Summary
For long QT syndrome (LQTS), recent progress in genome-sequencing technologies enabled the identification of rare genomic variants with diagnostic, prognostic, and therapeutic implications. However, pathogenic stratification of the identified variants remains challenging, especially in variants of uncertain significance. This study aimed to propose a phenotypic cell-based diagnostic assay for identifying LQTS to recognize pathogenic variants in a high-throughput manner suitable for screening. We investigated the response of LQT2-induced pluripotent stem cell (iPSC)-derived cardiomyocytes (iPSC-CMs) following IKr blockade using a multi-electrode array, finding that the response to IKr blockade was significantly smaller than in Control-iPSC-CMs. Furthermore, we found that LQT1-iPSC-CMs and LQT3-iPSC-CMs could be distinguished from Control-iPSC-CMs by IKs blockade and INa blockade, respectively. This strategy might be helpful in compensating for the shortcomings of genetic testing of LQTS patients.
Keywords: long QT syndrome, induced pluripotent stem cell, phenotype-based diagnosis, multi-electrode array, genome editing
Highlights
-
•
IKs blockade showed a smaller response of delayed repolarization in LQT1-iPSC-CMs
-
•
IKr blockade showed a smaller response of delayed repolarization in LQT2-iPSC-CMs
-
•
INa blockade markedly ameliorated prolonged repolarization in LQT3-iPSC-CMs
-
•
Ion-channel blockade response distinguished LQTS subtypes with high accuracy
The methods presented by the authors allowed recognition of long QT syndrome (LQTS) subtypes 1, 2, and 3 using specific ion-channel current blockade with a combination of patient-derived iPSCs and a multi-electrode array system. This strategy might potentially compensate for the shortcomings of genetic testing for LQTS, especially in patients who have variants of unknown significance or no identified mutations.
Introduction
Long QT syndrome (LQTS) is caused by hereditary cardiac channelopathies characterized by a prolonged QT interval and abnormal T-wave morphology on electrocardiograms and capable of precipitating malignant arrhythmia (i.e., Torsade de Pointes [TdP]), resulting in syncope and sudden death (Moss, 2003). Genetic tests are currently utilized to assist treatment selection and prognostication (Napolitano et al., 2005); however, the advent of high-output sequencing techniques using next-generation sequencing has allowed identification of an extremely large number of variants from both patients and healthy individuals. Given the extreme clinical importance of identifying pathogenic variants among those of uncertain significance (i.e., VUSs) (Horie, 2016), a phenotype-based high-throughput diagnostic test is required to identify clinically relevant genetic abnormalities.
Giudicessi and Ackerman (2013) suggested “current-centric” classification of LQTS-susceptibility genes, which is reasonable in terms of phenotype-based diagnosis and subsequent treatment selection. However, current-centric classification is difficult in clinical settings. Although the use of provocative tests, including exercise-stress tests and drug-infusion tests, has been proposed to predict the LQTS genotypes, it remains difficult to appropriately diagnose and manage decisions based on their results (Priori et al., 2013).
Human induced pluripotent stem cell (iPSC) technology is promising for cell transplantation and disease modeling for diagnosis, investigation of disease mechanisms, and identification of new drugs (Hamazaki et al., 2017, Inoue and Yamanaka, 2011, Takahashi et al., 2007). Because iPSCs retain the genetic information of the cells from which they are derived, cells differentiated from iPSCs can potentially recapitulate the phenotypic variation of each donor. Therefore, iPSC-derived cardiomyocytes (iPSC-CMs) potentially play a key role in various fields of regenerative medicine (Egashira et al., 2011) and might represent a powerful diagnostic tool for LQTS.
Here, we demonstrated that iPSC-CMs can be applied to phenotypic, cell-based, high-throughput screening for recognition and classification of LQTS by using iPSCs from patients with LQTS types 1, 2, and 3, which account for ∼90% of all LQTS.
Results
Clinical Phenotype of LQTS Patients Enrolled in this Study and Genetic Mutations
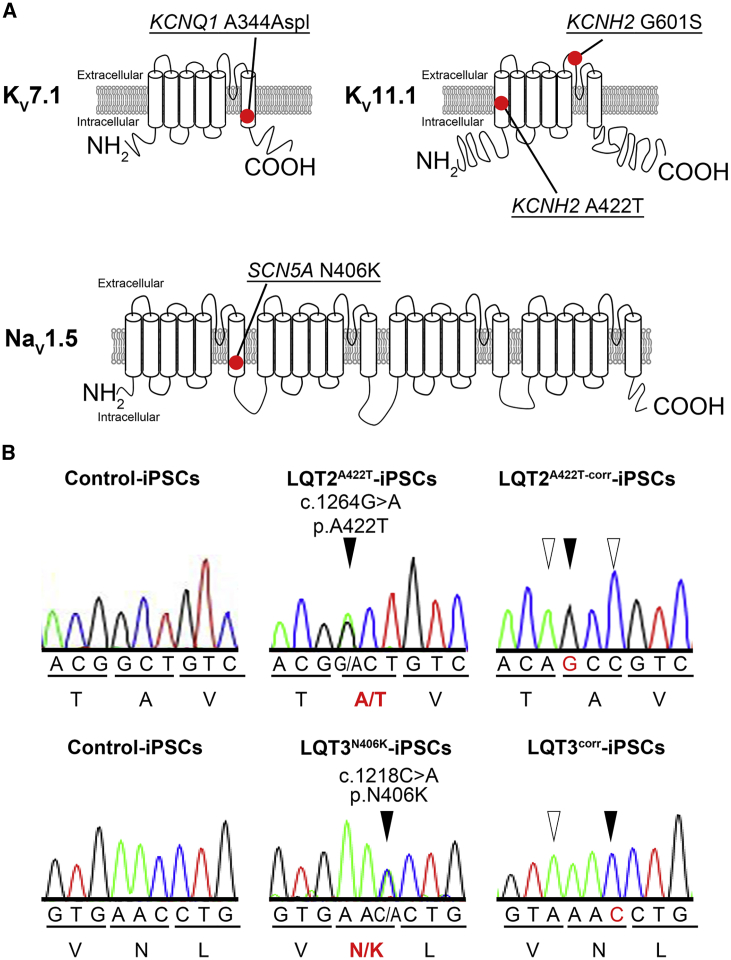
All patients enrolled in this study were symptomatic, except for an LQT2 patient harboring potassium voltage-gated channel subfamily H member 2 (KCNH2) p.G601S (Table 1). All mutations were located in the transmembrane or pore domains, thereby causing heterozygous missense mutations in KCNH2 and sodium voltage-gated channel alpha subunit 5 (SCN5A) and splicing errors in potassium voltage-gated channel subfamily Q member 1 (KCNQ1) (Figure 1A). The sequence of gene-corrected LQT2A422T-iPSCs (LQT2A422T-corr-iPSCs) and LQT3N406K-iPSCs (LQT3corr-iPSCs) was confirmed by Sanger sequencing (Figure 1B).
Table 1.
