Abstract
Chronic spontaneous urticaria (CSU) can occur in children and the clinical management is quite challenging. Here, we described a paediatric clinical case of CSU successfully managed by conventional therapy, including systemic steroids, cetirizine, anti-leukotriene drug and dietary restriction (for histamine-releasing foods). This patient showed neither atopy nor any allergic sensitisation; moreover, the autologous serum skin test resulted negative. This category of patients with no comorbidity and no evidence of atopy might benefit from the conventional drug management; however, a protracted course of steroid treatment with very slow and gradual tapering may be needed. This approach was successful and safe in our clinical case, but a careful follow-up, due to the potential side effects of steroids, should be recommended.
Keywords: paediatrics (drugs and medicines), medical management, dermatology
Background
Urticaria (or hives) is characterised with the appearance of erythaematous, itchy and raised skin lesions with variable size and shape. The single lesions are transient and usually disappear within 1–24 hours. However, urticarial rashes can have a relapsing course: chronic urticaria (CU) is defined by the recurrence of hives for more than 6 weeks, through a daily or almost daily exhibition of symptoms.1
Whereas acute urticaria is a common condition in children (up to 25% of the general paediatric population experience at least one episode during childhood), actually CU is observed much less frequently: indeed, a prevalence <1% has been estimated in children.2 3
The main pathophysiological event in urticaria is the release of histamine (and other inflammatory mediators) following the activation and degranulation of basophils and/or mast cells; however, the triggering factors and/or pathogenic mechanisms of CU can be variable among individuals (eg allergy, infections, physical factors, etc). Sometimes, CU cannot be traced to any specific environmental triggers and this occurrence is currently reported as chronic spontaneous urticaria (CSU).4
The association between CSU and some autoimmune disorders has been repeatedly described during the last few decades. Some authors identified the concomitant production of anti-IgE or anti-FcεRI antibodies in 30%–50% of patients with CSU, who showed a positive autologous serum skin test (ASST). This finding further supported the indication to use omalizumab in patients with CSU unresponsive to the conventional therapy, in addition to paediatric asthma.4 5
Here, we described the successful management of an adolescent developing CSU without any evident environmental trigger and comorbidity, through drug conventional therapy and dietary modifications.
Case presentation
A 14-year Caucasian boy (weight=47 kg, height=161 cm) required specialist medical attention because of the onset of several episodes of urticarial rashes for 2 months (figure 1), despite the therapy with anti-histamine drugs (eg, cetirizine 10 mg/day) and short courses of steroid therapy (prednisone 0.5 mg/kg for 5–7 days, including dose tapering). Therefore, he underwent a careful infectious and immunologic work-up before starting further therapy.
Figure 1.
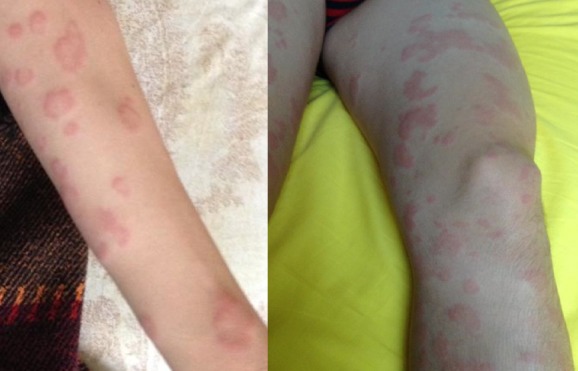
Severe and diffuse urticarial rash on arms and legs.
The personal medical history was unremarkable: no previous hospital admissions were reported and the physical development (including pubertal stage: G3, PH3, AH2, gonadal volume: 8 mL bilaterally) was regular. He was born at term by vaginal delivery after an uncomplicated pregnancy. Finally, in the family history, maternal autoimmune thyroiditis was the only relevant disease.
Importantly, no recent infections and/or febrile illnesses were reported. Moreover, the patient had no previous or known diagnosis of allergy. Therefore, based on a careful collection of the medical history, no infections or dietary, allergic, pharmacological and physical agents could be evidenced as potential trigger(s) of CU.
Investigations
The complete cell blood count never showed any significant abnormality, as well as the biochemistry (liver and renal function test, electrolytes, lactate dehydrogenase, creatine kinase and so on) and the inflammatory parameters (C-reactive protein, erythrocytes sedimentation rate). In particular, the eosinophils count was always below 300/mm3.
