Human neural progenitor cells have been grafted successfully into rhesus monkeys with spinal cord hemisection, resulting in anatomic integration and improved neurological function.
In the CNS, the spinal cord is a critically important conduit for sensory and motor signals. Relatively small spinal anatomic lesions can cause irreparable harm, as the ascending sensory and descending motor and autonomic pathways, as well as their connections to local inputs and outputs at each spinal level, all lie within just a few centimeters of one another. Furthermore, the spinal cord lies within a tight cylindrical space throughout most of its length, and any swelling after injury can lead to its being squeezed to the point of vascular compression. The collapse of spinal venous drainage, with its attendant feed-forward increase in intraspinal pressure, can lead to an eventual disruption of arterial flow. As a result, traumatic spinal cord injury (SCI) can quickly evolve into spinal ischemia, with infarction and irreversible tissue loss the tragic outcome. Our ability to intervene in this process has thus far proven limited; to date, no medical therapies exist for SCI, and no surgical treatments have proven effective save for decompressive laminectomy in very selected cases. As a result, even though spinal neural circuits are among the most well-described in neurobiology, that understanding has not yet translated to effective strategies for either their rescue or repair after traumatic injury1. In this issue of Nature Medicine, Tuszynski and colleagues2 report the amelioration of both tissue injury and neurological deficits after SCI in rhesus monkeys, by human neural progenitor cells delivered locally to the site of injury
In this study Tuszynski and colleagues build on recent studies that have evaluated the use of human neural progenitors in rodent SCI3,4, to now assess the efficacy of human neural progenitors in non-human primates as well. In their earlier studies in rodents, Tuszynski and colleagues reported that spinal NPCs proved better able to support corticospinal tract ingrowth in lesioned rodent spinal cord than analogous forebrain-derived cells 5. They also showed that the axons of newly produced neurons from implanted NPCs can project far into adult tissue, as has also been shown in rodent models of induced neurogenesis 6 It thus seems that newly-developing neurons can extend axons in the adult CNS, unconstrained by the inhibitory signals that might otherwise suppress axonal regeneration by resident mature neurons. However, the neuroanatomy of the human spinal cord differs greatly from that of rodents, so much so that non-human primate models are required to further assess the therapeutic potential of neural progenitor grafts. Marmoset monkeys were first used successfully for this purpose7, in studies that suggested the wisdom of moving to primates with more human-like size and neuroanatomy. Old-world monkeys such as the rhesus macaque share the bulk of the major tracts, patterns of connectivity and functional relationships that characterize the human spinal cord, and this is the host species that Tuszynski and colleagues choose to use for these experiments.
The investigators carried out hemisection of Rhesus monkey cervical spinal cords to model an especially severe form of human SCI, which may occur with penetrating injuries of the spinal cord or traumatic displacements of the vertebral column. Two weeks later, they grafted human NPCs derived from early human fetal spinal cords into the lesion sites (see Figure). The authors used early NPCs derived from human spinal cord as their donor cells; their past studies had indicated the importance of using spinal progenitors for this purpose, as opposed to the brain-derived cells used in many other past studies of neural stem cell grafts in SCI. In addition, they had previously reported that implanted human donor cells matured slowly in the recipient spinal cord3 - mimicking the prolonged maturation of human neurons and glia during human gestation – and so they knew to allow longer recovery times before evaluating the transplanted monkeys. Combining these elements in the design of their present study, they found that implanted human spinal NPCs proceeded to differentiate as neurons and glia - the latter mostly as astrocytes - as far out as 9 months after transplant. They also observed that there was little migration of the NPCs from their transplant sites. This observation distinguished NPCs from their closely related glial progenitor cells, an alternative cellular reagent that readily migrates widely throughout the CNS, and is hence appropriate for many disease targets, yet lacks the neuronal production capability of neural progenitor cells8.
Figure.
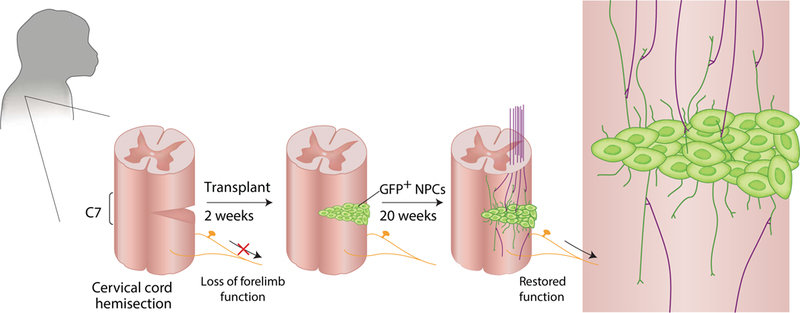
Rosenzweig et al., transplant human neural progenitor cells (NPCs) labeled with GFP into the hemisected monkey spinal cord, a model that mimics severe human SCI. They find that after 20 weeks, donor neurons extend fibers into the host spinal cord, while receiving inputs from the host as well, so that the graft can serve as a functional bridge across the injury site, resulting in partial restoration of motor function.