Information on Patients Enrolled in the Present Study
| LQTS Type | Mutation | Age (years) | Sex | Corrected QT Interval | Symptom |
|---|---|---|---|---|---|
| LQT1 | KCNQ1 | 11 | Male | 424 ms | Cardiac arrest |
| A344Aspl | 500 ms on exercise | Ventricular fibrillation | |||
| LQT2 | KCNH2 | 53 | Female | 493 ms | Syncope |
| A422T | |||||
| KCNH2 | 14 | Female | 480 ms | Asymptomatic | |
| G601S | |||||
| LQT3 | SCN5A | 20 | Female | 522 ms | Sudden death |
| N406K | |||||
| Healthy Control | 46 | Male | 443 ms | None | |
| 36 | Female | No QT prolongation | None | ||
Figure 1.
Introduction of iPSC Lines Used in This Study
(A) Summary of topologies of mutations of iPSC lines used in this study. LQT1A344Aspl carries a heterozygous KCNQ1 mutation (c.1032C > A, p.A344Aspl); LQT2A422T and LQT2A422T-corr are the isogenic pair harboring the heterozygous KCNH2 mutation (c.1264G > A, p.A422T) and the corrected sequence, respectively; LQT2G601S carries a heterozygous KCNH2 mutation (c.1801G > A, p.G601S); and LQT3N406K and LQT3corr are the isogenic pair harboring the heterozygous SCN5A mutation (c.1218C > A, p.N406K) and the corrected sequence, respectively.
(B) Sequence analysis of PCR-amplified genomic DNA of the isogenic pair of LQT2A422T and LQT2 A422T-corr and of LQT3N406K and LQT3corr, respectively. The gene-corrected cell lines harbor several silent mutations (white arrow head) to avoid further digestion by CRISPR/Cas9.
LQT2A422T- and LQT2G601S-iPSC-CMs Display Smaller Responses to IKr Blockade
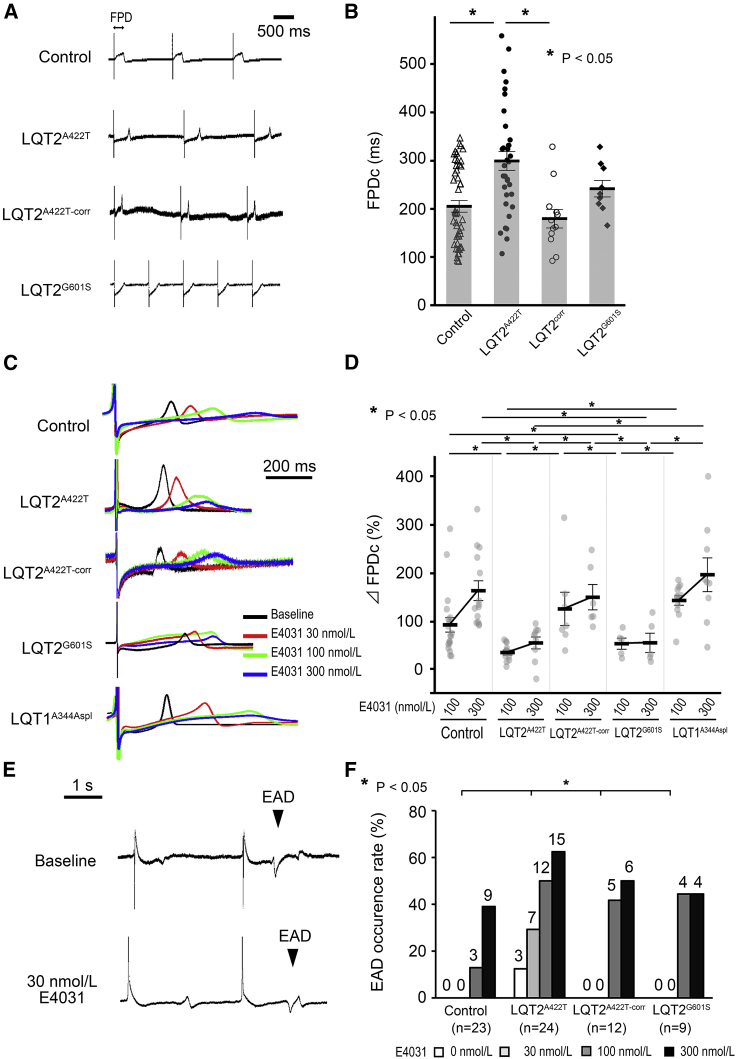
We hypothesized that IKr contribution to repolarization could be indirectly evaluated by assessing the field-potential (FP) response to IKr blockade. At baseline, the corrected LQT2A422T-iPSC-CM FP duration (FPDc) was significantly longer than that in Control- and LQT2 A422T-corr-iPSC-CMs (301.0 ± 15.7 ms, 202.2 ± 10.8 ms, and 180.2 ± 20.9 ms, respectively; p < 0.05) (Figures 2A and 2B). To verify our hypothesis, we blocked IKr using E4031 in LQT2G601S- and LQT1A344Aspl-iPSC-CMs. Figure 2C displays representative FP traces in each CM group treated with 30, 100, and 300 nmol/L E4031. To evaluate the contribution of blocked IKr to CM repolarization, we calculated %ΔFPDc of iPSC-CMs except for those with early afterdepolarizations (EADs). Upon administration of 100 and 300 nmol/L E4031, the %ΔFPDc of LQT2A422T- and LQT2G601S-iPSC-CMs was significantly smaller than that of Control-, LQT2A422T-corr-, and LQT1A344Aspl-iPSC-CMs (Figure 2D). These results suggested that %ΔFPDc upon IKr blockade reflected the density of IKr. Regarding the FPDc value, LQT1A344Aspl-iPSC-CMs showed the most prolonged FPDc among the five iPSC-CM lines (Figure S1A).
Figure 2.
Functional Analysis of iPSC-CMs Using MEA Following IKr Blockade
(A) Representative traces of FP in Control-, LQT2A422T-, LQT2A422T-corr-, and LQT2G601S-iPSC-CMs.
(B) FPDc baseline data in Control-, LQT2A422T-, LQT2corr-, and LQT2G601S-iPSC-CMs (independent experiments, n = 45, 35, 12, and 9 from independent differentiation experiments, n = 16, 11, 6, and 3, respectively; mean ± SEM; p = 0.001; one-way ANOVA). ∗p < 0.05, Fisher's LSD post hoc test.
(C) Representative traces of the FPD following administration of 30 nmol/L (red), 100 nmol/L (green), and 300 nmol/L (blue) E4031.
(D) Averaged FPDc ratio before and after E4031 treatment (%ΔFPDc) in Control-, LQT2A422T-, LQT2A422T-corr-, LQT2G601S-, and LQT1A344Aspl-iPSC-CMs. FPDc prolongation upon treatment with 100 and 300 nmol/L E4031 was smaller in LQT2A422T- and LQT2G601S-iPSC-CMs than in Control-, LQT2corr-, or LQT1A344Aspl-iPSC-CMs (independent experiments, n = 23, 24, 12, 9, and 12 from independent differentiation experiments, n = 8, 8, 6, 3, and 3 in Control-, LQT2A422T-, LQT2A422T-corr-, LQT2G601S-, and LQT1A344Aspl-iPSC-CMs, respectively; mean ± SEM; p = 0.025; two-way repeated measures ANOVA). ∗p < 0.05, Fisher's LSD post hoc test.
(E) Examples of EADs. EAD documented in a sample of LQT2A422T-iPSC-CMs before E4031 treatment (upper) and following 30 nmol/L E4031 treatment (lower).
