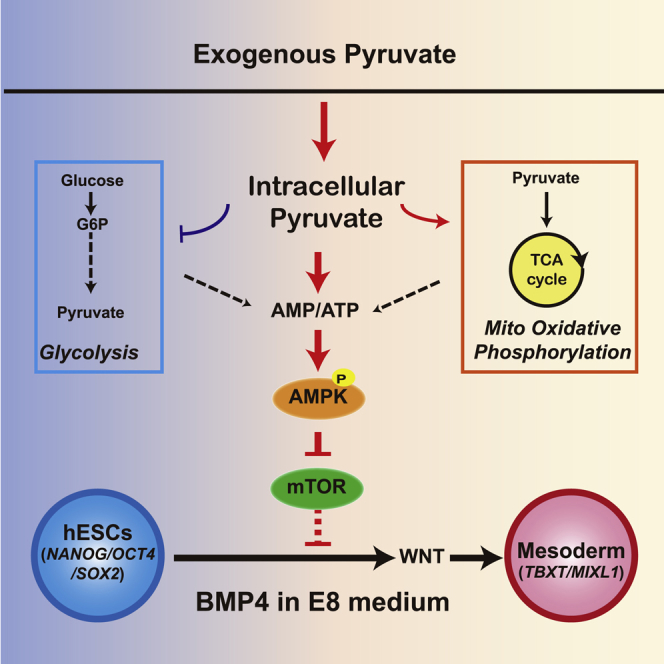
Summary
Pyruvate is a key metabolite in glycolysis and the tricarboxylic acid (TCA) cycle. Exogenous pyruvate modulates metabolism, provides cellular protection, and is essential for the maintenance of human preimplantation embryos and human embryonic stem cells (hESCs). However, little is known about how pyruvate contributes to cell-fate determination during epiblast stage. In this study, we used hESCs as a model to demonstrate that elevated exogenous pyruvate shifts metabolic balance toward oxidative phosphorylation in both maintenance and differentiation conditions. During differentiation, pyruvate potentiates mesoderm and endoderm lineage specification. Pyruvate production and its mitochondrial metabolism are required in BMP4-induced mesoderm differentiation. However, the TCA-cycle metabolites do not have the same effect as pyruvate on differentiation. Further study shows that pyruvate increases AMP/ATP ratio, activates AMPK, and modulates the mTOR pathway to enhance mesoderm differentiation. This study reveals that exogenous pyruvate not only controls metabolism but also modulates signaling pathways in hESC differentiation.
Keywords: hESCs, pyruvate, BMP4, glycolysis, TCA cycle, AMPK, WNT, metabolism, mesoderm, differentiation
Graphical Abstract
Highlights
-
•
Exogenous pyruvate enhances TCA-cycle metabolism in hESCs
-
•
Exogenous pyruvate promotes BMP4-induced mesoderm differentiation
-
•
Exogenous pyruvate influences differentiation through AMPK/mTOR pathways
In this report, Chen and colleagues demonstrate that pyruvate promotes mesoderm differentiation in human embryonic stem cells. Pyruvate elevates TCA-cycle metabolism and modulates AMPK and mTOR pathways to promote BMP4-induced mesoderm differentiation. This discovery highlights that pyruvate as a common metabolite can regulate signaling pathways in cell-fate determination.
Introduction
Balanced cellular metabolism is essential for cell survival and proliferation in embryogenesis and cancer biology. Metabolic balance is shifted when the cell fate is changed in somatic reprogramming or stem cell differentiation (Cliff and Dalton, 2017, Shyh-Chang and Daley, 2015, Teslaa and Teitell, 2015). Increasing evidence shows that the modulation of cellular metabolism affects self-renewal and cell-fate determination (Ito and Ito, 2016, Pavlova and Thompson, 2016, Zhang et al., 2012). Pyruvate is the keystone molecule in mammalian metabolism and has been widely used to culture human preimplantation embryos and human embryonic stem cells (hESCs) (Butcher et al., 1998, Conaghan et al., 1993, International Stem Cell Initiative et al., 2010). Here we investigated the impact of exogenous pyruvate on hESC metabolism, and further revealed its roles in signal transduction and cell-fate determination.
Pyruvate is the main product from glycolysis, and can be utilized in either lactate production or generation of acetyl-coenzyme A (CoA) for the tricarboxylic acid (TCA) cycle (Gray et al., 2014). In pluripotent stem cells, pyruvate is mainly converted to lactic acid via lactic acid fermentation, and the rest is utilized through the TCA cycle to produce energy in oxidative phosphorylation (Gray et al., 2014, Liu et al., 2018, Olson et al., 2016). In contrast, pyruvate is mainly metabolized through the TCA cycle in somatic cells (Pavlova and Thompson, 2016). The interference of pyruvate-related metabolism has a profound impact on autophagy, cell survival, and cell differentiation in stem cells (Garcia-Prat et al., 2017, Moussaieff et al., 2015b, Nagaraj et al., 2017). For example, the inhibition of glycolysis suppresses endogenous pyruvate production and induces differentiation (Burgess et al., 2014, Lunt and Vander Heiden, 2011, Moussaieff et al., 2015b, Vander Heiden et al., 2009). During gastrulation and stem cell differentiation, the balance of pyruvate usage is shifted from glycolysis to mitochondrial oxidative metabolism (Butcher et al., 1998, Conaghan et al., 1993, International Stem Cell Initiative et al., 2010). All the evidence suggests that pyruvate plays a central role in stem cell regulation.
Cells in our body are constantly under the influence of extracellular pyruvate, which can be imported through its transporters for intracellular metabolism (Halestrap et al., 1990, Poole and Halestrap, 1993). Exogenous pyruvate has been widely used in clinical practice and basic research. In clinical practice, systemic administration of pyruvate is used as a therapeutic intervention for cardiac, neurological, and acid-base disorders (Salahudeen et al., 1991). In basic research, up to 40 mM pyruvate has been used in cell culture, and many basic culture media contain 0.5–1 mM pyruvate (Chavez-Perez et al., 2011, Hereng et al., 2011, Watanabe et al., 2017). Pyruvate is used in all preimplantation embryo and hESC media (Butcher et al., 1998, Conaghan et al., 1993, International Stem Cell Initiative et al., 2010), and displays cellular protection to stem cells (Gonzalez et al., 2005, Hereng et al., 2011, Ramos-Ibeas et al., 2017, Salahudeen et al., 1991). In our laboratory we use E8 medium, which contains 0.5 mM pyruvate, to maintain hESCs.
Exogenous pyruvate is important in early embryogenesis. Pyruvate uptake from media is essential for preimplantation embryos (Butcher et al., 1998). Pyruvate regulates the nuclear localization of TCA-cycle enzymes and is required for the genomic activation in mammalian zygote (Nagaraj et al., 2017). At the same time, pyruvate also plays a role in epigenetic modification. Pyruvate is converted to acetyl-CoA, which contributes to histone acetylation (Moussaieff et al., 2015b, Pietrocola et al., 2015). Elevated exogenous pyruvate also promotes the nuclear translocation of pyruvate dehydrogenase (PDH) for histone acetylation (Sutendra et al., 2014). In addition, pyruvate generates α-ketoglutarate (α-KG) in the TCA cycle, which is involved in DNA methylation (Carey et al., 2015, Moussaieff et al., 2015b, TeSlaa et al., 2016). Given its essential roles in embryogenesis, it would be important to explore the regulatory functions of pyruvate in hESC maintenance and differentiation.
