Abstract
BACKGROUND
Positive family history is a risk factor for development of colorectal cancer. Despite numerous studies on the topic, the absolute risk in patients with a positive family history remains unclear and therefore studies are lacking to validate non-invasive screening methods in individuals with positive family history.
AIM
To quantify the risk of colorectal cancer in individuals with a positive family history.
METHODS
A comprehensive electronic literature search was performed using PubMed from January 1955 until November 2017, EMBASE from 1947 until 2018, and Cochrane Library without date restrictions. Two independent reviewers conducted study selection, data extraction and quality assessment. A meta-analysis of Mantel-Haenzel relative risks was performed using the random effects model. Newcastle-Ottawa scale was used to score the quality of selected papers. Funnel plot and Egger’s regression test was performed to detect publication bias. Subgroup analysis was performed comparing Asian and non-Asian studies. Sensitivity analyses were performed to rule out the effect of the timing of the study, overall quality, the main outcome and the effect of each individual study in overall result.
RESULTS
Forty-six out of 3390 studies, including 906981 patients were included in the final analysis. 41 of the included studies were case-control and 5 were cohort. A positive family history of colorectal cancer in first-degree relatives was associated with significantly increased risk of colorectal cancer with a relative risk of 1.87 (95%CI: 1.68-2.09; P < 0.00001). Cochrane Q test was significant (P < 0.00001, I2 = 90%). Egger’s regression test showed asymmetry in the funnel plot and therefore the Trim and Fill method was used which confirmed the validity of the results. There was no difference between Asian versus non-Asian studies. Results remained robust in sensitivity analyses.
CONCLUSION
Individuals with a positive family history of colorectal cancer are 1.87 times more likely to develop colorectal cancer. Screening guidelines should pay specific attention to individuals with positive family history and further studies need to be done on validating current screening methods or developing new modalities in this high-risk population.
Keywords: Colorectal cancer, Risk, Family history
Core tip: The increased risk of developing colorectal cancer in individuals with a positive family history remains unknown. Many independent studies have provided different numerical risks with relatively large differences between the values. Here, we have performed a systematic review and meta-analysis to provide a more accurate estimate of this increased risk in an attempt to aid future guideline making and help implement preventative measures for at-risk individuals.
INTRODUCTION
Colorectal cancer is the third most common cancer and the fourth leading cancer-related cause of death worldwide[1]. Most colorectal cancer seems to have a stepwise progression from precancerous lesions[2]. As an example, the number, size and physical characteristics of adenomas can determine the likelihood of malignant transformation[3]. Presence of advanced colorectal adenomas characterized by a large size (greater than 1 cm), high multiplicity (more than 3 adenomas), villous mor-phology and high grade dysplasia results in higher risk of developing colorectal cancer[4]. The incidence of colorectal cancer is expected to increase in the future, leading to an additional 1.1 million deaths by the year 2030[5]. Given the morbidity and mortality associated with this cancer, it is important for clinicians to understand the quantitative risk associated with various risk factors.
Several environmental and hereditary factors are known as the risk factors for colorectal cancer[6]. Some of these include previous history of inflammatory bowel diseases (ulcerative colitis and Crohn’s disease), high amounts of processed meat in the diet, high body fat, cigarette smoking and low fruit and vegetable consumption[7]. In addition, patients with inherited conditions such as, hereditary non-polyposis colorectal cancer (HNPCC) and familial adenomatous polyposis (FAP) as well those with a positive family history of colorectal cancer in relatives are at a higher risk of developing this condition[8]. An old meta-analysis of 27 studies attempted to determine the risk associated with colorectal cancer in individuals with a positive family history of the condition, however, many newer studies have been published and the recommended methodology to perform conventional meta-analysis has since significantly changed specially in the area of risk of bias assessment[9].
Several case-control and cohort studies from different regions around the world have attempted to quantify the risk of familial colorectal cancer[9]. However, substantial variability is present amongst the estimated risks in different publications. Therefore, despite availability of multiple screening modalities for colorectal precancerous and cancerous lesions such as colonoscopy, fecal occult blood test (FOBT), and fecal immunochemical test (FIT), guidelines either lack specific recommendations for preventative screening in individuals with a positive family history of colorectal cancer or make conditional recommendation based upon quality evidence[10-12]. Therefore, the aim of this study was to systematically review these papers and perform a meta-analysis according to Cochrane Group Methodology to provide a more accurate estimate for the risk of colorectal cancer associated with a positive family history of the disease in first-degree relatives of the patient[13].
MATERIALS AND METHODS
Registration
The study protocol was registered (CRD42018094964) in the International prospective register of systematic reviews (PROSPERO).
Search strategy
Comprehensive electronic searches of PubMed from January 1955 until November 2017, EMBASE from 1947 until 2018, and Cochrane Library without date restrictions were performed using a highly sensitive search strategy to identify studies with MeSH headings and text words which included (1) Family, (2) Colorectal Cancer, (3) Medical History. No language restriction was applied. In addition, the bibliography of selected articles were manually searched to find any additional studies for our meta-analysis.
Inclusion criteria
Case-control studies were included if they involved colon, rectal or colorectal cancer patients as cases and non-colorectal cancer patients as controls. The exposure of interest was a positive family history of colorectal cancer in first-degree relatives of patients. Additionally, cohort studies were eligible for inclusion if they followed individuals with positive and negative family history of colorectal cancer in first-degree relatives and assessed the patients for the outcome of colorectal cancer. Studies which did not clearly define relatives as first-degree relatives were also included, however, we planned to do a sensitivity analysis to investigate their effect on overall result.
Exclusion criteria
Abstracts, studies with insufficient data that did not allow for independent calculation of relative risk, paediatric studies, as well as duplicate studies were excluded. Moreover, we excluded studies which relied on the same patient databases and medical records during overlapping patient recruitment periods to avoid du-plications. Studies which included patients with known hereditary conditions (FAP and HNPCC) or inflammatory bowel diseases were excluded. Studies that reported family history without specifying colorectal cancer were not included in the analysis.
Outcome measure
The main outcome of interest in this meta-analysis was the relative risk of colorectal cancer in first-degree relatives of patients. We independently calculated relative risk based on original data presented in the studies[14].
Reliability
In order to reduce the risk of selection bias, two independent reviewers performed the literature search, data extraction and quality assessment. In cases where an agreement could not be reached, a third reviewer was involved.
Risk of bias
Newcastle-Ottawa scale (NOS) for the assessment of risk of bias in non-randomized studies was sued to assess the quality of the included studies[15]. The score ranged from 0 to 9 based on three categories: Selection, comparability and exposure/ outcome[15]. We defined a score greater than 5 as high quality and any score equal or less than 5 was considered low.
