Abstract
Recent technological advancements have enabled the creation of portable, low-cost, and unobtrusive sensors with tremendous potential to alter the clinical practice of rehabilitation. The application of wearable sensors to movement tracking has emerged as a promising paradigm to enhance the care provided to patients with neurological or musculoskeletal conditions. These sensors enable quantification of motor behavior across disparate patient populations and emerging research shows their potential for identifying motor biomarkers, differentiating between restitution and compensation motor recovery mechanisms, remote monitoring, tele-rehabilitation, and robotics. Moreover, the big data recorded across these applications serve as a pathway to personalized and precision medicine. This paper presents state-of-the-art and next generation wearable movement sensors, ranging from inertial measurement units to soft sensors. An overview of clinical applications is presented across a wide spectrum of conditions that have potential to benefit from wearable sensors, including stroke, movement disorders, knee osteoarthritis, and running injuries. Complementary applications enabled by next-generation sensors that will enable point-of-care monitoring of neural activity and muscle dynamics during movement are also discussed.
I. INTRODUCTION
Rapid advancements in electronics and computing have created an opportunity and responsibility1 to translate these technological advances to the field of rehabilitation. In particular, wearable sensors have emerged as a promising technology with substantial potential to benefit a wide range of individuals, from patients living with mobility deficits to high performance athletes recovering from an injury. Wearable sensors provide precise, quantitative measurements of human movement, enabling tracking of the effects of disease or injury through their influence on the movement system. Importantly, the portability of wearable sensors allow their use in free-living environments, thus providing more ecologic and rich data related to health and disability. Wearable sensors provide an opportunity for the collection of big data across clinical and real-world settings, enabling the growth of personalized and precision medicine2.
The field of wearable sensors has seen exponential growth during the last decade; however, widespread clinical use of this promising technology has yet to be realized. Clinical applications of wearable sensors include remote monitoring3, mobile health3,4, and expansion of health metrics beyond traditional clinical settings5. This focused review begins with a summary of the state-of-the-art in wearable movement sensors and their current applications to neurologic and orthopedic rehabilitation, followed by emerging clinical applications. We conclude with an overview of next-generation sensor technologies that expand motion sensing through hybrid sensors, neural interfaces, and soft sensors.
2. LITERATURE SELECTION
To characterize the (i) state-of-the-art, (ii) emerging, and (iii) next generation wearable sensor technologies utilized in the fields of neurologic and orthopedic rehabilitation, a literature search was performed using Medline, Pubmed, and CINAHL databases. Studies published from 2013 – 2018 were the focus of this search. Our search delimiters included studies published in English and studies with adult human participants. Discussion was steered toward stroke and movement disorders to exemplify applications in neurological rehabilitation, and knee osteoarthritis and running to exemplify applications in orthopedic rehabilitation. Sample keywords and their combinations included: sensors, rehabilitation, stroke, Parkinson’s disease (PD), Huntington’s disease (HD), osteoarthritis (OA), and running.
3. REVIEW OF EVIDENCE
Recovery of motor function is a major goal of neurologic and orthopedic rehabilitation. Rehabilitation interventions facilitate motor learning by leveraging repetitive, progressive, and task-specific motor practice provided in sensory-enriched environments6—treatment parameters that enhance activity-dependent plasticity in the central nervous system7. Precise measurements of motor behavior over different timescales may assist in exploring and optimizing motor learning. Wearable motion sensors enable the objective measurement of body orientation, motion, direction, and physiological state during movement in ecological settings8, thus providing clinicians with data that can be used to guide and enhance rehabilitation activities.
State-of-the-art technology
Force-based sensors are commonly integrated with footwear to measure the interaction of the body with the ground during walking9. These sensors include load-sensitive switches or force-sensitive resistors that characterize gait based on the configuration of the sensors. A single sensor attached to the heel allows detection of heel strike and heel off phases of gait, while multiple sensors within an insole enable examination of walking strategies10, center of pressure translations11, and the estimation of vertical ground reaction forces throughout the gait cycle12. Force-based sensors are also used to drive auditory13 and visual14 biofeedback during gait training13,14. Limitations of force-based sensors include their susceptibility to mechanical wear over time, limited direct measurements to events during stance phase9, and potential drift secondary to humidity and temperature inside the shoe15 that may influence data quality.
Gyroscopes measure the rate of change of angular motion by detecting the Coriolis forces that act on a moving mass in a rotating reference frame. These forces are proportional to the rate of angular rotation of the limb. Gyroscopes are secured on to body segments in line with the plane of the movement that is being measured16, and tri-axial gyroscopes allow three-dimensional measurements. Particular strengths of gyroscope sensors are that their measurements are not influenced by gravitational forces17 and vibrations during heel strike do not distort the signal18.
Accelerometers measure body movements based on the rate of change of speed. The measurement principle underlying accelerometry is commonly explained by a mass-spring system19. Based on the displacement of the mass element, the resultant acceleration is derived19. While there are several classes of accelerometers, the most commonly used in rehabilitation research are strain gauge, capacitive, piezoresistive, and piezoelectric19. Accelerometers used in rehabilitation commonly have 1 to 3 sensing axes, which allow motion detection in one- to three-dimensional space. Accelerometers are commonly used for continuous monitoring of gait, mobility, and activities of daily living. Accelerometer signals can be used to compute position or velocity; however, drift from integration reduces data quality18. Additional limitations associated with the use of accelerometers include poor reliability when measuring non-dynamic events20 and the influence of gravity on the acceleration signal9. Various signal processing strategies are being developed to improve data quality9.
Magnetometers are devices that detect the earth’s gravitation vector. Their measurements provide compass heading information and a reference measure for body orientation relative to gravity9. Because magnetometers are insensitive to acceleration during dynamic movements, their use alongside accelerometers allow separation of gravitational components from kinematic acceleration data. Moreover, given the qualities and limitations of gyroscopes, accelerometers, and magnetometers, these sensor types are often combined in self-contained devices called inertial measurement units (IMUs) to optimize measurement capabilities. Force-based sensors offer additional insight into a wearer’s interaction with the environment, and have been used alongside IMUs as well. By and large, limitations in the quality of individual sensor signals can be addressed with advanced processing and intelligent algorithms21. The following section overviews applications of these sensors across the neurological and orthopedic domains.
State-of-the-art clinical applications
Wearable sensors are portable, low-cost, and unobtrusive tools that provide objective, quantitative, and continuous information about motor behavior in a range of environments. Clinically, wearable sensors have been utilized for assessment, including the instrumentation of common mobility tests22, identification of pathological movement23,24, characterization of disease stage25, falls management26,27, and activity recognition (AR). They have also been used to augment treatments, such as enabling biofeedback-based gait training12,28,29. This section cites specific examples of these clinical applications (Table 1).
Table 1.
Clinical applications of state-of-the-art technology in select neurological and orthopedic populations.
