Abstract
Human glial progenitor cells (hGPCs) can engraft, expand, and differentiate into functional oligodendrocytes and astrocytes when transplanted neonatally into murine hosts, in which they outcompete the host glial pool to ultimately colonize and dominate the recipient brains. When congenitally hypomyelinated mutants are used as hosts, the donor hGPCs generate myelinogenic oligodendrocytes as well as astrocytes, so that the recipient mice develop a largely humanized white matter, with entirely human-derived myelin. In addition, by neonatally engrafting hGPCs derived from patient- and disease-specific pluripotent stem cells, glial chimeric mice may be produced in which large proportions of all macroglial cells are not only human, but patient-and disease-specific. Human glial chimeric mice thus provide intriguing preparations by which to investigate the species-specific contributions of human glia to both cognition and human-selective neurodegenerative and neuropsychiatric diseases, as well as the potential for therapeutic glial cell replacement in these disorders. This review presents an overview of the uses, characteristics and limitations of the human glial chimeric brain model, while providing a step-by-step protocol for the establishment of these mice.
Keywords: glial progenitor, oligodendrocyte progenitor, stem cell, cell transplant, mouse models
Over the past two decades, we and others have explored the use of glial progenitor cells as engraftable cellular therapeutics for the treatment of glial disorders, in particular those characterized by oligodendrocyte loss and consequent demyelination (reviewed in (Goldman, 2016, 2017). The intended therapeutic targets of this approach are broad, and include pediatric disorders of myelin formation and maintenance, such as the hereditary leukodystrophies and lysosomal storage disorders (Goldman, 2011; Osorio and Goldman, 2016b), as well as the adult-onset disorders of myelin integrity, such as multiple sclerosis and the age-related white matter diseases of vascular insufficiency (Franklin and Goldman, 2015; Goldman et al., 2012). With these therapeutic purposes in mind, a variety of increasingly refined methods have been established for isolating human glial progenitor cells, and for modeling their behavior and instructing their fates, both in vitro and in vivo, the latter via transplantation-based strategies.
Like their rodent counterparts (Raff et al., 1983), human GPCs may generate both astrocytes and oligodendrocytes (Windrem et al., 2004), and in some contexts neurons as well (Belachew et al., 2003; Nunes et al., 2003); yet despite their multilineage competence, they have been most often referred to as oligodendrocyte progenitor cells (OPCs), and also as NG2 cells, the latter designation based on their expression of the NG2 chondroitin sulfate proteoglycan (Nishiyama et al., 2009; Nishiyama et al., 1996). While these terms are effectively synonymous, in this review these cells will be referred to as glial progenitor cells, GPCs. Human GPCs (hGPCs) can be reliably and efficiently isolated from human brain tissue by selecting for glial gangliosides recognized by the A2B5 antibody, with concurrent negative selection by depletion of polysialylated NCAM-defined neuroblasts (Nunes et al., 2003; Roy et al., 1999; Windrem et al., 2004). They may also – and more specifically - be isolated on the basis of the CD140a-defined ectodomain of the PDGFα receptor, which includes all of the potentially oligoneogenic cells of the human brain (Sim et al., 2011). Progressively more oligodendrocyte-biased phenotypes may be further selected, based upon the concurrent expression of the CD9 tetraspanin, or of the oligodendrocytic sulfatide recognized by the O4 antibody (Douvaras et al., 2014; Piao et al., 2015). Whether derived from adult or fetal human brain, each of these phenotypes has now proven efficient, in a variety of laboratories and model systems, in remyelinating structurally-demyelinated or hypomyelinated tissue.
Human GPCs may be transplanted to restore lost myelin
Beginning with pioneering studies by Gumpel and Lachapelle, and later by Blakemore and colleagues (Blakemore et al., 1990; Gumpel et al., 1987; Lachapelle et al., 1983), the ability of transplanted oligodendroglia and their progenitors to effect myelin repair has been studied in a number of model systems (Archer et al., 1997; Archer et al., 1994; Duncan, 2005; Yandava et al., 1999); broadly reviewed in (Ben-Hur and Goldman, 2008; Franklin and ffrench-Constant, 2008). Efforts focusing on the specific use of GPCs rather than their derived oligodendrocytes, and emphasizing the use of human GPCs in particular, are more recent (Windrem et al., 2004; Windrem et al., 2002; Windrem et al., 2008). Many of these studies have assessed the myelinogenic competence of GPCs in the shiverer mouse (MBP shi/shi), a congenitally hypomyelinated mouse deficient in myelin basic protein (Popko et al., 1987; Readhead et al., 1987). Using immunodeficient shiverer mice (rag2−/− × MBP shi/shi) as hosts, we developed a multi-site delivery procedure by which donor GPCs could be introduced into the major presumptive white matter tracts of newborn mice (Windrem et al., 2008). This approach resulted in the widespread engraftment of donor hGPCs throughout the entire CNS, with infiltration of the forebrain, brainstem and cerebellum, and ultimately the spinal cord and roots (Figure 1). The donor hGPCs then produced both oligodendrocytes and astrocytes, resulting in the dense and efficient central myelination of these otherwise unmyelinated mice. This in turn was associated with their substantially prolonged survival, with frank rescue and phenotypic recovery of a large minority (Windrem et al., 2008). Indeed, whereas untreated shiverers invariably die by 20–21 weeks of age, a fraction of neonatally-engrafted mice achieved normal lifespans of over 2 years, suggesting the potential power of this approach towards therapeutic remyelination.
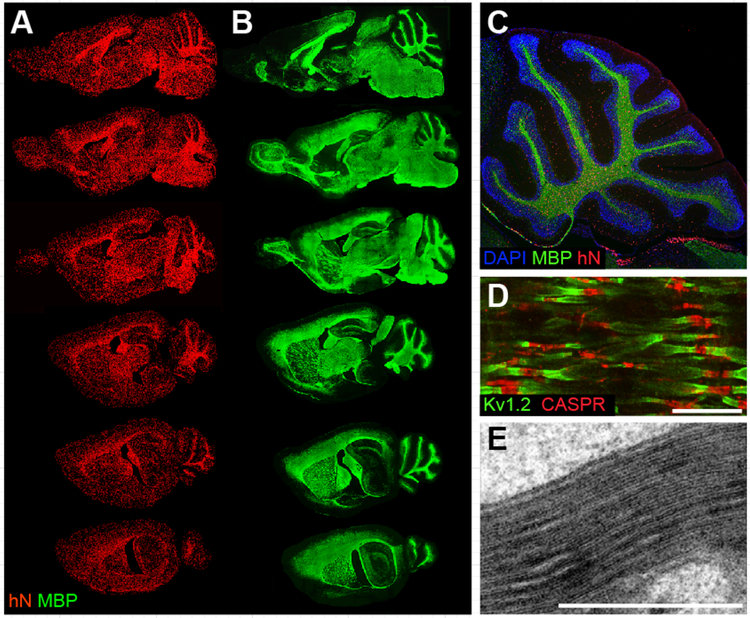
Figure 1. Engrafted hGPCs chimerize and myelinate the congenitally unmyelinated shiverer brain.
