Summary
During probing and blood feeding, haematophagous mosquitoes inoculate a mixture of salivary molecules into their vertebrate hosts’ skin. In addition to the anti‐haemostatic and immunomodulatory activities, mosquito saliva also triggers acute inflammatory reactions, especially in sensitized hosts. Here, we characterize the oedema and the cellular infiltrate following Aedes aegypti mosquito bites in the skin of sensitized and non‐sensitized BALB/c mice by flow cytometry. Ae. aegypti bites induced an increased oedema in the ears of both non‐sensitized and salivary gland extract‐ (SGE‐)sensitized mice, peaking at 6 hr and 24 hr after exposure, respectively. The quantification of the total cell number in the ears revealed that the cellular recruitment was more robust in SGE‐sensitized mice than in non‐sensitized mice, and the histological evaluation confirmed these findings. The immunophenotyping performed by flow cytometry revealed that mosquito bites were able to produce complex changes in cell populations present in the ears of non‐sensitized and SGE‐sensitized mice. When compared with steady‐state ears, the leucocyte populations significantly recruited to the skin after mosquito bites in non‐sensitized and sensitized mice were eosinophils, neutrophils, monocytes, inflammatory monocytes, mast cells, B‐cells and CD4+ T‐cells, each one with its specific kinetics. The changes in the absolute number of cells suggested two cell recruitment profiles: (i) a saliva‐dependent migration; and (ii) a migration dependent on the immune status of the host. These findings suggest that mosquito bites influence the skin microenvironment by inducing differential cell migration, which is dependent on the degree of host sensitization to salivary molecules.
Keywords: Aedes aegypti, eosinophils, IL‐5, inflammation, mosquito bites, saliva
Abbreviations
- i.p.
intraperitoneal
- PBS
phosphate‐buffered saline
- SGE
salivary gland extract
- t‐SNE
t‐distributed stochastic neighbour embedding
Introduction
Mosquitoes are insects that belong to the order Diptera, family Culicidae, and include approximately 3500 species worldwide of which three genera are clinically relevant to humans: Anopheles, Culex and Aedes.1, 2, 3 Aedes aegypti (Linnaeus, 1762) is a cosmopolitan mosquito species originated from the Old World, and currently found in tropical and subtropical regions, having been introduced in the Americas by sailing ships from Africa.4, 5 In this context, Ae. aegypti is known to be the primary vector of arboviruses that cause emerging and re‐emerging diseases, such as yellow fever, dengue, chikungunya and Zika.6, 7, 8, 9, 10, 11
Both male and female Ae. aegypti need sugar, acquired mainly from nectar, which serves as a source of energy for flight and basal metabolism. However, this kind of meal contributes little to fertility;12 therefore, blood feeding performed by females is important for the ovary maturation and eggs development.7, 13 In order to successfully feed, mosquitoes need to locate the blood vessels in the vertebrate skin through a process known as ‘probing’ and often make several attempts by changing the bite site. Once the blood vessel is located, feeding can occur in the lacerated vessel or in the haematoma around it.14 Hence, the first challenge faced by the mosquito after the physical disruption of the skin is haemostasis, a complex and redundant system that includes coagulation, platelet aggregation and vasoconstriction that quickly inhibits blood ingestion by the mosquito. Nevertheless, during the evolutionary process with their vertebrate hosts, Ae. aegypti have developed a salivary cocktail with anticoagulant, antiplatelet and vasodilator components that neutralize the effects of host haemostasis, making the blood feeding possible.15, 16, 17, 18, 19, 20
During the probing and blood feeding, this salivary gland secretion is deposited in the host tissue. Thus, in addition to the trauma caused by the insertion of the mosquito mouthparts into the skin, a cutaneous reaction to mosquito bites may occur due to effects of saliva inoculation.21 The initial studies showing the differences in the cutaneous reactions to mosquito bites among individuals were published almost a century ago,22, 23 and the first works describing the immunological nature of skin reactions to mosquito bites were published in the following decades.24, 25, 26 Furthermore, Hudson et al. (1960) demonstrated that mosquitoes that had their salivary glands ducts cut were still able to probe and acquire a blood meal but, despite the mechanical damage observed in these cases, no cutaneous reactions were observed, reinforcing the essential role of mosquito saliva for the development of skin reactions.27
The typical course of sensitization to mosquito bites and its association with skin macroscopic reactions is well established,21, 24, 28 although unusual extreme reactions are also described.29, 30, 31 The cutaneous cellular infiltrate following mosquito bites has been evidenced by classical studies in humans and other species using histopathology and immunohistochemistry techniques. So far, very few works have characterized in detail the skin inflammatory profile following Ae. aegypti bites over time or in hosts with different immunological history of mosquito exposure.32, 33, 34 The literature also lacks contemporary approaches to assess this subject. Thus, the aim of the present study was to evaluate the kinetics of oedema and leucocyte recruitment in the ears of mice after Ae. aegypti bites. Flow cytometry was used as a tool for quantitative and qualitative immunophenotyping analyses of the cellular infiltrate of non‐sensitized and salivary gland extract‐ (SGE‐)sensitized mice.
Materials and methods
Mice
BALB/c mice (female, 6–12 weeks old) were bred and maintained at the Isogenic Breeding Unit (Department of Immunology, Institute of Biomedical Sciences, University of São Paulo, Brazil) under specific pathogen‐free conditions. For the experiments, animals were divided into groups containing three–eight mice. All experiments involving laboratory animals were evaluated and approved by the Institutional Animal Care and Use Committee from the Institute of Biomedical Sciences, University of São Paulo, under the protocol number 121/2014. All procedures were in accordance with the Brazilian National Law number 11,794, Decree 6,899 and normative resolutions from the Conselho Nacional de Controle de Experimentação Animal (CONCEA), the federal agency that regulates all research activities involving animal use in the country. None of the animals used in the present work became ill or died prior to the experimental endpoint.
Mosquitoes and SGE
Aedes aegypti mosquitoes (male and female) were bred in an insectary at the Department of Parasitology, ICB/USP, Brazil, where they were fed and mated as previously described.35 Five‐ to eight‐day‐old female adult mosquitoes were used for the experimental mice sensitization and SGE preparation as described.36
Mice sensitization with SGE and challenge by mosquito bites
Animals were sensitized by intraperitoneal (i.p.) inoculation of SGE [5 μg/animal, once a day, diluted in 0·5 ml phosphate‐buffered saline (PBS)] for 5 consecutive days. After 30 days of the first inoculation animals were anaesthetized, and the dorsal region of each ear was exposed to five adult female Ae. aegypti for 30 min. To do that, mosquitoes were placed inside a cylindrical plastic container (5 cm diameter) covered with tulle fabric, according to the methodology adapted from Barros et al.37 A tape protected the tulle fabric region where the head of the anaesthetized animals was placed, so that only the ears were exposed to the mosquitoes (Fig. 1a). A control group received PBS i.p. for 5 consecutive days (0·5 ml/animal, once a day) and was subjected to the same protocol of mosquito bite exposure. A group of naïve mice neither inoculated nor exposed to mosquitoes was included in some experiments to represent the steady‐state condition.
Figure 1.
