Summary
Generation of functional β cells from pluripotent sources would accelerate diagnostic and therapeutic applications for diabetes research and therapy. However, it has been challenging to generate competent β cells with dynamic insulin-secretory capacity to glucose and incretin stimulations. We introduced transcription factors, critical for β-cell development and function, in differentiating human induced pluripotent stem cells (PSCs) and assessed the impact on the functionality of derived β-cell (psBC) progeny. A perifusion system revealed stepwise transduction of the PDX1, NEUROG3, and MAFA triad (PNM) enabled in vitro generation of psBCs with glucose and GLP-1 responsiveness within 3 weeks. PNM transduction upregulated genes associated with glucose sensing, insulin secretion, and β-cell maturation. In recipient diabetic mice, PNM-transduced psBCs showed glucose-responsive insulin secretion as early as 1 week post transplantation. Thus, enhanced pre-emptive β-cell specification of PSCs by PNM drives generation of glucose- and incretin-responsive psBCs in vitro, offering a competent tissue-primed biotherapy.
Keywords: iPSC, reprogramming, β-cell regeneration, transcription factor, PDX1, NEUROG3, MAFA
Highlights
-
•
PNM transduction facilitates generation of glucose- and GLP1-responsive β cells
-
•
Stepwise PNM improves glucose sensing, insulin secretion, and β-cell maturation
-
•
PNM-transduced psBCs showed glucose-responsive insulin secretion in diabetic mice
In this article, Ikeda Yasuhiro and colleagues show that stepwise transduction of the triad of transcription factors PDX1, NEUROG3, and MAFA (PNM) enabled in vitro generation of glucose- and GLP-1-responsive β cells from iPSCs within 3 weeks. PNM transduction improves glucose sensing, insulin secretion, and β-cell maturation of generated cells. PNM-transduced β cells showed glucose-responsive insulin secretion as early as 1 week post transplantation in diabetic mice.
Introduction
Diabetes affects more than 442 million adults worldwide (WHO, 2016), and this number is estimated to reach 642 million by 2040 (Ogurtsova et al., 2017). Whereas type 1 diabetes (T1D) results from autoimmune destruction of pancreatic β cells, type 2 diabetes (T2D) is caused by peripheral insulin resistance, β-cell dysfunction, and β-cell loss (Butler et al., 2003). Transplantation of stem cell-derived insulin-producing cells could provide a cell therapy option to restore β-cell loss or dysfunction in patients with diabetes (Holditch et al., 2014). Because of the capacity for unlimited self-renewal and pluripotent lineage specification, human pluripotent stem cells (PSCs) have been extensively studied for β-cell generation potential (Holditch et al., 2014). For the generation of β-like insulin-producing cells from PSCs guided differentiation protocols have been developed, which model the key stages of pancreatic development in vitro, including definitive endoderm, foregut, pancreatic endoderm, and endocrine progenitor stages (D'Amour et al., 2006, Nostro et al., 2011, Basford et al., 2012, Rezania et al., 2012). However, most protocols typically yield populations of multihormonal PSC-derived β cells (psBCs) lacking glucose responsiveness (D'Amour et al., 2006, Basford et al., 2012, Thatava et al., 2013, Cogger et al., 2017), and generation of more mature, glucose-responsive psBCs has required prolonged in vivo or in vitro maturation steps (Bruin et al., 2015a, Bruin et al., 2015b, Kroon et al., 2008, Rezania et al., 2012).
Insulin secretion in vivo occurs in two distinct phases, with the first phase (0–5 min) corresponding to the release of the stored pool of insulin granules and the second phase corresponding to the release of newly formed insulin granules (Curry and MacLachlan, 1987, Pfeifer et al., 1981), and identifying the first-phase temporal insulin profile is essential for determination of proper functionality of β cells since lack of first-phase insulin-secretory response is characteristic of immature and/or dysfunctional β cells (Dhawan et al., 2015, Gerich, 2002). The dynamic perifusion system allows evaluation of temporal insulin secretion profiles in response to glucose and other secretagogs. In contrast, commonly used static glucose-stimulated insulin secretion (GSIS) assays preclude detection of the critical first-phase insulin secretion. In static GSIS assays, islets are also “bathed” with its secretory products such as insulin, amylin, and glucagon, which can affect insulin secretion and islet function and thus potentially alter the results. Another important feature of functional β cells is their responsiveness to glucagon-like peptide 1 (GLP-1), an incretin hormone regulating glucose homeostasis (Kim and Egan, 2008). Impairment of GLP-1-induced insulin secretion is frequently found in patients with T2D (Kjems et al., 2003). Recently, several groups have demonstrated highly efficient generation of insulin-producing β cells with various key mature β-cell features from PSCs (Pagliuca et al., 2014, Rezania et al., 2014, Russ et al., 2015). However, stem cell-derived β cells did not show notable glucose and incretin responsiveness by the dynamic perifusion system or were analyzed only by static GSIS assays that do not detect the first-phase GSIS.
Studies have identified transcription factors critical for β-cell development, maturation, or function. PDX1 is expressed at the 5- to 6-somite stage and is mandatory for pancreatic organogenesis (Miki et al., 2012). PDX1 expression is followed by induction of NKX2.2 (Sussel et al., 1998) and downstream NKX6.1 (Sander et al., 2000) in pancreatic progenitor cells, which play critical roles in β-cell differentiation. In uncommitted progenitors in the developing pancreas, NEUROG3 is required for the specification of the endocrine lineage (Gradwohl et al., 2000). Specifically, transient NEUROG3 expression induces various transcription factors important for endocrine cell-lineage differentiation and β-cell function, including NEUROD1, ARX, PAX6, and ISL1 (Collombat et al., 2003). In the later stages of β-cell differentiation, MAFA and MAFB regulate β-cell formation and maturation (Artner et al., 2010). In particular, MAFA binds to a conserved insulin enhancer element RIPE3b/C1-A2 and enhances insulin gene expression as well as glucose-responsive insulin secretion (Aguayo-Mazzucato et al., 2011). In developing and mature β cells, PDX1 also binds insulin promoter to regulate insulin expression (Iype et al., 2005). Moreover, ESRRG is induced in adult β cells and plays a key role in β-cell metabolic maturation (Yoshihara et al., 2016).
Previously, we have reported inconsistent induction of PDX1 and that NKX6.1 is responsible for intrapatient variations among induced PSC (iPSC) clones in their β-cell differentiation propensities (Thatava et al., 2013). Weak in vitro induction of NKX6.1 also leads to lower maturation of psBCs in vivo (Rezania et al., 2013). We therefore hypothesized that improved β-cell specification by the introduction of key transcription factors would facilitate generation of glucose-responsive psBCs in vitro. In this study, we demonstrate generation of glucose- and GLP-1-responsive psBCs in vitro through improved β-cell specification by stepwise introduction of PDX1, NEUROG3, and MAFA (PNM) in differentiating iPSC progeny.