A-E, Myelination of congenitally hypomyelinated shiverer mice by human fetal tissue-derived glial progenitor cells. A-B. Representative sagittal images of an engrafted shi/shi × rag2−/− brain, sacrificed at 1 year of age. A, Human donor cells identified by an anti-human nuclear antibody (hN; red). B, Donor-derived myelin basic protein (MBP; green) in sections adjacent or nearly so to matched sections in A. All major white matter tracts heavily express MBP (which is all donor-derived in MBP-null shiverer mice). C, Sagittal view through cerebellum of a year-old engrafted shi/shi × rag2−/− brain. All cells were stained with DAPI (blue); donor cells were identified by human nuclear antigen (hN, red), and donor–derived myelin by MBP (green). D, Reconstituted nodes of Ranvier in the cervical spinal cord of a transplanted and rescued 1-year-old shi/shi × rag2−/− mouse, showing paranodal Caspr protein and juxtaparanodal potassium channel Kv1.2, symmetrically flanking each node. Untransplanted shiverer brains do not have organized nodes of Ranvier and, hence, cannot support saltatory conduction (Caspr, red; Kv1.2, green) E. Electron micrograph of a 16-week-old shiverer mouse implanted perinatally with hGPCs, shows a shiverer axon with a densely compacted myelin sheath.
Scale: D, 5 μm. E, 1 μm. Images from Windrem et al., 2008 (A-B); Goldman et al., 2012 (C-D); Windrem et al., 2004 (E); figure adapted from (Osorio and Goldman, 2016a).
In the shiverer model, donor hGPCs mature in a highly context-dependent fashion, such that those donor cells that engraft presumptive white matter develop as myelinogenic oligodendrocytes and fibrous astrocytes. In contrast, those cells that invade the cortical and subcortical gray matter and differentiate do so as astrocytes (Windrem et al., 2004). Moreover, the differentiation of hGPCs differs depending upon whether the cells have been transplanted into normally-myelinated or congenitally-hypomyelinated recipients: in normally-myelinated hosts, transplanted hGPCs colonize both the gray and white matter, ultimately differentiating as astrocytes or remaining as glial progenitors(Windrem et al., 2014). In contrast, hGPCs transplanted into shiverer mice first preferentially expand in the callosal and capsular white matter, giving rise therein to new oligodendrocytes as well as astrocytes and new hGPCs (Figures 1 and 2).
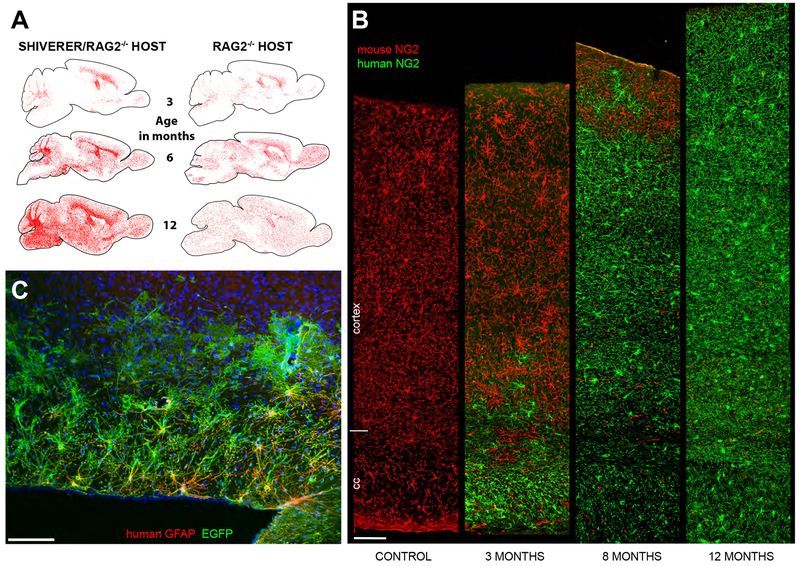
Figure 2. Human GPCs out-compete and ultimately replace resident mouse GPCs.
A, hGPCs neonatally transplanted into either congenitally hypomyelinated shiverer × rag2−/− (left column), or into normally myelinated rag2−/− (right) mouse brain, disperse and expand broadly throughout the brain as a function of age. hGPCs reach higher density in white matter than gray matter of the hypomyelinated shiverer (left), in contrast to their relatively uniform distribution in normally myelinated brain (right). Red dots indicate individual human donor GPCs, as labeled by human nuclear antigen (left and right columns). B, Progressive replacement of host mouse GPCs, by donor hGPCs, as identified by species-specific antibodies against NG2, compared to an unengrafted control mouse (left-most column). By one year, hGPCs have replaced mouse GPCs throughout the entire depth of the cortex. C, EGFP-tagged human astrocytes in the corpus callosum, cortex, and fimbria in a 2-year old rag2−/− mouse. The hGPCs were labeled with an EGFP-expressing lentivirus (green) in vitro, prior to transplantation. Dorsal is toward the top and rostral to the left in this sagittal section. (EGFP, green; human-specific GFAP, red).
Scale: 100 μm. A and B, from (Windrem et al., 2014).
Yet even more stunning than their broad colonization, the implanted hGPCs not only integrate into the recipient brains, but over time predominate; they first displace and then ultimately replace the resident glial populations of their hosts. In both shiverers and myelin wild-type recipients, colonization of the cortical and subcortical gray by migrating hGPCs is followed first by their selective expansion, and then by the inexorable displacement and in situ death of resident murine glial progenitors (Windrem et al., 2014). As a result of this apparent competition between resident mouse GPCs and the invading human GPCs, by a year of age the donor hGPCs are typically distributed in a relatively uniform manner throughout the forebrain white and gray matter, while mouse GPCs are scarce, and often absent from the now human glial-dominant forebrains. Importantly, this competitive advantage of human over murine glial progenitors is evident in wild-type as well as in shiverer mice; in both recipient environments, the human glial progenitors typically out-compete their murine counterparts.