| Clinical application | Sensor (model), associated technology | Findings | |
|---|---|---|---|
| Assessment | Clinical Instrumentation | IMU (Physilog®, GaitUp, Lausanne, Switzerland) |
High reliability and low measurement error for most measures taken when used for instrumented TUG test in individuals post-stroke31 |
| Falls Management | Phone-based IMU (Xperia Ray SO-03C, Sony Mobile Communications Inc., Tokyo, Japan) |
Able to identify differences in kinematic gait variables in those post-stroke with and without a history of falls35 | |
| IMU (Opal, APDM Inc., Portland, Oregon, USA) |
Able to identify differences in dynamic gait stability between stroke cohort and control, and variables that may play a significant role in increased fall risk36 | ||
| Identification of Pathologic Motor Features | IMU (Kinesia ONE™, Great Lakes NeuroTechnologies Inc., Cleveland, OH) |
High test-retest reliability and sensitivity in measuring bradykinesia, hypokinesia and dysrhythmia in those with PD32 | |
| iPod-based IMU (iPod®, Cupertino, California, USA) |
Able to detect significant differences in trunk control during static activities in people with HD compared to controls; found amplitude of thoracic and pelvic trunk movements was significantly greater in participants with HD33 | ||
| Activity Recognition | IMU (Physilog®, GaitUp, Lausanne, Switzerland |
Excellent ability to classify (90.4%) basic activities common to daily life in individuals post-stroke (e.g. lying, sitting, standing, walking, stair walking and taking an elevator)37 | |
| Step Watch Activity Monitor™ (Orthocare Innovations, Seattle Washington, USA) |
Able to characterize activity levels without relying on self-report data or clinician opinion38; assess real-world performance39; and guide community-based treatments using goal setting40 for individuals post-stoke | ||
| Phone-based IMU (Blackberry Z10, Waterloo, Ontario, CAN) |
Good sensitivity and specificity in detecting immobile (standing, sitting, laying) versus mobile (walking) states, but poor ability to classify more complicated movements (walking up stairs and other small movements)4 in people with PD | ||
| Characterization of Disease Stage | IMU (Opal inertial sensors, APDM, Inc., Portland, OR, USA) |
High correlation between disease severity and turning velocity, duration, and step number in those with PD tracked over 7 days34 | |
| Treatment | Biofeedback | Force sensor Smart Shoes, custom made IMUs Smart Pants, custom made |
Significant improvements in balance, mobility, strength, and range of motion comparable to improvements seen with therapist cueing only; suggesting the potential use in at home training for those with PD and post-stroke17 |
| IMU (TecnoBody®, Dlamine BG, ITL) |
Improved BBS score, and reduced mediolateral sway during standing in participants with PD who received biofeedback with Gamepad during training42 | ||
| Force sensor (not specified) Pager motor (not specified) |
Reduced KAM by 14.2% in people with OA43 | ||
IMU = Inertial Measurement Unit; TUG = Timed Up and Go Test; PD = Parkinson’s Disease; HD = Huntington’s Disease; BBS = Berg Balance Scale; OA = Osteoarthritis; KAM = Knee Adduction Moment
Stroke
Advanced signal processing approaches have enabled IMU instrumentation of popular clinical tests such as the 10-meter walk test27 and the Timed Up-and-Go test22, providing clinically-relevant data on movement quality in addition to the traditional outcome of “time to complete”. Moreover, advanced AR algorithms have enabled IMU data to be used to identify and quantify gross movements with high sensitivity and specificity30. For example, data extracted from IMUs located in mobile phones have differentiated stroke survivors who are fallers from those who are not based on an estimate of inter-stride variability26. These analyses, however, have been limited when used to quantify more complex movements4, motivating further work in this area. Accelerometer-based step activity monitors have also been used to monitor physical activity in the home and the community, providing ecologically-valid mobility data for the development of treatment-based classifications31, the assessment of real-world performance32, and to guide community-based treatment programs33.
Wearable sensors have also enabled novel gait training approaches, such as biofeedback-based interventions. For example, a custom body-worn sensor system comprised of force sensors and IMUs was used to provide kinematic biofeedback during gait training, leading to improvements in balance, mobility, strength, and range of motion that were comparable to the treatment benefits obtained through therapist-directed gait training12. These results demonstrate the potential for wearable sensors to provide effective gait intervention without direct oversight by a clinician (e.g., in real world settings).
Parkinson’s disease (PD)
Like in stroke, AR algorithms have enabled IMU data to be used to identify pathological motor features characteristic of PD. For example, periods of motor fluctuations between mobile and immobile states (i.e., on-off periods) in levodopa-treated individuals were detected using IMU data analyzed with an advanced AR algorithm34. Other studies have demonstrated how IMUs may be useful in tracking primary physical symptoms of PD, such as tremor35, dyskinesias, and bradykinesia23, and in tracking disease progression25. For example, IMUs have been used to differentiate between tremor-dominant and non-tremor-dominant patients with PD35. Mancini et. al. tracked features of turning performance (e.g., velocity, duration, and step number) for seven days, revealing a high correlation between disease severity and turning mobility25. Additional studies have shown that IMU-enabled continuous monitoring of baseline gait metrics can predict disease progression and gait decline 1- and 2-years later36. Moreover, a recent large study of 190 patients with PD and 101 age-matched controls shows the feasibility for large-scale clinical trials to use IMUs to robustly track spatiotemporal parameters of gait37.
Like in stroke, sensor-enabled biofeedback interventions have gained popularity as noninvasive training tools in PD rehabilitation. For example, wearable sensors have been used to facilitate the delivery of rhythmic auditory or haptic cues during gait training, an approach shown to enhance motor learning in persons with PD38. Similarly, IMUs have been used effectively to provide haptic and visual biofeedback related to kinematic data during balance and gait training in persons with PD28.
Knee Osteoarthritis
Wearable sensors have been used to understand population-level behavior in individuals with OA. Based on the Osteoarthritis Initiative (OAI), a large epidemiological study on knee OA that utilized wearable sensors to track physical activity in 1,111 adults, only 12.9% of men and 7.7% of women with knee OA met aerobic physical activity guidelines39. The study revealed that in people with knee OA, more sedentary behavior was associated with worse physical function40 and greater risk of future functional decline41. The Multicenter Osteoarthritis Study (MOST), another large epidemiological study enabled by wearable activity trackers, revealed that disease severity and knee pain were not predictive of physical activity levels42 and that older adults with high risk of knee OA did not meet Physical Activity Guidelines despite walking ≥ 10,000 steps per day43. These wearable sensor-enabled studies have yielded critical insights into the factors related to reduced physical activity in persons with knee OA and the effects of reduced physical activity on health.
For individuals with knee OA, the most common therapeutic application of wearable sensors is directed toward altering kinematics to reduce knee joint loading during walking. People with medial tibiofemoral OA walk with greater medial compartment loading compared to individuals with knee OA44. Greater medial compartment loading is implicated in more rapid disease progression45. There is thus significant interest in interventions that can reduce medial compartment loading. The knee adduction moment (KAM) during walking, measured using 3D motion capture, is commonly used as a surrogate for medial compartment loading44. There are several examples in the literature of wearable sensors being used to reduce KAM. Dowling et al., for example, developed an active feedback system fitted inside a shoe29. The system delivered haptic feedback if the pressure on the lateral aspect of the shoe exceeded a specific threshold, with the goal being to produce a subtle medial shift in weight bearing to reduce KAM. Use of this innovative biofeedback system led to a mean reduction of 14.2% in KAM. Although encouraging, this study was performed in healthy individuals, in a controlled lab environment, using expensive motion analysis instruments, and with a prototype version of the device. Significant work is needed to translate these systems to free-living conditions among people with knee OA.
Running
Up to 79% of runners are injured in a given year46. There is emerging interest in the role that impact mechanics may play in running injuries. Accumulating evidence shows associations between impact loading, as measured with a force plate, and injuries in runners. Indeed, vertical loadrates during the impact phase of running are associated with tibial stress fractures47. Runners with diagnosed injuries also have higher vertical loadrates compared to those who have never been injured48. Similarly, vertical loadrates are related to other common running injuries such as patellofemoral pain and plantar fasciitis48. While it is vertical loadrates that are related to running injuries, peak tibial acceleration during landing has been shown to be related to these loadrates49. Therefore, peak tibial acceleration, which can be measured with an accelerometer, has become a surrogate measure for vertical loadrates (Fig. 1A).
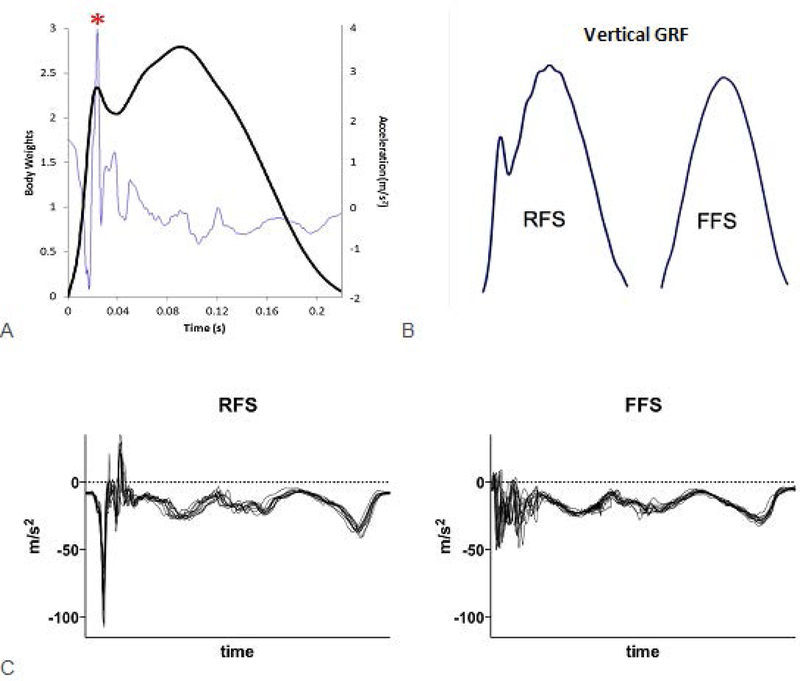
Figure 1.
