ABSTRACT
Background
Dairy product intake has been associated with decreased risk of type 2 diabetes (T2D) in cohort studies. However, results from clinical trials on T2D-related risk factors remain inconclusive.
Objective
The aim of this clinical trial was to evaluate the impact of high dairy product intake (HD) (≥4 servings/d) for 6 wk, compared with an adequate dairy product intake (AD) (≤2 servings/d), on glycemic and insulinemic parameters, insulin sensitivity, insulin secretion, and β-cell function in hyperinsulinemic adults.
Methods
In this crossover clinical trial, hyperinsulinemic adults were randomly assigned to HD or AD for 6 wk, then crossed over after a 6-wk washout period. Serum glucose, insulin, C-peptide, HOMA-IR, Matsuda index, insulinogenic index, and disposition index were measured and analyzed using a repeated-measures mixed model adjusted for age, sex, and BMI. Anthropometric measures were collected and food intake was evaluated using a validated FFQ.
Results
Nineteen men and 8 women completed the study (mean ± SD age: 55 ± 14 y; BMI: 31.3 ± 3.3 kg/m2. Dairy product intake was 5.8 servings/d in the HD condition and 2.3 servings/d in the AD condition after 6 wk. No difference was observed between HD and AD after 6 wk for all outcomes.
Conclusions
HD does not affect glycemic and insulinemic parameters, insulin sensitivity, insulin secretion, and β-cell function over AD in hyperinsulinemic adults. Additional larger and longer studies assessing T2D-related risk factors are required. This trial was registered at clinicaltrials.gov as NCT02961179.
Keywords: prediabetes, hyperinsulinemia, milk, cheese, yogurt, insulin resistance, insulin secretion, β-cell function
Introduction
Diabetes affected ∼8.8% of the world population in 2017, of which 90% is type 2 diabetes (T2D) (1, 2). The diagnosis of T2D is usually preceded by increased insulin resistance/reduced insulin sensitivity, which can be assessed by the HOMA-IR (3) or the Matsuda index during a 75-g 2-h oral-glucose-tolerance test (OGTT) (4). In response to insulin resistance, insulin secretion increases from pancreatic β-cells, which can be estimated using the insulinogenic index during an OGTT (5). Insulin secretion and insulin sensitivity are linked through a hyperbolic relation that represents the capacity of β-cells to compensate for whole-body insulin resistance, a capacity which can be estimated using the disposition index (6). A reduction of β-cell function is recognized as an early marker of T2D development in individuals at risk (7).
Diet has a central role in the prevention of T2D, notably through consumption of recommended amounts of fruits and vegetables or dietary fibers, for instance (1, 8). Dairy product intake has also been associated with a reduced risk of T2D in meta-analyses of cohort studies, especially total, low-fat, and fermented dairy products (9–11). Despite these potential benefits of dairy intake in prevention of T2D, results from clinical trials remain controversial. A systematic review of clinical trials by our group observed a modest increase in fasting glucose concentrations and no change in insulin concentrations or insulin resistance with the HOMA-IR in nondiabetic subjects after an increased dairy product intake, but the quality of evidence was low for all outcomes (12). These results are contrasting with observational evidence, biologically contradictory, but also of limited clinical significance regarding the variations observed (12). The controversies could be explained primarily with the lack of studies assessing T2D-related parameters and indexes as primary outcomes and focusing on a population with proper hyperinsulinemia or prediabetes. Further, great variability between studies could be due to other factors in study designs, such as various dairy serving sizes and types, length of intervention, level of control imposed on the diet administration, together with genetic and environmental differences between subjects (12).
Overall, the beneficial effects of dairy product intake on T2D-related glycemic parameters and indexes in subjects at risk of T2D remain inconclusive despite promising results in cohort studies. Therefore, the main goal of this clinical study was to test the hypothesis that high dairy product intake (HD) (≥4 servings/d) for 6 wk, compared with an adequate dairy product intake (AD) (≤2 servings/d), improves insulin sensitivity/resistance, insulin secretion, and β-cell function assessed by a 75-g 2-h OGTT in hyperinsulinemic or prediabetic adults.
Methods
Selection of participants
This randomized open-label crossover study (NCT02961179) took place at the Centre hospitalier universitaire (CHU) de Québec—Université Laval Research Center in Québec City, Canada, from February 2017 to July 2018. Caucasian men aged between 18 and 75 y or postmenopausal women [absence of menstruation for >12 mo, in order to limit potential effects of the menstrual cycle on data (13)] were recruited from the Québec City metropolitan area via poster advertisements, flyers, and email lists from Université Laval and from the Institute of Nutrition and Functional Foods. Eligibility criteria included a BMI (in kg/m2) between 25.0 and 39.9 and a stable body weight (weight change of <5% in the last 3 mo before screening), fasting insulin >90 pmol/L, fasting glucose <7.0 mmol/L, glycated hemoglobin (HbA1c) <6.5%, stable doses of lipid-lowering agents for >3 mo, and willingness to comply with the protocol. Subjects were excluded if they had a high dairy product consumption at baseline (approximately >2 servings/d) or aversion, allergy, or intolerance to dairy products; were tobacco users; had a diagnosis of T2D or any disease related to glucose metabolism; major surgery in the 3 mo before the study onset; inflammatory bowel disease or any other gastrointestinal disorder which may influence nutrient digestion and absorption; thyroid disease other than stable treated hypothyroidism; or altered liver activity (aspartate aminotransferase >2 times the upper limit of normal). Subjects were excluded if they received any drugs affecting lipid or glucose metabolism other than those used to treat dyslipidemia or hypertension. Before the study onset, participants were asked not to change their usual daily consumption of dairy products, namely ≤2 servings/d. Written informed consent was requested from all subjects before the beginning of the intervention. The study was conducted according to the principles of the Declaration of Helsinki and was approved by the ethics committee of the CHU de Québec.