Human donor glial progenitors dominate the competition
The selective expansion of the human glial population in the mouse appears at least in part to be a product of the more sustained proliferation of the transplanted human GPCs, which appear to retain cell-autonomous regulation of expansion, and expand in numbers by over 40-fold in the corpus callosum alone over the first year of life (Windrem et al., 2008). Yet the advantage of human GPCs in the mouse environment is clearly based on more than preferential expansion, since the murine GPCs are completely replaced by their human counterparts over time. Rather, the process of hGPC colonization seems overtly competitive. Human GPCs typically expand outwards from their periventricular and callosal points of introduction, in advancing waves that seem to both repulse and overwhelm resident murine progenitors, which proceed to die, largely in situ (Figure 2). The human GPCs ultimately attain a relatively uniform distribution, achieving apparent contact inhibition in a manner analogous to that reported developmentally by Bergles and colleagues (Hughes et al., 2013). While the molecular basis for the competitive dominance of the human GPCs is unknown, several recent studies have identified differential expression of both MYC and hedgehog-dependent pathways as contributing to clonal dominance during early ontogeny (Amoyel and Bach, 2014; Amoyel et al., 2014; Claveria et al., 2013). Further assessment of differential gene expression by mouse and human GPCs in vivo may permit the discovery of similar regulators of competition that favor the relative dominance of human over mouse GPCs when the two are in direct competition, a decidedly unnatural situation that might nonetheless provide us important insights into the differential expansion of favored glial cell populations in the developing human brain, and the signals that determine which among competing populations is ultimately dominant.
Regardless of its molecular basis, the domination of the host brains by human GPCs leads to the slow but inexorable glial humanization of these brains, as mature astrocytes undergo presumably normal turnover in adulthood, with astrocytic replacement from now-humanized resident progenitor pools. This process results in the substantial astrocytic humanization of these rodent brains, first by fibrous astrocytes of the white matter, and then by protoplasmic astroglia of both cortical and subcortical gray matter (Goldman et al., 2015; Windrem et al., 2014). In both shiverer and myelin wild-type hosts, the proportion of human astrocytes thus increases monotonically as a function of time, and is matched by a corresponding decrease in the proportion of mouse astrocytes. Over time, these brains thus become chimeric for human astrocytes, as well as for human glial progenitors, and in shiverer mice, this process includes replacement of host oligodendrocytes by their human counterparts as well. As a result, in one-year old shiverer mice neonatally engrafted with human GPCs, essentially all resident glial progenitor cells, all oligodendrocytes, and large proportions of all astrocytes are of human origin (Figure 1 and 2).
Human glia maintain uniquely hominid features in chimeras
Human astrocytes are larger and more complex than those of rodents; human cortical astrocytes can exhibit over triple the diameter, and over 10-fold the number of terminal processes, as rodent astrocytes (Oberheim et al., 2006; Oberheim et al., 2009). Human astrocytes have both structural features and functional competencies unique to hominids, and exhibit a range of astrocytic pleomorphism without precedent in infraprimate mammals. On that basis, we have postulated that the functional roles of glia have expanded during evolution, and especially so with the appearance of hominids (Oberheim et al., 2006; Oberheim et al., 2009). These evolutionary changes are of particular interest because astrocytes have been shown to play vital roles in information processing within the CNS (Araque et al., 1999; Kang et al., 1998). Astrocytes are required for synaptogenesis and maintenance of synaptic density (Ullian et al., 2001), and a number of specific astrocytic modulators of synaptic plasticity have been identified, including the glypicans (Allen et al., 2012), TNFα (Stellwagen and Malenka, 2006), and the neuroligins (Stogsdill et al., 2017), among others. Importantly, these ligands may be differentially expressed by human astroglia, potentially offering advantages to human astroglia in the regulation of synaptic plasticity, relative to infraprimate glia (Oberheim et al., 2009).
On the basis of these observations, we asked if the greater structural complexity of human astrocytes relative to those of rodents might be accompanied by functional differences. In particular, we considered whether human glial chimeric mouse brains, with their substantial colonization by human astroglia and their progenitors, might manifest functional distinctions from wild-type mice, and if so whether these functional differences might reflect aspects of human cognitive evolution (Han et al., 2013). This possibility was anticipated by the observation that human astrocytes propagate Ca2+ waves significantly more rapidly than rodents (Oberheim et al., 2009). To define the human-selective contributions of astrocytic complexity to network function, we therefore assessed the behavior of human glial chimeras to both matched unengrafted and allografted mice. To that end, hGPCs, pre-biased in vitro to astrocytic phenotype, were transplanted into neonatal immunodeficient mice, thereby establishing human glial chimeras with especially large complements of human astrocytes as well as GPCs. By 7–10 months of age, the majority of all forebrain OPCs and astrocytes in these mice were typically of human origin (Han et al., 2013). The engrafted human glia appeared to mature in a cell-autonomous fashion, in that the diameter, domain size and morphology of human astrocytes in the chimeric mouse brain each approximated that of astrocytes in the normal adult human brain.
Human glial chimeras for assessing the glial contribution to network and cognitive function
Since human glia appeared to maintain the cell-autonomous size, complexity and domain architecture of human astrocytes in the mouse environment, together with Maiken Nedergaard’s group we asked if human glial chimeric brains exhibited increased synaptic plasticity. To that end, we compared the threshold for inducing hippocampal long-term potentiation (LTP) in chimeric mice with that of littermate controls. We found that high frequency stimulation significantly potentiated the field EPSP slopes of neurons in the chimeric hippocampi, and indeed did so to a larger degree and for a substantially longer period, than in unengrafted littermate controls. Thus, human glial chimeras manifested substantially facilitated LTP (Han et al., 2013). Importantly, such stable, long-lasting changes in synaptic function are thought to be involved in learning and memory, of which LTP is typically considered an in vitro surrogate. Since hippocampal LTP was enhanced in chimeric mice, we next asked whether the human glial chimeras might have cognitive advantages over their unengrafted or mouse GPC-allografted counterparts. We found that the human glial chimeras indeed performed better than control mice across a variety of learning tasks, that included auditory fear conditioning, novel object and place recognition, and Barnes maze navigation. In all of these tests - but not in any test of social interactivity or primary perception - the human glial chimeras performed better and acquired new causal associations more quickly than did murine-allografted or untransplanted controls (Han et al., 2013). In short, they learned more rapidly, and at least along the axes of the tests performed, could be defined - if colloquially so - as smarter. As such, these glial chimeras may provide us a viable - if provocative - model by which to evaluate the species-specific contributions of human glia to human cognition.
hiPSC-derived glial chimeras as models of human genetic and inflammatory CNS disease
Most of the studies described thus far used hGPCs derived from brain tissue, of both adult and fetal origin. Yet GPCs and their derived astrocytes and oligodendrocytes may also be derived from pluripotential stem cells (PSCs) (Hu et al., 2009; Izrael et al., 2007; Wang et al., 2013). Bipotential oligodendrocyte-astrocyte progenitors may now be produced in quantity from both embryonic stem cells (hESCs) and induced pluripotent cells (iPSCs), typically in 3–4 months, a time course similar to that of their appearance from neural stem cells in early development (Douvaras et al., 2014; Piao et al., 2015; Stacpoole et al., 2013). The cells produced by these protocols are as efficient at myelinogenesis in vivo as their tissue-derived counterparts, myelinating most axons within the hypomyelinated shiverer forebrain, and rescuing a substantial fraction of neonatally-engrafted shiverer mice from early death (Wang et al., 2013) (Figure 3).
