Abstract
Sweet’s syndrome is an acute febrile neutrophilic dermatosis with classical clinical features. Systemic manifestations in Sweet’s syndrome including fever, arthralgia, myalgia and ocular involvement are common. Pulmonary involvement is a rare manifestation that has been reported previously in 34 cases and can be fatal if left untreated. We report a striking case of Sweet’s syndrome with respiratory failure secondary to bilateral pulmonary interstitial infiltrates, which rapidly responded to intravenous corticosteroid therapy. This case is an important reminder of the systemic manifestations of Sweet’s syndrome and highlights the value of collaboration between different medical specialities to optimise patient management and outcomes.
Keywords: dermatology, respiratory medicine, haematology (incl blood transfusion)
Background
Sweet’s syndrome was first described in 1964 by Sweet as an acute febrile neutrophilic dermatosis.1 It classically presents with abrupt onset of tender erythematous plaques, often with a pseudovesicular or psuedopustular appearance due to pronounced oedema, with characteristic rapid response to corticosteroid therapy.2 It may be associated with inflammatory and autoimmune disorders, malignancy (particularly haematopoetic disorders), medications, infections and pregnancy, although at least 50% of cases are estimated to be idiopathic.3
Systemic manifestations in Sweet’s syndrome are common, including fever in 40–80%, arthralgia in 30–60%, myalgia and ocular involvement such as conjunctivitis and episcleritis.2 3 Neutrophilic infiltration can rarely cause other extracutaneous complications, such as pulmonary involvement, aseptic meningitis, sterile osteomyelitis, hepatitis, renal failure, myositis and pancreatitis. Pulmonary involvement is a rare manifestation that has been previously reported in 34 cases. We report a striking case of Sweet’s syndrome with pulmonary involvement.
Case presentation
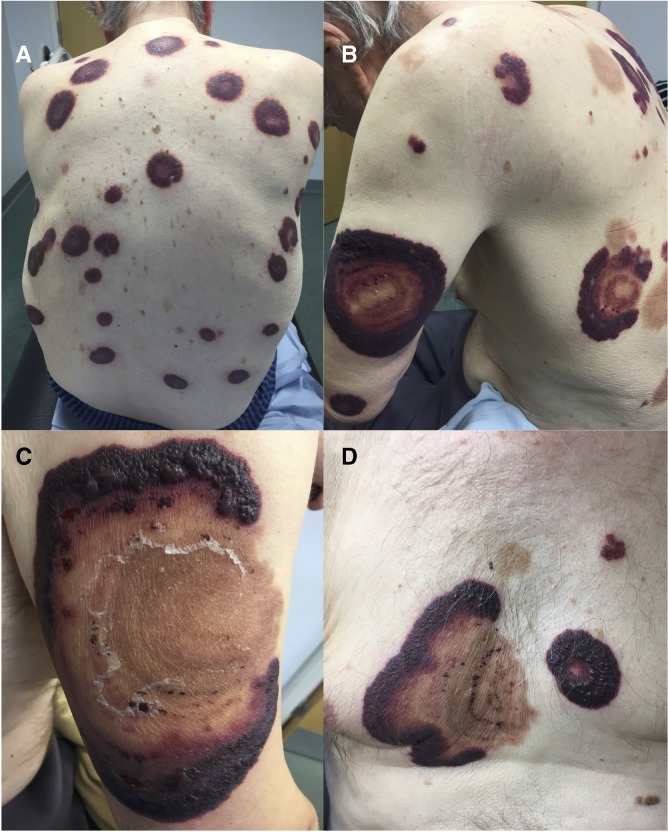
An 82-year-old man presented with a 2-week history of a widespread tender eruption on his chest, back and limbs. He was being treated for high-risk primary myelofibrosis with ruxolitinib (Janus kinase 1/2 inhibitor). On examination, there were multiple large tender purpuric plaques measuring 1–3 cm at the aforementioned sites (figure 1A). A clinical diagnosis of Sweet’s syndrome was made and he was commenced on 40 mg of oral prednisolone, with rapid clearance of lesions within 2 weeks. Histological examination of several skin biopsies confirmed the diagnosis of Sweet’s syndrome (figure 2A–D).
Figure 1.
(A) Multiple tender purpuric annular plaques on back at initial presentation. (B) Several weeks later, after initial clearance of plaques seen as hyperpigmented scars, recurrence of painful annular plaques on upper arms and back. (C) Recurrent annular plaque on upper arm with prominent vesiculation. (D) Recurrent lesions on mid chest.
Figure 2.