Publication bias
We did not restrict our search strategy based on language, risk of bias, sample size or geographical location of the study. A funnel plot analysis was also performed to assess the likelihood of publication bias[16]. Egger’s regression test was also performed to detect asymmetries in the funnel plot[17]. Comprehensive Meta-analysis Version 3.0 was used for Egger’s regression analysis for assessing asymmetries in the funnel plot and for Trim and Fill sensitivity analysis[18]. P values less than 0.05 were considered significant for the significance of asymmetry.
Statistical analysis
Review Manager 5.3 was used to perform a meta-analysis of random model Mantel-Haenzel relative risk for case control and cohort studies[18]. P values less than 0.05 were considered to be statistically significant. Higgins I2 and Cochran’s Q were used to measure heterogeneity as recommended by Cochrane Collaboration[19]. Additional subgroup and sensitivity analyses were planned a priori to investigate sources of heterogeneity in the result. Subgroup analysis was performed based on the geographical location of the study by separately analyzing Asian and non-Asian studies. Several sensitivity analyses were also conducted by excluding the largest included trial as well as each included study by turn to ensure none single study has significantly changed the conclusion of the study. P values less than 0.10 were considered statistically significant for heterogeneity. Additional subgroup and sensitivity analyses were planned a priori to investigate sources of heterogeneity in the result. Subgroup analysis was performed based on the geographical location of the study by separately analyzing Asian and non-Asian studies given different prevalence of colorectal in these two areas. Several sensitivity analyses were also conducted by excluding the largest included trial as well as each included study by turn to ensure none single study has significantly changed the conclusion of the study. results were presented with 95% confidence intervals whenever possible.
RESULTS
Characteristics of included studies
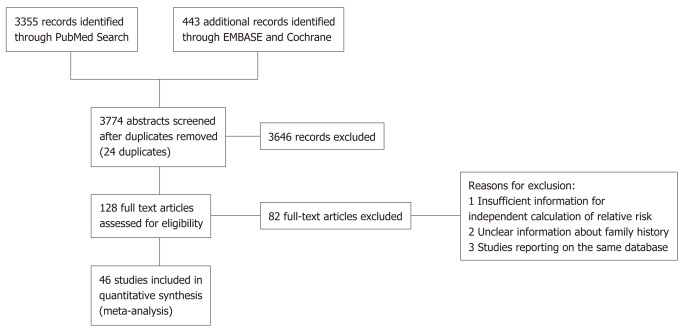
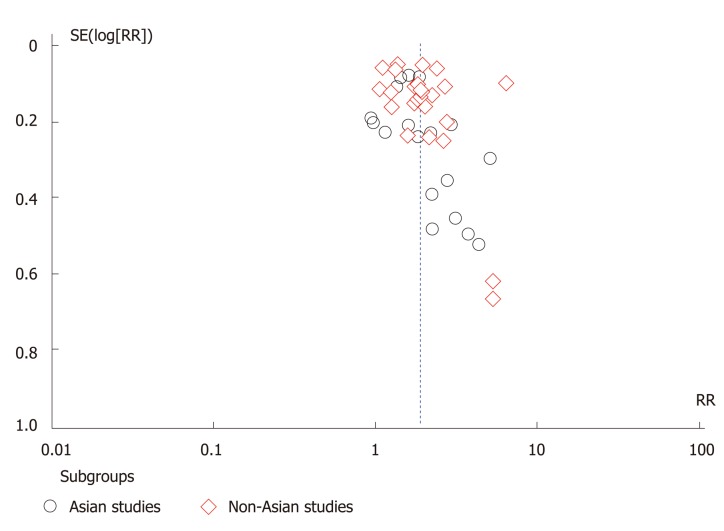
Of 3390 studies identified, 46 studies including 906981 patients were included in the final analysis. All studies with exception of one were written in the English[20]. Figure 1 depicts the PRISMA diagram for our literature search. 41 of the included studies were case control and 5 were prospective and retrospective cohort. In total, there were 47898 colorectal cancer patients and 320360 control subjects included in the case control studies. In addition, there were 68345 patients with a positive family history of colorectal cancer, and 470378 subjects without a family history of colorectal cancer. Table 1 contains detailed information about the studies included. We observed small visual asymmetry in the funnel plot (Figure 2) and Egger’s regression for the detection of asymmetry (Figure 3) in the funnel plot was statistically significant (P = 0.047).
Figure 1.
PRISMA diagram of the literature search conducted.
Table 1.
Characteristics of the included studies (n = 46)
| Study author | Year of publication | Type of study | Country of origin | Type of family history | Study period (start-end) | Type of outcome | Nos quality scale |
| Arafa et al[33] | 2011 | Case control | Jordan | FDR | 2008-2009 | Secondary | 6 |
| Bener et al[34] | 2010 | Case control | Qatar | FDR | 2003-2008 | Primary | 7 |
| Bonelli et al[35] | 1988 | Case control | Italy | FDR | 1980-1986 | Primary | 4 |
| Castiglione et al[36] | 2012 | Case control | Italy | FDR | 1995-2009 | Primary | 4 |
| Centonze et al[37] | 1993 | Case control | Italy | FDR | 1987-1989 | Primary | 8 |
| Crockett et al[38] | 2012 | Case control | United States | FDR | 2001-2006 | Secondary | 7 |
| Emami et al[39] | 2015 | Case control | Iran | FDR | N/A | Secondary | 5 |