After a primary telephone screening, subjects were invited to the CHU de Québec—Université Laval Research Center for a screening visit. Body weight was measured with a professional scale accurate to 0.1 kg (Health O Meter Professional, Sunbeam products, Inc.) and height was measured using a wall-mounted stadiometer with 1-mm accuracy (The Easy-Glide Bearing Stadiometer, Perspective Enterprises), with subjects in light clothing and without shoes. Fasting blood samples were collected and medical/sociodemographic questionnaires were administered to ensure eligibility. Serum glucose concentrations were measured using a hexokinase assay (14). Serum insulin concentrations were obtained using a chemiluminescence assay (Siemens Healthcare) (15). HbA1c was determined using a colorimetric method after an initial separation by ion exchange chromatography (16).
Dietary intervention
Eligible subjects were randomly assigned to either a high dairy product intake (HD) or an adequate dairy product intake (AD) for 6 wk, then changed groups after a 6-wk washout period. Random assignment was performed using a computer-generated sequence and fixed blocks composed of 10 participants. Allocation was not concealed. Both participants and research personnel were not blinded to interventions and outcomes.
During the HD intervention period, participants were instructed to consume 4–5 servings of dairy products daily, by replacing other foods in their diet in order to prevent weight gain. Examples of dairy products and serving sizes were suggested using the recommendations of Canada's Food Guide for Healthy Eating 2007 (17). No restriction regarding fat content was given to participants. Several exceptions were as follows: ice cream was considered in the total serving count (serving = 125 mL) but limited to 3 servings/wk; sour cream was considered in the total serving count (serving = 175 g) as well as coffee cream (serving = 250 mL); butter, whipped cream, and processed foods containing exclusively modified milk substances (frozen desserts, melted cheese products, etc.) were excluded from the dairy product serving count. Milk substitutes, including soy desserts or plant-based beverages (almond, cashew, rice, hemp, etc.), were not accepted in the daily serving count for dairy products. Written instructions relating to the types of dairy products to consume and examples of serving sizes were administered by a registered dietician. A variety of dairy products were given to subjects at the beginning of the HD intervention period. Participants were asked to avoid changing their lifestyle habits during the entire period of the study. During the AD intervention period, subjects had to consume ≤2 servings of dairy products daily, using the same instructions as the HD intervention period. During the washout period, participants were instructed to come back to their usual daily consumption of dairy products, namely ≤2 servings/d.
Clinical investigations
Anthropometric measures and dietary intake
Four visits were required at the CHU de Quebec—Université Laval Research Center after an 8-h overnight fast, at week 0 and week 6 for the first intervention period, and at week 12 and week 18 for the second intervention period. Measurements and clinical investigations were identical for each study visit. Body weight and BMI were collected; waist circumference was measured using the mean of 2 measures at the top of the iliac crest, while the subject was standing. Body composition was evaluated, in the fasting state and at the same time across visits, using a 4-electrode bioimpedance scale (InBody 520 Body Composition Analyzer). At each visit, subjects were instructed to complete a validated FFQ containing 91 items and 33 subquestions (18). Energy and nutrient intake were analyzed using the Nutrition Data System for Research and the Canadian Nutrient File 2015 (19). Foods consumed were categorized into the following food groups: fruits and vegetables, cereals and grains, dairy products, and meats and substitutes, as per Canada's Food Guide for Healthy Eating 2007 (17). Physical activity was assessed using an auto-administered questionnaire containing 10 items; however, the data were deemed unsuitable for analysis owing to multiple inconsistencies in reporting and missing values.
Primary outcomes: glycemic parameters and indexes
At each visit, participants undertook a 75-g 2-h OGTT after an overnight fast. Blood samples were collected through a venous catheter from an antecubital vein at time −15, 0, 15, 30, 45, 60, 90, and 120 min in spray-coated silica-containing tubes. Serum was separated by centrifugation at 1560 × g at room temperature for 10 min (Heraeus Clinifuge Centrifuge; Thermo Fisher Scientific Inc.). Samples were stored on dry ice until processed. Serum glucose, insulin, and C-peptide were measured at each time point during the OGTT. Chemiluminescence assays were used to measure C-peptide concentrations (15).