Pluripotency and differentiation are influenced by exogenous metabolites such as methionine, serine, acetate, and membrane-permeable α-KG analog (dimethyl α-KG, DM2OG) (Carey et al., 2015, Kilberg et al., 2016, Nagaraj et al., 2017, Shiraki et al., 2014, Wang et al., 2009). We and others have shown that some exogenous metabolites can also influence signaling cascades (Meng et al., 2018b, Carcamo et al., 2004). Because of pyruvate's critical roles in metabolism and early embryogenesis, we hypothesize that exogenous pyruvate can influence metabolism and signaling pathways in hESCs. In this report, we demonstrate that elevated exogenous pyruvate alters the metabolic profile and enhances oxidative metabolism. Pyruvate changes the expression of metabolic genes and the phosphorylation of metabolic enzymes. In differentiation, pyruvate potentiates mesodermal and endodermal lineage-specific differentiation. We further show that pyruvate stimulates mesoderm differentiation by modulating AMP kinase (AMPK) and mammalian target of rapamycin (mTOR) pathways. Our study highlights pyruvate as a key modulator that integrates metabolism and signal transduction in stem cell regulation.
Results
Exogenous Pyruvate Shifts Metabolic Balance in Undifferentiated hESCs
Considering pyruvate's central role in glycolysis and mitochondrial metabolism, we investigated how elevated exogenous pyruvate influences cellular activities in hESCs. To understand the interactions between different culture components, we chose chemically defined E8 medium as the platform to study pyruvate influence. All of the media contain 0.5 mM pyruvate in base medium, and extra pyruvate was added to test the impact of elevated exogenous pyruvate.
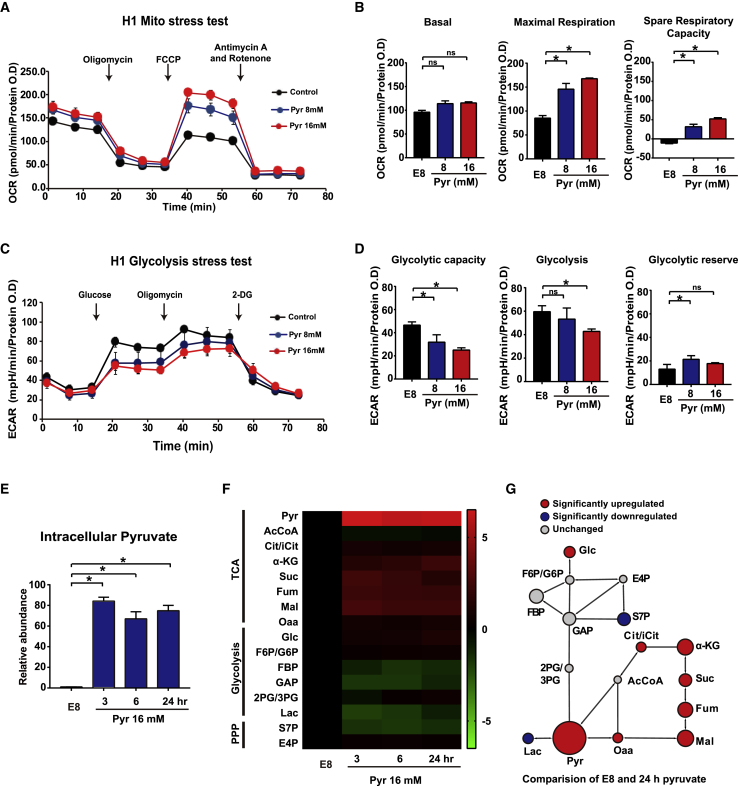
We first examined whether elevated exogenous pyruvate directly affects cellular metabolism in hESCs. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured in H1 hESCs using extracellular metabolic flux analyses conducted by the Seahorse Analyzer. Elevated exogenous pyruvate significantly increased maximal respiration and spare respiration capacity in the cell Mito stress test (Figures 1A and 1B). At the same time, ECAR was suppressed by elevated pyruvate in the cell glycolysis stress test (Figure 1C). Elevated exogenous pyruvate significantly suppressed glycolytic capacity and glycolysis (Figure 1D). We also tested metabolic changes in fibroblast cells, whereby pyruvate showed no effect on OCR and ECAR levels (Figures S1A and S1B).
Figure 1.
Exogenous Pyruvate Alters Metabolic Balance in hESC Maintenance
(A) Oxygen consumption rate (OCR) of H1 cells under different concentrations of pyruvate (Pyr), measured with Mito stress test. Pyruvate treatment was added during the assay (n ≥ 3 biological repeats).
(B) Basal respiration, maximal respiration, and spare respiratory capacity of H1 cells, calculated from Mito stress test results in (A) (n = 3 biological repeats, ∗p < 0.05; ns, not significant).
(C) Extracellular acidification rate (ECAR) of H1 cells under different concentrations of pyruvate (Pyr), measured with glycolysis stress test. Pyruvate treatment was added during the assay (n ≥ 3 biological repeats).
(D) Glycolytic capacity, glycolysis level, and glycolytic reserve of H1 cells, calculated from glycolysis stress test results in (B) (n = 3 biological repeats, ∗p < 0.05; ns, not significant).
(E) LC-MS analysis of intracellular pyruvate levels under exogenous pyruvate treatment. H1 cells were cultured with 16 mM pyruvate for 0, 3, 6, and 24 h in E8 medium before sample collection (n ≥ 3 biological repeats, ∗p < 0.05).
(F) Heatmap showing the intracellular levels of key metabolites in TCA cycle, glycolysis, and pentose phosphate (PPP) pathways under pyruvate treatment, measured by LC-MS. H1 cells were treated with 16 mM pyruvate for 3, 6, and 24 h. Metabolite levels at each time point were normalized to the levels in E8-cultured cells, and colors represent fold change.
(G) Cytoscape network map with the Metscape plug-in generated from LC-MS data in (F), showing the relative fold changes of metabolites after 16 mM pyruvate treatment for 24 h. Red nodes indicate significantly increased metabolite levels compared with control (E8), blue nodes indicate significantly decreased levels, and gray nodes indicate no significant changes. The size of nodes represents relative fold changes (n ≥ 3 biological repeats).
See also Figure S1.
We then assessed pyruvate's effect on metabolism by liquid chromatography-mass spectrometry (LC-MS). As expected, elevated exogenous pyruvate significantly increased the intracellular pyruvate level (Figure 1E), indicating that pyruvate was imported into hESCs. Further analysis by LC-MS showed that pyruvate treatment significantly increased almost all metabolites in the TCA cycle except for acetyl-coA. Meanwhile, most metabolites in glycolysis and pentose pathway were not significantly changed besides glucose (Glc), sedoheptulose 7-phosphate (S7P), and lactic acid (Lac) (Figures 1F and 1G). The increased TCA-cycle metabolites and decreased lactic acid were consistent with the metabolism test by Seahorse Analyzer (Figures 1A–1D). These data indicate that exogenous pyruvate significantly promote mitochondrial oxidative phosphorylation for energy generation in hESCs.
Lineage-Specific Effects of Exogenous Pyruvate during Differentiation
The shift of energy production from glycolysis to oxidative phosphorylation is often associated with the exit from self-renewal (Candelario et al., 2013, Gu et al., 2016, Shyh-Chang and Daley, 2015, Zhang et al., 2011). We examined whether the shifted metabolic balance by pyruvate could affect gene expression of pluripotency markers. Additional pyruvate did not significantly change the expression of NANOG, POU5F1, and SOX2 in hESCs maintained in E8 medium (Figure S1C).
We then examined whether pyruvate affects differentiation in different platforms (Figure S1D). In spontaneous differentiation, the expression of NANOG, POU5F1, and SOX2 decreased to a similar extent with or without extra pyruvate (Figure S1E), but elevated pyruvate increased the expression of TBXT (BRACHYURY, T), MIXL1, HHEX, EOMES, and SOX17 in mesoderm and endoderm lineages (Figure S1F), while suppressing the expression of ectodermal markers PAX6, PAX3, and SOX1 (Figure S1G). This suggests that pyruvate has distinctive effects on the spontaneous differentiation to different lineages.