| Fernandez et al[40] | 2002 | Case control | Italy | FDR | 1985-1992 | Secondary | 5 |
| Freedman et al[41] | 1996 | Case control | United States | FDR | 1982-1992 | Secondary | 6 |
| Fuchs (health professional cohort) et al[42] | 1994 | Prospective | United States | FDR | 1986-1992 | Primary | 3 |
| Fuchs (nurse health cohort) | 1994 | Prospective | United States | FDR | 1982-1990 | Primary | 3 |
| Grosso et al[43] | 2014 | Case control | Italy | Unclear | 2000-2012 | Secondary | 7 |
| Guo et al[44] | 2010 | Case control | China | At least one FDR or two or more SDR | 2007 | Secondary | 7 |
| Huang et al[45] | 2004 | Case control | Japan | FDR | 1988-1998 | Secondary | 2 |
| Ibáñez-sanz et al[46] | 2017 | Case control | Spain | FDR, SDR, TDR | 2008-2013 | Secondary | 7 |
| Il'yasova et al[47] | 2003 | Case control | United States | Unclear | 1996-2000 | Secondary | 7 |
| Jia et al[20] | 2007 | Case control | China | FDR | 2003-2005 | Secondary | 7 |
| Jo et al[48] | 2012 | Case control | South Korea | Unclear | 2004-2007 | Secondary | 3 |
| Kampman et al[49] | 2000 | Case control | United States | FDR | 1991-1994 | Secondary | 6 |
| Kim et al[50] | 2009 | Case control | South Korea | FDR | 2001-2004 | Secondary | 4 |
| Kotake et al[51] | 1995 | Case control | Japan | FDR | 1992-1994 | Primary | 6 |
| Kune et al[52] | 2009 | Case control | Australia | FDR | 1980-1981 | Primary | 7 |
| La vecchia et al[53] | 1996 | Case control | Italy | FDR | 1985-1992 | Secondary | 4 |
| Le merchand et al[54] | 1999 | Case control | United States | FDR | 1987-1991 | Secondary | 8 |
| Lee et al[55] | 2014 | Retrospective | Sweden | FDR: Sibling only | 1958-2009 | Primary | 5 |
| Lohsoonthorn et al[26] | 1995 | Case control | Thailand | FDR: Parents only | N/A | Primary | 7 |
| Mahmoudi et al[56] | 2014 | Case control | Iran | Unclear | 2009-2012 | Secondary | 4 |
| Mahmoudi et al[57] | 2016 | Case control | Iran | Unclear | 2008-2012 | Secondary | 4 |
| Minami et al[58] | 2003 | Case control | Japan | FDR | 1997-2001 | Secondary | 5 |
| Morois et al[59] | 2014 | Prospective | France | FDR | 1990-2008 | Secondary | 4 |
| Newcomb et al[60] | 1999 | Case control | United States | FDR | 1990-1991 | Primary | 7 |
| Otani et al[61] | 2006 | Case control | Japan | Unclear | 1990-2003 | Secondary | 7 |
| Pou et al[61] | 2012 | Case control | Argentina | FDR | 2006-2010 | Secondary | 7 |
| Rennert et al[62] | 2010 | Case control | Israel | FDR | N/A | Secondary | 5 |
| Rosenberg et al[63] | 1998 | Case control | United States | FDR | 1992 -1994 | Secondary | 8 |
| Russo et al[64] | 1998 | Case control | Italy | Unclear | 1992-1996 | Secondary | 4 |
| Samadder et al[65] | 2016 | Case control | United States | FDR | 2000-2010 | Secondary | 7 |
| Schoen et al[66] | 2015 | Prospective | United States | FDR | 1993-2001 | Primary | 5 |
| Senda-nakagawa herpacc (i) et al[67] | 2017 | Case control | Japan | FDR | 1988-2000 | Secondary | 5 |
| Senda-nakagawa herpacc (ii) | 2017 | Case control | Japan | FDR | 2001-2005 | Secondary | 5 |
| Seow et al[68] | 2002 | Case control | China | FDR | 1999-2000 | Secondary | 4 |
| Shang et al[69] | 2016 | Case control | Australia, Canada, United States | FDR | 1997-2012 | Secondary | 6 |
| Sun et al[70] | 2012 | Case control | Canada | Unclear | 1997-2003 | Secondary | 7 |
| Turati et al[71] | 2013 | Case control | Italy, Switzerland | FDR | 1991-2009 | Primary | 3 |
| Weigl et al[72] | 2016 | Case control | Germany | FDR | 2003-2014 | Secondary | 7 |
| Wells et al[73] | 2014 | Case control | United States | FDR: colon cancer only (not rectal) | 1993-1996 | Secondary | 5 |
FDR: First-degree relative; SDR: Second degree relative; TDR: Third degree relative; NOS: Newcastle Ottawa scale.
Figure 2.
Funnel plot of included studies separated based on the country of origin (Asian vs non-Asian).
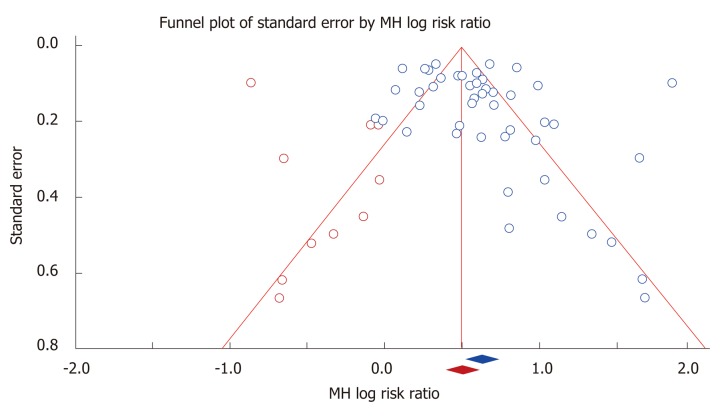
Figure 3.
Trim and Fill analysis of the funnel plot to adjust for asymmetries. Red dots indicate studies which were imputed.
