Abstract
The data and information presented here refer to the research article entitled: “Reactivating endogenous mechanisms of cardiac regeneration via paracrine boosting with the human amniotic fluid stem cell secretome” (Balbi et al., 2019, Apr 04). This dataset illustrates the in vitro paracrine effect exerted by the human amniotic fluid stem cell secretome on rodent neonatal cardiomyocytes, human endothelial progenitors and different subsets of cardiac progenitor cells. Cytokine/chemokine profiling of the human amniotic fluid stem cell secretome is provided as well. This data can provide useful insights in regenerative medicine as demonstrating the in vitro cardioprotective and proliferative secretory paracrine potential of human fetal stem cells.
Keywords: Paracrine effect, Human amniotic fluid stem cells, Cardiac progenitor cells, Neonatal cardiomyocytes, Endothelial progenitors, Proliferation, Cardioprotection, Angiogenesis
Specifications Table
| Subject area | Biology and Medicine |
| More specific subject area | Regenerative Medicine, Cardiovascular Disease |
| Type of data | Graph; Figures |
| How data was acquired |
Data was acquired by using: - BioTek Microplate Reader with Gen5 2.03 Software - Epifluorescence Axiolab microscope (Carl Zeiss, Oberkochen, Germany, with a Zeiss × 40 Achroplan objective); - Extended-ISIS Camera, Photonic Science, Millham, UK; - Axiovert microscope equipped with Axiovision software (Carl Zeiss, Oberkochen, Germany) - ImageXpress Micro high-content screening microscope equipped with MetaXpress software (Molecular Devices, California, USA). |
| Data format | Raw and analyzed by independent evaluators. Authors provided raw data in a supplementary excel file. |
| Experimental factors | Data acquired was analyzed by ImageJ and Prism Graph Pad software, and then plotted into graph with representative figures |
| Experimental features | Data was obtained by in vitro analysis including cytokine/chemokine Array Assay, MTT colorimetric Assay, Ca2+signaling recording, immunostaining, morphological analysis and Click-IT EdU Imaging kit staining. |
| Data source location |
University of Genova, Dept. of Experimental Medicine, Italy ICGEB Trieste, Italy University of Pavia, Italy Leiden University Medical Centre, Leiden, The Netherlands |
| Data accessibility | Data are available within the article |
Value of the data
|
1. Data
Data presented here offer a preliminary characterization of the modulatory soluble components within the whole of the human amniotic fluid stem cell (hAFS) secretome (Fig. 1 and Table 1). It also provides in vitro confirmation of the pro-survival cardioprotective and pro-angiogenic potential of hAFS-conditioned medium on target cells with cardiovascular relevance (Fig. 2), along with its paracrine proliferative effect on human cardiac progenitors and rodent neonatal cardiomyocytes (Fig. 3, Fig. 4). Indeed, this dataset supports the subsequent in vivo analyses carried out in the manuscript by Balbi C. et al. in the International Journal of Cardiology.
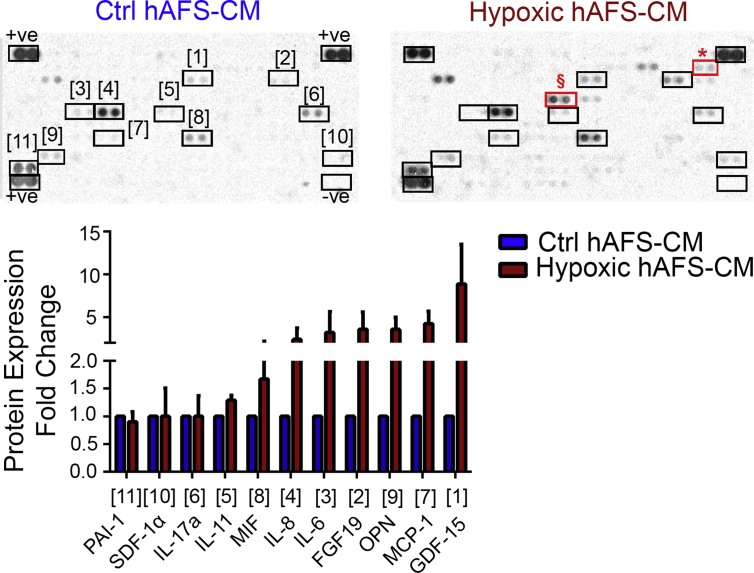
Fig. 1.
hAFS Secretome Profiling. Cytokine and chemokine array of hypoxic hAFS secretome (Hypoxic hAFS-CM) compared to normoxic control one (Ctrl hAFS-CM) assessed by quantification of positive pixels for each selected cytokine. Upper panel: representative images of array membranes in which numbers indicate the corresponding chemokine/cytokine as reported in the graph below. Red boxes indicate EMMPRIN (*) and IGFBP-2 (§), which are not expressed by Ctrl hAFS-CM. Values are expressed as fold change of Hypoxic hAFS-CM over Ctrl hAFS-CM and reported in Table 1. + ve: positive reference control; -ve: negative reference control.