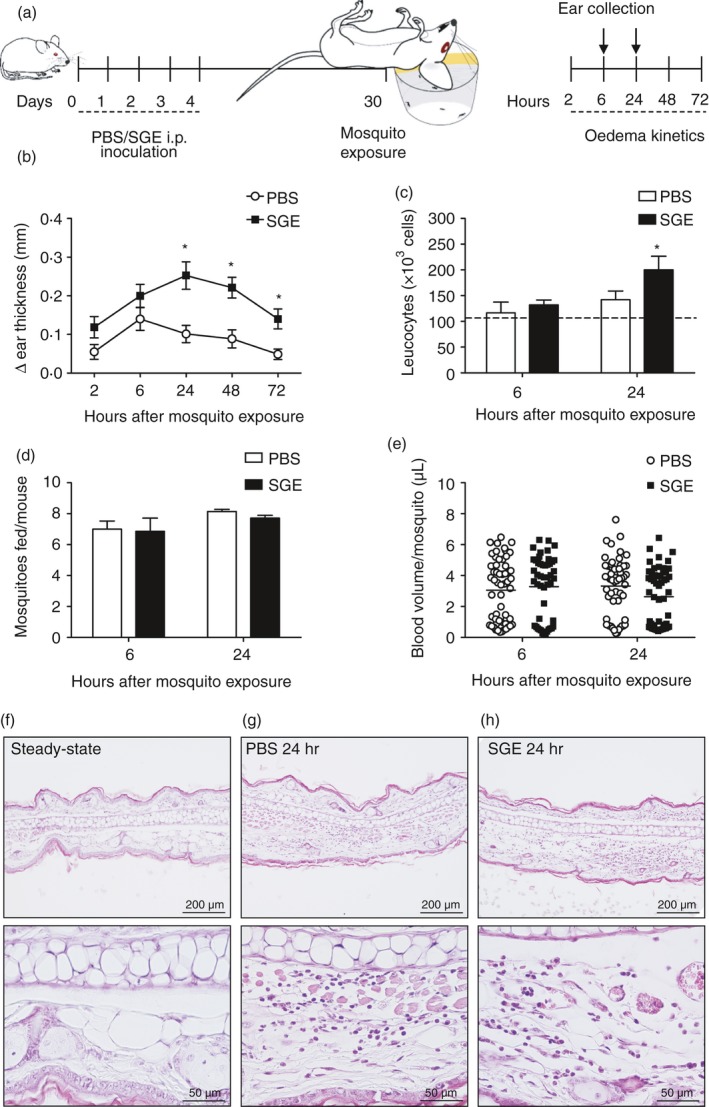
Kinetics of the skin oedema, cell counts and histological evaluation of the ear of non‐sensitized and salivary gland extract (SGE)‐sensitized mice after exposure to Aedes aegypti bites. (a) Experimental protocol: BALB/c mice were inoculated with phosphate‐buffered saline (PBS; 0·5 ml/animal, once a day) or sensitized with intraperitoneal (i.p.) injection of SGE (5 μg/animal, once a day, in 0·5 ml of PBS) for 5 consecutive days. Thirty days after the first inoculation, each ear was exposed to the bites of five female mosquitoes. The oedema was evaluated after 2, 6, 24, 48 and 72 hr, and the ears were collected after 6 and 24 hr for histological analysis and flow cytometry. (b) The kinetics of oedema was determined as the ‘Δ ear thickness’, representing the difference between measurements of the ear thickness after and before exposure in millimetres. (c) The number of total leucocytes in the ear was obtained by multiplying the total number of cells in the ear with the percentage of CD45+ events. The average number of cells in the steady‐state condition is shown as a dotted line. (d) The number of mosquitoes that fed on each mouse and (e) the blood volume ingested by individual mosquitoes is presented. (f–h) Ears were processed as described, embedded in paraffin and transversal sections of ~5 μm were stained with haematoxylin and eosin (100 and 400 ×): (f) steady‐state condition; (g) PBS‐treated group exposed to mosquito bites; (h) SGE‐sensitized group exposed to mosquito bites. *p ≤ 0·05 versus PBS group.
Time course of oedema and histological evaluation of the ear following the Aedes aegypti bites
Thirty days after the first PBS or SGE inoculation, the animals had their ears exposed to Ae. aegypti bites as described earlier. The ear thickness was manually measured with a precision caliper (Mitutoyo 7301, Kawasaki, Kanagawa, Japan) after 2, 6, 24, 48 and 72 hr of exposure. The results are expressed as the ‘Δ ear thickness’, representing the difference between measurement of the ear thickness after and before exposure in millimetres.
The ears of mice (sensitized or not with SGE) exposed to mosquito bites were removed and immersed in 10% phosphate‐buffered formalin for 24 hr, transferred to ethanol gradient for 30 min each gradient, following xylene for 30 min, and then embedded in paraffin. Tissue transversal sections of 5 μm were stained with haematoxylin and eosin, and evaluated by optical microscopy.
Evaluation of the blood‐feeding performance and volume ingested by Aedes aegypti
After exposure, the plastic containers having the mosquitoes were placed at −20° for 30 min, and an estimation of the blood volume ingested by each mosquito was performed as described.35 Briefly, dead mosquitoes were individually placed in microcentrifuge tubes (Axygen, Union City, CA), grinded with plastic pestles in 250 μl of distilled water and centrifuged for 5 min/300 g. In parallel, 20 μl of blood from each mouse was collected, diluted to 500 μl of distilled water and a serial dilution was performed (1:2 ratio) also in distilled water. Two‐hundred microlitres of blood dilutions and of debris‐free supernatants from mosquito homogenates were transferred to 96‐well plates and the absorbance was evaluated at 540 nm. Homogenates of non‐fed female mosquitoes were used as the assay's blank. A linear regression was produced by using the optical density values from blood dilutions and used as a ‘standard curve’ to estimate the blood ingested by each mosquito. The detection limit of this assay was 0·5 μl of blood. Based on these data, the number of mosquitoes that effectively fed on each mouse was also calculated.
Characterization of skin cellular infiltrate by flow cytometry
In another set of experiments, the ears of the animals exposed to mosquitoes (previously sensitized or not with SGE) were removed at 6 and 24 hr after exposure, and processed for cell phenotyping by flow cytometry. Naïve mice ears (not sensitized and not exposed to mosquito bites) were included as a steady‐state group. The ears were separated into ventral and dorsal dermal sheets, and cut into small fragments. To separate the cells from tissues, each pool of fragments was digested in a PBS solution containing 10% inactivated fetal bovine serum, 10 mg/ml collagenase Type IV (Sigma‐Aldrich/Merck KGaA, Darmstadt, Germany), 1 mg/ml deoxyribonuclease I (DNase I; Sigma‐Aldrich, St Louis, MO) and incubated for 40 min at 37° with agitation at 1250 rpm in a thermomixer (Eppendorf ThermoMixer®, Hamburg, Germany). After the incubation, the dermal sheets were separated from the epidermis, and the suspensions containing cells and ear fragments were pressed through a cell strainer (40 μm pore) with a syringe plunger.38 The resulting suspension was centrifuged at 400 g for 5 min, the supernatant was removed, and the cells were resuspended in 1 ml of flow cytometry buffer (PBS containing 1% fetal bovine serum) with anti‐mouse CD16/CD32 antibody (clone 2·4G2 – Bioxcell, West Lebanon, NH) to block Fc receptors.
After counting, the same number of cells from each animal was transferred to polypropylene tubes (12 × 75 mm) and incubated for 30 min at 4° in the dark with the following fluorescence‐conjugated antibodies for cell surface markers. The ‘myeloid’ panel was anti‐CD45 (clone 30‐F11 – Biolegend, San Diego, CA), anti‐CD11b (clone M10/70 – Biolegend), anti‐CD11c (clone N418 – Biolegend), anti‐Ly6C (clone HK1·4 – Biolegend), anti‐Ly6G (clone 1A8 – BD Biosciences, San Jose, CA), anti‐MHC class II (I‐A/I‐E; clone M5/114·15·2 – Biolegend), anti‐CD64 (clone X54‐5/7·1 – Biolegend), anti‐CCR2 (clone 475301 – R&D Systems, Minneapolis, MN), anti‐Siglec‐F (clone E5O‐2440 – BD Biosciences), anti‐CD117 (c‐Kit; clone: 2B8 – BD Biosciences) and anti‐FcɛRI (clone: MAR‐1 – BD Biosciences), used to characterize the following populations: eosinophils, neutrophils, monocytes, inflammatory monocytes, macrophages, dendritic cells and mast cells as described in Fig. S1. The ‘lymphoid’ panel was anti‐CD19 (clone: 1D3 – BD Biosciences), anti‐CD3 (clone: 145‐2C11 – BD Biosciences), anti‐CD4 (clone: RM4‐5 – BD Biosciences), anti‐CD8 (clone: 53‐6·7 – BD Biosciences), used to characterize B‐cells, CD4+ T‐cells (T helper cells), CD8+ T‐cells (cytotoxic T‐cells) and other T‐cells (non‐CD4+/non‐CD8+) as described in Fig. S2. After further washing, cells were resuspended in flow cytometry buffer and acquired by a FACSCanto II flow cytometer or a LSRFortessa X‐20 (BD Biosciences, Franklin Lakes, NJ). Data were analysed using the FlowJo software, version 10.5.3 (Tree Star, Ashland, OR) for calculating cell frequency, dimensionality reduction and visualization by t‐distributed stochastic neighbour embedding (t‐SNE).
IL‐5 determination
Six and twenty‐four hours after mosquito exposure, mice were deeply anaesthetized and blood samples were collected through cardiac puncture before the removal of the ear. Serum was separated and stored at −80° until use for cytokine determination. The levels of IL‐5 were assayed by OptEIA™ ELISA sets (BD Biosciences, San Diego, CA), according to the manufacturers’ recommendations. Values were expressed as pg/ml deduced from standard curves of recombinant IL‐5 cytokine ran in parallel (detection limit 15·6 pg/ml).
Statistical analysis
Statistical analysis of differences between means of experimental groups was performed using Student's t‐test or analysis of variance (ANOVA) followed by Tukey as a post‐test, with minimum established significance in p ≤ 0·05. All statistical analyses were performed by GraphPad Instat (GraphPad Software, La Jolla, CA).
Results
Aedes aegypti bites induce skin oedema and inflammation in the ear of sensitized and non‐sensitized mice
As depicted in Fig. 1a, the oedema and the cell infiltrate induced in the ears by mosquito bites were evaluated in SGE‐sensitized mice (‘SGE’ group) and compared with non‐sensitized mice inoculated with PBS only (‘PBS’ group). The kinetics of oedema revealed increasing ear thickness throughout the evaluation, peaking at 6 hr in the PBS group and 24 hr in the SGE group (Fig. 1b). While no significant differences between the groups were observed at 2 hr and 6 hr after the bites, the ear thickness was significantly greater in mice of the SGE group when compared with the PBS group at later time points (0·25 ± 0·10 mm versus 0·10 ± 0·06 mm at 24 hr: p ≤ 0·01; 0·22 ± 0·08 mm versus 0·09 ± 0·07 mm at 48 hr: p ≤ 0·01; 0·14 ± 0·07 mm versus 0·05 ± 0·04 mm at 72 hr: p ≤ 0·01, respectively).