Relative risk of colorectal cancer in first-degree relatives
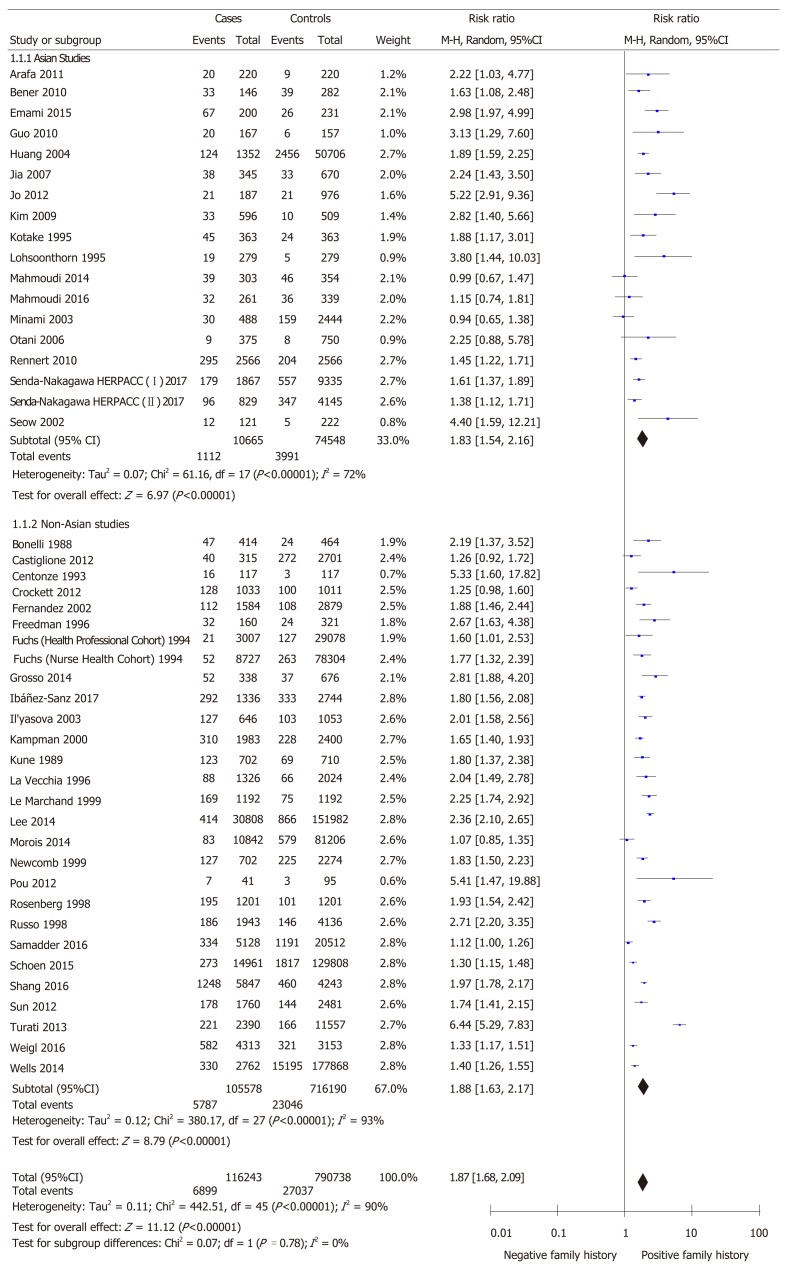
The relative risk of developing colorectal cancer in first-degree relatives of patients was 1.87 (95%CI: 1.68-2.09; P < 0.00001) using the random effects model to account for detected heterogeneity (Figure 4). We performed a subgroup analysis between Asian and non-Asian studies as hypothesized a priori. 18 studies were conducted in Asian countries and 28 studies were conducted in non-Asian countries. The relative risk of colorectal cancer in first-degree relatives was 1.83 (95%CI: 1.54-2.16; P < 0.00001) in Asian studies as compared to 1.88 (95%CI: 1.63-2.17; P < 0.00001) in non-Asian studies. Heterogeneity remained in both subgroups (P < 0.00001, I2 = 72 and I2 = 93% respectively). There was no significant difference in the relative risk between the subgroups (P = 0.78). Figure 4 depicts this meta-analysis.
Figure 4.
Relative risk of developing colorectal cancer in individuals with a first-degree relative. Subgroup analysis is conducted based on the geographical location where the study was conducted (Asian vs non-Asian).
Sensitivity analysis
Family history as the primary objective: Thirteen studies reported information about family history as their primary outcome and 33 studies reported information as their secondary outcomes. Heterogeneity persisted in studies with family history as primary or secondary outcome. The Mantel-Haenzel random effect relative risk was not significantly different between the two subgroups (P = 0.28).
Risk of bias: There were 23 high-quality studies and 23 low-quality studies. Heterogeneity was unaffected by quality of the included studies. The Mantel-Haenzel random effects relative risk was not significantly different between the two subgroups (P = 0.99).
Case control studies versus cohort studies: There were 41 case control studies and 5 cohort studies with non-significant difference in relative risk between the groups (P = 0.27). Design of studies did not affect the heterogeneity in the results.
Timing of the study: There were 12 studies published prior the year 2000 and 34 studies published after. The Mantel-Haenzel random effects relative risk was not significantly different between the subgroups (P = 0.14). Heterogeneity was not significant in studies published before 2000 (P = 0.16, I2 = 29%), and significant in studies published after 2000 (P < 0.00001, I2 = 92%).
Proximity of relative with positive history: Thirty-five studies reported family history only in first-degree relatives and 11 studies were either unclear or included other groups. Heterogeneity was unaffected by the family history information. The relative risk between the two subgroups was not statistically significant (P = 0.30).
Excluding each study in turn: Excluding none of the included studies significantly changed the results.
Trim and fill analysis: The adjusted Mantel-Haenzel random effects relative risk was 1.66 (95%CI: 1.47-1.87) in Trim and Fill analysis, which is not substantially different from the crude value for the measure. Figure 3 shows the visual representation of the funnel plot after the inclusion of imputations for possible missing studies.
DISCUSSION
Our meta-analysis of case control and cohort studies showed that patients with a positive family history of colorectal cancer in first-degree relatives have a 1.87-fold chance for the development of this condition compared to those without a family history. To our knowledge, this is the first comprehensive published meta-analysis estimating the relative risk for development of colorectal cancer in the context of positive family history in the last 15 years including more than 900000 patients. The only published meta-analysis included 26 studies published before the year 2000 to estimate the relative risk of colorectal cancer to be 2.25 in patients with a positive family history[9]. However, the authors only searched MEDLINE as opposed to multiple databases which could have led to selection bias. On the other hand they did not assess the studies for the risk of bias. In our study a subgroup analysis showed the risk of colorectal cancer to be 2.06 in a sensitivity analysis of studies published before the year 2000. These results could indicate the possibility of time lag publication bias whereby over time, with newer studies available, evidence indicates that the initial risk for familial colorectal cancer may have been overestimated[21]. Moreover, it is possible that studies published before the year 2000 included patients with hereditary conditions such as FAP and HNPCC due to lack of awareness or technological advances to detect those patients, therefore contributing to the overestimation of colorectal cancer risk in individuals with a positive family history. However, a sensitivity analysis did not show a significant difference in the overall risk in studies published before 2000 as compared to those published afterward.
We performed subgroup analysis based on the location of the study conducted. This subgroup analysis was based on the fact that colorectal cancer has a higher incidence in Europe and North America and it is less common in South and Central Asia[22]. According to the 2018 global burden of cancer report published by the World Health Association, the age standardized incidence of colorectal cancer is 17.7 per 100000 in Asia as opposed to 26.2 in North America, and 30 in Europe[22]. Additionally, the Western diet has been associated with an increased risk of colorectal cancer especially for those diagnosed at a younger age[23]. However, our analysis revealed that there was no significant difference between the two subgroups that may indicate that the role of family history has equal importance in Asian as compared to non-Asian populations. This finding may play an important role in developing recommendations regarding individuals with family history of colorectal cancer in screening guideline in Asian populations. One should note that the absolute risk might still be lower in an individual with Asian background given the overall lower prevalence despite similar relative risk.