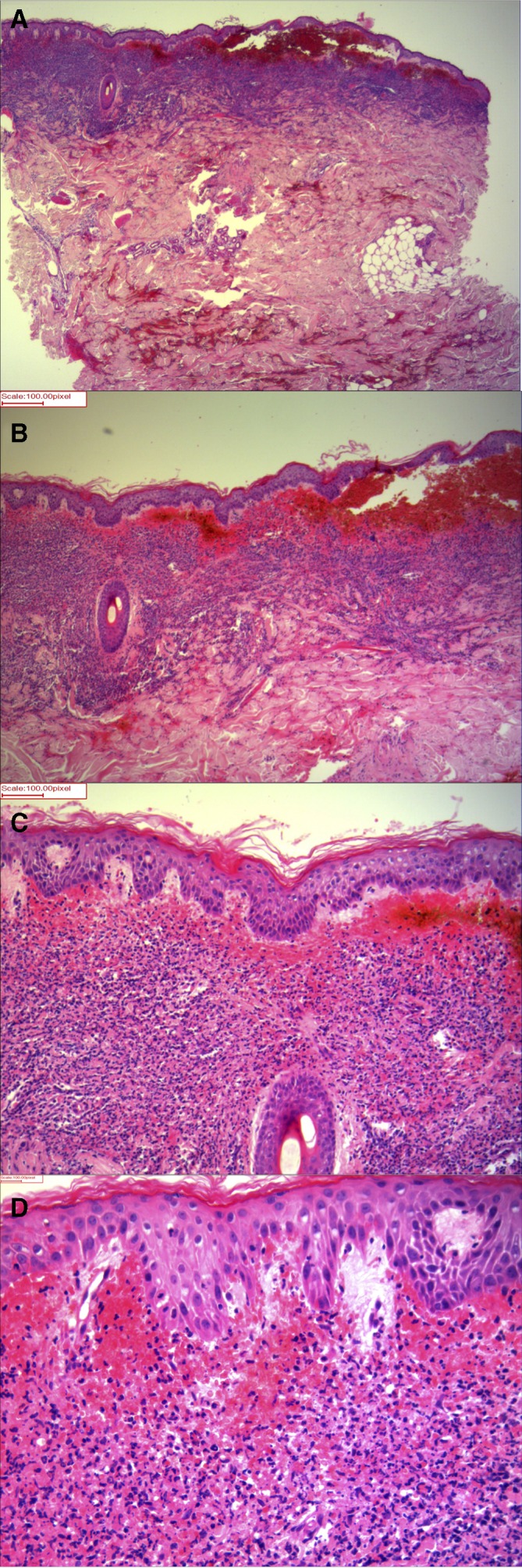
Histological examination of skin biopsy from left arm showing features of severe Sweet’s syndrome. (A, B) On low magnification, there is a dense superficial and mid dermal infiltrate and massive subepithelial oedema imparting a pseudobullous appearance. (C) On medium magnification, collection of purpura with dense inflammation in the dermis can be seen. (D) On high magnification, the dermal infiltrate is composed of neutrophils with marked red cell extravasation. There are prominent blood vessels with oedema of their walls but no areas of fibrinoid necrosis. H&E, original magnification (A, B) 10× (C) 20× and (D) 40×.
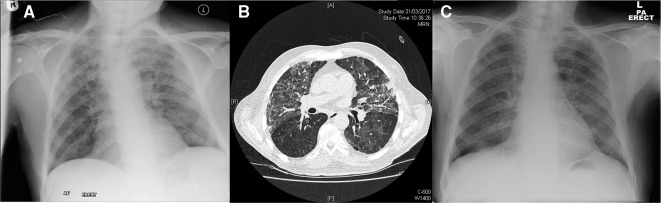
The dose of prednisolone was tapered down, but the eruption quickly recurred. He developed larger, vesicular tender plaques measuring up to 20 cm at new sites over his torso and limbs, with smaller lesions on his scalp, tongue and nose (figure 1B–D). He simultaneously complained of acute progressive shortness of breath requiring emergency admission with type one respiratory failure and severe hypoxia. Chest radiograph showed upper and mid zone lung consolidation (figure 3A). He was initially misdiagnosed with infective community-acquired pneumonia and treated with antibiotics. He continued to deteriorate despite broad-spectrum antibiotic treatment and underwent high-resolution CT (HRCT) chest imaging. This showed bilateral ground-glass opacities and interstitial thickening in the upper lung lobes (figure 3B).
Figure 3.
(A) Chest X-ray pretreatment showing bilateral upper and mid zone consolidation. (B) High-resolution CT chest showing bilateral upper lobe ground-glass opacities and interstitial thickening. (C) Chest X-ray 5 days after treatment with intravenous methylprednisolone showing almost complete clearing of bilateral infiltrates.
