Summary
Objective
Obesity is a major public health issue with significant impact on quality of life, morbidity and mortality rates. It is estimated that if the current trends continue, 18% of men and 21% of women worldwide will be obese by 2025. All the current therapies are not optimal due to limited efficacy or safety; thus, there is a need for additional devices for the treatment of obesity. This study aimed to examine the safety, tolerability, and efficacy of a biodegradable encapsulated Epitomee device for weight loss. The technology is based on absorbent pharmaceuticals polymers and bonding materials that self‐expand in the stomach to create a pH‐sensitive super absorbent gel structure for weight loss.
Methods
A prospective, 12‐week twice daily use of the encapsulated device in patients with body mass index of 27–40 kg m−2. Efficacy endpoints were the percent total body weight loss (%TBWL), proportion of participants with 5% TBWL and changes in cardio‐metabolic markers. Safety analysis included evaluation of adverse events, laboratory and endoscopic findings.
Results
Overall, 52 patients completed the study. TBWL per intension‐to‐treat analysis was 3.68 ± 3.07% (3.23 ± 2.69 kg) and 4.52 ± 2.97% (3.95 ± 2.57 kg) per protocol. No device serious adverse effects reported. The most common adverse events were headache (18.1%), viral infection (11.5%), abdominal discomfort (10.1%), bloating (7.9%), nausea and constipation (5% each) and flatulence (4.3%). Endoscopy in 26 patients revealed mild, asymptomatic gastric/duodenal erythema without erosions in five patients.
Conclusions
Twelve weeks of Epitomee capsules treatment combined with lifestyle counselling resulted in 3.68–4.52% of TBWL. With continued research, the Epitomee capsules have considerable potential to become a non‐invasive, safe and effective treatment option for weight loss.
Keywords: Obesity, weight loss, intragastric balloon
Introduction
Diet, physical activity and behavioural modification are the cornerstones of weight management. However, weight loss achieved by lifestyle modifications alone is often limited and difficult to maintain 1, 2, 3, 4, 5. Bariatric surgery demonstrated greater weight loss and health benefits in individuals with body mass index (BMI) ≥40 kg m−2 or with BMI ≥35 kg m−2 and comorbidities 6. In a meta‐analysis of 11 studies with 796 individuals, Gloy et al. reported that compared with non‐surgical treatment of obesity, bariatric surgery leads to greater body weight loss and higher remission rates of T2DM and metabolic syndrome 7.
Bariatric surgery is underutilized because of costs and insurance coverage issues and also because of the related morbidity and mortality. Consequently, there are increasing attempts to develop minimally invasive endoscopic procedures such as endoscopic sleeve gastroplasty, aspiration therapy or intragastric balloons that may bridge the gap between medical therapy and surgery 8. Several efficient intragastric balloons with lower complications rate were introduced 9, 10. More recently, Food and Drug Administration approved several endoscopic weight loss devices including the Reshape, the Orbera, the Obalon and the AspireAssist 11. Nevertheless, despite improvements in the design of the intragastric balloons, their implantation and/or removal requires endoscopic procedure under sedation, and their safety and efficacy remain questionable. Reported adverse events (AEs) included small bowel obstruction secondary to early balloon deflation, ulceration with gastrointestinal (GI) haemorrhage, gastric perforation and death 12.
The concept of expanding non‐caloric material in the stomach to produce satiety signals is a new area under investigation not yet well recognized among clinicians. Gelesis are non‐systemic orally administered capsules containing small hydrogel particles. The product's manufacturers state that by cross‐linking two components together, Gelesis increases satiety and reduces hunger 13, 14, 15.
Epitomee Medical (Caesarea, Israel) has developed a novel, orally self‐administered device (hereafter referred to as the Epitomee device [ED]) for induction of weight loss in patients with overweight and obesity. The ED is shown in Figure 1. The ED components include absorbent pharmaceuticals polymers and bonding materials that self‐expand in the stomach to create a pH‐sensitive super absorbent gel structure. Preliminary studies using gastric scintigraphy method 16 have shown a 40% reduction during the linear phase, in the gastric emptying rate following 10 d of ED treatment in healthy patients who are normal or overweight (unpublished data).