(F) Percentage of samples in which EADs occurred in Control-, LQT2A422T-, LQT2A422T-corr-, and LQT2G601S-iPSC-CMs. The number on top of each bar shows the number of arrhythmic events. ∗p < 0.05; Pearson's Chi-square test.
See also Figure S1.
During the prolonged FPD by IKr blockade, EADs were recorded in each cell line (Figure 2E), revealing that, upon less than 100 nmol/L E4031, the occurrence rate of EADs was significantly higher in LQT2A422T-iPSC-CMs than in Control-, LQT2A4222T-corr-, LQT2G601S-, or LQT1A344Aspl-iPSC-CMs (Figure 2F). These data suggested that LQT2A422T-iPSC-CMs were more vulnerable to arrhythmic events.
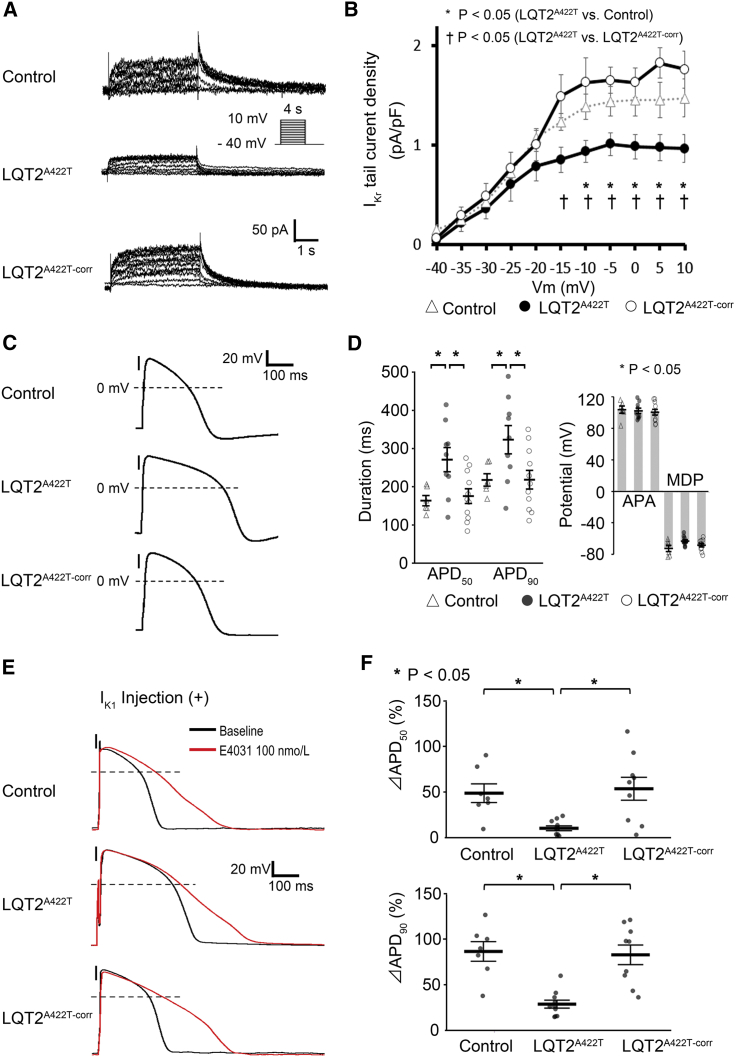
Patch-Clamp Results Show Reduced IKr and Attenuated Response to IKr Blockade in LQT2A422T-iPSC-CMs
To investigate the IKr in differentiated CMs, we performed patch-clamp electrophysiological analysis, with IKr current detected as an E4031-sensitive current (Figure 3A). IKr tail current densities in LQT2A422T-corr-iPSC-CMs increased significantly as compared with those in LQT2A422T-iPSC-CMs according to the whole-cell patch-clamp method (peak IKr density: LQT2A422T, 1.01 ± 0.11 pA/pF; LQT2 A422T-corr, 1.85 ± 0.16 pA/pF) (Figure 3B), indicating that gene correction normalized the reduced IKr observed in LQT2A422T-iPSC-CMs. We then evaluated action potential duration (APD) to assess the contribution of IKr to repolarization (Figure 3C), finding that the APD90 in Control-, LQT2A422T-, and LQT2 A422T-corr-iPSC-CMs was 215.8 ± 16.2 ms, 321.1 ± 37.0 ms, and 216.4 ± 24.4 ms, respectively (p < 0.05), whereas the AP amplitude and maximum diastolic potential (MDP) did not differ significantly (Figure 3D).
Figure 3.
Electrophysiological Properties of iPSC-CMs and AP Response to IKr Blockade
(A) Representative current traces of the IKr in Control-, LQT2A422T-, and LQT2A422T-corr-iPSC-CMs.
(B) Average current-voltage relationships for peak tail currents in Control-, LQT2A422T-, and LQT2A422T-corr-iPSC-CMs (independent experiments, n = 8, 6, and 5 from independent differentiation experiments, n = 4, 4, and 3, respectively; mean ± SEM; p = 0.029; two-way repeated measures ANOVA). ∗p < 0.05, Fisher's LSD post hoc test for Control versus LQT2A422T; †p < 0.05, LQT2A422T-corr versus LQT2A422T.
(C) Representative traces of AP with 1-Hz pacing.
(D) APD50 and APD90 in Control-, LQT2A422T-, and LQT2A422T-corr-iPSC-CMs (independent experiments, n = 6, 9, and 11, from independent differentiation experiments, n = 5, 6, and 5, respectively; mean ± SEM; p = 0.010 and p = 0.025 for APD50 and APD90, respectively; one-way ANOVA). ∗p < 0.05, Fisher's LSD post hoc test for APD50 and APD90.
(E) Representative AP traces changed by IKr blockade.
(F) Percentage of APD prolongation after E4031 treatment (%ΔAPD) at 1-Hz pacing in each cell line. %ΔAPD50 and %ΔAPD90 in Control-iPSC-CMs and LQT2A422T-corr-iPSC-CMs versus LQT2A422T-iPSC-CMs (independent experiments, n = 7, 10, and 10 from independent differentiation experiments, n = 5, 5, and 6 in Control-, LQT2A422T-, and LQT2A422T-corr-iPSC-CMs, respectively; mean ± SEM; p = 0.001; two-way repeated measures ANOVA). ∗p < 0.05, Fisher's LSD post hoc test.
See also Figure S2.
APA, action potential amplitude; MDP, maximum diastolic potential.
To confirm IKr blockade-mediated FPD prolongation, we evaluated APD prolongation upon IKr blockade. Because MDP elevation due to IKr blockage disturbed correct APD measurement in some samples (Figure S2A), MDP was fixed at approximately –80 mV by introducing an artificial IK1 current using the dynamic clamp method (Table 2; Figure S2B), thereby allowing the evaluation of APD prolongation in the presence of IKr blockade (Figures 3E and S2A). APD90 prolongation induced by treatment with a higher concentration of E4031 was significantly shorter in LQT2A422T-iPSC-CMs than in Control- and LQT2 A422T-corr-iPSC-CMs (28.4 ± 4.4% versus 85.8 ± 10.7% and 82.1 ± 10.7%, respectively; p < 0.05), with the APD50 showing a similar pattern of prolongation (10.4 ± 2.6% versus 48.6 ± 10.2% and 53.4 ± 12.5%, respectively; p < 0.05). These responses to E4031 were compatible with those observed in FPD experiments (Figure 3F). These data rigorously supported the multi-electrode array (MEA) results, showing that the response to IKr blockade reflected IKr density.