Results
Screening of β-Cell Transcription Factor(s) for Improved Glucose- and GLP-1-Responsive Insulin Secretion in psBCs
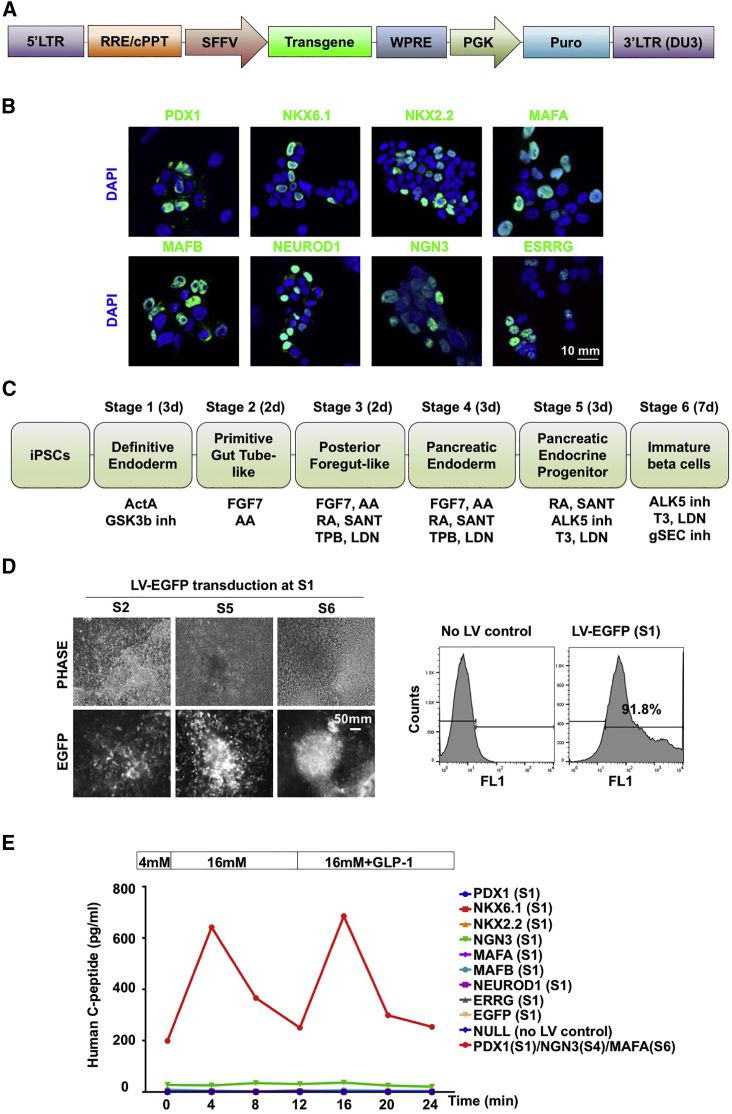
We produced lentiviral vectors carrying codon-optimized open reading frames (ORFs) of transcription factors critical for β-cell development and function, including PDX1, NKX6.1, NKX2.2, MAFA, MAFB, NEUROD1, NEUROG3, and ESRRG (Figure 1A). Vector titers were determined by puromycin selection, and the expression of encoded transgene proteins was verified in vector-infected 293T cells by immunostaining with specific antibodies (Figure 1B). Monolayer iPSCs underwent a guided differentiation process for 3 weeks (Figure 1C). When differentiating iPSC progeny at stage 1 (S1, day 2) was transduced by a control EGFP-expressing lentiviral vector at an approximate multiplicity of infection of 30, we found EGFP signals throughout the differentiation process from S2 to S6 (Figure 1D, left panel). Flow-cytometry analysis demonstrated that over 90% of cells were EGFP positive at the end of S6 (Figure 1D, right panel). Efficient EGFP transduction was also found when iPSC progeny was transduced at other stages.
Figure 1.
Screening of β-Cell Transcription Factor(s) for Improved Glucose- and GLP-1-Responsive Insulin Secretion in psBCs
(A) Schematic diagram of lentiviral vector constructs.
(B) Expression of individual β-cell factors (green). Scale bar, 10 mm.
(C) Summary of the six-stage differentiation strategy.
(D) EGFP expression monitored by UV microscope at S2, S5, and S6 (left panel). Representative fluorescence-activated cell sorting (FACS) plots for EGFP expression in control and lentiviral (LV)-EGFP transduced (S1) cells in the end of S6 stage (right panel). Phase, phase contrast. Scale bar, 50 mm.
(E) Secretion of human C peptide by psBCs in response to sequential stimulation of 4 mM, 16 mM, and 16 mM + GLP-1 glucose medium.
To determine whether the introduction of a single, key β-cell transcription factor could improve the glucose and GLP-1 responsiveness of psBCs, we first transduced S1 iPSC progeny with a single lentiviral vector carrying a β-cell factor. Since overexpression of PDX1, NEUROG3, and MAFA has been shown to transdifferentiate various cell types into insulin-producing cells (Zhu et al., 2017), we also transduced iPSC progeny with a combination of lentiviral vectors expressing the PNM triad at stages 1, 4, and 6, respectively. Perifusion experiments of S6 psBCs demonstrated very-low-level, non-glucose-responsive C-peptide secretion by unmodified (NULL) or EGFP vector-infected control cells (Figure 1E). Introduction of PDX1, NKX6.1, NKX2.2, MAFA, MAFB, NEUROD1, or ESRRG at S1 did not strongly affect C-peptide secretion or glucose and GLP-1 responsiveness. In contrast, introduction of NEUROG3 alone strongly enhanced the insulin-secretory capacity of resulting psBCs, from up to 0.08 pg/mL of C-peptide secretion in unmodified control psBCs and up to 36 pg/mL in NEUROG3-transduced psBCs (Figure S1). Nevertheless, NEUROG3 transduction alone did not improve glucose and GLP-1 responsiveness of resulting psBCs. Notably, stepwise transduction of PDX1, NEUROG3, and MAFA together (PNM) in differentiating iPSC progeny led within 3 weeks to the generation of psBCs with notable glucose and GLP-1 responsiveness, along with increased insulin-secretory capacity.
Stepwise PDX1, NEUROG3, and MAFA Transduction Facilitates Generation of Glucose- and GLP-1-Responsive psBCs
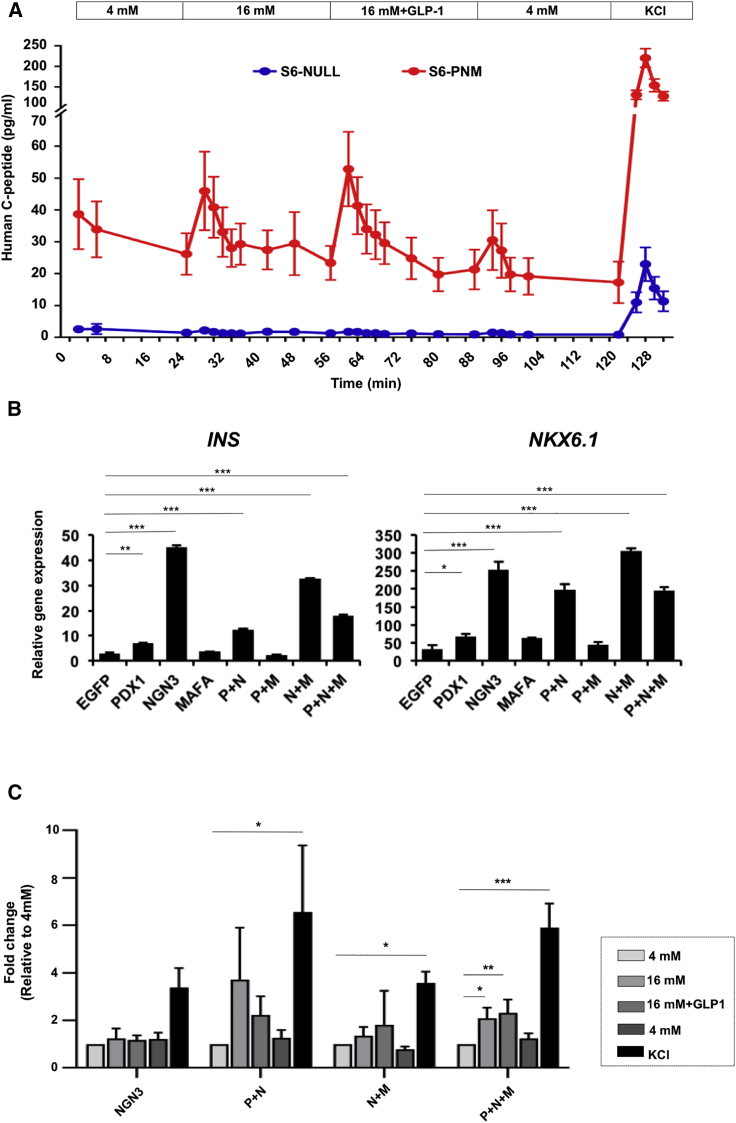
To assess the reproducibility of generation of glucose- and GLP-1-responsive psBCs by PNM transduction, we analyzed the dynamics of C-peptide secretion from S6 psBCs with or without PNM transduction. After confirming the expression of exogenous PDX1, NEUROG3, and MAFA transgenes in S6 psBCs (Figure S2A), we observed rapid responses in C-peptide secretion from PNM-transduced S6 psBCs upon sequential increases of glucose levels from 4 to 16 mM, addition of GLP-1 to 16 mM glucose, and in 30-mM KCl depolarization (Figure 2A). The robust reaction to high glucose solution highly resembled the pattern of human islets under the same conditions (Bentsi-Barnes et al., 2011) (Figure S2B). When we assessed the fold change of C-peptide levels within individual samples at the medium-changing time points, we found a 2.1-fold increase in 16-mM glucose stimulation at the 4-min time point (p = 0.03), when compared with the levels at the last time point in the previous stage. We also found immediate 2.2-fold increase in 16 mM glucose + GLP-1 (p < 0.001), no significant change on reversion to 4 mM (p = 0.146), and 10.7-fold increase in KCl treatment (p < 0.001). Additionally, one feature of immature fetal β cells is the exaggerated insulin secretion under low glucose (Blum et al., 2012). However, we observed repressed C-peptide secretion from PNM-transduced S6 psBCs in basal 4-mM glucose treatment. Moreover, PNM-transduced S6 psBCs also displayed a clear first- and second-phase insulin response upon 16-mM glucose treatment, which is another key feature of mature β cells (Song et al., 2000, Song et al., 2002). Together, our data suggested that PNM transduction promotes the differentiation of iPSCs to glucose-responsive, incretin (GLP-1)-responsive mature β cells in vitro.