(A) Vertical ground reaction force (dark line) with the tibial acceleration (light line) overlaid to demonstrate the similarity in timing of its peak with the vertical impact peak. (B) Vertical ground reaction force curves of a rearfoot strike (RFS) pattern and a forefoot strike (FFS) pattern. Note the distinct impact peak of the RFS pattern. (C) Tibial acceleration pattern for a RFS runner and a FFS runner. Pattern recognition can be used to distinguish footstrike pattern from these traces. Authors’ original work.
Wearable sensors may also assist in examining other gait characteristics that may contribute to running injuries, such as cadence and strike pattern. Among elite runners, achieving cadences near 180 steps per minute is thought to optimize performance50. Increased cadence has other benefits such as reduction in hip and knee energy absorption, patellofemoral stress, and hip adduction51,52. Further, increasing habitual cadences have demonstrated small reductions in vertical loadrates53. Strike pattern, on the other hand, influences the ground reaction forces applied to the body. Rearfoot strike (RFS) results in a very distinct impact peak in the vertical ground reaction force that is absent during forefoot strike (FFS)54 (Figure 1B). Transitioning to a FFS pattern has been shown to resolve chronic patellofemoral pain55 as well as chronic anterior compartment syndrome56. These distinct impact features can be seen in accelerometer data and can be used to differentiate between a RFS and a FFS pattern (Figure 1C).
Wearable sensors present an exciting opportunity both in the prevention and treatment of running-related injuries by affording the ability to provide real-time feedback to the individual. Many of the commercial IMUs provide information on cumulative loads which can be extremely helpful in preventing overload injuries in runners. Given the range of gait characteristics that can be measured (e.g., strike pattern, lower extremity angles, tibial shock, etc.) a wide variety of gait deviations can be addressed. Once the faulty aspect of gait is identified by the physical therapist, the runner can be instructed in how to alter the gait pattern. The therapist can then set audible signals to remind the patient to attend to their gait when it begins to degrade beyond a certain threshold. Feedback can then be gradually removed with time. Runners can first practice these gait changes in the clinic; however, wearable sensors allow the runner to translate the gait changes from the clinic into their natural running environment. This provides greater ecologic validity to the treatment, as well as can reduce the number of clinical visits needed, thereby reducing overall healthcare costs.
IMUs have important limitations to note when assessing running. Impact magnitudes during running can often exceed 16gs, which is the limit of some commercial devices. Similarly, accelerations during running include high frequency components that require adequate sampling frequencies (500–1000hz). These factors need to be considered when choosing IMU-based devices for running studies.
Clinimetric properties of sensors
The use of wearable sensors to inform neurologic and orthopedic rehabilitation practice warrants careful consideration of their clinimetric properties, which vary among devices57, conditions, measures, and environments58. Information on reliability, validity, and sensitivity is available for some devices, but not all. For example, wearable sensors used for running have been shown to provide acceptable, valid, and reliable values for some measures59; however, IMU-derived measures of tibial acceleration magnitudes and determinations of strike patterns require validation. For PD, a recent review of sensor characteristics concluded that only 9 out of the 73 devices considered could be recommended based on the availability and acceptability of their clinimetric properties57. Continued examination of the clinimetric properties of wearable sensor measurements may improve the standardization of data processing, definition of variables, and development of population-specific algorithms57,58.
Emerging clinical applications of commercially available technology
Emerging clinical applications using existing sensor technologies include their use (i) to identify biomarkers of disease onset and progression, (ii) to differentiate between restitution and compensatory mechanisms of motor recovery, (iii) to provide opportunities for tele-rehabilitation and big data collection, and (iv) in next-generation robotics.
Biomarkers
Tracking disease onset and progression is particularly valuable for those with chronic diseases. As such, there are increasing research efforts directed toward identification of biomarkers. A biomarker is a measurable characteristic that represents either a normal biologic process, pathologic process, or response to an intervention58. For example, there is emerging research on identifying motor biomarkers in genetic neurodegenerative diseases. The unobtrusive nature of wearable sensors, coupled with their ability to measure subtle changes in mobility in ecological settings, makes them a highly promising tool for detecting subclinical motor changes that can signal disease onset and progression. Evidence for this emerging application follows.
PD is characterized by dopamine depletion in the basal ganglia, which results in motor disturbances such as tremor, postural instability, bradykinesia, and gait impairment. While most cases of PD are idiopathic, a subset of the population can be explained by genetic factors, of which the most common mutation is leucine-rich repeat kinase 2 (LRRK2) – G2019S59. Accelerometers fixed on the low back have been used to identify increased stride time variability60, arm swing asymmetry, and trunk axial jerk in asymptomatic carriers of the LRRK2-G2019S mutation (i.e. at-risk for PD) during dual-task walking compared to healthy controls61.
The identification of motor biomarkers in HD is also an emerging area where wearable sensors have strong potential. HD is an autosomal-dominant neurodegenerative disease that is characterized by a combination of hyperkinetic and hypokinetic motor features62. Pharmaceutical and rehabilitative interventions are being developed to delay the clinical onset or slow down progression of HD63. These efforts, however, are attenuated due to limited knowledge of optimal clinical endpoints that are needed for clinical trials. There is emerging evidence for the use of wearable sensors to identify alterations in motor control, which may serve as a worthwhile endpoint. As in PD, an IMU fixed on the low back of individuals with pre-manifest HD and healthy controls was effective in detecting subclinical decrements in the sensory modulation of postural control64 and variability in trunk movement during walking65. Similarly, wearable iPOD sensors (IMU-based) fixed on the trunk and low back were able to detect abnormal trunk movements in persons with manifest HD compared to controls24. Despite these exciting preliminary findings that support the use of wearable sensors to identify and monitor biomarkers of disease onset and progression in movement disorders, larger, multi-site, and longitudinal studies are needed to catalyze this application.
Motor restitution versus compensation
An emerging clinical application of wearable sensor technologies is in differentiating restitution from compensation when assessing the nature of motor recovery66. Restitution refers to the re-appearance of movement patterns that were present prior to the injury, while compensation refers to the emergence of a new set of movement patterns post-injury resulting from substitution or adaptive mechanisms66. Elucidation of the mechanisms by which recovery occurs during rehabilitation allows for the development of computational models that can organize biological and behavioral data to inform clinical decision making66.
Researchers have also begun to use wearable sensor technologies and analytical techniques to look beyond gross functional and biomechanical recordings, with a focus on the neural control of movement. An example is the use of surface electromyography (sEMG) in the examination of motor modules during functional activities67 to identify neuromechanical differences between healthy and pathological movement68, evaluate the effects of neurorehabilitation intervention69, and assess changes in neuromotor control resulting from robotic intervention70. While more research is needed, this is a promising application of commercially available sensor technology. By differentiating between restitution and compensation mechanisms of recovery following neuromotor injury/dysfunction, sEMG analyses have potential to influence the prescription and evaluation of rehabilitative treatments.
Tele-rehabilitation
As our population ages and chronic disease rates and healthcare costs continue to rise, there is a demand for increased access to healthcare services and decreased costs. Tele-rehabilitation is a relatively new branch of telemedicine that prioritizes developing and optimizing telecommunication technologies for rehabilitation services (e.g., evaluation, monitoring, and treatment)71. The emerging use of wearable movement sensors to enable tele-rehabilitation services is both exciting and timely.