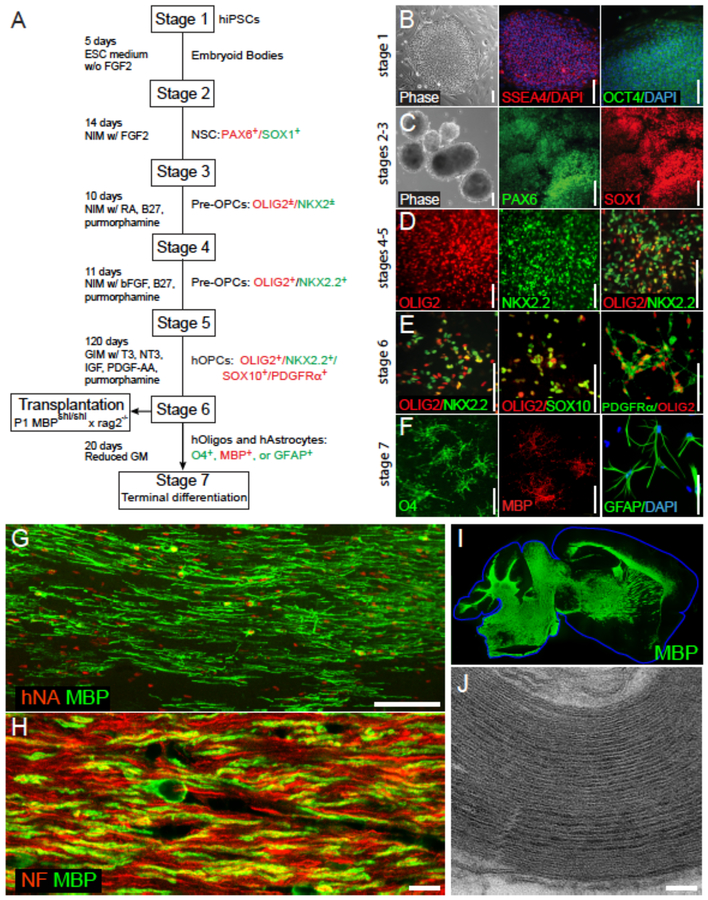
Figure 3. Human pluripotent stem cell-derived GPCs can remyelinate dysmyelinated hosts.
A, This schematic outlines the multi-stage protocol by which GPCs, oligodendrocytes and astrocytes may be generated from human pluripotent stem cells, whether embryonic stem cells (hESCs) or induced pluripotent stem cells (iPSCs). B-F show representative images taken at serial stages of glial differentiation, with the serial expression of selected marker proteins noted at each stage. G, 3 months after neonatal transplant into hypomyelinated shiverer mice, human induced pluripotent cell (hiPSC)-derived GPCs have matured as myelinating, myelin basic protein (MBP)-expressing oligodendrocytes (MBP, green; human nuclear antigen, red). H, The hiPSC–derived oligodendrocytes ensheath mouse axons (neurofilament, red; MBP, green). I, Human iPSC-derived oligodendrocytes can myelinate the entire brain of shiverer mice, which do not otherwise express MBP (green). J, The myelin generated by hiPSC oligodendrocytes is ultrastructurally normal, exhibiting major dense lines and thick myelin sheaths. The use of such serial and distinct stages of growth factor exposure, paired with more extended periods of differentiation, have led to the production of highly enriched populations of human GPCs, that are highly efficient at myelinogenesis in vivo while manifesting no evident tumorigenesis.
Scale: B-E, 100 μm; F, 25 μm; G, 100 μm; H, 10 μm; J, 100 nm. Images from (Wang et al., 2013); figure adapted from (Goldman and Kuypers, 2015) and (Osorio and Goldman, 2016a).
Importantly, while these protocols for producing hGPCs from pluripotent stem cells were developed to permit the production of hGPCs in clinically-useful quantities, they also enabled the establishment of chimeric mice using glia derived from patient and disease specific PSCs. These in turn may be used to assess not only the in vivo pathology of disease-derived glia, but also the extent to which that glial pathology may contribute to disease pathogenesis. By way of early example, Fossatti and colleagues produced hGPCs from iPSCs derived from patients with primary progressive multiple sclerosis (PPMS), and then transplanted the immature oligodendroglia derived from these cells into shiverer mice, in which the cells differentiated as myelinogenic cells that ensheathed resident axons (Douvaras et al., 2014; Fossati and Douvaras, 2014). While the mice did not manifest any stigmata of PPMS, the authors were nonetheless able to establish mice stably chimerized with PPMS-derived oligodendroglia, thereby providing a potential in vivo model for future studies of genetic vulnerability to PPMS. More broadly, this study established the potential value of mice chimerized with patient-specific hGPCs for identifying susceptibility factors for autoimmune inflammatory diseases of the CNS.
Human glial chimeras as hosts for infections unique to the human brain
Human glial chimeras may have applications beyond that of modeling the species-specific role of human glia in neurological function and dysfunction. In particular, these mice may permit us to better assess the biology of human-selective infectious and inflammatory diseases of the CNS. For instance, a number of pathogenic viruses of the human brain are gliotropic, and some are specifically human in their species-selectivity. Viruses such as the JCV polyomavirus, the cause of progressive multifocal leukoencephalopathy (PML), and human herpesvirus-6, another prominent viral encephalitis of immunocompromised individuals, have never been amenable to experimental study in vivo, since these are human glial-specific pathogens (Haley and Atwood, 2014). On that basis, we asked whether human glial chimerization might permit these animals to be infected with human gliotropic viruses in vivo, and if so whether these infected human glial chimeras might develop pathology replicating that of humans. By neonatally engrafting immunodeficient myelin-deficient rag2−/− × shi/shi mice with hGPCs, we established mice with a fully humanized white matter, which were then infected by intracerebral injection of JCV (Kondo et al., 2014). Within several weeks thereafter, the JCV-infected mice manifested oligodendrocytic loss with demyelination and astroglial proliferation, replicating thereby the cardinal features of human PML (Figure 4).