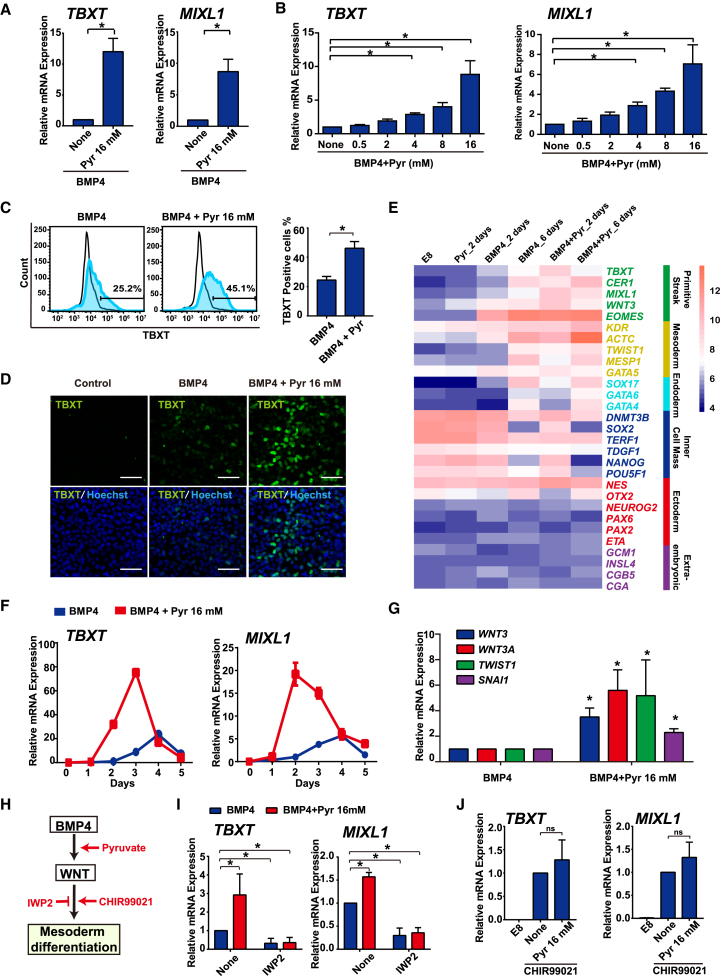
We further investigated the impact of exogenous pyruvate during lineage-specific differentiation, including mesoderm, endoderm, and ectoderm differentiation (Figure S1D). Elevated pyruvate suppressed the expression of PAX6 and SOX1 in ectoderm differentiation under the dual inhibition of transforming growth factor β (TGFβ) and bone morphogenetic protein (BMP) pathways (Figure S1H). In endoderm differentiation induced by activin A, endoderm marker SOX17 expression was significantly increased by elevated pyruvate according to flow-cytometry analysis (Figure S1I). In BMP4-driven mesoderm differentiation, elevated pyruvate significantly enhanced the expression of mesodermal markers TBXT and MIXL1 (Figure 2A). These results suggest that elevated exogenous pyruvate affects hESC differentiation in a lineage-specific manner. In the rest of this study we focused on the molecular mechanisms of pyruvate in mesoderm differentiation.
Figure 2.
Exogenous Pyruvate Potentiates Mesoderm Differentiation under BMP4 Induction through WNT Pathway
(A) Effect of pyruvate on BMP4-induced mesoderm differentiation. H1 cells were induced to mesoderm cell fate by BMP4 in E8 medium with or without pyruvate supplement and collected on day 2 for qRT-PCR analysis (n = 3 independent experiments, ∗p < 0.05).
(B) Dose response to pyruvate during mesoderm differentiation. H1 cells were induced toward mesoderm cell fate by BMP4 in the presence of pyruvate at different concentrations, and the expression of TBXT and MIXL1 was measured by qRT-PCR after 2 days. Data shown are normalized to day-0 levels (n = 3 independent experiments, ∗p < 0.05).
(C) FACS analysis of TBXT expression on day 3 of BMP4-induced differentiation with or without pyruvate treatment. Left: representative image of histogram. Right: bar graph showing mean ± SD of three independent experiments (∗p < 0.05).
(D) Immunostaining of TBXT on day 3 of BMP4-induced differentiation with or without pyruvate treatment. Control, no BMP4 treatment. Scale bars, 50 μm.
(E) Microarray analysis of gene expression in major lineages after 2 days and 6 days of pyruvate treatment during H1 maintenance or mesoderm induction.
(F) Time course of gene expression (TBXT/MIXL1) under pyruvate treatment during mesoderm differentiation, measured by qRT-PCR. Results are representative of three independent experiments.
(G) qRT-PCR analysis of WNT pathway-related genes during BMP4-induced differentiation with or without 16 mM pyruvate treatment for 2 days (n = 3 independent experiments, ∗p < 0.05).
(H) Schematic showing pyruvate modulation of BMP4- and WNT pathway-induced mesoderm differentiation. CHIR99021 is an activator of WNT pathway, and IWP2 is an inhibitor of WNT pathway.
(I) qRT-PCR analysis of the effect of WNT inhibitor IWP2 (3 μM) on mesoderm differentiation with or without pyruvate (Pyr) treatment for 2 days (n = 3 independent experiments, ∗p < 0.05).
(J) qRT-PCR analysis of the impact of pyruvate on 5 μM CHIR99021-induced mesoderm differentiation for 1 day (n = 3 independent experiments, not significant [ns]).
See also Figures S1 and S2.
Exogenous Pyruvate Potentiates Mesoderm Differentiation under BMP4 Induction
We further investigated how exogenous pyruvate affects mesoderm induction (Figure 2A). The expression of mesoderm markers TBXT and MIXL1 were significantly elevated by pyruvate in a dose-dependent manner, and the maximum effect was reached at 16 mM pyruvate (Figure 2B). The effect of pyruvate on mesoderm differentiation was validated in multiple hESC and induced pluripotent stem cell (iPSC) lines, including H9 hESCs, and ND1 and ND2 human iPSCs (Figure S1J). The positive impact by pyruvate was then confirmed by fluorescence-activated stem cell (FACS) analysis and immunostaining of TBXT expression in H1 cells (Figures 2C and 2D).
Global gene expression was evaluated with microarray analysis, and elevated pyruvate induced significant changes in gene expression during differentiation (Figure S2A). Under BMP4 treatment, elevated pyruvate significantly increased mesendoderm gene expression after treatment for 2 days and 6 days (Figure 2E). Pyruvate led to a distinctive expression profile in glycolysis- and TCA-cycle-related genes (Figures S2B and S2C). Interestingly, the pattern of metabolic gene expression was influenced by BMP4 treatment. Pyruvate also enhanced gene expression related to gastrulation and the development of various organs (Figure S2D). We also showed that pyruvate positively affected gene expression in various aspects of cellular functions, such as metabolism, cell adhesion, extracellular matrix signaling, and Sonic Hedgehog and WNT pathways (Figure S2E). These data were consistent with the modulation of metabolism and epithelial-to-mesenchymal transition during mesoderm differentiation.
To understand how the pyruvate effect is related to signal transduction, we examined the emergence of key mesodermal genes at specific time points. Elevated pyruvate not only enhanced TBXT and MIXL1 expression but also accelerated their emergence (Figure 2F). This indicates that pyruvate sensitized the cellular response to BMP4 during mesoderm cell-fate determination. We then investigated whether the elevated pyruvate could bypass the requirement of key signaling pathways. Even with additional pyruvate, mesodermal differentiation was suppressed by the inhibitors of ERK, BMP, or TGFβ pathways (Figure S2F), suggesting that pyruvate is not an active differentiation inducer but augments differentiation upon induction. This is also consistent with previous findings that the elevated pyruvate in E8 medium did not decrease pluripotency gene expression on its own (Figure S1C).
