Abstract
Background:
As a marine organism, soft corals can be utilized to be various bioactive substances, especially terpenoids and steroids. The soft corals family which produces bioactive generally come from clavulariidae, alcyoniidae, nephtheidae and xeniidae family.
Objective:
To investigate the bioactivity of Nitric Oxide (NO) inhibitor release from soft coral crude extracts of Sinularia sp. (SCA), Nephthea sp. (SCB), Sarcophyton sp. (SCC), Sarcophyton sp. (SCD), Sinularia sp. (SCE) and Sinularia sp. (SCF).
Materials and Methods:
Soft coral is collected from Palu Bay (Central Sulawesi). NO inhibitory release activity measured according to the Griess reaction. Soft corals sample macerated with 1:2 (w/v). Then, Soft coral extracts with the best NO Inhibitor activity partitioned with Dichloromethane, Ethyl acetate, and n-butanol. The bioactive of all crude extracts were identified by GC-MS to find compounds with anti-inflammatory potential.
Results:
Sarcophyton sp. (SCC) and Sinularia sp. (SCF) are able to inhibit NO concentrations of 0.22 ± 0.04 and 0.20 ± 0.04 μM at 20 mg/mL, respectively. The chemical constituents determined and showed the potential as anti-inflammatory in the crude of Sinularia sp. (SCA) were Octacosane (3.25%). In Nephthea sp., (SCB) were Cyclohexene, 6-ethenyl-6-methyl-1-(1-methylethyl)-3-(1-methylethylidene)-,(S)- (0.55%); Azulene, 1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethylidene)-, (1S-cis)- (0.53%); and 1,7,7-Trimethyl-2-vinylbicyclo[2.2.1]hept-2-ene (4.72%). In Sarcophyton sp, (SCC) were Eicosane (0.12%); Nonacosane (10.7%); 14(β)-Pregnane (0.87%); Octacosane 6.39%); and Tricosane (1.53%). In Sarcophyton sp. (SCD) were 14(β)-Pregnane (2.69%); and Octadecane (27.43%). In crude of Sinularia sp. (SCE) were Oleic Acid (0.63%); 7,10-Hexadecadienoic acid, methyl ester (0.54%); 14(β)-Pregnane (1.07%); 5,8,11,14-Eicosatetraenoic acid, ethyl ester, (all-Z)- (4.60%); Octacosane (7.75%); and 1,2-Benzisothiazole, 3-(hexahydro-1H-azepin-1-yl)-, 1,1-dioxide (1.23%). In the crude of Sinularia sp., (SCF) were Oxirane, decyl- (1.38%); Nonacosane (0.57%); Cyclohexanol, 5-methyl-2-(1-methylethenyl)- (0.61%); 14B-Pregnane (0.76%); and Tetratriacontane (1.02%).
Conclusion:
The extract of Sarcophyton sp. (SCC) and Sinularia sp. (SCF) showed the best NO inhibitory release activity. This study is making soft corals from Central Sulawesi, Indonesia can become a potential organism in the discovery and development of bioactive substances anti-inflammatory.
Keywords: Central Sulawesi, natural product, Nephthea, nitric oxide, Sarcophyton, Sinularia
1. INTRODUCTION
Indonesian marine organisms are so diverse and have potential as bioactive producer. Soft corals are well-known as various bioactive substances source, especially terpenoids and steroids with exceptional functionality [1-6]. Soft corals are included in cnidarian phylum, anthozoa class, octocorallia subclass and 60% of bioactive substances reportedly have the potential to produce drug compounds [7-9].
Soft corals are soft-bodied sessile invertebrates, having no physical defense systems that rely on chemical defense system to survive [2]. The percentage of drug raw materials from soft coral found at 11-17% [10]. Soft corals become a source of biologically active molecules and model compounds used in drug material [11, 12].
The soft corals family which produces bioactive generally come from Clavulariidae, Alcyoniidae, Nephtheidae, Xeniidae family. Family Alcyoniidae most reported as bioactive producer [13]. Soft corals were reported to produce a variety of unique bioactive substances, including sesquiterpenoid, diterpenoid and steroids compounds [14]. The bioactive substances isolated from soft corals had anti-inflammatory activity [15, 16].
The anti-inflammatory properties often isolated and applied to the treatment of fever, pain and inflammatory conditions [17]. From the literature, it was reported that soft corals produce potentially anti-inflammatory compounds by inhibiting and reducing the expression of iNOS protein [18-25]. iNOS (inducible nitric oxide synthase) is one of the pro-inflammatory proteins, which can affect the development of acute to be chronic inflammatory responses and is closely related to the development of chronic human disease, including Alzheimer's [26], atherosclerosis, arthritis [27], diabetes [28], inflammatory bowel disease [29], and cancer [30].
The production of nitric oxide (NO) by iNOS involves in the pathogenesis of inflammatory response [31]. NO concentration by iNOS expressed in macrophage cells is related to phagocytic activity [32]. The inhibitor of NO release in macrophages is one way to study the potential of anti-inflammatory agents. Increased levels of NO in chronic inflammatory affect various palogical conditions [33]. The activity and expression of the iNOS enzyme are related to the production of NO. Therefore, one way of developing anti-inflamma-tory agents is by controlling the levels of NO in macrophages [34].
The oceans of eastern Indonesia hold great potential in the marine organism biodiversity [35]. The great potential of the marine organism is theoretically also a potential for the discovery of bioactive substances from the marine organism [36]. However, there is not so many exploration of soft coral potential bioactive substances. Only 19 new compounds were isolated from soft coral and ascidians and have the potential pharmacological [37]. Based on the description above, this research examines NO-Inhibitor of six soft corals origin Palu Bay, Central Sulawesi, Eastern Indonesia. NO inhibitory release potency was measured by observing the inhibition of LPS-induced NO release. The aim of this research is to investigate the NO inhibitor release of soft corals crude extract Sinularia sp. (SCA), Nephthea sp. (SCB), Sarcophyton sp. (SCC), Sarcophyton sp. (SCD), Sinularia sp. (SCE) and Sinularia sp. (SCF) from Central Sulawesi, Indonesia, which is one oceans with high biodiversity.
2. MATERIALS AND METHODS
2.1. Chemical and Reagents
Dichloromethane (DCM with p.a grade and purity of purchased from Merck), methanol (MeOH p.a, Merck), Ethyl acetate (EtOAc p.a, Merck), n-butanol (BuOH p.a, Merck), dimethyl sulfoxide p.a, (Merck), Dulbecco’s modified Eagle’s medium (DMEM/F12) powder Gibco Life Technologies, 10% Fetal Bovine Serum (FBS), 1% penisilin-streptomicin, Lipopolysaccharide LPS E. coli O111:B4 (List Biological Laboratory, Inc.), Griess Reagent Kit for Nitrite Determination G-7921 (Thermo Fisher Scientific).
2.2. Animal Materials
Soft coral samples were collected from the coastal of Kabonga Besar Village, Donggala District, Palu Bay, Central Sulawesi, Eastern Indonesia at coordinates 43.31 South Latitude and 119.46 East Longitude in December, 2016. Sampling was done with equipment SCUBA at a depth of 3-5 m. Each soft coral sample was rinsed with seawater and immediately stored in ice. After arriving at the laboratory, samples of soft corals were cut into smaller sizes, put into containers and stored in a freezer immediately. This study was approved by Animal care and used committee with ethical clearance number 680-KEP-UB.
2.3. Extraction
A total of 350 g (wet weight) sample was macerated 1:2 w/v with methanol: dichloromethane (1: 1) for 48 hours [18, 38, 39]. Sample was filtered, evaporated (Rotary Vacuum Evaporator EYELA N-1100), dried, weighed and divided into several parts: SCA (4.08 g), SCB (5.21 g), SCC (2.99 g), SCD (8.59 g), SCE (7.12 g), and SCF (5.58 g).
The maceration was performed three times each of the soft coral samples. Soft coral extracts with the best NO inhibitor activity were partitioned with Dichloromethane, Ethyl acetate, and n-butanol for 24 hours. Then, evaporated and weighed, so obtained SCC DCM (0.93 g), SCC EtOAc (0.08 g), SCC BuOH (0.06 g), SCE DCM (2.32 g), SCE EtOAc (0.27 g), SCE BuOH (0.18 g), SCF DCM (1.64 g), SCF EtOAc (0.14 g), SCF BuOH (0.09 g). Each crude extracts were subjected to preliminary phytochemical screening and assays for NO inhibitory activity.
2.4. Phytochemical Screening
All crude extracts were subjected to initial phytochemical screening assay to check the presence of secondary metabolites using the standard conventional protocol described by Harborne [39].