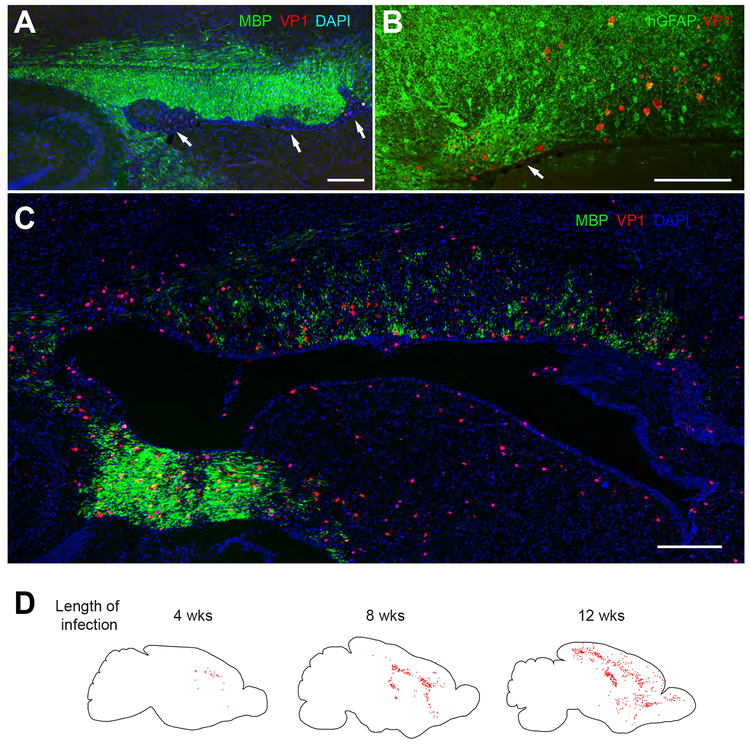
Figure 4. Human glial chimeric mice can develop a prototypic human demyelinating viral encephalitis.
Infection by the human-specific gliotropic JC polyomavirus (JCV) was observed and followed in human glial chimeras, after intracerebral viral injection at a single locus. Viral spread in vivo was tracked by immunostaining for the late JC viral VP1 antigen, as a function of time after infection. In these chimeric shiverers, essentially all myelinating oligodendrocytes, as well as most GPCs and large numbers of astrocytes, are human, and only human cells are infected by this virus. A-B, By 4 weeks after infection, focal regions of demyelination (A, arrows) and infection-associated astrogliosis (B, arrow) are noted in the forebrain white matter of human glial chimeras, here abutting the callosal wall of the lateral ventricle. C, By 11 weeks after infection, diffuse hypomyelination of the corpus callosum and capsules was noted. D, Sagittal sections of an infected chimera at each of 3 time-points; individual JCV–infected VP1+ cells are dot-mapped onto the schematic. Infected human cells became progressively more widespread with time, to include much of the forebrain white matter by 12 weeks post-infection, with marked cortical spread by that point as well. Scale: 200 μm (A, C); 100 μm (B). From (Kondo et al., 2014).
We further noted that in the JCV-infected human glial chimeras, that hGPCs and astrocytes were infected more rapidly than were oligodendrocytes, and viral replication was noted primarily in astrocytes and hGPCs rather than in oligodendrocytes, which instead exhibited viral T antigen-associated apoptotic death. By establishing human glial chimeras in wild-type rather than hypomyelinated hosts - which produced mice colonized with human astrocytes and GPCs but not oligodendrocytes - we then established that human astroglia were sufficient for JCV spread, and that astrocytes, rather than oligodendrocytes, were the principal targets for, and reservoir of, infection in vivo (Kondo et al., 2014).
This study established the utility of glial chimeras for studying the biology of human gliotropic viruses in vivo, and promises to be the first of many using human glial-transplanted animals to investigate human-specific brain infections in vivo. In particular, beyond these known gliotropic disorders, emerging threats that may target neural stem and progenitor cell populations as well as their derived glia, such as the Zika virus, Chikungunya virus, CMV and HIV, among others, might also be especially amenable to in vivo investigation using humanized glial chimeras. Indeed, our JCV-infected humanized mice revealed the limits of our knowledge of these human gliotropic encephalitides, and hence the promise afforded by human glial chimeras for fundamental mechanistic discovery as well as therapeutic modeling of these enigmatic and often dire disorders.
Humanized glial chimeras as models of neurodegenerative and neuropsychiatric disorders
Going forward, human glial chimeras may find great value in defining the relative contributions of glial pathology to diseases of the nervous system for which the relative roles of neurons and glia remain unclear or explored. In particular, the neurodegenerative and neuropsychiatric disorders involve both neuronal and glial pathology, and yet have traditionally been considered primary neuronal disorders; evident glial pathology such as hypomyelination has been assumed, often with little evidence, to have been secondary to neuronal loss. A host of neurodegenerative disorders, including ALS, Huntington disease, Alzheimer’s disease and others, have been reported to have significant glial involvement, and yet the specific contributions of glial dysfunction to disease pathogenesis have been difficult to establish. In vivo chimeras may allow causal glial pathology to be better defined in these disorders, and ultimately to be more specifically targeted. For instance, Benraiss and colleagues used mice rendered chimeric for mutant huntingtin-expressing GPCs to identify a critical role for glial pathology in the progression of Huntington disease (Benraiss et al., 2016). Similarly, Windrem and colleagues used glial chimeras produced from iPSC GPCs derived from patients with childhood-onset schizophrenia, to define a causal role for glial dysfunction in schizophrenia (Windrem et al., 2017). Remarkably, the disease-derived glial chimeric mice manifested not only the hypomyelination typically associated with childhood-onset schizophrenia, but also aspects of its associated behavioral pathology, attesting to the fidelity with which the humanized chimeras can recapitulate aspects of disease phenotype in vivo. More broadly, these studies demonstrated that - at least in neonates - normal human GPCs could replace disease-derived GPCs in vivo; by so doing, these findings served to expand the potential scope of cell replacement therapy beyond isolated dysmyelination, to include treatment of the neurodegenerative and neuropsychiatric disorders – conditions that just a few years ago would not even remotely have been considered potential targets of cell therapy (Goldman, 2016).