Table 2.
Parameters of Action Potentials under IK1 Injection with Dynamic Clamp in Each Cell Line
| Baseline |
100 nmol/L E4031 Administration |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | LQT2A422T | LQT2corr | p Value | Control | LQT2A422T | LQT2corr | p Value | |
| No. of cells | 8 | 14 | 13 | 7 | 10 | 10 | ||
| APD50 (ms) | 192.8 ± 12.4a | 261.3 ± 21.6 | 197.7 ± 17.4a | 0.026 | 278.7 ± 22.8b | 277.9 ± 29.5 | 301.9 ± 51.7 | 0.883 |
| APD90 (ms) | 236.0 ± 14.0a | 310.2 ± 22.1 | 237.8 ± 19.4a | 0.019 | 423.7 ± 25.1b | 384.8 ± 37.5 | 412.4 ± 58.4b | 0.833 |
| MDP (mV) | −81.4 ± 1.0 | −82.2 ± 0.4 | −81.1 ± 0.7 | 0.408 | −78.5 ± 1.6 | −81.6 ± 0.6 | −80.3 ± 0.7 | 0.102 |
| APA (mV) | 119.4 ± 2.1 | 124.3 ± 1.7 | 119.0 ± 1.8 | 0.066 | 121.8 ± 3.3 | 121.2 ± 2.4 | 120.0 ± 2.2 | 0.886 |
| Cm (pF) | 65.9 ± 7.9 | 66.5 ± 9.3 | 64.2 ± 10.3 | 0.990 | 61.6 ± 7.6 | 66.5 ± 11.5 | 64.2 ± 13.4 | 0.838 |
| Injected IK1 (pA/pF) | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.874 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.955 |
p Values were calculated using one-way ANOVA.
APA, action potential amplitude; Cm, membrane capacitance; MDP, maximum diastolic potential; pA, picoampere; pF, picofarad.
p < 0.05; Fisher's LSD post hoc test for Control or LQT2corr versus LQT2A422T.
p < 0.05; parameters of the same cell line before and after administration of 100 nmol/L E4031 were statistically compared using an unpaired Student's t test.
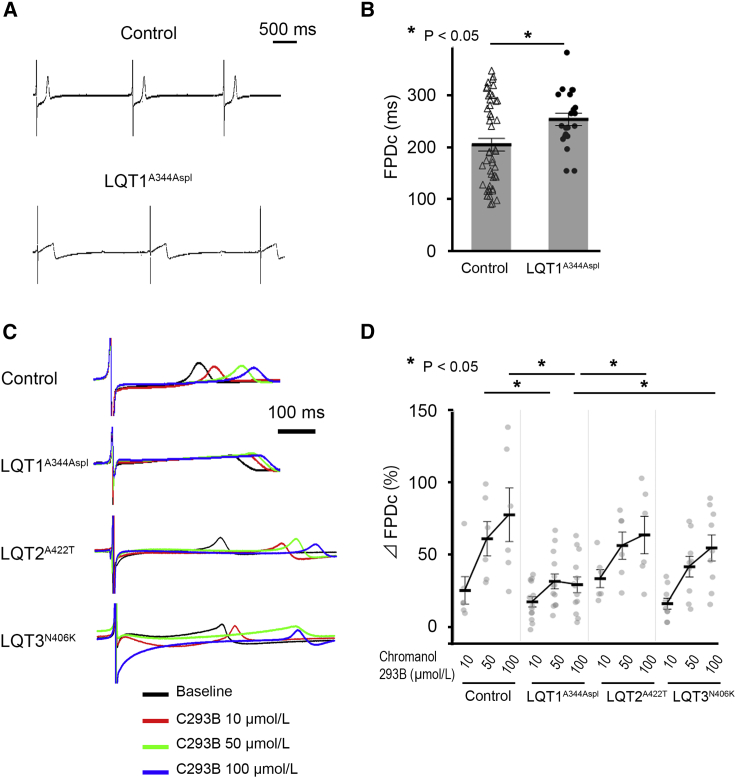
LQT1A344Aspl-iPSC-CMs Display an Attenuated Response to IKs Blockade
We then performed IKs blockade to elucidate IKs deficiency by using different concentrations of chromanol 293B. At baseline, the FPDc of LQT1A344Aspl-iPSC-CMs was longer than that of Control-iPSC-CMs (249.4 ± 14.0 ms and 202.2 ± 10.8 ms, respectively; p < 0.05) (Figures 4A and 4B). Figure 4C shows representative FP traces in each CM group treated with 10, 50, and 100 μmol/L chromanol 293B. Upon administration of 100 μmol/L chromanol 293B, LQT1A344Aspl-iPSC-CMs showed a significantly smaller %ΔFPDc than those observed in Control-, LQT2A422T-, and LQT3N406K-iPSC-CMs (Figure 4D). These results suggested that the effect of IKs blockade on FPD prolongation reflected the IKs density. Regarding the FPDc value, LQT2A422T-iPSC-CMs exhibited the longest FPDc among the three lines at 100 μmol/L chromanol 293B (Figure S1B).
Figure 4.
Functional Analysis of iPSC-CMs Using MEA Following IKs Blockade
(A) Representative traces of FP in Control- and LQT1A344Aspl-iPSC-CMs.
(B) FPDc at baseline was longer in LQT1A344Aspl-iPSC-CMs than in Control-iPSC-CMs (independent experiments, n = 45 and 21 from independent differentiation experiments, n = 16 and 6 in Control- and LQT1A344Aspl-iPSC-CMs, respectively; mean ± SEM; p < 0.05; unpaired Student's t test). ∗p < 0.05.
(C) Representative traces of the FPD following administration of 10 μmol/L (red), 50 μmol/L (green), and 100 μmol/L (blue) chromanol 293B in Control- and LQT1A344Aspl-iPSC-CMs.
(D) Averaged %ΔFPDc in each cell line. %ΔFPDc upon treatment with 100 μmol/L chromanol 293B was significantly smaller in LQT1A344Aspl-iPSC-CMs than in Control-, LQT2A422T-, and LQT3N406K-iPSC-CMs (independent experiments, n = 6, 13, 6, and 9 from independent differentiation experiments, n = 3, 5, 3, and 3 in Control-, LQT1A344Aspl-, LQT2A422T-, and LQT3N406K-iPSC-CMs, respectively; mean ± SEM; p = 0.001; two-way repeated measures ANOVA). ∗p < 0.05; Fisher's LSD post hoc test.