2.5. In Vitro NO Inhibitory Release Activity
In vitro anti-inflammatory assay followed instructions of of B.-W with modification [40-42]. Macrophage cells isolated from mice BALB/c were obtained from the Biomedical Center Laboratory, Faculty of Medicine, Brawijaya University. Isolation of macrophages from mice following the instructions of Zhang et al. with modifications [43].
Mice were cervical-dislocated and then placed in supine position, abdominal skin opened and cleaned the peritoneum sheath with 70% alcohol. Then, injected ±10 mL cold DMEM medium into the peritoneal cavity (wait ± 1 minute while pressed-press slowly). After that, the peritoneal fluid aspirated from the peritoneal cavity by tapping with two fingers of internal organs, fluids aspirated by syringe injection, selected in a non-fat and distant part of the intestine. Aspirates collected in a centrifuge tube and centrifuged (Biosan Centrifuge LMC-3000) at 800-1000 rpm at room temperature, 8-10 minutes. Then, the supernatant was removed and added to 1 ml of medium DMEM complete (containing 10% FBS) in pellet obtained.
The macrophage cells placed in TC plate 96 well (1 × 106 cell/well) were suspended in a DMEM medium containing 10% FBS, at 5% CO2 incubator, 37ºC for 2 hours. Inflammation in macrophages was induced by incubating them for 24 h in a medium containing LPS (0.01 µg/mL) without the presence of test extracts. The crude extracts SCA-SCF (5, 10, 20 mg/mL) were added to the cells 5 min before LPS challenge, respectively. The culture supernatant was collected to calculate the concentration of NO by Griess reaction and read using a microplate reader (Bio-Rad 550) absorbance of 570 nm.
2.6. Gas Chromatography and Mass Spectrometry (GC/MS)
For a quantitative analysis of bioactive profiles from all crude extracts, Hewlett-Packard (HP) 6890 GC MS was used with Agilent 19091S-433 HP-5MS column having 30 m length and 250 µm id. Helium was used as carrier gas at flow rate of 1 mL/min and oven temperature was set at 325°C. The initial oven temperature was 150°C which was held at 1°C/min. It ran for 10°C/min and was later increased to 240°C hold time for 2 min. The total run time was 22 minutes. The scan range was 50 - 550 amu. Structural assignments were based on analysis of fragmentation pattern of mass spectra and direct comparison of mass spectra with profiles in the National Institute of Standards and Technology (NIST) and Wiley library.
2.7. Statistical Analysis
All experimental measurement data were performed in three replicates and expressed as Mean ± SD (n = 3). The results of research were tested for statistical significance with One-way ANOVA. Differences were considered statistically significant at P <0.05. Statistical analysis was completed using The Microsoft Excel 2013 data processing program.
3. RESULTS
The procedure of identification of soft corals samples follows the instructions of Fabricus & Alderslade (2001). Based on monomorphic colony color, interior and surface sclerites, soft coral samples were identified as Sinularia sp. (SCA), Nephthea sp. (SCB), Sarcophyton sp. (SCC), Sarcophyton sp. (SCD), Sinularia sp. (SCE) and Sinularia sp. (SCF) as presented in Fig. (1).
Fig. (1).

Soft corals from Palu Bay, Central Sulawesi.
The phytochemical analysis of all soft corals crude extracts was presented in Table 1. The crude extracts by using solvents dichloromethane: methanol of soft corals were assayed to detect secondary metabolites. The chemical constituent analysis of all crude extracts indicated the presence of saponins, polyphenols (tannins), steroids, triterpenoids, alkaloids, and flavonoids.
Table 1. Phytochemical analysis of all soft corals crude extracts.
|
Chemical
Constituents |
Soft Corals Species | Standard | |||||
|---|---|---|---|---|---|---|---|
| Sinularia sp. (SCA) | Nephthea sp. (SCB) | Sarcophyton sp. (SCC) | Sarcophyton sp. (SCD) | Sinularia sp. (SCE) | Sinularia sp. (SCF) | ||
| Saponins | + | - | - | + | + | + | Stable foam formed for 15 minutes |
| Polyphenols (tannins) | + | + | + | + | - | + | Brown precipitate formed |
| Steroids | - | + | - | - | + | - | Green or blue colour produced (Lieberman-Buchard) |
| Triterpenoids | + | - | + | + | - | + | Brown or reddish-brown colour produced (Lieberman-Buchard) |
| Alkaloids | + | + | + | + | + | + | Orange precipitate formed (Dragendorff) |
| Flavonoids | + | - | - | - | + | - | Orange, pink or red colour produced |
+: Present; -: Absent.
Six soft corals crude extract activity had analyzed as an inhibitor of NO release in macrophages. NO inhibitor activity was then evaluated by LPS-induced NO production on peritoneal mice macrophage cells, as presented in Fig. (2).
Fig. (2).
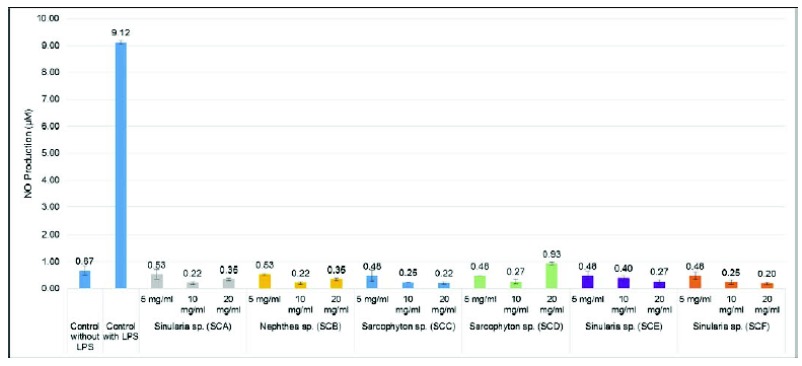
Inhibitory effects of all soft corals crude extracts on LPS-induced NO production in Mice Peritoneal Macrophages cell. Extracts were tested at 5 - 20 mg/ml. Data are presented as the means ± SD of three replications. Sarcophyton sp. (SCC) and Sinularia sp. (SCF) extracts showed the best NO inhibition activity. Based on statistical analysis, the difference of extract concentration affects the NO concentration, not the different types of soft corals.
Fig. (2) illustrates all soft coral crude extracts, showed the ability to inhibit NO production. Sarcophyton sp. (SCC) and Sinularia sp. (SCF) extracts showed the best NO inhibition activity with the concentration of NO, 0.22 ± 0.04 and 0.20 ± 0.04 μM at 20 mg/mL, respectively. On the other hand, NO concentration on negative control was 9.12 ± 0.07 μM. Sinularia sp. (SCA), Nephthea sp. (SCB) and Sarcophyton sp. (SCD) extracts increases NO concentration at 20 mg/mL. The results of statistical analysis obtain that the different types of soft corals did not affect the concentration of NO, while the difference of extract concentration affected. The different types of soft corals and concentration of the extract did not show any interaction on the concentration of NO. The results of analysis of variance presented in Table 2.
Table 2. Analysis of variance two-factor with replication ANOVA.
|
Source of
Variation |
SS | df | MS | F | P-value | F crit |
|---|---|---|---|---|---|---|
| Sample | 0.221 | 9 | 0.025 | 1.194 | 0.351 | 2.393 |
| Columns | 0.978 | 1 | 0.978 | 47.476 | 0.000 | 4.351 |
| Interaction | 0.109 | 9 | 0.012 | 0.585 | 0.794 | 2.393 |
| Within | 0.412 | 20 | 0.021 | - | - | - |
| Total | 1.720 | 39 | - | - | - | - |
Furthermore, soft coral extract Sarcophyton sp. (SCC), Sinularia sp. (SCE) and Sinularia sp. (SCF) was partitioned by polarity. Then, evaluated by LPS-induced NO production on peritoneal mice macrophage cells, as presented in Table 3.
Table 3. Inhibitory effects of SCC, SCE, and SCF partitioned extracts on LPS-induced NO production in Mice Peritoneal Macrophages cell. Extracts were tested at 5 - 20 mg/ml. Data are presented as the means ± SD.
| Fractions | NO Production (µM) | ||
|---|---|---|---|
| 5 mg/ml | 10 mg/ml | 20 mg/ml | |
| Control with LPS | 163.38 ± 3.79 | ||
| Control without LPS | 6.00 ± 0.92 | ||
| SCC DCM | 4.85 ± 0.17 | 6.31 ± 0.21 | 6.08 ± 0.01 |
| SCC EtOAc | 6.48 ± 0.25 | 6.46 ± 0.38 | 6.62 ± 0.34 |
| SCC BuOH | 8.46 ± 0.17 | 5.15 ± 0.21 | 4.77 ± 0.21 |
| SCE DCM | 6.54 ± 0.32 | 6.38 ± 0.17 | 6.77 ± 0.17 |
| SCE EtOAc | 6.38 ± 0.32 | 6.92 ± 0.17 | 7.08 ± 0.21 |
| SCE BuOH | 4.62 ± 0.17 | 4.54 ± 0.01 | 6.38 ± 0.17 |
| SCF DCM | 6.08 ± 0.01 | 7.23 ± 0.27 | 6.54 ± 0.17 |
| SCF EtOAc | 5.07 ± 0.50 | 5.84 ± 0.66 | 7.97 ± 0.28 |
| SCF BuOH | 6.23 ± 0.34 | 6.62 ± 0.21 | 5.69 ± 0.27 |
Table 3 illustrates SCC, SCE, and SCF partitioned extracts showed the ability to inhibit NO production. Sarcophyton sp. (SCC) DCM and Sinularia sp. (SCF) EtOAc extracts showed the best NO inhibition activity with the concentration of NO 4.85 ± 0.17 and 5.07 ± 0.50 μM at 5 mg/mL, respectively.