Figure 1.
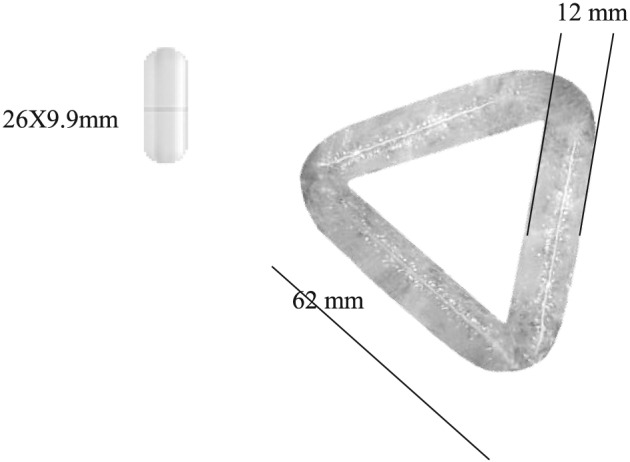
Epitomee device self‐expanding in the stomach after ingestion. AE, adverse event.
The weight reduction mechanism of ED is thought to be similar to those of other water‐soluble, fermentable fibres. We hypothesized that the rigidity of the device scaffold while mixed with ingested food is sensed by the stomach wall as if it is a large solid food mass sending satiety signals to the brain thus mimicking the stomach's ‘solid food emptying pattern’. The encapsulated device may promote satiety via activation of the gut–brain axis thereby leading to weight loss. The ED mechanism of action is purely mechanical and does not involve any chemical activity. The device has no calories, and it disintegrates to small particles within 30 min in the intestine. The particles are then secreted naturally through the GI tract. Like Gelesis, Epitomee capsule will be classified as a medical device by the Food and Drug Administration.
In the feasibility unpublished clinical trial, the safety of the ED was tested over a period of 4 weeks. Sixteen patients who are overweight/obese consumed one capsule once daily with a total of more than 600 encapsulated ED consumed cumulatively, resulting in minimal side effects of discomfort, nausea and heartburn (unpublished data). Based on the initial experience, the hypothesis was that the device will be tolerated well and safely over a 12‐week treatment period in patients with overweight and obesity, and a daily dose of two capsules will effectively result in patients' weight loss.
Methods
Study design and participants
A prospective, single‐centre, single arm, 12‐week phase II study was conducted at Assaf Harofeh Medical Center from June 2014 through August 2017. All participants provided written informed consent, and the protocol was approved by the local institutional review board of Assaf Harofeh Medical Center. Subjects were screened by means of medical history, physical examination and laboratory tests, which included complete blood count, chemistry profile, Thyroid Stimulating Hormone (TSH), insulin, lipid profile and HbA1C. All participants underwent an initial evaluation including consultations with a nutritionist and a gastroenterologist.
Participants were 25–61 years old with a BMI 27–40 kg m−2 with or without controlled hypertension and/or dyslipidaemia. Exclusion criteria included any cardiac, renal, hepatic or vascular disease, uncontrolled diabetes, inflammatory bowel disease, a history of GI surgery, weight change of 5% within 6 months prior to study onset and any history of seizures or serious psychiatric illness.
The first 22 patients enrolled underwent upper endoscopy, with routine use of conscious sedation at baseline before the first capsule intake and at the end of the study after the last capsule intake, in order to evaluate the gastric mucosa for any acute changes. The last four patients enrolled were treated with a slightly larger device prototype that had 30% more weight when fully expanded in the stomach and therefore were also examined by endoscopy.
Procedures and endpoints
Following screening, participants received ED capsule to be administered twice daily for 12 weeks. Each capsule should be swallowed with two cups of water about 30 min prior to lunch or dinner. Study visits occurred at baseline and every 2 weeks. Participants were asked to follow a hypocaloric diet (500 kcal d−1 deficit) and were advised to increase their physical activity. The diet was prescribed by the study dietician during the first visit of the patient to the clinic and was designed individually to fit the patient's daily activities. The lifestyle intervention was of light intensity and was developed in accordance with AHA/ACC/TOS 2013 Guidelines for the Management of Overweight and Obesity in Adults. The lifestyle counselling included phone calls from a dietician every 2 weeks to discuss with the patient adherence to the diet and study protocol and to ensure that the patient is being engaged in aerobic physical activity of at least 150 min per week. Weight and vital signs were measured at each visit. Blood tests were performed at baseline, after 6 weeks of treatment and at the end of the study. Study endpoints included safety evaluation of any AEs throughout the study period and the presence of acute mucosal lesions as well as the %TBWL at 12‐week post‐treatment. Additional secondary endpoints included the proportion of patients with at least 5% TBWL and changes in markers of cardio‐metabolic risk factors.