The peaks of ear's oedema for the PBS and the SGE groups (6 hr and 24 hr after exposure to mosquitos, respectively) were chosen for quantitative evaluations. At both time points, the number of cells in the ears of the PBS group was similar to those found in steady‐state ears. For the SGE group, no differences in the total cell number in the ears were observed after 6 hr of mosquito exposure, while after 24 hr the number was higher than that observed in the ears of the control group (Fig. 1c). To confirm whether the exposure to mosquito bites was similar in both groups, the number of mosquitoes that fed in each mouse (Fig. 1d) and the volume of blood ingested by each mosquito (Fig. 1d) was quantified, and no differences among the groups were found.
Next, we evaluated the histology of ear sections after 24 hr of mosquito exposure. Compared with the ear of naïve mice (‘Steady‐state’ – Fig. 1f), there was a slight inflammatory infiltrate in the dermal layer ear of mice from the PBS group upon mosquito exposure (Fig. 1g). On the other hand, the ear of the SGE group presented a significant increase in the inflammatory infiltrate and hyperplasia in the dermal layer following mosquito bites when compared with the other groups (Fig. 1h).
Aedes aegypti bites induce differential recruitment of myeloid and lymphoid cells to the ears of sensitized and non‐sensitized mice
The inflammatory cell infiltrate in the ears was characterized after 6 hr and 24 hr of mosquito exposure by flow cytometry. The cell surface markers chosen for the myeloid panel are described in Materials and methods, and the gating strategy performed on CD45+ live cells to characterize each cell population is presented in Fig. S1. The markers employed allowed us to identify seven major populations of myeloid cells as follows: eosinophils, neutrophils, monocytes, inflammatory monocytes, macrophages, dendritic cells and mast cells. To summarize the complexity of the myeloid composition in a two‐dimensional manner and display the proportion of each cell population related to the total (100%), a t‐SNE map of the above‐described populations was produced, according to the combination of markers expressed by each population (Fig. 2a). The whole data representing the proportion of each cell type are displayed in Fig. S3.
Figure 2.
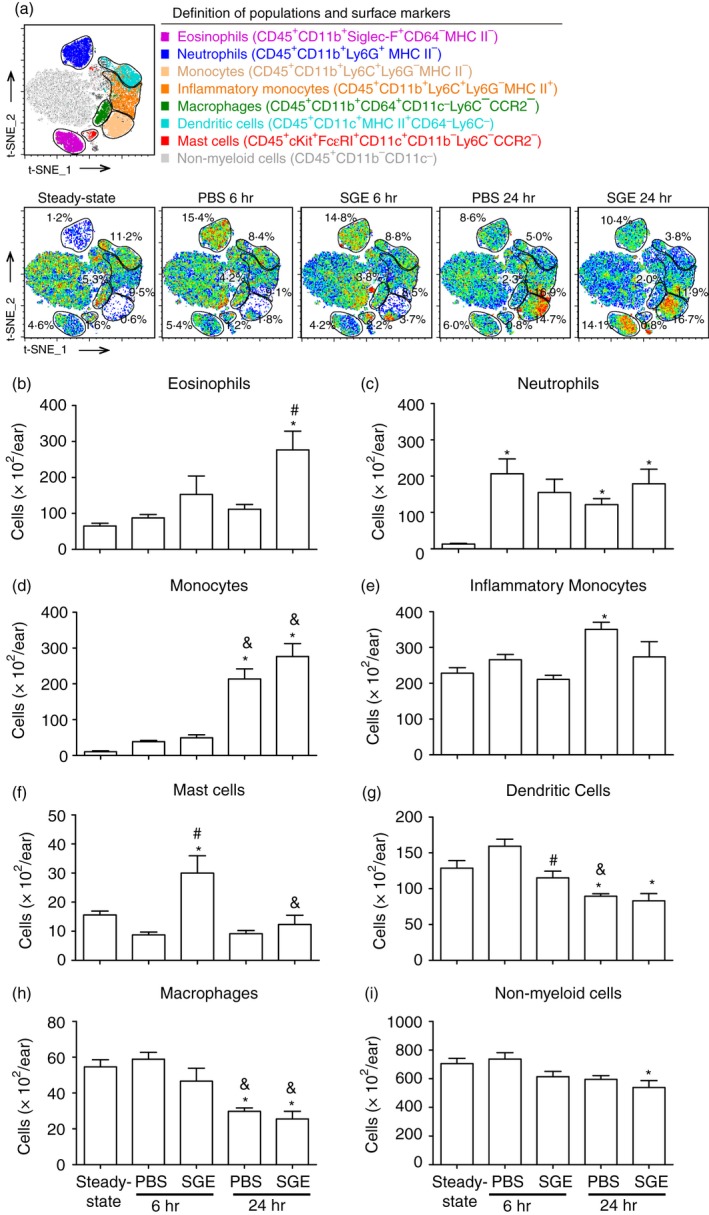
Immunophenotyping of the myeloid infiltrate in the ear of non‐sensitized and salivary gland extract (SGE)‐sensitized mice after exposure to Aedes aegypti bites. BALB/c mice were inoculated with phosphate‐buffered saline (‘PBS’ group) or sensitized with SGE (‘SGE’ group) followed by exposure of each ear to the bites of five female mosquitoes, as described in Materials and methods. The ears were collected after 6 and 24 hr, and analysed by flow cytometry. The ears of naïve mice were included as a reference to the steady‐state condition. (a) t‐Distributed stochastic neighbour embedding (t‐SNE) map and markers were used to characterize each cell population as follows: (b) eosinophils; (c) neutrophils; (d) monocytes; (e) inflammatory monocytes; (f) macrophages; (g) dendritic cells; (h) mast cells; (i) non‐myeloid cells. *p ≤ 0·05 versus steady‐state condition; # p ≤ 0·05 versus respective PBS group; & p ≤ 0·05 versus respective group after 6 hr.
Compared with the steady‐state condition (mice not exposed to mosquito bites), the absolute number of eosinophils remained about the same in the ears of the PBS group after 6 hr and 24 hr of mosquito exposure, while the ears of the SGE group presented a slight, non‐significant increase after 6 hr and a significant increase after 24 hr of mosquito exposure (Fig. 2b). Neutrophils were virtually absent in the steady‐state ears and increased in all experimental groups without differences among them (Fig. 2c). Regarding monocytes, their absolute number was similarly higher in the ears of both PBS and SGE groups after 6 hr of mosquito exposure, although the increase was not statistically significant. After 24 hr of mosquito exposure, the increase of monocytes was significantly more intense, but without statistical differences between PBS and SGE groups (Fig. 2d). Inflammatory monocytes were present in similar numbers of steady‐state ears after 6 hr of exposure. After 24 hr, an increase in the inflammatory monocytes was observed in the ears of the PBS group, but not of the SGE group (Fig. 2e). Recruitment of macrophages and dendritic cells presented a very similar kinetics over time; when compared with steady‐state ears, the absolute numbers of these cells in the ears of both exposed groups after 6 hr did not significantly change. However, after 24 hr less macrophages and dendritic cells were present in the ear of both mosquito‐exposed groups (Fig. 2f,g, respectively). The ears of the PBS group after 6 hr or 24 hr of mosquito exposure presented a similar number of mast cells to those found in steady‐state ears. On the other hand, these cells were significantly increased after 6 hr, returning to the level of steady‐state ears after 24 hr in the SGE group (Fig. 2h). The remaining cells, considered as non‐myeloid cells because of the lack of CD11b and excluding double‐positive cells for FcεRI and c‐Kit (mast cell markers), were present in similar numbers in the ears of PBS and SGE groups and at both time points when compared with ears of the steady‐state group (Fig. 2i).
The cell surface markers chosen for the lymphoid panel are described in Materials and methods, and the gating strategy performed on CD45+ cells to characterize each cell population is presented in Fig. S2. The markers employed allowed us to identify four populations of lymphoid cells as follows: B‐cells, CD4+ T‐cells, CD8+ T‐cells and a population of CD3+CD4−CD8− cells, depicted as ‘other T‐cells’. The complexity and the total number of lymphoid cells in the ear were much inferior than those for myeloid populations. Because of that, the t‐SNE map produced in this case did not include myeloid populations that would overshadow the analysis (Fig. 3a). Therefore, the proportion presented in the t‐SNE map and in Fig. S4 only reflects the lymphoid populations. Regarding CD4+ T‐cells, their numbers were similar among steady‐state ears and both mosquito‐exposed groups (PBS and SGE) after 6 hr. After 24 hr, the ears of the SGE group presented higher numbers of T helper cells than the PBS group or steady‐state ears (Fig. 3b). Very few numbers of CD8+ T‐cells were detected in the ears throughout the experiment, and no significant differences were observed when comparing the number of these cells between the exposed groups and the steady‐state condition (Fig. 3c). The other T‐cells detected in our evaluation were present in lower amounts in the ears of both exposed groups after 6 hr when compared with the steady‐state ears. After 24 hr, the ears of the PBS group still presented lower numbers of these cells, while the SGE group presented similar numbers of the steady‐state condition (Fig. 3d). After 6 hr of mosquito exposure, the ears of both PBS and SGE groups presented a very low and comparable number of B‐cells, similar to that found in the steady‐state ears. Otherwise, the ears of both exposed groups presented a significant and intense increase of B‐cells after 24 hr (Fig. 3e).