The study was extended by GC-MS analysis of soft coral crude extracts (SCA-SCF). The mass spectrum of each compound compared with that in the NIST and Wiley library. In the crude extract Sinularia sp. (SCA) there were 68 peaks, but only 52 peaks were detected with qualities above 85% and identified in 25 compounds.
Crude extract Nephthea sp. (SCB) there were 64 peaks, but only 35 peaks with qualities above 85% and identified in 33 compounds.
Crude extract Sarcophyton sp. (SCC) there were 31 peaks, but only 23 peaks with quality min 85% and identified in 18 compounds.
In the crude extract Sarcophyton sp. (SCD) there were 33 peaks, but only 26 peaks were detected with qualities above 85% and identified in 15 compounds.
Crude extract Sinularia sp. (SCE) there were 56 peaks, but only 44 peaks with qualities above 85% and identified in 35 compounds. In the crude extract Sinularia sp. (SCF) there were 29 peaks, but only 25 peaks with quality min 85% and identified in 22 compounds.
Based on GC-MS analysis and literature studies, there were 148 compounds identified in all crude extracts (SCA-SCF), and there were 22 compounds that have the potential to be anti-inflammatory. The chemical constituents identified by GC-MS analysis of all crude extracts that indicated as potentially anti-inflammatory presented in Table 4.
Table 4. The chemical constituents probabilities identified by GC-MS analysis of all crude extracts that were indicated as potentially anti-inflammatory.
| Compound | Molecular Formula | RT (min) | Area (%) |
Quality
(min 85) |
Library | References |
|---|---|---|---|---|---|---|
| Sinularia sp. (SCA) | ||||||
| Octacosane | C28H58 | 11.466 | 3.25 | 91 | NIST | [44] |
| Nephthea sp. (SCB) | ||||||
| Cyclohexene, 6-ethenyl-6-methyl-1-(1-methylethyl)-3-(1-methylethylid ene)-, (S)- |
C15H24 | 4.745 | 0.55 | 90 | NIST | [45] |
| Azulene, 1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethylidene)-, (1S-cis)- | C15H24 | 5.957 | 0.53 | 93 | NIST | [46] |
| 1,7,7-Trimethyl-2-vinylbicyclo[2.2.1]hept-2-ene | C12H18 | 6.334 | 4.72 | 90 | NIST | [47] |
| Sarcophyton sp. (SCC) | ||||||
| Eicosane | C20H42 | 7.357 | 0.12 | 86 | NIST | [48] |
| Nonacosane | C29H60 | 13.352 | 6.78 | 98 | NIST | [48] |
| 21.525 | 3.92 | 96 | NIST | |||
| 14(β)-Pregnane | C21H36 | 14.398 | 0.87 | 95 | Wiley | [49] |
| Octacosane | C28H58 | 17.450 | 6.39 | 99 | NIST | [44] |
| Tricosane | C23H48 | 18.250 | 0.32 | 95 | NIST | [48] |
| 19.227 | 1.21 | 93 | NIST | |||
| Sarcophyton sp. (SCD) | ||||||
| 14(β)-Pregnane | C21H36 | 6.997 | 0.11 | 99 | Wiley | [49] |
| 15.758 | 0.77 | 96 | Wiley | |||
| 15.953 | 0.98 | 96 | Wiley | |||
| 17.302 | 0.83 | 97 | Wiley | |||
| Octadecane | C18H38 | 10.798 | 13.06 | 95 | NIST | [44] |
| 18.273 | 9.27 | 97 | NIST | |||
| 21.977 | 5.10 | 95 | NIST | |||
| Sinularia sp. (SCE) | ||||||
| Oleic Acid | C18H34O2 | 7.351 | 0.63 | 86 | NIST | [50] |
| 7,10-Hexadecadienoic acid, methyl ester | C17H30O2 | 8.740 | 0.54 | 90 | NIST | [51] |
| 14(β)-Pregnane | C21H36 | 10.752 | 0.36 | 97 | Wiley | [49] |
| 17.296 | 0.71 | 95 | Wiley | |||
| 5,8,11,14-Eicosatetraenoic acid, ethyl ester, (all-Z)- | C22H36O2 | 12.381 | 4.60 | 95 | NIST | [51] |
| Octacosane | C28H58 | 18.256 | 7.75 | 91 | NIST | [44] |
| 1,2-Benzisothiazole, 3-(hexahydro-1H-azepin-1-yl)-, 1,1-dioxide | C13H16N2O2S | 18.759 | 1.23 | 91 | NIST | [48] |
| Sinularia sp. (SCF) | ||||||
| Oxirane, decyl- | C12H24O | 10.003 | 1.38 | 92 | NIST | [52] |
| Nonacosane | C29H60 | 10.363 | 0.13 | 94 | NIST | [48] |
| 12.346 | 0.44 | 90 | NIST | |||
| Cyclohexanol, 5-methyl-2-(1-methylethenyl)- | C10H18O | 10.906 | 0.61 | 86 | NIST | [53] |
| 14(β)-Pregnane | C21H36 | 11.060 | 0.26 | 90 | Wiley | [49] |
| 11.969 | 0.50 | 86 | Wiley | |||
| Tetratriacontane | C34H70 | 13.678 | 1.02 | 95 | NIST | [44] |
The results also showed an increase in NO production at the concentration of 20 mg/ml; this indicated that there were cytotoxic compounds. The chemical constituents of the GC-MS analysis in Table 5 showed compounds that indicated as cytotoxic.
Table 5. The chemical constituents Probabilities identified by GC-MS analysis of all crude extracts that were indicated as cytotoxic.
| Compound | Molecular Formula | RT (min) | Area (%) |
Quality
(min 85) |
Library | References |
|---|---|---|---|---|---|---|
| Sinularia sp. (SCA) | ||||||
| Heptadecanoic acid, 16-methyl-, methyl ester |
C19H38O2 | 10.969 | 1.60 | 93 | NIST | [54] |
| 1-Hexacosene | C26H52 | 11.792 | 2.52 | 97 | NIST | |
| 13.346 | 0.75 | 92 | NIST | |||
| Tricosane | C23H48 | 12.089 | 2.16 | 90 | NIST | [55] |
| 12.232 | 3.55 | 91 | NIST | |||
| 13.581 | 1.45 | 91 | NIST | |||
| 14.175 | 0.82 | 95 | NIST | |||
| 14.838 | 9.62 | 95 | NIST | |||
| 15.044 | 0.44 | 96 | NIST | |||
| 15.947 | 0.47 | 90 | NIST | |||
| Nephthea sp. (SCB) | ||||||
| Cyclohexane, 1-ethenyl-1-methyl-2, 4-bis(1-methylethenyl)- [1S-(1.al pha.2.beta.,4.beta.)]- |
C15H24 | 3.836 | 1.30 | 90 | NIST | [56] |
| (-)-Aristolene | C15H24 | 4.185 | 0.68 | 99 | NIST | [57] |
| Hexadecanoic acid, methyl ester | C17H34O2 | 9.060 | 1.22 | 97 | NIST | [58] |
| .beta.-caryophyllene | C15H24 | 9.226 | 0.34 | 92 | Wiley | [2] |
| Octadecanoic acid, methyl ester | C19H38O2 | 10.969 | 0.52 | 97 | NIST | [59] |
| Octadec-9-enoic acid | C18H34O2 | 11.106 | 0.97 | 99 | NIST | [60] |
| 5,8,11,14-Eicosatetraenoic acid, ethyl ester, (all-Z)- | C22H36O2 | 12.386 | 3.31 | 94 | NIST | [61] |
| Sarcophyton sp. (SCD) | ||||||
| 9,17-Octadecadienal, (Z)- | C18H32O | 8.334 | 0.14 | 92 | NIST | [62] |
| Hexadecanoic acid, methyl ester | C17H34O2 | 9.054 | 1.06 | 95 | NIST | [58] |
| 1-Hexacosene | C26H52 | 12.781 | 0.86 | 94 | NIST | [63] |
| Tricosane | C23H48 | 12.935 | 1.53 | 96 | NIST | [55] |
| 13.164 | 1.87 | 92 | NIST | |||
| Sinularia sp. (SCE) | ||||||
| Cyclohexane, 1-ethenyl-1-methyl-2, 4-bis(1-methylethenyl)- [1S-(1.al pha.2.beta.,4.beta.)]- |
C15H24 | 3.836 | 0.71 | 98 | NIST | [56] |
| 9.654 | 1.01 | 89 | NIST | |||
| 7-Pentadecyne | C15H28 | 9.134 | 0.94 | 98 | NIST | [64] |
| Tricosane | C23H48 | 12.986 | 2.86 | 91 | NIST | [55] |
| 1-Hexacosene | C26H52 | 14.061 | 0.76 | 94 | NIST | [63] |
| 17.181 | 1.50 | 90 | NIST | |||
| 20.302 | 0.18 | 95 | NIST | |||
| Sinularia sp. (SCF) | ||||||
| Acetamide, N-methyl-N-[4-[4-methoxy-1-hexahydropyridyl]-2-butynyl]- | C13H22N2O2 | 9.060 | 0.25 | 91 | Wiley | [65] |
| n-Eicosane | C20H42 | 10.980 | 0.41 | 93 | Wiley | [55] |
| Tricosane | C23H48 | 11.415 | 0.35 | 91 | NIST | [55] |
| 18.250 | 0.89 | 95 | NIST | |||
| 1-Hexacosene | C26H52 | 11.792 | 3.44 | 95 | NIST | [63] |
4. DISCUSSION
The analysis of the bioactive substances component of all soft coral crude extracts showed the presence of saponins, polyphenols (tannins), steroids, triterpenoids, alkaloids, and flavonoids. Phytochemical analysis of Sinularia sp. and Lobophytum sp. also reported the presence of alkaloids, Flavonoids, tannins, steroids, triterpenoids and saponins [38, 66]. Previous research has reported the terpenoid and steroids derivative bioactive compounds of the soft coral genus Lobophytum, Sarcophyton. Nephthea and Sinularia, showing potential anti-inflammatory biological activity. This research also gives information on anti-inflammatory of the soft coral extract of Nephthea sp., Sarcophyton sp., and Sinularia sp.