WNT activation is a key event in mesodermal commitment in BMP4-induced mesoderm differentiation (Figure 2H) (Kurek et al., 2015). Under BMP4 treatment, pyruvate enhanced the expression of key WNT pathway genes including WNT3, WNT3A, TWIST1, and SNAI1 (Figure 2G). Microarray data also showed increased gene expression in some components of the canonical WNT pathway (Figure S2G). qPCR analysis showed that the expression pattern of WNT receptor family members was also affected by pyruvate, BMP4, and their joint treatments (Figure S2H). The WNT inhibitor IWP2 significantly suppressed TBXT and MIXL1 expression (Figure 2I). Interestingly, additional pyruvate did not enhance mesodermal differentiation that was initiated by WNT pathway activator CHIR99021 (Figure 2J). This indicates that the pyruvate effect is stage specific. Pyruvate promotes BMP4-induced differentiation, but WNT activation bypasses the need for elevated pyruvate.
Exogenous Pyruvate Increases Metabolism in the TCA Cycle during Mesoderm Differentiation
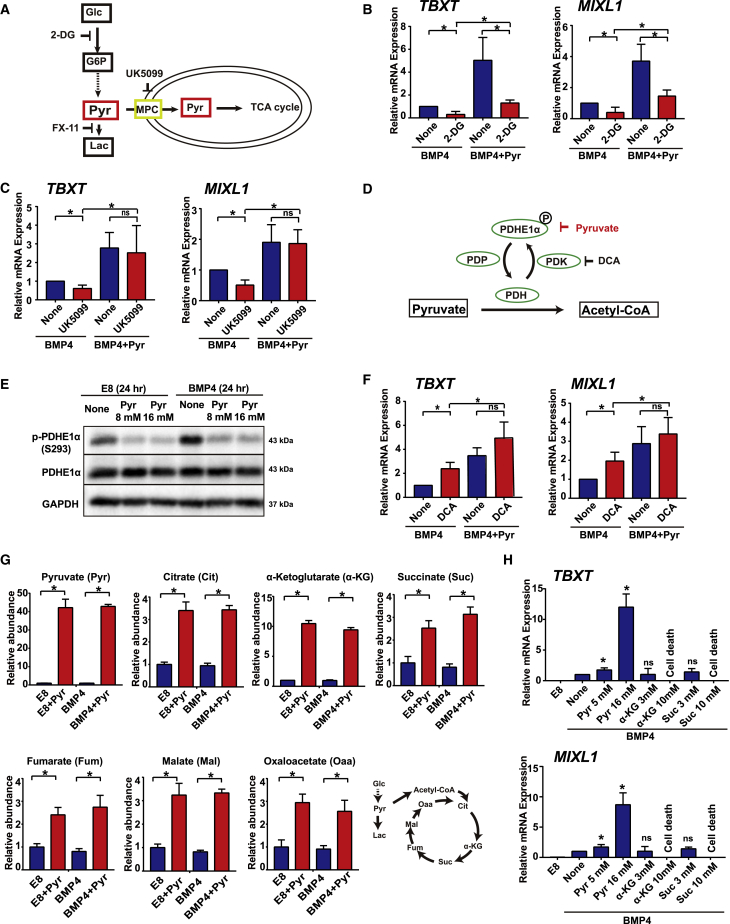
We examined whether mesoderm differentiation could be affected by the modulators of pyruvate-related metabolism (Figure 3A). We showed that the glycolysis inhibitor 2-deoxy-D-glucose (2-DG) significantly suppressed mesoderm marker gene expression induced by BMP4, and the addition of pyruvate partially rescued the phenotype (Figure 3B). In contrast, 2-DG did not have the same inhibitory effect on mesodermal differentiation induced by the WNT activator CHIR99021 (Figure S3A). We also used UK5099 to inhibit pyruvate transportation across mitochondrial membrane (McCommis and Finck, 2015). UK5099 significantly inhibited mesodermal induction, but elevated exogenous pyruvate fully rescued the differentiation phenotype (Figure 3C). At the same time, mesoderm differentiation is not affected by the inhibition of lactic acidosis by FX11, a lactate dehydrogenase A (LDHA) inhibitor (Figure S3B). These results suggest that pyruvate production and its mitochondrial metabolism are important for mesodermal induction.
Figure 3.
Exogenous Pyruvate Modulates TCA-Cycle Activity in Maintenance and Mesoderm Differentiation
(A) Schematic showing the glycolysis pathway and the functions of relevant inhibitors.
(B) qRT-PCR analysis of the impact of 3 mM 2-DG during BMP4-induced differentiation with or without 16 mM pyruvate treatment for 2 days (n = 3 independent experiments, ∗p < 0.05).
(C) qRT-PCR analysis of the impact of 3 μM UK5099 during BMP4-induced differentiation with or without 16 mM pyruvate treatment for 2 days (n = 3 independent experiments, ∗p < 0.05; ns, not significant).
(D) Schematic of pyruvate dehydrogenase (PDH) and the function of pyruvate and PDK inhibitor DCA.
(E) Western blotting analysis of PDHE1α phosphorylation during maintenance and differentiation, with or without 8 or 16 mM pyruvate treatment. Results shown are representative of three independent experiments.
(F) qRT-PCR analysis of the impact of 3 mM DCA during BMP4-induced differentiation with or without 16 mM pyruvate treatment for 2 days (n = 3 independent experiments, ∗p < 0.05; ns, not significant).
(G) Levels of key metabolites during maintenance and differentiation, measured by LC-MS analysis. H1 cells were cultured with or without 16 mM pyruvate for 24 h with or without BMP4 induction in E8 medium. Metabolite levels were normalized to the levels in control cells without BMP4 or pyruvate treatment (n = 3 biological repeats, ∗p < 0.05).
(H) Effects of metabolites on early mesoderm differentiation. Pyr, sodium pyruvate; α-KG, dimethyl 2-oxoglutarate; Suc, dimethyl succinate (n = 3 independent experiments, ∗p < 0.05; ns, not significant compared with control). None, no pyruvate treatment.
See also Figure S3.
After pyruvate is imported into mitochondria, it is converted to acetyl-CoA for the TCA cycle by the pyruvate dehydrogenase (PDH) complex. Phosphorylation of PDHE1α by pyruvate dehydrogenase kinase (PDK) leads to PDH inhibition and TCA-cycle suppression (Figure 3D). We showed that elevated pyruvate suppressed PDHE1α phosphorylation in a dose-dependent manner both in E8 medium and under BMP4-containing differentiation conditions (Figures 3E and S3C). This indicates that exogenous pyruvate induces a feedback to promote its own metabolism and generate more acetyl-CoA by suppressing PDH phosphorylation. The PDK inhibitor dichloroacetate (DCA) suppresses PDH phosphorylation and promotes pyruvate conversion to acetyl-CoA (Rodrigues et al., 2015, Ruggieri et al., 2015, Shen et al., 2013). It was also reported that DCA suppresses pluripotency by promoting the PDH cycle (Rodrigues et al., 2015). We showed that DCA significantly enhanced mesodermal differentiation (Figure 3F). Taken together, these results suggest that increased pyruvate metabolism in mitochondria is beneficial to mesodermal differentiation.