Investigations
Skin biopsy
Histological examination showed a dense neutrophilic infiltrate in the superficial and mid dermis, massive subepithelial dermal oedema with purpura and a lack of fibrinoid necrosis (figure 2A–D). These histological features confirmed the diagnosis of Sweet’s syndrome.
Laboratory investigations are summarised in table 1.
Table 1.
Summary of laboratory investigations.
| C reactive protein | 132 mg/L |
| Haemoglobin | 86 g/L |
| Mean cell volume | 87.4 fL |
| Platelet count | 15× 109/L |
| White ell count | 4.9×109/L |
| Creatinine | 148 μmol/L |
| Urea | 16.3 mmol/L |
| Liver function | Normal |
| Blood and urine cultures | Negative |
| Galactomannan antigen | Negative |
| Interferon gamma release assay | Negative |
| Hepatitis B surface antigen | Negative |
| Hepatitis C antibody | Negative |
| Anti-nuclear antibody | Negative |
| Glomerular basement membrane antibody | Negative |
| Anti-neutrophil cytoplastic antibody | Negative |
| HIV 1 and 2 antibody | Negative |
Chest radiograph
Upper and mid zone lung consolidation (figure 3A).
HRCT: Bilateral ground-glass opacities and interstitial thickening in the upper lung lobes (figure 3B).
Differential diagnosis
The initial cutaneous presentation of rapidly progressive multiple tender purpuric plaques, with oedema and vesiculation, was most in keeping with Sweet’s syndrome, which is commonly associated with myeloproliferative disorders. The striking purpuric appearance of the lesions is unusual for Sweet’s syndrome but is explained by the coexisting thrombocytopenia. We also considered vasculitis, but it usually presents with smaller purpuric papules at distal sites and would be extremely unusual while on ruxolitinib, as it inhibits both T cells and B cells. We also considered leukaemia cutis caused by infiltration of the skin with leukaemia cells, which usually presents with asymptomatic nodules and plaques. The figurate annular appearance of the lesions also made us consider erythema gyratum repens, which is a paraneoplastic phenomenon usually associated with lung cancer. These differentials were all excluded on skin biopsy.
Other differentials for a neutrophilic dermatosis in association with a haematological malignancy include bullous pyoderma gangrenosum (PG) and neutrophilic eccrine hidradenitis (NEH). Bullous PG presents with recurring superficial ulcerating lesions with bullae which can resemble those of Sweet’s and are corticosteroid responsive, but systemic features are usually absent and histology shows subepidermal haemorrhagic bullae and pustules.4 NEH usually occurs in patients with acute myeloid leukaemia as a reaction to chemotherapy, most commonly cytarabine and daunorubicin.5 NEH presents with oedematous erythematous or purpuric papules, nodules and plaques. Histology shows neutrophilic infiltration within and around eccrine glands with necrosis of eccrine epithelium.
The chest radiography findings were initially suggestive of an infective process. HRCT showed bilateral ground-glass opacities and interstitial thickening in the upper lung lobes, with a differential of vasculitis, atypical infection and other causes of pulmonary infiltration. The case was discussed between the respiratory, haematology and dermatology teams. As a diagnosis of cutaneous Sweet’s had already been made and the respiratory symptoms coincided with rapid progression of cutaneous lesions, the consensus was that his symptoms were in keeping with Sweet’s syndrome with pulmonary involvement. Atypical infection was felt to be unlikely given lack of other symptoms, lack of improvement despite broad-spectrum antibiotic treatment and negative serological tests and cultures.
Treatment
A diagnosis of Sweet’s syndrome with pulmonary involvement was made. He was treated with 500 mg of intravenous methylprednisolone over 3 days, which rapidly improved his chest symptoms with radiological clearance of pulmonary infiltrates after several days (figure 3C). Colchicine was added with a slow weaning course of prednisolone with good clinical response and complete resolution of his cutaneous lesions.
Outcome and follow-up
He remained clear of respiratory symptoms and cutaneous lesions on a combination of colchicine with prednisolone. He unfortunately died of progressive end-stage myelofibrosis several months later.