Figure 2.
PNM-Transduced psBCs Are Responsive to Glucose and GLP-1 In Vitro
(A) Secretion of human C peptide by PNM-transduced and untransduced control psBCs (n = 3 independent experiments). Data represent the means ± SEM.
(B) Levels of INS and NKX6.1 transcripts in S6 psBCs, relative to those of undifferentiated iPSCs (n = 3 independent experiments). Data represent the means ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Student’s unpaired t test.
(C) Fold changes (FC) of C-peptide levels at the 4-min time point in each stage relative to the levels at the last time point in the basal 4 mM glucose (n = 3 independent experiments). Data represent the means ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Comparison log2 fold change, one-sample t test.
To further assess the contributions of PDX1 (S1), NEUROG3 (S4), and MAFA (S6), we determined the impact of transduction of different combinations of P/N/M. All psBCs that included NEUROG3 (NEUROG3 alone, PDX1 + NEUROG3, NEUROG3 + MAFA, PDX1 + NEUROG3 + MAFA) showed increased levels of INS and NKX6.1 transcripts (Figure 2B). Transduction with PDX1 alone, MAFA alone, or both PDX1 and MAFA showed no significant effect (Figure 2B). When we assessed the GSIS of psBCs by the perifusion system, groups with NEUROG3 transduction exhibited higher baseline C-peptide secretion as well as higher total secretable C-peptide contents, whereas psBCs groups without NEUROG3 transduction showed little C-peptide secretion (Figure S2C). Among these high C-peptide-releasing groups containing NEUROG3 transduction, only the PNM transduction group displayed significantly higher C-peptide levels at 16 mM (p = 0.033), 16 mM + GLP-1 (p = 0.005), and upon KCl depolarization (p < 0.001) relative to the 4-mM glucose baseline (Figure 2C). psBCs transduced with both PDX1 and NEUROG3 showed a trend of enhanced C-peptide secretion upon high glucose stimulation, whereas transduction with NEUROG3 alone or MAFA + NEUROG3 led to no significant change in glucose-responsive C-peptide secretion. These data indicate that NEUROG3 transduction is critical in generating insulin-secreting β-like cells from iPSCs, while additional PDX1 and MAFA transduction is necessary to induce glucose responsiveness in psBCs.
Mature β-Cell Characteristics Found in PNM-Transduced psBCs
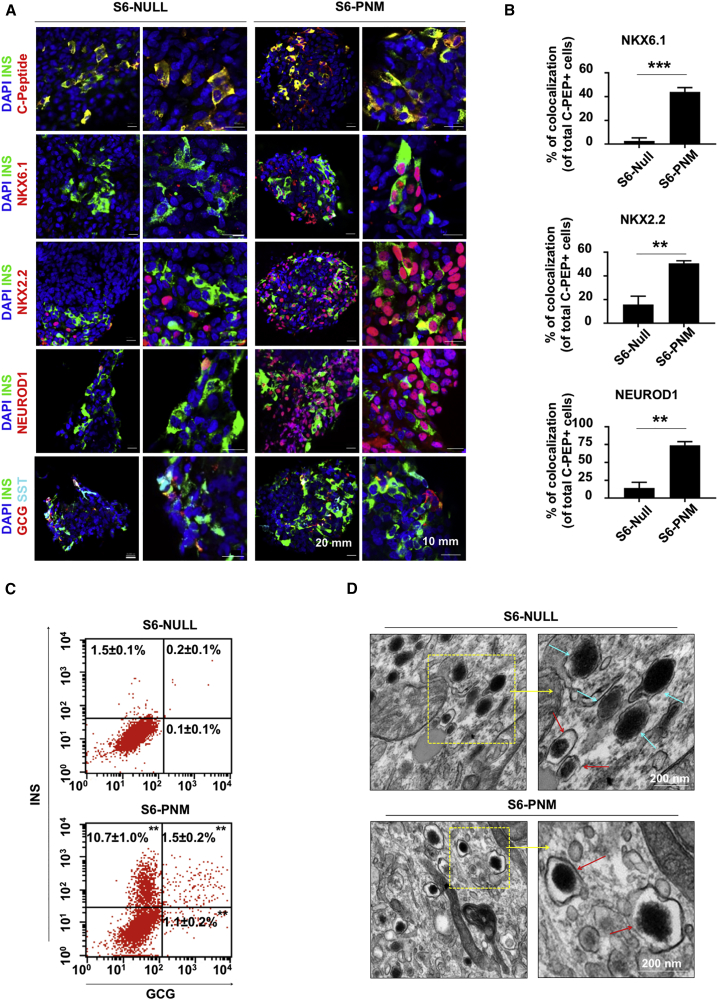
We next characterized the expression of β-cell markers in PNM-transduced (S6-PNM) and control (S6-NULL) psBCs. Immunohistochemistry revealed that C-peptide signals were frequently colocalized with insulin (INS) signals in both S6-PNM and S6-NULL psBCs (Figure 3A, top row). Pancreatic α-cell marker glucagon (GCG) and δ-cell marker somatostatin (SST) signals were also found, with some multihormonal psBCs in both groups (Figure 3A, bottom row). In contrast, widespread expression of NKX6.1, NKX2.2, and NEUROD1 was only seen in S6-PNM psBCs (Figure 3A, second to fourth rows). This resulted in more frequent coexpression of NKX6.1 (p < 0.001), NKX2.2 (p = 0.006), and NEUROD1 (p = 0.004) in insulin-positive cells in S6-PNM compared with S6-NULL psBCs (Figure 3B). These observations indicate that stepwise PNM transduction promotes the induction of key β-cell transcription factors NKX6.1, NKX2.2, and NEUROD1 in insulin-producing cells. Flow-cytometry analysis showed that S6-PNM populations contained 12.2% insulin-positive cells, compared with 1.7% in the S6-NULL population (Figure 3C).
Figure 3.
Induction of NKX6.1, NKX2.2, and NEUROD1 in PNM-Transduced psBCs
(A) Representative immunofluorescent staining images of psBCs. Scale bars, 20 mm and 10 mm.
(B) Quantification of colocalization of insulin and NKX6.1, insulin and NKX2.2, or insulin and NEUROD1 in psBCs (in total n = 1,890 insulin-positive cells were counted in S6-PNM, n = 770 insulin-positive cells were counted in S6-NULL from n = 3 independent experiments). Data represent the means ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Student’s unpaired t test.
(C) Representative FACS plots for insulin and glucagon expression in psBCs (n = 3 independent experiments). Data represent the means ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Student's unpaired t test.
(D) Representative insulin granules (red arrows) and glucagon granules (blue arrows). Scale bar, 200 nm.
We also performed transmission electron microscopy analysis to characterize ultrastructures of secretory granules in S6-NULL and S6-PNM psBCs (Figure 3D). Consistent with flow-cytometry analysis, we observed more cells with endocrine granules in S6-PNM psBCs than in S6-NULL psBCs. In S6-PNM psBCs, there were numerous insulin granules with characteristic electron-dense crystal cores surrounded by a light halo, similar to normal human β-cell insulin granules (Deconinck et al., 1972). In contrast, S6-NULL psBCs often presented both insulin granules and glucagon granules characterized by larger dense cores and grayer halo (Deconinck et al., 1972). These data further support the improved maturation pattern of psBCs upon stepwise PNM transduction.