LQT3N406K-iPSC-CMs Display a Greater Response to INa Blockade, which Was Normalized by Gene Correction
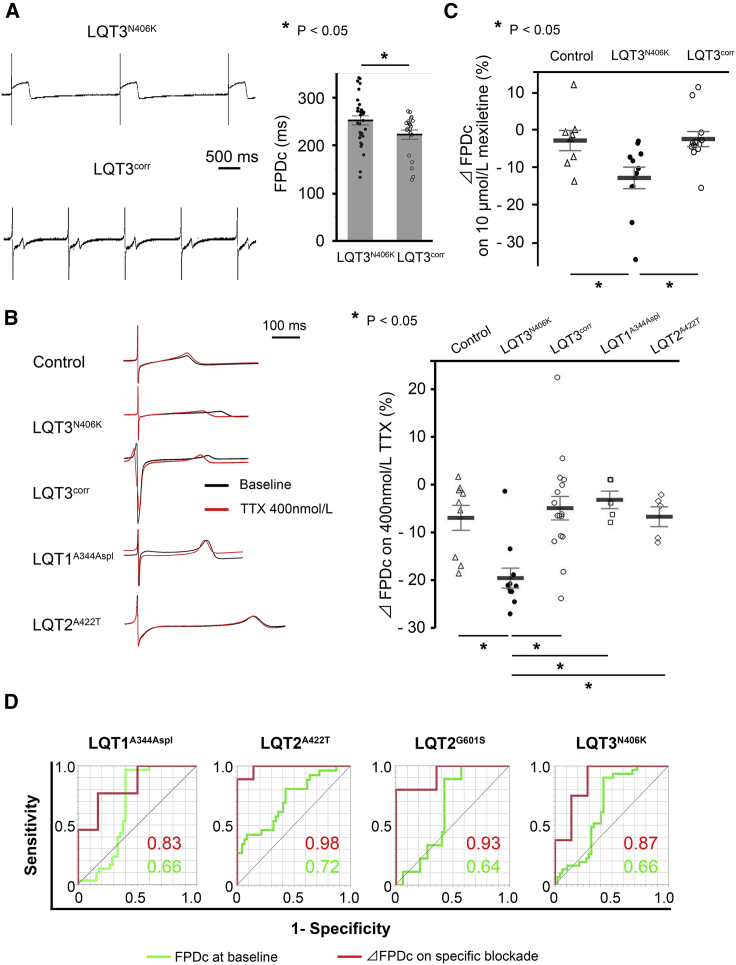
We then blocked INa using tetrodotoxin (TTX) in order to elucidate the excessive INa-Late response. At baseline, the FPDc of LQT3N406K-iPSC-CMs was longer than that of the corrected LQT3-iPSC-CMs (LQT3corr-iPSC-CMs) (Figure 5A). Figure 5B shows representative FP traces in each CM group treated with 400 nmol/L TTX. Upon administration of 400 nmol/L of TTX, the %ΔFPDc for Control-, LQT3N406K-, LQT3corr-, LQT1A344Aspl-, and LQT2A422T-iPSC-CMs was –6.8 ± 2.8%, –19.0 ± 2.6%, –4.8 ± 2.0%, –3.0 ± 3.8%, and –6.6 ± 3.6% (p < 0.05), respectively. A similar response was observed using mexiletine, another typical INa blocker (Figure 5C).
Figure 5.
Functional Analysis of iPSC-CMs Using MEA Following INa Blockade
(A) Representative traces of FP in LQT3N406K- and LQT3corr-iPSC-CMs (left). FPDc at baseline in LQT3N406K-iPSC-CMs was significantly shortened by gene correction (right) (independent experiments, n = 36 and 29 from independent differentiation experiments, n = 8 and 8 in LQT3N406K- and LQT3corr-iPSC-CMs, respectively; mean ± SEM; p < 0.05; unpaired Student's t test). ∗p < 0.05.
(B) Representative traces of the FP following administration of 400 nmol/L (red) TTX in Control-, LQT3N406K-, LQT3corr-, LQT1A344Aspl-, and LQT2A422T-iPSC-CMs (left). The response to treatment with 400 nmol/L TTX was significantly larger in LQT3N406K-iPSC-CMs than in Control-, LQT3corr-, LQT1A344Aspl-, and LQT2A422T-iPSC-CMs (right) (independent experiments, n = 9, 11, 17, 5, and 5 from independent differentiation experiments, n = 4, 5, 4, 3, and 3 in Control-, LQT3N406K-, LQT3corr-, LQT1A344Aspl-, and LQT2A422T-iPSC-CMs, respectively; mean ± SEM; p < 0.001; one-way ANOVA). ∗p < 0.05; Fisher's LSD post hoc test.
(C) Averaged %ΔFPDc on 10 μmol/L mexiletine in Control-, LQT3N406K-, and LQT3corr-iPSC-CMs (independent experiments, n = 7, 11, and 12 from independent differentiation experiments, n = 3, 3, and 5, respectively; mean ± SEM; p < 0.001; one-way ANOVA). ∗p < 0.05; Fisher's LSD post hoc test.
(D) Comparison of AUCs for baseline FPDc and %ΔFPDc upon specific current blockade for recognizing disease-specific iPSC-CMs. Specific current blockade enhanced the accuracy of recognizing disease-specific iPSC-CMs.
See also Table S1.
%ΔFPDc on Specific Current Blockade Is a Better Predictor for Estimating LQTS Subtype than Baseline FPDc in the iPSC-CM Model
To determine the accuracy of specific current blockade, receiver operating characteristic (ROC) curve analysis was performed in each cell line. Although the area under the ROC curves (AUCs) for baseline FPDc were <0.75, the AUCs for %ΔFPDc following specific current blockade were significantly improved, indicating that specific current blockade enhanced the detectability of the abnormal current (Figure 5D; Table S1).
Discussion
Among >15 different LQTS subtypes based on responsible pathogenic genes (Schwartz et al., 2013), LQT1, LQT2, and LQT3 are the most common. In LQT1, exercise or emotional stress often triggers arrhythmias, with β blockers the most effective at preventing these arrhythmic events (Moss et al., 2000, Priori et al., 2004, Schwartz et al., 2001). By contrast, in LQT2, drugs or hypopotassemia trigger TdP, and β blockers are less effective than in LQT1. In LQT3, TdP is triggered during sleep, and mexiletine represents a therapeutic option based on its suppression of excessive INa-Late. Therefore, precise classification of LQTS subtypes is clinically important for determining therapeutic strategies and predicting prognosis (Inoue and Yamanaka, 2011). In clinical settings, genetic testing is often performed to assist clinical diagnosis of LQTS; however, it identifies variants in LQTS-related genes in only 60% of cases (Kapa et al., 2009, Wehrens et al., 2002). In addition, not all identified variants are proven to be causative, with many having unknown pathogenicity in the absence of information regarding their electrophysiological consequences (VUSs). Moreover, abnormalities caused by variants in intronic or promoter regions might be overlooked by genetic testing alone. Therefore, a patient-oriented diagnostic system based on its specific phenotype plays an important role in compensating for the shortcomings of genetic testing.