It is not the goal of tele-rehabilitation to replace health professionals; rather, it is to elevate the level of care72. The remote monitoring afforded by wearable sensors allows for real-time movement tracking in real world settings. This enables the continuous sampling of activity, rather than a finite series of collections taken during periodic clinic visits. Continuous remote monitoring of movement data may be used by clinicians to map progress and develop personalized interventions. The transmission of these data to clinicians via wireless communication systems could increase patient access to clinicians by bypassing the need to physically travel to a clinic. Similarly, for those with progressive neurological conditions, personalized biofeedback or tele-therapy can be administered in the comfort of home or community settings. These data, coupled with supported human-computer interactions, could also enable an assessment of quality of task practice, as well as patient engagement and compliance with home-based interventions (e.g., exercise programs). There is limited, moderate evidence showing that tele-rehabilitation results in comparable improvements with that of conventional therapy. Additional research is needed to extend the evidence base73. Research is also needed to determine the reliability and validity of the wearable sensor data that might be utilized through tele-rehabilitation approaches73. Furthermore, challenges in privacy and security of information exist, warranting consideration of enhanced security protections based on policy, regulatory76, and security protocols77.
Robotics
Wearable sensors have played an important role in enabling the development of next-generation assistive and rehabilitation robots. For example, during the last decade, portable rigid exoskeletons have emerged as an exciting tool to enable individuals who cannot walk, to walk again74. These powerful systems utilize sensors such as encoders or potentiometers to measure their movement and provide an estimate of limb movement—information that is used to modulate the forces delivered to the wearer. Such sensors, however, are not compatible with a new class of wearable robots that are made from soft and compliant materials75. IMUs and force sensors have been shown to be more easily integrated into these soft robotic exosuits, enabling their emergence for a variety of biomedical applications, including reducing the energy used during healthy walking76 and running77, and restoring more normal walking after stroke78,79.
Next-generation wearable sensors
Non-invasive monitoring of neural activity
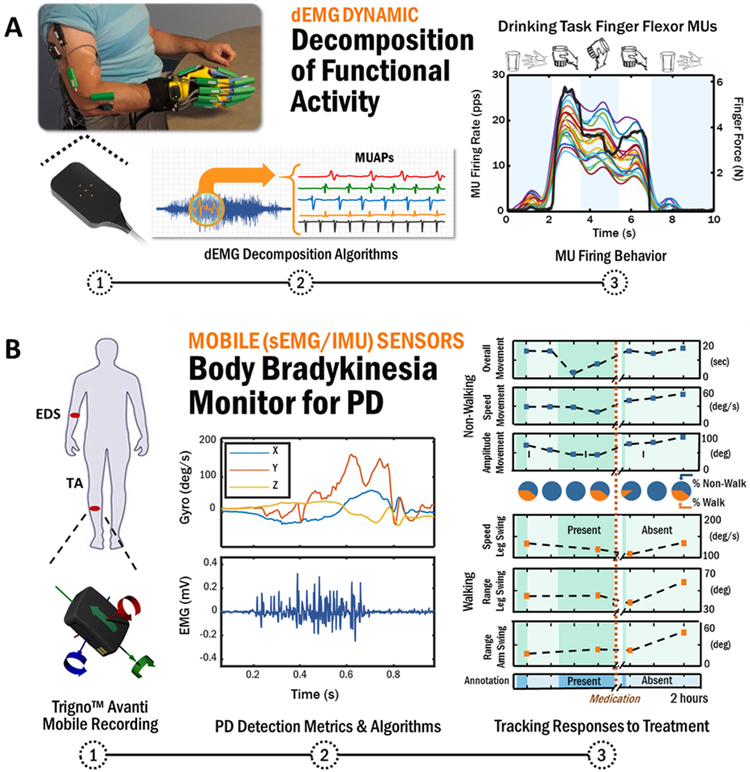
Extending our discussion of sEMG-enabled assessment of motor module analyses, complementary sensor modalities are emerging that enhance movement measurement by monitoring underlying neural control mechanisms. Indeed, motor impairments arise from changes in both neural control and the degradation of mechanical properties of muscles, and the relative contribution of each may be unique for each individual. Inherent to the control of movement are the firings of individual motoneurons that propagate towards the neuromuscular junction where their activation and rate coding regulate muscle contraction force and quality of movement. Deficits in motoneuron control are known to underlie neurological80,81 and musculoskeletal82 conditions, but have been difficult to discern using traditional techniques based on needle EMG recordings83, which are invasive, yield the firings of relatively few motoneurons, and are not practical beyond monitoring highly constrained activities that result from isometric muscle contractions. With the advance of neural sensors and their underlying artificial intelligence concepts, methods for extracting motoneuron firing behavior from noninvasive sEMG during isometric contractions84, and more recently during functional activities of everyday life85, have been made possible (Figure 2A).
Figure 2:
(A) Schematic of advanced sEMG sensor technology that can extract the firings of individual motor unit activity during functional tasks which can be used to study the underlying mechanisms of human movement in health and disease as well as provide a non-invasive neural interface as a real-time controller of a prosthetic or similar robotic device. (B) A schematic illustrating the use of hybrid sensor technology to autonomously monitor changes in the presence and severity of body bradykinesia in response to dopamine replacement medication in a person with Parkinson’s disease. Authors’ original work.
Recent work in this area has shown that groups of motoneurons are regulated differently when multiple muscles function in synergy to perform a functional task86 and that abnormal motoneuron firing behavior underlies motor impairments following stroke80,81. Assessing motoneuron recruitment patterns across neurological and orthopedic populations could provide valuable insight in determining whether rehabilitation efforts that target abnormalities in movement also have a measurable effect on reversing underlying deficits in motoneuron firing behavior.
Another emerging application of this technology includes assessing activation patterns of motoneurons specific to different training interventions. For example, a recent study showed that subjects were able to selectively activate different populations of motoneurons and thereby exercise components of the muscle with greater fatigue-resistance capabilities87. Subjects were able to increase the activation of relatively larger motoneurons that control higher-forces with respect to relatively smaller motoneurons that control lower-forces, in some cases by as much as 40%. Research is continuing to expand on these exciting preliminary findings to provide a basis for new strength training protocols to mitigate muscle weakness in patient populations with muscle atrophy from normal aging, musculoskeletal injury, or long-term bed-rest88.
Hybrid Sensors for Monitoring Muscle Activity and Movement
Recent technological advancements have enabled integration of miniaturized sensor components into on-chip electronic systems with ultra-low power consumption. This has fostered the development of “hybrid” wearable sensors that combine in a single encapsulation (i) motion sensing and (ii) EMG sensing of muscle activity. Hybrid sensors can be particularly advantageous for monitoring quality of movement when assessing and treating motor impairments. Indeed, the ability to measure characteristics of both the wearer’s movement and the underlying muscle activity responsible for regulating the movement provides a more holistic assessment of movement dysfunction. Hybrid sensors in use today for movement monitoring include an EMG recording component and a motion component, such as an accelerometer or IMU89,90
The feasibility of this technology was initially evaluated for automated detection of functional activities of daily living in individuals with stroke91. Using a minimal subset of four hybrid sensors (combined sEMG and accelerometer sensors located on both upper arms, one forearm, and one thigh), activities related to feeding, grooming, dressing, transferring, locomotion, and toileting were detected with a mean sensitivity of 95.0% and a mean specificity of 99.7%. Significant improvements in sensitivity and specificity resulted when both sEMG and accelerometer data were included, highlighting the value of a hybrid sensor approach for this application. Preliminary work in stroke demonstrated that a hybrid sEMG and accelerometer sensor could differentiate voluntary from spastic contractions92. Hybrid sensing has also been shown to be effective for the automated detection of the involuntary movements associated with PD during unscripted activities of daily living89,90. Indeed, the use of one hybrid sensor (sEMG and accelerometer) per symptomatic limb was sufficient in achieving 94.9% sensitivity and 97.1% specificity for autonomous tracking of tremor and dyskinesia in that limb in response to levodopa treatment.
