Abstract
Fungal infections are a major cause of animal and plant morbidity and mortality worldwide. Effective biological therapeutics could complement current antifungal drugs, but understanding of their in vivo mechanisms has been hampered by technical barriers to intravital imaging of host-pathogen interactions. Here we characterize the fungal infection of zebrafish as a model to understand the mechanism-of-action for biological antifungal therapeutics through intravital imaging of these transparent animals. We find that non-specific human IgG enhances phagocytosis by zebrafish phagocytes in vivo. Polyclonal anti-Candida antibodies enhance containment of fungi in vivo and promote survival. Analysis suggests that early phagocytic containment is a strong prognostic indicator for overall survival. Although polyclonal anti-Candida antibodies protect against disease, this is not necessarily the case for individual monoclonal anti-Candida antibodies. Thus, the zebrafish appears to provide a useful model host for testing if a biological therapeutic promotes phagocytosis in vivo and enhances protection against candidemia.
1.1. Introduction
Fungal infections are a major cause of animal and plant morbidity and mortality worldwide (Brown et al., 2012). In healthy communities with good access to health care, opportunistic fungi such as Candida and Aspergillus account for the majority of lethal fungal infections of humans. These infections often result from iatrogenic immunocompromising events such as surgical procedures, allogeneic bone marrow transplantation, and use of in-dwelling catheters. Although several classes of antifungal drugs are approved for use in the United States, nearly one-third of all cases of disseminated candidiasis result in death (Enoch et al., 2006; Horn et al., 2009).
Work over several decades has sought to develop effective vaccination protocols and biological therapeutics to complement the currently available chemotherapeutics. Recent work has led to the discovery of two different antibodies that can prevent infection in mouse models of candidiasis. The Cassone group identified an anti-β-glucan mouse IgG antibody with efficacy in preventing murine disseminated candidiasis and with in vitro killing activity (Torosantucci et al., 2005). The Cutler group identified and characterized an anti-mannan mouse IgM antibody with efficacy in a murine model (Han et al., 2001; Xin and Cutler, 2011; Xin et al., 2008). Nevertheless, the development of monoclonal antibodies for protection against candidemia has not yet realized its potential (Bromuro et al., 2002; Casadevall et al., 1998).
Therapeutic antibodies can act through a number of different mechanisms to block microbial pathogenesis, including direct neutralization, enhancement of complement deposition and/or activation, augmentation of opsonic uptake, activation of natural killer cells, and blockade of adhesion (Ramsland et al., 2015). While many of these activities can be and have been evaluated in vitro with in the presence of host cells, these in vitro evaluations do not fully recapitulate the host environment (Golay and Introna, 2012). Mechanistic in vivo mouse studies are limited in resolution, but genetic knockout experiments suggest that complement is a crucial component in the protective activity of both an IgM and an IgG monoclonal antibody specific for Candida (Han et al., 2001). Thus, the in vivo mechanism(s) of action of these antibodies remain obscure, in part because of the technical barriers to intravital imaging of host-pathogen interactions during fungal infection.
Zebrafish larvae represent an attractive model to discern mechanism, as their immune mechanisms are largely conserved with other vertebrates and it is possible to non-invasively image particle- and microbe-phagocyte interactions within an infected animal (Tobin et al., 2012). Opsonic recognition in fish occurs, as in mammals, through antibodies, complement and mannose-binding lectin pathways (Bengten and Wilson, 2015; Leiro et al., 1996; Leiro et al., 2008; Nakao et al., 2011; Zhang and Cui, 2014). In addition, scavenger receptors act as broad specificity non-opsonic receptors (Froystad et al., 1998). However, the roles and mechanisms of opsonic protection to infection remain only partially understood in the zebrafish and other teleosts.
Here we sought to characterize the use of a zebrafish model to evaluate mechanism-of-action for biological antifungal therapeutics. We assayed the ability of zebrafish to utilize mammalian antibodies for phagocytosis and the ability of anti-Candida antibodies to prevent lethal disseminated infection in these fish. We found that some mammalian IgG antibodies can promote phagocytosis of unopsonized beads in fish, and that polyclonal rabbit anti-Candida antibodies protect zebrafish against disseminated C. albicans disease. Intravital imaging revealed that these antibodies resulted in greater early phagocyte containment of C. albicans at the infection site. Non-invasive longitudinal studies further show that early containment is a strong prognostic indicator for protection against lethal disease. Although rabbit anti-Candida antibodies are effective in promoting phagocytosis and protecting against disease, the monoclonal mouse IgM anti-Candida antibody that demonstrated efficacy in a murine systemic infection model, neither protects against disseminated disease nor promotes phagocytosis. Thus, this zebrafish infection model appears to provide a useful in vivo model for the ability of a biological therapeutic to promote phagocytosis in vivo and may be useful for assaying effectiveness in protection against candidiasis. Furthermore, the tight correlation between early phagocytosis and overall survival highlights how early containment could play an important role in innate immunity to Candida.