As regards the infectious work-up, cytomegalovirus, Epstein-Barr virus, Mycoplasma pneumoniae and parvovirus B19 serology showed no evidence of recent or concomitant infections. The anti-streptolysin-O titre resulted to be mildly elevated (266 IU/mL, n.v. 20–250 IU/mL), but no progressive increase was noticed and, importantly, the pharyngeal swab resulted negative for group A Streptococcus pyogenes, after microbiological culture. Finally, despite the absence of any gastrointestinal complaints, the patient was investigated for stool parasites and for Helicobacter pylori antigen. All resulted all negative.
As regards the immunological aspects, no antibody deficiency (IgM, IgG and IgA) emerged and the negativity for IgA antibodies to tissue transglutaminase (anti-tTG IgA) allowed to rule out celiac disease as well. Importantly, total serum IgE levels were negligible (4 IU/mL) and no food and environmental allergen sensitisation was detected by skin prick tests and/or specific serum IgE. Interestingly, anti-nucleus antibody (ANA) resulted to be positive at low titre (1:160), but anti-dsDNA and antibodies to extractable nuclear antigens (ENA) were all negative. Moreover, no complement deficit was detected (C3=115 mg/dL, n.v. 90–180; C4=19 mg/dL, n.v. 10–40; C1q Inhibitor=170 mg/L, n.v. 100–250).
Considering the positive family history, the thyroid function was investigated: fT4=0.80 ng/dL (v.n. 0.6–1.2), TSH=3.797 mUI/L (n.v. 0.340–4.5). Despite these normal values, the patient showed positive anti-peroxidase antibody (33.1 UI/mL, n.v. <9), whereas anti-thyroglobulin antibody was negative. However, the cervical ultrasound did not show any signs of thyroiditis or other gland abnormalities (eg, nodules).
Finally, the patient also underwent the ASST: 50 µL of undiluted and sterile autologous serum was injected intra-dermally along with positive (histamine) and negative (saline) controls. No skin reaction was elicited around the site of serum injection: therefore, the result was negative and no further in vitro tests to detect anti-IgE or anti-FcεRI (high affinity IgE receptor) antibody were requested. Importantly, the skin biopsy was considered, but the patient declined this procedure before beginning the treatment described later.
Differential diagnosis
The differential diagnosis of CSU, as regards the aetiological aspect, is quite wide and challenging. Indeed, based on the anamnestic and physical findings, several investigations should be performed in order to reasonably exclude infectious, allergic, neoplastic and physical factors. As previously mentioned, 30%–50% of CSU patients can produce anti-IgE or anti-FcεRI antibodies and this finding resulted to be variably associated with concomitant autoimmune diseases, which should be included in the differential diagnosis. Regardless of their presence, some rheumatic diseases may manifest with urticaria-like skin manifestations, characterised with severe pruritus as a prominent feature. Skin biopsy is usually an important diagnostic tool, in order to rule out urticarial vasculitis.4 6–8 In addition, even celiac disease was excluded: it is quite prevalent in the general population, especially children and may manifest with extra-intestinal manifestations only (including variable and recurrent skin diseases).9 10
Treatment
The patient needed a protracted systemic steroid treatment. Indeed, in addition to cetirizine (10 mg/day, corresponding 0.2 mg/kg/day) and montelukast (5 mg/day, corresponding to 0.1 mg/kg/day), he initially received prednisone (1 mg/kg/day) for 7–10 days; then, the dose was tapered very slowly over 6 weeks, due to the previous relapsing clinical course. Indeed, before starting this therapy (namely, during the previous 2–3 months), the patient developed several episodes of diffuse urticaria, which required hospital admission and intravenous therapy (with methyl-prednisolone and chlorphenamine) two times. An additional and important recommendation was the avoidance of histamine-releasing foods. After withdrawal of the steroid therapy, both anti-histamine and anti-leukotriene therapies were maintained: montelukast was stopped after 6 weeks and cetirizine was continued until 4 months after the last urticarial attack. The dietary regimen free of histamine-releasing foods (in particular: cheese, raw and grilled sausages, ham, dried and grilled fish, preserved foods and so on) was extended for several months more and those aliments were reintroduced gradually one by one.
Outcome and follow-up
After 8 months, the patient returned to all his normal lifestyle with no drug therapy. Importantly, he has never relapsed over a follow-up period of more than 3 years. Importantly, no adverse events have been reported and, in particular, no symptoms or signs of osteoporosis and ocular disease occurred during the follow-up. However, the potential side effects of prolonged use of steroids must be carefully considered when this therapy is prescribed.