Table 1.
Cytokine and chemokine profiling of hAFS secretome obtained following following 24h 1% O2 hypoxic preconditioning and compared to normoxic conditions (Ctrl hAFS-CM).
| Ctrl hAFS-CM | Hypoxic hAFS-CM | |
|---|---|---|
| PAI-1 | 1 | 0.90 ± 0.18 |
| IL-17α | 1 | 1.00 ± 0.36 |
| SDF-1α | 1 | 1.00 ± 0.51 |
| IL-11 | 1 | 1.29 ± 0.11 |
| MIF | 1 | 1.67 ± 0,49 |
| IL-8 | 1 | 2.39 ± 1.32 |
| IL-6 | 1 | 3.20 ± 2.41 |
| OPN | 1 | 3.55 ± 1.43 |
| FGF-19 | 1 | 3.58 ± 1.98 |
| MCP-1 | 1 | 4.23 ± 1.45 |
| GDF-15 | 1 | 8.87 ± 4.64 |
Values are assessed by quantification of positive pixels for each selected cytokine on the array membrane and are expressed as mean ± s. e.m of the fold change in the cytokine/chemokine expression of hypoxic hAFS secretome (Hypoxic hAFS-CM) over control normoxic hAFS-CM (Ctrl hAFS-CM) of n = 3 experiments; FGF-19: Fibroblast Growth Factor 19; GDF-15: Growth/differentiation factor 15; IL-6: Interleukin-6; IL-11: Interleukin-11; IL-17α: Interleukin- 17α: MCP-1: Monocyte Chemotactic Protein-1; MIF: Macrophage migration inhibitory factor; OPN: Osteopontin; SDF-1α: Stromal cell-Derived Factor 1-alpha; PAI-1: Plasminogen Activator Inhibitor-1.
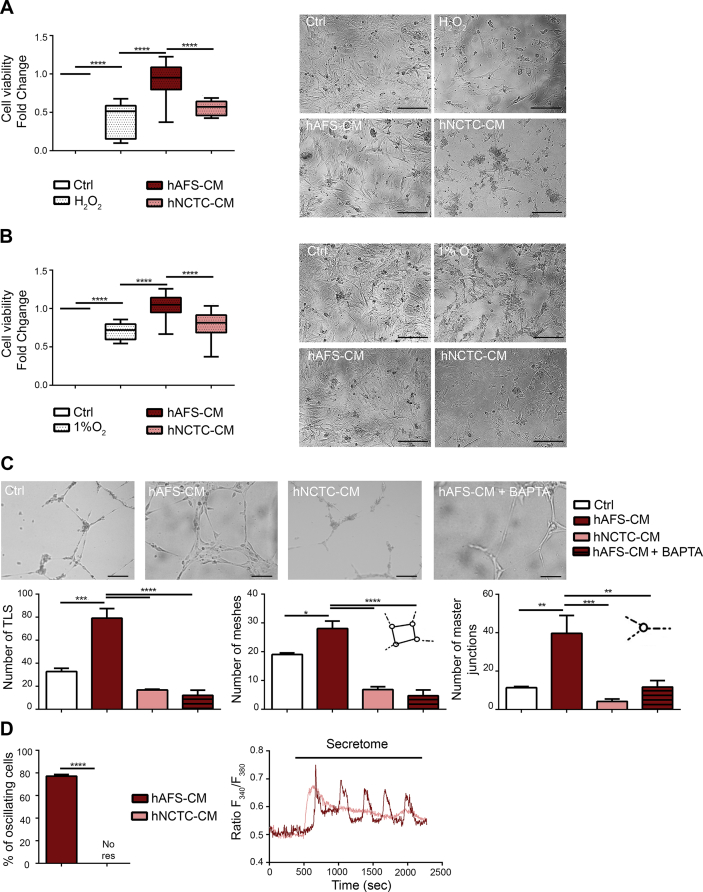
Fig. 2.
