Abstract
Soft tissue sarcomas account for about 1% of malignant tumours. More than 50 subtypes of these tumours have been described with some being extremely rare, namely malignant peripheral nerve sheath tumours (MPNST). The authors present a case of a man aged 81 years with a medical history of an adenocarcinoma of the rectum, which was referred to our clinic due to a growing painless mass on the right arm. An MRI showed a well-delimited encapsulated mass involving the long head of the biceps muscle. Biopsy findings revealed a spindle cell tumour with cytonuclear pleomorphism. The patient underwent wide tumour excision and was successfully reconstructed with a latissimus dorsi functional muscle transfer. The presence of two pulmonary nodules on CT scan staging implied a lung biopsy that showed rectum primary tumour metastases. With these additional findings, the pathology department reassessed the case and reclassified the arm tumour as an MPNST, synchronous with pulmonary adenocarcinoma metastases of the rectum.
Keywords: plastic and reconstructive surgery, surgical oncology, radiotherapy
Background
Soft tissue sarcomas (STS) account for about 1% of malignant tumours. There is no age group predilection, occurring from adolescence to adulthood. Sixty per cent of STS cases arise in the limbs, with the distal femoral area around the knee joint being the most common affected region.1
More than 50 subtypes of STS have been described with some being extremely rare, namely malignant peripheral nerve sheath tumours (MPNST).2 3 These can be sporadic (40% of the cases), associated with neurofibromatosis type I (NF1) (50%) or radiation therapy (RT) induced (10%).4 A patient with NF1 has a risk 4600 times higher of having an MPNST, with a wide age range of 7–63 years.5
The present case has several particularities such as an extremely rare presentation, namely the presence of two synchronous tumours and the presence of an MPNST in a patient without NF, which was successfully reconstructed with a pedicled latissimus muscle transfer.
The message here is that the differentiation between STS is critical because the different subtypes have distinctive approach strategies and prognosis, and that every reader should think about the diagnosis of an MPNST even in patients without NF.
To the authors’ knowledge, no other case with such particularities has yet been published. The authors think that these data deserve to be shared among the medical community.
Case presentation
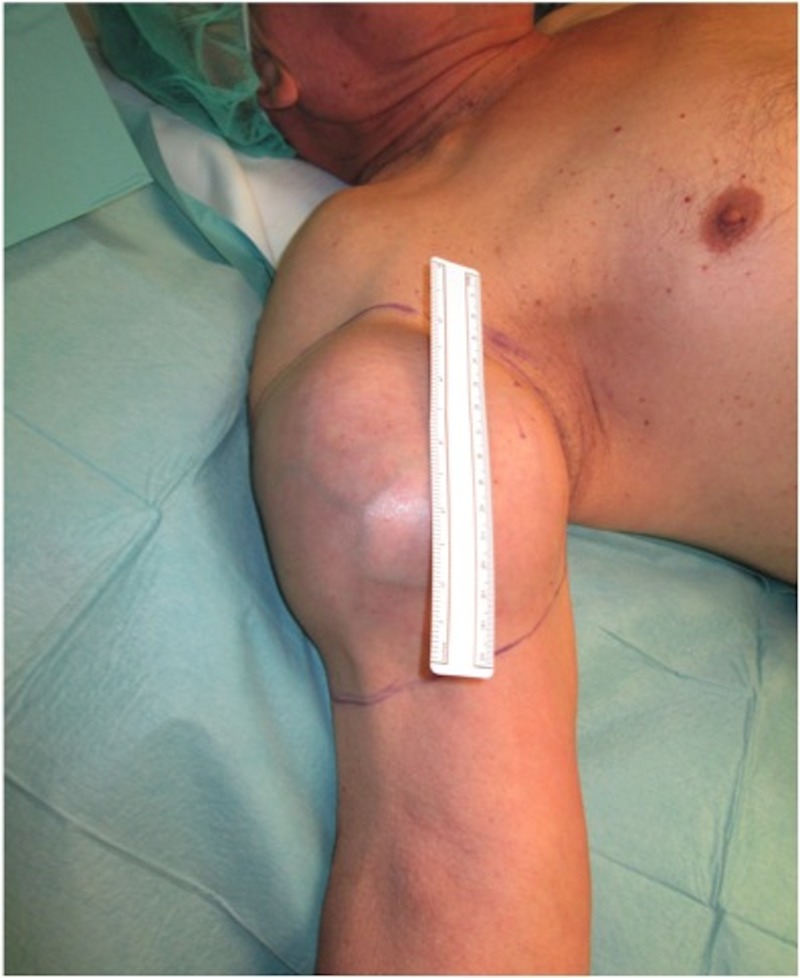
A man aged 81 years was referred to our clinic due to a 3-month history of an extensive, adherent, stiff, painless, progressive growing mass on the right arm (figure 1). He had a medical history of arterial hypertension, renal lithiasis and an adenocarcinoma of the rectum, diagnosed at the age of 79, treated with neoadjuvant therapy (RT and chemotherapy) and abdominoperineal amputation.