NO is a potential mediator of physiological processes, with functions related to cell signaling and vasodilation, protect the organs from ischemic damage, and also shows antimicrobial and antitumor activity [67]. NO is a mediator synthesized by the enzyme NO-synthase (NOS) [68], which can be divided into two types: constitutive isoform (NOS endothelial and neuronal NOS) and an inducible isoform (iNOS). iNOS is regulated by inflammatory mediators (LPS, cytokines) and increased levels of NO by iNOS was directly involved in the pathogenesis of inflammatory response [31].
NO endothelial-derivate induce vascular relaxation (vasodilation) and platelet aggregation and adhesion inhibitors [69]. Some research results report the importance of anti-inflammatory agents that can control NO, as cardioprotective and hypotensive agents [70, 71]. In the chronic inflammatory stage, iNOS produces NO as an inflammatory mediator, causing vasodilation and edema at the site of inflammation [72]. Thus, by inhibiting NO production directly it also inhibits the expression of the iNOS enzyme and this is one way in the treatment of inflammation.
Chemical constituents of the six crude extracts showed the potential of each extract which could be developed as a potential anti-inflammatory agent. In the extract of Sinularia sp. (SCA) there are 3.25% compounds that have the potential as anti-inflammatory agents; Nephthea sp. (SCB) of 5.80%; Sarcophyton sp. (SCC) of 19.61%; Sarcophyton sp. (SCD) of 30.12%; Sinularia sp. (SCE) of 15.82%; and Sinularia sp. (SCF) of 4.34%.
However, there was increased NO concentrations at 20 mg/ml, indicating the presence of substances as NO activators and cytotoxic effects if at high levels (Wanzola et al., 2010). Table 5 shows the chemical constituents GC-MS analysis indicated cytotoxic. There are 23.38% of SCA extracts which are cytotoxic; SCB has 8.34%; SCD has 5.46%; SCE has 7.96%; while SCF has 5.34%. This data confirms that crude extracts of Sarcophyton sp. (SCC) are best developed as an anti-inflammatory agent because from the compound profile the results of a GC-MS analysis are not obtained by compounds that have cytotoxic properties. Compounds with cytotoxic properties can damage cells and can increase NO concentration as an immune response in its role to kill tumor or cancer cells. In the inflammatory response, by inhibiting NO level, it is associated with inhibition of iNOS expression, in this case it can prevent the acute inflammatory response from becoming chronic.
The soft coral genus Sarcophyton is a rich source cembraneterpen [73-76]. About 100 references have reported the results of research on secondary metabolites with various biological variations of the genus Sarcophyton [77]. Previous research has isolated seven compounds from Sarcophyton crassocaule which indicate efficacy as an anti-inflammatory because it can inhibit the expression of iNOS protein in RAW267.7 macrophage cells were stimulated LPS [19]. Six compounds were isolated from S. ehrenbergii also able to reduce the expression of iNOS protein [78]. Soft corals S. pauciplicatum also reportedly able to reduce the expression of iNOS [79].
In the octocorallia subclass of the genus Nephthea, there are a variety of species, which produce various sesquiterpenes, diterpenes, and steroids compounds [15, 80, 81]. Genus Nephthea is a famous coral reef organisms with a wonderful source of terpenoids with various biological activities and widespread throughout the world, especially in the Indo-Pacific region [61, 82]. Previous research has presented the genus Nephthea proven to produce compounds capable of inhibiting the accumulation of iNOS protein expression, such as N. columnaris produces Columnariols A and B [18]; N. erecta produces erectathiol [25]; and N. chabroli produces 4-methylated steroids, nebrosteroids A-E, G-H [83], nebrosteroids M, 19-oxygenatedsteroids, nebrosteroids I-J, K-L [25].
Sinularia genus has proven to be a rich source of bioactive steroids [1, 4]; diterpenoids [84-86], sesquiterpenoids [87-89] and cembranoids [90, 91] with various biological activities [92]. The many previous studies have reported that compounds isolated from soft coral genus Sinularia have been shown to inhibit or reduce the pro-inflammatory protein expression of iNOS and NO production in macrophages, among others Cembrane-based diterpenoids compound were isolated from S. triangular [84]; Secosterol isolated from S. granosa [23]; Sinularioside from Sinularia sp. origin of Bunaken Marine Park North Sulawesi [93]; Crassarosterosides A and C were isolated from S. crassa [94]; Eight diterpenoid compounds from S. flexibilis [95]; Crassarines F and H were isolated from S. crassa which significantly inhibited iNOS protein [96]; 11-dehydrosinulariolide, sinulariolide and 11-epi-sinulariolide acetate from S. discrepans [97]; Thioflexibilolide A was isolated from S. flexibilis [98]; Gyrosanolides A-C and gyrosanin A were isolated from S. gyrosa [16, 99]. Flexibilisolide A and flexilarin from S. granosa and S. querciformis [100]; (+)-11,12-epoxysarcophytol A potentially anti-inflammatory of S. gibberosa [101]. Grandilobatins D was isolated from S. grandilobata [102].
Chemical constituents from the results of GC-MS analysis showed variations in chemical compounds with bioactivity potential from soft coral extracts. These compounds are thought to interact with each other to provide biological effects. Bioactive substances from natural products can work in synergy between compounds with one another [103]. Natural products can work through multi-compound and multi-target synergistic modes [104].
The chemical compounds identified with the GC-MS analysis in soft coral extracts also show potential as antioxidants. Sarcophyton sp. (SCC) there are 13.39%, i.e. 1-Hexadecanethiol [105]; 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexame-thyl-, (all-E)- [106]; 2-Dodecen-1-yl(-) succinic anhydride [107]; and Octacosane [44]. Sinularia sp. (SCF) has 19.81%, i.e. Cyclohexanol, 5-methyl-2-(1-methylethenyl)- [53]; Nonadecane [108]; Bacchotricuneatin c [59]; Tetrapentacontane, 1,54-dibromo-; Hexatriacontane [109]; and Longifolenaldehyde [110]. These compounds can become a natural anti-inflammatory-mechanism of action of bioactive antioxidants, namely by providing electrons, so that free radical molecules are unstable. Bioactive antioxidants can prevent oxidative stress through the scavenging of free radicals. Inflammation can be inhibited by preventing stress oxidative in cells [111]. The author also determined the antioxidant potential (Scavenging DPPH Method) of the six soft coral extracts in other research. The results showed that all six extracts could scavenge DPPH radicals. This research has presented at the International Conference on Fisheries and Marine, Airlangga University October 6th, 2018 (The manuscript is also temporary the process of publishing proceedings).