We observed substantial heterogeneity in the results which persisted despite various sensitivity analyses except for the subgroup of studies published before the year 2000. We used random model effect analysis to reduce the effect of heterogeneity on our results. We also performed several sensitivity analyses to explain the statistical heterogeneity. Several factors may explain the observed heterogeneity. Various environmental and lifestyle factors such as physical activity, smoking and alcohol consumption also impact the likelihood of developing colorectal cancer[7]. Our meta-analysis was limited by the primary information provided and we were not able to calculate an adjusted relative risk for familial colorectal cancer based on the abovementioned factors. Consequently, it is possible that inherent differences between the study subjects in other risk factors could have led to the presence of heterogeneity in the results as one might expect from such a large meta-analysis. In addition, evidence from previous studies shows that the familial risk of colorectal cancer may also be site dependent which could have also contributed to heterogeneity in the results[9]. Moreover, the familial risk of colorectal cancer is also dependent on the number of relatives affected which could have led to heterogeneity in the results[9].
There are other possible shortcomings in this study due to intrinsic nature of each meta-analysis. Firstly, there is a possibility for selection bias. Although we did not restrict the language of the initial literature search and used a sensitive strategy to include all the critical studies, it is possible that some eligible studies may not have been included. Only one of the included studies was not published in the English language[20]. However, in this case we were able to access duplicate publication of the same results in English[24]. In addition to selection bias, given that most of the included studies were retrospective in design, there is a possibility of recall bias. It is possible that patients may have provided incorrect family history information[25]. Indeed, the Newcastle-Ottawa scale tool revealed that only 1 included study used a blinded trained interview as a method of determining patient family history information[26].
Another potential source of bias is publication bias which led us to perform Trim and Film sensitivity analysis. We limited our search to published articles and excluded abstracts. We observed small visual asymmetry in the funnel plot and Egger’s test was significant for asymmetry. It is important to mention that the presence of asymmetry in a funnel plot does not necessarily indicate publication bias and could be caused by other reporting biases[17]. Since we were unable to offer other possibilities than publication bias for the asymmetry of the funnel plot, we decided to perform Trim and Fill analysis. Our results remained robust with the Trim and Fill analysis with the adjusted relative risk overlapping greatly with the crude relative risk. These analyses indicate that although publication bias is a possibility in this meta-analysis, it could not have substantially affected the results.
The cause for the increased risk of colorectal cancer in patients with a positive family history is not well defined, but it can be attributed to both genetic and environmental factors[27]. Some known environmental factors for colorectal cancer include poor nutritional practices such as a diet rich in fats and red meat, smoking, obesity, low physical activity and heavy alcohol consumption[27]. Recent advances in cancer research has recognized the individual variability in biological markers in cancer patients, leading to the emergence of pathological molecular epidemiology[28,29]. According to this emerging field, it is possible that specific environmental factors such as dietary choices, physical activity and alcohol consumption contribute to the incidence and prognosis of specific forms of colorectal cancer categorized through the presence or absence of pathological molecular markers. For instance, it is well established that mutations within KRAS and BRAF oncogenes lead to an increased risk of developing colorectal cancer through the activation of the epidermal growth factor receptor. A recent case case-control study of 959 Chinese CRC cases found that one’s mutational status is associated with variables such as sex, smoking status, serum carbohydrate antigen 19-9 and carcinoembryonic antigen[30]. According to the findings of this paper, colorectal cancer tumours with mutated KRAS or BRAF were associated with higher levels of serum carbohydrate antigen 19-9 and carcino-embryonic antigen which are considered to be indicative of poor prognosis and survival in CRC patients[30]. Moreover, another pathological molecular epidemiology study determined that having a first degree relative with CRC is significantly associated with having wild type KRAS[31]. Many of the studies looking at specific subsets of CRC patients are recent and still substantial variability between individual papers is present, making it exceedingly difficult to perform a meta-analysis with high clinical importance. Over the next decade, as newer studies in the field of molecular pathological epidemiology become available, an updated meta-analyses can potentially examine specific subsets of colorectal cancer, such as those with mutated KRAS and BRAF to further explore the role of family history as compared or in combination of other factors demonstrated by molecular epidemiology studies.
Future studies should aim to determine how these environmental factors act in conjunction with genetic factors to affect patients with a family history.
In conclusion, we have found that patients with a positive family history of colorectal cancer in first-degree relatives are at a significantly higher risk of developing the disease. These findings could be used for the development of guidelines for screening and preventative programs for patients of colorectal cancer relatives in all populations. The development of such guidelines could yield population-wide health benefits, as national organizations such as the American Cancer Society, currently focus on individuals at an average risk of colorectal cancer as opposed to those at an increased risk for their guidelines[32]. In addition, although some organizations, such as United States. Multi-society Task Force on Colorectal Cancer, have produced guidelines directed at high-risk populations, they require further validation by more recent studies[12]. Despite development of multiple non-invasive modalities to screen average-risk individuals, none has been validated in a rigorous study in individuals with positive family history. Therefore, the results of our meta-analysis might provide grounds for future studies to develop better screening methods as compared to colonoscopy in this population[10-12].
ARTICLE HIGHLIGHTS
Research background
Colorectal cancer is one of the most common and dangerous malignancies which is likely caused by a combination of environmental and genetic factors. Although it has been long known that individuals with a positive family history of colorectal cancer are at an increased risk of developing this cancer, a robust quantitative estimate of this increased risk is not available in the medical literature with large variability between individual studies.
Research motivation
Estimating the increased risk of individuals with a positive family history of colorectal cancer could be crucial for the development of preventative and screening guidelines for these individuals. The currently existing screening guidelines for individuals with a positive family history are not based on high quality evidence or absent all-together.
Research objectives
The objective of this report was to accurately estimate the risk of developing colorectal cancer in patients with a positive family history.
Research methods
This project was a meta-analysis of case-control and cohort studies of colorectal cancer patients. Data from individual papers was extracted to independently calculate a relative risk of colorectal cancer in patients with a positive family history.
Research results
We found that a positive family history of colorectal cancer in first-degree relatives is associated with significantly increased risk of colorectal cancer with a relative risk of 1.87 (95%CI: 1.68-2.09; P < 0.00001). Future research should aim to determine the influence of environmental factors such as diet and exercise on the familial risk of developing colorectal cancer.
Research conclusions
We found that individuals with a positive family history of colorectal cancer have almost 2-fold higher chance of developing this cancer. To our knowledge, this is the first manuscript in the past decade which estimated the risk of familial colorectal cancer. Our results can substantially contribute to the development of new screening guidelines for individuals with a positive family history.