2. Materials and Methods
2.1. Zebrafish care and maintenance
Zebrafish were housed in recirculating systems (Aquatic Habitats, Apopka, FL) at the University of Maine Zebrafish Facility. All zebrafish care and husbandry were performed as previously described (Brothers et al., 2013). Zebrafish care protocols and experiments were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health under the University of Maine-approved Institutional Animal Care and Use Committee (IACUC) protocols A2012-11-03 and A2015-11-03. Larvae were grown at a density of 150 fish per dish in 150 mm Petri dishes containing 150 ml of E3 media (5 mM sodium chloride, 0.174 mM potassium chloride, 0.33 mM calcium chloride, 0.332 mM magnesium sulfate, 2 mM HEPES in nanopure water, pH=7). The transgenic zebrafish strain used was Tg(mpx:EGFP)i114, which expresses enhanced green fluorescent protein (EGFP) in neutrophils (Renshaw et al., 2006). E3 media was supplemented with 0.3 mg/L methylene blue for the first 6 hours to prevent microbial growth. Larvae were transferred to E3 containing 0.02 mg/ml of 1-phenyl-2-thiourea (PTU) (Sigma-Aldrich, St. Louis, MO) to prevent pigmentation and incubated at 33°C.
2.2. Bead opsonization and preparation
Polystyrene beads at 1% w/v (Spherotech, Lake Forest, IL, 2.5–4.5 μm diameter fluorescent beads, FP-3056–2) were opsonized with rabbit IgG, mouse IgG, human IgG, and human Fab (Jackson ImmunoResearch, West Grove, PA) at antibody concentrations of 250 μg/ml, by incubation in the dark for one hour at 37°C, then washed three times in phosphate-buffered saline (PBS) and resuspended in PBS to a final concentration of 1.5 × 107 beads/ml, using a hemacytometer to achieve the desired concentration.
2.3. Fungal strains, growth conditions, and antibody co-injection
Engineered red fluorescent C. albicans strain CAF2.1-dTom-NATr (CAF2-dTomato) (Gratacap et al., 2013) was grown on yeast-peptone-dextrose (YPD) agar (Difco, Livonia, MI; 20 g/L peptone, 10 g/L yeast extract, 20 g/L glucose, 2% agar). For infections, liquid cultures of C. albicans were grown overnight in YPD at 30°C on a roller drum (New Brunswick Scientific, Edison, NJ). The overnight-cultured C. albicans were washed twice with PBS and resuspended in 5% w/v polyvinylpyrrolidone (PVP) (Sigma-Aldrich, St. Louis, MO) to a final concentration of 1 × 107 cfu/ml. PVP ensures a consistent small-volume injection dose (Detrich et al., 2010). Separate aliquots of the resuspension were mixed with either rabbit anti-Candida IgG pAb (USBiological), mouse anti-Candida IgM mAb (Regeneron Pharmaceuticals, Inc., Tarrytown, NY) or mock opsonized with rabbit anti-GFP IgG pAb (USBiological, Salem, MA) at a final antibody concentration of 10 μg/ml. For co-injection experiments C. albicans were mixed with antibodies twenty minutes prior to injection. Monoclonal mouse IgM anti-Candida antibody recognizing β-mannan (B6.1) was purified by Regeneron Pharmaceuticals, Inc. (Tarrytown, NY) using standard methods from hybridoma licenced through Ligocyte Pharmaceuticals, Inc.
2.4. Hindbrain injections and drug treatment
Zebrafish at the prim25 stage (at approximately 36 hours post fertilization; staged according to the method of Kimmel, et al. (Kimmel et al., 1995)), were manually dechorionated, and anesthetized in Tris-buffered tricaine methane sulfonate (Tricaine; 200 mg/ml; Western Chemicals, Inc., Frendale, WA) prior to injection. For injections, approximately 5 nL of opsonized polystyrene bead suspension at 1.5 × 107 beads/ml in PVP, or 2 nL of C. albicans suspension at 1.0 × 107 cfu/ml was microinjected through the otic vesicle into the hindbrain ventricle to achieve a dose of approximately 15 beads or 15 fungal cells at the site of injection. Within one hour post-injection, larvae were screened using a Zeiss Axiobserver Z1 microscope equipped with Vivatome system (Carl Zeiss Microimaging, Thornwood, NJ) for selection of larvae containing 13–17 beads/fungal cells at the site of injection (SOI). Larvae were subsequently incubated at 33°C in E3 media containing PTU. In experiments to test the relationship between extracellular fungal number and mortality, larvae were preincubated for 1 hour pre-infection with either DMSO or diphenyleneiodonium (Enzo, Farmingdale NY) as described (Brothers et al., 2013).
2.5. Fluorescence microscopy
At four hours post injection (HPI) larvae were anesthetized in Tricaine and immobilized in 0.5% low-melting-point agarose (Lonza, Walkersville, MD) in E3 media containing Tricaine, and placed in a 96-well glass-bottom imaging dish (Greiner Bio-One, Monroe, NC; 655892). Confocal images were acquired using an Olympus IX-81 inverted microscope with an FV-1000 laser scanning confocal system (Olympus, Waltham, MA). The EGFP and dTomato fluorescent proteins were detected by laser/optical filters at 40x magnification (NA 0.9) for excitation/emission at 488/505–525 nm (SDM 560) and 543/560–620 nm (SDM 640), respectively. Larvae were imaged by confocal microscopy in 24-well coverslip-bottom plates (Mattek, Ashland, MA) in a small volume of egg water (deionized water with 60 mg/L Instant Ocean salts, Spectrum Brands, Mentor, OH) plus anaesthetic (200 μg/ml MS-222). Filter sets were at 543/610 and 488/510 excitation/emission wavelengths for dTomato and EGFP, respectively, and a 40x/0.75 N.A. UPlanFL N objective were used to collect Z-stacks of 31–59 slices at an interslice distance of 2 μm. Z-stack images of the hindbrain ventricle were scored for the following at the site of infection: 1) Number of intracellular Candida, 2) Number of extracellular Candida, 3) Number of neutrophils recruited, 4) Number of neutrophils that had phagocytosed Candida, and 5) Number of macrophage-like cells that had phagocytosed Candida. Images were collected and processed using Fluoview (Olympus, Waltham, MA) and Photoshop (Adobe Systems, Inc., San Jose, CA), and subsequently analyzed with Fluoview to quantify bead/phagocyte interaction at the site of injection. After imaging, larvae were euthanized by Tricaine overdose.