Insulin resistance was estimated using the HOMA-IR index: HOMA-IR = [insulin (pmol/L) × glucose (mmol/L)]/135 (3). Insulin sensitivity from the OGTT was assessed using the Matsuda index: 10,000/√ {[fasting glucose (mmol/L) × fasting insulin (pmol/L)] × [mean glucose OGTT (mmol/L) × mean insulin OGTT (pmol/L)]} (4). The incremental AUCs for glucose, insulin, and C-peptide during the OGTT were calculated using the trapezoidal equation. First-phase insulin response to glucose during the OGTT was calculated using the insulinogenic index: [insulin 30 min (pmol/L) − insulin 0 min (pmol/L)]/[glucose 30 min (mmol/L) − glucose 0 min (mmol/L)] (5). β-Cell function was estimated using the disposition index, calculated as follows: AUC insulin/AUC glucose × Matsuda index (6). Insulin concentrations for several points during the OGTT were removed owing to important hemolysis; index equations were adjusted using available data (20).
Statistical analyses
The minimum group size (n = 24) was calculated to provide 80% power to detect an anticipated difference of 11% in insulin sensitivity over 6 wk, as measured by the Matsuda index (SD: 1.6), at P < 0.05 (21). The recruitment goal was fixed at 33 participants to account for 20–25% dropout.
Statistical analyses were conducted using SAS/Stat software version 9.4 (SAS Institute Inc.). Skewness (±1) and/or kurtosis (±4) were used to assess the normality of distribution. Variables were transformed using the log10 or squared root in case of abnormal distribution. Comparison of baseline characteristics between participants who dropped out and those who completed the study was conducted using 2-sample independent t tests and chi-squared tests. Comparison between groups was conducted using a mixed model with repeated measures for crossover designs (22). The model included the variables treatment (HD or AD), visit number (1–4), and selected covariables (age, sex, and BMI) as fixed effects. Subjects were included as the random effect and visits were included in the repeated statement. The interaction treatment × visit was tested for all dependent variables. Multiple comparisons between visits were conducted using Tukey's post hoc test. Data are expressed as arithmetic means ± SDs unless otherwise stated, with statistical significance set at P < 0.05.
Results
Description of participants
The participant flow diagram is presented in Figure 1. From the 396 people contacted by phone for eligibility, 68 were screened and 34 were eligible to participate in the study. One participant withdrew before the onset of the trial. From the 33 adults who were randomly assigned, 6 dropped out for reasons not related to the dairy intervention. No difference was observed between dropouts and completers. The data presented are from the 27 subjects who completed the study. Characteristics of the participants are presented in Table 1. No difference was observed between subjects initially randomly assigned to the HD or the AD intervention at the beginning of the study. Included participants had a mean age of 55 ± 14 y (min–max = 28–70 y) and mean BMI of 31.3 ± 3.2. All participants had fasting hyperinsulinemia and 11 met the criteria for prediabetes (2 h glucose post-OGTT ≥7.8 mmol/L and/or fasting glucose ≥6.1 mmol/L) (23).
FIGURE 1.
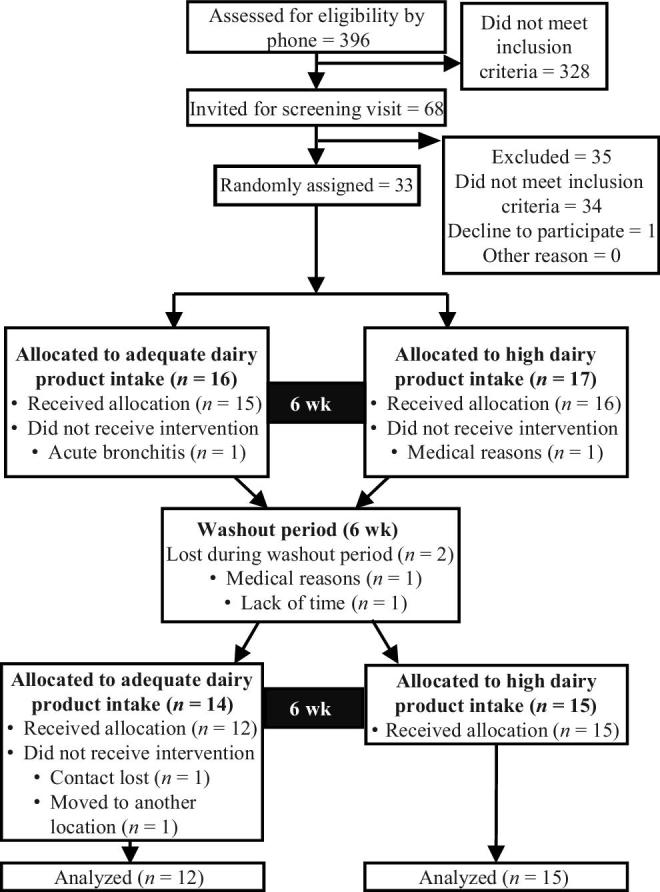
Flow diagram of participants.
TABLE 1.