Research perspective
More research is required to gain a better understanding of the influence of environmental factors on the familial risk of colorectal cancer. In addition, future projects should determine whether the number of first degree relatives affected and their age of initial diagnosis has an effect on the increased risk of this cancer.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Yaghoobi M’s research is partly supported by an Internal Career Award by the Department of Medicine, McMaster University.
Data sharing statement: No additional data is available.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Peer-review started: March 20, 2019
First decision: April 11, 2019
Article in press: July 19, 2019
P-Reviewer: Cubiella J, Ogino S S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
Contributor Information
Parsa Mehraban Far, Division of Medicine, Queen’s University, Kingston, ON K7L 3N6, Canada.
Abdulaziz Alshahrani, Division of Gastroenterology, McMaster University, Hamilton, ON L8S 4K1, Canada.
Mohammad Yaghoobi, Division of Gastroenterology, McMaster University, Hamilton, ON L8S 4K1, Canada; The Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, ON L8S 4K1, Canada. yaghoob@mcmaster.ca.
References
- 1.International Agency for Research on Cancer WHO. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide [Internet] 2012 [cited 2018 Apr 18] Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. [Google Scholar]
- 2.Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009–1013. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 3.Toll AD, Fabius D, Hyslop T, Pequignot E, DiMarino AJ, Infantolino A, Palazzo JP. Prognostic significance of high-grade dysplasia in colorectal adenomas. Colorectal Dis. 2011;13:370–373. doi: 10.1111/j.1463-1318.2010.02385.x. [DOI] [PubMed] [Google Scholar]
- 4.Cottet V, Jooste V, Fournel I, Bouvier AM, Faivre J, Bonithon-Kopp C. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut. 2012;61:1180–1186. doi: 10.1136/gutjnl-2011-300295. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 6.Lauby-Secretan B, Vilahur N, Bianchini F, Guha N, Straif K International Agency for Research on Cancer Handbook Working Group. The IARC Perspective on Colorectal Cancer Screening. N Engl J Med. 2018;378:1734–1740. doi: 10.1056/NEJMsr1714643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, Berry DA. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24:1207–1222. doi: 10.1007/s10552-013-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strate LL, Syngal S. Hereditary colorectal cancer syndromes. Cancer Causes Control. 2005;16:201–213. doi: 10.1007/s10552-004-3488-4. [DOI] [PubMed] [Google Scholar]
- 9.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 10.Leddin D, Lieberman DA, Tse F, Barkun AN, Abou-Setta AM, Marshall JK, Samadder NJ, Singh H, Telford JJ, Tinmouth J, Wilkinson AN, Leontiadis GI. Clinical Practice Guideline on Screening for Colorectal Cancer in Individuals With a Family History of Nonhereditary Colorectal Cancer or Adenoma: The Canadian Association of Gastroenterology Banff Consensus. Gastroenterology. 2018;155:1325–1347.e3. doi: 10.1053/j.gastro.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 12.Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson DJ. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112:1016–1030. doi: 10.1038/ajg.2017.174. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Blackwell. [Google Scholar]
- 14.Tripepi G, Jager KJ, Dekker FW, Wanner C, Zoccali C. Measures of effect: relative risks, odds ratios, risk difference, and 'number needed to treat'. Kidney Int. 2007;72:789–791. doi: 10.1038/sj.ki.5002432. [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos PTM. Ottawa Hospital Research Institute [Internet] 2014 [cited 2018 Apr 19] Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 16.Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y, Williams JW, Jr, Meerpohl J, Norris SL, Akl EA, Schünemann HJ. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol. 2011;64:1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Sedgwick P, Marston L. How to read a funnel plot in a meta-analysis. BMJ. 2015;351:h4718. doi: 10.1136/bmj.h4718. [DOI] [PubMed] [Google Scholar]
- 18.Collaboration TC. Incorporating heterogeneity into random-effects models [Internet] 2016 [cited 2018 Apr 19] Available from: http://handbook-5-1.cochrane.org/chapter_9/9_5_4_incorporating_heterogeneity_into_random_effects_models.htm. [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia HR, He XL, Zhu ZZ, Jin XX, Wang AZ, Huang HY, Zhu J, Yu GB, Zhu GS. [TP53 gene polymorphisms and colorectal cancer risk in Chinese population] Zhonghua Yi Xue Za Zhi. 2007;87:1448–1451. [PubMed] [Google Scholar]
- 21.Cochrane Methods Bias - Reporting biases [Internet] 2018 [cited 2018 Apr 21] Available from: http://methods.cochrane.org/bias/reporting-biases.
- 22.Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 23.Slattery ML, Potter JD, Ma KN, Caan BJ, Leppert M, Samowitz W. Western diet, family history of colorectal cancer, NAT2, GSTM-1 and risk of colon cancer. Cancer Causes Control. 2000;11:1–8. doi: 10.1023/a:1008913619957. [DOI] [PubMed] [Google Scholar]
- 24.Zhu ZZ, Wang AZ, Jia HR, Jin XX, He XL, Hou LF, Zhu G. Association of the TP53 codon 72 polymorphism with colorectal cancer in a Chinese population. Jpn J Clin Oncol. 2007;37:385–390. doi: 10.1093/jjco/hym034. [DOI] [PubMed] [Google Scholar]
- 25.Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43:87–91. doi: 10.1016/0895-4356(90)90060-3. [DOI] [PubMed] [Google Scholar]
- 26.Lohsoonthorn P, Danvivat D. Colorectal cancer risk factors: a case-control study in Bangkok. Asia Pac J Public Health. 1995;8:118–122. doi: 10.1177/101053959500800211. [DOI] [PubMed] [Google Scholar]