To understand how pyruvate metabolism through the TCA cycle could affect differentiation, we examined the metabolite profile 24 h after pyruvate treatment in either maintenance or differentiation conditions. In both conditions, exogenous pyruvate treatment significantly increased the intracellular levels of TCA-cycle metabolites, including citrate, α-ketoglutarate, succinate, fumarate, malate, and oxaloacetate (Figure 3G). We then inspected whether the metabolites in the TCA cycle could affect mesoderm differentiation. Dimethyl α-KG (α-KG) and dimethyl succinate (Suc) did not significantly enhance mesoderm differentiation as strongly as pyruvate, and caused cell death at high concentration (Figure 3H), implying that pyruvate does not regulate differentiation through its TCA-cycle metabolites. Researchers also reported that histone acetylation influences hESC differentiation (Moussaieff et al., 2015b). We used acetate and butyrate to promote histone acetylation, and also applied anacardic acid to inhibit histone acetylation. We found that none of them enhanced mesoderm differentiation, similar to pyruvate (Figure S3D). These data indicate that histone acetylation is not the main pathway that is activated by pyruvate in mesoderm differentiation.
Exogenous Pyruvate Increases AMP/ATP Ratio and Activates AMPK Pathway in Mesoderm Differentiation
While exploring pyruvate's impact on differentiation, we noticed that mesoderm differentiation was enhanced by pretreatment with exogenous pyruvate prior to BMP4 exposure (Figure 4A). This suggests that the elevated pyruvate leads to a long-lasting effect that allows the cells to respond to BMP4 more strongly. Microarray analysis showed that exogenous pyruvate promoted the expression of carbohydrate and lipid metabolism in maintenance E8 medium (Figure S4A). Some genes related to signaling pathways were also upregulated, even though the cells did not differentiate (Figure S4B). This suggests that pyruvate may lead to a feedback control signal that regulates metabolism, with long-term implications.
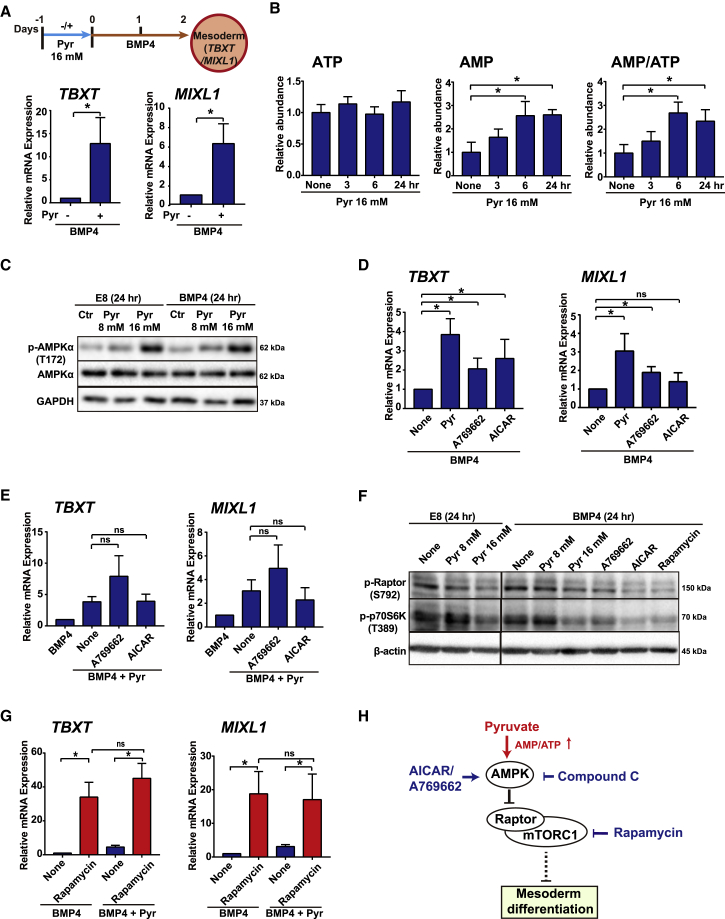
Figure 4.
Exogenous Pyruvate Increases AMP/ATP Ratio and Activates the AMPK Pathway in Mesoderm Differentiation
(A) Effect of pyruvate pretreatment on TBXT/MIXL1 expression. hESCs were pretreated with 16 mM pyruvate in E8 medium for 24 h (day −1 to day 0) before the induction to mesoderm by BMP4 for 2 days (day 0 to day 2). TBXT and MIXL1 expression were analyzed by qRT-PCR on day 2 (n = 3 independent experiments, ∗p < 0.05).
(B) LC-MS analysis of intercellular ATP, AMP, and AMP/ATP ratio under pyruvate treatment. H1 cells were cultured with 16 mM pyruvate for 0, 3, 6, and 24 h in E8 medium. Metabolite levels were normalized to the levels in control cells without pyruvate treatment (n ≥ 3 biological repeats, ∗p < 0.05).
(C) Western blotting analysis of pyruvate (8 or 16 mM) effects on AMPK phosphorylation. H1 cells were subjected to pyruvate treatment for 24 h during maintenance (E8) or differentiation (BMP4). Results shown are representative of three independent experiments.
(D and E) Effect of AMPK activators during BMP4-induced differentiation with (E) or without (D) 16 mM pyruvate treatment for 2 days. AICAR, 1 mM; A769662, 10 μM (n = 3 independent experiments, ∗p < 0.05; ns, not significant).
(F) Western blotting analysis of pyruvate effects on mTOR pathway. H1 cells were treated with pyruvate (8 or 16 mM) for 24 h both in E8 and in BMP4-induced differentiation. AMPK activators (A769662, 10 μM and AICAR, 1 mM) and mTOR inhibitor (rapamycin, 50 nM) were added during BMP4-induced differentiation for 24 h. Results shown are representative of three independent experiments.
(G) qRT-PCR analysis of the effect of mTOR pathway inhibitors during BMP4-induced differentiation for 2 days. Rapamycin, 50 nM (n = 3 independent experiments, ∗p < 0.05; ns, not significant).
(H) Proposed model of pyruvate action. mTOR is a central regulator of mesoderm differentiation, and pyruvate enhances differentiation by suppression of mTOR through AMPK.
See also Figure S4.
Considering pyruvate's role in metabolism, we conducted additional LC-MS analysis on cellular metabolites in hESCs (Figure S4C). We noticed that pyruvate treatment increased the AMP level, and consequently elevated the AMP/ATP ratio after 3–24 h of treatment (Figure 4B). We showed that elevated pyruvate increased AMPK phosphorylation in a dose-dependent manner in both maintenance and differentiation conditions (Figure 4C). These data suggest that elevated pyruvate activates the AMPK pathway in hESCs.
Next, we examined whether AMPK activation could affect mesoderm differentiation. AMPK activators AICAR and A769662 significantly increased mesodermal differentiation under BMP4 induction (Figure 4D). At the same time, AMPK activators did not significantly increase mesodermal differentiation in the presence of elevated pyruvate, suggesting that they work in the same pathway (Figure 4E). We also found that the AMPK inhibitor Compound C inhibited TBXT/MIXL1 expression regardless of pyruvate treatment (Figure S4D). These data suggest that mesoderm differentiation is enhanced by AMPK activated by pyruvate treatment.
Finally, we analyzed whether AMPK's downstream effectors are involved in mesoderm differentiation (Figure 4F). AMPK suppresses the mTOR pathway (Gwinn et al., 2008), so we examined the pyruvate impact on mTOR signaling. We showed that the phosphorylation of the mTOR regulator Raptor (S792) was significantly suppressed by pyruvate and AMPK activators, implying that the mTOR pathway was inhibited by pyruvate-AMPK through Raptor. At the same time, the phosphorylation of mTOR substrate p70S6K (T389) was also suppressed by pyruvate and AMPK activators, confirming that mTOR was inhibited by pyruvate and AMPK activators. These data suggest that pyruvate suppressed mTOR activity through AMPK in hESCs. We then examined whether mTOR inhibition could play a role in mesoderm differentiation. We found that the mTOR inhibitor rapamycin significantly increased mesodermal gene expression, and pyruvate did not provide an additive effect to enhance the differentiation (Figures 4G and S4E). These results are consistent with previous reports that mTOR inhibition promotes mesoderm cell fate in hESCs (Zhou et al., 2009, Jung et al., 2016, Nazareth et al., 2016, Meng et al., 2018a), and implies that pyruvate probably works in the same pathway as rapamycin in promoting mesoderm cell fate. Taken together, our data suggest that elevated exogenous pyruvate potentiates mesoderm differentiation through AMPK activation and mTOR inhibition (Figure 4H).
