Abstract
The potential of phenolic compounds of medicinal plants including Astragalus danicus L is determined by but not limited to their antioxidant activity. Their anti-inflammatory, antitumor, and other useful properties are known, which allows using these phytochemicals within preventive activities to reduce the risk of many serious diseases. Chromatographic analysis of the Astragalus danicus L. biomaterial from the plant samples collected in three regions of the Kemerovo region (Western Siberia, Russia) established the presence of compounds of flavonols (isorhamnetin glucoside, kaempferol glucoside), flavones (apigenin 7-glucoside), phenylpropanoids (chlorogenic acid) in the aerial part of plants. The total content of phenolic compounds in plant samples ranged from 100.75 ± 3.87 mg/g (Yashkinsky district) to 190.95 ± 7.34 mg/g (Belovsky district). The content of chlorogenic acid in the studied samples was from 0.14 ± 0.01 mg/g to 1.16 ± 0.04 mg/g. Isorhamnetin glucoside was found only in samples of plants from two districts - Prokopievsky (41.39 ± 1.58 mg/g) and Belovsky (95.0 ± 3.66 mg/g). The content of glucosides of kaempferol ranged from 0.38 ± 0.01 mg/g to 0.55 ± 0.02 mg/g. Its content is almost twice as high as the content in the well-known analogues of Astragalus. Apigenin-7-glucoside was isolated in Astragalus samples for the first time, in a small amount (3.34 ± 0.13 mg/g) in a sample of plants of one growing zone. Studies have confirmed that the content of flavonoids in plants significantly depends not only on the genetic characteristics of plants, but also on the hydrothermal regime, the climatic conditions of different botanical and geographical areas of the habitat. This work shows that Astragalus danicus L. growing in Kemerovo region is a promising raw material for pharmacological preparations.
Keywords: Biochemistry, Plant biology
1. Introduction
Secondary metabolites play an important role in the adaptation of plants to the environment, and also determine their bioactive potential. The bioactive characteristics of plants are formed by antioxidant, antimicrobial, anti-inflammatory, antiviral and other chemotherapeutic actions of their secondary metabolites [1].
The Astragalus L. genus (Astragalus) belongs to the Fabaceae family and is one of the most numerous in species diversity and polymorphism. It includes about 3,000 species, most of which are found in central and southwestern regions of Asia [2]. Numerous studies of plants of the Astragalus L. genus indicate a great interest in its representatives as sources of biologically active substances [3]. For various types of Astragalus, such compounds as flavonoids, saponins, alkaloids, polysaccharides, sterols, higher fatty acids, vitamins, essential oils, macro-and microelements are distinguished [4, 5, 6, 7]. Some species have antioxidant, antitumor, antibacterial, antiviral, tonic activities, others are used to treat nephritis, diabetes, cancer [8, 9, 10, 11, 12, 13]. The potential of plants is determined not only by species, but also by geographical and climatic conditions of growth. Plants of the same species collected in different regions and/or different years may significantly differ in biological activity. Moreover, plants grown under extreme conditions often demonstrate the unique qualities of their metabolites. Therefore, studies of phytochemical compounds in medicinal plants are still relevant and promising.
One of the interesting areas of research in the Astragalus L. genus is the screening of phenolic compounds, various groups of flavonoids and other compounds with antioxidant activity. The presence of flavonols (isorhamnetin, kaempferol), flavones (apigenin 7-glucoside), phenylpropanoids (chlorogenic acid) was identified in the biomaterial of plants of the Astragalus danicus species from different habitats.
Isorhamnetin is considered to be anticarcinogen. It is established that isorhamnetin inhibits mitosis and, accordingly, the activity of MAP and EPK kinases [14]. Formulations containing isorhamnetin from Persicaria hydropiper are promising for the treatment of cardiomyopathy, obesity [15, 16]. Isorhamnetin is contained in the fruit of sea buckthorn (Hippophae rhamnoides L.), and its effectiveness in the treatment of ischemic diseases and circulatory disorders is experimentally proved [17].
Kaempferol has a wide range of beneficial properties, including initiating apoptosis of ovarian cancer cells as a result of activation of the tumor suppressor (p53 protein) and apoptosis proteins [18]. Its antioxidant activity increases the body's resistance to oxidative stress. A significant decrease in the immune response of leukocytes with the use of kaempferol for the treatment of autoimmune diseases, atherosclerosis is found [19, 20]. A significant amount of kaempferol is found in apples, wormwood, cabbage, gooseberry, strawberries, mustard, horseradish, parsley [13].
Apigenin is also an anticarcinogen that inhibits the growth of breast, skin, prostate, and thyroid cancer cells [21, 22]. It may be used to maintain the health of people with asthmatic diseases [23].
Phenylpropanoids, to which chlorogenic acid belongs, possess antioxidant, immunomodulatory, choleretic, hepatoprotective, antibacterial and antiviral activity. It has been suggested that chlorogenic acid may have potential antitumor activity and be used in cancer therapy [24, 25, 26].
The aim of the work is to estimate the quantitative content of phenolic compounds (flavonoids) in the above-ground part of medicinal plants Astragalus danicus collected in different regions of the Kemerovo region (Western Siberia, Russia).