In Vitro Cardioprotective and Angiogenic Paracrine Effects driven by the hAFS Secretome. A) mNVCM viability following H2O2 oxidative stress with or without pre-incubation with 80μg/ml of hAFS-CM or hNCTC-CM, compared to untreated healthy cells (Ctrl) and evaluated by MTT assay. All values are expressed as mean ± s. e.m of at least n = 3 experiments as fold change over Ctrl condition (H2O2: 0.41 ± 0.07; hAFS-CM: 0.93 ± 0.04; hNCTC-CM: 0.55 ± 0.03; ****p < 0.0001). On the right, mNVCM representative pictures: untreated healthy cells (Ctrl), cells exposed to oxidative stress without any secretome priming (H2O2), cells pre-incubated with hAFS-CM and exposed to oxidative stress (hAFS-CM), and cells pre-incubated with hNCTC-CM and exposed to oxidative stress (hNCTC-CM); scale bar 100μm. B) mNVCM viability after 1% O2 hypoxic injury, with or without pre-incubation with 80μg/ml of hAFS-CM or hNCTC-CM, compared to untreated healthy cells (Ctrl) and evaluated by MTT assay. All values are expressed as mean ± s. e.m of at least n = 3 experiments as fold change over Ctrl condition (1% O2: 0.71 ± 0.02; hAFS-CM: 1.04 ± 0.03; hNCTC-CM: 0.78 ± 0.05; ****p < 0.0001). On the right, representative pictures of mNVCM: untreated healthy cells (Ctrl), cells exposed to 1% O2 without any secretome priming (1% O2), cells pre-incubated with hAFS-CM and exposed to 1% O2 (hAFS-CM) and cells pre-incubated with hNCTC-CM and exposed to 1% O2 (hNCTC-CM); scale bar 100μm. C) Tubulogenesis assay on hECFC with or without (Ctrl) treatment with 80μg/ml of hAFS-CM (hAFS-CM), hNCTC-CM (hNCTC-CM) or hAFS-CM in presence of Ca2+ signalling inhibitor BAPTA (hAFS-CM + BAPTA). Digital images of endothelial tubes were obtained by bright-field light microscopy 10 hours after plating cells on Cultrex-coated wells; scale bar: 50μm. All values are expressed as mean ± s. e.m of at least n = 3 experiments. From left to right: number of total TLS per picture (Ctrl: 32.67 ± 2.91; hAFS-CM: 79.33 ± 8.41; hNCTC-CM: 16.80 ± 0.58; hAFS-CM + BAPTA: 12.00 ± 4.62; ***p < 0.001, p = 0.0001, ****p < 0.0001); number of meshes per picture (Ctrl: 19.00 ± 0.58; hAFS-CM: 28.00 ± 2.65; hNCTC-CM: 6.80 ± 1.02; hAFS-CM + BAPTA: 4.67 ± 2.03; *p < 0.05, (p = 0.0185), ****p < 0.0001); number of master junctions per pictures (Ctrl: 11.33 ± 0.67; hAFS-CM: 39.67 ± 9.26; hNCTC-CM: 4.20 ± 1.24; hAFS-CM + BAPTA: 11.67 ± 3.33; **p < 0.01, (p = 0.0059), ***p < 0.001, (p = 0.0005), hNCTC-CM versus hAFS-CM; **p < 0.01, (p = 0.0064), hAFS-CM + BAPTA versus hAFS-CM). D) Percentage of hECFC displaying an oscillatory increase in [Ca2+]i in response to different treatments; all values are expressed as mean ± s. e.m in 195 cells analysed in the hAFS-CM group and 102 cells for hNCTC-CM group. (hAFS-CM: 77.23 ± 7.72; hNCTC-CM: no response; ****p < 0.0001). Right panel: representative tracings of the increase in [Ca2+]i induced by secretome treatment in hECFC. Ctrl: control untreated cells; H2O2: hydrogen peroxide; hAFS-CM: human Amniotic Fluid Stem Cell-Conditioned Medium; hNCTC-CM: human keratinocyte NCTC cell-Conditioned Medium; hECFC: human Endothelial Colony Forming Cells; TLS: Tube-Like Structure length.