Baseline characteristics of included subjects1
| Characteristics | Initially randomly assigned to AD (n = 15) | Initially randomly assigned to HD (n = 12) | Total (n = 27) |
|---|---|---|---|
| Sex, n men/total | 12/15 | 7/12 | 19/27 |
| Age, y | 56 ± 9 | 55 ± 14 | 55.5 ± 14 |
| Body weight, kg | 91.5 ± 15.9 | 88.7 ± 14.4 | 90.2 ± 15 |
| BMI, kg/m2 | 31.2 ± 3.2 | 31.5 ± 3.2 | 31.3 ± 3.2 |
| Waist circumference, cm | 110 ± 8 | 108 ± 10 | 109 ± 9 |
| Body fat mass, kg | 30.3 ± 8.7 | 31.8 ± 8.2 | 30.9 ± 8.3 |
| Lean body mass, kg | 61.7 ± 12.8 | 57.4 ± 12.9 | 59.8 ± 12.8 |
| Lean dry mass, kg | 16.4 ± 3.5 | 15.3 ± 3.5 | 15.9 ± 3.4 |
| Body fat, % | 32.9 ± 7.9 | 35.9 ± 8.3 | 34.3 ± 8 |
| Systolic blood pressure, mm Hg | 138 ± 14 | 139 ± 11 | 139 ± 13 |
| Diastolic blood pressure, mm Hg | 82 ± 12 | 78 ± 10 | 80 ± 11 |
| Serum fasting glucose, mmol/L | 5.3 ± 0.5 | 5.4 ± 0.4 | 5.3 ± 0.5 |
| Serum fasting insulin, pmol/L | 108 ± 40 | 122 ± 63 | 114 ± 51 |
| Insulin resistance, HOMA-IR | 4.2 ± 1.5 | 5 ± 2.8 | 4.5 ± 2.2 |
| Glucose 2 h post-OGTT, mmol/L | 6.8 ± 3.1 | 8 ± 2.3 | 7.3 ± 2.8 |
| Whole blood glycated hemoglobin, % | 5.5 ± 0.7 | 5.6 ± 0.2 | 5.6 ± 0.3 |
| Hyperinsulinemic only, n | 14 | 10 | 24 |
| Altered fasting glucose concentrations (≥6.1 mmol/L), n | 2/15 | 1/12 | 3/27 |
| Glucose intolerance (based on 2-h OGTT), n | 5/15 | 5/12 | 10/27 |
| Prediabetes,2n | 5/15 | 6/12 | 11/27 |
n = 27. Values are means ± SDs unless otherwise indicated. No difference was found between the randomly assigned groups at the beginning of the study (2-sample independent t tests). AD, adequate dairy product intake; HD, high dairy product intake; OGTT, oral-glucose-tolerance test.
Prediabetes is characterized by altered fasting glucose and/or glucose intolerance (23).
Anthropometric measures and dietary intake
Anthropometric measures and dietary intake are presented in Tables 2 and 3. No difference was observed between HD and AD after 6 wk for all anthropometric variables. Mean dairy product intake after the HD intervention was 5.8 ± 2.0 servings/d compared with 2.3 ± 1.2 servings/d after the AD intervention. Comparison between groups showed higher calcium intake and lower PUFAs after HD compared with AD (Table 3).
TABLE 2.
Anthropometric measures before and after an AD and HD in hyperinsulinemic adults1
| AD | HD | Changes between groups | |||
|---|---|---|---|---|---|
| 0 wk | 6 wk | 0 wk | 6 wk | P value | |
| Body weight, kg | 90.5 ± 15 | 90.4 ± 15.1 | 90.1 ± 14.9 | 90.5 ± 14.9 | 0.95 |
| BMI, kg/m2 | 31.5 ± 3.3 | 31.4 ± 3.3 | 31.3 ± 3.1 | 31.5 ± 3.2 | 0.93 |
| Waist circumference, cm | 110 ± 9 | 109 ± 9 | 109 ± 9 | 108 ± 9 | 0.66 |
| Body fat mass, kg | 31.3 ± 8.7 | 31.2 ± 7.7 | 30.7 ± 7.9 | 31.7 ± 7.7 | 0.88 |
| Lean body mass, kg | 59.7 ± 12.9 | 59.6 ± 12.8 | 59.8 ± 13.1 | 59.7 ± 13.2 | 0.94 |
| Lean dry mass, kg | 15.9 ± 3.5 | 15.9 ± 3.4 | 15.9 ± 3.6 | 15.9 ± 3.6 | 0.93 |
| Body fat, % | 34.5 ± 8.2 | 34.6 ± 7.4 | 34.2 ± 8.1 | 34.7 ± 7.9 | 0.93 |
n = 27. Values are means ± SDs. Differences between groups after 6 wk were analyzed using a mixed model with treatment, visit, and treatment × visit as fixed attributes adjusted for age, sex, and BMI, with subjects as the random statement and visits as the repeated statement. AD, adequate dairy product intake; HD, high dairy product intake.
TABLE 3.