We further explored how elevated pyruvate could be used in hESC applications. We used BMP4 treatment to initiate mesoderm differentiation and then directed cell fate toward cardiomyocytes through WNT inhibition and heparin (Lin et al., 2017). We found that BMP4 alone was inefficient for cardiomyocyte induction, while cardiomyocyte differentiation was significantly improved when pyruvate was applied during BMP4 induction (Figures S4F and S4G). Pyruvate probably improves cardiac differentiation through enhanced WNT expression and mesoderm induction, which allows more efficient cardiomyocyte differentiation at a later stage. We believe that pyruvate can potentially be utilized in other lineage-specific differentiation methods in the near future.
Discussion
Pyruvate is a keystone metabolite and its associated metabolism has long been implicated in embryogenesis and carcinogenesis, but it has not been considered as a modulator of differentiation or signal transduction. This study reveals that elevated exogenous pyruvate shifts metabolic balance, regulates kinase cascades, and profoundly affects lineage-specific differentiation in hESCs.
Metabolic balance is associated with pluripotency and differentiation (Shyh-Chang and Daley, 2015, Ito and Ito, 2016). In comparison with hESCs, mesoderm cells have differential gene expression and demonstrate increased oxidative phosphorylation (Moussaieff et al., 2015b). We found that pyruvate metabolism not only was essential for mesoderm induction, but also shifted hESC metabolism quickly in a matter of hours. We think that in regular BMP4-initiated differentiation, metabolic balance shift follows the induction of cell differentiation and subsequently reinforces cell differentiation. Such a process requires multiple steps and requires a couple of days to execute. The elevation of oxidative phosphorylation by pyruvate allows the cells to respond to BMP4 more quickly and strongly, which leads to accelerated mesodermal differentiation (Figure 2F). Pyruvate accelerated WNT gene expression (Figure 2G) and altered the expression pattern of WNT receptors (Figures S2G and S2H), both of which probably contributed to enhanced mesoderm differentiation. When the WNT pathway is directly activated by CHIR99021, cells are not as responsive to 2-DG and pyruvate as in BMP4-induced differentiation (Figures 2J and S3A). We further showed that elevated pyruvate induces mesendoderm differentiation through inhibition of the mTOR pathway, which is consistent with previous reports that mTOR inhibitor enhances mesendoderm differentiation through lineage-specific metabolic flux (Cliff et al., 2017, Nazareth et al., 2016, Zhou et al., 2009). We also noticed that pyruvate altered metabolic gene expression differentially depending on whether BMP4 was present. These data indicate that the effect of pyruvate is dependent on cell signaling, which could be caused either by the combinatory effect of metabolism and signal transduction or by cell-type-specific responses due to BMP4-induced differentiation. It would be interesting to further explore how metabolism and cell signaling pathways interact to coordinate cell-fate decisions.
With elevated concentration, metabolites such as nicotinamide can play regulatory roles not only in metabolism but also in signal transduction, and we explored whether pyruvate has similar effects in signaling pathways (Meng et al., 2018b). Exogenous pyruvate has been implicated in many cellular processes, but most studies focus on its role in metabolism and reactive oxygen species suppression (Gray et al., 2014, Hereng et al., 2011, Ramos-Ibeas et al., 2017, Watanabe et al., 2017). Here we show that pyruvate modulates kinase cascades in signal transduction. This affects the phosphorylation status of multiple crucial proteins, such as PDHE1α and AMPK. We further demonstrate that pyruvate potentiates BMP4-induced differentiation through AMPK activation and mTOR inhibition. Based on the findings in this report, we propose that regulation of signaling pathways could be an important function of pyruvate. It would be interesting to re-examine the cellular events influenced by pyruvate to find out whether the control of signal transduction events is involved as potential underlying mechanisms.
Cell fate can be affected through epigenetic modifications by metabolites, such as acetate and α-KG (Atlasi and Stunnenberg, 2017, Carey et al., 2015, Moussaieff et al., 2015a, Moussaieff et al., 2015b, Pietrocola et al., 2015, TeSlaa et al., 2016). We tested whether pyruvate promoted differentiation through epigenetic modulation. To our surprise, pyruvate demonstrated a much stronger effect on mesoderm differentiation, and neither acetate nor dimethyl α-KG affected differentiation in the same way as pyruvate (Figures 3H and S3D). If pyruvate promoted mesoderm differentiation by enhancing histone acetylation similarly to other metabolites, we expected to see other activators of the process enhance mesoderm differentiation in a similar manner. However, our data showed that acetate, histone deacetylase (HDAC) inhibitor butyrate, and histone acetyltransferase (HAT) inhibitor showed no significant effect (Figure S3D). This suggests that pyruvate did not exert its function on mesoderm differentiation mainly through histone acetylation. These data also demonstrate that metabolites in the same metabolic pathways may have differential impact on differentiation through different mechanisms. It would be interesting to know how epigenetic modification could be differentially affected by these nutrients.
In this report, we also demonstrate that pyruvate is an efficient tool for regulation of cellular function. Exogenous pyruvate is efficiently imported intracellularly to influence metabolism and signal transduction in embryos and stem cells. In comparison with pyruvate, other metabolites, such as α-KG and succinate, have to be chemically modified to increase their membrane permeability, and they also led to cell death at high concentrations (Figure 3H). In contrast, pyruvate is easy to access and can be used at high concentration for routine research and stem cell applications. As an example, we show that the potentiated differentiation by pyruvate could be used to improve cardiomyocyte differentiation. Pyruvate-treated cells gave rise to a significantly higher yield of cardiomyocytes in BMP4-initiated cardiac differentiation (Figures S4F and S4G). Considering its unique characteristics in hESC regulation, pyruvate will be a useful tool to modulate metabolism and improve stem cell lineage-specific differentiation and other applications.
Experimental Procedures
Key Resources Table
The key resources table is available in Supplemental Information.
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, G.C. (guokaichen@umac.mo).
Experimental Model and Subject Details
Human iPSC Lines
hESC lines (H1, H9) and human iPSC lines (ND1, ND2) were used in this study. A SOX17-GFP H9 cell line was used for endoderm differentiation (Wang et al., 2011). The use of hESCs and hiPSCs was approved by the Institutional Review Board at the University of Macau.
hESC Culture Maintenance and Passaging
Cells were maintained in home-made E8 medium (DMEM/F12, L-ascorbic acid, selenium, transferrin, insulin, fibroblast growth factor 2 [FGF2], and TGFβ) following published protocols (Chen et al., 2011). In brief, cells were cultured in E8 medium (with 1× penicillin/streptomycin) on Matrigel-coated (Corning, 354230) plates. Confluent (∼80%) cells were passaged every 3–4 days using the DPBS-EDTA method as described by Beers et al. (2012). ROCK inhibitor Y-27632 (5 μM) was used during cell passaging.
Unless otherwise stated, all the pyruvate concentrations in the article refer to the amount of pyruvate added to the medium (surplus to the 0.5 mM pyruvate in E8 medium).
Spontaneous Differentiation
Spontaneous differentiation was conducted on the monolayer platform. Confluent (30%–50%) cells were cultured in E6 medium (E8 components minus FGF2 and TGFβ) with 1× penicillin/streptomycin for 12 days in the presence or absence of 8 mM pyruvate. Medium was changed every day. Expression of pluripotency and lineage-specific marker genes (NANOG, POU5F1, SOX2, TBXT, MIXL1, SOX17, PAX6) were tested via qRT-PCR.