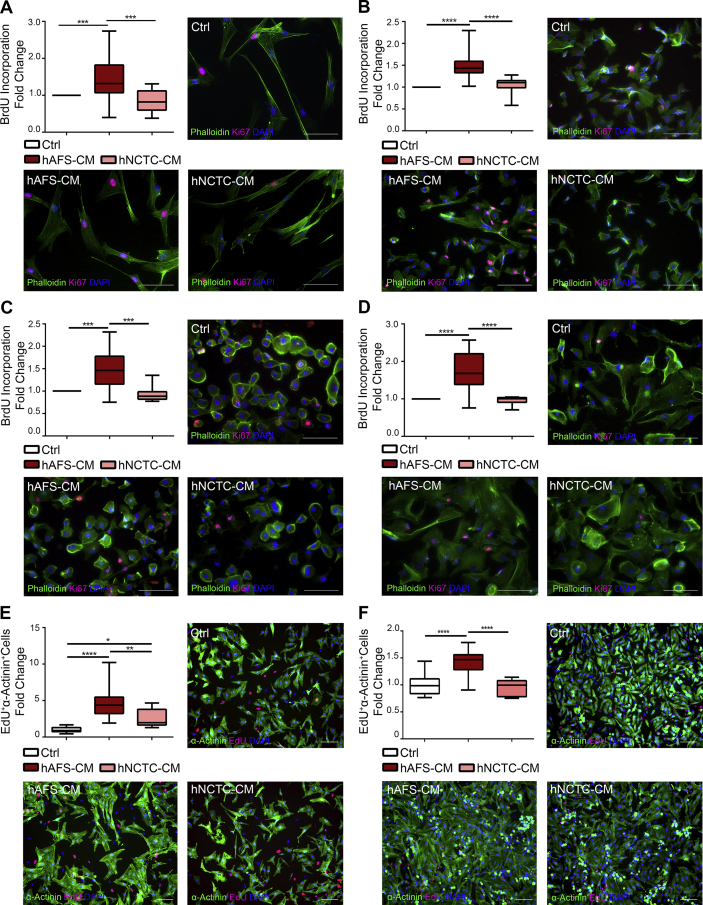
Fig. 3.
In Vitro Proliferative Paracrine Effect on human CPC and rat NVCM by hAFS Secretome. A-D) Evaluation of proliferative response from different human CPC subpopulations after treatment with 80μg/ml of either hAFS-CM or hNCTC-CM, compared to untreated cells (Ctrl) by BrdU ELISA. All values are expressed as mean ± s. e.m of at least n = 3 experiments as fold change over Ctrl condition, with representative pictures of cells in control conditions (Ctrl) or following treatment with either hAFS-CM or hNCTC-CM and stained with Ki67 (pink), phalloidin (green) and DAPI (blue); scale bar 200μm in all pictures but for A) which is 100μm. A) Adult hCPC (hAFS-CM: 1.47 ± 0.11; hNCTC-CM: 0.86 ± 0.09; ***p < 0.001, (p = 0.0001 hAFS-CM versus Ctrl; p = 0.0002 hNCTC versus hAFS-CM). B) fSca-1+ hCPC (hAFS-CM: 1.47 ± 0.05; hNCTC-CM: 1.05 ± 0.07; ****p < 0.0001). C) Adult hEPDCc (hAFS-CM: 1.50 ± 0.07; hNCTC-CM: 0.94 ± 0.06. ***p < 0.001, p = 0.0003, hAFS-CM versus Ctrl and p = 0.0002 as versus hNCTC-CM). D) Adult hEPDCs (hAFS-CM: 1.70 ± 0.09; hNCTC-CM: 0.96 ± 0.03; ****p < 0.0001). E-F) Analysis of proliferation of rNVCM exposed to 80μg/ml of either hAFS-CM or hNCTC-CM compared to untreated cells (Ctrl). All values are expressed as mean ± s. e.m of at least n = 3 experiments and evaluated as fold change over control condition (Ctrl) of EdU- and cardiac α-Actinin-positive cells with representative pictures of cells in control conditions (Ctrl) or following treatment with either hAFS-CM or hNCTC-CM and stained with EdU (red), cardiac α-Actinin (green) and DAPI (blue), scale bar 100μm. E) rNVCM isolated from 2-days-old rat hearts, n = 3 experiments (Ctrl: 1.00 ± 0.09; hAFS-CM: 4.63 ± 0.34; hNCTC-CM: 2.60 ± 0.43; ****p < 0.0001, **p < 0.01, p = 0.0028, *p < 0.05, p = 0.0407). F) rNVCM isolated from 5-days-old rat hearts, n = 3 experiments (Ctrl: 1.00 ± 0.07; hAFS-CM: 1.43 ± 0.05; hNCTC-CM: 0.96 ± 0.05 ****p < 0.0001). BrDU: 5-Bromo-2′-DeoxyUridine; EdU: 5-Ethynyl-2′-deoxyUridine; αActinin: sarcomeric alpha actinin; DAPI: 4′,6-DiAmidino-2-PhenylIndole.