2.6. Statistics
Parametric unpaired T-tests or non-parametric Mann-Whitney tests were conducted on data (Graphpad Prism 6, Graphpad Software, Inc., La Jolla, CA) to analyze the statistical significance of differences between the opsonized and mock-opsonized treatment groups, depending on whether the data were normally distributed. For multiple-comparisons, one-way ANOVA was used with post-test analysis (Graphpad Prism). For imaging experiments in which the number of fish examined per cohort in an individual experiment was small due to technical limitations, data was analyzed by Fisher’s Exact Test with two or three groups and appropriate post-test analysis using Graphpad Prism.
3. Results
3.1. Human IgG promotes macrophage phagocytosis of microspheres in the zebrafish hindbrain.
The zebrafish larva offers a transparent vertebrate model with significant similarities to the human immune system, and holds promise as a tool for pre-clinical evaluation of therapeutics (Sullivan and Kim, 2008; Tobin et al., 2012). Because there is a significant clinical need for new anti-fungal therapies, we sought to use this model to characterize the mechanism of action for potentially therapeutic antibodies. We first tested whether mammalian antibodies can stimulate phagocytosis in zebrafish. We coated polystyrene microspheres with IgG antibodies of human, rabbit, or mouse origin, using human Fab as a control, and injected them into the hindbrain ventricle as previously described (Fig. 1A; Brothers et al., 2011). We used mpx:EGFP transgenic zebrafish that have green neutrophils so we could characterize the phagocyte types involved in response to microspheres (Brothers et al., 2011; Renshaw et al., 2006). Previous work has showed that EGFP-negative phagocytes are macrophages, so we will refer to EGFP-negative cells that have engulfed fungi as macrophages (Ellett et al., 2011; Gray et al., 2011). We ensured a consistent inoculum across groups by screening within the first 30 minutes post-injection and imaged representative fish four hours post-injection (Fig. 1B). Quantification of immune cell interactions with coated microspheres revealed that only coating with human IgG resulted in significantly enhanced phagocytosis of microspheres in comparison to the Fab-coated control (Fig. 1C). Furthermore, the percentage of antibody-coated microspheres both in contact with and contained within a phagocyte was significantly higher in the human IgG-opsonized group as compared to the Fab control group (Fig. 1D). Additionally, we categorized the microsphere-phagocyte interaction by immune cell type, and observed that opsonized microspheres are primarily phagocytosed by non-neutrophil mononuclear cells, likely macrophages (Fig. 1E). Representative images demonstrate that inocula were similar but there was greater phagocytosis of beads coated with human IgG (Fig. 1F–I). These experiments demonstrate that opsonization of polystyrene microspheres with human IgG enhances zebrafish immune cell phagocytosis, specifically by macrophages. These initial results suggested that opsonization of microbes with polyclonal antibodies might also enhance immune cell activity and phagocytosis in this in vivo infection model.
Fig. 1. Opsonizing polystyrene beads with human IgG increases bead-phagocyte interaction at SOI.
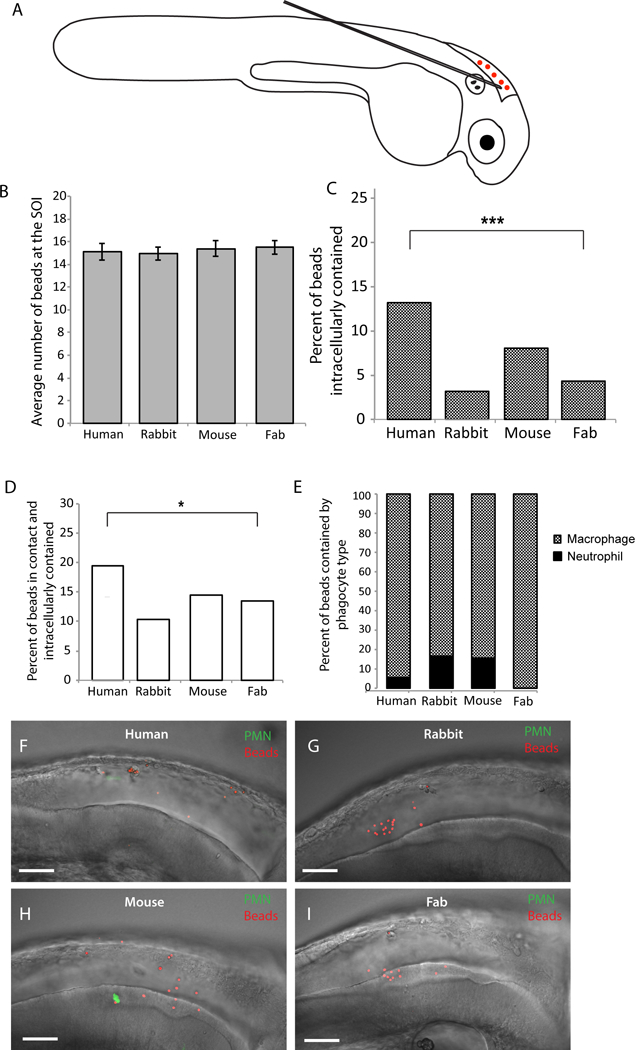
Prim25 stage mpx:EGFP transgenic zebrafish larvae (staged as described by Kimmel et al., 1995) with EGFP-expressing neutrophils and non-fluorescent macrophages (Renshaw et al., 2006) were injected into the hindbrain with polystyrene beads opsonized with human IgG, rabbit IgG, mouse IgG, and human IgG Fab fragment. (A) Microinjection of a 5 nL volume into the hindbrain ventricle through the otic vesicle was performed as described (Brothers and Wheeler, 2012), and inoculum was verified by screening larvae immediately post-injection. (B) Inoculum size within one hour post-injection is consistent