Discussion
The diagnosis of CSU in children is less common than in adults.4 In 2000, Greaves et al reported three paediatric cases.11 In 2004, Brunetti et al described 93 children with CU: 47% cases were traced to a specific cause, such as physical urticaria (20%), infectious urticaria (18%), allergic urticaria (3%) or other clear triggering factors (5%). Therefore, the remaining patients were diagnosed with ‘idiopathic’ CU, namely CSU. Half of them, corresponding to 30% of the whole cohort, showed a positive ASST.12
In 2006, the study by Du Toit et al from South Africa, including 80 children with CU, suggested that ASST could not be considered a reliable surrogate of in vitro studies to investigate the presence of anti-IgE or anti-FcεRI antibody. However, the real clinical significance of these autoantibodies has not been fully elucidated, yet.13 Indeed, both IgE-specific and FcεRI-specific autoantibodies have been described in several and variable clinical settings (eg, atopic dermatitis, asthma and so on), and even in healthy individuals.14 Moreover, according to the study published in 2011 by Sahiner et al from Turkey, who investigated 100 children affected with CSU through a wide panel of laboratory investigations (including ASST in 45 patients, whose 46% showed a positive reaction), no prognostic difference was demonstrated, according to the ASST results.15 Chansakulporn et al described the natural history of paediatric CSU in a perspective study including 92 children: >50% and almost 70% of cases achieved the complete remission of symptoms within 3 and 5 years, respectively. Hence, paediatric CSU seems to have a favourable course in general and, importantly, ASST positive patients showed no prognostic difference.16
According to the European Medicinal Agency recommendations, unresponsive CSU can be eligible to the treatment with omalizumab as add-on therapy in children older than 12 years.4 However, in children with poor evidence of IgE-mediated disease, as in the present case, this biological therapy should be probably considered only after the failure of all conventional drugs, including anti-histamines, anti-leukotrienes and appropriate course of systemic steroids. Conversely, uncontrolled CSU patients should be considered for the biological therapy, considering the potential modulatory effect that omalizumab plays on basophils and mast cells homeostasis, regardless of the presence of anti-IgE and/or FcεRI antibody.17 18
In our case, we achieved a successful result without omalizumab, but we had to implement a protracted steroid therapy for 6 weeks, with very low and gradual dose tapering until the complete withdrawal. Of course, this is not the usual therapeutic approach in this clinical setting, according to the updated guidelines for CU,19 which was published after our experience with this patient.
A protracted (and closely monitored) systemic steroid therapy, as described above, might be an option in those patients who do not respond to non-steroidal therapies only or need repeated short courses of systemic steroids to control disease flares, especially if they do not tolerate increased doses of anti-histamines. Moreover, as already mentioned, omalizumab is usually considered in clinical cases whereby there are convincing evidences that the disease is IgE-mediated, but our patient had no increase of total or specific serum IgE.
In conclusion, we reported an example of successful management of paediatric CSU with conventional (not biological) therapy, after a careful work-up that excluded any significant comorbidity and IgE sensitisation/excessive production. Moreover, in presence of a relapsing course with frequent flares that require repeated short cycles of systemic steroids, a very gradual and slow tapering of the therapy may contribute to achieve the individual tolerance with prolonged remission of symptoms. However, such an approach needs a close follow-up, in order to detect potential side effects.
Learning points.
A protracted steroid therapy with gradual dose tapering can be considered in chronic spontaneous urticaria (CSU) patients unresponsive to first-line therapies and frequent flares.
Autologous serum skin test-negative paediatric CSU may be successfully treated with conventional drug therapy.
The systemic steroid therapy should be considered only in selected cases, due to potential side effects.
