparison to manual segmentation. Hum. Brain Mapp. 2013;34:2313–2329. doi: 10.1002/hbm.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagonabarraga J., Kulisevsky J., Strafella A.P., Krack P. Apathy in Parkinson's disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14:518–531. doi: 10.1016/S1474-4422(15)00019-8. [DOI] [PubMed] [Google Scholar]
- Paoli R., Botturi A., Ciammola A., Silani V., Prunas C., Lucchiari C. Neuropsychiatric burden in Huntington's disease. Brain Sci. 2017;7:67. doi: 10.3390/brainsci7060067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L., Lansdall C., Rowe J. The neuroanatomical and neurochemical basis of apathy and impulsivity in frontotemporal lobar degeneration. Curr. Opin. Behav. Sci. 2018;22:14–20. doi: 10.1016/j.cobeha.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters F., Perani D., Herholz K., Holthoff V., Beuthien-Baumann B., Sorbi S. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 2006;21:373–379. doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- Reijnders J.S.A.M., Scholtissen B., Weber W.E.J., Aalten P., Verhey F.R.J., Leentjens A.F.G. Neuroanatomical correlates of apathy in Parkinson's disease: a magnetic resonance imaging study using voxel-based morphometry. Mov. Disord. Off. J. Mov. Disord. Soc. 2010;25:2318–2325. doi: 10.1002/mds.23268. [DOI] [PubMed] [Google Scholar]
- Reyes S., Viswanathan A., Godin O., Dufouil C., Benisty S., Hernandez K. Apathy: a major symptom in CADASIL. Neurology. 2009;72:905–910. doi: 10.1212/01.wnl.0000344166.03470.f8. [DOI] [PubMed] [Google Scholar]
- Riley J.D., Moore S., Cramer S.C., Lin J.J. Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy Behav. 2011;21:80–87. doi: 10.1016/j.yebeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert P., Onyike C.U., Leentjens A.F.G., Dujardin K., Aalten P., Starkstein S. Proposed diagnostic criteria for apathy in Alzheimer's disease and other neuropsychiatric disorders. Eur. Psychiatry. 2009;24:98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Robinson J.L., Laird A.R., Glahn D.C., Blangero J., Sanghera M.K., Pessoa L. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage. 2012;60:117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas H.D., Salat D.H., Lee S.Y., Zaleta A.K., Pappu V., Fischl B. Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity. Brain. 2008;131:1057–1068. doi: 10.1093/brain/awn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C.A., Aylward E.H., Wild E.J., Langbehn D.R., Long J.D., Warner J.H. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 2014;10:204–216. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- Roth R.M., Flashman L.A., Saykin A.J., McAllister T.W., Vidaver R. Apathy in schizophrenia: reduced frontal lobe volume and neuropsychological deficits. Am. J. Psychiatry. 2004;161:157–159. doi: 10.1176/appi.ajp.161.1.157. [DOI] [PubMed] [Google Scholar]
- Sen P.N., Basser P.J. A model for diffusion in white matter in the brain. Biophys. J. 2005;89:2927–2938. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierpowska J., Gabarrós A., Fernandez-Coello A., Camins À., Castañer S., Juncadella M. Morphological derivation overflow as a result of disruption of the left frontal aslant white matter tract. Brain Lang. 2015;142:54–64. doi: 10.1016/j.bandl.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockeel P., Dujardin K., Devos D., Denève C., Destée A., Defebvre L. The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: validation in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2006;77:579–584. doi: 10.1136/jnnp.2005.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein S.E., Leentjens A.F.G. The nosological position of apathy in clinical practice. J. Neurol. Neurosurg. Psychiatry. 2008;79:1088–1092. doi: 10.1136/jnnp.2007.136895. [DOI] [PubMed] [Google Scholar]
- Steventon J.J., Trueman R.C., Rosser A.E., Jones D.K. Robust MR-based approaches to quantifying white matter structure and structure/function alterations in Huntington's disease. J. Neurosci. Methods. 2016;265:2–12. doi: 10.1016/j.jneumeth.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi S.J., Scahill R.I., Owen G., Durr A., Leavitt B.R., Roos R.A. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12:637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- Tekin S., Cummings J.L. Frontal–subcortical neuronal circuits and clinical neuropsychiatry: an update. J. Psychosom. Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Thompson J.C., Snowden J.S., Craufurd D., Neary D. Behavior in Huntington's disease: dissociating cognition-based and mood-based changes. J. Neuropsychiatr. Clin. Neurosci. 2002;14:37–43. doi: 10.1176/jnp.14.1.37. [DOI] [PubMed] [Google Scholar]
- Thompson J., Harris J., Sollom A., Stopford C., Howard E., Snowden J. Longitudinal evaluation of neuropsychiatric symptoms in Huntington's disease. J. Neuropsychiatr. Clin. Neurosci. 2012;24:53–60. doi: 10.1176/appi.neuropsych.11030057. [DOI] [PubMed] [Google Scholar]
- Thu D.C.V., Oorschot D.E., Tippett L.J., Nana A.L., Hogg V.M., Synek B.J. Cell loss in the motor and cingulate cortex correlates with symptomatology in Huntington's disease. Brain J. Neurol. 2010;133:1094–1110. doi: 10.1093/brain/awq047. [DOI] [PubMed] [Google Scholar]
- Tippett L.J., Waldvogel H.J., Thomas S.J., Hogg V.M., van Roon-Mom W., Synek B.J. Striosomes and mood dysfunction in Huntington's disease. Brain J. Neurol. 2007;130:206–221. doi: 10.1093/brain/awl243. [DOI] [PubMed] [Google Scholar]
- van Duijn E., Craufurd D., Hubers A.A.M., Giltay E.J., Bonelli R., Rickards H. Neuropsychiatric symptoms in a European Huntington's disease cohort (REGISTRY) J. Neurol. Neurosurg. Psychiatry. 2014;85:1411–1418. doi: 10.1136/jnnp-2013-307343. [DOI] [PubMed] [Google Scholar]
- Von Der Heide R.J., Skipper L.M., Klobusicky E., Olson I.R. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Phan K.L., Liberzon I., Taylor S.F. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Yi H.-G., Maddox W.T., Mumford J.A., Chandrasekaran B. The role of corticostriatal systems in speech category learning. Cereb Cortex N. Y. 2016;26:1409–1420. doi: 10.1093/cercor/bhu236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A.B., Penney J.B., Starosta-Rubinstein S., Markel D.S., Berent S., Giordani B. PET scan investigations of Huntington's disease: cerebral metabolic correlates of neurological features and functional decline. Ann. Neurol. 1986;20:296–303. doi: 10.1002/ana.410200305. [DOI] [PubMed] [Google Scholar]
- Zamboni G., Huey E.D., Krueger F., Nichelli P.F., Grafman J. Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates. Neurology. 2008;71:736–742. doi: 10.1212/01.wnl.0000324920.96835.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material