Figure 3.
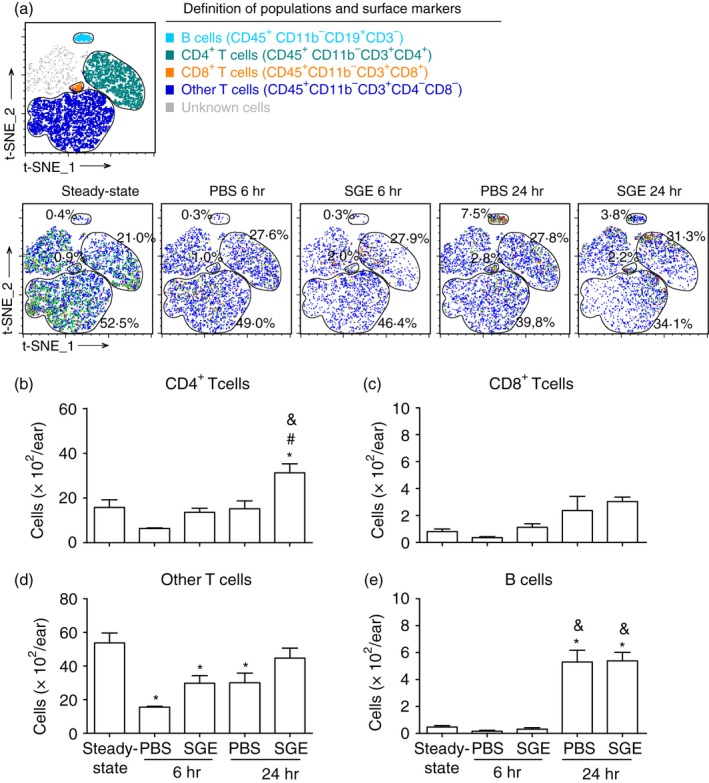
Immunophenotyping of the lymphoid infiltrate in the ear of non‐sensitized and salivary gland extract (SGE)‐sensitized mice after exposure to Aedes aegypti bites. BALB/c mice were inoculated with phosphate‐buffered saline (‘PBS’ group) or sensitized with SGE (‘SGE’ group) followed by exposure of each ear to the bites of five female mosquitoes, as described in Materials and methods. The ears were collected after 6 and 24 hr, and analysed by flow cytometry. The ears of naïve mice were included as a reference to the steady‐state condition. (a) t‐Distributed stochastic neighbour embedding (t‐SNE) map and markers were used to characterize each cell population as follows: (b) CD4+ T‐cells; (c) CD8+ T‐cells; (d) other T‐cells; (e) B‐cells; *p ≤ 0·05 versus steady‐state condition; # p ≤ 0·05 versus respective PBS group; & p ≤ 0·05 versus respective group after 6 hr.
Figure 4 presents a summary of the differences observed for myeloid and lymphoid cell populations in the ears of non‐sensitized and SGE‐sensitized mice after 6 hr and 24 hr, comparing their absolute numbers with steady‐state ears. The comparison did not take into account statistical significance, but the proportion of increase/decrease in the absolute number of each cell population. A two‐ to fourfold increase was represented by one arrow up; four‐ to eightfold increase, two arrows up; more than eightfold increase, three arrows up. On the contrary, a decrease of more than half‐fold was represented by one arrow down. An increase of up to 1·9‐fold and a decrease of less than half‐fold in the absolute number of the cells were considered as similar to the steady‐state condition (≅). Based on these parameters, two inflammatory profiles were evidenced. The first profile seems to be dependent on the presence of Ae. aegypti saliva per se, but independent of the immune status of the mice (sensitized or non‐sensitized). Despite specific time and intensity kinetics, neutrophils, monocytes, B‐cells and CD8+ T‐cells fit in this category. The second profile seems to be heavily dependent on the immune status of the mice (i.e. previous sensitization to salivary molecules); eosinophils, mast cells and CD4+ T‐cells fit in this category.
Figure 4.
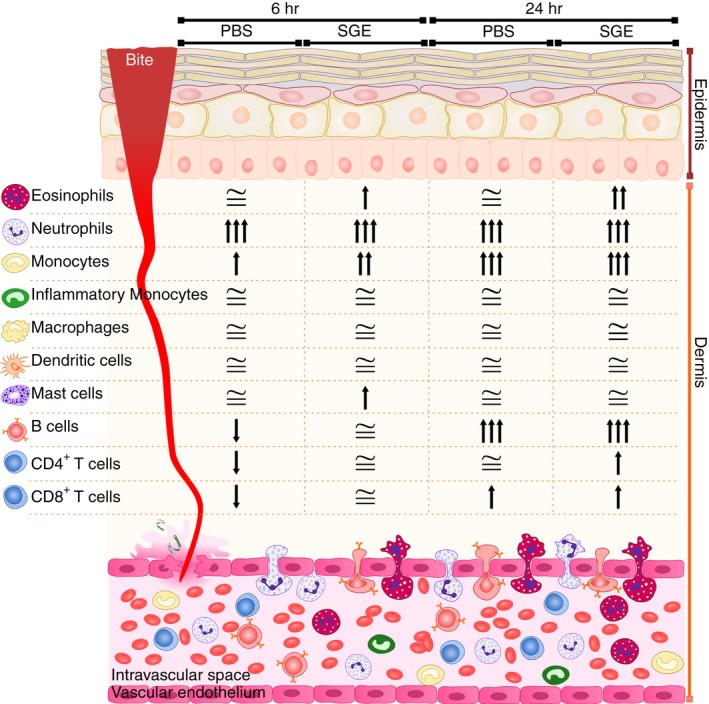
Changes on each leucocyte population in the ear of non‐sensitized and salivary gland extract (SGE)‐sensitized mice after exposure to Aedes aegypti bites related to the steady‐state condition. BALB/c mice were inoculated with phosphate‐buffered saline (‘PBS’ group) or sensitized with SGE (‘SGE’ group) followed by exposure of each ear to the bites of five female mosquitoes, as described in Materials and methods. The ears were collected after 6 and 24 hr, and analysed by flow cytometry. The ears of naïve mice were included as a reference to the steady‐state condition. The fold‐change was calculated by the ratio of the absolute number of each cell population from experimental groups and the steady‐state condition of the correspondent population: ‘↑’ represents two‐ to fourfold increase; ‘↑↑’ represents four‐ to eightfold increase, ‘↑↑↑’ represents more than eightfold increase; ‘↓’ represents more than half‐fold decrease; ‘≅’ represents the interval between a decrease of less than half‐fold and an increase of up to 1·9‐fold in the absolute number of cells.
IL‐5 levels in serum correlate with eosinophil infiltration in the ears
Knowing that IL‐5 is the most important cytokine involved in terminal differentiation and proliferation of eosinophil precursors, as well as in the activation and accumulation of eosinophils in the blood,39 the levels of this cytokine were evaluated in the serum of naïve mice and compared with the serum of PBS and SGE groups following mosquito exposure. Almost undetectable levels of IL‐5 were found in the serum of steady‐state mice, as well as in the serum of the PBS and SGE groups after 6 hr of mosquito exposure. The same was observed in the serum of the PBS group after 24 hr of exposure, while in the serum of the SGE group, a significant increase of IL‐5 was observed when compared with all previous groups (Fig. 5).
Figure 5.
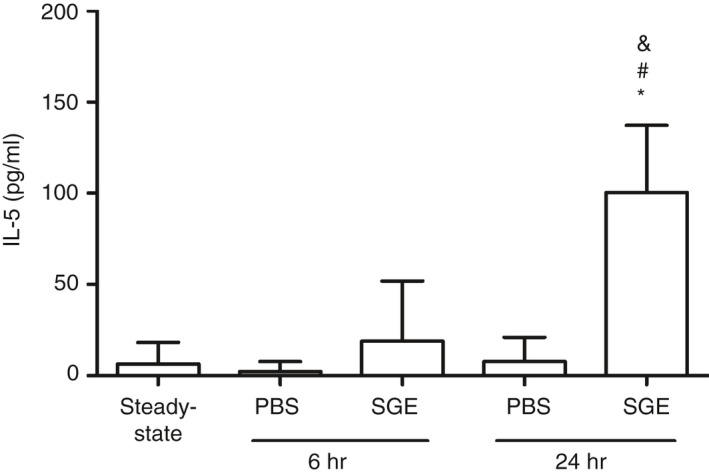
Exposure to Aedes aegypti bites promotes increased IL‐5 levels in the serum of salivary gland extract (SGE)‐sensitized mice. BALB/c mice were inoculated with phosphate‐buffered saline (‘PBS’ group) or sensitized with SGE (‘SGE’ group) followed by exposure of each ear to the bites of five female mosquitoes, as described in Materials and methods. Blood samples were collected from each mouse before death, and the IL‐5 levels were determined by ELISA. *p ≤ 0·05 versus steady‐state condition; # p ≤ 0·05 versus respective PBS group; & p ≤ 0·05 versus respective group after 6 hr.