Global Gene Expression Profiling Identifies Accelerated Induction of Glucose Sensing and Insulin Secretion Genes upon PNM Transduction
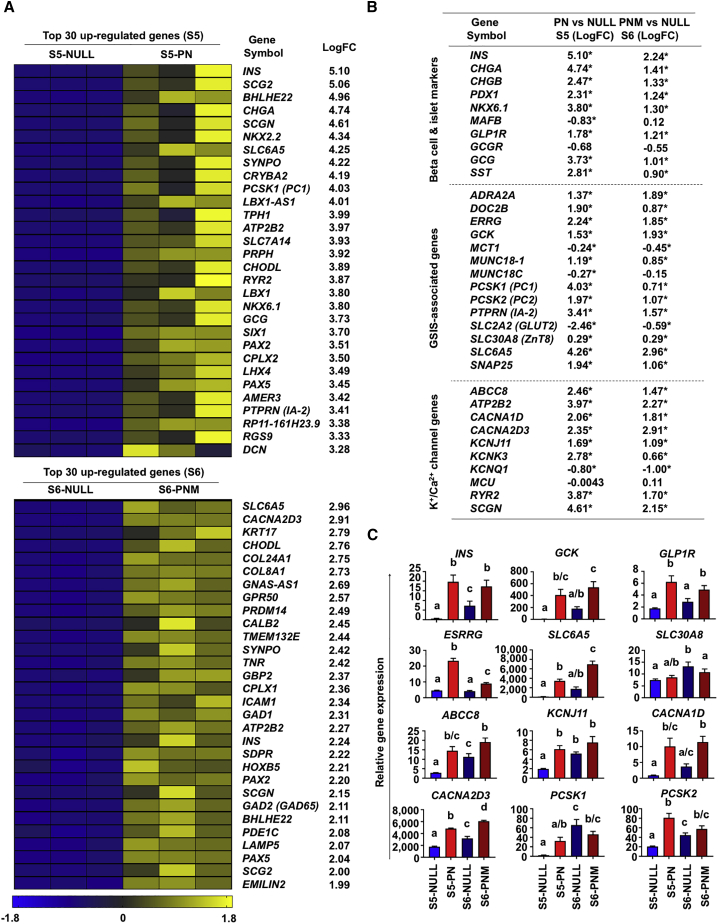
To further understand the impact of PNM transduction on iPSC differentiation into psBCs, we performed next-generation RNA-sequencing analyses using RNA samples from psBCs at the end of S5 (control S5-NULL cells versus S5-PN cells, 13 days after differentiation, before S6 MAFA transduction) as well as psBCs at the end of S6 (control S6-NULL cells versus S6-PNM, 20 days after differentiation). The top 30 upregulated genes for S5-PN and S6-PNM identified key genes relevant to β-cell development and function including INS, NKX2.2, NKX6.1, PAX2, and PCSK1 (PC1), as well as insulin granule exocytosis, such as CHGA, SCG2/CHGC, SCGN, CPLX1, and CPLX2. The major T1D antigen genes, including INS, IA-2 (PTPRN), and GAD65 (GAD2), were also prioritized. Other notable genes identified in both S5 and S6 include SLC6A5/GLYT2, which controls a glycine-insulin autocrine feedback, BHLHE22, regulating insulin gene expression (Melkman-Zehavi et al., 2011), and a plasma membrane Ca2+-ATPase, ATP2B2/PMCA2, which affects GSIS and β-cell proliferation (Pachera et al., 2015) (Figure 4A). Further analysis of sets of genes, associated with β-cell/islet maturation, GSIS, L-type voltage-dependent calcium channel (VDCC), and ATP-sensitive potassium channel (KATP channel), also identified significant upregulation of genes underlying β-cell functionality, including GCK, ESRRG, CHGB, SLC30A8, ABCC8, KCNJ11, and CACNA2D3 (Figure 4B). In addition, GCG and SST expression was also upregulated, especially at S5, suggesting accelerated endocrine differentiation by PDX1 and NEUROG3 transduction. qRT-PCR analysis verified upregulation of key β-cell factors in S5-PN and S6-PNM psBCs (Figure 4C). Of note, when the top 30 downregulated genes were identified for S5-PN and S6-PNM (Figure S3A), α-fetoprotein (AFP), an extraembryonic endoderm or hepatoblast marker, was found as the most prominently suppressed gene upon PNM transduction, suggesting improved β-cell specification by PNM transduction in derived iPSC progeny. Further comparison between S5-PN and S6-PNM psBCs showed critical genes related to insulin secretion, β-cell maturation/function, and ionic channels upregulated in S6-PNM psBCs (Figures S3B and S3C). The upregulated differentially expressed genes in S6-PNM psBCs were enriched in signaling pathways including lysosome, axon guidance, insulin secretion, glutamatergic synapse, insulin signaling, endocrine, and other factor-regulated calcium reabsorption, confirming the extra benefits of MAFA transduction at S6 (Figure S3D).
Figure 4.
Global Gene Expression Profiles of PN-Transduced S5 and PNM-Transduced S6 psBCs
(A) Heatmaps of top 30 upregulated genes. LogFC stands for the log2 fold change of expression level relative to control.
(B) Summary of differentially expressed genes. LogFC stands for the log2 fold change of expression level relative to control. ∗p < 0.05. Student's unpaired t test.
(C) qRT-PCR analysis for critical β-cell genes (n = 3 independent experiments). Data represent the means ± SEM. Different letters represent statistically significant differences between two groups throughout four groups; one-way ANOVA with Tukey’s test for multiple comparisons.
PNM Transduction in Other iPSC Lines
To evaluate the robustness of PNM transduction to generate glucose- and incretin-responsive psBCs from other iPSC lines, we differentiated an additional five iPSC lines and assessed the GSIS of derived psBCs. Two iPSC lines demonstrated dynamic glucose and GLP-1 responsiveness upon PNM transduction, while three other clones showed no enhanced glucose responsiveness (Figure S4), likely due to extensive clonal variations in β-cell differentiation propensity among iPSC lines (Thatava et al., 2013).
PNM-Modified psBCs Demonstrate Glucose-Responsive Insulin Secretion In Vivo
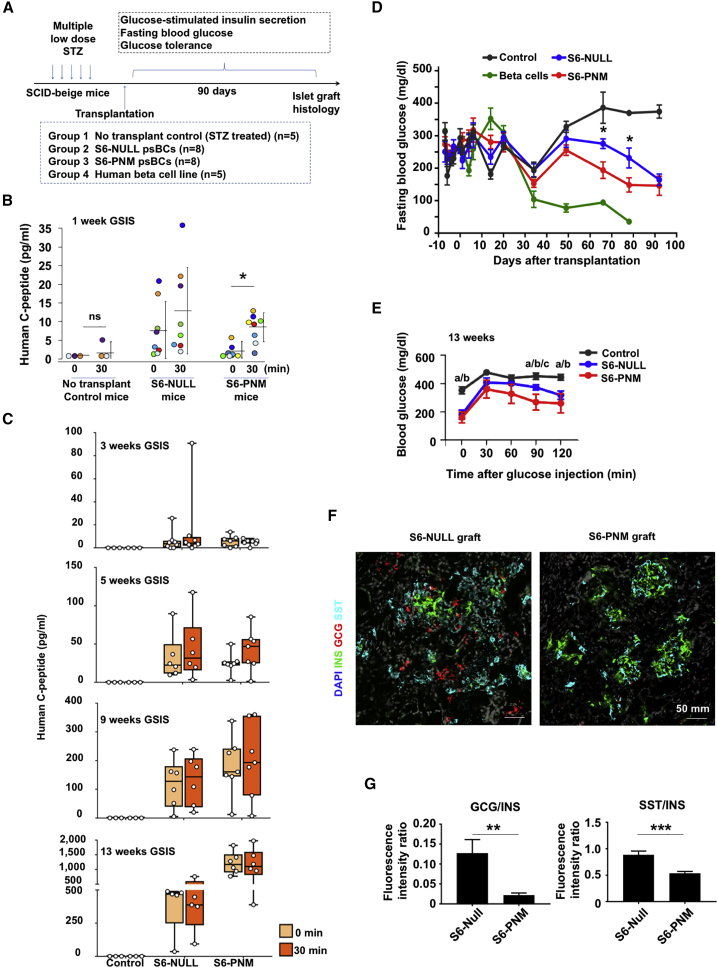
To evaluate the glucose-responsive insulin-secretory capacity of PNM-modified psBCs in vivo, we transplanted approximately 50 million S6 psBCs into the kidney capsules of immunodeficient SCID-beige mice with streptozotocin-induced diabetes (Figure 5A). We also transplanted a human β-cell line as a control. When glucose-responsive insulin secretion was monitored upon intraperitoneal glucose administration, we found low levels of human C peptide in circulation in S6-NULL and S6-PNM recipient mice 1 week after transplantation. Importantly, levels of circulating human C peptide were significantly increased 30 min after glucose challenge in S6-PNM recipient mice (p = 0.003) (Figure 5B), although S6-NULL recipient mice did not show in vivo glucose responsiveness. No notable circulating human C peptide was seen in control mice, while mice transplanted with the human β-cell line showed no glucose responsiveness, but higher levels of human C peptide before (average 15.1 pg/mL) and after (average 18.4 pg/mL) glucose challenge. These data demonstrate the glucose-responsive insulin-secretory capacity of PNM-transduced psBCs in vivo, as early as 1 week post transplantation.