Statistical analyses
Statistical analysis was performed to determine the device's clinical performance based on treatment efficacy and safety for various outcome measures reported during the study. The data were analysed by using basic descriptive statistics and comparison tests, where applicable. Measured variables are listed individually and, if appropriate, tabulated by descriptive statistics. To reduce bias, the primary endpoint analysis was performed using the intention‐to‐treat (ITT) population. Two patients who dropped out within their first week (the patients regret participation in the study) and did not show for the first follow‐up visit (Figure 2) were not included in the ITT analysis (modified ITT [mITT]). For the mITT analysis, the last observation carried forward (LOCF) methodology was used. Additional supportive analyses included the following: analysis of the ‘per protocol’ population and longitudinal or repeated measures analysis to assess impact of ‘time post‐treatment’ upon results. Subgroup analysis was also performed on the last four patients enrolled who were treated with a slightly larger device prototype. Patient and treatment‐related factors were used for covariate analyses to investigate their impact on safety and efficacy. Such factors included the following: age, gender, baseline weight, BMI and waist circumference, blood pressure, blood lipid profile, fasting glucose levels and HbA1C. The relation between baseline characteristics and primary efficacy measurement (weight reduction and %TBWL at 12 weeks follow‐up visit) were analysed using Pearson correlation coefficient. Additionally, the %TBWL of men versus women was compared using non‐parametric test.
Figure 2.
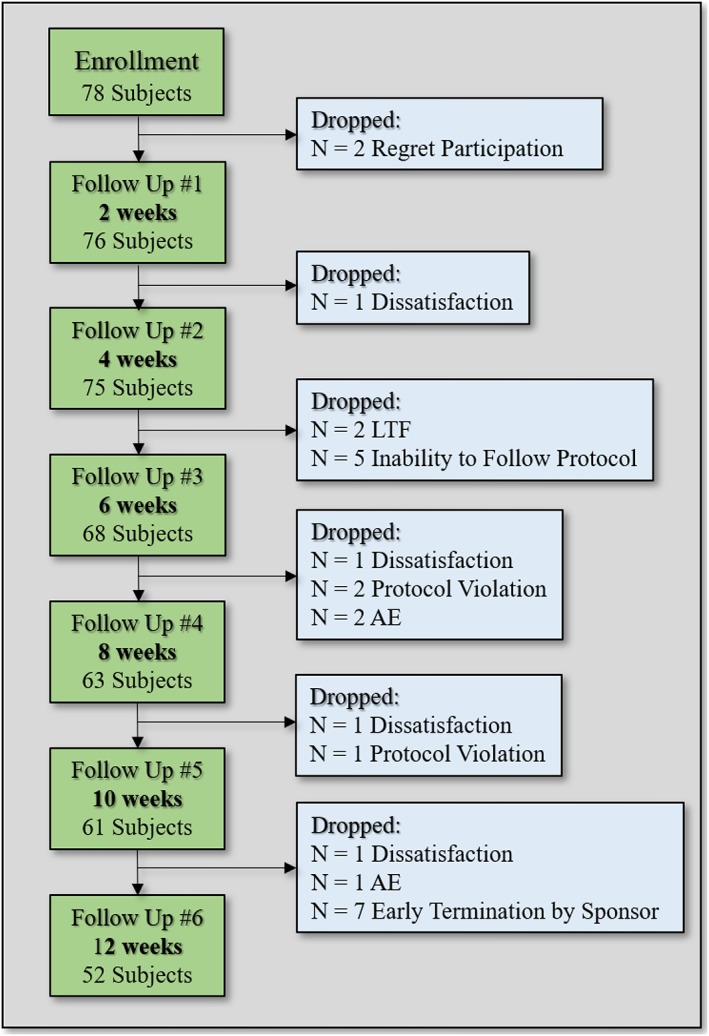
Patients' accountability.