Dietary intake before and after an AD and HD in hyperinsulinemic adults1
| AD | HD | Changes between groups repeated | |||
|---|---|---|---|---|---|
| 0 wk | 6 wk | 0 wk | 6 wk | P value | |
| Food groups | |||||
| Dairy products, servings/d | 2.9 ± 2.1 | 2.3 ± 1.2 | 2.6 ± 1.9 | 5.8 ± 2 | 0.0005*† |
| Fruits, servings/d | 2.6 ± 2.4 | 2.2 ± 2 | 2.2 ± 2 | 2.1 ± 1.4 | 0.67* |
| Vegetables, servings/d | 3.7 ± 2.3 | 3.3 ± 2.3 | 3.8 ± 3.8 | 3.2 ± 1.7 | 0.74* |
| Grains and cereals, servings/d | 4.7 ± 2.6 | 4.4 ± 2.2 | 4.6 ± 2.5 | 4 ± 2.2 | 0.63 |
| Meat and substitutes, servings/d | 3.1 ± 1.6 | 3 ± 1.4 | 2.9 ± 1.4 | 2.5 ± 1.2 | 0.17 |
| Dietary intake | |||||
| Fat, % kcal/d | 36.2 ± 4.8 | 36.2 ± 6.7 | 36.1 ± 6 | 34.8 ± 4.9 | 0.47 |
| SFAs, % kcal/d | 12.7 ± 2.9 | 12.3 ± 3.7 | 12.6 ± 3.1 | 14.3 ± 3.4 | 0.17* |
| MUFAs, % kcal/d | 14.4 ± 2.1 | 14.5 ± 2.9 | 14.2 ± 2.5 | 12.9 ± 2 | 0.06 |
| PUFAs, % kcal/d | 6.3 ± 1.5 | 6.6 ± 1.5 | 6.5 ± 1.9 | 5.1 ± 1 | 0.02* |
| Protein, % kcal/d | 17.8 ± 4.4 | 17.4 ± 4.2 | 17.9 ± 3.1 | 19.9 ± 3.5 | 0.20 |
| Carbohydrate, % kcal/d | 45.3 ± 6.9 | 45 ± 8.4 | 45.7 ± 7.4 | 44.9 ± 6.4 | 0.79 |
| Energy, kcal/d | 2384 ± 1095 | 2098 ± 762 | 2193 ± 1029 | 2439 ± 888 | 0.64 |
| Cholesterol, mg/d | 316 ± 170 | 280 ± 107 | 278 ± 125 | 323 ± 139 | 0.96 |
| Dietary fibers, g/d | 24.1 ± 10.1 | 22.5 ± 11.2 | 23.5 ± 13.6 | 22.2 ± 10.1 | 0.70* |
| Vitamin D, µg/d | 27.6 ± 30.6 | 27.2 ± 34.4 | 24.4 ± 26.9 | 28.3 ± 31.7 | 0.93 |
| Calcium, mg/d | 1368 ± 739 | 1156 ± 408 | 1283 ± 707 | 2196 ± 651 | 0.002*† |
| Potassium, mg/d | 3715 ± 1345 | 3353 ± 1251 | 3560 ± 1686 | 4211 ± 1222 | 0.11* |
| Sodium, mg/d | 3199 ± 1539 | 2801 ± 1001 | 3061 ± 1458 | 3494 ± 1223 | 0.23* |
n = 27. Values are means ± SDs. Differences between groups after 6 wk were analyzed using a mixed model with treatment, visit, and treatment × visit as fixed attributes adjusted for age, sex, and BMI, with subjects as the random statement and visits as the repeated statement. *P < 0.05 for treatment × visit; †P < 0.05 for visit. AD, adequate dairy product intake; HD, high dairy product intake.
Glycemic parameters and insulin sensitivity, insulin secretion, and β-cell function
Glycemic parameters and indexes are presented in Table 4. No difference was observed between HD and AD after 6 wk for all glycemic parameters and indexes.
TABLE 4.
Biochemical values before and after an AD and HD in hyperinsulinemic adults1
| AD | HD | Changes between groups | |||
|---|---|---|---|---|---|
| 0 wk | 6 wk | 0 wk | 6 wk | P value | |
| Serum fasting glucose, mmol/L | 5.3 ± 0.5 | 5.3 ± 0.6 | 5.3 ± 0.5 | 5.4 ± 0.5 | 0.89 |
| Serum fasting insulin, pmol/L | 121 ± 58 | 129 ± 77 | 111 ± 52 | 127 ± 68 | 0.73 |
| HOMA-IR | 4.8 ± 2.5 | 5.2 ± 3.8 | 4.4 ± 2.3 | 5.2 ± 3.4 | 0.77 |
| Serum glucose 2 h post-OGTT, mmol/L | 7.4 ± 2.9 | 7.1 ± 2.4 | 7 ± 2.3 | 7.3 ± 2.8 | 0.86 |
| Serum AUC glucose, mmol/L × h | 1.8 ± 0.3 | 1.8 ± 0.3 | 1.7 ± 0.3 | 1.8 ± 0.3 | 0.56 |
| Serum AUC insulin, pmol/L × h | 154 ± 81 | 160 ± 87 | 161 ± 76 | 158 ± 270 | 0.56 |
| Serum AUC C-peptide, pmol/L × h | 480 ± 103 | 487 ± 133 | 472 ± 125 | 474 ± 101 | 0.69 |
| Matsuda index | 6.6 ± 3.2 | 6.8 ± 4.4 | 7.2 ± 4.8 | 6.2 ± 3.1 | 0.96 |
| Insulinogenic index | 214 ± 108 | 209 ± 111 | 199 ± 82 | 240 ± 152 | 0.67 |
| Disposition index | 506 ± 259 | 511 ± 243 | 537 ± 270 | 493 ± 225 | 0.46 |
n = 27. Values are means ± SDs. Differences between groups after 6 wk were analyzed using a mixed model with treatment, visit, and treatment × visit as fixed attributes adjusted for age, sex, and BMI, with subjects as the random statement and visits as the repeated statement. AD, adequate dairy product intake; HD, high dairy product intake; OGTT, oral-glucose-tolerance test.