In this study, we successfully distinguished LQT1, LQT2, and LQT3 based on the phenotype displayed by iPSC-CMs to specific current blockade. In addition, we demonstrated that this could be performed using MEA for increased accessibility to the method, as well as its enabling higher throughput, which is optimal for screening systems. To verify the accuracy of this protocol, we generated an ROC curve showing significantly improved %ΔFPDcs upon specific current blockade as compared with FPDcs acquired at baseline, thereby supporting the ability of the method to recognize disease-specific iPSC-CMs. For LQT2, we performed voltage-clamp and current-clamp assays to support the MEA data, and confirmed that gene correction recovered IKr density, which correlated with the normalized IKr blockade response of the FPDc in LQT2corr-iPSC-CMs. Consistent with these results, Holzem et al. (2016) demonstrated that the reduced IKr blockade response reflected reduced IKr expression, as shown via optical imaging of perfused left-ventricular wedge preparations in patients with heart failure. In addition to LQT2, we also clarified that LQT1A344Aspl-iPSC-CMs displayed an attenuated response to IKs blockade, and that LQT3N406K-iPSC-CMs displayed a greater response to INa blockade, which was normalized by gene correction. Of significant note, each type of LQT-iPSC-CMs showed specific response which reflected the impaired ion currents as follows: LQT1A344Aspl-iPSC-CMs showed smaller %ΔFPDc upon IKs blockade while they showed normal %ΔFPDc upon IKr blockade. In contrast, LQT2A422T-iPSC-CMs showed smaller %ΔFPDc upon IKs blockade while they showed normal %ΔFPDc upon IKr blockade. These results strongly suggested that our system has a potential to distinguish the subtypes of LQTS. Regarding IKs blockade, no significant difference in %ΔFPDc on 10 μmol/L chromanol 293B was observed. Given the half maximal inhibitory concentration for KCNQ1/KCNE1 is 16.1 ± 1.8 μmol/L (Bett et al., 2006), IKs was only partially blocked. We speculated that small portion of IKs blockade might be masked by repolarization reserve in iPSC-CMs.
In addition, upon IKr blockade, some of the LQT2A422T-iPSC-CMs showed triggered activities at baseline or 30 nmol/L E4031, whereas no arrhythmic events were recorded in other clones, potentially because, compared with the other clones including LQT2G601S-iPSC-CMs, LQT2A422T-iPSC-CMs had less repolarization reserve composed of ion currents except IKr. Moreover, this might indicate why, upon IKr blockade, LQT2G601S-iPSC-CMs were less vulnerable to EADs than LQT2A422T-iPSC-CMs. Interestingly, these results seem to be consistent with the clinical history of donor patients, in which, while the KCNH2 p.G601S carrier remained asymptomatic, the KCNH2 p.A422T carrier had episodes of syncope. Further investigation of various mutations, including both pathogenic and non-pathogenic variants, is needed to elucidate this relationship.
Development of iPSC technology has provided increased opportunity for investigating monogenic disorders, such as LQTS, and iPSC-CMs have proven capable of recapitulating clinical phenotypes. However, most previous experiments were performed using labor-intensive techniques, such as patch-clamp methods. On the other hand, MEA, which is also used in neuronal science, improves the throughput of electrophysiological examination and enables CMs to exist in a less-invasive state relative to patch clamping. Therefore, protocols combining iPSC-CMs and the MEA system could potentially offer a less labor-intensive and higher-throughput method for analyzing disease-specific human CMs and could potentially lead to applications enabling clinical diagnosis of LQTS.
This study might offer novel insight into the utility of iPSC-CMs for phenotype-based diagnosis of LQTS. This method has several powerful advantages. First, although next-generation sequencing technology provides information about a large number of rare genetic variants, the pathophysiological significance of such variants is often uncertain. Therefore, the method presented in this study might be helpful in uncovering pathophysiological mechanisms for individuals, especially patients with VUSs or compound mutations, as well as genotype-negative patients. Second, this method enables a more comprehensive and practical diagnosis of LQTS. IKr-mediated LQTS includes LQT2, as well as LQT6 (potassium voltage-gated channel subfamily E regulatory subunit 2), whereas IKs-mediated LQTS includes not only LQT1 but also LQT5 (potassium voltage-gated channel subfamily E regulatory subunit 1) and LQT11 (A-kinase anchoring protein 9), as well as INa-mediated LQTS, including LQT3, 9 (caveolin 3), LQT10 (sodium voltage-gated channel beta subunit 4), and LQT12 (syntrophin alpha 1). Therefore, this current-centric classification4 provides us with the clinical ability for selective treatment. For example, mexiletine should be recommended for patients with INa-mediated LQTS, whereas β blockers would be predicted to be effective in all patients with IKs-mediated LQTS. To reveal whether this protocol is more reliable, it is necessary to evaluate the accuracy of this protocol using other cell lines. Nevertheless, the protocol presented here offers important clinical implications in the diagnosis and treatment of LQTS.
This study certainly includes some limitations. We applied Fridericia's formula for FPD correction. However, it is not clear whether the correction formula was valid for iPSC-CMs or not. We used only a small number of iPSC lines with already known pathogenic mutations and did not test iPSCs from LQTS patients carrying VUSs or mutations in the non-coding regions, or patients with no detected mutations. We should also use iPSC lines from persons, without phenotypes, despite carrying pathogenic mutations. In addition, the lack of isogenic controls for LQT1A344Asppl line, the lack of blind tests, the comparisons not performed at the same day of culture, and the variable quality and purity of the iPSC-CMs should be noted as limitations in this study. As such, this study is only a prototype and further investigation is required.
In conclusion, this study showed that the multiple subtypes of LQT could be potentially distinguished by specific ion-channel blockade using the MEA system with patient-derived iPSCs, and that this protocol might serve as a novel method to compensate for the shortcomings of genetic testing of LQTS patients, especially in patients who have VUSs or no identified mutations.
Experimental Procedures
iPSC Generation, and CM Differentiation and Purification
iPSCs were generated from LQT patients and healthy controls, as reported previously (Spencer et al., 2014, Wuriyanghai et al., 2018). All protocols were approved by the Committee on Human Research at Kyoto University (Kyoto, Japan) and conformed to the principles of the Declaration of Helsinki. iPSCs were differentiated into CMs using the previously described “GiWi” protocol (Figure S3A) (Lian et al., 2013). Differentiated CMs were purified in glucose-depleted lactate medium, as described previously (Tohyama et al., 2013), and iPSC-CMs were analyzed on days 60 through 100 (Figure S3B). Details are shown in the Supplemental Information.
Genome Editing
The target site was set to cover the mutation site, and single-guide RNA was constructed. Annealed oligo was inserted into the PX459 (Addgene, Cambridge, MA, USA) plasmid digested by BpiI. The targeting vector was designed to include a floxed puromycin-resistant drug cassette flanked by 1.5-kb homology arms, the 3′ arm of which included the gene-correcting site. The targeting vector was constructed by assembling PCR products of the homology arms, drug-resistant cassette, and backbone pENTR vector with Gibson Assembly (NEB, Ipswich, MA, USA). Thereafter, several point mutations were inserted to avoid further digestion of the targeting site.
The procedures associated with introduction of the vectors were performed, as reported previously (Li et al., 2016). In brief, 5 μg of the CRISPR/Cas9 vector and the targeting vector was introduced into 1.0 × 106 iPSCs using an NEPA 21 electroporator (NEPA GENE, Ichikawa, Japan). Several days after electroporation, drug selection was initiated with 0.7 mg/mL puromycin. Puromycin-resistant cells were dispersed into single cells and dissociated onto a dish. Several days thereafter, single colonies were picked and screened by PCR. Drug-cassette-positive cells were then expanded, and their floxed sites were removed by Cre excision. Sanger sequencing was performed to confirm the sequence of the targeted site and possible off-target sites. Primers used for genome editing are listed in the Supplemental Information (Tables S2 and S3).