Discussion
The skin is the primary barrier between the host and the environment, restricting water loss, protecting against chemical and physical insults, and preventing the entry of microorganisms. Among many physiological functions, the skin is recognized nowadays as an immunological organ, and provides the first line of defense against microbial pathogens.40, 41, 42, 43, 44 Mosquitoes, like many other haematophagous arthropods, possess an adapted feeding apparatus capable of breaking the skin to search for blood. During intradermal probing to find blood vessels, mosquitoes thrust their mouthparts repeatedly in the skin causing local inflammation. The saliva released in the process presents compounds with biological activities that may sensitize the host over time, triggering further immunological reactions in subsequent contacts.45
The evaluation of ear thickness following Ae. aegypti bites revealed a similar initial oedema in non‐sensitized (‘PBS’ group) and SGE‐sensitized (‘SGE’ group) mice, with a particular kinetics thereafter. In the PBS group the peak of oedema occurred after 6 hr and decreased afterwards, while in the SGE group the peak occurred 24 hr after exposure to Ae. aegypti. Nonetheless, after 6 hr of exposure to mosquitos, both PBS and SGE groups presented similar cell numbers to those found in a steady‐state condition. On the other hand, after 24 hr of exposure to mosquitoes, total leucocytes significantly increased only in the ear of the SGE group when compared with a steady‐state ear. The mosquito's blood‐feeding performance was comparable in all groups, confirming that the general changes observed were due to differences in the immune status of the animals. Taken together, these findings suggest that the increase of ear thickness after 6 hr of exposure was probably due to the plasmatic extravasation caused by the salivary components and not to cell migration, whereas the oedemas found after 24 hr of mosquito exposure in both groups were strongly correlated with the cellular infiltrate in the skin. Our results are in line with an early study evaluating skin histopathological changes following Ae. aegypti bites in humans.32 In this work, skin biopsies at 30 min and 6 hr after mosquito exposure showed a stronger oedema when compared with biopsies after 24 hr, but the cellular infiltrate was more intense at 24 hr than in the initial stages.32
The complexity of the cellular components of the skin immune system has been revealed by the effort of many research groups.43 These works complement classic histological studies by uncovering the molecular surface markers of skin immune cells, their origin (haematopoietic or not) and function. Flow cytometry has been an essential tool to accomplish these tasks, and new analysis algorithms have been recently developed to cluster cytometric data in high‐dimensional space.46, 47, 48, 49 One of these algorithms, called t‐SNE, performs an analysis that allows the visualization of high‐dimensional data in a two‐dimensional representation, using colour as a third dimension for evaluation of additional features of the cells.50 By employing two panels of antibodies for different cell surface markers combined with side‐scatter and forward‐scatter parameters (Figs S1 and S2) followed by t‐SNE analysis, we were able to perform a general identification of 10 major populations of immune cells: eosinophils, neutrophils, monocytes, inflammatory monocytes, macrophages, mast cells, dendritic cells, B‐cells, CD4+ and CD8+ T‐cells. The t‐SNE derived map clearly segregated different cell populations in space and provided the specific proportions for each cell population. These relative proportions displayed in the t‐SNE maps were mirrored by the absolute number of cells, except for the SGE group after 24 hr of mosquito exposure that had proportionally more cells and, therefore, presented more intense phenotypes compared with the other groups. The statistical analysis showed that all these populations increased or decreased in mosquito‐exposed mice at least in one time point evaluated when compared with the steady‐state condition. However, from a quantitative viewpoint, these changes segregated into two different profiles: a saliva‐dependent cell recruitment that was independent on the host immune status; and an immune‐dependent cell recruitment that occurs only on SGE‐sensitized hosts.
Regarding the cells represented by the first profile, neutrophils seem to be ubiquitously present in the bite site of most arthropods evaluated so far, including mosquitoes,32, 51 sand flies,52, 53 fleas,54, 55 soft56 and hard ticks,57, 58 among others. However, the presence of neutrophils in the skin following arthropod bites seems to be detrimental to the host in some cases: (i) the skin lesion created during tick feeding is dependent on neutrophils;59 (ii) neutrophils recruited by sand fly bite capture Leishmania major, die by apoptosis and are phagocytized by macrophages, acting as Trojan horses by enabling the parasite to establish a productive infection.60 For Ae. aegypti, the neutrophils that migrate in response to the bite are thought to coordinate a local innate immune network that recruits myeloid cells permissive to virus infection.61 Interestingly, neutrophil depletion in all the above situations reverted the respective phenotype, reducing the skin damage and collagen destruction in the case of tick infestation, and the pathogen infectivity in the case of Leishmania and viral infection.
To our surprise, monocytes (but not inflammatory monocytes) were another cell population indistinctly recruited to the ear in response to mosquito bite. To our knowledge, there is only one report in the literature regarding the recruitment of monocytes (and neutrophils) to the dermis induced by Ae. aegypti SGE. This recruitment increases when dengue virus is inoculated, and becomes even more intense when the mosquito SGE is administered in the presence of enhancing antibodies against the virus.62 For other mosquito species, the exposure of mice to Culex pipiens induced a significant increase of monocytes and other cell types in the blood, although the skin infiltrate was not evaluated in the study.63 On the contrary, both bites and SGE of Anopheles stephensi were able to reduce the monocyte recruitment to the skin and to the lymph nodes induced by injection of Plasmodium berghei sporozoites.64 Further studies are needed to understand the phenotypic dynamics of monocytes–inflammatory monocytes–macrophages in the skin following Ae. aegypti bites, and the biological meaning of our findings.
Although in very small numbers, the presence of B‐cells in the skin inflammatory infiltrate following mosquito bite is not entirely surprising. The antibody‐independent functions of these cells have been recently addressed, especially cytokine production and effector/regulatory roles.65, 66 A growing body of evidence suggests the existence of skin‐resident B‐cells, and these cells are recognized nowadays as an important population of the cutaneous immune system, participating in skin homeostasis and in inflammatory conditions associated to infection, disease and tumours through proinflammatory and immunoregulatory actions.67 In a previous work, we described an increase of B‐cell numbers in the bronchoalveolar lavage fluid of mice sensitized by mosquito bites and intranasally challenged with the mosquito SGE, but this phenotype was not observed in the lung tissue.37 Few works have described the presence of inflammatory B‐cells in skin infiltrates following exposure to haematophagous arthropods,68, 69 while others failed to observe a significant upregulation of these cells upon arthropod infestation.70, 71 In our knowledge, however, this is the first report showing and quantifying B‐cells in the skin of Ae. aegypti‐exposed mice.
On the cells represented by the second profile identified, eosinophils were strongly recruited following exposure to Ae. aegypti. In fact, eosinophils represented the cell type that proportionally increased the most in response to mosquito bites in the ears of SGE‐sensitized mice. The eosinophilic inflammation has been demonstrated by histopathology in different host species exposed to Ae. aegypti.32, 72, 73 In addition, a study by Chen et al.74 showed that natural sensitization of BALB/c mice to Ae. aegypti mosquito lead to immediate and delayed cutaneous reactions, increased IgE and IgG1 levels, as well as T‐cell proliferation, suggesting the development of a Th2 response. This may explain the specific presence of CD4+ T‐cells in the ear of SGE‐sensitized mice exposed to mosquito bites, although the phenotype of this T‐cell population remains to be characterized. Indeed, we have already demonstrated that mosquito bites sensitize mice towards a Th2 response; the intranasal challenge of these mice with salivary antigens promotes the production of IL‐4, IL‐5 and IL‐13, and the migration of eosinophil to their lungs. In addition, we observed increased T‐cell numbers in the bronchoalveolar lavage fluid but not in the lung tissue of bite‐sensitized mice challenged with the mosquito SGE.37 Many other works have shown the presence of eosinophils in the arthropod bite site of different host species.75, 76, 77, 78, 79 Of note, the proportion of eosinophils and neutrophils in the skin following bite may vary depending on the haematophagous arthropod studied, on the vertebrate host species and on their immune status (naïve versus immune/sensitized), a finding also observed in our study.