2. Materials and methods
2.1. Materials
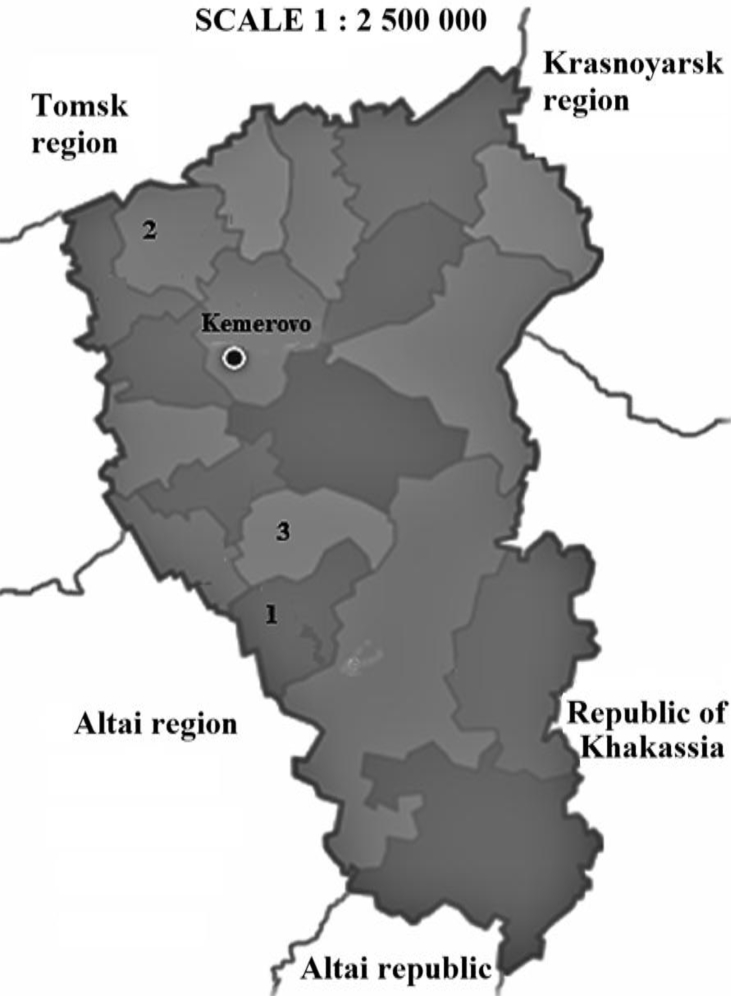
Samples of the aerial part of Astragalus danicus Retz were collected in three districts (collection zone 1 - Prokopievsky district, collection zone 2 - Yashkinsky district, collection zone 3 - Belovsky district) of the Kemerovo region (Western Siberia, Russia) July 6–7, 2018 (Fig. 1). In accordance with the botanical and geographical zoning, the Yashkinsky district (3.5K кm2) belongs to the Tomsk taiga-forest-steppe region, the Belovsky (3.2K кm2) and Prokopievsky districts (3.4K кm2) belong to the Insko-Tomsky taiga-forest-steppe region. The species biomaterial was confirmed by I. V. Tarasova, head of the herbarium of Kemerovo State University. Astragalus danicus of Western Siberia grows to 30 cm, well-adapted throughout the meadow, steppe communities, in sparse birch and pine forests. The flowering phase is observed in June–July, fruiting − in July–August. The collected biomass amounted to 2,000 g, 2,500 g, and 2,500 g, respectively, in the Yashkinsky, Belovsky and Prokopievsky districts. The shoot/leaves/flowers ratio of the collected biomass averaged 4:2:1 for each of the collection area.
Fig. 1.
Places of plant sample collection: 1 - Prokopyevsky district; 2 - Yashkinsky district; 3 - Belovsky district.
2.2. Preparation of plant extracts
To determine the flavonoids and total phenolic contents according to Gursoy et al. [27], the averaged sample of the aerial part of plants (shoot, leaves, flowers at a ratio of 2:2:1) was collected after drying the field samples to an air-dry state (500 g). The plant material was crushed and subjected to extraction with methyl carbinol (the ratio of plant material/extractant 1:10, temperature 10 °C) for 2 days under constant stirring using a 200×g shaker until complete extraction of biologically active substances [28, 29, 30, 31, 32, 33]. The resulting tincture was a clear liquid of dark greenish-brown color with a characteristic odor. Extracts were stored in the refrigerator (in the dark, at a temperature of 4–6 °C) until analysis. All chemicals (analytical or better grade) used in this study were purchased from Fluka/Sigma-Aldrich (Sigma-Aldrich Rus, Moscow, Russia) or were obtained from the Institute of Biotechnology of Kemerovo State University (Kemerovo, Russia).
2.3. Preparative and analytical HPLC
Preparative and analytical HPLC was performed on a Prominence liquid chromatograph (Shimadzu LC-20b Japan) with diode-array detection according to Sarikurkcu et al. [34]. The chromatographic column Kromasil C18 had a size of 250 × 4.6 mm (particle size of the sorbent 5 microns). A mixture of water with o-phosphoric acid (pH = 4.6) (A), acetonitrile (B) was used as the mobile phase. Gradient elution mode (% B) was 0–20 min with a gradient change of 10–20% and 20–60 min - 20–50%. The eluent flow rate was 1.0 ml/min, the column thermostat temperature was 35 °C. During the preparative accumulation, the acid was not added to the mobile phase. Detection was carried out at a wavelength of 365 nm. The outputs of certain compounds were calculated on the basis of pre-constructed calibration curves.