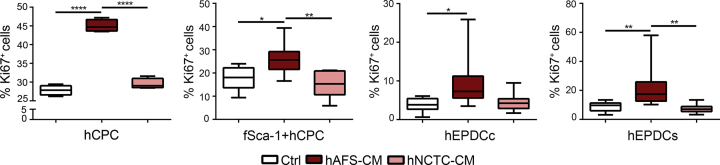
Fig. 4.
Evaluation of Ki67 expression on human CPC stimulated in vitro by the hAFS secretome. Evaluation of proliferative response from different CPC subpopulations after treatment with 80μg/ml of either hAFS-CM or hNCTC-CM, compared to untreated cells (Ctrl) by Ki67 staining. All values are expressed as mean ± s. e.m of the percentage of Ki67-positive cells of at least n = 3 experiments. hCPC (Ctrl: 27.82 ± 0.66%; hAFS-CM: 45.01 ± 0.82%; hNCTC-CM: 29.50 ± 0.71%. ****p < 0.0001). fSca-1+ hCPC (Ctrl: 17.73 ± 2.13%; hAFS-CM: 26.52 ± 1.46%; hNCTC-CM: 15.13 ± 2.44%. *p < 0.05 p = 0.011; **p < 0.01, p = 0.0011). hEPDCc (Ctrl: 3.87 ± 0.60%; hAFS-CM: 9.57 ± 1.23%; hNCTC-CM: 4.52 ± 0.95%; *p < 0.05, p = 0.0131). hEPDCs (Ctrl: 8.83 ± 1.20%; hAFS-CM: 21.17 ± 2.08%; hNCTC-CM: 7.37 ± 1.01%; **p < 0.01, p = 0.0083 and p = 0.0018).
2. Experimental design, materials and methods
For detailed Methods please refer to Balbi C. et al. in the International Journal of Cardiology [1].
2.1. Cell culture
hAFS were obtained and cultured as described in Balbi C. et al. in the International Journal of Cardiology [1]. Human NCTC 2544 (hNCTC) keratinocytes were purchased (Interlab Cell Line Collection, Genova, Italy) and cultured in MEM/Earl's Balanced Salt Solution (MEM/EBSS) with 10% FBS, 1% non-essential aminoacids, 1% L-glutamine, and 1% penicillin/streptomycin (all EuroClone, Italy).
Human adult cardiac progenitor cells (hCPC) were obtained as previously reported [2], from atrial appendage specimens as clinical waste, at the Division of Cardiac Surgery, San Martino Hospital (Genova, Italy), following written informed consent and according to local ethical committee authorization (P.R.007REG2013). Briefly, cardiac tissue was cut into fragments of approximately 0.5 mm in PBS and trypsin solution was added for 10 minutes, then fragments were placed in a culture dish as primary tissue explant culture in Iscove Modified Dulbecco's Medium (EuroClone, Milano, Italy) with 20% FBS, 1% L-glutamine, 1% penicillin/streptomycin, (all Thermo Fisher Scientific, Waltham, Massachusetts). Cells migrating from explants were collected after 2–3 weeks. Human fetal Sca-1+ CPC and human adult epicardium derived progenitor cells (hEPDC) were obtained as previously reported [3], [4], [5] from human heart tissue, following written informed consent and according to local Medical Ethics Committee at Leiden University Medical Center (P08.087). hCPC were cultured in Iscove Modified Dulbecco's Medium (EuroClone, Milano, Italy) with 20% FBS, 1% L-glutamine, 1% penicillin/streptomycin, (all Thermo Fisher Scientific, Waltham, Massachusetts). Human fetal Sca-1+ CPC (fSca-1+ hCPC) were obtained from human foetal heart tissue, after elective abortion without medical indication from 10 to 22 weeks of gestation and sorted for Sca-1 cross-reactivity (MACS MicroBead Kit, Miltenyi Biotechnology, Bergisch Gladbach, Germany) as previously described [3], [4] and cultured on 0.1% gelatin-coated dishes in M199 (Gibco-Thermo Fisher Scientific, Waltham, Massachusetts)/EGM (3:1) supplemented with 10% FBS (Gibco-Thermo Fisher Scientific, Waltham, Massachusetts), 10 ng/ml basic fibroblast growth factor (bFGF), 5 ng/ml epithelial growth factor (EGF), 5 ng/ml insulin-like growth factor (IGF-1) and 5 ng/ml hepatocyte growth factor (HGF). hEPDC were obtained from human atrial samples obtained during cardiac surgery, and isolated by separating the epicardium from the underlying myocardium [5]. Briefly, the tissue was processed into small pieces and digested in a 0.25% Trypsin/EDTA solution (Serva, Heidelberg, Germany). Cells were cultured in 1:1 Dulbecco's modified Eagle's medium (DMEM-glucose low; Invitrogen, Carlsbad, California) and Medium 199 (M199; Invitrogen, Carlsbad, California) supplemented with 10% heat-inactivated FCS (Gibco-Thermo Fisher Scientific, Waltham, Massachusetts), and 100 U/ml penicillin/streptomycin (Gibco-Thermo Fisher Scientific, Waltham, Massachusetts). To avoid hEPDC undergoing epithelial-to-mesenchymal transition (EMT) while maintaining cobble-like morphology (hEPDCc), the ALK5-kinase inhibitor SB431542 (5–10μm; Tocris Bioscience, Bristol, UK) was added to the culture medium. hEPDC activating epithelial-to-mesenchymal transition (EMT) as not treated with such inhibitor, acquired a more elongated, fibroblast-like, spindle morphology (hEPDCs).