The NO is a signaling molecule that has an important role in the pathogenesis of inflammation. Excessive NO production by iNOS was detected in several inflammatory diseases [112]. NO is considered a pro-inflammatory mediator that induces inflammation because of excessive production under abnormal conditions [113]. Therefore, one pathway discovery of an anti-inflammatory agent by observing inhibition of NO levels produced by iNOS pro-inflammatory proteins [114-116].
CONCLUSION
Based on the assay results, extract of Sarcophyton sp. (SCC) and Sinularia sp. (SCF) showed the best NO inhibitory release activity. Purification and characterization of compounds from both can then be done so that the compounds that act as anti-inflammatory can be known. This study is exclusively making soft corals from Central Sulawesi, Indonesia can become a potential organism in the discovery and development of bioactive substances anti-inflammatory.
ACKNOWLEDGEMENTS
Thanks to the Dean of the Faculty of Fisheries and Marine Sciences Brawijaya University, Director of the Palu Fisheries and Marine Institute, and Head of Biomedical Central Laboratory who has facilitated, so that this research can be done.
LIST OF ABBREVIATIONS
- iNOS
Inducible Nitric Oxide Synthase
- NO
Nitric Oxide
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All the reported experimental procedures on animals were approved by the Animal Care and Used Committee with ethical clearance number 680-KEP-UB, Indonesia.
HUMAN AND ANIMAL RIGHTS
No humans were used in the study. All the reported experiments on animals were in accordance with the Committee for the update of the Guide for the Care and Use of Laboratory Animals.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the [Universitas Brawijaya, Doctoral Dissertation: Wendy Alexander Tanod, supervised by Yenny Risjani] at [http://ub.ac.id], reference number [In press, 2019].
FUNDING
Thanks to the Ministry of Research, Technology and Higher Education who have provided scholarship and research dissertation doctoral grants in 2018 (No. 1170/K9/KT.03/2018).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hu L.C., Yen W.H., Su J.H., Chiang M.Y.N., Wen Z.H., Chen W.F., Lu T.J., Chang Y.W., Chen Y.H., Wang W.H., Wu Y.C., Sung P.J. Cembrane derivatives from the soft corals, Sinularia gaweli and Sinularia flexibilis. Mar. Drugs. 2013;11(6):2154–2167. doi: 10.3390/md11062154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W., Li Y., Guo Y. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B. 2012;2(3):227–237. [Google Scholar]
- 3.Fattorusso E., Romano A., Taglialatela-Scafati O., Janib Achmad M., Bavestrello G., Cerrano C. Lobozoanthamine, a new zoanthamine-type alkaloid from the Indonesian soft coral Lobophytum sp. Tetrahedron Lett. 2008;49(14):2189–2192. [Google Scholar]
- 4.Lu Y., Huang C.Y., Lin Y.F., Wen Z.H., Su J.H., Kuo Y.H., Chiang M.Y., Sheu J.H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008;71(10):1754–1759. doi: 10.1021/np8003563. [DOI] [PubMed] [Google Scholar]
- 5.Duh C.Y., El-Gamal A.A.H., Chu C.J., Wang S.K., Dai C.F. New cytotoxic constituents from the Formosan soft corals Clavularia viridis and Clavularia violacea. J. Nat. Prod. 2002;65(11):1535–1539. doi: 10.1021/np0201873. [DOI] [PubMed] [Google Scholar]
- 6.Duh C.Y., Wang S.K., Weng Y.L. Brassicolene, a novel cytotoxic diterpenoid from the Formosan soft coral Nephthea brassica. Tetrahedron Lett. 2000;41(9):1401–1403. [Google Scholar]
- 7.Sheu J.H., Ahmed A.F., Shiue R.T., Dai C.F., Kuo Y.H. Scabrolides A-D, four new norditerpenoids isolated from the soft coral Sinularia scabra. J. Nat. Prod. 2002;65(12):1904–1908. doi: 10.1021/np020280r. [DOI] [PubMed] [Google Scholar]
- 8.Higa T., Tanaka J., Ohtani I.I., Musman M., Roy M.C., Kuroda I. Bioactive compounds from coral reef invertebrates. Pure Appl. Chem. 2001;73(3):589–593. [Google Scholar]
- 9.Coll J.C. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 1992;1002:613–631. [Google Scholar]
- 10.Leewis R.J., Janse M. Advances in coral husbandry in public Aquariums. Arnhen, The Netherlands: Burger’s Zoo; 2008. p. 2. [Google Scholar]
- 11.Carté B.K. Biomedical potential of marine natural products. BioSci. 1996;46(4):271–286. [Google Scholar]
- 12.Coll J.C., Bowden B.F., Tapiolas D.M., Willis R.H., Djura P., Streamer M., Trott L. Studies of Australian soft corals-XXXV. The terpenoid chemistry of soft corals and its implications. Tetrahedron. 1985;41(6):1085–1092. [Google Scholar]
- 13.Putra M.Y. Bioactive marine natural products from the Indonesian soft coral Sinularia sp. (order Alcyonacea, family Alcyoniidae). 2012 PhD Thesis. [Google Scholar]
- 14.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2013;30(2):237–323. doi: 10.1039/c2np20112g. [DOI] [PubMed] [Google Scholar]
- 15.Wei W.C., Sung P.J., Duh C.Y., Chen B.W., Sheu J.H., Yang N.S. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs. 2013;11(10):4083–4126. doi: 10.3390/md11104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng S.Y., Chuang C.T., Wen Z.H., Wang S.K., Chiou S.F., Hsu C.H., Dai C.F., Duh C.Y. Bioactive norditerpenoids from the soft coral Sinularia gyrosa. Bioorg. Med. Chem. 2010;18(10):3379–3386. doi: 10.1016/j.bmc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Rainsford K.D. Anti-inflammatory drugs in the 21st century. Inflammation in the pathogenesis of chronic diseases. Subcell. Biochem. 2007;42:3–27. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao T.H., Sung C.S., Lan Y.H., Wang Y.C., Lu M.C., Wen Z.H., Wu Y.C., Sung P.J. New anti-inflammatory cembranes from the cultured soft coral Nephthea columnaris. Mar. Drugs. 2015;13(6):3443–3453. doi: 10.3390/md13063443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin W.Y., Chen B.W., Huang C.Y., Wen Z.H., Sung P.J., Su J.H., Dai C.F., Sheu J.H. Bioactive cembranoids, sarcocrassocolides P-R, from the Dongsha Atoll soft coral Sarcophyton crassocaule. Mar. Drugs. 2014;12(2):840–850. doi: 10.3390/md12020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thao N.P., Luyen B.T.T., Ngan N.T.T., Song S.B., Cuong N.X., Nam N.H., Kiem P.V., Kim Y.H., Minh C.V. New anti-inflammatory cembranoid diterpenoids from the Vietnamese soft coral Lobophytum crassum. Bioorg. Med. Chem. Lett. 2014;24(1):228–232. doi: 10.1016/j.bmcl.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Roy P.K., Roy M.C., Taira J., Ueda K. Structure and bioactivity of a trisnorditerpenoid and a diterpenoid from an Okinawan soft coral, Cespitularia sp. Tetrahedron Lett. 2014;55(8):1421–1423. [Google Scholar]
- 22.Yin J., Zhao M., Ma M., Xu Y., Xiang Z., Cai Y., Dong J., Lei X., Huang K., Yan P. New casbane diterpenoids from a South China Sea soft coral, Sinularia sp. Mar. Drugs. 2013;11(2):455–465. doi: 10.3390/md11020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C.Y., Su J.H., Duh C.Y., Chen B.W., Wen Z.H., Kuo Y.H., Sheu J.H. A new 9,11-secosterol from the soft coral Sinularia granosa. Bioorg. Med. Chem. Lett. 2012;22(13):4373–4376. doi: 10.1016/j.bmcl.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Lin W.Y., Su J.H., Lu Y., Wen Z.H., Dai C.F., Kuo Y.H., Sheu J.H. Cytotoxic and anti-inflammatory cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. 2010;18(5):1936–1941. doi: 10.1016/j.bmc.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 25.Cheng S.Y., Huang Y.C., Wen Z.H., Chiou S.F., Wang S.K., Hsu C-H., Dai C.F., Duh C.Y. Novel sesquiterpenes and norergosterol from the soft corals Nephthea erecta and Nephthea chabroli. Tetrahedron Lett. 2009;50(7):802–806. [Google Scholar]