2.4. Thin-layer chromatography
Thin layer chromatography was performed on Sorbfil PTSH-AF-A plates followed by densitometry (densitometer with Sony (Handycam HDR-CX405) photo-recording system, LLC “IMID”, Russia). Chromatographic zones were cut out and further analyzed.
2.5. Spectrophotometric studies
Spectrophotometric studies were performed on a spectrophotometer SF-2000 (OKB "Spectr", St. Petersburg, Russia); IR spectroscopy was performed on a FSM-1202 instrument (InfraSpek, St. Petersburg, Russia). IR spectra were recorded in potassium bromide disks (Fluka, Germany). The results were processed using the FSpec software (version 4.0.0.2 for Windows®, LLC “Monitoring”, Russia). Spectra were recorded in the range of 4000–400 1/cm with a resolution of 4 1/cm.
2.6. Extraction and fractionation
The crushed vegetable raw materials (500 g) were extracted with 70% ethanol at a ratio of 1:10 in an ultrasonic bath (100 W, frequency 35 kHz) at 40 °C twice for 30 min over 2 h. Alcohol extraction was filtered through membrane filters (pore size 0.2 μm). The filtrate was concentrated to a water residue using a rotary evaporator. Next, the filtrate was subjected to liquid-phase extraction with hexane (fraction 1) and a mixture of ethyl acetate-ethanol in a ratio of 5:1 (fraction 2). Fraction 2 was chromatographed on a Sephadex LH-20 sorbent (10 × 350 mm column) on a Bio-Logic liquid chromatograph (Bio-Rad, United States), eluted with isopropanol at a gradient of from 20 to 90%.
2.7. Analytical standards
Isorhamnetin 3-glucopyranoside (Isorhamnetin-3-O-β-D glucopyranosyl-(1→2)-β-D glucopyranosyl-(1→6)-β-D-glucopyranoside, ≥90%, 00960590), kaempferol 3-glucoside (3,4′,5,7-Tetrahydroxyflavone 3-glucoside, 3-(β-D-Glucopyranosyloxy)-5,7-dihydroxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one, 3-Glucosylkaempferol, Astragalin, Kaempferol 3-β-D-glucopyranoside, Kaempferol 3-glucoside, ≥90%, 68437), apigenin 7-glucoside (4′,5,7-Trihydroxyflavone 7-glucoside, ≥97%, 44692), chlorogenic acid (1,4,5-Trihydroxycyclohexanecarboxylic acid 3-(3,4-dihydroxycinnamate), ≥95%, C3878) were purchased from Fluka/Sigma-Aldrich (Sigma-Aldrich Rus, Moscow, Russia).
For chromatographic determination of substances, a mixed initial solution was prepared which contained 1 mg/ml of each of isorhamnetin glucoside, kaempferol glucoside, apigenin 7-glucoside, chlorogenic acid in ethanol immediately before the experiment. To create a calibration curve, working standard solutions were prepared by successive dilution of the mixed initial solution with ethanol to final concentrations from 0.1 to 100 μg/ml. The solutions were chromatographed and eluted as described above. The content of each of isorhamnetin glucoside, kaempferol glucoside, apigenin 7-glucoside, chlorogenic acid in the extracts was calculated on the basis of the preset calibration curves between the areas of the peaks and concentrations of standard solutions.
2.8. Statistical analysis
All experiments were performed three times. Data processing was carried out by standard methods of mathematical statistics. Homogeneity of the sampling effects was checked using the Student's t-test. The data were subjected to analysis of variance (ANOVA) using Statistica 10.0 (StatSoft Inc., 2007, USA). Differences between means were considered significant when the confidence interval is smaller than 5% (p ≤ 0.05).
3. Results and discussion
In our study, the total content of phenolic substances in samples of Astragalus danicus was 181.84 ± 6.98 mg/g, 100.75 ± 3.87 mg/g and 190.95 ± 7.34 mg/g from 1-3 collection area, accordingly.