among groups. (C-I) Images of the hindbrain ventricle were acquired via confocal microscopy at 4 hpi. Bead and phagocyte numbers were quantified based on 2 μm Z-stacks using a 40x/0.75 N.A. UPlanFL N objective. (C, D) A two-tailed Fisher’s exact test indicates a significant difference in the percentage of beads observed inside a phagocyte (C, p <.0001) and in contact with a phagocyte and intracellular (D, p < 0.05) between human IgG and human Fab opsonized groups. (E) Opsonized polystyrene beads are phagocytosed primarily by GFP-negative macrophages. Representative images reveal more bead-phagocyte interaction in fish injected with human IgG opsonized beads (F) as compared to beads opsonized with rabbit IgG (G), mouse IgG (H) and human Fab fragment (I). Images were acquired at 1024 × 600 pixels, and the number of slices for panels F, G, H, and I are 58, 53, 45, and 59, respectively. Movies including all of the slices are included as Supplemental Data Files M1E, M1F, M1G and M1H. Data are pooled from seven independent experiments. Although technical constraints limited cohort sizes in each experiment, reproducible differences were found from experiment to experiment. Number of fish scored; Human IgG n=18; Rabbit IgG n=17; Mouse IgG n=14; Human Fab fragment n=26. Scalebar = 50 μm.
3.2. Co-injection of polyclonal anti-Candida antibodies enhances early phagocytosis of C. albicans in zebrafish.
To test the ability of microbe-specific antibodies to enhance early phagocytosis in the zebrafish, we co-injected rabbit polyclonal anti-Candida or mock anti-GFP antibodies with CAF2-dTomato C. albicans in the hindbrain ventricle of mpx:EGFP transgenic zebrafish and measured phagocytosis. We first determined a concentration of polyclonal antibody that provided for specific recognition, comparing binding to yeast by utilizing a fluorescent secondary antibody and quantifying fluorescence by flow cytometry. We found that 10 μg/ml was the highest concentration of antibody that provided for maximal opsonization while eliminating non-specific binding by the mock anti-GFP antibodies (Suppl Fig 1). We then co-injected anti-Candida or anti-GFP antibodies at this concentration with CAF2-dTomato fungal cells and measured phagocytosis. Within the first 30 minutes post-injection, fish were imaged, fungal cells were counted, and only fish injected with the proper inoculum (15 +/− 5 cells) were used. Fish were then imaged at 4 hpi at high resolution by confocal microscopy and the following parameters were scored in the hindbrain ventricle: intracellular fungi, extracellular fungi, neutrophil recruitment, neutrophil-phagocytosed fungi, or fungi phagocytosed by macrophage-like cells.
We found that early interaction between C. albicans and phagocytes is altered by co-injection of Candida-specific antibodies. Preliminary counts established that fungal inocula were comparable between groups (Fig. 2A). However, there was a significantly higher percentage of total fungal cells that were intracellular when they were co-administered with the Candida-specific antibodies. Parsing the location of intracellular C. albicans relative to phagocyte type revealed that, with antibody co-administration, macrophages but not neutrophils phagocytosed both a higher proportion of fungi (Fig. 2B) and a higher average number of fungi per infected fish (Fig. 2C). The total number of extracellular fungal cells was significantly lower with co-injection of Candida-specific antibody (mean +/− SEM; 8.1 +/− 2.9 vs. 16.3 +/− 2.7; anti-Candida vs. anti-GFP). Thus, the higher percentage of intracellular fungi is attributable primarily to a significantly lower number of extracellular fungal cells rather than a higher number of intracellular fungal cells. This suggests that intracellular fungi were killed or prevented from replicating over the first four hours of infection.
Fig. 2. Opsonization with anti-Candida polyclonal antibody enhances phagocytosis in vivo.