Discussion
Pulmonary involvement in Sweet’s syndrome manifests as a neutrophilic alveolitis with corticosteroid-responsive culture-negative pulmonary infiltrates.6 A recent review article identified 34 previously reported cases of Sweet’s syndrome with pulmonary involvement.7 Cutaneous and pulmonary symptoms are usually concomitant, although occasionally skin lesions can precede lung involvement by months or years. Patients present with dry cough and dyspnoea. Chest X-ray usually shows diffuse pulmonary infiltrates, and unilateral or bilateral interstitial infiltrates can be confirmed on chest CT. In some cases, pulmonary opacities and pleural effusions have also been described.6 7 The majority of cases show prompt improvement following oral or intravenous corticosteroid therapy. Other immunosuppressant therapy with colchicine or dapsone has been described in six cases.7 Sweet’s syndrome with pulmonary involvement can progress to fatal respiratory failure and there are five reported cases of death from acute respiratory distress syndrome.8–12
In patients with malignant disease, Sweet’s syndrome is known to be aggressive and resistant to treatment and can be a sign of progressive disease, as in our patient. A similar case of Sweet’s syndrome as a terminal event in a patient with ruxolitinib-treated myelofibrosis has been described previously by Chaterjee et al.13 Ruxolitinib is not a recognised cause or treatment of Sweet’s syndrome.5
In summary, we present a striking case of Sweet’s syndrome associated with respiratory failure secondary to bilateral pulmonary interstitial infiltrates, which rapidly responded to intravenous corticosteroid therapy. Pulmonary involvement is a rare but recognised complication of Sweet’s syndrome that can potentially be fatal if not adequately recognised and treated. This case is an important reminder to physicians to always consider the rare systemic manifestations of Sweet’s syndrome. Additionally, collaboration between medical specialities, in this case dermatology, haematology and respiratory, is paramount to optimise patient management and outcomes.
Learning points.
Sweet’s syndrome presents with abrupt onset of tender erythematous plaques, often with a pseudovesicular or pseudopustular appearance, and is often associated with haematopoietic disorders. In patients with malignant disease, aggressive Sweet’s syndrome can be a sign of progressive disease.
Sweet’s syndrome can rarely be associated with pulmonary involvement, which presents with dry cough and dyspnoea, with interstitial infiltrates and/or pleural effusion seen on radiography.
Sweet’s syndrome with pulmonary involvement responds rapidly to corticosteroid therapy but can be fatal if left untreated.
Collaboration between medical specialities is paramount to optimise patient management and outcomes.
Footnotes
Contributors: SHM wrote the manuscript. SMH, ZUH and DPM critically revised the manuscript and approved the final version for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol 1964;76:349–56. 10.1111/j.1365-2133.1964.tb14541.x [DOI] [PubMed] [Google Scholar]
- 2. Fett DL, Gibson LE, Su WP. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc 1995;70:234–40. 10.4065/70.3.234 [DOI] [PubMed] [Google Scholar]
- 3. Cohen PR. Sweet' s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis 2007;2:34 10.1186/1750-1172-2-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol 1996;34:395–409. 10.1016/S0190-9622(96)90428-4 [DOI] [PubMed] [Google Scholar]
- 5. Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol 2018;79:987–1006. 10.1016/j.jaad.2017.11.064 [DOI] [PubMed] [Google Scholar]
- 6. Astudillo L, Sailler L, Launay F, et al. Pulmonary involvement in Sweet’s syndrome: a case report and review of the literature. Int J Dermatol 2006;45:677–80. 10.1111/j.1365-4632.2006.02585.x [DOI] [PubMed] [Google Scholar]
- 7. Fernandez-Bussy S, Larbaca G, Cabello F, et al. Sweets syndrome with pulmonary involvement: case report and review of the literature. Resp Med Case Rep 2012;6:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takimoto CH, Warnock M, Golden JA. Sweet’s syndrome with lung involvement. Am Rev Respir Dis 1991;143:177–9. 10.1164/ajrccm/143.1.177 [DOI] [PubMed] [Google Scholar]
- 9. Komiya I, Tanoue K, Kakinuma K, et al. Superoxide anion hyperproduction by neutrophils in a case of myelodysplastic syndrome. Association with Sweet’s syndrome and interstitial pneumonia. Cancer 1991;67:2337–41. [DOI] [PubMed] [Google Scholar]
- 10. Thurnheer R, Stammberger U, Hailemariam S, et al. Bronchial manifestation of acute febrile neutrophilic dermatosis (Sweet’s syndrome). Eur Respir J 1998;11:978–80. 10.1183/09031936.98.11040978 [DOI] [PubMed] [Google Scholar]
- 11. Robbins CM, Mason SE, Hughey LC. Sweet syndrome with pulmonary involvement in a healthy young woman. Arch Dermatol 2009;145:344–6. 10.1001/archdermatol.2008.610 [DOI] [PubMed] [Google Scholar]
- 12. Aparicio V, Gil P, Juárez A, et al. [Fatal sweet with pulmonary involvement]. Rev Clin Esp 2010;210:96–7. 10.1016/j.rce.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 13. Chatterjee B, Rqieh U, Greaves P, et al. Sweet syndrome as terminal event in ruxolitinib-treated myelofibrosis. Br J Haematol 2015;169:307 10.1111/bjh.13334 [DOI] [PubMed] [Google Scholar]