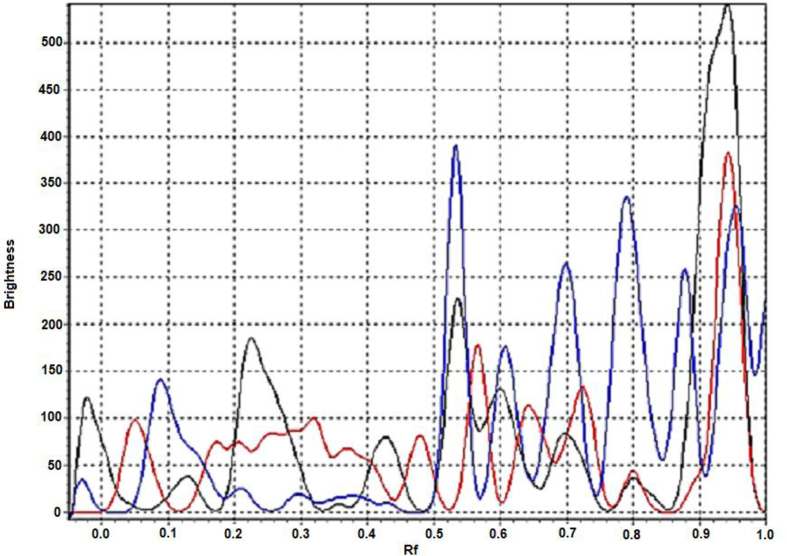
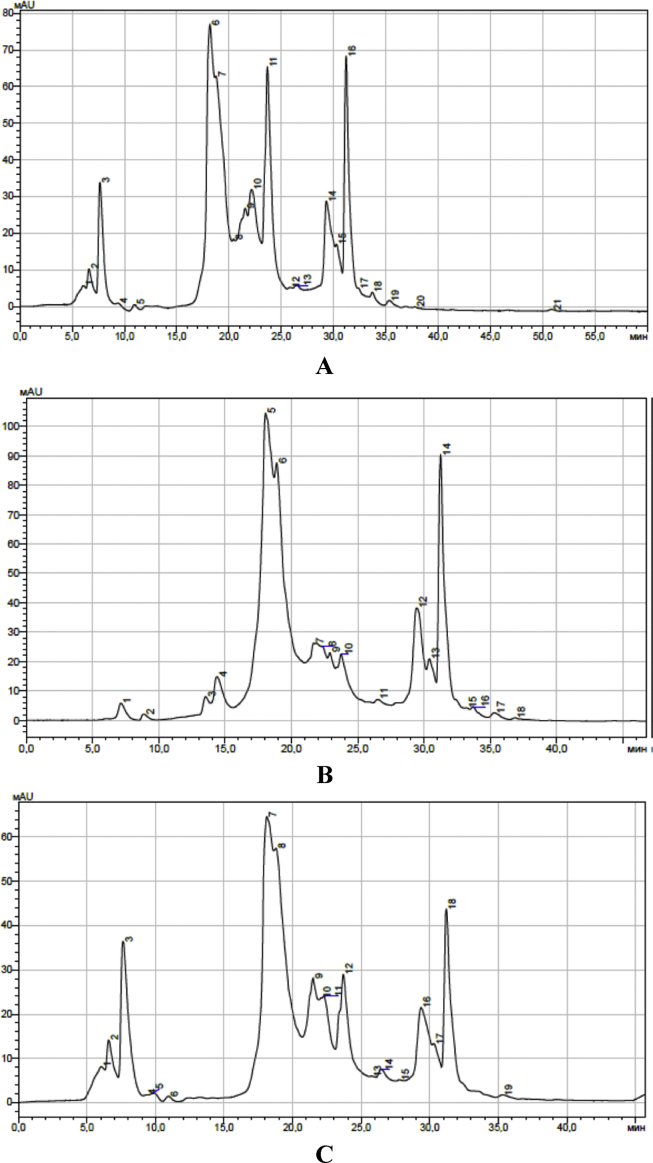
Isorhamnetin glucoside is characterized by the value Rf 0.7. It is found only in biological samples of area 1 (41.39 ± 1.58 mg/g) and area 3 (95.0 ± 3.66 mg/g) (Fig. 2). The content of the glucosides of isorhamnetin is clearly traced on HPLC chromatograms (Fig. 3). The content was 1.55 ± 0.06 mg/g in samples from area 1 (peak 6–7, Fig. 3A), 2.28 ± 0.08 mg/g in samples from area 2 (peak 5–6, Fig. 3B) and 0.92 ± 0.04 mg/g in plant material from area 3 (peak 7–8, Fig. 3C).
Fig. 2.
Densitogram of Astragalus danicus samples after developing by diazoreactive: black line – Prokopyevsky district, red line – Yashkinsky district, blue line – Belovsky district.
Fig. 3.
Chromatogram of Astragalus danicus alcohol extract sample: A collection area 1; B collection area 2; C collection area 3.
We have found a variation in the indices of the glucosides of kaempferol (Rf 0.57) from 0.38 ± 0.01 mg/g in samples from area 1 to 0.55 ± 0.02 mg/g from area 2. The intermediate value of the indicator identified in the sample from the collection area 3–0.41 ± 0.02 mg/g. The elution of kaempferol glucoside is shown in chromatograms (peak 16, Fig. 3A, peak 14, Fig. 3B and peak 18, Fig. 3C).