The future
Human glial chimeras are already proving of great utility in defining the glial contributions to neurological disease, and the specific role of human glia in both cognition and cognitive pathology. Yet they may also prove of great value in screening agents, both small molecules and biologics, whose effects on glial cells require assessment, or that might be specifically directed at glial molecular targets. Indeed, as chimerization of experimental animals with multiple phenotypes becomes possible – with pluripotent stem cell-derived human microglia (Abud et al., 2017; Douvaras et al., 2017; Pandya et al., 2017) and vascular cells, human peripheral immune cells (Arainga et al., 2016), and even with human neurons (Brustle et al., 1998; Chen et al., 2016; Keyoung et al., 2001) – and as our ability to produce these other phenotypes on a disease-specific basis becomes more mature - one can readily envision the real-time assessment of the interactions of multiple neural phenotypes in vivo, in a largely humanized system. This capability should allow the deployment and benefit of far more precise and informative animal models of human neurological disease than any yet available.
To capitalize upon these opportunities, we offer the following protocols, and welcome any questions from those engaged in the deployment and use of this technology.
The method: How to make a chimera
Materials
1. Preparation of Cells
Cell Scraper
2x scalpels – size 11
HBSS, no calcium, no magnesium (HBSS−/−) at 25°C – At least 12ml per 6 well plate of adherent cells
100 micron cell strainer
Dissection microscope
10cm cell tissue culture treated plate
2× 15ml tubes
50ml tube
Hemocytometer
15ml tube per 6-well plate
Centrifuge
0.2ml PCR tube
HBSS, no calcium, no magnesium (HBSS−/−) at 4°C – 15ml
1.5ml tube with screw top.
Ice Bucket
20/200 μl pipette closely calibrated to the Hamilton syringe to be used for transplantation
2. Transplantation of hGPCs into neonatal mice
HBSS−/− at 4°C – 15ml
70% ethanol
Stereotaxic frame with digital micrometer (or other apparatus to secure neonatal mouse head)
Heating Block
Dissection microscope
Fiber optic light
10μl Hamilton Gastight syringe with attached micropipette
3× 10 ml syringe
2× 0.22 micron syringe filter
3× 30 gauge needle
3× 26 gauge needle
Sterile cotton swab
Sterile sheet
Sterile gauze
Alcohol prep pads
Povidone-iodine prep pad
Paper towel
P1000 pipet tip box
Methods
1. Preparation of Cells
Detachment and dissection of hESC or hIPSC derived hGPCs
Adherent hESC or hIPSC hGPCs must first be detached and large colonies dissected.
Remove media from wells to be used.
Add 1ml of HBSS−/− to each well.
Using cell scraper, gently detach cells into HBSS−/−.
Tilt plate and ensure cells are suspended in HBSS−/− by scraping into pool of liquid.
Pipette out cells into 15ml tube.
Repeat steps 2–5 to ensure maximal recovery of cells.
Allow cells to settle in tube.
Remove supernatant leaving ~200μl of media on top.
Pipette up and down with a p1000 pipette ~5 times to break up clusters.
Transfer cells to the middle of a 10cm tissue culture treated plate (Note 1).
Examine cells under a dissecting microscope in a sterile hood.
Dissect larger cell aggregates until they are <100 microns in size with two size 11 scalpels.
Collect cells in a 15 ml tube
Wash with HBSS−/− as needed to collect all cells from the plate. Ideally until total collected volume = 12ml total.
Pre-wet 100-micron cell strainer with 1ml of HBSS−/− on 50ml tube.
Transfer 12ml of cells through 100-micron cell strainer.
Add 1ml of HBSS−/− to tube cells were in to wash and then pass them through the cell strainer.
Washing and resuspension of cells
If using hESC or hIPSC-derived hGPCs, begin at step 2. Sorted human fetal cells can either be used directly after sorting (FACS or MACS), or can be left on ultra-low attachment plates overnight in glial media.
For sorted fetal cells, transfer cells in media to a 15ml tube and add HBSS−/− to 14ml
Remove 10μl and count cells on hemocytometer.
Centrifuge cells for 10 minutes at 200 × g.
Carefully remove supernatant.
Resuspend in 100μl HBSS−/−.
Transfer cell suspension to 0.2ml pcr tube.
Wash 15ml tube with 100μl of HBSS−/−.
Transfer wash to 0.2ml pcr tube.
Centrifuge for 10 minutes at 200 × g.
Completely remove supernatant.
Resuspend cells in HBSS−/− to achieve desired concentration for injections (e.g. 100,000 cells/μl) (Note 2).
Place pcr tube in 1.5ml tube and keep on ice until ready to use.
2. Transplantation of Cells
Transplantation of cells into neonatal mice is best accomplished at P1.
2.1. Equipment preparation
Place sterile sheet where stereotaxic apparatus will be set up.
Set up stereotaxic apparatus under dissection microscope.
Set up fiber optic lighting to illuminate injection area.
Spray down working area with 70% ethanol and wipe down to dry.
Fill one 10ml syringe with 70% ethanol
Fill one 10ml syringe with HBSS−/−
Attach a 0.22μm syringe filter to each syringe.
Attach 26G needle to each syringe filter
Set heating block to 45°C
Sterilize P1000 pipet tip box with 70% ethanol and place on top of heating block.
Moisten sterile gauze with sterile HBSS−/− and place into box.
Fasten Hamilton syringe on stereotactic apparatus.
Flush Hamilton syringe micropipette with 1mL of 70% ethanol by inserting filled ethanol syringe into the top of the Hamilton syringe
- Flush Hamilton syringe with 1mL of HBSS−/− as you did in Step 12.
- If air bubbles persist in syringe, flush again with HBSS−/−
Place plunger into Hamilton syringe taking care that air bubbles do not form
Depress plunger to 4μl mark.
Using a cotton swab, remove excess HBSS−/− from the tip of the Hamilton syringe needle. Use a downward motion as to not damage the needle.
Draw 1μl of air into the syringe to create a separation air bubble.
2.2. Animal preparation
Carry out these procedures within a biosafety hood; practice proper sterile techniques.
Remove top of cage.
Place a couple of food pellets at the opposite end of the cage from the nest as to lure the mother away.
- When the mother leaves the nest, remove half of the pups placing them in the humidified warming chamber.
- Never remove more than half of the pups at a time as to not stress the mother.
Remove one pup from humidified warming chamber.
Wipe pup down with povidone-iodine
Place pup in a glove-barriered hole in the ice within ice bucket.
Cover the pup with ice and wait 2–4 minutes for the pup to be properly anesthetized.
Remove the pup from the ice and wipe the pup down with an ethanol wipe.
Place the pup into the stereotactic device securing their head.
2.3. Loading of Cells
Triturate the cell suspension gently using the P20 pipette to homogenize; avoid creating air bubbles.
- Withdraw an appropriate volume for your injection type into the Hamilton syringe:
- 2μl for forebrain injection
- 3μl for forebrain + hindbrain injection
Return tube of cells to ice while injecting each mouse.