Figure 1.
Large mass of the anterior compartment of the right arm (110 mm of great diameter).
On physical examination, he presented a large mass on the anterior compartment of the right arm, with no skin alterations, namely retractions or ulcerations, no palpable lymphatic nodes and without sensory or motor deficits.
Investigations
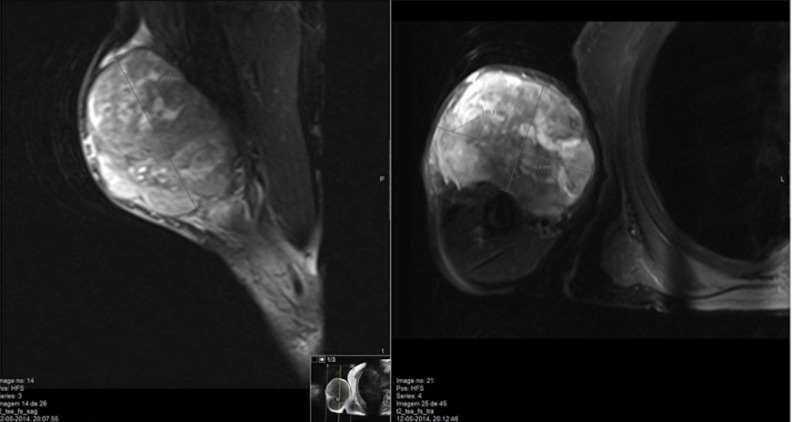
An MRI performed 2 months before the consultation revealed a well-delimited encapsulated mass with 80×85×60 mm, involving the long head of the biceps muscle and surrounding fat, with homogeneous iso-signal intensity on a T1-weighted image and heterogeneous high signal intensity on a T2-weighted image, suggesting a malignant lesion. No osseous or neurovascular invasion was present (figure 2); Doppler evaluation showed high vascularisation. Previously, cytological and histological studies revealed characteristic findings of a spindle cell tumour with citonuclear pleomorphism. Immunohistochemical analysis was inconclusive due to insufficient sample size. A staging brain, thoracic and abdominopelvic CT scan was performed giving the suspicion of a soft tissue sarcoma (STS), namely leiomyosarcoma or rhabdomyosarcoma. Two pulmonary nodules (16 and 19 mm) were found in distinct lobules (biopsy results were inconclusive), without lymph node or organ involvement. An MRI of the arm was repeated, which demonstrated an increasing size of the mass (110×111×77 mm), with the same cleavage planes.
Figure 2.
MRI showing a well-delimited encapsulated mass, with 110×111×77 mm, apparently within the biceps muscle and surrounding fat.