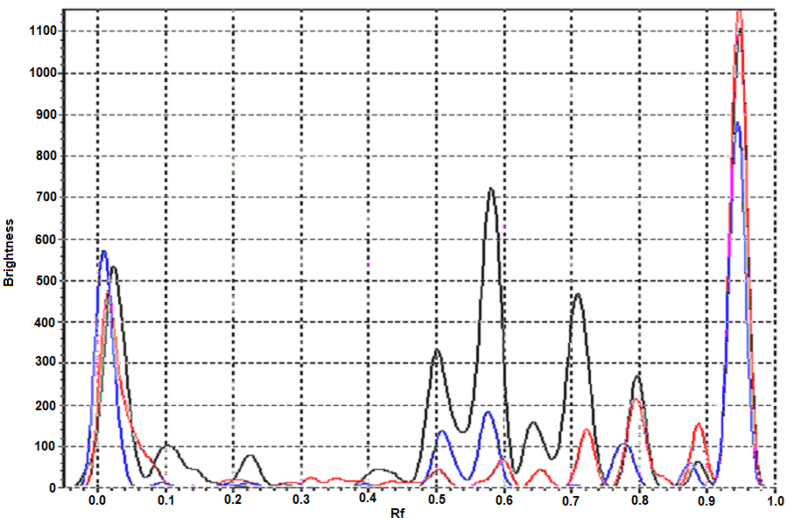
According to the content (Fig. 4) of chlorogenic acid (Rf 0.51), the investigated samples of Astragalus danicus differ significantly from 0.14 ± 0.01 mg/g (area 2) to 1.16 ± 0.04 mg/g (area 1). The sample from collection area 3 was 0.81 ± 0.03 mg/g.
Fig. 4.
Densitogram of Astragalus danicus L. samples (365 nm): black line – Prokopyevsky district, red line – Yashkinsky district, blue line – Belovsky district.
In plant biomass of Astragalus danicus, apigenin 7-glucoside was isolated in a small amount (3.34 ± 0.13 mg/g) only in the sample from the collection area 2.
Jaradat et al. [35] have established that among the Astragalus L. species growing in Palestine (Astragalus aleppicus, Astragalus angustifolius, Astragalus annularis and Astragalus boeticus) A. Boeticus is characterized by a high content of phenols, certain groups of flavonoids and tannins. These plants have a high antioxidant and antimicrobial activity against Staphylococcus aureus, Escherichia coli, American Pseudomonas, as well as against methicillin-resistant strains of Staphylococcus aureus and white candidiasis.
Astragalus complanatus, which is one of the main raw materials for Chinese medicine, has been well studied. Researchers continue to expand the list of polysaccharides isolated from it [36]. Typically, seeds and roots are used as the basis of biological samples.
Semmar et al [37] studied the Astragalus caprinus species, widespread in Tunisia. The main flavonoids in the samples were kaempferol glucoside (about 80% of the total flavonoids) or quercetin polyglucoside, depending on the region of the biomaterial collection. The authors confirmed the difference in the content of antioxidant components, depending on the geographical location of the collection of plants.
Our research confirms that the bioactive components in a plant depend on the conditions of the growing areas. The two studied areas (Belovsky and Prokopyevsky districts of the Kemerovo region) are geographically close, but not all the samples of plants have the same phenolic content. The climate of the Kemerovo region is extremely continental: winters are cold and long, while summers are short though warm. In January, the average air temperature is −17 … −20 °C, while in July, it goes up to +17 ... +18 °C. The air temperature reaches its highest of +38 °C in summer. The lowest winter temperatures are −54 °C in the southern part of the region and −57 °C in the northern part. The average annual precipitation is between 300 mm/year in the plains and foothills and 1,000 mm/year and more in mountainous regions. The frost-free period lasts from 100 days in the northern part of the region to 120 days in the South of the Kuznetsk Basin. For the collection of Astragalus danicus samples, the annual average temperature was +1.8 °C, +0.3 °C, +1.8 °C; the monthly average temperature in January was from −18°С to −22°С, from −22 °C to −24 °C, from – +18 °C to +22 °C; the monthly average temperature in July was from +17°С to +22°С, from +18 °C to +24 °C, from +18 °C to +22 °C; the precipitation amount was from 350 – 450 mm/year (in the foothills and mountainous regions – up to 600 mm/year), from 550 to 650 mm/year and 370–495 mm/year in the Prokopievsky, Yashkinsky, and Belovsky districts respectively. The air temperature goes up as high as +38 °C in summer and as low as −50 °C in winter. According to the temperature regime, area 1 and area 3 are similar, while area 2 is somewhat cooler. By the amount of precipitation, the division is similar. The average of 400 mm/year for area 1 and 3, and 600 mm/year for area 2. In 2018, the year of collection of plant samples, there were no sharp differences in weather conditions, except for those already noted. From April 20, the air temperature went above freezing during the day and at night. In early- and mid-May, there were several days with ground frosts at night. June, when flowering began, was hot and dry in all the areas, with an average temperature of +25.2–25.3 °C and +22.7 °C during the day and +19.1–19.3 °C and +17.9 °C at night, for areas 1, 3, and 2 respectively.
The total content of phenolic compounds and isorhamnetin glucoside, in particular, in these two areas are approximately the same (p > 0.05). Minor differences in the content of kaempferol glucoside depending on the growing area, approximately 1.4 times compared to the content in the samples of plants of the Yashkinsky district. The detection of apigenin 7-glucoside in only one area and isorhamnetin glucoside in the other two confirms the territorial uniqueness of these zones, including for the growing conditions of plants.