Figure 5.
PNM-Transduced psBCs Show Glucose-Responsive Insulin Secretion 1 Week Post Transplantation
(A) Experimental design for in vivo psBC analysis.
(B and C) Fasting and 30 min (following IPGTT) human C-peptide levels in mice. C-peptide levels from individual m are shown on scale bar-and-whisker plots. Student's paired t test. ns, not significant.
(D) Fasting blood glucose levels after transplantation in mice. Data represent the means ± SEM. Comparison of S6-PNM with S6-NULL, ∗p < 0.05. Student's unpaired t test.
(E) Blood glucose levels during IPGTT in mice. Data represent the means ± SEM. “a” represents a statistically significant difference between control and S6-PNM, “b” represents a difference between control and S6-NULL, “c” represents a difference between S6-NULL and S6-PNM; one-way ANOVA with Tukey’s test for multiple comparisons.
(F) Representative immunofluorescent staining images of psBC grafts. Scale bar, 50 mm.
(G) Fluorescence intensity ratios of GCG to INS and SST to INS in images of S6-NULL (n = 22 images, 5–6 images per mouse, 4 mice were analyzed) and S6-PNM (n = 23 images, 5–6 images per mouse, 4 mice were analyzed) grafts. Data represent the means ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Student's unpaired t test.
Fasting human C-peptide levels in S6-PNM-transplanted mice gradually increased over time, from 3 pg/mL at 1 week to 1,172 pg/mL at 13 weeks post transplantation (Figure S5A). GSIS was not detected at 3, 5, 9, and 13 weeks (Figure 5C). Similar results were found in S6-NULL-transplanted mice (Figure 5C). However, fasting circulating C-peptide levels were significantly lower in S6-NULL than those observed in S6-PNM at weeks 11 (p = 0.047) and 13 (p = 0.048) (Figure S5A). Similarly, although blood glucose levels after 16 h of fasting remained high in both S6-NULL and S6-PNM psBCs-transplanted mice for 3 weeks, S6-NULL- and S6-PNM-recipient mice started to show lower blood glucose levels than controls at 8 weeks post transplant (Figure 5D). The blood glucose levels were significantly lower in S6-PNM mice than those in S6-NULL mice at 9 (p = 0.022) and 11 (p = 0.047) weeks post transplant (Figure 5D). To assess the impact of psBC transplantation on the glucose tolerance in vivo, we also conducted an intraperitoneal glucose tolerance test (IPGTT) at 7 and 13 weeks post transplant. There was no difference observed in glucose levels among diabetic controls, S6-NULL recipient, and S6-PNM recipient mice at 7 weeks (Figure S5B). However, at 13 weeks, S6-PNM recipient mice displayed lower glucose levels than S6-NULL at 90 min (p = 0.003), indicating superior glucose regulation by S6-PNM over S6-NULL psBCs (Figure 5E).
Prospectively, 13 weeks after transplantation, we harvested the kidneys with psBC grafts from the recipient mice. Further analysis revealed that the insulin-positive cells in S6-PNM and S6-NULL grafts were largely monohormonal. However, S6-PNM grafts contained rich clusters of insulin-positive cells, whereas in S6-NULL grafts the insulin-positive cells were scattered (Figure 5F). Notably, insulin-positive cells in S6-NULL were accompanied by significantly more glucagon-positive (p = 0.007) and somatostatin-positive cells (p < 0.001) compared with S6-PNM (Figure 5G), suggesting that the less mature S6-NULL cells may further adopt α-cell and δ-cell destiny in vivo.
Discussion
Despite extensive progress in human PSC differentiation protocols, in vitro generation of functional psBCs with dynamic glucose and incretin responsiveness has been an ongoing challenge limiting the derivation of competent cell therapies (Holditch et al., 2014). Most protocols failed to show the first-phase insulin secretion of their β-cell products upon glucose and GLP-1 stimulations. Additionally, GSIS of psBCs has been assessed mainly by static GSIS assays, which do not monitor first-phase GSIS and can be affected by varying metabolites secreted by the cells during the long incubation period with glucose. Here we assessed psBCs in a perifusion system that mimics the physiological glycemic environment and detects the first-phase GSIS. Our data demonstrated that stepwise introduction of key transcription factors PDX1, NEUROG3, and MAFA achieved reproducible generation of glucose- and GLP-1-responsive psBCs in vitro. PNM transduction accelerated psBC maturation through enhanced induction of key β-cell factors related to glucose sensing and insulin secretion. PNM transduction also improved β-cell specification, implicated by potent induction of important β-cell factors, such as NEUROD1, NKX6.1, NKX2.2, GCK, and GLP1R, while suppressing the extraembryonic endoderm or hepatoblast marker AFP. Our study therefore suggests PNM transduction facilitates in vitro generation of functional β cells from human PSCs through improved β-cell specification. The pre-achieved organotypic phenotype offered a proficient biotherapeutics capable to reverse symptoms of diabetes post transplantation.
Neonatal β cells are characterized by poor glucose responsiveness with a generalized immaturity of the metabolic pathways, and only gain robust glucose responsiveness several weeks after birth (Jermendy et al., 2011). Human psBCs typically show characteristics reminiscent of neonatal β cells. Various studies have demonstrated generation of glucose-responsive psBCs through in vivo maturation (Kroon et al., 2008, Rezania et al., 2014), with more accelerated maturation in rats than mice (Bruin et al., 2015a, Bruin et al., 2015b). Those observations indicate the roles of extracellular stimuli for acquisition of mature β-cell fate. Accordingly, the field has been searching for small molecules/growth factors that can induce maturation of psBCs, and identified factors, such as thyroid hormone or β-cellulin, that have been included in prolonged in vitro psBC maturation processes (Aguayo-Mazzucato et al., 2013, Pagliuca et al., 2014, Rezania et al., 2014, Russ et al., 2015). We showed that PNM transduction facilitates generation of glucose- and GLP-1-responsive psBCs in 3 weeks without prolonged in vitro culture or in vivo additional maturation steps, implying that PNM transduction can substitute critical external stimuli provided through in vivo maturation or prolonged in vitro maturation steps. Considering that NEUROG3 alone can strongly enhance expression of INS and NKX6.1 and insulin-secretory capacity, this further supports that NEUROG3 transduction plays the key role in improved iPSC differentiation into mature β cells upon PNM transduction (McGrath et al., 2015) Additionally, transduction of MAFA, the major transcription factor for β-cell maturation in neonatal β cells, contributes to the accelerated psBC maturation. However, single MAFA transduction at S1 or transduction of S4 NEUROG3 plus S5 MAFA failed to induce glucose responsiveness in psBCs, indicating that MAFA and NEUROG3 transduction are not sufficient for reproducible in vitro psBC maturation. In contrast, psBCs with dual PDX1 and NEUROG3 transduction showed a trend of GSIS, suggesting an important role of PDX1 transduction in accelerated psBC maturation. Since PDX1 deficiency impairs GSIS through mitochondrial dysfunction (Gauthier et al., 2009), it is plausible that PDX1 transduction contributes to GSIS in psBCs in addition to improved pancreatic specification. Use of an inducible transgene expression system for PNM transduction/shutdown will clarify the contributions of each factor to improved iPSC differentiation or psBC maturation.
Previous studies have demonstrated that simultaneous transduction of PDX1, NEUROG3, and MAFA achieves transdifferentiation of pancreatic exocrine, liver, or intestine cells into insulin-producing cells (Zhu et al., 2017). However, these (re)differentiation strategies appeared to be insufficient in generating authentic β cells that display β-cell morphology and robust glucose and incretin responsiveness. Recently, Saxena et al. (2016) reported improved psBC generation from iPSCs through the introduction of two expression constructs; one for bicistronic PDX1-MAFA fusion protein linked by a self-cleaving 2A peptide and another for NEUROG3. Derived psBCs showed glucose responsiveness by a static GSIS assay. In contrast to prior studies, our study delivered PNM at different steps during iPSCs-to-β-cell differentiating process based on known expressing patterns of each factor (Brissova et al., 2002, Gradwohl et al., 2000, Holland et al., 2002, Matsuoka et al., 2003, Matsuoka et al., 2004). Specifically, we delivered PNM at stages 1, 4, and 6, respectively. Our stepwise gene delivery strategy strongly enhanced iPSC differentiation into mature β cells, resulting in generation of functional psBCs with clear glucose and GLP-1 responsiveness, demonstrated by a real-time GSIS assay underscoring the permissive timeline of sequential tissue-specific programming.