Hybrid sensors that combine sEMG and IMU sensing hold even greater opportunities for wearable activity monitoring of movement disorders. The availability of angular velocity measurement in such a hybrid sensor proved highly effective in providing the first whole-body bradykinesia detector for PD (with an average accuracy of 95.0% for combined walking and non-walking activities) during unconstrained activities of daily living before and after levodopa therapy90 (Figure 2B). Similar technology has been shown to be effective when assessing the quality of movement in stroke93, and to monitor athletic performance for prevention of injury94.
Soft sensors
Advances in materials science have enabled explorations into the development of soft sensors and their applications to rehabilitation. Soft sensors can be placed in locations not possible with current movement monitoring devices. For example, stretchy sensors can be placed on the arch of runners with plantar fasciitis. Because runners with plantar fasciitis often have weak intrinsic foot muscles95, there is resultant flattening of the arch and increased strain on the plantar fascia. Stretchy sensors can provide feedback to the runner when their arch is lowering too much, reminding them to engage those muscles.
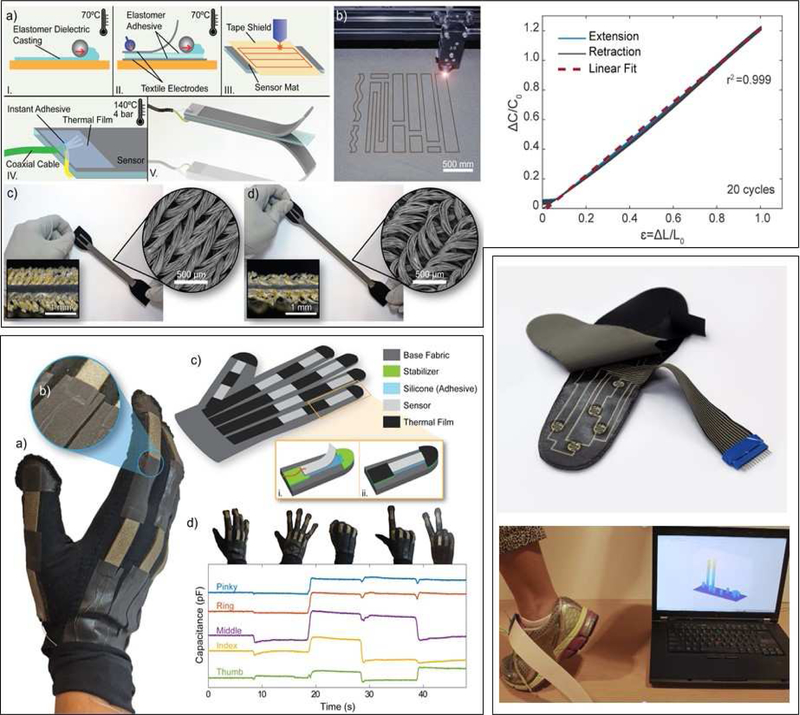
Because placement of sensors could be a source of imprecision in measurement96, the prospect of soft, textile-based sensors that could be worn like clothing is very attractive. For example, ultra-thin, ultra-light, and stretchable sEMG sensors that look similar to a temporary tattoo and are mechanically unnoticeable to the user, are being tested for use in evaluating exercise performance during rehabilitation97. In addition, elastomeric soft sensors have been integrated into a wearable sensing suit to measure hip, knee, and ankle kinematics98. Such sensing garments could be used for continuous kinematic monitoring in the community. More recently, an alternative stretchable, capacitive sensor has been developed with conductive knit fabrics as the electrode layer and a dielectric layer made from silicone elastomer99 (Figure 3 top). These sensors can be rapidly customized via a layered manufacturing process using a film applicator and laser cutting has demonstrated scalable, fast, low-cost production and arbitrary shaping of both strain99 and pressure sensors100. The textile-based nature of these sensors make them much more suitable for integration into apparel than existing sensor technologies. It has been demonstrated that these sensors can be integrated into a glove for measuring finger movements99 (Figure 3, bottom left) and grip force100. Additional promising initial results with other textile-compatible sensors demonstrate the ability to measure tension101 and applied pressure102 in wearable devices. Apart from making the transduction mechanism compatible with apparel, developments have also focused on creating conductive traces within textile materials to eliminate wiring and enable systems to be washable. Figure 3 (bottom right) highlights adaptations of this early work to develop an insole for measuring contact pressure.
Figure 3.
Preliminary work towards textile-based sensors. Top. Capacitive fabric-based stretch soft sensor for measuring joint kinematics. Bottom left, demonstration of sensors in soft robotic glove for measuring finger movement. Bottom right, ongoing work to develop pressure sensing insole using conductive textile traces and electrodes combined with a printed piezoresistive film. Authors’ original work.
Limitations
First, this review does not provide a comprehensive, systematic review of the literature, rather a focused discussion of the current and emerging sensor technologies and their clinical applications. Therefore, studies presenting similar technologies and clinical applications may not have been included. Second, the clinical applications discussed are limited to stroke, PD, HD, OA, and running populations. While these are only a few of the conditions that utilize and can benefit from wearable movement sensors, the conditions chosen illustrate the large spectrum of individuals with varying degrees of capabilities that may benefit from existing and emerging sensor technologies.
Conclusions
The central goal of physical rehabilitation is to facilitate the re-acquisition of movement abilities after injury or onset of disease. Motor behavior is viewed as an output of the movement system based on its encompassing interaction with cardiovascular, pulmonary, endocrine, integumentary, nervous, and musculoskeletal systems103, thus movement data has high potential in examining health and disease across systems. Wearable sensors are a promising rehabilitation technology because of their precision, noninvasiveness, and easy deployment compared to other methods. Their complementary measurement of kinematic motion, neural activity, and muscle dynamics offers a targeted approach for assessing and treating a variety of neurologic and orthopedic conditions. Additionally, more widespread monitoring of movement in clinical and ecological settings and across different rehabilitation timescales may serve as a pathway to the development of computational models of recovery and precision medicine. Finally, advancements in materials science are allowing for the development of next-generation sensors that can record biologic movements from device interfaces that are more fully transparent to the wearer.
ACKNOWLEDGEMENTS
We are grateful for the consultation and technical input provided by Jaehyun Bae.