Treatment
One month after the initial consultation, the patient underwent wide tumour excision, with preservation of the brachial artery, cubital and median nerves, with sacrifice of biceps muscle, musculocutaneous nerve, cephalic and basilic veins. The extemporaneous evaluation showed negative margins for tumour, with the nearest surgical margin of 2 mm (deep/osseous margin). The resulting defect (150×130 mm; 195 mm2) was immediately reconstructed with a pedicled innervated (thoracodorsal nerve) latissimus dorsi muscle (LDMC) flap. The muscle was distally anchored to the remaining biceps tendon, with a non-resorbable suture, mimetising the biceps excursion, and then covered with split-thickness skin graft. The procedure was uneventful. Postoperative splinting of the upper right limb and functional early rehabilitation were performed.
The pathology findings were compatible with a spindle cell tumour with clear cells, with high mitotic index (>60 mitosis per 10 high-power fields) and an extensive area of necrosis, without involvement of the epidermis or muscular planes. Immunohistochemical analysis indicated positive staining for CAM 5.2, AE1/AE3, CD10 (cytokeratins) and vimentin (figure 3) and negative staining for CK7, CK20, CK19, HBM-45, Melan-A, S100, PAX-8, racemase, C-kit, MDM-2, desmine and CD34. Due to these findings, a CT scan was taken to clarify primary or metastatic origin of the arm malignant spindle cell tumour.
Figure 3.
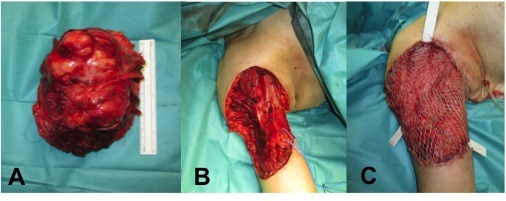
(A) Wide tumour resection; (B) resulting defect of 150×130 mm; 195 mm2; (C) functional muscle transfer reconstruction with latissimus dorsi muscle flap, covered with a split-thickness skin graft.
A CT-guided biopsy of the two mentioned pulmonary nodules was repeated. Histological examination revealed characteristics of a tubular adenocarcinoma of intestinal type, positive for immunostain CDX-2, compatible with metastasis of the rectum primary tumour.
After the additional clinical information and staging exams, the pathology department reassessed the case for tumour reclassification. In light of those results, and in the presence of positive staining for S100 protein (figure 4) and absence of Melan-A, HMB-45, podoplanin and GFAP expression, clear cell sarcoma and malignant peripheral nerve sheath tumour were the most likely diagnoses. To outline the final diagnosis, a cytogenetic study was performed. No EWSR1 (22q12) gene rearrangement was detected, which is present in about 90% of clear cell sarcomas, allowing the diagnosis of an MPNST.
Figure 4.
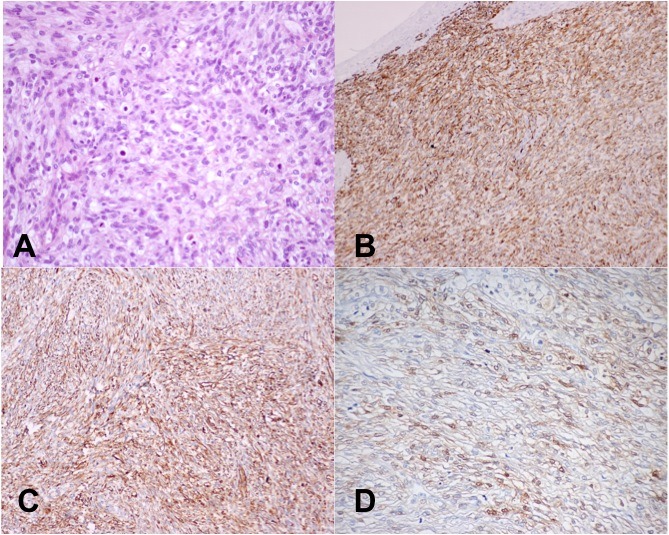
Histopathological findings: (A) spindle cell tumour with clear cells showing high mitotic index (200x); (B) immunochemistry: diffuse positivity for CAM5.2 (100x); (C) immunochemistry: strong and diffuse positivity for vimentin (100x); (D) immunochemistry: positive staining for S100 protein.