Apparently, it is more severe climatic conditions of area 2 that provided the formation of apigenin 7-glucoside in the Astragalus danicus samples, while there are no reports of apigenin 7-glucoside in the samples from other territories that do not have significant frosts (Turkey, Tunisia, Palestine, Lithuania, etc.) [35, 36, 37, 38, 39]. More precipitation in area 2 located in the North of the Kemerovo region, contributed to lesser accumulation of kaempferol glucoside in the Astragalus danicus samples.
Arumugam et al. [38] studied the content of antioxidant compounds and their activity of Astragalus ponticus, examining in detail the samples of extracts of various plant parts. The percentage yield of various parts of plant extracts ranged from 7.87 to 17.73%. The content of total phenolic compounds and flavonoids ranges from 13.35 ± 0.63 to 26.34 ± 0.50 mg of the extract GAEs/g (gallic acid equivalent per g of fluid extract) and 9.94 ± 0.13 to 49.13 ± 0.51 mg of the extract REs/g, respectively. The highest concentration was from the leaves, and the second highest - from the aerial parts of plants. Comparing to the data obtained in the work [39] with phenol content in leaves and flowers of Astragalus glycyphyllos 25.99 and 23.71 mg GAEs/g extract and flavonoids 21.00 and 16.71 mg REs/g extract, the authors claim that their plant samples are not inferior in content, but have accumulated a large antioxidant activity.
Our study confirms that samples of Astragalus danicus growing in the Kemerovo Region are not inferior to those of plants of other Astragalus species and other places of growth in the total phenol content (25.23 ± 0.61 mg GAEs/g extract, 13.98 ± 0.54 mg GAEs/g extract and 26.50 ± 0.62 mg GAEs/g extract from 1–3 collection area).
Phytochemical analysis [38] of extracts of samples of Astragalus ponticus determined the presence of syringic acid with the highest concentration in flowers, p-coumaric acid in foliage, ferulic acid in flowers and aerial part of the plant, benzoic acid in foliage, o-coumaric acid in foliage, hesperidin in foliage, luteolin in foliage. The main flavonoid in biosamples is luteolin, its content exceeded almost 4.5 times exceeded the known highest content. Flavonoids (isorhamnetin glucoside, kaempferol glucoside, apigenin 7-glucoside and chlorogenic acid) of our samples of Astragalus danicus plants in samples [39] were not identified, but previously determined concentrations of these compounds in various samples of Astragalus are given: kaempferol - 0.05–15.0 mg/l, apigenin - 0.17–11.0 mg/l, chlorogenic acid - 0.35–45.0 mg/l. According to the results of our research, Astragalus samples collected in the Kemerovo Region had the highest content of kaempferol glucoside 11.00 ± 1.60 mg/l, chlorogenic acid 23.0 ± 1.65 mg/l, isorhamnetin glucoside 27.76 ± 1.19 mg/l.
4. Conclusions
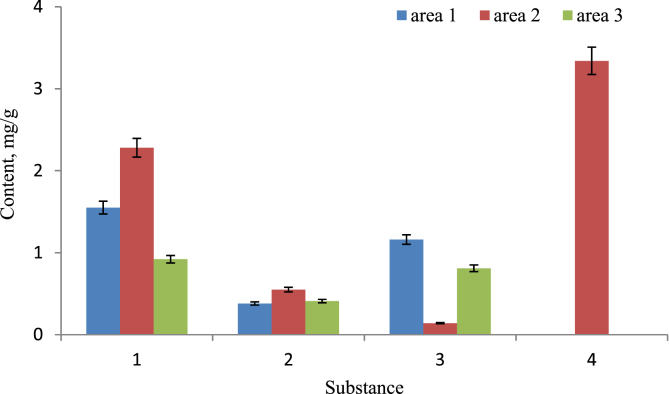
As a result of chromatographic analysis of Astragalus danicus L. biomaterial, representing the population of a species from three districts of the Kemerovo region, it was established that the plants contain flavonoids in the form of flavonol compounds (isorhamnetin glucoside, kaempferol glucoside), flavones (apigenin 7-glucoside), phenylpropanoids (chlorogenic acid) (Fig. 5). In the Astragalus samples of the Kemerovo region, kaempferol glucoside and chlorogenic acid are found in average concentrations in comparison with samples of the species growing in other territories. The content of isorhamnetin glucoside is almost twice as high as the known analogues. Apigenin 7-glucoside was isolated for the first time in Astragalus samples. The obtained data confirm that the content of flavonoids significantly depends on the habitat of the species and is associated with the macro- and microelement composition of the soil and the hydrothermal regime of different botanical-geographical zones. And the samples of Astragalus danicus L. plants collected in the Kemerovo Region act as a unique pharmaceutical raw material for the production of biologically active substances of prophylactic and therapeutic drugs.
Fig. 5.
The content of phenolic compounds of Astragalus danicus L. samples from Prokopyevsky district (area 1), Yashkinsky district (area 2), Belovsky district (area 3): 1 - isorhamnetin glucoside; 2 - kaempferol glucoside; 3 - apigenin 7-glucoside; 4 - chlorogenic acid.
Declarations
Author contribution statement
Olga Babich, Alexander Prosekov, Lyubov Dyshlyuk: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Alexandra Zaushintsena, Andrey Sukhikh: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Svetlana Ivanova: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Russian Science Foundation [project number 18-75-10066].