Discussion
Results from this crossover clinical trial showed no difference in insulin resistance, insulin sensitivity, insulin secretion, and β-cell function between HD and AD interventions after 6 wk in hyperinsulinemic subjects, suggesting an overall neutral effect of both HD and AD on T2D-related glycemic parameters and indexes. Furthermore, no difference was observed in anthropometric measures between HD and AD after 6 wk.
The results of this study contrast with observational evidence supporting a beneficial effect of high dairy products in the prevention of T2D (11); yet, similar results were observed in a 6-wk randomized crossover trial, in which liquid low-fat dairy products (milk and yogurt) or sugar-sweetened products were administered to 33 subjects with abdominal obesity. No change was observed in glycemic parameters or insulin resistance after 6 wk of dairy intake in comparison with baseline values. However, the disposition index was higher in the dairy group than in the sugar-sweetened product group after 6 wk (24). In another clinical study, 1 L semi-skimmed milk or 1 L noncalorie soft drink was administered to 60 overweight or obese subjects for 6 mo. No difference was observed between groups for insulin concentrations or the Matsuda index (25). Contrasting results between observational and clinical studies might be explained primarily by the relatively short length of intervention in clinical trials (26). The development toward T2D can take many years after the first declaration of hyperinsulinemia, suggesting longer clinical trials are required to properly assess the effect of dairy intake in prevention of the disease. In addition, the absence of studies assessing T2D diagnosis as a hard-point outcome is a critical limit of current literature assessing dairy product intake and should be addressed in future clinical trials. In sum, increasing dairy product intake for 6 wk does not seem to improve insulin sensitivity, insulin secretion, or β-cell function in hyperinsulinemic subjects.
Although increasing dairy production intake did not affect T2D-related glycemic parameters and indexes in the present study, large interindividual variability was observed in response to dairy product intake for insulin resistance using the HOMA-IR and insulin sensitivity using the Matsuda index, as presented in Figure 2. Large variations in response to dairy products can result in statistical analyses that argue for a null effect, although some subjects are significantly affected positively or negatively by the dietary treatment. The large variation in the individual response to dairy products may be explained primarily by some methodological elements, namely the differences between subjects according to their choices in the types of dairy products and fat content they consumed, the other foods chosen during the intervention, age, and health status. Another possible explanation for the variation in response in some subjects could be the large CVs for HOMA-IR and the Matsuda index (14.4% and 20.4%, respectively, for impaired glucose tolerance subjects) (27). In addition, increasing interest is given to the interindividual variability in response to diet according to the genetic background of subjects, known as gene–diet interactions. Specific genes associated with T2D have been shown to have gene–diet interactions with T2D-related risk factors (28, 29). Thus, individual response to dairy products might be partially due to genetic variability between subjects (30, 31). For instance, a cross-sectional study realized in 210 healthy Canadians identified an interaction between dairy product intake and a variation of the glucokinase gene (GCK) (rs1799884, G > A, minor allele frequency = 0.17), which has been associated with impaired glucose metabolism and HOMA-IR levels (32, 33). Dairy product intake >2.2 servings/d was associated with a beneficial effect on the HOMA-IR in subjects with the “A” allele for rs1799884 in the GCK gene (36% of the population), whereas dairy intake <2.2 servings/d was associated with deteriorated HOMA-IR (33). However, no difference in the HOMA-IR was observed in the “G” carriers after dairy product intake, which represented 64% of the population (33). Despite these results, studies assessing gene–diet interactions with dairy products are scarce, especially regarding T2D and T2D-related biochemical parameters. In sum, exploring the causes of existing interindividual variability is essential in clinical trials because differences in response to dairy products might influence group results and might contribute to the controversies observed in clinical trials.
FIGURE 2.
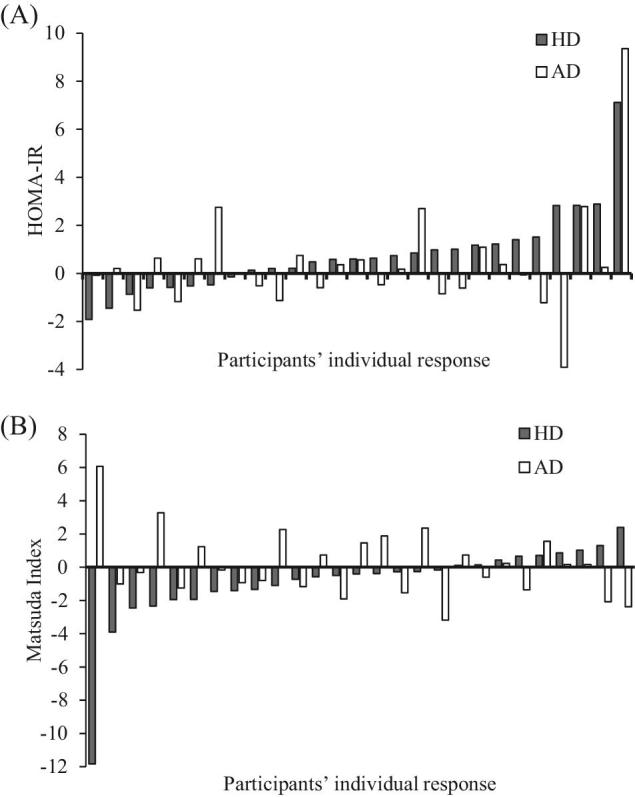
Interindividual variability in response to HD and AD for insulin resistance and insulin sensitivity in ascending order for HD. (A) Changes in the HOMA-IR; (B) changes in the Matsuda index. n = 27. Data are presented as the individual differences between before and after the intervention. AD, adequate dairy product intake; HD, high dairy product intake.