Treatment‐related complications were monitored and reported; if applicable, their incidence rate was calculated and analysed by type, severity, action taken and outcome. Descriptive statistics, 95% confidence interval and significance tests (parametric [t‐test] and non‐parametric tests [Wilcoxon signed rank test for paired data]) were applied to compare the mean values of patients' weight, BMI, waist circumference, change in weight and %TBWL on an ITT basis, using 0.05 for the two‐sided type 1 error assumption.
Results
Seventy‐eight healthy overweight and obese patients (27 < BMI < 40), 58 women (74%) and 20 men (26%), were enrolled. Patients' demographics are presented in Table 1. Although the study protocol was intended for 12 weeks of treatment, the treatment protocol was terminated at week 10 for seven patients due to the holiday season. Two patients regret their participation and dropped in their first week into the trial.
Table 1.
Patients' demographics
| Efficacy measure | Mean ± SD | Range |
|---|---|---|
| Age (years) | 41 ± 7 | 25–51 |
| Gender (male/female) | 20/58 | 26/74 |
| Total body weight (kg) | 89.03 ± 11.44 | 71.3–121.3 |
| Body mass index (units) | 32.5 ± 2.7 | 27.7–39.9 |
| Waist circumference (cm) | 105.7 ± 7.5 | 92.5–122.7 |
| Systolic blood pressure | 124 ± 13 | 101–161 |
| Diastolic blood pressure (mmHg) | 77 ± 9 | 59–100 |
| Fasting Triglycerides (TG) (mg dL−1) | 125 ± 64 | 26–364 |
| Fasting cholesterol (mg dL−1) | 189 ± 33 | 112–258 |
| Fasting glucose (mg dL−1) | 91 ± 9 | 67–111 |
| Haemoglobin A1C (%) | 5.3 ± 0.3 | 4.7–6.2 |
Overall, 52 patients (67%) completed the full 12 weeks of twice daily use of ED (per protocol analysis), and 24 patients (31.5%) withdrew prematurely. Patients who dropped out consumed ED capsules up to their time point of exit from the study and are included in the mITT analysis.
Efficacy
The trajectory of weight loss is displayed in Figure 3. The mean (±SD) TBWL of the mITT population by LOCF analysis revealed a mean TBWL loss of 3.23 ± 2.69 kg and a mean %TBWL of 3.68 ± 3.07% (mITT; P < 0.001; Table 2). The 52 patients who completed the full 12 weeks of treatment demonstrated a mean ± SD TBWL of 3.95 ± 2.57 kg (P < 0.001), equivalent to 4.52 ± 2.97% (P < 0.001) (per protocol analysis; Table 3). Moreover, 31% of the study population (mITT) and 42% of study completers achieved significant weight loss of more than 5% TBWL within 12 weeks of treatment. TBWL at week 12 was accompanied with improvement of various cardio‐metabolic parameters, including reductions in patients' BMI and waist circumference (Tables 3 and 4). Subgroup analysis was performed on the last four patients who were treated with a slightly larger device prototype. The mean (±SD) %TBWL of the mITT population (LOCF analysis) excluding the last four patients (n = 72) was 3.8 ± 3.1% as opposed to a mean (±SD) %TBWL of 1.4 ± 2.6% that was demonstrated by the last four patients (LOCF analysis). Three out of these four patients completed the full 12 weeks of treatment with mean ± SD %TBWL of 1.0 ± 3.0% as opposed to the 49 completers patients from the mITT that achieved a mean ± SD %TBWL of 4.7 ± 2.9%. Due to the small number of patients treated with the larger device, prototype statistical testing for significant differences between the two groups could not be performed.
Figure 3.