Mesoderm Differentiation
hESCs were differentiated under 20 ng/mL BMP4 (R&D Systems, 314-BP) induction in E8 medium for 2 days (or as specified in time-course experiments) with or without 16 mM pyruvate. Medium was changed every day. Expression of T/MIXL1 was tested via qRT-PCR.
Endoderm Differentiation
A SOX17-GFP H9 cell line was used for endoderm differentiation (Wang et al., 2011). After differentiation induction by CHIR99021 (5 μM) for 1 day, hESCs were treated with 10 ng/mL activin A (R&D Systems, 338-AC) for 3 days in differentiation medium (E8 medium minus FGF2, TGFβ, and insulin). Pyruvate (16 mM) was added during activin A treatment. Medium was changed every day. Expression of SOX17-GFP was examined using an Accuri C6 flow cytometer.
Ectoderm Differentiation
Cells were treated with 10 μM SB431542 (Selleck, S1067) + 100 nM LDN193189 (Selleck, S2618) for 4 days in E6 medium with or without 16 mM pyruvate. Medium was changed every day. Expression of PAX6 were tested via qRT-PCR.
Cardiac Differentiation
Cardiac differentiation from hESCs was carried out following published protocols (Meng et al., 2018b). Expression of TNNT2/NKX2-5 was tested via qRT-PCR.
Method Details
Method details are available in Supplemental Information.
Statistical Analysis
Seahorse data and LC-MS data are presented as mean ± SD of three or more biological repeats. All other data are presented as mean ± SD of three independent experiments unless otherwise specified. Student's t test was used for statistical analysis, and p < 0.05 was considered statistically significant.
Author Contributions
G.C. and C.S. conceived and designed the study. C.S., W.L., Y. Zhang, and Y.M. conducted maintenance and differentiation experiments. F.X. and C.S. conducted LC-MS metabolome analysis. C.S. and Z.R. conducted metabolic and signal pathway analysis. Z.R., S.L., E.C., Y. Zhao, and C.S. prepared samples for microarray and conducted data analysis. C.S., W.L., and G.C. wrote the manuscript. Most authors contributed to the editing and proofreading of the manuscript.
Acknowledgments
We thank Dr. Chu-Xia Deng and Dr. Ren-he Xu for their comments and suggestions. This project was supported by University of Macau Multi-Year Research Grants MYRG2018-00135-FHS, MYRG2015-00228-FHS, and MYRG2015-00229-FHS, and also by Macao Science and Technology Development Fund FDCT/131/2014/A3 and FDCT/056/2015/A2. We would like to thank the technical support by the Genomics, Bioinformatics and Single Cell Core Facility and the Bioimaging and Stem Cell Core Facility at the Faculty of Health Sciences, University of Macau.
Published: July 25, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.06.003.
Accession Numbers
The accession number for the microarrays data reported in this article is GEO: GSE130249.
Supplemental Information
References
- Atlasi Y., Stunnenberg H.G. The interplay of epigenetic marks during stem cell differentiation and development. Nat. Rev. Genet. 2017;18:643–658. doi: 10.1038/nrg.2017.57. [DOI] [PubMed] [Google Scholar]
- Beers J., Gulbranson D.R., George N., Siniscalchi L.I., Jones J., Thomson J.A., Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat. Protoc. 2012;7:2029–2040. doi: 10.1038/nprot.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R.J., Agathocleous M., Morrison S.J. Metabolic regulation of stem cell function. J. Intern. Med. 2014;276:12–24. doi: 10.1111/joim.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher L., Coates A., Martin K.L., Rutherford A.J., Leese H.J. Metabolism of pyruvate by the early human embryo. Biol. Reprod. 1998;58:1054–1056. doi: 10.1095/biolreprod58.4.1054. [DOI] [PubMed] [Google Scholar]
- Candelario K.M., Shuttleworth C.W., Cunningham L.A. Neural stem/progenitor cells display a low requirement for oxidative metabolism independent of hypoxia inducible factor-1alpha expression. J. Neurochem. 2013;125:420–429. doi: 10.1111/jnc.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcamo J.M., Pedraza A., Borquez-Ojeda O., Zhang B., Sanchez R., Golde D.W. Vitamin C is a kinase inhibitor: dehydroascorbic acid inhibits I kappa B alpha kinase beta. Mol. Cell Biol. 2004;24:6645–6652. doi: 10.1128/MCB.24.15.6645-6652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey B.W., Finley L.W.S., Cross J.R., Allis C.D., Thompson C.B. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Perez V.A., Strasberg-Rieber M., Rieber M. Metabolic utilization of exogenous pyruvate by mutant p53 (R175H) human melanoma cells promotes survival under glucose depletion. Cancer Biol. Ther. 2011;12:647–656. doi: 10.4161/cbt.12.7.16566. [DOI] [PubMed] [Google Scholar]
- Chen G., Gulbranson D.R., Hou Z., Bolin J.M., Ruotti V., Probasco M.D., Smuga-Otto K., Howden S.E., Diol N.R., Propson N.E. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff T.S., Dalton S. Metabolic switching and cell fate decisions: implications for pluripotency, reprogramming and development. Curr. Opin. Genet. Dev. 2017;46:44–49. doi: 10.1016/j.gde.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff T.S., Wu T., Boward B.R., Yin A., Yin H., Glushka J.N., Prestegaard J.H., Dalton S. MYC controls human pluripotent stem cell fate decisions through regulation of metabolic flux. Cell Stem Cell. 2017;21:502–516.e9. doi: 10.1016/j.stem.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaghan J., Handyside A.H., Winston R.M., Leese H.J. Effects of pyruvate and glucose on the development of human preimplantation embryos in vitro. J. Reprod. Fertil. 1993;99:87–95. doi: 10.1530/jrf.0.0990087. [DOI] [PubMed] [Google Scholar]
- Garcia-Prat L., Sousa-Victor P., Munoz-Canoves P. Proteostatic and metabolic control of stemness. Cell Stem Cell. 2017;20:593–608. doi: 10.1016/j.stem.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Gonzalez S.V., Nguyen N.H., Rise F., Hassel B. Brain metabolism of exogenous pyruvate. J. Neurochem. 2005;95:284–293. doi: 10.1111/j.1471-4159.2005.03365.x. [DOI] [PubMed] [Google Scholar]
- Gray L.R., Tompkins S.C., Taylor E.B. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W., Gaeta X., Sahakyan A., Chan A.B., Hong C.S., Kim R., Braas D., Plath K., Lowry W.E., Christofk H.R. Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell. 2016;19:476–490. doi: 10.1016/j.stem.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., Turk B.E., Shaw R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A.P., Poole R.C., Cranmer S.L. Mechanisms and regulation of lactate, pyruvate and ketone body transport across the plasma membrane of mammalian cells and their metabolic consequences. Biochem. Soc. Trans. 1990;18:1132–1135. doi: 10.1042/bst0181132. [DOI] [PubMed] [Google Scholar]