- 26.Newcombe E.A., Camats-Perna J., Silva M.L., Valmas N., Huat T.J., Medeiros R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflammation. 2018;15(1):276. doi: 10.1186/s12974-018-1313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCartney-Francis N.L., Song X., Mizel D.E., Wahl S.M., Sharon M.W. Selective inhibition of inducible nitric oxide synthase exacerbates erosive joint disease. J. Immunol. 2001;166(4):2734–2740. doi: 10.4049/jimmunol.166.4.2734. [DOI] [PubMed] [Google Scholar]
- 28.Kaplanski G., Marin V., Montero-Julian F., Mantovani A., Farnarier C. IL-6: A regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24(1):25–29. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 29.Hu G.P., Yuan J., Sun L., She Z.G., Wu J.H., Lan X.J., Zhu X., Lin Y.C., Chen S.P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs. 2011;9(4):514–525. doi: 10.3390/md9040514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marris E. Marine natural products: Drugs from the deep. Nature. 2006;443(7114):904–905. doi: 10.1038/443904a. [DOI] [PubMed] [Google Scholar]
- 31.Ianaro A., O’Donnell C.A., Di Rosa M., Liew F.Y. A nitric oxide synthase inhibitor reduces inflammation, down-regulates inflammatory cytokines and enhances interleukin-10 production in carrageenin-induced oedema in mice. Immunology. 1994;82(3):370–375. [PMC free article] [PubMed] [Google Scholar]
- 32.Risjani Y. Yunianta; Couteau, J.; Minier, C. Cellular immune responses and phagocytic activity of fishes exposed to pollution of volcano mud. Mar. Environ. Res. 2014;96:73–80. doi: 10.1016/j.marenvres.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Hinz B., Brune K. Cyclooxygenase-2-10 years later. J. Pharmacol. Exp. Ther. 2002;300(2):367–375. doi: 10.1124/jpet.300.2.367. [DOI] [PubMed] [Google Scholar]
- 34.Kwon T.H., Yoon I.H., Shin J.S., Lee Y.H., Kwon B.J., Lee K.T., Lee Y.S. Synthesis of indolyl-3-acetonitrile derivatives and their inhibitory effects on nitric oxide and PGE2 productions in LPS-induced RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2013;23(9):2571–2574. doi: 10.1016/j.bmcl.2013.02.114. [DOI] [PubMed] [Google Scholar]
- 35.Tapilatu Y.H. Status of drug discovery research based on marine organisms from Eastern Indonesia. Proc. Chem. 2015;14:484–492. [Google Scholar]
- 36.Yunianta Y., Risjani Y. Methanolic extract of Sargassum crystaefolium induce the apoptosis of human mammary carcinoma cells. Phycologia. 2017;56(4):838. [Google Scholar]
- 37.Chasanah E. Marine biodiscovery research in indonesia: Challenges and rewards. J. Coastal Dev. 2008;12(1):1–12. [Google Scholar]
- 38.Putra M.Y., Murniasih T., Swasono R.T., Wibowo J.T., Saputri A.N.C., Widhiana M.R., Arlyza I.S. Secondary metabolites and their biological activities in Indonesian soft coral of the genus Lobophytum. Asian Pac. J. Trop. Biomed. 2016;6(11):909–913. [Google Scholar]
- 39.Harborne J.B. Phytochemical Methods; A Guide to Modern Techniques of Plant Analysis. Published in the USA by Chapman and Hall in association with Methuen, Inc. 1998. [Google Scholar]
- 40.Chen B.W., Wang S.Y., Huang C.Y., Chen S.L., Wu Y.C., Sheu J.H. Hirsutalins I-M, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. Tetrahedron. 2013;69(10):2296–2301. [Google Scholar]
- 41.Ravipati A.S., Zhang L., Koyyalamudi S.R., Jeong S.C., Reddy N., Bartlett J., Smith P.T., Shanmugam K., Münch G., Wu M.J., Satyanarayanan M., Vysetti B. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012;12:173–187. doi: 10.1186/1472-6882-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fattorusso E., Luciano P., Putra M.Y., Taglialatela-Scafati O., Ianaro A., Panza E., Bavestrello G., Cerrano C. Chloroscabrolides, chlorinated norcembranoids from the Indonesian soft coral Sinularia sp. Tetrahedron. 2011;67(41):7983–7988. [Google Scholar]
- 43.Zhang X., Goncalves R., Mosser D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008;14:1–18. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakr R.O., El-Naa M.M., Zaghloul S.S., Omar M.M. Profile of bioactive compounds in Nymphaea alba L. leaves growing in Egypt: Hepatoprotective, antioxidant and anti-inflammatory activity. BMC Complement. Altern. Med. 2017;17(1):52. doi: 10.1186/s12906-017-1561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komalavalli T., Packia Lincy M., Muthukumarasamy S., Mohan V.R. Determination of bioactive components of Asystasia travancorica Bedd (Acanthaceae) by GC-MS Analysis. Int. J. Pharm. Clin. Res. 2014;6(2):155–158. [Google Scholar]
- 46.Sahi N.M. Evaluation of insecticidal activity of bioactive compounds from Eucalyptus citriodora against Tribolium castaneum. Int. J. Pharmacogn. Phytochem. Res. 2016;8(8):1256–1270. [Google Scholar]
- 47.Okagu I.U., Ngwu U.E., Odenigbo C.J. Bioactive constituents of methanol extract of Xylopia aethiopica (UDA) fruits from Nsukka, Enugu State, Nigeria. OAlib. 2018;5(3):1–11. [Google Scholar]
- 48.Kim J.E., Park K.M., Lee S.Y., Seo J.H., Yoon I.S., Bae C.S., Yoo J.C., Bang M.A., Cho S.S., Park D.H. Anti-inflammatory effect of Allium hookeri on carrageenan-induced air pouch mouse model. PLoS One. 2017;12(12):e0190305. doi: 10.1371/journal.pone.0190305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chao C.H., Wen Z.H., Su J.H., Chen I.M., Huang H.C., Dai C.F., Sheu J.H. Further study on anti-inflammatory oxygenated steroids from the octocoral Dendronephthya griffini. Steroids. 2008;73(14):1353–1358. doi: 10.1016/j.steroids.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Folmer F., Jaspars M., Solano G., Cristofanon S., Henry E., Tabudravu J., Black K., Green D.H., Küpper F.C., Aalbersberg W., Feussner K., Dicato M., Diederich M. The inhibition of TNF-α-induced NF-kappaB activation by marine natural products. Biochem. Pharmacol. 2009;78(6):592–606. doi: 10.1016/j.bcp.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Hassan M.H.A., Mohammed R., Hetta M.H., Abdelaziz T.A., El-Gendy A.O., Sleim M.A. Biological and chemical investigation of the soft coral Lobophytum pauciflorum collected from the Egyptian Red Sea. Int. J. Pharmacogn. Phytochem. Res. 2016;8(6):906–911. [Google Scholar]
- 52.Pandiyan R., Subbiah L., Palanisamy S., Ariyamuthu S., Muthusamy T., Velu R.K. A wide array on anti-inflammatory study in an ethanolic extract of Pupalia lappaceae juss. (amaranthaceae) by using wistar rats. Arch. Appl. Sci. Res. 2009;1(2):150–158. [Google Scholar]
- 53.Salem M.Z.M., Elansary H.O., Ali H.M., El-Settawy A.A., Elshikh M.S., Abdel-Salam E.M., Skalicka-Woźniak K. Bioactivity of essential oils extracted from Cupressus macrocarpa branchlets and Corymbia citriodora leaves grown in Egypt. BMC Complement. Altern. Med. 2018;18(1):23. doi: 10.1186/s12906-018-2085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saravanakumar K., Vivek R., Sithranga Boopathy N., Yaqian L., Kathiresan K., Chen J. Anticancer potential of bioactive 16-methylheptadecanoic acid methyl ester derived from marine trichoderma. J. Appl. Biomed. 2015;13(3):199–212. [Google Scholar]
- 55.Ahmed H.H., Abd-Rabou A.A., Hassan A.Z., Kotob S.E. Phytochemical analysis and anti-cancer investigation of Boswellia serrata bioactive constituents in vitro. Asian Pac. J. Cancer Prev. 2015;16(16):7179–7188. doi: 10.7314/apjcp.2015.16.16.7179. [DOI] [PubMed] [Google Scholar]