Mouse and rat NVCM isolation was performed in compliance with specific authorization (protocol 384/2016-PR, 792/2015-PR and EEC Council Directive 86/609, OJL 358, 12 December 1987). Mouse NVCM (mNVCM) were obtained as in [6] via enzymatic digestion from 2-days old mouse hearts (C57Bl/6 mouse) using a 0.125 mg/ml collagenase type II (Worthington Biochemicals, Lakewood, New Jersey) solution under constant stirring; cells were seeded on 1% gelatin coating solution (Sigma-Aldrich, St. Louis, Missouri, US) in complete plating medium (69% Dulbecco's Modified Eagle Medium, DMEM, 15% M199, 10% horse serum, 5% FBS, 100U/ml of penicillin and 100mg/ml of streptomycin and 1% L-glutammine, Gibco-Thermo Fisher Scientific, Waltham, Massachusetts and Sigma-Aldrich, St. Louis, Missouri, US). Rat NVCM (rNVCM) were obtained according to [9]. Briefly, 1- and 5-days-old (Wistar rat) hearts were digested by a 2 mg/ml trypsin (Gibco-Thermo Fisher Scientific, Waltham) and 20 μg/ml DNase II buffer solution (Sigma-Aldrich, St. Louis, Missouri), under slow stirring. A pre-plating step was performed to remove stromal cells. rNVMC were plated on Primaria cell culture multiwell plates (Corning, Tewksbury, Massachusetts) in complete medium (high glucose DMEM supplemented with 5% FBS, 20mg/ml vitamin B12 and with 100U/ml of penicillin and 100 μg/ml of streptomycin, respectively, from Gibco-Thermo Fisher Scientific, Waltham, Massachusetts and Sigma-Aldrich, St. Louis, Missouri, US). hECFC were isolated following written informed consent and ethical committee authorization (protocol n.20110004143, IRCCS Policlinico San Matteo Foundation, Pavia) and plated on collagen-coated culture dishes (BD Bioscience, Franklin Lake, New Jersey) in growth medium (EGM-2 Lonza, Basel, Switzerland) supplemented with endothelial basal medium (EBM-2), 5% FBS, recombinant human (rh) epithelial growth factor (rhEGF), rh vascular endothelial growth factor (rhVEGF), rh fibroblast growth factor-basic (rhFGF-B), rh insulin-like growth factor 1 (rfIGF-1), ascorbic acid and heparin. HUVEC (Human Umbilical Vein Endothelial Cells) were cultured in endothelial EGM-2 cultured medium as previously reported (Lonza, Basel, Switzerland) [7].
2.2. Collection of cell-conditioned medium
Cell-conditioned media from hAFS and hNCTC (namely hAFS-CM and hNCTC-CM, respectively) were obtained according to the hypoxic preconditioning protocol previously described by our group [6]. Cells were washed with PBS solution and incubated for 24h in serum-free medium (4.5g/l glucose DMEM, 1% L-glutamine, and 1% penicillin/streptomycin) under hypoxic condition (1% O2, 5% CO2 at 37 °C in a hypoxic incubator, Eppendorf, Hamburg, Germany). hAFS-CM and hNCTC-CM were concentrated using ultrafiltration membranes with a 3kDa selective cut-off (Amicon Ultra-15, Millipore, Burlington, Massachusetts). Protein amount of hAFS-CM and hNCTC-CM was evaluated by BCA (BicinChoninic Acid) Protein Assay Kit (Pierce, Thermo Fisher Scientific, Waltham, Massachusetts, US), following manufacturer's instructions in order to define cell-conditioned medium concentration that was used as 80μg/ml. hNCTC-CM was used as comparative negative control for in vitro experiments.