Mast cell degranulation is a pivotal event to immediate and delayed hypersensitivity to mosquito bites in sensitized hosts,80, 81 although a direct effect of An. stephensi saliva on these cells was also demonstrated in non‐sensitized hosts.82 In addition, mast cells are also involved in neutrophil recruitment during delayed hypersensitivity reactions,83 and the T‐cell‐dependent recruitment of neutrophils is reduced in mast cell‐deficient mice,84 reinforcing the role of these cells to the inflammatory environment created by mosquito saliva. In contrast, part of the immunomodulatory activities of An. stephensi saliva in vivo seem to be dependent on mast cells.85 The only study evaluating the direct effect of Ae. aegypti salivary components on mast cell biology in vitro revealed that the antigen‐specific degranulation of these cells was not affected in the presence of SGE, but the release of TNF‐α was decreased under the same conditions.86 Given the importance of mast cells on mosquito–host interactions, the interplay of these cells with Ae. aegypti saliva is recognized and it has been recently revisited in the context of dengue infection.87
A direct comparison of our findings and those of the literature is difficult because most studies evaluating inflammatory cells in the skin of mice and humans exposed to Ae. aegypti bites, or inoculated with the mosquito SGE, focused on specific cell populations and usually in the context of viral infection. For example, the histological analysis of the ear of naïve BALB/c mice exposed to bites of West Nile virus‐infected Ae. aegypti revealed a mild inflammation, while the ear of pre‐exposed mice presented an intense mononuclear and neutrophil infiltrate following the bites of infected mosquitoes.88 In another study, C57BL/6 mice lacking the IFN‐α/β receptors inoculated with the dengue virus alone or in the presence of Ae. aegypti SGE presented a modest recruitment of inflammatory neutrophils and monocytes to the dermis. However, under antibody‐enhancing conditions, the presence of SGE in the viral inoculum increased the recruitment of these cells to the tissue.62
An important aspect to be highlighted is that while some studies, such as the present one, evidence the proinflammatory nature of the salivary secretion,21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 a number of other studies describe anti‐inflammatory activities of saliva,89, 90 including some molecules associated to this phenotype.91, 92 This apparent ambiguity concerning the comprehension of vector saliva properties at the skin level may reflect an evolutionary battlefield between the mosquito and the vertebrate host. The salivary proteins are recognized by the cutaneous innate and adaptive immune system, and trigger an inflammatory reaction at the skin level (and less commonly at a systemic level) that produces the two profiles of cell recruitment discussed above. On the other hand, mosquitoes evolved salivary molecules that control inflammation in order to allow a successful blood feeding, as demonstrated by the mechanisms of action described for such salivary components. The intensity of the cutaneous reactions demonstrated in experimental models or in human studies reflects this arm‐wrestle dispute in which the anti‐inflammatory molecules may prevent the inflammation to become so intense that would turn the acquisition of a blood meal virtually impossible.
Finally, the model of skin inflammation in the ear has been used by several research groups because it requires small quantities of inoculi/stimuli.93, 94, 95, 96 Associated with the flow cytometry, a powerful tool for cell phenotyping, in addition to t‐SNE maps, a modern analysis that represent highly complex interactions in a simplified display, the present approach allowed us to partially characterize the inflammatory infiltrate in the skin and improve our understanding of the cellular changes caused by Ae. aegypti bite in non‐sensitized and sensitized hosts. Further studies will expand this universe by focusing on non‐classic and scarcer cell populations, such as subpopulations of macrophages, dendritic cells, non‐classic lymphocytes and innate lymphoid cells. Such knowledge may help to comprehend the local microenvironment found by the viruses transmitted by the mosquito during the blood meal and to establish strategies to block the infection.
Disclosure
The authors declare no commercial or financial conflict of interest.
Author contributions
MOH and ASN conceived the research, designed the experiments, and wrote the manuscript; MOH, LSN, JBA, MSB, DMF and ASN performed the experiments; MOH, JBA, DMF and ASN analysed the data and performed statistical analysis; MOH, JBA, MLC, APL, DMF and ASN discussed the data; MLC, APL, DMF and ASN contributed with reagents and materials/analysis tools. All authors read and approved the final manuscript.
Supporting information
Figure S1. Gating strategy for the flow cytometric analysis of myeloid populations in mouse ear.
Figure S2. Gating strategy for the flow cytometric analysis of lymphoid populations in mouse ear.
Figure S3. Relative proportion of the myeloid infiltrate in the ear of non‐sensitized and SGE‐sensitized mice after exposure to Ae. aegypti bites.
Figure S4. Relative proportion of the lymphoid infiltrate in the ear of non‐sensitized and SGE‐sensitized mice after exposure to Ae. aegypti bites.
Acknowledgements
The authors thank the CEFAP‐USP Core Facility for some flow cytometry performed, and Ms Sandra Alexandre Alves from Departamento de Imunologia, Instituto de Ciências Biomédicas, Universidade de São Paulo, Brazil, for technical assistance. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES ‐ Finance Code 001); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq ‐ Grant # 134636/2015‐5), Fundação de Amparo à Pesquisa do Estado de São Paulo ‐ Brasil (FAPESP ‐ Grant # 2009/09892‐6 and # 2015/25364‐0); and Núcleo de Pesquisa em Moléculas Bioativas de Artrópodes Vetores ‐ Brasil (NAP‐MOBIARVE ‐ Grant # 12.1.17661.1.7).
Contributor Information
Denise M. Fonseca, Email: denisefonseca@usp.br
Anderson Sá‐Nunes, Email: sanunes@usp.br.
References
- 1. Harbach RE. The Culicidae (Diptera): a review of taxonomy, classification and phylogeny. Zootaxa 2007; 1668:591–638. [Google Scholar]
- 2. Harbach RE, Howard TM. Index of currently recognized mosquito species (Diptera: Culicidae). Eur Mosq Bull 2007; 23:1–66. [Google Scholar]
- 3. Harbach R. Mosquito Taxonomic Inventory [Internet]. 2018. p. 16. URL http://mosquito-taxonomic-inventory.info/sites/mosquito-taxonomic-inventory.info/files/Valid Species %28composite Aedes%29_4.pdf [accessed 31 August 2018]
- 4. Consoli RAG, De Oliveira RL. Principais Mosquitos de Importância Sanitária no Brasil. Rio de Janeiro: Editora FIOCRUZ, 1994: 228. [Google Scholar]
- 5. ECDC . Aedes aegypti ‐ Factsheet for experts [Internet]. European Centre for Disease Prevention and Control. 2016. p. 10. URL https://ecdc.europa.eu/en/disease-vectors/facts/mosquito-factsheets/aedes-aegypti [accessed 31 August 2018]
- 6. Black WC, Bennett KE, Gorrochótegui‐Escalante N, Barillas‐Mury CV, Fernández‐Salas I, Muñoz ML et al Flavivirus susceptibility in Aedes aegypti . Arch Med Res 2002; 33:379–88. [DOI] [PubMed] [Google Scholar]
- 7. Christophers SR. Aedes aegypti (L.) the Yellow Fever Mosquito: Its Life History, Bionomics and Structure. London: Cambridge University Press, 1960: 132. [Google Scholar]
- 8. Dick GWA, Kitchen SF, Haddow AJ. Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–20. [DOI] [PubMed] [Google Scholar]
- 9. Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ et al Genome sequence of Aedes aegypti, a major arbovirus vector. Science 2007; 316:1718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zanluca C, de Melo VCA, Mosimann ALP, dos Santos GIV, dos Santos CND, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re‐emerging virus. Lancet 2012; 379:662–71. [DOI] [PubMed] [Google Scholar]
- 12. Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol 1995; 40:443–74. [DOI] [PubMed] [Google Scholar]
- 13. Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol 2005; 35:661–75. [DOI] [PubMed] [Google Scholar]
- 14. James AA, Rossignol PA. Mosquito salivary glands: parasitological and molecular aspects. Parasitol Today 1991; 7:267–71. [DOI] [PubMed] [Google Scholar]