- 56.Barrero A.F., Herrador M.M., Quílez del Moral J.F., Arteaga P., Meine N., Pérez-Morales M.C., Catalán J.V. Efficient synthesis of the anticancer β-elemene and other bioactive elemanes from sustainable germacrone. Org. Biomol. Chem. 2011;9(4):1118–1125. doi: 10.1039/c0ob00467g. [DOI] [PubMed] [Google Scholar]
- 57.Akaberi M., Iranshahy M., Iranshahi M. Review of the traditional uses, phytochemistry, pharmacology and toxicology of giant fennel (Ferula communis L. subsp. communis). Iran. J. Basic Med. Sci. 2015;18(11):1050–1062. [PMC free article] [PubMed] [Google Scholar]
- 58.Byju K., Anuradha V., Rosmine E., Sankar H.S.H., Gopinath A., Peter K.J.P., Kumar T.R.G., Vasundhara G., Kumar N.C., Nair S.M. DPPH scavenging property of active principles from soft coral Sarcophytonf flexuosum Tixier-Durivault. Pharm. Chem. J. 2015;49(3):178–182. [Google Scholar]
- 59.Ashraf A., Sarfraz R.A., Anwar F., Shahid S.A., Alkharfy K.M. Chemical composition and biological activities of leaves of Ziziphus mauritiana L. Native to Pakistan. Pak. J. Bot. 2015;47(1):367–376. [Google Scholar]
- 60.Joel E.L., Valentin Bhimba B. Evaluation of secondary metabolites from mangrove associated fungi Meyerozyma guilliermondii. Alexandria J. Med. 2013;49(3):189–194. [Google Scholar]
- 61.Hu J., Yang B., Lin X., Zhou X., Yang X., Long L., Liu Y. Chemical and biological studies of soft corals of the Nephtheidae family. Chem. Biodivers. 2011;8(6):1011–1032. doi: 10.1002/cbdv.201000105. [DOI] [PubMed] [Google Scholar]
- 62.Mathan S., Smith A.A., Kumaran J., Prakash S. Anticancer and antimicrobial activity of Aspergillus protuberus SP1 isolated from marine sediments of South Indian coast. Chin. J. Nat. Med. 2011;9(4):286–292. [Google Scholar]
- 63.Lavilla C.A., Jr, Uy M.M., Ohta S. Cytotoxic long-chain alkene and terpene isolated from the methanol extract of the air-dried leaves of Pipturus arborescens C.B. Rob. J. Multidiscip. Stud. 2014;3(1):16–26. [Google Scholar]
- 64.Sianipar N.F., Purnamaningsih R. Enhancement of the contents of anticancer bioactive compounds in mutant clones of rodent tuber (Typhonium flagelliforme Lodd.) based on GC-MS analysis. Pertanika, J. Trop. Agric. Sci. 2018;41(1):305–320. [Google Scholar]
- 65.Ahmad B., Khan I., Bashir S., Azam S. Chemical composition and antifungal, phytotoxic, brine shrimp cytotoxicity, insecticidal and antibacterial activities of the essential oils of Acacia modesta. J. Med. Plants Res. 2012;6(31):4653–4659. [Google Scholar]
- 66.Apri R., Zamani N.P., Effendi H. Exploration of soft coral as antioxidant at Pongok Island, South Bangka. J. Fish. Mar. Technol. 2013;4(2):211–217. [Google Scholar]
- 67.Balboa E.M., Conde E., Moure A., Falqué E., Domínguez H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013;138(2-3):1764–1785. doi: 10.1016/j.foodchem.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 68.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 69.Bhardwaj A., Huang Z., Kaur J., Knaus E.E. Rofecoxib analogues possessing a nitric oxide donor sulfohydroxamic acid (SO2NHOH) cyclooxygenase-2 pharmacophore: Synthesis, molecular modeling, and biological evaluation as anti-inflammatory agents. ChemMedChem. 2012;7(1):62–67. doi: 10.1002/cmdc.201100393. [DOI] [PubMed] [Google Scholar]
- 70.El-Gamal M.I., Lee W.S., Shin J.S., Oh C.H., Lee K.T., Choi J., Myoung N., Baek D. Synthesis of new tricyclic and tetracyclic fused coumarin sulfonate derivatives and their inhibitory effects on LPS-induced nitric oxide and PGE2 productions in RAW 264.7 Macrophages: Part 2. Arch. Pharm. (Weinheim) 2016;349(11):853–863. doi: 10.1002/ardp.201600243. [DOI] [PubMed] [Google Scholar]
- 71.Bhardwaj A., Batchu S.N., Kaur J., Huang Z., Seubert J.M., Knaus E.E. Cardiovascular properties of a nitric oxide releasing rofecoxib analogue: Beneficial anti-hypertensive activity and enhanced recovery in an ischemic reperfusion injury model. ChemMedChem. 2012;7(8):1365–1368. doi: 10.1002/cmdc.201200234. [DOI] [PubMed] [Google Scholar]
- 72.Yun H.Y., Dawson V.L., Dawson T.M. Neurobiology of nitric oxide. Crit. Rev. Neurobiol. 1996;10(3-4):291–316. doi: 10.1615/critrevneurobiol.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]
- 73.Eltahawy N.A., Ibrahim A.K., Radwan M.M., ElSohly M.A., Hassanean H.A., Ahmed S.A. Cytotoxic cembranoids from the Red Sea soft coral, Sarcophyton auritum. Tetrahedron Lett. 2014;55(29):3984–3988. [Google Scholar]
- 74.Hegazy M.E.F., Mohamed T.A., Abdel-Latif F.F., Alsaid M.S., Shahat A.A., Paré P.W. Trochelioid A and B, new cembranoid diterpenes from the Red Sea soft coral Sarcophyton trocheliophorum. Phytochem. Lett. 2013;6(3):383–386. [Google Scholar]
- 75.Blunt J.W., Copp B.R., Hu W.P., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2008;25(1):35–94. doi: 10.1039/b701534h. [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi J., Ohizumi Y., Nakamura H., Yamakado T., Matsuzaki T., Hirata Y. Ca-antagonistic substance from soft coral of the genus Sarcophyton. Experientia. 1983;39(1):67–69. doi: 10.1007/BF01960632. [DOI] [PubMed] [Google Scholar]
- 77.Liang L.F., Guo Y.W. Terpenes from the soft corals of the genus Sarcophyton: Chemistry and biological activities. Chem. Biodivers. 2013;10(12):2161–2196. doi: 10.1002/cbdv.201200122. [DOI] [PubMed] [Google Scholar]
- 78.Cheng S.Y., Wen Z.H., Chiou S.F., Tsai C.W., Wang S.K., Hsu C.H., Dai C.F., Chiang M.Y., Wang W.H., Duh C.Y. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2009;72(3):465–468. doi: 10.1021/np800362g. [DOI] [PubMed] [Google Scholar]
- 79.Thao N.P., Luyen B.T.T., Sun Y.N., Song S.B., Thanh N.V., Cuong N.X., Nam N.H., Kiem P.V., Kim Y.H., Minh C.V. NF-κB inhibitory activity of polyoxygenated steroids from the Vietnamese soft coral Sarcophyton pauciplicatum. Bioorg. Med. Chem. Lett. 2014;24(13):2834–2838. doi: 10.1016/j.bmcl.2014.04.103. [DOI] [PubMed] [Google Scholar]
- 80.Wang S.K., Puu S.Y., Duh C.Y. New steroids from the soft coral Nephthea chabrolii. Mar. Drugs. 2013;11(2):571–580. doi: 10.3390/md11020571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishii T., Matsuura H., Zhaoqi Z., Vairappan C.S. A new 4alpha-methylated sterol from a Nephthea sp. (Nephtheidae) Bornean soft coral. Molecules. 2009;14(9):3360–3366. doi: 10.3390/molecules14093360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amir F., Koay Y.C., Yam W.S. Chemical constituents and biological properties of the marine soft coral Nephthea : A review (Part 1). Trop. J. Pharm. Res. 2012;11(3):485–498. [Google Scholar]
- 83.Huang Y.C., Wen Z.H., Wang S.K., Hsu C.H., Duh C.Y. New anti-inflammatory 4-methylated steroids from the Formosan soft coral Nephthea chabroli. Steroids. 2008;73(11):1181–1186. doi: 10.1016/j.steroids.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 84.Su J.H., Wen Z.H. Bioactive cembrane-based diter-penoids from the soft coral Sinularia triangular. Mar. Drugs. 2011;9(6):944–951. doi: 10.3390/md9060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su J.H., Ahmed A.F., Sung P.J., Chao C.H., Kuo Y.H., Sheu J.H. Manaarenolides A-I, diterpenoids from the soft coral Sinularia manaarensis. J. Nat. Prod. 2006;69(8):1134–1139. doi: 10.1021/np050483q. [DOI] [PubMed] [Google Scholar]
- 86.Rudi A., Aknin M., Gaydou E.M., Kashman Y., Kashman Y. Several new isoprenoids from the soft coral Sinularia erecta. J. Nat. Prod. 1998;61(7):872–875. doi: 10.1021/np9705064. [DOI] [PubMed] [Google Scholar]