2.3. Cytokine and chemokine profiling of cell secretome
Cytokine and chemokine profiles of cell secretome was performed by a cytokine array assay (Proteome Profiler™ Human XL Cytokine Array kit; R&D System, Minnesota, US) following manufacturer's instructions. Analysis was performed on 2.5 μg of total protein content from cell conditioned medium and images of spotted array membranes acquired on X-ray film. Images were analyzed by ImageJ (https://imagej.nih.gov/jj/) with the protein Array Analyzer Plug-in.
2.4. In vitro analysis of hAFS secretome cardio-active potential
2.4.1. Cardioprotective potential
mNVCM were primed in serum-free conditions (SF) with hAFS-CM versus hNCTC-CM for 3h, then exposed for 4h to 150 μM H2O2 solution or under 1% O2 atmosphere and then cultured in complete medium for the following 24h. Cell viability was assessed by MTT assay using a 150μg/ml MTT solution (Sigma-Aldrich, Missouri).
2.4.2. Angiogenic effect
For Ca2+ signaling, hECFC were cultured on a coverslip and loaded with 4μM Fura-2 acetoxymethyl ester solution (Fura-2/AM; 1 mM stock in dimethyl sulfoxide) in physiological salt solution (PSS: 150mM NaCl, 6mM KCl, 1.5mM CaCl2, 1mM MgCl2, 10mM Glucose, 10mM Hepes with 7.4 pH) for 1 hour at room temperature. Cells were observed by an epifluorescence Axiolab microscope (Carl Zeiss, Oberkochen, Germany, with a Zeiss × 40 Achroplan objective). hECFC were excited alternately at 340 and 380nm, and the emitted light detected at 510nm. Custom software, working in the LINUX environment, was used to drive camera (Extended-ISIS Camera, Photonic Science, Millham, UK) and filter wheel, and to plot on-line the fluorescence from 10 up to 50 rectangular “regions of interest” (ROI). Adjacent ROIs never superimposed. [Ca2+]i was monitored by measuring the ratio of the mean fluorescence emitted at 510 nm when exciting alternatively at 340 and 380 nm (shortly termed “ratio”) for each ROI. Ratio measurements were performed and plotted on-line every 3s. Experiments were performed at room temperature (22 °C).
Early passage (P2–P3) hECFC were cultured in basal medium EBM-2 supplemented with 2% FBS in Cultrex (Trevigen, Gaithersburg, Maryland)-coated 96 well plates, in the presence of either hAFS-CM or hNCTC-CM for 24 hours. Capillary network formation was assessed starting from 4 up to 24 hours later. The angiogenic response was measured by evaluating both dimensional and topological parameters. Length of endothelial tube-like structures (TLS), number of polygon structures established by TLS, referred to as meshes and indicative of endothelial cell migration, and number of master junctions were measured from acquired bright field pictures by using the Angiogenesis Analyzer plugin of ImageJ (Gilles Carpentier, Faculte’ des Sciences et Technologie, Universite’ Paris Est, Creteil Val de Marne, France). Micrographs were captured by using an Olympus IX71 inverted microscope (Olympus Europa GmbH, Hamburg, Germany) equipped with a CPlan F1 10 × /0.30 objective. Three different sets of experiments, each performed in duplicate, were carried out. To evaluate the effect of Ca2+ signaling, the same protocol was repeated by priming hECFC with hAFS-CM in the presence of BAPTA (30μM solution for 2 hours), a membrane-permeable chelator used to prevent Ca2+−dependent processes [7], [8].
2.4.3. Proliferative potential on human CPC and rNVCM
hCPC, fSca-1+ hCPC, hEPDCc and hEPDCs were primed with hAFS-CM versus hNCTC-CM over-night. All CPC populations were incubated for the following 24h in complete medium with 10μM bromodeoxyuridine (BrdU). The hAFS-CM proliferative effect was also evaluated on human CPC populations by BrdU colorimetric assay (Roche, Basel, Switzerland) according to the manufacturer's instructions. CPC proliferation was also analysed by Ki67 (Millipore, Burlington, Massachusetts) and phalloidin staining (LifeTechnology, Carlsbad, California). Cells were treated with hAFS-CM versus hNCTC-CM for 3h, fixed with 4% PFA and processed by immunostaining. Images were acquired on an Axiovert microscope equipped with Axiovision software (Carl Zeiss, Oberkochen, Germany).