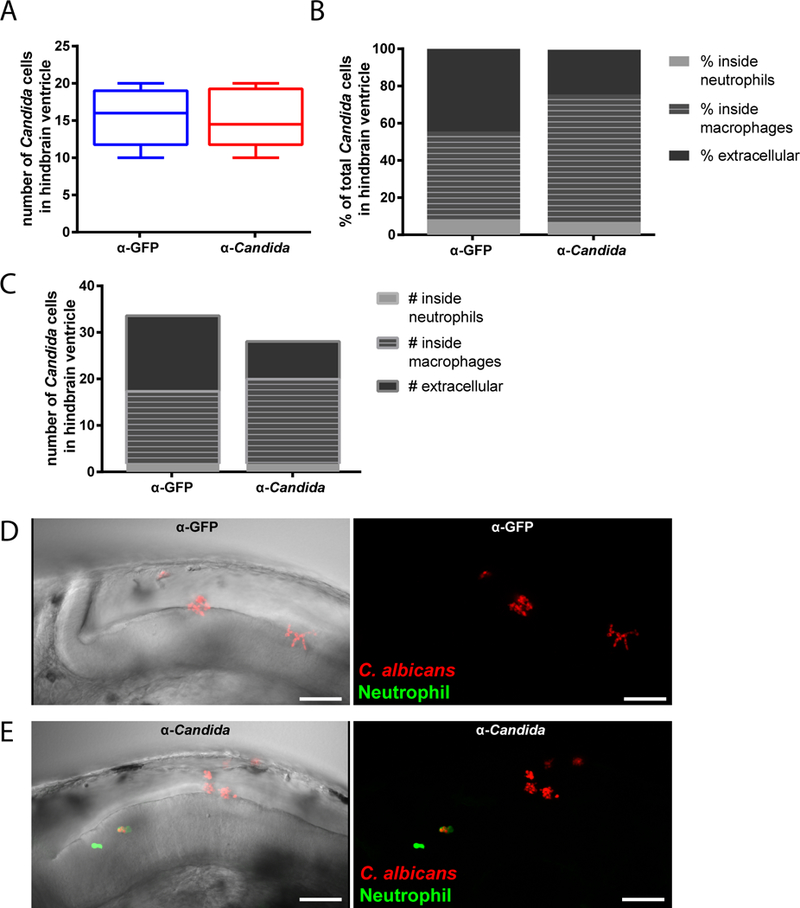
(A-E) Zebrafish of the mpx:EGFP line at the prim25 stage were infected in the hindbrain with 10–15 cells of CAF2-dTomato C. albicans, with screening within 30 minutes for confirming dose. C. albicans was co-injected with either anti-Candida or anti-GFP PAb at a final concentration of 10 μg/ml. Images were acquired via confocal microscopy at 4 hpi. Fungal and phagocyte numbers were quantified based on 2 μm Z-stacks using a 40x/0.75 N.A. UPlanFL N objective. (A) Initial inoculum of C. albicans cells in hindbrain ventricle (within 30 minutes of inoculation). (B) Average percent of total C. albicans cells in zebrafish hindbrain ventricle that are either inside neutrophils or macrophages or extracellular at 4 hpi. Significant difference between treatment groups in the percentages of total Candida phagocytosed by macrophages (p < 0.05; t-test), total phagocytosed by neutrophils (p < 0.05; Mann-Whitney), and total percent extracellular (p < 0.05; Mann-Whitney). (C) Average number of C. albicans inside neutrophils or macrophages or extracellular at 4 hpi. The only significant difference between treatment groups was in the number of extracellular Candida (p < .01; Mann-Whitney). (D-E) Representative images of the hindbrain ventricle in zebrafish larvae infected with C. albicans opsonized with (D) anti-GFP or (E) anti-Candida PAb at 36 hpf (scalebar = 50 μm). Images were acquired at 1024 × 600 pixels, with 35 slices for panel D and 52 slices for panel E. Movies including all of the slices are included as Supplemental Data Files M2D and M2E. Data are combined from three independent experiments, n=18 larvae per treatment group, whiskers = minimum and maximums. Although technical constraints limited cohort sizes in each experiment, reproducible differences were found from experiment to experiment.
On the host side, there was overall low recruitment of neutrophils in all animals and consequently low phagocytosis by neutrophils (mean +/− SEM; 4.0 +/− 1.26 vs. 2.4 +/− 0.74 neutrophils per fish; anti-Candida vs. anti-GFP). The relatively minor importance of neutrophils in phagocytosis may also be due to the nature of the infection site, as neutrophils tend to be relatively limited in bacterial phagocytosis in fluid-filled spaces (Colucci-Guyon et al., 2011). There was no difference in the number of macrophage-like cells at the infection site that had phagocytosed C. albicans, despite the fact that more fungal cells resided within macrophage-like cells with addition of the anti-Candida antibody (mean +/− SEM; 3.75 +/− 0.46 vs. 3.42 +/− 0.76 macrophage-like cells with internalized fungi per fish; anti-Candida vs. anti-GFP). Taken together, these results suggest that the polyclonal anti-C. albicans antibody enhances early phagocytic efficiency at the injection site.