Insulin is secreted through granule exocytosis. In response to glucose metabolism, inhibition of KATP channels and elevation of intracellular calcium levels lead to insulin vesicle fusion to the β-cell membrane with insulin secretion disorders precipitated by KATP channelopathy (Gauthier and Wollheim, 2008, Olson and Terzic, 2010, Takahashi et al., 2010). Intriguingly, PNM modification led to upregulation of genes involved in insulin release and VDCC- and KATP-channel activities, such as ABCC8 (Babenko et al., 2006), KCNJ11 (Gloyn et al., 2004), CACNA1D (Reinbothe et al., 2013), and SCGN (Yang et al., 2016). Other genes critical in glucose sensing or insulin secretion were also largely elevated, including SLC6A5 (Yan-Do et al., 2016), ESRRG (Yoshihara et al., 2016), SLC30A8 (Wijesekara et al., 2010), and GCK (Matschinsky et al., 2006). Intriguingly, SLC6A5 was identified as one of the top upregulated genes upon PNM transduction. SLC6A5 regulates the expression of glycine transporter 2 (GlyT2), which is expressed on human β cells and mediates the transportation of glycine, a neurotransmitter critical for coordinating insulin secretion by modulating the electrical activity of β cells via ionic channels and receptors. Additionally, PNM transduction enhanced GLP1R expression, which likely sensitized derived psBCs to incretin stimulation. These observations highlight the multifaceted effects of PNM transduction during iPSC differentiation into functional psBCs. Comparing our protocol with previously reported protocols is warranted in order to further understand the mechanism and inform future studies.
Previously, we have reported prominent intrapatient variations in psBC differentiation propensities among iPSC clones from T1D patients (Thatava et al., 2013). We found that PNM transduction led to more consistent generation of mature psBCs from iPSCs. Since PNM transduction strongly enhanced expression of major T1D antigen genes, such as INS/Proinsulin, PTPRN/IA-2, and GAD2/GAD65, PNM-modified psBCs from T1D patients would provide excellent research tools to model patient-specific autoimmune responses to β cells in vitro.
We also found that single transduction of PDX1, MAFA, NEUROD1, NKX2.2, NKX6.1, MAFB, or ESRRG alone did not improve insulin secretion or glucose responsiveness of psBCs. This is likely due to insufficient β-cell specification in the absence of trans-supplementation of NEUROG3. It is noteworthy that endogenous NEUROD1, NKX2.2, NKX6.1, and ESRRG were all highly upregulated upon PNM transduction, suggesting limited additive β-cell maturation effects by additional NEUROD1, NKX2.2, NKX6.1, and ESRRG transduction in PNM-modified psBCs.
We acknowledge that a yield of 12% insulin-positive cells upon PNM transduction is relatively low when compared with previous studies mainly using embryonic stem cells (Kroon et al., 2008, Pagliuca et al., 2014, Rezania et al., 2014). This is largely due to the high clonal and intrapatient variations in iPSC differentiation into insulin-producing cells, as we and others have described (Tateishi et al., 2008, Maehr et al., 2009, Thatava et al., 2013). It is also notable that introduction of NEUROG3 alone strongly enhanced induction of insulin gene; however, derived cells failed to show glucose responsiveness (Figures 2B and 2C). Very recently we found a small molecule that strongly enhanced iPSC differentiation into insulin-positive β cells (J.M.T. et al., unpublished data [manuscript in preparation]). With this small molecule, we were able to make up to 20% insulin-positive cells from iPSC clone BM9 without PNM introduction. However, these cells did not show glucose responsiveness by the perifusion system. These observations suggest that generation of insulin-producing cells and induction of glucose responsiveness in insulin-positive cells are regulated by independent mechanisms. With our stepwise PNM transduction strategy, we clearly demonstrated that PNM addition improved the differentiation efficiency and, more importantly, improved the quality of derived β cells, which marks considerable progress compared with previous studies. It is also noteworthy that our data provide an important implication for future translational applications. Improved PNM expression by clinically relevant strategies, such as RNA transfection or addition of small molecules or cytokines to enhance expression of endogenous PNM factors, together with PSC lines with high β-cell differentiation propensity, would allow more mature β-cell generation from PSCs for transplantation.
After transplanting the PNM-transduced psBCs into immunodeficient diabetic mice, we were able to detect glucose-responsive human C-peptide secretion as early as 1 week after surgery, indicating the functionality of psBCs in vivo. However, the levels of C-peptide secretion were low and we found no effects on glucose handling or fasting blood glucose levels until 9 weeks post transplantation, possibly due to slower vascularization (Mattsson et al., 2002) and adaptation of the transplanted cells. Nevertheless, PNM-transduced cells exhibited better diabetes-reversal effects, better glucose-clearance ability, and richer expression of insulin in excised grafts, indicating a therapeutic effect superior to that of S6-NULL psBCs. Unexpectedly, we did not see obvious GSIS by 30-min IPGTT tests in S6-PNM psBCs. We speculate that sustained NEUROG3 expression in PNM-transduced psBCs had negative impacts on long-term psBC function. Further analysis using an inducible NEUROG3-expression vector will elucidate the influence of NEUROG3 expression on sustained psBC functionality in vivo.
In summary, our study demonstrates successful generation of glucose- and GLP-1-responsive psBCs in vitro using a streamlined multifactor approach to ensure pre-emptive differentiation and lasting organotypic orientation after transplantation. While lineage-specification protocols have been implemented in regenerative clinical development strategies within and beyond (Terzic and Behfar, 2016) endocrinology, adoption of a reproducible and scalable platform for derivation of competent and proficient biologics would further accelerate diagnostic and therapeutic applications.
Experimental Procedures
Cells
Commercially available iPSC lines IISH2i-BM9 (WiCell, Madison, WI), and in-house iPSC lines #A, #B, #C, #D, and #E were tested for β-cell propensity.
Lentiviral Vectors
Lentiviral vector genome plasmid, pSIN-CSGW-PKG-puro, which supports EGFP expression and puromycin selection, was kindly provided by Dr. Paul Lehner (Cambridge Institute for Medical Research). Codon-optimized ORF sequences for β-cell factors, including PDX1, NEUROG3, NKX2.2, NKX6.1, NEUROD1, MAFA, MAFB, and ESRRG, were designed and synthesized (GenScript, Piscataway, NJ), and cloned into the place of the EGFP gene in pSIN-CSGW-PKG-puro with the unique BamHI and XhoI sites.
Guided Differentiation and Stepwise Lentiviral Vector Transduction
The basic differentiation strategy without viral vector transduction was a modified version of the previously published protocols. The detailed differentiation protocol is available in Supplemental Information.
qRT-PCR
For qRT-PCR, total RNA from differentiated iPSCs at indicated time points was isolated using TRIzol according to the manufacturer’s instructions. cDNAs were then synthesized by reverse transcription from 200 ng of total RNA. The sequences of the primers used for qRT-PCR are listed in Table S1.
RNA Sequencing
For RNA sequencing, a total of 200 ng of RNA from differentiated iPSCs at indicated time points was isolated using an RNeasy Mini Kit. Library preparation (TruSeq mRNA v2 [TMRNA]) and next-generation sequencing and analysis (standard secondary analysis pipeline, MAPRSeq) were performed in the Mayo Clinic Sequencing and Bioinformatics Cores. GEO: GSE133731.
Immunofluorescence Staining
Lentiviral vector-infected 293T cells, undifferentiated iPSCs, and cryosections of mouse kidneys with the grafts were fixed with 4% paraformaldehyde (PFA) for 20 min. After fixation, cells were washed once with PBS and permeabilized with 0.3% Triton X-100 in PBS for 10 min. Cells were then washed with PBS twice and blocked with 5% fetal bovine serum (FBS) in PBS for 1 h. Cells were incubated overnight with specific antibodies at 4°C, followed by incubation with secondary antibodies for 1 h at room temperature.