- 15. Champagne DE, Ribeiro JMC. Sialokinin I and II: vasodilatory tachykinins from the yellow fever mosquito Aedes aegypti . Proc Natl Acad Sci USA 1994; 91:138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calvo E, Mizurini DM, Sa A. Alboserpin, a factor Xa inhibitor from the mosquito vector of yellow fever, binds heparin and membrane phospholipids and exhibits antithrombotic Activity. J Biol Chem 2011; 286:27 998–28 010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calvo E, Tokumasu F, Marinotti O, Villeval J, Ribeiro JMC, Francischetti IMB. Aegyptin, a novel mosquito salivary gland protein specifically binds to collagen and prevents its interaction with glycoprotein vi, integrin α2β1 and von Willebrand factor. J Biol Chem 2007; 282:2051–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Champagne DE, Smartt CT, Ribeiro JM, James AA. The salivary gland‐specific apyrase of the mosquito Aedes aegypti is a member of the 5’‐nucleotidase family. Proc Natl Acad Sci USA 1995; 92:694–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stark KR, James AA. Isolation and characterization of the gene encoding a novel factor xa‐directed anticoagulant from the yellow fever mosquito, Aedes aegypti . J Biol Chem 1998; 273:20 802–9. [DOI] [PubMed] [Google Scholar]
- 20. Watanabe RMO, Soares TS, Morais‐zani K, Tanaka‐azevedo AM, Maciel C, Capurro ML et al Biochimie A novel trypsin Kazal‐type inhibitor from Aedes aegypti with thrombin coagulant inhibitory activity. Biochimie 2010; 92:933–9. [DOI] [PubMed] [Google Scholar]
- 21. Feingold BENF, Benjamin E, Michaeli D. The allergic responses to insect bites. Annu Rev Entomol 1968; 13:137–58. [Google Scholar]
- 22. Boycott AE. Sensitisation to insect bites. Univ Coll Hosp Mag 1928; 13:200–2. [Google Scholar]
- 23. Gordon RM. The susceptibility of the individual to the bites of Stegomyia calopus . Ann Trop Med Parasitol 1922; 16:229–34. [Google Scholar]
- 24. Mellanby K. Man's reaction to mosquito bites. Nature 1946; 158:554. [DOI] [PubMed] [Google Scholar]
- 25. McKiel JA. Sensitization to mosquito bites. Can J Zool 1959; 37:341–51. [Google Scholar]
- 26. Killby VA, Silverman PH. Hypersensitive reactions in man to specific mosquito bites. Am J Trop Med Hyg 1967; 16:374–80. [DOI] [PubMed] [Google Scholar]
- 27. Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science 1960; 131:1730–1. [DOI] [PubMed] [Google Scholar]
- 28. Peng Z, Li H, Simons FER. Immunoblot analysis of salivary allergens in 10 mosquito species with worldwide distribution and the human IgE responses to these allergens. J Allergy Clin Immunol 1998; 101:498–505. [DOI] [PubMed] [Google Scholar]
- 29. Engler RJ. Mosquito bite pathogenesis in necrotic skin reactors. Curr Opin Allergy Clin Immunol 2001; 1:349–52. [DOI] [PubMed] [Google Scholar]
- 30. Kulthanan K, Wongkamchai S, Triwongwaranat D. Mosquito allergy: clinical features and natural course. J Dermatol 2010; 37:1025–31. [DOI] [PubMed] [Google Scholar]
- 31. Simons FER, Peng Z. Skeeter syndrome. J Allergy Clin Immunol 1999; 104:705–7. [DOI] [PubMed] [Google Scholar]
- 32. Goldman L, Rockwell E, Richfield DF. Histopathological studies on cutaneous reactions to the bites of various arthropods. Am J Trop Med Hyg 1952; 1:514–25. [DOI] [PubMed] [Google Scholar]
- 33. Wilson AB, Clements AN. The nature of the skin reaction to mosquito bites in laboratory animals. Int Arch Allergy 1965; 26:294–314. [DOI] [PubMed] [Google Scholar]
- 34. French FE, West AS. Skin reaction specificity of guinea pig immediate hypersensitivity to bites of four mosquito species. J Parasitol 1971; 57:396–400. [PubMed] [Google Scholar]
- 35. Maciel C, Fujita A, Gueroni DI, Ramos AD, Capurro ML, Sá‐Nunes A. Evans blue as a simple method to discriminate mosquitoes’ feeding choice on small laboratory animals. PLoS ONE 2014; 9:e110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bizzarro B, Barros MS, Maciel C, Gueroni DI, Lino CN, Campopiano J et al Effects of Aedes aegypti salivary components on dendritic cell and lymphocyte biology. Parasit Vectors 2013; 6:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barros MS, Gomes E, Gueroni DI, Ramos AD, Mirotti L, Florsheim E et al Exposure to Aedes aegypti bites induces a mixed‐type allergic response following salivary antigens challenge in mice. PLoS ONE 2016; 11:e0155454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Belkaid Y, Jouin H, Milon G. A method to recover, enumerate and identify lymphomyeloid cells present in an inflammatory dermal site: a study in laboratory mice. J Immunol Methods 1996; 199:5–25. [DOI] [PubMed] [Google Scholar]
- 39. Rogerio AP, Sá‐nunes A, Faccioli LH. The activity of medicinal plants and secondary metabolites on eosinophilic inflammation. Pharmacol Res 2010; 62:298–307. [DOI] [PubMed] [Google Scholar]
- 40. Streilein JW. Skin‐Associated Lymphoid Tissues (SALT): Origins and functions. J Invest Dermatol 1983; 120:12–6. [DOI] [PubMed] [Google Scholar]
- 41. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol 2009; 9:679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol 2014; 14:289–301. [DOI] [PubMed] [Google Scholar]
- 43. Bos J. Skin Immune System: Cutaneous Immunology and Clinical Immunodermatology. New York: CRC Press, 2004: 840. [Google Scholar]
- 44. Erin Chen Y, Fischbach MA, Belkaid Y. Skin microbiota‐host interactions. Nature 2018; 553:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ribeiro JMC. Role of saliva in blood‐feeding by arthropod. Ann Rev Entomol 1987; 32:463–78. [DOI] [PubMed] [Google Scholar]
- 46. Aghaeepour N, Finak G, Consortium TF, Consortium TD, Hoos H, Mosmann TR et al Critical assessment of automated flow cytometry data analysis techniques. Nat Methods 2013; 10:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saeys Y, Van Gassen S, Lambrecht BN. Computational flow cytometry : helping to make sense of high‐dimensional immunology data. Nat Rev Immunol 2016; 16:449–62. [DOI] [PubMed] [Google Scholar]
- 48. Kvistborg P, Gouttefangeas C, Aghaeepour N, Cazaly A, Chattopadhyay PK, Chan C et al Thinking outside the gate: Single‐cell assessments in multiple dimensions. Immunity 2015; 42:591–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weber LM, Robinson MD. Comparison of clustering methods for high‐dimensional single‐cell flow and mass cytometry data. Cytom Part A 2016; 89:1084–96. [DOI] [PubMed] [Google Scholar]
- 50. Amir ED, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC et al viSNE enables visualization of high dimensional single‐cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol 2013; 31:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karppinen A, Rantala I, Vaalasti A, Palosuo T, Reunala T. Effect of cetirizine on the inflammatory cells in mosquito bites. Clin Exp Allergy 1996; 26:703–9. [PubMed] [Google Scholar]
- 52. Teixeira C, Gomes R, Oliveira F, Meneses C, Gilmore DC, Elnaiem DEA et al Characterization of the early inflammatory infiltrate at the feeding site of infected sand flies in mice protected from vector‐transmitted Leishmania major by exposure to uninfected bites. PLoS Negl Trop Dis 2014; 8:e3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Silva F, Gomes R, Prates D. Inflammatory cell infiltration and high antibody production in BALB/c mice caused by natural exposure to Lutzomyia longipalpis bites. Am J Trop Med Hyg 2005; 72:94–8. [PubMed] [Google Scholar]
- 54. Gross TL, Halliwell RE. Lesions of experimental flea bite hypersensitivity in the dog. Vet Pathol 1985; 22:78–81. [DOI] [PubMed] [Google Scholar]
- 55. Bosio CF, Viall AK, Jarrett CO, Gardner D, Rood MP, Hinnebusch BJ. Evaluation of the murine immune response to Xenopsylla cheopis flea saliva and its effect on transmission of Yersinia pestis . PLoS Negl Trop Dis 2014; 8:e3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johnston CM, Brownt SJ. Cutaneous and systemic cellular responses induced by the feeding of the argasid tick Ornithodoros parkeri . Int J Parasitol Parasites 1985; 15:621–8. [DOI] [PubMed] [Google Scholar]
- 57. Krinsky WL, Brown SJ, Askenase PW. Ixodes dammini: induced skin lesions in guinea pigs and rabbits compared to erythema chronicum migrans in patients with lyme arthritis. Exp Parasitol 1982; 53:381–95. [DOI] [PubMed] [Google Scholar]
- 58. Brown SJ, Knapp FW. Amblyomma americanum: sequential histological analysis of adult feeding sites on guinea pigs. Exp Parasitol 1980; 49:303–18. [DOI] [PubMed] [Google Scholar]
- 59. Tatchell R, Moorhouse D. Neutrophils: their role in the formation of a tick feeding lesion. Science 1970; 167:1002–3. [DOI] [PubMed] [Google Scholar]