DNA duplication in rNVCM was assessed by incubating cells with hAFS-CM versus hNCTC-CM and after 12 hours, 10μM EdU was added (Life Technology, Carlsbad, California). After additional 20 hours cells were fixed and stained for α -actinin and EdU. rNVCM were fixed with 4% PFA and stained by mouse anti-sarcomeric α-Actinin (Abcam) and Click-IT EdU-594 Imaging kit to reveal EdU incorporation (Life Technology, Carlsbad, California) as previously described [9]. Images were acquired and computed at the ICGEB High-Throughput Screening Facility, Trieste, Italy (http://www.icgeb.org/high-throughput-screening.html).
2.5. Statistical analysis
Results are presented as mean ± s.e.m. (standard error of mean) of at least three (n = 3) independent replicated experiments. Comparisons were drawn by one-way ANOVA followed by post-hoc Tukey's multiple test or by unpaired t-test when appropriate and analysed by Prism Version 6.0a GraphPad Software with statistical significance set at *p < 0.05.
Acknowledgements
S.B. was funded by Programma Giovani Ricercatori “Rita Levi Montalcini” from Italian Ministry of Research and Education; A.M.S. was supported by a Leiden University Medical Center (LUMC) research fellowship and a Dekker Fellowship (senior scientist, 2017T059) from the Dutch Heart Foundation. This study contributes to the main aims and goals of the Horizon 2020 COST Action CA17116 SPRINT- International Network for Translating Research on Perinatal Derivatives into Therapeutic Approaches (S. Bollini COST Action Member).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2019.104324.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Balbi C., Lodder K., Costa A., Moimas S., Moccia F., van Herwaarden T., Rosti V., Campagnoli F., Palmeri A., De Biasio P., Santini F., Giacca M., Goumans M.J., Barile L., Smits A.M., Bollini S. Reactivating endogenous mechanisms of cardiac regeneration via paracrine boosting with the human amniotic fluid stem cell secretome. Int. J. Cardiol. 2019 Jul 15;287:87–95. doi: 10.1016/j.ijcard.2019.04.011. 10.1016/j.ijcard.2019.04.011 Epub 2019 Apr 4. [DOI] [PubMed] [Google Scholar]
- 2.Barile L., Lionetti V., Cervio E., Matteucci M., Gherghiceanu M., Popescu L.M., Torre T., Siclari F., Moccetti T., Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 3.van Vliet P., Roccio M., Smits A.M., van Oorschot A.A.M., Metz C.H.G., van Veen T.A.B., Sluijter J.P.G., Doevendans P.A., Goumans M.-J. Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth. Heart J. 2008;16:163–169. doi: 10.1007/BF03086138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moerkamp A.T., Lodder K., van Herwaarden T., Dronkers E., Dingenouts C.K.E., Tengström F.C., van Brakel T.J., Goumans M.-J., Smits A.M. Human fetal and adult epicardial-derived cells: a novel model to study their activation. Stem Cell Res. Ther. 2016;7:174. doi: 10.1186/s13287-016-0434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dronkers E., Moerkamp A.T., van Herwaarden T., Goumans M.-J., Smits A.M. The isolation and culture of primary epicardial cells derived from human adult and fetal heart specimens. J. Vis. Exp. 2018 doi: 10.3791/57370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazzarini E., Balbi C., Altieri P., Pfeffer U., Gambini E., Canepa M., Varesio L., Bosco M.C., Coviello D., Pompilio G., Brunelli C., Cancedda R., Ameri P., Bollini S. The human amniotic fluid stem cell secretome effectively counteracts doxorubicin-induced cardiotoxicity. Sci. Rep. 2016;6:29994. doi: 10.1038/srep29994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dragoni S., Reforgiato M., Zuccolo E., Poletto V., Lodola F., Ruffinatti F.A., Bonetti E., Guerra G., Barosi G., Rosti V., Moccia F. Dysregulation of VEGF-induced proangiogenic Ca2+ oscillations in primary myelofibrosis-derived endothelial colony-forming cells. Exp. Hematol. 2015;43:1019–1030. doi: 10.1016/j.exphem.2015.09.002. e3. [DOI] [PubMed] [Google Scholar]
- 8.Lodola F., Laforenza U., Cattaneo F., Ruffinatti F.A., Poletto V., Massa M., Tancredi R., Zuccolo E., Khdar D.A., Riccardi A., Biggiogera M., Rosti V., Guerra G., Moccia F. VEGF-induced intracellular Ca2+ oscillations are down-regulated and do not stimulate angiogenesis in breast cancer-derived endothelial colony forming cells. Oncotarget. 2017;8:95223–95246. doi: 10.18632/oncotarget.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eulalio A., Mano M., Dal Ferro M., Zentilin L., Sinagra G., Zacchigna S., Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.