2.4. Injection of Cells in the Forebrain
Remove the 30g needle from its sheath to bend the needle tip to a 90° angle.
- Using the bent needle, create holes in the skull at these four sites (Note 3):
- Front: 1.0mm posterior from bregma, ±1.0mm from the midline
- Back: 2.4mm posterior from bregma, ±1.0mm from the midline
Remove bent needle and insert injection needle into the punctured hole stopping at the level of the skull (Note 4).
- Lower the needle to the following depths:
- Anterior: 1.0 mm
- Posterior: 0.9 mm
Inject 0.5μl in each site. Each site should be injected in 1 second.
Keep needle in the brain for 5 seconds following the injection and then retract it (Note 5).
2.5. Injection of Cells in the Hindbrain
If significant engraftment of the hindbrain is desirable (e.g. for enhanced survival of the shiverer mouse), adding a fifth injection site into the hindbrain is advised.
Reposition pup in the stereotactic apparatus so that its head is down at a 90° angle to its body and secure its head.
Locate the cisterna magna, a soft spot below the base of the skull resembling a shallow circle.
Create a hole at the midline using the same technique as before with the bent needle.
Remove the bent needle and lower the injection needle into the punctured hole stopping at the level of the skull.
Lower the needle to a depth of 1.1mm.
Inject 1μl over the course of 2 seconds.
Allow the needle to remain in the brain for 5 seconds following the injection
Retract the injection needle (Note 5).
2.6. Recovery of pups and clean up
Remove pup from the injection holder.
Wipe pup down with alcohol prep pad.
Place the pup in the humidified recovery chamber.
When all pups are awake within the humidified chamber, return them to their nest while the mother is away.
Remove Hamilton syringe plunger.
Flush the Hamilton syringe with 1ml HBSS−/−
Flush the Hamilton syringe with 1ml of 70% ethanol.
Attach 26G needle to empty 10ml syringe and fill with air.
Flush out the remaining ethanol.
Use the cotton swab to remove any additional fluid from the tip of the pipette.
Unmount the Hamilton syringe.
Utilizing this protocol will maximize the migration, survival, and engraftment of transplanted hGPCs. They will remain as hGPCs or differentiate into astrocytes or myelinating oligodendrocytes in a context-dependent manner. Time following injection before significant engraftment is reached will vary depending on mouse model and population of cells injected.
Notes
Dissection of clusters will be more easily achieved if the volume of liquid transferred to the plate is low enough to maintain surface tension. Avoid transferring bubbles, as they will interfere with visualization.
Depending upon the composition of cellular populations, the mouse model, and the speed of delivery, the number of cells injected will vary. Ideally, a volume of more than 2μl into the neonatal forebrain should not be exceeded, as this may lead to irreparable tissue damage and disruption of the host tissue architecture.
While pressing down on the skull with the bent syringe, the tissue will depress. The skull has been punctured once it pops back up. Do not go deeper than this, as that will risk tissue damage.
Verification that the needle is penetrating the brain can be obtained by confirming that the skull does not depress as the needle is lowered. If it does snap back, then the skull puncture may not be sufficiently large. Step 2.4.2 may then need to be repeated, or adjustment of the syringe site may be necessary to assure proper injection of cells directly into the brain at the proper depth.
In rare cases, backflow may be observed. Nonetheless, in these instances, the vacuum created by removing the needle will often pull the cells back into the intended location.
Acknowledgements:
Supported by NINDS, NIMH, the Mathers Charitable Foundation, the Adelson Medical Research Foundation, CHDI, the Novo Nordisk Foundation and the Lundbeck Foundation.
Footnotes
Conflict statement: Dr. Goldman has a patent on chimeric mouse models, US patent US7524491B2; the patent is owned by the University of Rochester, and the author receives no income from it.
Citations
- Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, et al. (2017). iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 94, 278–293 e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, and Barres BA (2012). Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoyel M, and Bach EA (2014). Cell competition: how to eliminate your neighbours. Development 141, 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoyel M, Simons BD, and Bach EA (2014). Neutral competition of stem cells is skewed by proliferative changes downstream of Hh and Hpo. The EMBO journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arainga M, Su H, Poluektova LY, Gorantla S, and Gendelman HE (2016). HIV-1 cellular and tissue replication patterns in infected humanized mice. Scientific reports 6, 23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, and Haydon PG (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22, 208–215. [DOI] [PubMed] [Google Scholar]
- Archer D, Cuddon P, Lipsitz D, and Duncan I (1997). Myelination of the canine central nervous sytem by glial cell transplantation: A model for repair of human myelin disease. Nature medicine 3, 54–59. [DOI] [PubMed] [Google Scholar]
- Archer DR, Leven S, and Duncan ID (1994). Myelination by cryopreserved xenografts and allografts in the myelin-deficient rat. Experimental neurology 125, 268–277. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, and Gallo V (2003). Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. Journal of Cell Biology 161, 169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T, and Goldman SA (2008). Prospects of cell therapy for disorders of myelin. Ann N Y Acad Sci 1142, 218–249. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Wang S, Herrlinger S, Li X, Chandler-Militello D, Mauceri J, Burm H, Toner M, Osipovitch M, Xu Q, et al. (2016). Human glia can both induce and rescue aspects of phenotype in Huntington Disease. Nature communications 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF, Crang AJ, and Franklin RJ (1990). Transplantation of glial cell cultures into areas of demyelination in the adult CNS. Prog Brain Res 82, 225–232. [DOI] [PubMed] [Google Scholar]
- Brustle O, Choudhary K, Karram K, Huttner A, Murray K, Dubois-Dalcq M, and McKay RD (1998). Chimeric brains generated by intraventricular transplantation of fetal human brain cells into embryonic rats. Nature biotechnology 16, 1040–1044. [DOI] [PubMed] [Google Scholar]
- Chen C, Kim WY, and Jiang P (2016). Humanized neuronal chimeric mouse brain generated by neonatally engrafted human iPSC-derived primitive neural progenitor cells. JCI Insight 1, e88632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claveria C, Giovinazzo G, Sierra R, and Torres M (2013). Myc-driven endogenous cell competition in the early mammalian embryo. Nature 500, 39–44. [DOI] [PubMed] [Google Scholar]
- Douvaras P, Sun B, Wang M, Kruglikov I, Lallos G, Zimmer M, Terrenoire C, Zhang B, Gandy S, Schadt E, et al. (2017). Directed Differentiation of Human Pluripotent Stem Cells to Microglia. Stem cell reports 8, 1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvaras P, Wang J, Zimmer M, Hanchuk S, O’Bara MA, Sadiq S, Sim FJ, Goldman J, and Fossati V (2014). Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem cell reports 3, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I (2005). Oligodendrocytes and stem cell transplantation: their potential in the treatment of leukoencephalopathies. J Inher Metab Dis 28, 357–368. [DOI] [PubMed] [Google Scholar]
- Fossati V, and Douvaras P (2014). Generating induced pluripotent stem cells for multiple sclerosis therapy. Regenerative medicine 9, 709–711. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, and Goldman SA (2015). Glial Disease and Repair-Remyelination. Cold Spring Harbor perspectives in biology 7, a020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJM, and ffrench-Constant C (2008). Remyelination in the CNS: from biology to therapy. Nature reviews Neuroscience 9, 839–855. [DOI] [PubMed] [Google Scholar]