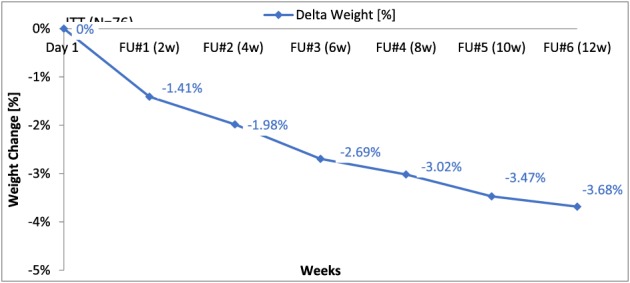
Total body weight loss (kg/%) along the study course (intention‐to‐treat analysis).
Table 2.
Clinical study results for efficacy measurements (intention‐to‐treat analysis)
| Efficacy measure | Pre‐treatment (mean ± SD) | Post‐treatment (mean ± SD) | Delta | P value |
|---|---|---|---|---|
| Total body weight (kg) | 89.4 ± 11.5 | 86.18 ± 11.9 | −3.23 ± 2.69 | <0.001 |
| Total body loss (%) | NA | NA | 3.68 ± 3.07 | <0.001 |
| Body mass index (units) | 32.65 ± 2.7 | 31.45 ± 2.93 | −1.19 ± 0.99 | <0.001 |
| Waist circumference (cm) | 1,054 ± 7.5 | 101.7 ± 7.6 | −3.7 ± 3.7 | <0.001 |
NA, not applicable.
Table 3.
Clinical study results for efficacy measurements (per protocol analysis)
| Efficacy measure | Pre‐treatment (mean ± SD) | Post‐treatment (mean ± SD) | Delta | P value |
|---|---|---|---|---|
| Total body weight (kg) | 89.2 ± 12.2 | 85.3 ± 12.6 | −3.95 ± 2.57 | <0.001 |
| Total body loss (%) | NA | NA | 4.52 ± 2.97 | <0.001 |
| Body mass index (units) | 32.61 ± 2.85 | 31.15 ± 2.98 | −1.46 ± 0.96 | <0.001 |
| Waist circumference (cm) | 106.0 ± 7.7 | 101.59 ± 7.92 | −4.41 ± 3.34 | <0.001 |
NA, not applicable.
Table 4.
Distribution of the adverse effects
| Event | n (%) |
|---|---|
| Any AE* | 138 |
| Mild | 131 (95.7) |
| Moderate | 5 (3.6) |
| Severe | 1 (0.7) |
| Withdrawal due to AE | 3 (2.1) |
| System organ class AEs | |
| Sudden hearing loss | 1 (0.7) |
| Gastrointestinal | 59 (42.8) |
| Abdominal pain/discomfort | 14 (10.1) |
| Bloating | 11 (7.9) |
| Constipation | 7 (5) |
| Nausea | 7 (5) |
| Flatulence | 6 (4.3) |
| Heartburn | 5 (3.6) |
| Diarrhoea | 2 (1.4) |
| Hiccups | 2 (1.4) |
| Eructation | 2 (1.4) |
| Blood in stool | 2 (1.4) |
| Vomiting | 1 (0.7) |
| Infections | 24 (17.4) |
| Viral infection | 16 (11.5) |
| Sore throat | 6 (4.3) |
| Pharyngitis | 1 (0.7) |
| Allergy | 1 (0.7) |
| Nervous system | 32 (23.2) |
| Headache | 25 (18.1) |
| Migraine | 3 (2.1) |
| Dizziness | 2 (1.4) |
| Fatigue | 2 (1.4) |
| Urinary disorders | 2 (1.4) |
| Urinary bladder surgery | 1 (0.7) |
| Urinary tract infection | 1 (0.7) |
| Other | 21 (15.2) |
| Back pain | 5 (3.6) |
| Muscle strain | 1 (0.7) |
| Arthralgia | 5 (3.6) |
| Pre‐menopausal pain | 5 (3.6) |
| Toothache | 2 (1.4) |
| Dry mouth | 2 (1.4) |
| Nail fungus | 1 (0.7) |
Patients may report more than one event.
AE, adverse event.