Footnotes
Contributors: DP managed the patient. DP conceived and wrote the article. AL and IB helped in writing the article. GLM provided intellectual contribution.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Zuberbier T, Asero R, Bindslev-Jensen C, et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy 2009;64:1417–26. 10.1111/j.1398-9995.2009.02179.x [DOI] [PubMed] [Google Scholar]
- 2. Sánchez-Borges M, Asero R, Ansotegui IJ, et al. Diagnosis and treatment of urticaria and angioedema: a worldwide perspective. World Allergy Organ J 2012;5:125–47. 10.1097/WOX.0b013e3182758d6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsakok T, Du Toit G, Flohr C. Pediatric urticaria. Immunol Allergy Clin North Am 2014;34:117–39. 10.1016/j.iac.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 4. Poddighe D, De Amici M, Marseglia GL. Spontaneous (Autoimmune) chronic urticaria in children: current evidences, diagnostic pitfalls and therapeutic management. Recent Pat Inflamm Allergy Drug Discov 2016;10:34–9. 10.2174/1872213X10666160219163502 [DOI] [PubMed] [Google Scholar]
- 5. Poddighe D, Brambilla I, Licari A, et al. Omalizumab in the therapy of pediatric asthma. Recent Pat Inflamm Allergy Drug Discov 2018;12:103–9. 10.2174/1872213X12666180430161351 [DOI] [PubMed] [Google Scholar]
- 6. Kolkhir P, Borzova E, Grattan C, et al. Autoimmune comorbidity in chronic spontaneous urticaria: a systematic review. Autoimmun Rev 2017;16:1196–208. 10.1016/j.autrev.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 7. Poddighe D, Cavagna L, Brazzelli V, et al. A hyper-ferritinemia syndrome evolving in recurrent macrophage activation syndrome, as an onset of amyopathic juvenile dermatomyositis: a challenging clinical case in light of the current diagnostic criteria. Autoimmun Rev 2014;13:1142–8. 10.1016/j.autrev.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 8. Kolkhir P, Pogorelov D, Olisova O, et al. Comorbidity and pathogenic links of chronic spontaneous urticaria and systemic lupus erythematosus--a systematic review. Clin Exp Allergy 2016;46:275–87. 10.1111/cea.12673 [DOI] [PubMed] [Google Scholar]
- 9. Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients 2018;10:800 10.3390/nu10070800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Silvestri A, Capittini C, Poddighe D, et al. HLA-DQ genetics in children with celiac disease: a meta-analysis suggesting a two-step genetic screening procedure starting with HLA-DQ β chains. Pediatr Res 2018;83:564–72. 10.1038/pr.2017.307 [DOI] [PubMed] [Google Scholar]
- 11. Greaves MW. Chronic urticaria in childhood. Allergy 2000;55:309–20. 10.1034/j.1398-9995.2000.00116.x [DOI] [PubMed] [Google Scholar]
- 12. Brunetti L, Francavilla R, Miniello VL, et al. High prevalence of autoimmune urticaria in children with chronic urticaria. J Allergy Clin Immunol 2004;114:922–7. 10.1016/j.jaci.2004.07.042 [DOI] [PubMed] [Google Scholar]
- 13. Du Toit G, Prescott R, Lawrence P, et al. Autoantibodies to the high-affinity IgE receptor in children with chronic urticaria. Ann Allergy Asthma Immunol 2006;96:341–4. 10.1016/S1081-1206(10)61245-8 [DOI] [PubMed] [Google Scholar]
- 14. Fiebiger E, Hammerschmid F, Stingl G, et al. Anti-FcepsilonRIalpha autoantibodies in autoimmune-mediated disorders. Identification of a structure-function relationship. J Clin Invest 1998;101:243–51. 10.1172/JCI511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sahiner UM, Civelek E, Tuncer A, et al. Chronic urticaria: etiology and natural course in children. Int Arch Allergy Immunol 2011;156:224–30. 10.1159/000322349 [DOI] [PubMed] [Google Scholar]
- 16. Chansakulporn S, Pongpreuksa S, Sangacharoenkit P, et al. The natural history of chronic urticaria in childhood: a prospective study. J Am Acad Dermatol 2014;71:663–8. 10.1016/j.jaad.2014.05.069 [DOI] [PubMed] [Google Scholar]
- 17. Antia C, Baquerizo K, Korman A, et al. Urticaria: a comprehensive review: treatment of chronic urticaria, special populations, and disease outcomes. J Am Acad Dermatol 2018;79:617–33. 10.1016/j.jaad.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 18. Saini SS, Kaplan AP. Chronic spontaneous urticaria: the Devil’s Itch. J Allergy Clin Immunol Pract 2018;6:1097–106. 10.1016/j.jaip.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dressler C, Rosumeck S, Werner RN, et al. Executive summary of the methods report for ‘The EAACI/GA2 LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. The 2017 Revision and Update’. Allergy 2018;73:1145–6. 10.1111/all.13414 [DOI] [PubMed] [Google Scholar]