- 60. Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S et al In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 2008; 321:970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pingen M, Bryden SR, Pondeville E, Fazakerley JK, Graham GJ, Mckimmie CS et al Host inflammatory response to mosquito bites enhances the severity of arbovirus infection article. Immunity 2016; 44:1455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schmid MA, Glasner DR, Shah S, Michlmayr D. Mosquito saliva increases endothelial permeability in the skin, immune cell migration, and dengue pathogenesis during antibody‐dependent enhancement. PLoS ONE 2016; 12:e1005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Abdel‐Hamid YM, Wahba MM. Detection of haematologic effects of mosquito biting using an animal model. J Egypt Soc Parasitol 2006 Dec; 36:937–44. [PubMed] [Google Scholar]
- 64. Schneider BS, Mathieu C, Peronet R, Me S. Anopheles stephensi saliva enhances progression of cerebral malaria in a murine model. Vector Born Zoonotc Dis 2011; 11:423–32. [DOI] [PubMed] [Google Scholar]
- 65. Lund FE. Cytokine‐producing B lymphocytes – key regulators of immunity. Curr Opin Immunol 2008; 20:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shen P, Fillatreau S. Antibody‐independent functions of B cells: A focus on cytokines. Nature 2015; 15:441–51. [DOI] [PubMed] [Google Scholar]
- 67. Egbuniwe IU, Karagiannis SN, Nestle FO, Lacy KE. Revisiting the role of B cells in skin immune surveillance. Trends Immunol 2015; 36:102–11. [DOI] [PubMed] [Google Scholar]
- 68. Castelli E, Caputo V, Morello V, Tomasino RM. Local reactions to tick bites. Am J Dermatopathol 2008; 30:241–8. [DOI] [PubMed] [Google Scholar]
- 69. Robbertse L, Annette S, Jane S, Barnard A, Leisewitz A, Ernst J et al Ticks and tick‐borne diseases comparison of the differential regulation of T and B‐lymphocyte subsets in the skin and lymph nodes amongst three cattle breeds as potential mediators of immune‐resistance to Rhipicephalus microplus . Ticks Tick Borne Dis 2018; 9:976–87. [DOI] [PubMed] [Google Scholar]
- 70. Bowles VM, Grey ST, Brandon MR. Cellular immune responses in the skin of sheep infected with larvae of Lucilia cuprina, the sheep blowfly. Vet Parasitol 1992; 44:151–62. [DOI] [PubMed] [Google Scholar]
- 71. Mbow ML, Rutti B, Brossard M. Infiltration of CD4+ CD8+ T cells, and expression of ICAM‐1, Ia antigens, IL‐1 alpha and TNF‐alpha in the skin lesion of BALB/c mice undergoing repeated infestations with nymphal Ixodes ricinus ticks. Immunology 1994; 596–602. [PMC free article] [PubMed] [Google Scholar]
- 72. French FE. Aedes aegypti: histopathology of immediate skin reactions of hypersensitive guinea pigs resulting from bites. Exp Parasitol 1972; 32:175–80. [DOI] [PubMed] [Google Scholar]
- 73. Rockwell EM, Johnson P. The insect bite reaction. II Evaluation of the allergic reaction. J Invest Dermatol 1952; 19:137–55. [DOI] [PubMed] [Google Scholar]
- 74. Chen YL, Simons FER, Peng Z. A mouse model of mosquito allergy for study of antigen‐specific IgE and IgG subclass responses, lymphocyte proliferation, and IL‐4 and IFN‐gamma production. Int Arch Allergy Immunol 1998; 116:269–77. [DOI] [PubMed] [Google Scholar]
- 75. Larrivee DH, Benjamini E, Feingold BF, Shimizu M. Histologic stages studies of allergic of guinea reactivity pig to skin: flea different bites. Exp Parasitol 1964; 15:491–502. [DOI] [PubMed] [Google Scholar]
- 76. Fettelschoss‐Gabriel A, Fettelschoss V, Thoms F, Giese C, Daniel M, Olomski F et al Treating insect‐bite hypersensitivity in horses with active vaccination against IL‐5. J Allergy Clin Immunol 2018; 142:1194–205. [DOI] [PubMed] [Google Scholar]
- 77. Lin HL, Lin JN, Chen CW, Kuo LC, Lee WC. Eosinophilic cellulitis after honeybee sting. J Formos Med Assoc 2009; 108:964–6. [DOI] [PubMed] [Google Scholar]
- 78. Anderson JM, Moore IN, Nagata BM, Ribeiro JMC, Valenzuela JG, Sonenshine DE. Ticks, Ixodes scapularis, feed repeatedly on white‐footed mice despite strong inflammatory response: an expanding paradigm for understanding tick‐host interactions. Front Immunol 2017; 8:e1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Belkaid Y, Valenzuela JG, Kamhawi S, Rowton E, Sacks DL. Delayed‐type hypersensitivity to Phlebotomus papatasi sand fly bite : an adaptive response induced by the fly? Proc Natl Acad Sci USA 2000; 97:6704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Peng Z, Rasic N, Liu Y, Simons F. Mosquito saliva – specific IgE and IgG anti‐ bodies in 1059 blood donors. J Allergy Clin Immunol 2002; 110:816–7. [DOI] [PubMed] [Google Scholar]
- 81. Reunala T, Brummer‐Korvenkontio H, Palosuo T. Are we really allergic to mosquito bites? Ann Med 1994; 26:301–6. [DOI] [PubMed] [Google Scholar]
- 82. Demeure CE, Brahimi K, Hacini F, Marchand F, Péronet R, Huerre M et al Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J Immunol 2005; 174:3932–40. [DOI] [PubMed] [Google Scholar]
- 83. Biedermann BT, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G et al Mast cells control neutrophil recruitment during T cell‐mediated delayed‐type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med 2000; 192:1441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Askenase PW, Van LH, Ron Y, Theoharides TC, Nordlund JJ, Scovern H et al Defective elicitation of delayed‐type hypersensitivity in W/Wv and SI/SId mast cell‐deficient mice. J Immunol 1983; 101:2687–94. [PubMed] [Google Scholar]
- 85. Depinay N, Hacini F, Beghdadi W, Depinay N, Beghdadi W, Peronet R et al Mast cell‐dependent down‐regulation of antigen‐specific immune responses by mosquito bites. J Immunol 2006; 176:4141–6. [DOI] [PubMed] [Google Scholar]
- 86. Bissonnette EY, Rossignol P, Befus AD. Extracts of mosquito salivary gland inhibit tumour necrosis factor alpha release from mast cells. Parasit Vectors 1993; 15:27–33. [DOI] [PubMed] [Google Scholar]
- 87. Rathore APS, John ALS. Immune responses to dengue virus in the skin. Open Biol 2018; 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schneider BS, Mcgee CE, Jordan JM, Stevenson HL, Soong L, Higgs S. Prior exposure to uninfected mosquitoes enhances mortality in naturally‐transmitted West Nile Virus infection. PLoS ONE 2007; 2:e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sales‐campos H, Reis P, Souza D, José P, Daniel A, Nardini V et al International immunopharmacology Aedes aegypti salivary gland extract ameliorates experimental in flammatory bowel disease. Int Immunopharmacol 2015; 26:13–22. [DOI] [PubMed] [Google Scholar]
- 90. Barros M, Lara P, Fonseca M, Moretti E, Filgueira L, Martins J et al Aedes aegypti saliva impairs M1‐associated proinflammatory phenotype without promoting or affecting M2 polarization of murine macrophages. Parasit Vectors 2019; 12:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jin L, Guo X, Shen C, Hao X, Sun P, Li P et al Salivary factor LTRIN from Aedes aegypti facilitates the transmission of Zika virus by interfering with the lymphotoxin‐β receptor. Nat Immunol 2018; 19:342–53. [DOI] [PubMed] [Google Scholar]
- 92. Calvo E, Mans BJ, Ribeiro MC, Andersen JF. Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein. Proc Natl Acad Sci USA 2009; 106:3728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ashraf MI, Shahzad M, Shabbir A. Oxyresveratrol ameliorates allergic airway inflammation via attenuation of IL‐4, IL‐5, and IL‐13 expression levels. Cytokine 2015; 76:375–81. [DOI] [PubMed] [Google Scholar]
- 94. Cochez PM, Michiels C, Hendrickx E, Van BA, Lemaire MM, Dauguet N et al AhR modulates the IL‐22‐producing cell proliferation/recruitment in imiquimod‐induced. Eur J Immunol 2016; 46:1449–59. [DOI] [PubMed] [Google Scholar]
- 95. Moon PD, Han NR, Ryu KJ, Kang SW, Go JH, Jang JB et al A novel compound 2‐(4‐{2‐[(phenylthio)acetyl]carbonohydrazonoyl}phenoxy)acetamide downregulates TSLP through blocking of caspase‐1/NF‐κB pathways. Int Immunopharmacol 2016; 38:420–5. [DOI] [PubMed] [Google Scholar]
- 96. Pohin M, Guesdon W, Mekouo AAT, Rabeony H, Paris I, Atanassov H et al Oncostatin M overexpression induces skin inflammation but is not required in the mouse model of imiquimod‐induced psoriasis‐like inflammation. Eur J Immunol 2016; 46:1737–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gating strategy for the flow cytometric analysis of myeloid populations in mouse ear.
Figure S2. Gating strategy for the flow cytometric analysis of lymphoid populations in mouse ear.
Figure S3. Relative proportion of the myeloid infiltrate in the ear of non‐sensitized and SGE‐sensitized mice after exposure to Ae. aegypti bites.
Figure S4. Relative proportion of the lymphoid infiltrate in the ear of non‐sensitized and SGE‐sensitized mice after exposure to Ae. aegypti bites.