Safety
Epitomee capsules (approximately 10,000 capsules overall) were safely swallowed by all subjects, during 714 treatment weeks. There was no device‐related serious AEs. The most common AEs were headache (18.1%), viral infection (11.5%) abdominal discomfort (10.1%), bloating (7.9%), nausea, (5%) constipation (5%) and flatulence (4.3%). Vomiting occurred in one patient. Of the 24 patients who withdrew from the study, only three discontinued treatment due to AEs: two patients from constipation and one from a non‐related AE (sudden hearing loss, requiring hospitalization) (Table 4). Other reasons for patients' withdrawals included dissatisfaction (four patients), loss to follow up (two patients) and noncompliance with the study protocol (five patients were unable to follow the protocol requirements, and three patients violated the protocol) (Figure 2). Endoscopy in 26 enrolled subjects, 12 weeks after repeated device use and 24 h post the last capsule ingestion, revealed complete device evacuation from the stomach of all patients, with no device aggregation and absence of acute mucosal lesions as assessed by an independent safety committee. Mild non‐significant and asymptomatic gastric or duodenal erythema without erosions was observed in five patients, without the need for medical intervention.
Discussion
The results of the present study demonstrate the high tolerability and a favourable safety profile of the Epitomee capsules. Furthermore, 42% of patients completing 12 weeks of treatment with the device along with lifestyle intervention had clinically significant weight loss (≥5%).
Multiple studies demonstrated that modest weight losses (5% to 7%) of initial weight are sufficient to produce significant clinically relevant improvement in cardiovascular risk factors in patients with overweight and obesity with T2DM 17, 18, 19, 20, 21, 22. Epitomee capsules like other GI devices are intended to provide an additional option of modest weight loss in patients with overweight and obesity with the potential to improve compliance as compared to pharmacotherapy and without the need for surgery or endoscopic procedure. It is generally accepted in the medical field that the more invasive the treatment is (e.g. bariatric surgery), the greater the efficacy is as a trade‐off for safety. The safety profile for intragastric balloons raised significant concerns about whether the modest weight loss efficacy warrants the potential risks associated with their implantation 12. Although the magnitude of weight loss observed with Epitomee capsules is less than other GI devices, the device favourable safety and tolerability profile may encourage patients to continue treatment over a longer period of time without jeopardizing safety. Thus, Epitomee capsules like Gelesis could play an important role in establishing a new category of modest weight loss long‐term treatments that facilitate patients' compliance and safety.
The main limitation of our study is the lack of a control condition where the lifestyle counselling is given with placebo. We acknowledge that we have no observations beyond 12 weeks to compare with other studies of 6–12 months. Furthermore, because the study was not powered to study obesity‐related comorbidities, most metabolic parameters were normal at baseline, and participants with diabetes were specifically excluded. Thus, there are very few patients that could be studied for treatment effects regarding improved glycaemic control and lipid profiles in pre‐diabetic and hyperlipidaemic patients, as well as the normalization of blood pressure in hypertensive patients.
Conclusion
Although the long‐term safety and efficacy of the capsules are not yet established, ED brings a new concept for weight loss with preliminary promising results. With continued research, the Epitomee capsules have considerable potential to become a novel, non‐invasive, safe and effective treatment option for weight loss. A large, randomized, placebo‐controlled trial evaluating the Epitomee capsules' long‐term safety and efficacy is in progress currently. Ultimately, as for other novel therapies, it will be important to couple the adoption of the novel therapy with neuroendocrine evaluation, lifestyle change, high quality diet, increased physical activity and psychological support, in order to ensure sustained treatment success.
Conflict of Interest Statement
E. B. has participated on an advisory board and was a consultant for Epitomee Medical. Epitomee Medical provided study devices and collaborated with the investigators in the design of the study, interpretation of the data, and the preparation, review and approval of the manuscript.
Shirin H., Richter V., Matalon S., Abramowich D., Maliar A., Shachar E., Moss S. F., and Broide E. (2019) Safety, tolerability and efficacy of a novel self‐use biodegradable device for management of obesity, Obesity Science & Practice, 5, 376–382. 10.1002/osp4.343.