3.3. Early phagocytosis efficiency is highly predictive for survival from hindbrain ventricle infection of C. albicans.
Increased early C. albicans containment at the injection site has previously been shown to be associated with survival, and conversely poor early engulfment is related to enhanced mortality (Brothers et al., 2013). However, the predictive value of non-invasive imaging has not been examined in detail. To more closely examine the relationship between failed early phagocytosis and mortality, relative to other measures of immune response, we measured a number of fungal and host variables over the first four hours of infection through longitudinal time-course confocal imaging. Infections and scoring were performed essentially as described above, although no antibody co-injection was used and fish were instead treated with either vehicle or the NADPH oxidase inhibitor diphenyleneiodonium (DPI), as previously described (Brothers et al., 2013). This inhibitor was used to reduce early phagocyte recruitment and enhance susceptibility to infection, increasing the range of host responses with varying phagocytosis efficiency thereby providing a better test set for exploring the relationship between fungal containment and survival. To analyze this data, we first plotted the relationship between the number of extracellular fungi at 4 hpi and the ultimate fate of the fish in the first 24 hpi. We found a strong and statistically significant difference in the number of extracellular fungal cells in fish that survived versus died (Fig. 3A). To identify a choice-point between survival and death, we then plotted the data cumulatively (Fig. 3B). For surviving fish, the plot indicates the percentage of surviving fish with X or more extracellular fungi. Thus, 0% of surviving fish have greater than 20 extracellular fungi. For dead fish, the plot indicates the percentage of dead fish with X or fewer extracellular fungi. Thus, 0% of dead fish have fewer than 5 extracellular fungi. This analysis demonstrated that the overwhelming majority of fish that died (91%) had more than 11 extracellular fungi, while all had at least 7 extracellular fungi at 4 hpi (Fig. 3B). Conversely, the overwhelming majority of fish that survived (85%) had 12 or fewer extracellular fungi at 4 hpi. Previous work had used an arbitrary level of 5 or greater extracellular fungi to stratify those fish with effective versus ineffective early phagocytosis (Brothers et al., 2013). This more detailed analysis suggests that the break-point between survival and mortality is closer to 10–12 extracellular fungi at 4 hpi.
Fig. 3. Inefficient early phagocytosis is a strong predictor of mortality in the zebrafish hindbrain infection model.
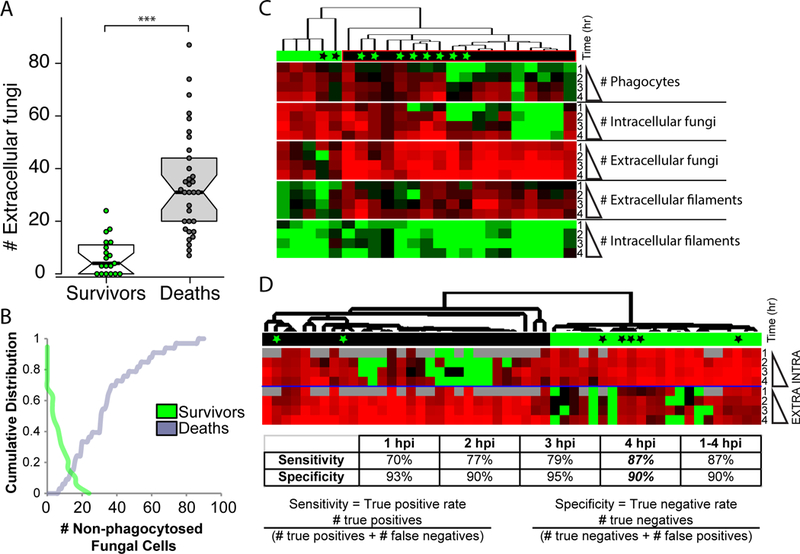
(A-D) Zebrafish of the mpx:EGFP line at the prim25 stage were infected in the hindbrain with 10–15 CAF2-dTomato C. albicans, with screening within 30 minutes for confirming dose. Fish were longitudinally imaged by confocal microscopy from 1–4 hpi and images were scored for fungal and host phenotype: fungal cells were scored as either intracellular/extracellular and yeast/filament; phagocytes in the hindbrain with ingested fungal cells and/or EGFP fluorescence were also counted. Fish were then removed from low-melt agarose with tricaine and kept until 24 hpi, when they were scored for survival. (A) Surviving and dead fish were scored for the number of extracellular fungi they had had at 4 hpi. Box-plots are shown; T-test was used for statistics, *** p<0.001. (B) Cumulative distributions of the number of extracellular fungi at 4 hpi for surviving (green) and dead (gray) fish are plotted. For surviving fish, the plot indicates the percentage of surviving fish with X or more extracellular fungi. Thus, 0% of surviving fish have greater than 20 extracellular fungi. For dead fish, the plot indicates the percentage of dead fish with X or fewer extracellular fungi. Thus, 0% of dead fish have fewer than 5 extracellular fungi. (C) Quantitative phenotypes at 1–4 hpi for individual fish were hierarchically clustered. Green bar above indicates cluster with greater survival and black bar above indicates cluster with greater mortality. Black asterisks indicate dead fish within survival cluster and green asterisks indicate survivors within death cluster. Red indicates high number and green indicates low number. (D) Only intracellular and extracellular fungal cell number was used in hierarchical clustering. Red indicates high number and green low number. Gray indicates lack of data. Black and green bars and asterisks are as in panel C. The discriminatory power of clustering for predicting mortality using data for each time point or the combined dataset (1–4 hpi) is shown in the table below. Sensitivity is calculated as (# true positives)/(# true positives + # false negatives). Specificity is calculated as (# true negatives)/(# true negatives + # false positives).
To examine the contribution of other early immune and fungal factors to infection outcome, we hierarchically clustered all of the data collected. However, incorporating this additional data resulted in a less precise divide for stratification of fish at 4 hpi (Fig. 3C). Instead, clustering based only on the number of intracellular and extracellular fungi provided a crisp divide between fish destined to survive and die (Fig. 3D). Clustering these data using the same parameters but limiting the data to that of individual time points (1, 2, 3, or 4 hpi) revealed that the data at 4 hpi is equivalent to using all of the data between 1 and 4 hpi for clustering. In this case, we achieved a sensitivity (true positive rate) of 87% combined with a specificity (true negative rate) of 90%. Taken together, these data demonstrate the power of using phagocytosis efficiency as an early non-invasive test that reliably predicts survival to 24 hpi. The predictive value of early phagocytosis efficiency for survival in this fungal infection model suggests that enhancing early phagocytosis could improve outcomes.