This study has strengths, beginning with the free-living context and the administration of a wide variety of dairy products. The reproduction of real-life conditions grants an increased generalization of results for people at risk of T2D. On the other hand, excluding premenopausal women created disparities in age range and sex representation in the current study, which represent a potential cofounder and a limit of generalization. Further, the crossover design helps to reduce the intergroup variability between subjects for both HD and AD. However, the liberty in food choices greatly limits the control of the research team on other potential active foods in the diet and on the types of dairy products, which might have accentuated the interindividual variability. Further, the relatively small intervention period could have been too short to properly assess insulin secretion and β-cell function; thus, results could have been different with a longer administration of dairy products. In addition, data collected on physical activity could not be utilized, which might represent a potential important cofounding element on glucose- and insulin-related outcomes. Finally, the absence of a control group with no dairy intake (or with less than the recommended intake) prevents us from assessing any dose–response effects on glycemic parameters of dairy consumption.
In conclusion, the results of the present study suggest that a high dairy intake (≥4 servings/d) for 6 wk does not affect insulin sensitivity or T2D-related glycemic parameters over an adequate dairy intake in hyperinsulinemic adults. Additional larger and longer-term studies assessing T2D and T2D-related glycemic parameters as primary outcomes are required. Furthermore, additional attention should be given to exploring the environmental and genetic factors surrounding interindividual variability in clinical trials.
Acknowledgements
We thank Andréa Taschereau-Charron, Sarah Chouinard-Castonguay, Élise Cant, Valérie-Ève Julien, and Camille Lambert for their precious help with the participants’ visits. The authors’ responsibilities were as follows—PJ, SJW, CG, and IR: designed the research and provided essential materials; SOC: conducted the research, analyzed the data, performed the statistical analysis, and wrote the paper; IR: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by Canadian Institutes of Health Research (CIHR) Institute of Nutrition, Metabolism and Diabetes Project Bridge Grant 2016-17 (to IR), a CIHR and Diabète Québec scholarship (to SOC), a Junior 2 research Scholar from the Fonds de recherche du Québec—Santé and a Diabetes Canada New Investigator Award (to CG), and a Junior 1 research Scholar from the Fonds de recherche du Québec—Santé (to IR).
Author disclosures: SOC, PJ, SJW, CG, and IR, no conflicts of interest.
Food isolation bags for dairy product transportation were given by the Dairy Farmers of Canada.
Abbreviations used: AD, adequate dairy product intake; CHU, Centre hospitalier universitaire; HbA1c, glycated hemoglobin; HD, high dairy product intake; OGTT, oral-glucose-tolerance test; T2D, type 2 diabetes.
References
- 1. International Diabetes Federation. DF diabetes atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 2. Ramachandran A, Snehalatha C, Nanditha A. Classification and diagnosis of diabetes. In Holt RI Cockram CS Flyvbjerg A, Goldstein BJ, editors. Textbook of diabetes. 5th ed. Oxford, UK: Wiley-Blackwell; 2017. p. 23–8. [Google Scholar]
- 3. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–19. [DOI] [PubMed] [Google Scholar]
- 4. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–70. [DOI] [PubMed] [Google Scholar]
- 5. Seltzer HS, Allen EW, Herron AL Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 1967;46:323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity 2008;16:1901–7. [DOI] [PubMed] [Google Scholar]
- 7. Bergman RN, Finegood DT, Kahn SE. The evolution of β-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest 2002;32:35–45. [DOI] [PubMed] [Google Scholar]
- 8. Ma RCW, Tong PCY. Epidemiology of type 2 diabetes. In: Holt RI Cockram CS Flyvbjerg A, Goldstein BJ, editors. Textbook of diabetes. 5th ed. Oxford, UK: Wiley-Blackwell; 2017. p. 43–64. [Google Scholar]
- 9. Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013;98:1066–83. [DOI] [PubMed] [Google Scholar]
- 10. Gao D, Ning N, Wang C, Wang Y, Li Q, Meng Z, Liu Y, Li Q. Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response meta-analysis. PLoS One 2013;8:e73965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr 2016;103:1111–24. [DOI] [PubMed] [Google Scholar]
- 12. O'Connor S, Turcotte A-F, Gagnon C, Rudkowska I. Increased dairy product intake modifies plasma glucose levels and glycated hemoglobin: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr 2019;10:262–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheu WH-H. Alteration of insulin sensitivity by sex hormones during the menstrual cycle. J Diabetes Investig 2011;2:258–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Passey R, Fuller J, Gillum R, Urry F, Gilles ML. Evaluation and comparison of 10 glucose methods and the reference method recommended in the proposed product class standard (1974). Clin Chem 1977;23:131–9. [PubMed] [Google Scholar]
- 15. Rauhut MM. Chemiluminescence. In: Grayson M, editor. Kirk-Othmer concise encyclopedia of chemical technology. 3rd ed. Hoboken, NJ: John Wiley and Sons; 1985. p. 247. [Google Scholar]
- 16. Sudhakar Nayak S, Pattabiraman TN. A new colorimetric method for the estimation of glycosylated hemoglobin. Clin Chim Acta 1981;109:267–74. [DOI] [PubMed] [Google Scholar]
- 17. Health Canada. Eating well with Canada's Food Guide. HC Pub. 4651 Ottawa, Ontario, Canada: Queen's Printer; 2007. [Google Scholar]
- 18. Goulet J, Nadeau G, Lapointe A, Lamarche B, Lemieux S. Validity and reproducibility of an interviewer-administered food frequency questionnaire for healthy French-Canadian men and women. Nutr J 2004;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Health Canada. [Internet], Canadian nutrient file 2015, Heatlh Canada, Ottawa. 2018[cited 26 October, 2018]. Available from: https://aliments-nutrition.canada.ca/cnf-fce/switchlocale.do?lang=en&url=t.search.recherche.