Acknowledgement of financial support/conflict of interest: This work was partially funded by the National Institutes of Health (Award numbers: 1KL2TR001411, 1ULT1TR001430, 1K01AR069720, and R01HD088619) and National Science Foundation (CNS-1446464). Dr. Walsh reports grants and personal fees from ReWalk Robotics, outside the submitted work. In addition, Dr. Walsh has multiple patents related to soft robotic technologies. Dr. Roy is employed by Delsys Inc and has multiple patents related to wearable sensor technologies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cook AM. Ethical issues related to the use/non-use of assistive technologies. Dev Disabil Bull. 2009;37(2):127–152. https://files.eric.ed.gov/fulltext/EJ920692.pdf. [Google Scholar]
- 2.Dhawan AP. Collaborative paradigm of preventive, personalized, and precision medicine with point-of-care technologies. IEEE J Transl Eng Heal Med. 2016;4(February). doi: 10.1109/JTEHM.2016.2635126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobkin BH. Behavioral self-management strategies for practice and exercise should be included in neurologic rehabilitation trials and care. Curr Opin Neurol. 2016;29(6):693–699. doi: 10.1097/WCO.0000000000000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capela NA, Lemaire ED, Baddour N, Rudolf M, Goljar N, Burger H. Evaluation of a smartphone human activity recognition application with able-bodied and stroke participants. J Neuroeng Rehabil. 2016;13(1):1–10. doi: 10.1186/s12984-016-0114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien MK, Shawen N, Mummidisetty CK, et al. Activity recognition for persons with stroke using mobile phone technology: toward improved performance in a home setting. J Med Internet Res. 2017;19(5):e184. doi: 10.2196/jmir.7385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Döbrössy MD, Dunnett SB. The influence of environment and experience on neural grafts. Nat Rev Neurosci. 2001;2(12):871–879. doi: 10.1038/35104055 [DOI] [PubMed] [Google Scholar]
- 7.Jonsdottir J, Cattaneo D, Recalcati M, et al. Task-oriented biofeedback to improve gait in individuals with chronic stroke: Motor learning approach. Neurorehabil Neural Repair. 2010;24(5):478–485. doi: 10.1177/1545968309355986 [DOI] [PubMed] [Google Scholar]
- 8.Hadjidj A, Souil M, Bouabdallah A, Challal Y, Owen H. Wireless sensor networks for rehabilitation applications : challenges and opportunities. J Netw Comput Appl. 2013;36:1–15. [Google Scholar]
- 9.Reuterbories J, Spaich EG, Larsen B, Andersen OK. Methods for gait event detection and analysis in ambulatory systems. Med Eng Phys. 2017;32(6):783–790. doi: 10.1016/j.medengphy.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 10.Munoz-Organero M, Parker J, Powell L, Mawson S. Assessing walking strategies using insole pressure sensors for stroke survivors. Sensors (Switzerland). 2016;16(10). doi: 10.3390/s16101631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolan KJ, Yarossi M, McLaughlin P. Changes in center of pressure displacement with the use of a foot drop stimulator in individuals with stroke. Clin Biomech. 2015;30(7):755–761. doi: 10.1016/j.clinbiomech.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 12.Byl N, Zhang W, Coo S, Tomizuka M. Clinical impact of gait training enhanced with visual kinematic biofeedback: Patients with Parkinson’s disease and patients stable post stroke. Neuropsychologia. 2015;79:332–343. doi: 10.1016/j.neuropsychologia.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 13.Owaki D, Sekiguchi Y, Honda K, Ishiguro A, Izumi SI. Short-term effect of prosthesis transforming sensory modalities on walking in stroke patients with hemiparesis. Neural Plast. 2016;2016. doi: 10.1155/2016/6809879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan V, Khoo I, Marayong P, DeMars K, Cormack J. Gait training in chronic stroke using walk-even feedback device: a pilot study. Neurosci J. 2016;2016:1–8. doi: 10.1155/2016/6808319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurkmans HLP, Bussmann JBJ, Selles RW, Horemans HLD, Benda E. Validity of the Pedar Mobile system for vertical force measurement during a seven-hour period. 2006;39:110–118. doi: 10.1016/j.jbiomech.2004.10.028 [DOI] [PubMed] [Google Scholar]
- 16.Tong K, Granat MH. A practical gait analysis system using gyroscopes. Med Eng Phys. 1999;21(2):87–94. doi: 10.1016/S1350-4533(99)00030-2 [DOI] [PubMed] [Google Scholar]
- 17.Summa S, Tosi J, Taffoni F, et al. Assessing bradykinesia in Parkinson’s disease using gyroscope signals In: Internation Conference on Rehabilitation Robotics (ICORR). London, UK; 2017:1556–1561. [DOI] [PubMed] [Google Scholar]
- 18.Mayagoitia RE, Nene AV, Veltink PH Accelerometer and rate gyroscope measurement of kinematics: An inexpensive alternative to optical motion analysis systems. J Biomech. 2002;35(4):537–542. doi: 10.1016/S0021-9290(01)00231-7 [DOI] [PubMed] [Google Scholar]
- 19.Kavanagh JJ, Menz HB. Accelerometry : A technique for quantifying movement patterns during walking. 2008;28:1–15. doi: 10.1016/j.gaitpost.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 20.Dorsch AK, Thomas S, Xu X, Kaiser W, Dobkin BH. SIRRACT: An international randomized clinical trial of activity feedback during inpatient stroke rehabilitation enabled by wireless sensing. Neurorehabil Neural Repair. 2015;29(5):407–415. doi: 10.1177/1545968314550369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haji Ghassemi N, Hannink J, Martindale C, et al. Segmentation of gait sequences in sensor-based movement analysis: a comparison of methods in Parkinson’s Disease. Sensors. 2018;18(1):145. doi: 10.3390/s18010145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wüest S, Aminian F, Aminian K, Gonzenbach R, de Bruin E. Reliability and validity of the inertial sensor-based Timed “Up and Go” test in individuals affected by stroke. J Rehabil Res Dev. 2016;53(5):599–610. [DOI] [PubMed] [Google Scholar]
- 23.Heldman DA, Espay AJ, LeWitt PA, Giuffrida JP. Clinical versus machine; reliability and responsiveness of motor endpoints in Parkinson’s disease. Parkinsonism Relat Disord. 2013;31(9):1713–1723. doi: 10.1109/TMI.2012.2196707.Separate [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kegelmeyer DA, Kostyk SK, Fritz NE, et al. Quantitative biomechanical assessment of trunk control in Huntington’s disease reveals more impairment in static than dynamic tasks. J Neurol Sci. 2017;376:29–34. doi: 10.1016/j.jns.2017.02.054 [DOI] [PubMed] [Google Scholar]
- 25.Mancini M, El-Gohary M, Pearson S, et al. Continuous monitoring of turning in Parkinson’s disease: rehabiliation potential. NeuroRehabilitation. 2015;37(1):3–10. doi: 10.3233/NRE-151236.Continuous [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isho T, Tashiro H, Usuda S. Accelerometry-based gait characteristics evaluated using a smartphone and their association with fall risk in people with chronic stroke. J Stroke Cerebrovasc Dis. 2015;24(6):1305–1311. doi: 10.1016/j.jstrokecerebrovasdis.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 27.Bergamini E, Iosa M, Belluscio V, Morone G, Tramontano M, Vannozzi G. Multi-sensor assessment of dynamic balance during gait in patients with subacute stroke. J Biomech. 2017;61:208–215. doi: 10.1016/j.jbiomech.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 28.Carpinella I, Cattaneo D, Bonora G, et al. Wearable sensor-based biofeedback training for balance and gait in Parkinson disease: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98(4):622–630.e3. doi: 10.1016/j.apmr.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 29.Dowling AV, Fisher DS, Andriacchio TP. Gait modification via verbal instruction and an active feedback system to reduce peak knee adduction moment. J Biomech Eng. 2010;132(7). [DOI] [PubMed] [Google Scholar]
- 30.Massé F, Gonzenbach RR, Arami A, Paraschiv-Ionescu A, Luft AR, Aminian K. Improving activity recognition using a wearable barometric pressure sensor in mobility-impaired stroke patients. J Neuroeng Rehabil. 2015;12(1):1–15. doi: 10.1186/s12984-015-0060-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulk GD, He Y, Boyne P, Dunning K. Predicting home and community walking activity poststroke. Stroke. 2017:406–412. doi: 10.1161/STROKEAHA.116.015309 [DOI] [PubMed] [Google Scholar]