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Shariff N., Sudarshana M., Umesha S., Hariprasad P. Antimicrobial activity of Rauvolfia tetraphylla and Physalis minima leaf and callus extracts. Afr. J. Biotechnol. 2006;5–10:946–950. [Google Scholar]
- 2.Myakshina T.A. Asian Russia: Composition and Chorology. Abstract of the dissertation of the candidate of biological sciences; Novosibirsk: 2012. Section Xiphidium Bunge of the genus Astragalus L. [Google Scholar]
- 3.Sergalieva M.U., Mazhitova M.V., Samotrueva M.A. Plants of the genus astragalus: prospects for use in pharmacy. Astrakhan Med. J. 2015;2:17–31. [Google Scholar]
- 4.Kozak M.F., Skvortsova I.A. Prospects for the use of Astragalus of the Astrakhan region as a source of medicinal raw materials. Elect. Scient. Educ. Bull. “Health Educ. XXI Century”. 2012;14–8:181–182. [Google Scholar]
- 5.Lobanova I.E. The dynamics of the content of ascorbic acid in the organs of sweet-leaved astragalus and the rank of spring. Siberian J. Agric. Sci. 2010;4:19–23. [Google Scholar]
- 6.Turtueva T.A., Nikolaev G.G., Gulyaev S.M., Zhalsanov Yu.V. The amino acid composition of the roots of Astragalus membranaceus (Fish.) Bunge. Bull. Buryat State Univ. 2013;12:75–77. [Google Scholar]
- 7.Pan H., Fang C., Zhou T., Wang Q., Chen J. Accumulation of calycosin and its 7-Obeta-D-glucoside and related gene expression in seedlings of Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao induced by low temperature stress. Plant Cell Rep. 2007;26:1111–1120. doi: 10.1007/s00299-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 8.Park Y.J., Thwe A.A., Li X., Kim Y.J., Kim J.K., Arasu M.V., Al-Dhabi N.A., Park S.U. Triterpene and flavonoid biosynthesis and metabolic profiling of hairy roots, adventitious roots, and seedling roots of Astragalus membranaceus. J. Agric. Food Chem. 2015;63:8862–8869. doi: 10.1021/acs.jafc.5b02525. [DOI] [PubMed] [Google Scholar]
- 9.Boual Z., Pierre G., Delattre C., Benaoun F., Petit E., Gardarin C., Michaud P., Ould El Hadj M.D. Mediterranean semi-arid plant Astragalus armatusas a source of bioactive galactomannan. Bioact. Carbohydr. Diet. Fibre. 2015;5–1:10–18. [Google Scholar]
- 10.Li S.G., Chen Y., Zhang Y.Q. Effects of Astragalus polysaccharide on nephritis induced by cationic bovine serum albumin in rats. Zhong Yao Cai. 2010;33–12:1913–1916. [PubMed] [Google Scholar]
- 11.Auyeung K.K., Woo P.K., Law P.C., Ko J.K. Astragalus saponins modulate cell invasiveness and angiogenesis in human gastric adenocarcinoma cells. J. Ethnopharmacol. 2012;141–2:635–641. doi: 10.1016/j.jep.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Li W., Zheng H., Bukuru J., De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004;92–1:1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Calderon-Montano J.M., Burgos-Moron E., Perez-Guerrero C., Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.E., Lee D.E., Lee K.W., Son J.E., Seo S.K., Li J., Jung S.K., Heo Y.S., Mottamal M., Bode A.M., Dong Z., Lee H.J. Isorhamnetin suppresses skin cancer through direct inhibition of MEK1 and PI3-K. Cancer Prev. Res. 2011;4:582–591. doi: 10.1158/1940-6207.CAPR-11-0032. [DOI] [PubMed] [Google Scholar]
- 15.Boubaker J., Bhouri W., Ben Sghaier M., Ghedira K., Dijoux Franca M.G., Chekir-Ghedira L. Ethyl acetate extract and its major constituent, isorhamnetin 3-O-rutinoside, from Nitraria retusa leaves, promote apoptosis of human myelogenous erythroleukaemia cells. Cell Prolif. 2011;44:453–461. doi: 10.1111/j.1365-2184.2011.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S.H., Kim B., Oh M.J., Yoon J., Kim H.Y., Lee K.J., Lee J.D., Choi K.Y. Persicaria hydropiper (L.) spach and its flavonoid components, isoquercitrin and isorhamnetin, activate the Wnt/beta-catenin pathway and inhibit adipocyte differentiation of 3T3-L1 cells. Phytother Res. 2011;25:1629–1635. doi: 10.1002/ptr.3469. [DOI] [PubMed] [Google Scholar]
- 17.Suryakumar G., Gupta A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.) J. Ethnopharmacol. 2011;138–2:268–278. doi: 10.1016/j.jep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Kim T.H., Ku S.K., Bae J.S. Inhibitory effects of kaempferol-3-O-sophoroside on HMGB1-mediated proinflammatory responses. Food Chem. Toxicol. 2012;50:118–1123. doi: 10.1016/j.fct.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Luo H., Rankin G.O., Li Z., Depriest L., Chen Y.C. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011;128:513–519. doi: 10.1016/j.foodchem.2011.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nirmala P., Ramanathan M. Effect of kaempferol on lipid peroxi-dation and antioxidant status in 1,2-dimethyl hydrazine induced colorectal carcinoma in rats. Eur. J. Pharmacol. 2011;654:75–79. doi: 10.1016/j.ejphar.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Yin F., Giuliano A.E., Law Van R.E., Herle A. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001;21:413–420. [PubMed] [Google Scholar]