- Goldman SA (2011). Progenitor cell-based treatment of the pediatric myelin disorders. Archives of neurology 68, 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA (2016). Stem and Progenitor Cell-Based Therapy of the Central Nervous System: Hopes, Hype, and Wishful Thinking. Cell Stem Cell 18, 174–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA (2017). Progenitor cell-based treatment of glial disease. Prog Brain Res 231, 165–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, and Kuypers NJ (2015). How to make an oligodendrocyte. Development 142, 3983–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nedergaard M, and Windrem MS (2012). Glial progenitor cell-based treatment and modeling of neurological disease. Science (New York, NY) 338, 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nedergaard M, and Windrem MS (2015). Modeling cognition and disease using human glial chimeric mice. Glia 63, 1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumpel M, Lachapelle F, Gansmuller A, Baulac M, Baron van Evercooren A, and Baumann N (1987). Transplantation of human embryonic oligodendrocytes into shiverer brain. Ann N Y Acad Sci 495, 71–85. [DOI] [PubMed] [Google Scholar]
- Haley SA, and Atwood WJ (2014). An animal model for progressive multifocal leukoencephalopathy. The Journal of clinical investigation 124, 5103–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. (2013). Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Du ZW, and Zhang SC (2009). Differentiation of human oligodendrocytes from pluripotent stem cells. Nature protocols 4, 1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, and Bergles DE (2013). Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nature neuroscience 16, 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, and Revel M (2007). Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neuroscience 34, 310–323. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, and Nedergaard M (1998). Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nature neuroscience 1, 683–692. [DOI] [PubMed] [Google Scholar]
- Keyoung HM, Roy NS, Benraiss A, Louissaint A Jr., Suzuki A, Hashimoto M, Rashbaum WK, Okano H, and Goldman SA (2001). High-yield selection and extraction of two promoter-defined phenotypes of neural stem cells from the fetal human brain. Nature biotechnology 19, 843–850. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Windrem MS, Zou L, Chandler-Militello D, Schanz SJ, Auvergne RM, Betstadt SJ, Harrington AR, Johnson M, Kazarov A, et al. (2014). Human glial chimeric mice reveal astrocytic dependence of JC virus infection. The Journal of clinical investigation 124, 5323–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachapelle F, Gumpel M, Baulac M, Jacque C, Duc P, and Baumann N (1983). Transplantation of CNS fragments into the brain of shiverer mutant mice: extensive myelination by implanted oligodendrocytes. I. Immunohistochemical studies. Developmental neuroscience 6, 325–334. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, and Zhu X (2009). Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nature Reviews Neuroscience 10, 9–22. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, and Stallcup WB (1996). Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. Journal of neuroscience research 43, 299–314. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, Kang J, Nedergaard M, and Goldman SA (2003). Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature medicine 9, 439–447. [DOI] [PubMed] [Google Scholar]
- Oberheim N, Wang X, Goldman SA, and Nedergaard M (2006). Astrocytic complexity distinguishes the human brain. Trends in Neurosciences 29, 1–10. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, et al. (2009). Uniquely hominid features of adult human astrocytes. J Neurosci 29, 3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio MJ, and Goldman SA (2016a). Cell therapy for pediatric disorders of glia In Translational Neuroscience, Tuszynski M, ed. (Springer; ), pp. 275–296. [Google Scholar]
- Osorio MJ, and Goldman SA (2016b). Glial progenitor cell-based treatment of the childhood leukodystrophies. Experimental neurology 283, 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya H, Shen MJ, Ichikawa DM, Sedlock AB, Choi Y, Johnson KR, Kim G, Brown MA, Elkahloun AG, Maric D, et al. (2017). Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nature neuroscience 20, 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D, et al. (2015). Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitors Remyelinate the Brain and Rescue Behavioral Deficits following Radiation. Cell Stem Cell 16, 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popko B, Puckett C, Lai E, Shine HD, Readhead C, Takahashi N, Hunt SW 3rd, Sidman RL, and Hood L (1987). Myelin deficient mice: expression of myelin basic protein and generation of mice with varying levels of myelin. Cell 48, 713–721. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, and Noble M (1983). A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303, 390–396. [DOI] [PubMed] [Google Scholar]
- Readhead C, Popko B, Takahashi N, Shine HD, Saavedra RA, Sidman RL, and Hood L (1987). Expression of a myelin basic protein gene in transgenic shiverer mice: correction of the dysmyelinating phenotype. Cell 48, 703–712. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, Braun PE, and Goldman SA (1999). Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci 19, 9986–9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, McClain CR, Schanz SJ, Protack TL, Windrem MS, and Goldman SA (2011). CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nature biotechnology 29, 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole SR, Spitzer S, Bilican B, Compston A, Karadottir R, Chandran S, and Franklin RJ (2013). High yields of oligodendrocyte lineage cells from human embryonic stem cells at physiological oxygen tensions for evaluation of translational biology. Stem cell reports 1, 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, and Malenka RC (2006). Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, Ejikeme T, Ji RR, and Eroglu C (2017). Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 551, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, and Barres BA (2001). Control of synapse number by glia. Science (New York, NY) 291, 657–661. [DOI] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, et al. (2013). Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12, 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, Roy NS, and Goldman SA (2004). Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nature medicine 10, 93–97. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Osipovitch M, Liu Z, Bates J, Chandler-Militello D, Zou L, Munir J, Schanz S, McCoy K, Miller RH, et al. (2017). Human iPSC glial mouse chimeras reveal glial contributions to schizophrenia. Cell Stem Cell 21, 195–208 e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Roy NS, Wang J, Nunes M, Benraiss A, Goodman R, McKhann GM, and Goldman SA (2002). Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res 69, 966–975. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, et al. (2008). Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell 2, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Morrow C, Munir J, Chandler-Militello D, Wang S, and Goldman SA (2014). A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J Neurosci 34, 16153–16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandava BD, Billinghurst LL, and Snyder EY (1999). “Global” cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proceedings of the National Academy of Sciences of the United States of America 96, 7029–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]