Flow Cytometry
iPSC-derived psBCs were dispersed into single-cell suspension. Cells were incubated overnight with specific antibodies at 4°C. After washing three times with 5% FBS, cells were stained with secondary antibodies. Cells were then washed and filtered through a 35-μm mesh Falcon tube, and analyzed using the LSR-II flow cytometer (BD Biosciences). Analysis of the results was performed using FlowJo software.
Electron Microscopy
iPSC-derived psBCs were fixed at room temperature. Cell samples were processed and analyzed by transmission electron microscopy at the Mayo Clinic Microscopy and Cell Analysis Core.
In Vitro GSIS Assay
We used an islet perifusion system (Biorep Technologies, Miami Lakes, FL). Detailed methods are available in Supplemental Information.
Mice Transplantation Studies
All animal experiments were performed in accordance with Mayo Clinic International Animal Care and Use Committee regulations. Immunodeficient Fox Chase SCID-Beige mice, aged 8–10 weeks, were purchased from Charles River Laboratory. Detailed methods are available in Supplemental Information.
Intraperitoneal Glucose Tolerance Test
For measurement of glucose-handling capacity in vivo, mice were fasted (16 h) and blood glucose was tested at 0, 30, 60, 90, and 120 min after intraperitoneal injection of D-(+)-glucose at 2 g/kg body weight.
Sample Size and Statistical Analysis
All data represent the means ± SEM of three to nine samples, as indicated in the figure legends. Group comparisons were analyzed by unpaired or paired t tests, one-sample t test, and one-way ANOVA with Tukey’s test using IBM SPSS Statistics v.22. Bar graphs, heatmaps, curves, and box-and-whisker plots were generated with GraphPad Prism7 and Excel 2010. Detailed methods are available in Supplemental Information.
Data Availability
All relevant data are available from the corresponding author on request.
Author Contributions
Y.Z. carried out all the experiments, analyzed the data, and prepared the manuscript. J.M.T. contributed to lentiviral production, animal experiments, and results interpretation. C.A.S. provided help in cell culture and data analysis. Q.L. provided advice on the manuscript and contributed to figure preparation. Z.Z. commented on the manuscript. K.R. and A.V.M. assisted with the perifusion experiments and provided generous advice on results interpretation. A.T., D.W., and Y.C.K. discussed the results and commented on the manuscript. Y.I. supervised the project, oversaw the preparation of the manuscript, commented on and revised the manuscript, and provided funding for all related work. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank Brian Lu and Salma Morsy for their advice on experimental techniques. This work is supported by Sheikh Khalifa Bin Zayed Al Nahyen Regenerative Diabetes Research Program, Mayo Center for Regenerative Medicine, Regenerative Medicine Minnesota, Vann Family Fund in Diabetes Research, Kendrick Foundation, Paul A. and Ruth M. Schilling Medical Research Endowment Fund, and NIH 1R43GM112316-01A.
Published: August 1, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.07.006.
Supplemental Information
References
- Aguayo-Mazzucato C., Koh A., El Khattabi I., Li W.C., Toschi E., Jermendy A., Juhl K., Mao K., Weir G.C., Sharma A. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia. 2011;54:583–593. doi: 10.1007/s00125-010-2026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C., Zavacki A.M., Marinelarena A., Hollister-Lock J., El Khattabi I., Marsili A., Weir G.C., Sharma A., Larsen P.R., Bonner-Weir S. Thyroid hormone promotes postnatal rat pancreatic beta-cell development and glucose-responsive insulin secretion through MAFA. Diabetes. 2013;62:1569–1580. doi: 10.2337/db12-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artner I., Hang Y., Mazur M., Yamamoto T., Guo M., Lindner J., Magnuson M.A., Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko A.P., Polak M., Cave H., Busiah K., Czernichow P., Scharfmann R., Bryan J., Aguilar-Bryan L., Vaxillaire M., Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N. Engl. J. Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- Basford C.L., Prentice K.J., Hardy A.B., Sarangi F., Micallef S.J., Li X., Guo Q., Elefanty A.G., Stanley E.G., Keller G. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012;55:358–371. doi: 10.1007/s00125-011-2335-x. [DOI] [PubMed] [Google Scholar]
- Bentsi-Barnes K., Doyle M.E., Abad D., Kandeel F., Al-Abdullah I. Detailed protocol for evaluation of dynamic perifusion of human islets to assess beta-cell function. Islets. 2011;3:284–290. doi: 10.4161/isl.3.5.15938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B., Hrvatin S.S., Schuetz C., Bonal C., Rezania A., Melton D.A. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat. Biotechnol. 2012;30:261–264. doi: 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M., Shiota M., Nicholson W.E., Gannon M., Knobel S.M., Piston D.W., Wright C.V., Powers A.C. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J. Biol. Chem. 2002;277:11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- Bruin J.E., Asadi A., Fox J.K., Erener S., Rezania A., Kieffer T.J. Accelerated maturation of human stem cell-derived pancreatic progenitor cells into insulin-secreting cells in immunodeficient rats relative to mice. Stem Cell Reports. 2015;5:1081–1096. doi: 10.1016/j.stemcr.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin J.E., Saber N., Braun N., Fox J.K., Mojibian M., Asadi A., Drohan C., O'Dwyer S., Rosman-Balzer D.S., Swiss V.A. Treating diet-induced diabetes and obesity with human embryonic stem cell-derived pancreatic progenitor cells and antidiabetic drugs. Stem Cell Reports. 2015;4:605–620. doi: 10.1016/j.stemcr.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Cogger K.F., Sinha A., Sarangi F., McGaugh E.C., Saunders D., Dorrell C., Mejia-Guerrero S., Aghazadeh Y., Rourke J.L., Screaton R.A. Glycoprotein 2 is a specific cell surface marker of human pancreatic progenitors. Nat. Commun. 2017;8:331. doi: 10.1038/s41467-017-00561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P., Mansouri A., Hecksher-Sorensen J., Serup P., Krull J., Gradwohl G., Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry D.L., MacLachlan S.A. Synthesis-secretion coupling of insulin: effect of aging. Endocrinology. 1987;121:241–247. doi: 10.1210/endo-121-1-241. [DOI] [PubMed] [Google Scholar]
- D'Amour K.A., Bang A.G., Eliazer S., Kelly O.G., Agulnick A.D., Smart N.G., Moorman M.A., Kroon E., Carpenter M.K., Baetge E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Deconinck J.F., Van Assche F.A., Potvliege P.R., Gepts W. The ultrastructure of the human pancreatic islets. II. The islets of neonates. Diabetologia. 1972;8:326–333. doi: 10.1007/BF01218493. [DOI] [PubMed] [Google Scholar]
- Dhawan S., Tschen S.I., Zeng C., Guo T., Hebrok M., Matveyenko A., Bhushan A. DNA methylation directs functional maturation of pancreatic beta cells. J. Clin. Invest. 2015;125:2851–2860. doi: 10.1172/JCI79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier B.R., Wiederkehr A., Baquie M., Dai C., Powers A.C., Kerr-Conte J., Pattou F., MacDonald R.J., Ferrer J., Wollheim C.B. PDX1 deficiency causes mitochondrial dysfunction and defective insulin secretion through TFAM suppression. Cell Metab. 2009;10:110–118. doi: 10.1016/j.cmet.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier B.R., Wollheim C.B. Synaptotagmins bind calcium to release insulin. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1279–E1286. doi: 10.1152/ajpendo.90568.2008. [DOI] [PubMed] [Google Scholar]
- Gerich J.E. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes. 2002;51(Suppl 1):S117–S121. doi: 10.2337/diabetes.51.2007.s117. [DOI] [PubMed] [Google Scholar]
- Gloyn A.L., Pearson E.R., Antcliff J.F., Proks P., Bruining G.J., Slingerland A.S., Howard N., Srinivasan S., Silva J.M., Molnes J. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- Gradwohl G., Dierich A., LeMeur M., Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holditch S.J., Terzic A., Ikeda Y. Concise review: pluripotent stem cell-based regenerative applications for failing beta-cell function. Stem Cells Transl. Med. 2014;3:653–661. doi: 10.5966/sctm.2013-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland A.M., Hale M.A., Kagami H., Hammer R.E., MacDonald R.J. Experimental control of pancreatic development and maintenance. Proc. Natl. Acad. Sci. U S A. 2002;99:12236–12241. doi: 10.1073/pnas.192255099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iype T., Francis J., Garmey J.C., Schisler J.C., Nesher R., Weir G.C., Becker T.C., Newgard C.B., Griffen S.C., Mirmira R.G. Mechanism of insulin gene regulation by the pancreatic transcription factor Pdx-1: application of pre-mRNA analysis and chromatin immunoprecipitation to assess formation of functional transcriptional complexes. J. Biol. Chem. 2005;280:16798–16807. doi: 10.1074/jbc.M414381200. [DOI] [PubMed] [Google Scholar]