This clinical case was discussed by a multidisciplinary team, and radiation adjuvant therapy was proposed (28 sessions; 50.4 Gy) followed by oral chemotherapy with capecitabine, based on the final diagnosis.
Outcome and follow-up
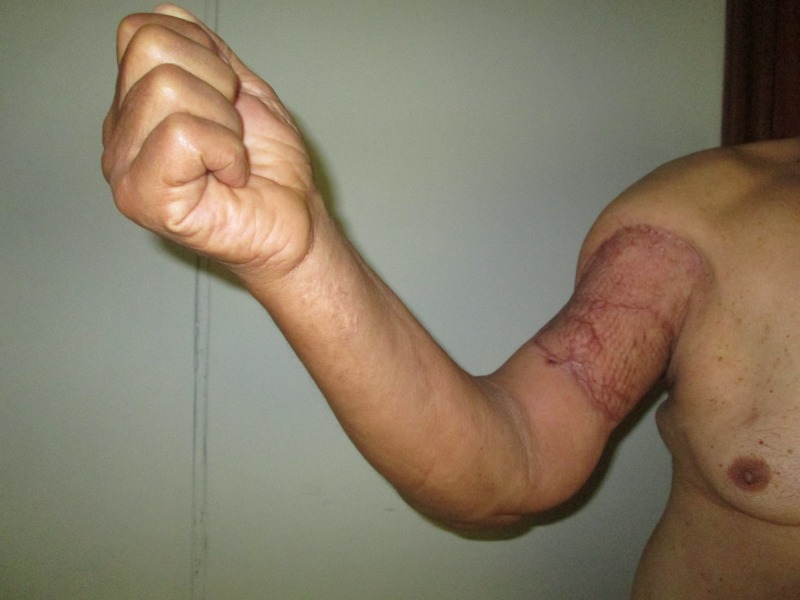
After a follow-up of 12 months, the patient had adequate arm coverage with good limb function, without any significant limitation on daily activities (figure 5). The motor examination showed: forearm pronation of 80o and supination of 60o; elbow flexion of 80o and extension of 0o; shoulder abduction of 90o and adduction of 30o; shoulder flexion of 100o and extension of 20o. Tone and power of the upper limb was preserved; biceps reflex was absent but the brachioradialis and triceps reflexes were normal. The sensory examination showed no changes except loss of sensation along the radial aspect of the forearm.
Figure 5.
Postoperative of 12 months: good arm coverage with no limb functional limitation.
The patient developed no lymphoedema after the surgery. No signs of recurrence were documented. A comparative QuickDASH test of the preoperative and postoperative of 12 months was performed, showing a reduction of 63.6 to 13.6.
Discussion
MPNST are STS derived from the Schwann cells or the pluripotent cells of the neural crest. These tumours are extremely rare, with an incidence of 0.001% in general population. Nonetheless, they can rise to 50% in patients with NF1.
Patients aged between 20 and 50 years are the most affected and peripheral nerve trunk roots, extremities and the head and neck regions are the typical MPNST locations. Metastasis of MPNST occurs in about 40% of patients, most frequently to the lung. The diagnosis is usually made by the association of macroscopic, histological and immunohistological studies.3
The pathogenesis of MPNST is not well known, but an NF1 gene inactivation is crucial for tumour growth.2 3
The histopathological diagnosis of MPNST is difficult in patients without NF1 or prior RT because they lack specific morphological characteristics, shared with other tumours. Their immunohistochemical profile is not distinct, making the diagnosis more challenging.6
Thus, most cases of MPNST in the literature were diagnosed and treated in patients with NF1.