Electrophysiological Recordings
MEA Recording
CMs were dissociated with 1 mg/mL collagenase B (Roche, Roswell, GA, USA) and Accumax (Innova Cell Technologies, San Diego, CA, USA). CM suspension (2 μL of 1.5 × 104 cells/μL) was placed onto the Matrigel-coated electrode (MED-P515A; Alpha MED Scientific, Osaka, Japan) (Asakura et al., 2015). After several hours, 1 mL RPMI/B27 medium containing 10% fetal calf serum was added. After 2 to 3 days, the medium was replaced with RPMI/B27 medium. After 6 to 20 days, the FP of spontaneously beating CMs was recorded. FP signals were digitally sampled at 20 kHz through 0.1-Hz high-pass and 10-kHz low-pass filters using the MED64 system (Alpha MED Scientific). FPD was defined as the interval between a positive or negative spike and a subsequent positive deflection, and inter-spike interval (ISI) was defined as the interval between adjacent spikes. These parameters were automatically measured and analyzed using Möbius QT (Alpha MED Scientific). More than 30 beats were recorded, and the FPDs and ISIs of the final 30 beats were averaged, as described previously (Asakura et al., 2015), which was standardized by Fridericia's formula (FPDc = FPD/ISI1/3) to minimize influence of a wide range of ISIs on FPDs (Table S4). EAD was defined as relatively slow negative spikes during the repolarizing phase, with samples displaying irregular beating excluded when calculating FPD and ISI. EAD-positive samples were defined as showing more than 5 EADs among 30 beats. The prolongation rate of FPDc (%ΔFPDc) was calculated as follows: %ΔFPDc (%) = [(FPDc after blockade – FPDc at baseline)/FPDc at baseline × 100]. For specific current blockade, we used E4031 (Wako Pure Chemicals, Osaka, Japan), chromanol 293B (Sigma-Aldrich, St. Louis, MO, USA), TTX (Alomone Labs, Jerusalem, Israel), and mexiletine (Sigma-Aldrich, Tokyo, Japan). Drug was gently administered by taking half of the medium out, diluting it, and then returning it to the solution. For measurement under stable conditions, FPD was measured 30 to 60 min after drug administration. All the data were acquired from at least three independent experiments.
Patch-Clamp Recording
CMs dissociated with Accutase (Thermo Fisher Scientific) were adhered onto Matrigel-coated glass coverslips for 5 days before recording AP in current-clamp mode using the perforated patch technique, whereas IKr was recorded from single cells in voltage-clamp mode using the ruptured whole-cell patch technique (Ma et al., 2011). The pipette solution comprised (in mM): 150 KCl, 5 NaCl, 2 CaCl2, 5 EGTA, 10 HEPES, and 5 MgATP (pH 7.2, adjusted with KOH), with amphotericin B added during AP recording (0.3 mg/mL, final concentration). The extracellular solution comprised (in mM): 150 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 15 glucose, 15 HEPES, and 1 Na-pyruvate (pH 7.4, adjusted with NaOH), with 2 μM nifedipine added during IKr recording.
Current-clamp recordings were sampled and filtered at 10 kHz. To evaluate AP prolongation by E4031, IK1 current was artificially injected using the dynamic clamp technique, as reported previously (Bett et al., 2013). Detailed dynamic clamp procedures are described in the following section. Ventricular-type CMs were defined as those exhibiting an APD90/APD50 < 1.4. E4031 administration (100 nM) was performed in ventricular-type CMs.
Before adding nifedipine for IK measurement, AP was recorded to identify ventricular-type CMs. Voltage-clamp recordings were then sampled at 2 kHz and filtered at 1 kHz. The voltage-clamp trace was obtained as follows: after a −40-mV holding pulse, step pulses were applied from −40 to 10 mV in 5-mV increments for 4 s and with a 10-s cycle length. The tail current was measured at the peak immediately after the depolarizing pulse. The measured current was standardized based on the patched cell-membrane capacitance. Therefore, IKr was defined as the E4031-sensitive current upon addition of 500 nM E4031 to the extracellular solution. Pipettes pulled from thin-walled borosilicate glass capillaries (TW150-4; World Precision Instruments, FL, USA) with a PP-830 puller (Narishige, Tokyo, Japan) exhibited between 4.0 and 7.0 MΩ resistance in voltage clamp and between 3.0 and 5.0 MΩ resistance in current clamp. All recordings were performed at between 35°C and 37°C. Data were acquired with an Axon 700B MultiClamp, Digidata 1440A digitizer hardware, and pCLAMP 10.4 software (Molecular Devices, Sunnyvale, CA, USA), as required. All the data were acquired from at least three independent experiments.
Real-Time IK1 Injection Using the Dynamic Clamp Technique
As described previously (Bett et al., 2013), an analog and digital I/O board PCIe-DAS1602/16 (Measurement Computing Corporation, Norton, MA, USA) was used to transfer the signal of membrane voltage to the IK1 signal using the same source code. In addition, 1 pA/pF of peak IK1 was injected to fix the MDP at approximately –80 mV in our iPSC-CMs before and after E4031 administration. IK1 was adjusted using a potentiometer (custom-made by Inter Medical, Nagoya, Japan). The IK1 equation was optimized for ventricular cells according to previous reports (Bett et al., 2013, Koumi et al., 1995, Van Putten et al., 2015). The equation we used was as follows: IK1 = 0.5 × (Vm + 85)/(1 + e[0.0896(Vm + 85)]).
Statistical Analysis
JMP Pro 13 (SAS, Cary, NC, USA) was used for statistical analysis. Data are presented as the mean ± SEM. An unpaired Student's t test or one-way analysis of variance (ANOVA), followed by Fisher's least significant difference (LSD) test, was used for two- or more than two-group comparisons. For repetitive measurements, we performed a two-way repeated measures ANOVA, followed by Fisher's LSD test. Pearson's chi-square test was used to determine independence of categorical data. Results were considered statistically significant at p < 0.05.
Author Contributions
Conceptualization, D.Y., S.B., and T.M.; Methodology, D.Y., S.B., T.M., and T.H.; Investigation, D.Y., H.S., T.H., K.A., K.M., H.K., Y.W., and Y.Y.; Writing –– Original Draft, D.Y.; Writing – Review & Editing, S.B., T.M., K.U., B.R.C., and M.H.; Funding Acquisition, S.B., T.M., and M.H.; Resources, S.B., T.M., K.H., B.R.C., M.H., and T.H.; Supervision, S.B., T.M., M.H., J.T., and T.H.
Acknowledgments
We thank Akitsu Hotta (Kyoto University, Kyoto, Japan) for providing the plasmid used to construct the targeting vector, Takashi Yamamoto (Hiroshima University, Hiroshima, Japan) for technical advice on genome editing, Tadahiko Matsumoto (Kyoto University, Kyoto, Japan) for Southern blot analysis, Takashi Ashihara (Shiga University of Medical Science, Otsu, Japan), Yukiko Himeno (Ritsumeikan University, Kusatsu, Japan) and Akira Amano (Ritsumeikan University, Kusatsu, Japan) for in silico technical advice on the dynamic clamp, Yasuhiko Tomita (Public health, Hyogo Health Service Association, Kobe, Japan) for recruitment of a patient, and Kyoko Yoshida (Kyoto University, Kyoto, Japan) for maintaining the cells. DNA sequencing and immunofluorescence analyses were performed at the Medical Research Support Center, Graduate School of Medicine, Kyoto University. This work was supported by the Japan Society for the Promotion of Science KAKENHI under grant number JP17K10141 to S.B. and 25461054 to T.M., and by grants from the Japan Agency for Medical Research and Development, AMED under grant number 17930004 to M.H. and 17ek09294h0001to T.M., the SENSHIN Medical Research Foundation and the Uehara Memorial Foundation. Bruce R Conklin: A founder of Tenaya Therapeutics (https://www.tenayatherapeutics.com/), a company focused on finding treatments for heart failure. Holds equity in Tenaya, and Tenaya provides research support for heart failure-related research.