- 20. Faulenbach MV, Wright LA, Lorenzo C, Utzschneider KM, Goedecke JH, Fujimoto WY, Boyko EJ, McNeely MJ, Leonetti DL, Haffner SM et al.. Impact of differences in glucose tolerance on the prevalence of a negative insulinogenic index. J Diabetes Complications 2013;27:158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L, Bosi E, Piatti P. l-arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism 2013;62:255–64. [DOI] [PubMed] [Google Scholar]
- 22. Hansson P, Holven KB, Øyri LKL, Brekke HK, Biong AS, Gjevestad GO, Raza GS, Herzig K-H, Thoresen M, Ulven SM. Meals with similar fat content from different dairy products induce different postprandial triglyceride responses in healthy adults: a randomized controlled cross-over trial. J Nutr 2019;149:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Punthakee Z, Goldenberg R, Katz P. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada: definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes 2018;42:S10–15. [DOI] [PubMed] [Google Scholar]
- 24. Maki KC, Nieman KM, Schild AL, Kaden VN, Lawless AL, Kelley KM, Rains TM. Sugar-sweetened product consumption alters glucose homeostasis compared with dairy product consumption in men and women at risk of type 2 diabetes mellitus. J Nutr 2015;145:459–66. [DOI] [PubMed] [Google Scholar]
- 25. Engel S, Tholstrup T, Bruun JM, Astrup A, Richelsen B, Raben A. Effect of high milk and sugar-sweetened and non-caloric soft drink intake on insulin sensitivity after 6 months in overweight and obese adults: a randomized controlled trial. Eur J Clin Nutr 2018;72:358–66. [DOI] [PubMed] [Google Scholar]
- 26. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009;32(Suppl 2):S151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Utzschneider KM, Prigeon RL, Tong J, Gerchman F, Carr DB, Zraika S, Udayasankar J, Montgomery B, Mari A, Kahn SE. Within-subject variability of measures of beta cell function derived from a 2 h OGTT: implications for research studies. Diabetologia 2007;50:2516–25. [DOI] [PubMed] [Google Scholar]
- 28. Smith CE, Tucker KL, Arnett DK, Noel SE, Corella D, Borecki IB, Feitosa MF, Aslibekyan S, Parnell LD, Lai C-Q et al.. Apolipoprotein A2 polymorphism interacts with intakes of dairy foods to influence body weight in 2 U.S. populations. J Nutr 2013;143:1865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Luis DA, Aller R, Izaola O, Pacheco D. Role of rs9939609 FTO gene variant in weight loss, insulin resistance and metabolic parameters after a high monounsaturated vs a high polyunsaturated fat hypocaloric diets. Nutr Hosp 2015;32:175–81. [DOI] [PubMed] [Google Scholar]
- 30. Ortega Á, Berná G, Rojas A, Martín F, Soria B. Gene-diet interactions in type 2 diabetes: the chicken and egg debate. Int J Mol Sci 2017;18:1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cornelis MC. Gene-diet interactions in type 2 diabetes. Curr Nutr Rep 2014;3:302–23. [Google Scholar]
- 32. Da Silva MS, Chartrand D, Vohl M-C, Barbier O, Rudkowska I. Dairy product consumption interacts with glucokinase (GCK) gene polymorphisms associated with insulin resistance. J Pers Med 2017;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muller YL, Piaggi P, Hoffman D, Huang K, Gene B, Kobes S, Thearle MS, Knowler WC, Hanson RL, Baier LJ et al.. Common genetic variation in the glucokinase gene (GCK) is associated with type 2 diabetes and rates of carbohydrate oxidation and energy expenditure. Diabetologia 2014;57:1382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