- 87.Chen D., Cheng W., Liu D., van Ofwegen L., Proksch P., Lin W. Capillosananes S-Z, new sesquiterpenoids from the soft coral Sinularia capillosa. Tetrahedron Lett. 2014;55(19):3077–3082. [Google Scholar]
- 88.Chao C.H., Hsieh C.H., Chen S.P., Lu C.K., Dai C.F., Sheu J.H. Sinularianins A and B, novel sesquiterpenoids from the Formosan soft coral Sinularia sp. Tetrahedron Lett. 2006;47(33):5889–5891. [Google Scholar]
- 89.Chao C.H., Hsieh C.H., Chen S.P., Lu C.K., Dai C.F., Wu Y.C., Sheu J.H. Novel cyclic sesquiterpene peroxides from the Formosan soft coral Sinularia sp. Tetrahedron Lett. 2006;47(13):2175–2178. [Google Scholar]
- 90.Lai D., Li Y., Xu M., Deng Z., van Ofwegen L., Qian P., Proksch P., Lin W. Sinulariols A-S, 19-oxygenated cembranoids from the Chinese soft coral Sinularia rigida. Tetrahedron. 2011;67(33):6018–6029. [Google Scholar]
- 91.Duh C.Y., Wang S.K., Tseng H.K., Sheu J.H., Chiang M.Y. Novel cytotoxic cembranoids from the soft coral Sinularia flexibilis. J. Nat. Prod. 1998;61(6):844–847. doi: 10.1021/np980021v. [DOI] [PubMed] [Google Scholar]
- 92.Liang C.H., Wang G.H., Hung W.J., Lin R.J., Cheng D.L., Chou T.H. Apoptosis effect of Sinularia leptoclados, S. depressan and S. inflate extracts in human oral squamous cell carcinomas. J. Taiwan Institute Chem. Eng. 2010;41(1):86–91. [Google Scholar]
- 93.Putra M.Y., Ianaro A., Panza E., Bavestrello G., Cerrano C., Fattorusso E., Taglialatela-Scafati O. Sinularioside, a triacetylated glycolipid from the Indonesian soft coral Sinularia sp., is an inhibitor of NO release. Bioorg. Med. Chem. Lett. 2012;22(8):2723–2725. doi: 10.1016/j.bmcl.2012.02.102. [DOI] [PubMed] [Google Scholar]
- 94.Chao C.H., Chou K.J., Huang C.Y., Wen Z.H., Hsu C.H., Wu Y.C., Dai C.F., Sheu J.H. Steroids from the soft coral Sinularia crassa. Mar. Drugs. 2012;10(2):439–450. doi: 10.3390/md10020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shih H.J., Tseng Y.J., Huang C.Y., Wen Z.H., Dai C.F., Sheu J.H. Cytotoxic and anti-inflammatory diterpenoids from the Dongsha Atoll soft coral Sinularia flexibilis. Tetrahedron. 2012;68(1):244–249. [Google Scholar]
- 96.Chao C.H., Chou K.J., Huang C.Y., Wen Z.H., Hsu C.H., Wu Y.C., Dai C.F., Sheu J.H. Bioactive cembranoids from the soft coral Sinularia crassa. Mar. Drugs. 2011;9(10):1955–1968. doi: 10.3390/md9101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu Y., Su H.J., Chen Y.H., Wen Z.H., Sheu J.H., Su J.H. Anti-inflammatory cembranoids from the Formosan soft coral Sinularia discrepans. Arch. Pharm. Res. 2011;34(8):1263–1267. doi: 10.1007/s12272-011-0804-x. [DOI] [PubMed] [Google Scholar]
- 98.Chen B.W., Chao C.H., Su J.H., Huang C.Y., Dai C.F., Wen Z.H., Sheu J.H. A novel symmetric sulfur-containing biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 2010;51(44):5764–5766. [Google Scholar]
- 99.Cheng S.Y., Chuang C.T., Wang S.K., Wen Z.H., Chiou S.F., Hsu C.H., Dai C.F., Duh C.Y. Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. J. Nat. Prod. 2010;73(6):1184–1187. doi: 10.1021/np100185a. [DOI] [PubMed] [Google Scholar]
- 100.Lu Y., Huang C-Y., Lin Y., Wen Z., Kuo Y., Chiang M.Y., Sheu J.H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. Chem. Pharm. Bull. (Tokyo) 2010;58(4):464–466. doi: 10.1248/cpb.58.464. [DOI] [PubMed] [Google Scholar]
- 101.Ahmed A.F., Tai S.H., Wen Z.H., Su J.H., Wu Y.C., Hu W.P., Sheu J.H.A.A. C-3 methylated isocembranoid and 10-oxocembranoids from a formosan soft coral, Sinularia grandilobata. J. Nat. Prod. 2008;71(6):946–951. doi: 10.1021/np7007335. [DOI] [PubMed] [Google Scholar]
- 102.Ahmed A.F., Wen Z.H., Su J.H., Hsieh Y.T., Wu Y.C., Hu W.P., Sheu J.H. Oxygenated cembranoids from a Formosan soft coral Sinularia gibberosa. J. Nat. Prod. 2008;71(2):179–185. doi: 10.1021/np070356p. [DOI] [PubMed] [Google Scholar]
- 103.Ulrich-Merzenich G., Panek D., Zeitler H., Vetter H., Wagner H. Drug development from natural products: Exploiting synergistic effects. Indian J. Exp. Biol. 2010;48(3):208–219. [PubMed] [Google Scholar]
- 104.Long F., Yang H., Xu Y., Hao H., Li P. A strategy for the identification of combinatorial bioactive compounds contributing to the holistic effect of herbal medicines. Nature Publishing Group. 2015;5:1–11. doi: 10.1038/srep12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Faridha B.I., Mohankumar R., Jeevan M., Ramani K. GC-MS analysis of bio-active molecules derived from Paracoccus pantotrophus FMR19 and the antimicrobial activity against bacterial pathogens and MDROs. Indian J. Microbiol. 2016;56(4):426–432. doi: 10.1007/s12088-016-0609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Su J-H., Ahmed A.F., Sung P.J., Wu Y-C., Sheu J-H. Meroditerpenoids from a Formosan soft coral Nephthea chabrolii. J. Nat. Prod. 2005;68(11):1651–1655. doi: 10.1021/np050278a. [DOI] [PubMed] [Google Scholar]
- 107.Rawal J.R., Sonawani P.R. Determination of bioactive components of Cynodon dactylon by GC-MS analysis & it’s in vitro antimicrobial activity. Int. J. Pharm. Life Sci. 2016;7(1):4880–4885. [Google Scholar]
- 108.Bhardwaj A., Shakil N.A., Jha V., Gupta R.K. Screening of nutritional, phytochemical, antioxidant and antibacterial activity of underutilized seeds of Scirpus articulatus the basis of Khubahi Ramdana Industry. J. Pharmacogn. Phytochem. 2014;3(4):11–20. [Google Scholar]
- 109.Zubair M., Hassan S., Rizwan K., Rasool N., Riaz M., Zia-Ul-Haq M., De Feo V. Antioxidant potential and oil composition of Callistemon viminalis leaves. ScientificWorldJournal. 2013;2013:489071. doi: 10.1155/2013/489071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Al-Abd N.M., Nor Z.M., Mansor M., Zajmi A., Hasan M.S., Azhar F., Kassim M. Phytochemical constituents, antioxidant and antibacterial activities of methanolic extract of Ardisia elliptica. Asian Pac. J. Trop. Biomed. 2017;7(6):569–576. [Google Scholar]
- 111.Arulselvan P., Fard M.T., Tan W.S., Gothai S., Fakurazi S., Norhaizan M.E., Kumar S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016;2016:1–15. doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hämäläinen M. Inducible Nitric Oxide Synthase as a Target of Treatment Modalities. 2008. [Google Scholar]
- 113.Sharma J.N., Al-Omran A., Parvathy S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15(6):252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 114.Momi S., Monopoli A., Alberti P.F., Falcinelli E., Corazzi T., Conti V., Miglietta D., Ongini E., Minuz P., Gresele P. Nitric oxide enhances the anti-inflammatory and anti-atherogenic activity of atorvastatin in a mouse model of accelerated atherosclerosis. Cardiovasc. Res. 2012;94(3):428–438. doi: 10.1093/cvr/cvs100. [DOI] [PubMed] [Google Scholar]
- 115.Wallace J.L. Nitric oxide as a regulator of inflammatory processes. Mem. Inst. Oswaldo Cruz. 2005;100(Suppl. 1):5–9. doi: 10.1590/s0074-02762005000900002. [DOI] [PubMed] [Google Scholar]
- 116.Hämäläinen M., Lilja R., Kankaanranta H., Moilanen E. Inhibition of iNOS expression and NO production by anti-inflammatory steroids. Reversal by histone deacetylase inhibitors. Pulm. Pharmacol. Ther. 2008;21(2):331–339. doi: 10.1016/j.pupt.2007.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the article is available in the [Universitas Brawijaya, Doctoral Dissertation: Wendy Alexander Tanod, supervised by Yenny Risjani] at [http://ub.ac.id], reference number [In press, 2019].