- 32.French MA, Moore MF, Pohlig R, et al. Self-efficacy mediates the relationship between balance/walking performance, activity, and participation after stroke. Top Stroke Rehabil. 2016;23(2):77–83. doi: 10.1080/10749357.2015.1110306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danks KA, Roos MA, Mccoy D, Reisman DS, Program MS. A step activity monitoring program improves real world walking activity post stroke. Disabil Rehabil. 2015;36(26):2233–2236. doi: 10.3109/09638288.2014.903303.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez-Molinero A, Samà A, Pérez-Martínez DA, et al. Validation of a Portable Device for Mapping Motor and Gait Disturbances in Parkinson’s Disease. JMIR mHealth uHealth. 2015;3(1):e9. doi: 10.2196/mhealth.3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delrobaei M, Memar S, Pieterman M, Stratton TW, McIsaac K, Jog M. Towards remote monitoring of Parkinson’s disease tremor using wearable motion capture systems. J Neurol Sci. 2018;384(August):38–45. doi: 10.1016/j.jns.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 36.Cavanaugh JT, Ellis TD, Earhart GM, Ford MP, Foreman KB, Dibble LE. Toward understanding ambulatory activity decline in Parkinson disease. Phys Ther. 2015;95(8):1142–1150. doi: 10.2522/ptj.20140498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlachetzki JCM, Barth J, Marxreiter F, et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS One. 2017;12(10):1–18. doi: 10.1371/journal.pone.0183989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieuwboer A, Rochester L, Müncks L, Swinnen SP. Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Park Relat Disord. 2009;15(SUPPL. 3):53–58. doi: 10.1016/S1353-8020(09)70781-3 [DOI] [PubMed] [Google Scholar]
- 39.Dulop DD, Song J, Semanik PA, et al. Initiative : Are guidelines being met ? Arthritis Rheumatol. 2012;63(11):3372–3382. doi: 10.1002/art.30562.Objective [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J, Chang RW, Ehrlich-Jones L, et al. Sedentary behavior and physical function: Objective evidence from the osteoarthritis initiative. Arthritis Care Res. 2015;67(3):366–373. doi: 10.1002/acr.22432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semanik PA, Lee J, Song J, et al. Accelerometer-monitored sedentary behavior and observed physical function loss. Am J Public Health. 2015;105(3):560–566. doi: 10.2105/AJPH.2014.302270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White DK, Tudor-Locke C, Felson DT, et al. Do radiographic disease and pain account for why people with or at high risk of knee osteoarthritis do not meet physical activity guidelines? Arthritis Rheum. 2013;65(1):139–147. doi: 10.1002/art.37748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White DK, Tudor-Locke C, Felson DT, et al. Walking to meet physical activity guidelines in knee osteoarthritis: Is 10,000 steps enough? Arch Phys Med Rehabil. 2013;94(4):711–717. doi: 10.1016/j.apmr.2012.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar D, Manal KT, Rudolph KS. Knee joint loading during gait in healthy controls and individuals with knee osteoarthritis. Osteoarthr Cartil. 2013;21(2):298–305. doi: 10.1016/j.joca.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennell KL, Bowles KA, Wang Y, Cicuttini F, Davies-Tuck M, Hinman RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis. 2011;70(10):1770–1774. doi: 10.1136/ard.2010.147082 [DOI] [PubMed] [Google Scholar]
- 46.RN Van Gent, Siem D, M Van Middelkoop, AG Van Os, Koes BW. Incidence and determinants of lower extremity running injuries in long distance runners: a systematic review. 2007;(January 2006):469–480. doi: 10.1136/bjsm.2006.033548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zadpoor AA, Nikooyan AA. Clinical Biomechanics The relationship between lower-extremity stress fractures and the ground reaction force : A systematic review. JCLB. 2011;26(1):23–28. doi: 10.1016/j.clinbiomech.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 48.Davis IS, Bowser BJ, Mullineaux DR. Greater vertical impact loading in female runners with medically diagnosed injuries: a prospective investigation. Br J Sports Med. 2015;50(14):887–892. [DOI] [PubMed] [Google Scholar]
- 49.Hennig EM, Lafortune MA. Relationships between ground reaction force and tibial bone acceleration parameters. Int J Sport Biomech. 1991;7:303–309. [Google Scholar]
- 50.Daniels J Daniel’s Running Formula. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 51.Heiderscheit BC, Chumanov ES, Michalski MP, Wille CM, Ryan MB. Effects of step rate manipulation on joint mechanics during running. Med Sci Sports Exerc. 2011;43(2):296–302. doi: 10.1249/MSS.0b013e3181ebedf4.Effects [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenhart RL, Thelen DG, Wille CM, Chumanov ES, Heidershceit BC. Increasing running step rate reduces patellofemoral joint forces. Med Sci Sports Exerc. 2014;46(3):557–564. doi: 10.1249/MSS.0b013e3182a78c3a.Increasing [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willy RW, Buchenic L, Rogacki K, Ackerman J, Schmidt A, Wilson JD. In field gait retraining and mobile monitoring to address running biomechanics associated with tibial stress fracture. Scand J Med Sci Sports. 2015;26(2):197–205. [DOI] [PubMed] [Google Scholar]
- 54.Rice HM, Jameson ST, Davis IS. Footwear matters: Influence of footwear and foot strike on loadrates during running. Med Sci Sports Exerc. 2016;48(12):2462–2468. [DOI] [PubMed] [Google Scholar]
- 55.Cheung RT, Davis IS. Landing pattern modification to improve patellofemoral pain in runners: a case series. J Orthop Sport Phys Ther. 2011;41(12):914–919. [DOI] [PubMed] [Google Scholar]
- 56.Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated With chronic exertional compartment syndrome. Am Coll Sport Med. 2012;40(5):1060–1067. [DOI] [PubMed] [Google Scholar]
- 57.Godinho C, Domingos J, Cunha G, et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. J Neuroeng Rehabil. 2016;13(1):1–10. doi: 10.1186/s12984-016-0136-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansson D, Malmgren K, Alt Murphy M. Wearable sensors for clinical applications in epilepsy, Parkinson’s disease, and stroke: a mixed-methods systematic review. J Neurol. 2018;(123456789):1–13. doi: 10.1007/s00415-018-8786-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Provot T, Chiementin X, Oudin E, Bolaers F, Murer S. Validation of a high sampling rate inertial measurement unit for acceleration during running. Sensors (Basel). 2017;17(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 69AD;3(3):89–95. doi: 10.1067/mcp.2001.113989 [DOI] [PubMed] [Google Scholar]
- 61.Kumari U, Tan EK. LRRK2 in Parkinson’s disease: genetic and clinical studies from patients. 2009;276:6455–6463. doi: 10.1111/j.1742-4658.2009.07344.x [DOI] [PubMed] [Google Scholar]
- 62.Mirelman A, Gurevich T, Giladi N, Bar-shira A, Orr-urtreger A, Hausdorff JM. Gait alterations in healthy carriers of the LRRK2 G2019S mutation. Ann Neurol. 2011;69:193–211. doi: 10.1002/ana.22165 [DOI] [PubMed] [Google Scholar]
- 63.Mirelman A, Bernad-Elazari H, Thaler A, et al. Arm swing as a potential new prodromal marker of Parkinson’s disease. Mov Disord. 2016;31(10):1527–1534. doi: 10.1002/mds.26720.Arm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berardelli A, Noth J, Thompson PD, et al. Pathophysiology of Chorea and Bradykinesia in Huntington ‘s Disease. 1999;14(3):398–403. [DOI] [PubMed] [Google Scholar]
- 65.Tabrizi SJ, Scahill RI, Owen G, et al. Articles Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington ‘s disease in the TRACK-HD study : analysis of 36-month observational data. 2013;4422(13):1–13. [DOI] [PubMed] [Google Scholar]
- 66.Porciuncula F, Marder KS, Wasserman P, Rao AK. Sensory modulation of postural control in Huntington’s disease. Mov Disord. 2017;32(Supplement 2):S192–3. [Google Scholar]
- 67.Collett J, Esser P, Khalil H, et al. Insights into gait disorders: Walking variability using phase plot analysis, Huntington’s disease. Gait Posture. 2014;40(4):694–700. doi: 10.1016/j.gaitpost.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 68.Reinkensmeyer DJ, Burdet E, Casadio M, et al. Computational neurorehabilitation: modeling plasticity and learning to predict recovery. J Neuroeng Rehabil. 2016;13(1):42. doi: 10.1186/s12984-016-0148-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010;103(2):844–857. doi: 10.1152/jn.00825.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veerbeek JM, Koolstra M, Ket JCF, EEH Van Wegen, Kwakkel G Effects of augmented exercise therapy on outcome of gait and gait-related activities in the first 6 months after stroke a meta-analysis. Stroke. 2011;42:3311–3315. doi: 10.1161/STROKEAHA.111.623819 [DOI] [PubMed] [Google Scholar]