3.4. Co-injection with polyclonal antibodies enhances host survival in a dose-dependent manner
Because we found that co-injection with a polyclonal anti-Candida antibody increases early phagocytosis (Fig. 2), we hypothesized that this antibody preparation would also protect fish from overall mortality. We measured survival in fish injected and imaged as described above. We found that co-injection with this antibody at the concentration that promotes phagocytosis and early containment (as shown in Fig. 2A) led to significant protection from mortality (Fig. 4A). The survival curve of larvae infected with opsonized Candida was significantly different to that of mock-opsonized Candida infected larvae (p = 0.0017; Fig. 4B). Larvae that were infected with opsonized Candida had a higher end point survival of 70.3% compared to that of larvae infected with mock-opsonized Candida of 34.3% (Fig. 4B). Further testing with lower doses of antibody revealed a dose-dependent protection from long-term mortality for the anti-Candida as compared to the non-specific antibody (Fig. 4B–C).
Fig. 4. Dose-responsive antibody-mediated protection from disseminated candidiasis.
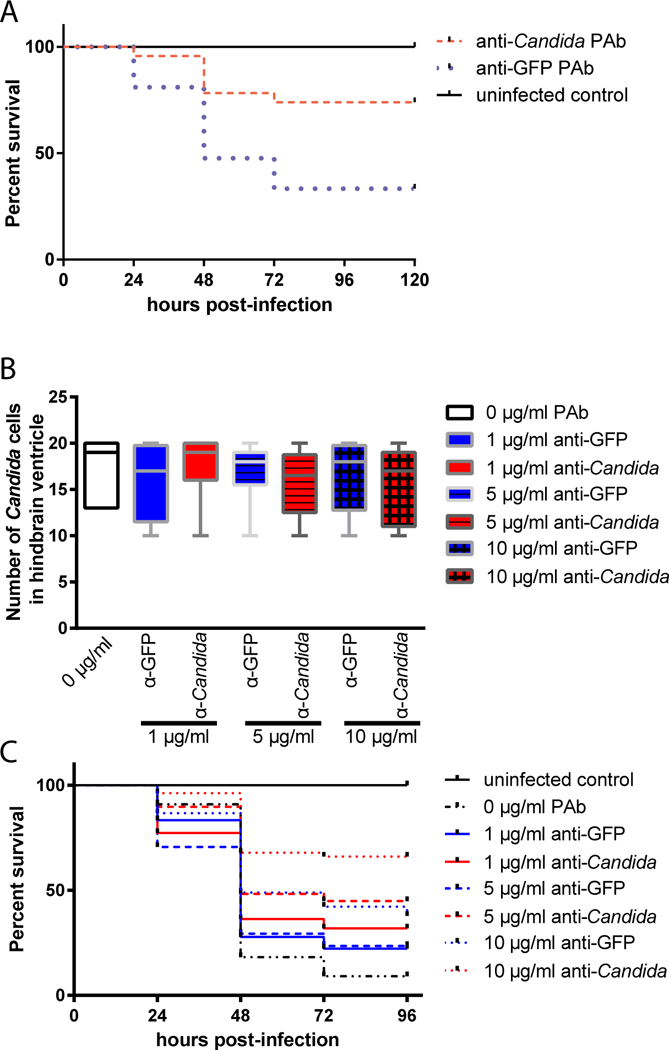
(A-C) Zebrafish of the mpx:EGFP line at the prim25 stage were infected in the hindbrain with 10–15 cells of CAF2-dTomato C. albicans, with screening within 30 minutes for confirming dose. Images were acquired via confocal microscopy at 4 hpi. Fungal and phagocyte numbers were quantified based on 2 μm Z-stacks using a 40x/0.75 N.A. UPlanFL N objective. (A) Average Survival Kaplan-Meier curves of larvae infected with C. albicans, co-injected with either anti-Candida or anti-GFP pAb at 10 μg/ml. Initial inoculum was consistent and is shown in Fig. 2A. After screening, fish were observed until 96 hpi for survival. Results pooled from two independent experiments. Difference between anti-Candida and anti-GFP is significant (p < 0.01 by Log-Rank; n=23 anti-Candida, 21 anti-GFP, 7 uninfected) (B-C) Dose-response test (2 trials combined; number of zebrafish per treatment group = 32, 11, 18, 22, 17, 29, 45 and 53 respectively for uninfected, 0 μg/ml, 1 μg/ml anti-GFP, 1 μg/ml anti-Candida, 5 μg/ml anti-GFP, and 5 μg/ml anti-Candida, 10 μg/ml anti-GFP, and 10 μg/ml anti-Candida; note that 10 μg/ml data from two trials in 4A was comparable and was therefore pooled with 2 experiments performed with all doses). C. albicans was co-injected with either anti-Candida or anti-GFP pAb at 1–10 μg/ml. (B) Initial inoculum of C. albicans Caf2-dTomato cells in zebrafish larvae hindbrain ventricle. No significant difference between inoculum in each treatment group; p > 0.05 by ANOVA. (C) Survival Kaplan-Meier curves of zebrafish larvae infected with C. albicans, anti-Candida IgG or anti-GFP IgG at 0, 1, 5 and 10 μg/ml over 96 h.p.i. Survival curves of the combined data from duplicate trials (for 0, 1, and 5 μg/ml) or four trials (10 μg/ml). Significant differences between anti-Candida and mock at 10 μg/ml (p < 0.01) and between anti-Candida and un-opsonized at 1 μg/ml (p < 0.05, Log-Rank of Kaplan-Meier).