References
- 1. Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev 2018; 39: 79–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alamuddin N, Wadden TA. Behavioral treatment of the patient with obesity. Endocrinol Metab Clin North Am 2016; 45: 565–580. [DOI] [PubMed] [Google Scholar]
- 3. Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation 2012; 125: 1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ross KM, Qiu P, You L, Wing RR. Characterizing the pattern of weight loss and regain in adults enrolled in a 12‐week internet‐based weight management program. Obesity 2018; 26: 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saunders KH, Umashanker D, Igel LI, Kumar RB, Aronne LJ. Obesity pharmacotherapy. Med Clin North Am 2018; 102: 135–148. [DOI] [PubMed] [Google Scholar]
- 6. Vella A, Ma J. What has bariatric surgery taught us about the role of the upper gastrointestinal tract in the regulation of postprandial glucose metabolism? Front Endocrinol 2018; 9: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non‐surgical treatment for obesity: a systematic review and meta‐analysis of randomised controlled trials. BMJ 2013; 347: f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar N. Endoscopic therapy for weight loss: gastroplasty, duodenal sleeves, intragastric balloons, and aspiration. World J Gastrointest Endosc 2015; 7: 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rashti F, Gupta E, Ebrahimi S, Shope TR, Koch TR, Gostout CJ. Development of minimally invasive techniques for management of medically‐complicated obesity. World J Gastroenterol 2014; 20: 13424–13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martins Fernandes FA Jr, Carvalho GL, Lima DL, et al. Intragastric balloon for overweight patients. JSLS 2016; 20():e2015.00107: e2015.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marrone AK, Antonino MJ, Silverstein JS, et al. The regulatory perspectives on endoscopic devices for obesity. Gastrointest Endosc Clin N Am 2017; 27: 327–341. [DOI] [PubMed] [Google Scholar]
- 12. Tate CM, Geliebter A. Intragastric balloon treatment for obesity: FDA safety up‐dates. Adv Ther 2017; 35: 1–4. [DOI] [PubMed] [Google Scholar]
- 13. Urban LE, Audet D, Ron ES, Sannino A, Zohar Y, Heshmati HM. Short‐term administration of a novel hydrogel (Gelesis200) increases fullness and satiety in subjects who are overweight or have obesity: first‐in‐human STAGE study. Poster presented at European Congress on Obesity 2017. May 17‐20, Porto, Portugal.
- 14. Heshmati HM, Tacchino R, Ron E, Sannino A, Zohar Y. Attiva, A novel superabsorbent biodegradable hydrogel, increases the feeling of satiety in humans. In: Program of the 19th Annual Meeting and Clinical Congress of the American Association of Clinical Endocrinologists. April 21‐25, 2010; Boston MA.
- 15. Astrup A, Kristensen M, Gnessi L, et al. Oral administration of Gelesis100, a novel hydrogel, significantly decreases body weight in overweight and obese subjects. In: Program of the Endocrine Society 96th Annual Meeting. June 21‐24, 2014; Chicago, IL.
- 16. Grybäck P, Hermansson G, Lyrenäs E, Beckman KW, Jacobsson H, Hellström PM. Nationwide standardization and evaluation of scintigraphic gastric emptying: reference values and comparisons between subgroups in a multicentre trial. Eur J Nucl Med 2000;27(6):647‐655, Nationwide standardisation and evaluation of scintigraphic gastric emptying: reference values and comparisons between subgroups in a multicentre trial. [DOI] [PubMed] [Google Scholar]
- 17. Khaodhiar L, Cummings S, Apovian CM. Treating diabetes and prediabetes by focusing on obesity management. Curr Diab Rep 2009; 9: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feldstein AC, Nichols GA, Smith DH, et al. Weight change in diabetes and glycemic and blood pressure control. Diabetes Care 2008; 31: 1960–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep 2015; 4: 287–302. [DOI] [PubMed] [Google Scholar]
- 20. Ridderstrale M, Gudbjornsdottir S, Eliasson B, Nilsson PM, Cederholm J. Obesity and cardiovascular risk factors in type 2 diabetes: results from the Swedish National Diabetes Register. J Intern Med 2006; 259: 314–322. [DOI] [PubMed] [Google Scholar]
- 21. Gilis‐Januszewska A, Piwońska‐Solska B, Lindström J, et al. Determinants of weight outcomes in type 2 diabetes prevention intervention in primary health care setting (the DE‐PLAN project). BMC Public Health 2018; 18: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 1992; 16: 397–415. [PubMed] [Google Scholar]