Published: August 01, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.06.007.
Supplemental Information
References
- Asakura K., Hayashi S., Ojima A., Taniguchi T., Miyamoto N., Nakamori C., Nagasawa C., Kitamura T., Osada T., Honda Y. Improvement of acquisition and analysis methods in multi-electrode array experiments with iPS cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods. 2015;75:17–26. doi: 10.1016/j.vascn.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Bett G.C.L., Morales M.J., Beahm D.L., Duffey M.E., Rasmusson R.L. Ancillary subunits and stimulation frequency determine the potency of chromanol 293B block of the KCNQ1 potassium channel. J. Physiol. 2006;576:755–767. doi: 10.1113/jphysiol.2006.116012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett G.C.L., Kaplan A.D., Lis A., Cimato T.R., Tzanakakis E.S., Zhou Q., Morales M.J., Rasmusson R.L. Electronic “expression” of the inward rectifier in cardiocytes derived from human-induced pluripotent stem cells. Heart Rhythm. 2013;10:1903–1910. doi: 10.1016/j.hrthm.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira T., Yuasa S., Fukuda K. Induced pluripotent stem cells in cardiovascular medicine. Stem Cells Int. 2011;2011:348960. doi: 10.4061/2011/348960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicessi J.R., Ackerman M.J. Genotype- and phenotype-guided management of congenital long QT syndrome. Curr. Probl. Cardiol. 2013;38:417–455. doi: 10.1016/j.cpcardiol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki T., Rouby N., Fredette N.C., Santostefano K.E., Terada N. Concise review: induced pluripotent stem cell research in the era of precision medicine. Stem Cells. 2017;35:545–550. doi: 10.1002/stem.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzem K.M., Gomez J.F., Glukhov A.V., Madden E.J., Koppel A.C., Ewald G.A., Trenor B., Efimov I.R. Reduced response to IKr blockade and altered hERG1a/1b stoichiometry in human heart failure. J. Mol. Cell Cardiol. 2016;96:82–92. doi: 10.1016/j.yjmcc.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M. Molecular genetics have opened a new era for arrhythmia research but also Pandora׳s box? J. Arrhythm. 2016;32:313–314. doi: 10.1016/j.joa.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Yamanaka S. The use of induced pluripotent stem cells in drug development. Clin. Pharmacol. Ther. 2011;89:655–661. doi: 10.1038/clpt.2011.38. [DOI] [PubMed] [Google Scholar]
- Kapa S., Tester D.J., Salisbury B.A., Harris-Kerr C., Pungliya M.S., Alders M., Wilde A.A., Ackerman M.J. Genetic testing for long-QT syndrome distinguishing pathogenic mutations from benign variants. Circulation. 2009;120:1752–1760. doi: 10.1161/CIRCULATIONAHA.109.863076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumi S., Backer C.L., Arentzen C.E. Characterization of inwardly rectifying K+ channel in human cardiac myocytes. Alterations in channel behavior in myocytes isolated from patients with idiopathic dilated cardiomyopathy. Circulation. 1995;92:164–174. doi: 10.1161/01.cir.92.2.164. [DOI] [PubMed] [Google Scholar]
- Li H.L., Gee P., Ishida K., Hotta A. Efficient genomic correction methods in human iPS cells using CRISPR-Cas9 system. Methods. 2016;101:27–35. doi: 10.1016/j.ymeth.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Lian X., Zhang J., Azarin S.M., Zhu K., Hazeltine L.B., Bao X., Hsiao C., Kamp T.J., Palecek S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Guo L., Fiene S.J., Anson B.D., Thomson J.A., Kamp T.J., Kolaja K.L., Swanson B.J., January C.T. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 2011;301 doi: 10.1152/ajpheart.00694.2011. H2006–E2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A.J., Zareba W., Hall W.J., Schwartz P.J. Effectiveness and limitations of β-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- Moss A.J. Long QT syndrome. JAMA. 2003;289:2041–2044. doi: 10.1001/jama.289.16.2041. [DOI] [PubMed] [Google Scholar]
- Napolitano C., Priori S.G., Schwartz P.J., Bloise R., Ronchetti E., Nastoli J., Bottelli G., Cerrone M., Leonardi S. Genetic testing in the long QT syndrome: development and validation of an efficient approach to genotyping in clinical practice. JAMA. 2005;294:2975–2980. doi: 10.1001/jama.294.23.2975. [DOI] [PubMed] [Google Scholar]
- Priori S.G., Napolitano C., Schwartz P.J., Grillo M., Bloise R., Ronchetti E., Moncalvo C., Tulipani C., Veia A., Bottelli G. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- Priori S.G., Wilde A.A., Horie M., Cho Y., Behr E.R., Berul C., Blom N., Brugada J., Chiang C.E., Huikuri H. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Schwartz P.J., Priori S.G., Spazzolini C., Moss A.J. Genotype-phenotype correlation in the long-QT syndrome. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- Schwartz P.J., Ackerman M.J., George A.L., Wilde A.A.M. Impact of genetics on the clinical management of channelopathies. J. Am. Coll. Cardiol. 2013;62:169–180. doi: 10.1016/j.jacc.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer C.I., Baba S., Nakamura K., Hua E.A., Sears M.A., Fu C.C., Zhang J., Balijepalli S., Tomoda K., Hayashi Y. Calcium transients closely reflect prolonged action potentials in iPSC models of inherited cardiac arrhythmia. Stem Cell Reports. 2014;3:269–281. doi: 10.1016/j.stemcr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;107:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tohyama S., Hattori F., Sano M., Hishiki T., Nagahata Y., Matsuura T., Hashimoto H., Suzuki T., Yamashita H., Satoh Y. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Van Putten R.M.E.M., Mengarelli I., Guan K., Zegers J.G., van Ginneken A.C., Verkerk A.O., Wilders R. Ion channelopathies in human induced pluripotent stem cell derived cardiomyocytes: a dynamic clamp study with virtual IK1. Front. Physiol. 2015;6:7. doi: 10.3389/fphys.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens X.H., Vos M.A., Doevendans P.A., Wellens H.J.J. Novel insights in the congenital long QT syndrome. Ann. Intern. Med. 2002;137:981–992. doi: 10.7326/0003-4819-137-12-200212170-00012. [DOI] [PubMed] [Google Scholar]
- Wuriyanghai Y., Makiyama T., Sasaki K., Kamakura T., Yamamoto Y., Hayano M., Harita T., Nishiuchi S., Chen J., Kohjitani H. Complex aberrant splicing in the induced pluripotent stem cell-derived cardiomyocytes from a patient with long QT syndrome carrying KCNQ1-A344Aspl mutation. Heart Rhythm. 2018;15:1566–1574. doi: 10.1016/j.hrthm.2018.05.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.