- 27.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamada T, Keum NN, Nishihara R, Ogino S. Molecular pathological epidemiology: new developing frontiers of big data science to study etiologies and pathogenesis. J Gastroenterol. 2017;52:265–75. doi: 10.1007/s00535-016-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Qiu T, Ling Y, Guo L, Li L, Ying J. Molecular pathological epidemiology of colorectal cancer in Chinese patients with KRAS and BRAF mutations. Oncotarget. 2015;6:39607–39613. doi: 10.18632/oncotarget.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonsalves WI, Mahoney MR, Sargent DJ, Nelson GD, Alberts SR, Sinicrope FA, Goldberg RM, Limburg PJ, Thibodeau SN, Grothey A, Hubbard JM, Chan E, Nair S, Berenberg JL, McWilliams RR Alliance for Clinical Trials in Oncology. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Cancer Society Guideline for Colorectal Cancer Screening [Internet] [cited 2019 Jan 1] Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html. [Google Scholar]
- 33.Arafa MA, Waly MI, Jriesat S, Al Khafajei A, Sallam S. Dietary and lifestyle characteristics of colorectal cancer in Jordan: a case-control study. Asian Pac J Cancer Prev. 2011;12:1931–1936. [PubMed] [Google Scholar]
- 34.Bener A, Moore MA, Ali R, El Ayoubi HR. Impacts of family history and lifestyle habits on colorectal cancer risk: a case-control study in Qatar. Asian Pac J Cancer Prev. 2010;11:963–968. [PubMed] [Google Scholar]
- 35.Bonelli L, Martines H, Conio M, Bruzzi P, Aste H. Family history of colorectal cancer as a risk factor for benign and malignant tumours of the large bowel. A case-control study. Int J Cancer. 1988;41:513–517. doi: 10.1002/ijc.2910410407. [DOI] [PubMed] [Google Scholar]
- 36.Castiglione G, Visioli CB, Zappa M, Grazzini G, Mallardi B, Mantellini P. Familial risk of colorectal cancer in subjects attending an organised screening programme. Dig Liver Dis. 2012;44:80–83. doi: 10.1016/j.dld.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Centonze S, Boeing H, Leoci C, Bonfiglio C, Guerra V, Misciagna G. Familial risk of colo-rectal cancer in a low incidence area in southern Italy. Eur J Epidemiol. 1993;9:26–32. doi: 10.1007/BF00463086. [DOI] [PubMed] [Google Scholar]
- 38.Crockett SD, Long MD, Dellon ES, Martin CF, Galanko JA, Sandler RS. Inverse relationship between moderate alcohol intake and rectal cancer: analysis of the North Carolina Colon Cancer Study. Dis Colon Rectum. 2011;54:887–894. doi: 10.1007/DCR.0b013e3182125577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emami N, Saadat I, Omidvari S. Susceptibility to Colorectal Cancer and Two Genetic Polymorphisms of XRCC4. Pathol Oncol Res. 2015;21:881–885. doi: 10.1007/s12253-015-9905-z. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez E, La Vecchia C, Talamini R, Negri E. Joint effects of family history and adult life dietary risk factors on colorectal cancer risk. Epidemiology. 2002;13:360–363. doi: 10.1097/00001648-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Freedman AN, Michalek AM, Marshall JR, Mettlin CJ, Petrelli NJ, Black JD, Zhang ZF, Satchidanand S, Asirwatham JE. Familial and nutritional risk factors for p53 overexpression in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:285–291. [PubMed] [Google Scholar]
- 42.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331:1669–1674. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 43.Grosso G, Biondi A, Galvano F, Mistretta A, Marventano S, Buscemi S, Drago F, Basile F. Factors associated with colorectal cancer in the context of the Mediterranean diet: a case-control study. Nutr Cancer. 2014;66:558–565. doi: 10.1080/01635581.2014.902975. [DOI] [PubMed] [Google Scholar]
- 44.Guo X, Zhang L, Wu M, Wang N, Liu Y, Er L, Wang S, Gao Y, Yu W, Xue H, Xu Z, Wang S. Association of the DNMT3B polymorphism with colorectal adenomatous polyps and adenocarcinoma. Mol Biol Rep. 2010;37:219–225. doi: 10.1007/s11033-009-9626-z. [DOI] [PubMed] [Google Scholar]
- 45.Huang XE, Hirose K, Wakai K, Matsuo K, Ito H, Xiang J, Takezaki T, Tajima K. Comparison of lifestyle risk factors by family history for gastric, breast, lung and colorectal cancer. Asian Pac J Cancer Prev. 2004;5:419–427. [PubMed] [Google Scholar]
- 46.Ibáñez-Sanz G, Díez-Villanueva A, Alonso MH, Rodríguez-Moranta F, Pérez-Gómez B, Bustamante M, Martin V, Llorca J, Amiano P, Ardanaz E, Tardón A, Jiménez-Moleón JJ, Peiró R, Alguacil J, Navarro C, Guinó E, Binefa G, Fernández-Navarro P, Espinosa A, Dávila-Batista V, Molina AJ, Palazuelos C, Castaño-Vinyals G, Aragonés N, Kogevinas M, Pollán M, Moreno V. Risk Model for Colorectal Cancer in Spanish Population Using Environmental and Genetic Factors: Results from the MCC-Spain study. Sci Rep. 2017;7:43263. doi: 10.1038/srep43263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Il'yasova D, Martin C, Sandler RS. Tea intake and risk of colon cancer in African-Americans and whites: North Carolina colon cancer study. Cancer Causes Control. 2003;14:767–772. doi: 10.1023/a:1026371307954. [DOI] [PubMed] [Google Scholar]
- 48.Jo J, Nam CM, Sull JW, Yun JE, Kim SY, Lee SJ, Kim YN, Park EJ, Kimm H, Jee SH. Prediction of Colorectal Cancer Risk Using a Genetic Risk Score: The Korean Cancer Prevention Study-II (KCPS-II) Genomics Inform. 2012;10:175–183. doi: 10.5808/GI.2012.10.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kampman E, Slattery ML, Caan B, Potter JD. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk (United States) Cancer Causes Control. 2000;11:459–466. doi: 10.1023/a:1008914108739. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Kim DH, Lee BH, Kang SH, Lee HJ, Lim SY, Suh YK, Ahn YO. Folate intake and the risk of colorectal cancer in a Korean population. Eur J Clin Nutr. 2009;63:1057–1064. doi: 10.1038/ejcn.2009.37. [DOI] [PubMed] [Google Scholar]
- 51.Kotake K, Koyama Y, Nasu J, Fukutomi T, Yamaguchi N. Relation of family history of cancer and environmental factors to the risk of colorectal cancer: a case-control study. Jpn J Clin Oncol. 1995;25:195–202. [PubMed] [Google Scholar]