- Jermendy A., Toschi E., Aye T., Koh A., Aguayo-Mazzucato C., Sharma A., Weir G.C., Sgroi D., Bonner-Weir S. Rat neonatal beta cells lack the specialised metabolic phenotype of mature beta cells. Diabetologia. 2011;54:594–604. doi: 10.1007/s00125-010-2036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Egan J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems L.L., Holst J.J., Volund A., Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52:380–386. doi: 10.2337/diabetes.52.2.380. [DOI] [PubMed] [Google Scholar]
- Kroon E., Martinson L.A., Kadoya K., Bang A.G., Kelly O.G., Eliazer S., Young H., Richardson M., Smart N.G., Cunningham J. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Maehr R., Chen S., Snitow M., Ludwig T., Yagasaki L., Goland R., Leibel R.L., Melton D.A. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl. Acad. Sci. U S A. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschinsky F.M., Magnuson M.A., Zelent D., Jetton T.L., Doliba N., Han Y., Taub R., Grimsby J. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes. 2006;55:1–12. [PubMed] [Google Scholar]
- Matsuoka T.A., Artner I., Henderson E., Means A., Sander M., Stein R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc. Natl. Acad. Sci. U S A. 2004;101:2930–2933. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T.A., Zhao L., Artner I., Jarrett H.W., Friedman D., Means A., Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol. Cell. Biol. 2003;23:6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson G., Jansson L., Carlsson P.O. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51:1362–1366. doi: 10.2337/diabetes.51.5.1362. [DOI] [PubMed] [Google Scholar]
- McGrath P.S., Watson C.L., Ingram C., Helmrath M.A., Wells J.M. The basic helix-loop-helix transcription factor NEUROG3 is required for development of the human endocrine pancreas. Diabetes. 2015;64:2497–2505. doi: 10.2337/db14-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkman-Zehavi T., Oren R., Kredo-Russo S., Shapira T., Mandelbaum A.D., Rivkin N., Nir T., Lennox K.A., Behlke M.A., Dor Y. miRNAs control insulin content in pancreatic beta-cells via downregulation of transcriptional repressors. EMBO J. 2011;30:835–845. doi: 10.1038/emboj.2010.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki R., Yoshida T., Murata K., Oki S., Kume K., Kume S. Fate maps of ventral and dorsal pancreatic progenitor cells in early somite stage mouse embryos. Mech. Dev. 2012;128:597–609. doi: 10.1016/j.mod.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Nostro M.C., Sarangi F., Ogawa S., Holtzinger A., Corneo B., Li X., Micallef S.J., Park I.H., Basford C., Wheeler M.B. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Olson T.M., Terzic A. Human K(ATP) channelopathies: diseases of metabolic homeostasis. Pflugers Arch. 2010;460:295–306. doi: 10.1007/s00424-009-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachera N., Papin J., Zummo F.P., Rahier J., Mast J., Meyerovich K., Cardozo A.K., Herchuelz A. Heterozygous inactivation of plasma membrane Ca(2+)-ATPase in mice increases glucose-induced insulin release and beta cell proliferation, mass and viability. Diabetologia. 2015;58:2843–2850. doi: 10.1007/s00125-015-3745-y. [DOI] [PubMed] [Google Scholar]
- Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M.A., Halter J.B., Porte D., Jr. Insulin secretion in diabetes mellitus. Am. J. Med. 1981;70:579–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- Reinbothe T.M., Alkayyali S., Ahlqvist E., Tuomi T., Isomaa B., Lyssenko V., Renstrom E. The human L-type calcium channel Cav1.3 regulates insulin release and polymorphisms in CACNA1D associate with type 2 diabetes. Diabetologia. 2013;56:340–349. doi: 10.1007/s00125-012-2758-z. [DOI] [PubMed] [Google Scholar]
- Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A., O'Dwyer S., Quiskamp N., Mojibian M., Albrecht T. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- Rezania A., Bruin J.E., Riedel M.J., Mojibian M., Asadi A., Xu J., Gauvin R., Narayan K., Karanu F., O'Neil J.J. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A., Bruin J.E., Xu J., Narayan K., Fox J.K., O'Neil J.J., Kieffer T.J. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31:2432–2442. doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- Russ H.A., Parent A.V., Ringler J.J., Hennings T.G., Nair G.G., Shveygert M., Guo T., Puri S., Haataja L., Cirulli V. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34:1759–1772. doi: 10.15252/embj.201591058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M., Sussel L., Conners J., Scheel D., Kalamaras J., Dela Cruz F., Schwitzgebel V., Hayes-Jordan A., German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- Saxena P., Heng B.C., Bai P., Folcher M., Zulewski H., Fussenegger M. A programmable synthetic lineage-control network that differentiates human IPSCs into glucose-sensitive insulin-secreting beta-like cells. Nat. Commun. 2016;7:11247. doi: 10.1038/ncomms11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.H., Kjems L., Ritzel R., McIntyre S.M., Johnson M.L., Veldhuis J.D., Butler P.C. Pulsatile insulin secretion by human pancreatic islets. J. Clin. Endocrinol. Metab. 2002;87:213–221. doi: 10.1210/jcem.87.1.8181. [DOI] [PubMed] [Google Scholar]
- Song S.H., McIntyre S.S., Shah H., Veldhuis J.D., Hayes P.C., Butler P.C. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J. Clin. Endocrinol. Metab. 2000;85:4491–4499. doi: 10.1210/jcem.85.12.7043. [DOI] [PubMed] [Google Scholar]
- Sussel L., Kalamaras J., Hartigan-O'Connor D.J., Meneses J.J., Pedersen R.A., Rubenstein J.L., German M.S. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hatakeyama H., Okado H., Noguchi J., Ohno M., Kasai H. SNARE conformational changes that prepare vesicles for exocytosis. Cell Metab. 2010;12:19–29. doi: 10.1016/j.cmet.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Tateishi K., He J., Taranova O., Liang G., D'Alessio A.C., Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J. Biol. Chem. 2008;283:31601–31607. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- Terzic A., Behfar A. Stem cell therapy for heart failure: Ensuring regenerative proficiency. Trends Cardiovasc. Med. 2016;26:395–404. doi: 10.1016/j.tcm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatava T., Kudva Y.C., Edukulla R., Squillace K., De Lamo J.G., Khan Y.K., Sakuma T., Ohmine S., Terzic A., Ikeda Y. Intrapatient variations in type 1 diabetes-specific iPS cell differentiation into insulin-producing cells. Mol. Ther. 2013;21:228–239. doi: 10.1038/mt.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2016. Global Report on Diabetes.https://www.who.int/diabetes/global-report/en/ [Google Scholar]
- Wijesekara N., Dai F.F., Hardy A.B., Giglou P.R., Bhattacharjee A., Koshkin V., Chimienti F., Gaisano H.Y., Rutter G.A., Wheeler M.B. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53:1656–1668. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan-Do R., Duong E., Manning Fox J.E., Dai X., Suzuki K., Khan S., Bautista A., Ferdaoussi M., Lyon J., Wu X. A glycine-insulin autocrine feedback loop enhances insulin secretion from human beta-cells and is impaired in type 2 diabetes. Diabetes. 2016;65:2311–2321. doi: 10.2337/db15-1272. [DOI] [PubMed] [Google Scholar]
- Yang S.Y., Lee J.J., Lee J.H., Lee K., Oh S.H., Lim Y.M., Lee M.S., Lee K.J. Secretagogin affects insulin secretion in pancreatic beta-cells by regulating actin dynamics and focal adhesion. Biochem. J. 2016;473:1791–1803. doi: 10.1042/BCJ20160137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara E., Wei Z., Lin C.S., Fang S., Ahmadian M., Kida Y., Tseng T., Dai Y., Yu R.T., Liddle C. ERRgamma is required for the metabolic maturation of therapeutically functional glucose-responsive beta cells. Cell Metab. 2016;23:622–634. doi: 10.1016/j.cmet.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Liu Q., Zhou Z., Ikeda Y. PDX1, NEUROGENIN-3 and MAFA: critical transcription regulators for beta cell development and regeneration. Stem Cell Res. Ther. 2017;8:240. doi: 10.1186/s13287-017-0694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available from the corresponding author on request.