The primary treatment for MPNST is wide tumour excision. The size and location of the defect, exposure of vital structures, along with the need for adjuvant RT, will dictate the type of reconstruction.7
A recent case report of a patient with NF1 and an MPNST of the left shoulder was successfully treated with a pedicle fillet flap of the upper arm.8 Pedicled or free flaps allow coverage of large defects, avoiding complications related to skin grafting, which can postpone adjuvant treatments.
Goertz et al reviewed 65 patients with MPNST, 75% involving extremities; in 14 cases a tendon transfer and flap coverage was necessary, but no details were provided concerning the procedure.9
Choi et al presented a case of a patient aged 54 years with a history of NF1 with an MPNST of the upper arm and forearm. Debulking surgery was done because the tumour was not encapsulated, and the remaining defect was reconstructed with local advancement flap.10
Miyamoto et al presented another case of a patient with NF1 with a recurrent MPNST on the right shoulder. The defect was successfully reconstructed with a pedicled LDMC flap and a free anterolateral thigh flap, because the medial side of the defect could not be covered with LDMC flap alone.5
Lohman et al conducted a review of 100 consecutive patients with STS of the upper extremity that underwent surgery. Among the patients with tumours located at the proximal arm and elbow, six underwent free flap reconstruction (LDMC, rectus abdominis, parascapular) and seven underwent transposition flap (LDMC, pectoralis major, triceps).11
A Medline (PubMed) research was done using the following term: ‘malignant peripheral nerve sheath tumor of the extremities’, which yielded 102 results (between 2000 and 2017). Of these, only the papers which included reconstructive techniques where analysed. Table 1 shows a review of some of the cases of MPSNT of the extremities.
Table 1.
A review of some of the cases of MPNST of the extremities
| Surgery type | Complications | Follow-up | |
| Singla et al
8
(n=1) |
Fillet flap | N/A | N/A |
| Goertz et al
9
(n=49) |
Resection Resection+functional reconstruction Amputation |
N/A | MRI of the local area and X-ray of the chest every 3 months after surgery for the first 2 years |
| Choi et al
10
(n=1) |
Debulking+local advancement flap | No complications | N/A |
| Miyamoto et al
5
(n=1) |
Wide excision+pedicled LDMC flap+free ALT flap | No complications | N/A |
| Lohman et al
11
(n=7) |
Transposition flaps Free flaps |
Wound dehiscence Osteomyelitis Haematoma Flap necrosis |
Mean follow-up: 33 months* |
*No information available about the imaging procedures performed.
ALT, alterolateral thigh; LDMC, latissimus dorsi muscle; n, sample size; N/A, not available.
The role of RT and chemotherapy for MPNST has not yet been fully defined. Adjuvant RT is often endorsed for intermediate or high-grade lesions, >5 cm or with marginal excision, to promote local control. Some trials with conventional chemotherapy have shown negligible results.12
Local recurrence rates of MPNST can reach 65%. Although sporadic lesions of MPNST have commonly been associated with a better prognosis than the NF1-associated cases, a recent study concluded there were no statistically differences in the global survival rate.13 Some factors associated with poor prognosis include tumour size (>5 cm), involved margins, high-grade, p53 mutations and S100β negativity.14 15
Our patient had no clinical signs of NF1 such as café au lait spots or multiple neurofibromas, neither a family history of the disease.
The patient was staged by CT scanning that revealed two pulmonary nodules, which were first interpreted as possible metastasis of the arm tumour. The pulmonary biopsy findings allowed us to conclude that those lesions were lung metastases of the rectum adenocarcinoma. At the same time, with the diagnosis of malignant spindle cell tumour on the arm, we had to clarify its primary or metastatic origin. The last possibility was excluded by CT scan imaging.
After clinical, imaging and immunohistochemical supplementary information, we concluded that the patient had simultaneously two different histological tumours/synchronous tumours.