- Hereng T.H., Elgstoen K.B., Cederkvist F.H., Eide L., Jahnsen T., Skalhegg B.S., Rosendal K.R. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011;26:3249–3263. doi: 10.1093/humrep/der317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Stem Cell Initiative. Akopian C.V., Andrews P.W., Beil S., Benvenisty N., Brehm J., Christie M., Ford A., Fox V., Gokhale P.J., Healy L. Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cell. Dev. Biol. Anim. 2010;46:247–258. doi: 10.1007/s11626-010-9297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Ito K. Metabolism and the control of cell fate decisions and stem cell renewal. Annu. Rev. Cell Dev. Biol. 2016;32:399–409. doi: 10.1146/annurev-cellbio-111315-125134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.H., Kang K.W., Kim J., Hong S.C., Park Y., Kim B.S. CXCR2 inhibition in human pluripotent stem cells induces predominant differentiation to mesoderm and endoderm through repression of mTOR, beta-catenin, and hTERT activities. Stem Cells Dev. 2016;25:1006–1019. doi: 10.1089/scd.2015.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilberg M.S., Terada N., Shan J. Influence of amino acid metabolism on embryonic stem cell function and differentiation. Adv. Nutr. 2016;7:780S–789S. doi: 10.3945/an.115.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek D., Neagu A., Tastemel M., Tuysuz N., Lehmann J., van de Werken H.J., Philipsen S., van der Linden R., Maas A., van I.W.F. Endogenous WNT signals mediate BMP-induced and spontaneous differentiation of epiblast stem cells and human embryonic stem cells. Stem Cell Reports. 2015;4:114–128. doi: 10.1016/j.stemcr.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Linask K.L., Mallon B., Johnson K., Klein M., Beers J., Xie W., Du Y., Liu C., Lai Y. Heparin promotes cardiac differentiation of human pluripotent stem cells in chemically defined albumin-free medium, enabling consistent manufacture of cardiomyocytes. Stem Cells Transl. Med. 2017;6:527–538. doi: 10.5966/sctm.2015-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Ren Z., Lu K., Song C., Cheung E.C.W., Zhou Z., Chen G. The suppression of medium acidosis improves the maintenance and differentiation of human pluripotent stem cells at high density in defined cell culture medium. Int. J. Biol. Sci. 2018;14:485–496. doi: 10.7150/ijbs.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt S.Y., Vander Heiden M.G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- McCommis K.S., Finck B.N. Mitochondrial pyruvate transport: a historical perspective and future research directions. Biochem. J. 2015;466:443–454. doi: 10.1042/BJ20141171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D.L., Frank A.R., Jewell J.L. mTOR signaling in stem and progenitor cells. Development. 2018;145 doi: 10.1242/dev.152595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Ren Z., Xu F., Zhou X., Song C., Wang V.Y., Liu W., Lu L., Thomson J.A., Chen G. Nicotinamide promotes cell survival and differentiation as kinase inhibitor in human pluripotent stem cells. Stem Cell Reports. 2018;11:1347–1356. doi: 10.1016/j.stemcr.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaieff A., Kogan N.M., Aberdam D. Concise review: energy metabolites: key mediators of the epigenetic state of pluripotency. Stem Cells. 2015;33:2374–2380. doi: 10.1002/stem.2041. [DOI] [PubMed] [Google Scholar]
- Moussaieff A., Rouleau M., Kitsberg D., Cohen M., Levy G., Barasch D., Nemirovski A., Shen-Orr S., Laevsky I., Amit M. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015;21:392–402. doi: 10.1016/j.cmet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Nagaraj R., Sharpley M.S., Chi F.T., Braas D., Zhou Y.G., Kim R., Clark A.T., Banerjee U. Nuclear localization of mitochondrial TCA cycle enzymes as a critical step in mammalian zygotic genome activation. Cell. 2017;168:210–223.e11. doi: 10.1016/j.cell.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth E.J.P., Rahman N., Yin T., Zandstra P.W. A multi-lineage screen reveals mTORC1 inhibition enhances human pluripotent stem cell mesendoderm and blood progenitor production. Stem Cell Reports. 2016;6:679–691. doi: 10.1016/j.stemcr.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson K.A., Schell J.C., Rutter J. Pyruvate and metabolic flexibility: illuminating a path toward selective cancer therapies. Trends Biochem. Sci. 2016;41:219–230. doi: 10.1016/j.tibs.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrocola F., Galluzzi L., Bravo-San Pedro J.M., Madeo F., Kroemer G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 2015;21:805–821. doi: 10.1016/j.cmet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Poole R.C., Halestrap A.P. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol. 1993;264(4 Pt 1):C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Ramos-Ibeas P., Barandalla M., Colleoni S., Lazzari G. Pyruvate antioxidant roles in human fibroblasts and embryonic stem cells. Mol. Cell. Biochem. 2017;429:137–150. doi: 10.1007/s11010-017-2942-z. [DOI] [PubMed] [Google Scholar]
- Rodrigues A.S., Correia M., Gomes A., Pereira S.L., Perestrelo T., Sousa M.I., Ramalho-Santos J. Dichloroacetate, the pyruvate dehydrogenase complex and the modulation of mESC pluripotency. PLoS One. 2015;10:e0131663. doi: 10.1371/journal.pone.0131663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri V., Agriesti F., Scrima R., Laurenzana I., Perrone D., Tataranni T., Mazzoccoli C., Lo Muzio L., Capitanio N., Piccoli C. Dichloroacetate, a selective mitochondria-targeting drug for oral squamous cell carcinoma: a metabolic perspective of treatment. Oncotarget. 2015;6:1217–1230. doi: 10.18632/oncotarget.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahudeen A.K., Clark E.C., Nath K.A. Hydrogen peroxide-induced renal injury. A protective role for pyruvate in vitro and in vivo. J. Clin. Invest. 1991;88:1886–1893. doi: 10.1172/JCI115511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y.C., Ou D.L., Hsu C., Lin K.L., Chang C.Y., Lin C.Y., Liu S.H., Cheng A.L. Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. Br. J. Cancer. 2013;108:72–81. doi: 10.1038/bjc.2012.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki N., Shiraki Y., Tsuyama T., Obata F., Miura M., Nagae G., Aburatani H., Kume K., Endo F., Kume S. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 2014;19:780–794. doi: 10.1016/j.cmet.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Shyh-Chang N., Daley G.Q. Metabolic switches linked to pluripotency and embryonic stem cell differentiation. Cell Metab. 2015;21:349–350. doi: 10.1016/j.cmet.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Sutendra G., Kinnaird A., Dromparis P., Paulin R., Stenson T.H., Haromy A., Hashimoto K., Zhang N., Flaim E., Michelakis E.D. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell. 2014;158:84–97. doi: 10.1016/j.cell.2014.04.046. [DOI] [PubMed] [Google Scholar]
- TeSlaa T., Chaikovsky A.C., Lipchina I., Escobar S.L., Hochedlinger K., Huang J., Graeber T.G., Braas D., Teitell M.A. Alpha-ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 2016;24:485–493. doi: 10.1016/j.cmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslaa T., Teitell M.A. Pluripotent stem cell energy metabolism: an update. EMBO J. 2015;34:138–153. doi: 10.15252/embj.201490446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Alexander P., Wu L., Hammer R., Cleaver O., McKnight S.L. Dependence of mouse embryonic stem cells on threonine catabolism. Science. 2009;325:435–439. doi: 10.1126/science.1173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Shirai T., Namkoong H., Zhang H., Berry G.J., Wallis B.B., Schaefgen B., Harrison D.G., Tremmel J.A., Giacomini J.C. Pyruvate controls the checkpoint inhibitor PD-L1 and suppresses T cell immunity. J. Clin. Invest. 2017;127:2725–2738. doi: 10.1172/JCI92167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Rodriguez R.T., Wang J., Ghodasara A., Kim S.K. Targeting SOX17 in human embryonic stem cells creates unique strategies for isolating and analyzing developing endoderm. Cell Stem Cell. 2011;8:335–346. doi: 10.1016/j.stem.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Khvorostov I., Hong J.S., Oktay Y., Vergnes L., Nuebel E., Wahjudi P.N., Setoguchi K., Wang G., Do A. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30:4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Nuebel E., Daley G.Q., Koehler C.M., Teitell M.A. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 2012;11:589–595. doi: 10.1016/j.stem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Su P., Wang L., Chen J., Zimmermann M., Genbacev O., Afonja O., Horne M.C., Tanaka T., Duan E. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc. Natl. Acad. Sci. U S A. 2009;106:7840–7845. doi: 10.1073/pnas.0901854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.