- 71.Ferrante S, Bejarano NC, Ambrosini E, et al. A personalized multi-channel FES controller based on muscle synergies to support gait rehabilitation after stroke. Front Neurosci. 2016;10(September). doi: 10.3389/fnins.2016.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobs DA, Koller JR, Steele KM, Ferris DP. Motor modules during adaptation to walking in a powered ankle exoskeleton. 2018:1–15. doi: 10.1186/s12984-017-0343-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winters JM. Telerehabilitation research: emerging opportunities. Annu Rev Biomed Eng. 2002;4(1):287–320. doi: 10.1146/annurev.bioeng.4.112801.121923 [DOI] [PubMed] [Google Scholar]
- 74.Polisena J, Coyle D, Coyle K, McGill S. Home telehealth for chronic disease management: A systematic review and an analysis of economic evaluations. Int J Technol Assess Health Care. 2009;25(3):339–349. doi: 10.1017/S0266462309990201 [DOI] [PubMed] [Google Scholar]
- 75.Chen J, Jin W, Zhang X-X, Xu W, Liu X-N, Ren C-C. Telerehabilitation approaches for stroke patients: systematic review and meta-analysis of randomized controlled trials. 2015. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 76.Hall JL, McGraw D. For telehealth to succeed, privacy and security risks must be identified and addressed. Health Aff. 2014;33(2):216–221. doi: 10.1377/hlthaff.2013.0997 [DOI] [PubMed] [Google Scholar]
- 77.Rezaeibagha F, Mu Y. Practical and secure telemedicine systems for user mobility. J Biomed Inform. 2018;78(December):24–32. doi: 10.1016/j.jbi.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 78.Esquenazi A, Talaty M, Packel A, Saulino M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am J Phys Med Rehabil. 2012;91(11):911–921. doi: 10.1097/PHM.0b013e318269d9a3 [DOI] [PubMed] [Google Scholar]
- 79.Veale AJ, Xie SQ. Towards compliant and wearable robotic orthoses: A review of current and emerging actuator technologies. Med Eng Phys. February 2016. doi: 10.1016/j.medengphy.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 80.Panizzolo FA, Galiana I, Asbeck AT, et al. A biologically-inspired multi-joint soft exosuit that can reduce the energy cost of loaded walking. J Neuroeng Rehabil. 2016;13(1):43. doi: 10.1186/s12984-016-0150-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee G, Kim J, Panizzolo FA, et al. Reducing the metabolic cost of running with a tethered soft exosuit. Sci Robot. 2017;2(6). [DOI] [PubMed] [Google Scholar]
- 82.Awad LN, Bae J, O’Donnell K, et al. A soft robotic exosuit improves walking in patients after stroke. Sci Transl Med. 2017;9(400):eaai9084. doi: 10.1126/scitranslmed.aai9084 [DOI] [PubMed] [Google Scholar]
- 83.Bae J, Siviy C, Rouleau M, et al. A lightweight and efficient portable soft exosuit for paretic ankle assistance in walking after stroke In: IEEE International Conference on Robotics and Automation. Brisbane, Australia; 2018. [Google Scholar]
- 84.Chou L-W, Palmer JA, Binder-Macleod S, Knight CA. Motor unit rate coding is severely impaired during forceful and fast muscular contractions in individuals post stroke. J Neurophysiol. 2013;109(12):2947–2954. doi: 10.1152/jn.00615.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li X, Wang Y-C, Suresh NL, Rymer WZ, Zhou P. Motor unit number reductions in paretic muscles of stroke survivors. IEEE Trans Inf Technol Biomed. 2011;15(4):505–512. doi: 10.1109/TITB.2011.2140379 [DOI] [PubMed] [Google Scholar]
- 86.Kallenberg LAC, Hermens HJ. Motor unit action potential rate and motor unit action potential shape properties in subjects with work-related chronic pain. Eur J Appl Physiol. 2006;96(2):203–208. doi: 10.1007/s00421-004-1215-1 [DOI] [PubMed] [Google Scholar]
- 87.Falla D, Lindstrøm R, Rechter L, Farina D. Effect of pain on the modulation in discharge rate of sternocleidomastoid motor units with force direction. Clin Neurophysiol. 2010;121(5):744–753. doi: 10.1016/j.clinph.2009.12.029 [DOI] [PubMed] [Google Scholar]
- 88.De Luca CJ, Adam A, Wotiz R, Donald Gilmore L, Hamid Nawab S, Luca D. Decomposition of surface EMG signals. J Neurophysiol. 2006;96:1646–1657. doi: 10.1152/jn.00009.2006 [DOI] [PubMed] [Google Scholar]
- 89.De Luca CJ, Chang S-S, Roy SH, Kline JC, Nawab SH. Decomposition of surface EMG signals from cyclic dynamic contractions. J Neurophysiol. 2015;113(6):1941–1951. doi: 10.1152/jn.00555.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kline J, Contessa P, Roy S, De Luca G. Coordination of motor unit firings during cyclic activities of the upper-limb In: Proc. ISB 2017 Congress XXVI. Brisbane, Australia; 2017. [Google Scholar]
- 91.De Luca CJ, Kline JC, Contessa P. Transposed firing activation of motor units. J Neurophysiol. 2014;112(4):962–970. doi: 10.1152/jn.00619.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pope ZK, Hester GM, Benik FM, DeFreitas JM. Action potential amplitude as a noninvasive indicator of motor unit-specific hypertrophy. J Neurophysiol. 2016;115(5):2608–2614. doi: 10.1152/jn.00039.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roy SH, Cole BT, Gilmore LD, et al. High-resolution tracking of motor disorders in Parkinson’s disease during unconstrained activity. Mov Disord. 2013;28(8):1080–1087. doi: 10.1002/mds.25391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roy S, Shiwani B, Kline J, et al. Autonomous tracking of body bradykinesia during unconstrained activities in Parkinson’s disease. Movement Disorders, ed. Mov Disord. 2017;(32):448–449. doi: 10.1002/mds.27087 [DOI] [Google Scholar]
- 95.Roy SH, Cheng MS, Chang S-S, et al. A Combined sEMG and Accelerometer System for Monitoring Functional Activity in Stroke. IEEE Trans Neural Syst Rehabil Eng. 2009;17(6):585–594. doi: 10.1109/TNSRE.2009.2036615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghaffari R, Jayarman A, Lonini L, Rogers JA, Shawen N. Automatic detection of spasticity from flexible wearable sensors. In: UbiComp/ISWC Adjunct.; 2017. [Google Scholar]
- 97.van Meulen FB, Klaassen B, Held J, et al. Objective evaluation of the quality of movement in daily life after stroke. Front Bioeng Biotechnol. 2016;3:210. doi: 10.3389/fbioe.2015.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gilgien M, Spörri J, Kröll J, Crivelli P, Müller E. Mechanics of turning and jumping and skier speed are associated with injury risk in men’s World Cup alpine skiing: a comparison between the competition disciplines. Br J Sports Med. 2014;48(9):742–747. doi: 10.1136/bjsports-2013-092994 [DOI] [PubMed] [Google Scholar]
- 99.Cheung RTH, Sze LKY, Mok NW, Ng GYF. Intrinsic foot muscle volume in experienced runners with and without chronic plantar fasciitis. J Sci Med Sport. 2016;19:713–715. doi: 10.1016/j.jsams.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 100.Willemsen ATM, van Alsté JA, Boom HBK. Real-time gait assessment utilizing a new way of accelerometry. J Biomech. 1990;23(8):859–863. doi: 10.1016/0021-9290(90)90033-Y [DOI] [PubMed] [Google Scholar]
- 101.Constantinescu G, Jeong J-W, Li X, et al. Epidermal electronics for electromyography: An application to swallowing therapy. Med Eng Phys. 2016;38(8):807–812. doi: 10.1016/J.MEDENGPHY.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 102.Menguc Y, Park Y, Pei H, et al. Wearable soft sensing suit for human gait measurement. Int J Rob Res. 2014;33(14):1748–1764. [Google Scholar]
- 103.Atalay A, Sanchez V, Atalay O, et al. Batch fabrication of customizable silicone-textile composite capacitive strain sensors for human motion tracking. 2017;1700136:1–8. doi: 10.1002/admt.201700136 [DOI] [Google Scholar]
- 104.Atalay O, Atalay A, Gafford J, Walsh C. A highly sensitive capacitive-based soft pressure sensor based on a conductive fabric and a microporous dielectric layer. Adv Mater Technol. 2018;3(1):1700237. doi: 10.1002/admt.201700237 [DOI] [Google Scholar]
- 105.Araromi OA, Walsh CJ, Wood RJ. Hybrid carbon fiber-textile compliant force sensors for high-load sensing in soft exosuits In: International Conference on Intelligent Robots and Systems (IROS). Vancouver, Canada; 2017. [Google Scholar]
- 106.Araromi OA, Walsh CJ, Wood RJ. Fabrication of stretchable composites with anisotropic electrical conductivity for compliant pressure transducers. In: IEEE Sensors.; 2016. [Google Scholar]
- 107.Amercian Physical Therapy Association. Physical Therapist Practice and The Movement System.; 2015.