Concerning the MPNST, as with the other STS, limb-sparing surgery is currently the cornerstone. Wide tumour excision followed by immediate functional flap reconstruction and RT for safer margins is recommended.16 Successful reconstruction is measured by preservation of patient health, limb function, limb sensation and cosmetic and stable wound coverage.17 18 All these assumptions were achieved in this case, except limb sensation of the skin grafted area.
Concerning the lung metastases of the rectal adenocarcinoma, due to patient’s age and the location of the metastases, it was decided to perform only oral chemotherapy.
Due to several particularities and extremely rare presentation, namely the presence of two synchronous tumours and the rare MPNST presentation without NF, which was successfully reconstructed, the authors think that these data deserve to be shared among the medical community.
Patient’s perspective.
When I first noticed I had a mass in my arm I went to my general surgeon consultation. He had treated my rectum cancer so I trusted him. The doctor decided I should get an MRI. After he saw the result, he told me it would be better to do a biopsy of the mass. By this time, I was already having some difficulties to perform my everyday activities like eating and dressing because of the size of the mass, although I had no pain. The biopsy showed I had a tumour. The doctor told me I had to get a CT of my body to see if the tumour had already spread. At this point, I started to be very anxious because I already have had a tumour 2 years before. The CT showed something in my lungs. A biopsy was performed but it was not conclusive. At this point, my general surgeon sent me to a plastic surgery consultation. The doctor decided to repeat the MRI which showed that the tumour was growing. At this point, the plastic surgeon explained me it would be better to excise the tumour as it was growing and it was limiting my daily living activities. He made me aware of all the risk of the surgery such as nerve damage. He explained me the reconstruction he would perform, trying to be as functional as possible. I decided to go through with it. When I woke up from the surgery the doctor told me it had been a success. I had a splint in my arm for the first week. The doctors decided I should repeat the biopsy of the pulmonary nodules, which showed metastasis of the tumour I had 2 years before. My case was discussed by other doctors and they decided I should do radiation therapy and chemotherapy which I accepted. These treatments were aggressive but at the end I did very well. During this period, I also did physiotherapy to my arm which allowed me to start progressively doing all my daily activities without any restrictions. I would like to thank to all the doctors that helped me to fight against another tumour and to be able to have a quality of life similar to the one I had before the surgery.
Learning points.
Malignant peripheral nerve sheath tumours (MPSNT) are extremely rare soft tissue sarcomas (STS) and most of them are associated with neurofibromatosis type I (NF1).
The histopathological diagnosis of MPNST is difficult in patients without NF1 or prior radiation therapy (RT) because they lack specific morphological characteristics, shared with other tumours; their immunohistochemical profile is not distinct, making the diagnosis more challenging.
The primary treatment for MPNST is wide tumour excision; the size and location of the defect, exposure of vital structures, along with the need for adjuvant RT, will dictate the type of reconstruction.
The role of RT and chemotherapy for MPNST has not yet been fully defined.
Although sporadic lesions of MPNST have commonly been associated with a better prognosis than the NF1-associated cases, a recent study concluded there were no statistically differences in the global survival rate.