- 52.Kune GA, Kune S, Watson LF, Bahnson CB. Personality as a risk factor in large bowel cancer: data from the Melbourne Colorectal Cancer Study. Psychol Med. 1991;21:29–41. doi: 10.1017/s0033291700014628. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez E, La Vecchia C, Decarli A. Attributable risks for pancreatic cancer in northern Italy. Cancer Epidemiol Biomarkers Prev. 1996;5:23–27. [PubMed] [Google Scholar]
- 54.Le Marchand L, Wilkens LR, Hankin JH, Kolonel LN, Lyu LC. Independent and joint effects of family history and lifestyle on colorectal cancer risk: implications for prevention. Cancer Epidemiol Biomarkers Prev. 1999;8:45–51. [PubMed] [Google Scholar]
- 55.Lee M, Czene K, Rebora P, Reilly M. Patterns of changing cancer risks with time since diagnosis of a sibling. Int J Cancer. 2015;136:1948–1956. doi: 10.1002/ijc.29239. [DOI] [PubMed] [Google Scholar]
- 56.Mahmoudi T, Karimi K, Arkani M, Farahani H, Nobakht H, Dabiri R, Asadi A, Vahedi M, Zali MR. Lack of associations between Vitamin D metabolism-related gene variants and risk of colorectal cancer. Asian Pac J Cancer Prev. 2014;15:957–961. doi: 10.7314/apjcp.2014.15.2.957. [DOI] [PubMed] [Google Scholar]
- 57.Mahmoudi T, Karimi K, Karimi N, Farahani H, Nobakht H, Dabiri R, Vahedi M, Zali MR. Association of adiponectin receptor 1 gene - 106 C > T variant with susceptibility to colorectal cancer. Meta Gene. 2016;9:210–214. doi: 10.1016/j.mgene.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minami Y, Tateno H. Associations between cigarette smoking and the risk of four leading cancers in Miyagi Prefecture, Japan: a multi-site case-control study. Cancer Sci. 2003;94:540–547. doi: 10.1111/j.1349-7006.2003.tb01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morois S, Cottet V, Racine A, Clavel-Chapelon F, Carbonnel F, Bastide N, Boutron-Ruault MC. Colonoscopy reduced distal colorectal cancer risk and excess cancer risk associated with family history. Cancer Causes Control. 2014;25:1329–1336. doi: 10.1007/s10552-014-0438-7. [DOI] [PubMed] [Google Scholar]
- 60.Newcomb PA, Taylor JO, Trentham-Dietz A. Interactions of familial and hormonal risk factors for large bowel cancer in women. Int J Epidemiol. 1999;28:603–608. doi: 10.1093/ije/28.4.603. [DOI] [PubMed] [Google Scholar]
- 61.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S Japan Public Health Center-Based Prospective Study Group. Plasma C-reactive protein and risk of colorectal cancer in a nested case-control study: Japan Public Health Center-based prospective study. Cancer Epidemiol Biomarkers Prev. 2006;15:690–695. doi: 10.1158/1055-9965.EPI-05-0708. [DOI] [PubMed] [Google Scholar]
- 62.Rennert G, Rennert HS, Pinchev M, Gruber SB. A case-control study of levothyroxine and the risk of colorectal cancer. J Natl Cancer Inst. 2010;102:568–572. doi: 10.1093/jnci/djq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenberg L, Louik C, Shapiro S. Nonsteroidal antiinflammatory drug use and reduced risk of large bowel carcinoma. Cancer. 1998;82:2326–2333. doi: 10.1002/(sici)1097-0142(19980615)82:12<2326::aid-cncr5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 64.Russo A, Franceschi S, La Vecchia C, Dal Maso L, Montella M, Conti E, Giacosa A, Falcini F, Negri E. Body size and colorectal-cancer risk. Int J Cancer. 1998;78:161–165. doi: 10.1002/(sici)1097-0215(19981005)78:2<161::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 65.Samadder NJ, Curtin K, Pappas L, Boucher K, Mineau GP, Smith K, Fraser A, Wan Y, Provenzale D, Kinney AY, Ulrich C, Burt RW. Risk of Incident Colorectal Cancer and Death After Colonoscopy: A Population-based Study in Utah. Clin Gastroenterol Hepatol. 2016;14:279–86.e1-2. doi: 10.1016/j.cgh.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schoen RE, Razzak A, Yu KJ, Berndt SI, Firl K, Riley TL, Pinsky PF. Incidence and mortality of colorectal cancer in individuals with a family history of colorectal cancer. Gastroenterology. 2015;149:1438–1445.e1. doi: 10.1053/j.gastro.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakagawa-Senda H, Ito H, Hosono S, Oze I, Tanaka H, Matsuo K. Coffee consumption and the risk of colorectal cancer by anatomical subsite in Japan: Results from the HERPACC studies. Int J Cancer. 2017;141:298–308. doi: 10.1002/ijc.30746. [DOI] [PubMed] [Google Scholar]
- 68.Seow A, Quah SR, Nyam D, Straughan PT, Chua T, Aw TC. Food groups and the risk of colorectal carcinoma in an Asian population. Cancer. 2002;95:2390–2396. doi: 10.1002/cncr.10971. [DOI] [PubMed] [Google Scholar]
- 69.Shang J, Reece JC, Buchanan DD, Giles GG, Figueiredo JC, Casey G, Gallinger S, Thibodeau SN, Lindor NM, Newcomb PA, Potter JD, Baron JA, Hopper JL, Jenkins MA, Win AK. Cholecystectomy and the risk of colorectal cancer by tumor mismatch repair deficiency status. Int J Colorectal Dis. 2016;31:1451–1457. doi: 10.1007/s00384-016-2615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun Z, Liu L, Wang PP, Roebothan B, Zhao J, Dicks E, Cotterchio M, Buehler S, Campbell PT, McLaughlin JR, Parfrey PS. Association of total energy intake and macronutrient consumption with colorectal cancer risk: results from a large population-based case-control study in Newfoundland and Labrador and Ontario, Canada. Nutr J. 2012;11:18. doi: 10.1186/1475-2891-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turati F, Edefonti V, Bosetti C, Ferraroni M, Malvezzi M, Franceschi S, Talamini R, Montella M, Levi F, Dal Maso L, Serraino D, Polesel J, Negri E, Decarli A, La Vecchia C. Family history of cancer and the risk of cancer: a network of case-control studies. Ann Oncol. 2013;24:2651–2656. doi: 10.1093/annonc/mdt280. [DOI] [PubMed] [Google Scholar]
- 72.Weigl K, Jansen L, Chang-Claude J, Knebel P, Hoffmeister M, Brenner H. Family history and the risk of colorectal cancer: The importance of patients' history of colonoscopy. Int J Cancer. 2016;139:2213–2220. doi: 10.1002/ijc.30284. [DOI] [PubMed] [Google Scholar]
- 73.Wells BJ, Kattan MW, Cooper GS, Jackson L, Koroukian S. Colorectal cancer predicted risk online (CRC-PRO) calculator using data from the multi-ethnic cohort study. J Am Board Fam Med. 2014;27:42–55. doi: 10.3122/jabfm.2014.01.130040. [DOI] [PMC free article] [PubMed] [Google Scholar]