- 22.Shukla S., Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm. Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel D., Shukla S., Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (Review) Int. J. Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 24.Huang Y., Chen H., Zhou X., Wu X., Hu E., Jiang Z. Inhibition effects of chlorogenic acid on benign prostatic hyperplasia in mice. Eur. J. Pharmacol. 2017;809:191–195. doi: 10.1016/j.ejphar.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Shao P., Zhang J.F., Chen X.X., Sun P.L. Microwave-assisted extraction and purification of chlorogenic acid from by-products of Eucommia Ulmoides Oliver and its potential anti-tumor activity. J. Food Sci. Technol. 2015;52:4925–4934. doi: 10.1007/s13197-014-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sortino R.M., Romanelli S.M., Knoll G.A., Banerjee I.A. Development of peptide conjugated chlorogenic acid nanoassemblies for targeting tumorigenic cells. Soft Mater. 2015;13:150–159. [Google Scholar]
- 27.Gursoy N., Sarikurkcu C., Tepe B., Solak M.H. Evaluation of antioxidant activities of 3 edible mushrooms: Ramaria flava (Schaef.: Fr.) Quel., Rhizopogon roseolus (Corda) TM Fries., and Russula delica Fr. Food Sci. Biotechnol. 2010;19:691–696. [Google Scholar]
- 28.Bourezzane S., Haba H., Long C., Benkhaled M. Chemical composition and antioxidant activity of Astragalus monspessulanus L. growing in semiarid areas of Algeria. J. Serb. Chem. Soc. 2018;83–1:31–38. [Google Scholar]
- 29.Qi L., Liu C.-Y., Wu W.-Q., Gu Z.-L., Guo C.-Y. Protective effect of flavonoids from Astragalus complanatus on radiation induced damages in mice. Fitoterapia. 2011;82:383–392. doi: 10.1016/j.fitote.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Ma C., Tao G., Tang J., Lou Z., Wang H., Gu X., Hu L., Yin M. Preparative separation and purification of rosavin in Rhodiola rosea by macroporous adsorption resins. Separ. Purif. Technol. 2009;69:22–28. [Google Scholar]
- 31.Ji S., Li R., Wang Q., Miao W.-J., Li Z.-W., Si L.-L., Qiao X., Yu S.-W., Zhou D.-M., Ye M. Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J. Ethnopharmacol. 2015;176:475–484. doi: 10.1016/j.jep.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Yu K., Gong Y., Lin Z., Cheng Y. Quantitative analysis and chromatographic fingerprinting for the quality evaluation of Scutellaria baicalensis Georgi using capillary electrophoresis. J. Pharm. Biomed. Anal. 2007;43:540–548. doi: 10.1016/j.jpba.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiedenfeld H., Dumaa M., Malinowski M., Furmanowa M., Narantuya S. Phytochemical and analytical studies of extracts from Rhodiola rosea and Rhodiola quadrifida. Die Pharmazie. 2007;62:308–311. [PubMed] [Google Scholar]
- 34.Sarikurkcu C., Kirkan B., Ozer M.S., Ceylan O., Atilgan N., Cengiz M., Tepe B. Chemical characterization and biological activity of Onosma gigantean extracts. Ind. Crops Prod. 2018;115:323–329. [Google Scholar]
- 35.Jaradat N.A., Zaida A.N., Abuzant A., Khalaf S., Abu-Hassan N. Phytochemical and biological properties of four Astragalus species commonly used in traditional Palestinian medicine. Eur. J. Integr. Med. 2017;9:1–8. [Google Scholar]
- 36.Xue B., Li J., Chai Q., Liu Z., Chen L. Effect of total flavonoid fraction of Astragalus complanatus R. Brown on angiotensin II-induced portal-vein contraction in hypertensive rats. Phytomedicine. 2008;15–9:759–762. doi: 10.1016/j.phymed.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Semmar N., Jay M., Chemli R. Chemical diversification trends in Astragalus caprinus (Leguminosae), based on the flavonoid pathway. Biochem. Syst. Ecol. 2001;29–7:727–738. doi: 10.1016/s0305-1978(00)00105-8. [DOI] [PubMed] [Google Scholar]
- 38.Arumugam R., Kirkan B., Sarikurkcu C. Phenolic profile, antioxidant and enzyme inhibitory potential of methanolic extracts from different parts of Astragalus ponticus Pall. South Afr. J. Bot. 2019;120:268–273. [Google Scholar]
- 39.Butkute B., Dagilyte A., Benetis R., Padarauskas A., Ceseviciene J., Olsauskaite V., Lemeziene N. Mineral and phytochemical profiles and antioxidant activity of herbal material from two temperate Astragalus species. BioMed Res. Int. 2018;11 doi: 10.1155/2018/6318630. [DOI] [PMC free article] [PubMed] [Google Scholar]