Footnotes
Contributors: RN: planning, conducting, reporting, conception and design, acquisition of data. RV-F: planning, conducting, reporting. RH: analysis and interpretation of data. ÁS: analysis and interpretation of data.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. van Vliet M, Kliffen M, Krestin GP, et al. Soft tissue sarcomas at a glance: clinical, histological, and MR imaging features of malignant extremity soft tissue tumors. Eur Radiol 2009;19:1499–511. 10.1007/s00330-008-1292-3 [DOI] [PubMed] [Google Scholar]
- 2. Park JH, Kang CH, Kim CH, et al. Highly malignant soft tissue sarcoma of the extremity with a delayed diagnosis. World J Surg Oncol 2010;8:84 10.1186/1477-7819-8-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nikumbh DB, Suryawanshi KH, Dravid NV, et al. Giant Sporadic Low Grade Malignant Peripheral Nerve Sheath (MPNST) of Left Thigh. J Clin Diagn Res 2013;7:1155–8. 10.7860/JCDR/2013/5511.3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yuan Z, Xu L, Zhao Z, et al. Clinicopathological features and prognosis of malignant peripheral nerve sheath tumor: a retrospective study of 159 cases from 1999 to 2016. Oncotarget 2017;8:104785–95. 10.18632/oncotarget.18975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyamoto S, Fujiki M, Sakisaka M, et al. Combined Use of the Latissimus Dorsi Musculocutaneous Flap and the Anterolateral Thigh Flap to Reconstruct an Extensive Shoulder Defect in an NF-1 Patient. Plast Reconstr Surg Glob Open 2016;4:e670 10.1097/GOX.0000000000000644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Owosho AA, Estilo CL, Huryn JM, et al. A Clinicopathologic Study of Head and Neck Malignant Peripheral Nerve Sheath Tumors. Head Neck Pathol 2018;12 10.1007/s12105-017-0841-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guha D, Davidson B, Nadi M, et al. Management of peripheral nerve sheath tumors: 17 years of experience at Toronto Western Hospital. J Neurosurg 2018;128:1–9. 10.3171/2017.1.JNS162292 [DOI] [PubMed] [Google Scholar]
- 8. Singla P, Kachare SD, Fitzgerald TL, et al. Reconstruction using a pedicled upper arm fillet flap after excision of a malignant peripheral nerve sheath tumor: A case report. World J Clin Cases 2014;2:899–902. 10.12998/wjcc.v2.i12.899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goertz O, Langer S, Uthoff D, et al. Diagnosis, treatment and survival of 65 patients with malignant peripheral nerve sheath tumors. Anticancer Res 2014;34:777–84. [PubMed] [Google Scholar]
- 10. Choi SK, Kim CK, Kim SH, et al. A Case of Malignant Peripheral Nerve Sheath Tumor with Neurofibromatosis Type 1. Journal of Korean Society for Microsurgery 2017;26:23–5. 10.15596/ARMS.2017.26.1.23 [DOI] [Google Scholar]
- 11. Lohman RF, Nabawi AS, Reece GP, et al. Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 2002;94:2256–64. 10.1002/cncr.10419 [DOI] [PubMed] [Google Scholar]
- 12. Zehou O, Fabre E, Zelek L, et al. Chemotherapy for the treatment of malignant peripheral nerve sheath tumors in neurofibromatosis 1: a 10-year institutional review. Orphanet J Rare Dis 2013;8:127 10.1186/1750-1172-8-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kolberg M, Høland M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol 2013;15:135–47. 10.1093/neuonc/nos287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fan Q, Yang J, Wang G. Clinical and molecular prognostic predictors of malignant peripheral nerve sheath tumor. Clin Transl Oncol 2014;16:191–9. 10.1007/s12094-013-1061-x [DOI] [PubMed] [Google Scholar]
- 15. Wang T, Yin H, Han S, et al. Malignant peripheral nerve sheath tumor (MPNST) in the spine: a retrospective analysis of clinical and molecular prognostic factors. J Neurooncol 2015;122:349–55. 10.1007/s11060-015-1721-5 [DOI] [PubMed] [Google Scholar]
- 16. Megerle K, Sauerbier M. Reconstructive treatment of soft tissue sarcoma of the upper extremity. J Hand Surg Am 2011;36:1241–7. 10.1016/j.jhsa.2011.04.017 [DOI] [PubMed] [Google Scholar]
- 17. Saint-Cyr M, Langstein HN. Reconstruction of the hand and upper extremity after tumor resection. J Surg Oncol 2006;94:490–503. 10.1002/jso.20486 [DOI] [PubMed] [Google Scholar]
- 18. Panigrahi S, Mishra S, Das S, et al. Primary malignant peripheral nerve sheath tumor at unusual location. J Neurosci Rural Pract 2013;4:83 10.4103/0976-3147.116480 [DOI] [PMC free article] [PubMed] [Google Scholar]