Recovery from spinal cord injuries is dependent upon the re-establishment of appropriate neuronal connectivity. Tuszynski and colleagues discovered that the newly developing neurons rising from their transplanted neural progenitors extended axons, both rostrally and caudally from the graft site. While large numbers of axons penetrated the adjacent spinal parenchyma, most extended no more than 2 mm. Nonetheless, a small number of donor axons appeared to extend as far as 5 cm, exhibiting long-distance projection through the descending lateral corticospinal tracts.
Given the limitations of sample size inherent in working with primates, the authors could not assess the relative contributions of the different donor-derived axons to functional improvement. Nonetheless, the monkeys did show a restoration of forelimb functions, such as object manipulation, beyond what would typically be expected following surgical hemisection. Whereas a set of 4 lesioned monkeys who underwent unsuccessful grafts manifested little functional change beyond their first peri-operative month, 5 successfully-engrafted monkeys tested for as long as 9 months all showed some degree of progressive improvement. These improvements included partially restored segmental forelimb function, accompanied by improved long-tract function and locomotion as well. Notably, as important as the investigators’ 5 successful transplants were, their failures in this study were equally informative as they identified the importance of draining the lesion site of cerebrospinal fluid, and of suspending the donor cells in a growth factor-laden fibrin matrix as critical steps in ensuring successful donor cell engraftment.
On the basis of these promising data, the authors proposed that neurons arising within the grafts established connections with ingrowing transected fibers, while projecting their own axons out into the host spinal cord. They postulated that by so doing, the grafts formed multi-synaptic bridges by which ingrowing axons could use graft-derived neurons as functional intermediaries with which to communicate with their distant targets. As such, those monosynaptic pathways lost to SCI - including those of both ascending sensory and descending motor tracts - might be restored through polysynaptic, graft-mediated relays. While speculative in regards to these monkeys, this concept of the grafted cells providing a neuronal bridge for restoring long-distance, if polysynaptic, connectivity has been advanced in a number of studies of rodent SCI5,9–11, most recently with the use of rabies tracing to more precisely infer the synaptic relationships of host and donor neurons12. In the latter case, the donor neurons indeed appeared to serve as synaptically-coupled bridges between the proximal and distal portions of transected spinal cord. Further studies would benefit from ablating the grafted neurons, to prove that the monkey’s motor improvement depended directly on the engrafted cells and their vectorially-specific connections with the host.
Spinal cord injuries are varied in nature, as are their clinical presentations and prognosis; no two are the same. Patients with incomplete and non-transective injuries can improve over time, and their improvement may be as significant as it is unpredictable. But if there is one common theme, it is that patients with frank transections or severe contusions with tissue cavitation are left with permanent injuries, with severe impairment and little improvement over time. These patients are thus likely to be the greatest beneficiaries of a neural progenitor-based treatment strategy, and the first in whom it is likely to be assessed. By convincingly demonstrating that transplant-based neural circuit reconstruction in the injured spinal cord can be effective, and doing so in non-human primates to boot, Tuszynski and colleagues have thus significantly advanced the cause of neuronal replacement therapy for spinal repair, and brought this exciting strategy one step closer to the clinic.
Supplementary Material
References
- 1.Dobkin BH & Havton LA Basic advances and new avenues in therapy of spinal cord injury. Annu Rev Med 55, 255–282 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenzweig E, … & Tuszynki M Restorative effects of human neural stem cell grafts to the primate spinal cord. Nature medicine this issue(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu P, et al. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. The Journal of clinical investigation 127, 3287–3299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu P, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150, 1264–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadoya K, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nature medicine 22, 479–487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benraiss A, et al. Sustained mobilization of endogenous neural progenitors delays disease progression in a transgenic model of Huntington’s disease. Cell Stem Cell 12, 787–799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi Y, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PloS one 7, e52787 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman SA Stem and progenitor cell-based therapy of the central nervous system: Hopes, hype, and wishful thinking. Cell Stem Cell 18, 174–188 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonner JF, et al. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci 31, 4675–4686 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings BJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proceedings of the National Academy of Sciences of the United States of America 102, 14069–14074 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadoya K, et al. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron 64, 165–172 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler AF, Lee-Kubli C, Kumamaru H, Kadoya K & Tuszynski MH Comprehensive monosynaptic rabies virus mapping of host connectivity with neural progenitor grafts after spinal cord injury. Stem cell reports 8, 1525–1533 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