3.5. A mannan-directed monoclonal antibody does not improve infection outcome in disseminated candidiasis in zebrafish
We attempted to extend these studies to determine if a previously characterized anti-Candida monoclonal antibody would be effective in enhancing early phagocytosis and/or overall survival in the context of this zebrafish larval infection model. We used an IgM anti-Candida antibody previously described to react with mannan epitopes on the fungal cell surface and protect mice from disseminated candidiasis (Han and Cutler, 1995). C. albicans was co-injected with control anti-GFP or monoclonal anti-Candida IgM, and inocula were validated to ensure equivalent doses (Suppl. Fig. S2A). Larvae were scored for survival for four days post-infection, but there was no protection provided by this antibody in the larval fish model (Suppl. Fig. S2B). Taken together, these experiments suggest that this single monoclonal antibody may not be effective in enhancing survival in this zebrafish model of C. albicans infection, but do not exclude the possibility that other monoclonal antibodies may be effective.
4. Discussion
Humoral immunity is a crucial component in adaptive immunity and successful vaccination. Although antibodies play a number of well-recognized protective roles, technical obstacles limit our ability to characterize how they actually promote protection in the context of a whole animal infection model. The zebrafish larval model offers the advantages of a small and transparent vertebrate host with an immune system that is similar to man (Carradice and Lieschke, 2008; Meeker and Trede, 2008; Tobin et al., 2012; Torraca et al., 2014). Here, we examined the possibility for using these animals to characterize the ability of antibodies to enhance phagocytosis of Candida albicans and thereby promote resistance to disease. We found that polyclonal anti-Candida antibodies can promote containment and survival in this model, whereas the single monoclonal tested was not effective in promoting survival.
We first demonstrated that opsonization of polystyrene microspheres with human IgG enhances zebrafish immune cell activity and phagocytosis, specifically by macrophages. This may result from phagocytosis through scavenger receptors, complement receptors, and/or antibody receptors. Scavenger receptor-mediated phagocytosis has been demonstrated for protein-coated beads in rainbow trout (Froystad et al., 1998). Complement- and antibody-mediated phagocytosis by macrophages has also been demonstrated in teleosts (Leiro et al., 1996; Leiro et al., 2008). Intriguingly, opsonization with human serum also enhances phagocytosis of beads by salmon macrophages in a process dependent on complement deposition (Johnson and Smith, 1984). Although an Fc gamma receptor homolog has not yet been found in zebrafish, expansions of related receptors may allow for a more broad-specificity phagocytosis of antibody-opsonized targets (Akula et al., 2014). In the context of an artificial polystyrene substrate, rabbit and mouse IgGs may not interact as efficiently as human IgGs with zebrafish receptors on phagocytes or elicit sufficient complement deposition to increase uptake. Given the evolutionary conservation of polysaccharide pattern recognition receptors, it is possible that coating microspheres with fungal cell wall polysaccharides as well as IgG will enhance their recognition and phagocytosis through a combination of opsonic and non-opsonic receptors that more closely mimics a fungal pathogen (Netea et al., 2015). It will also likely be informative to test if morpholino knock-down of complement pathway components affects phagocytosis of different IgG-coated microspheres.
We found that mammalian antibodies enhance immune cell activity and phagocytosis in this in vivo infection model, similar to the IgG-coated microspheres. Closer analysis made possible using intravital imaging in the zebrafish revealed that, in the presence of anti-Candida polyclonal antibodies, macrophages but not neutrophils phagocytose a higher proportion of fungi. Taken together, these results demonstrate that the polyclonal anti-C. albicans antibody tested in these studies can enhance early phagocytic efficiency at the injection site. It is notable that antibody-enhanced phagocytosis of fungi has not been demonstrated before in vivo to our knowledge, and this highlights the utility of the zebrafish model to measure specific immune responses in the complex in vivo environment. Efficient recognition and ingestion of particles requires crosstalk among phagocytic receptors (Freeman and Grinstein, 2014). Thus, enhanced phagocytosis of Candida in the presence of anti-Candida antibodies may result from recognition by a combination of scavenger receptors, complement receptors, and/or antibody receptors. Similar to the mammalian situation, fish complement binds well to yeast (Ichiki et al., 2012). It is possible that opsonization with polyclonal antibodies enhances this complement deposition and phagocytosis.
Previous work had used an arbitrary level of extracellular fungi to stratify those fish with effective versus ineffective early phagocytosis (Brothers et al., 2013). The more detailed studies presented here suggest that the break-point between survival and mortality is closer to 10–12 extracellular fungi at 4 hpi. Taken together, these data demonstrate the power of using phagocytosis efficiency as an early non-invasive test that can be used to predict survival at 24 hpi. This may be especially important in the hindbrain ventricle model of infection, in which phagocytosis blocks hyphal germination of C. albicans and thereby prevents lethal invasive growth (Brothers et al., 2013). In addition, phagocytosis provides a critical break on infection progression in other zebrafish infection models in which it has been monitored non-invasively in vivo (Bojarczuk et al., 2016; Clay et al., 2007). The importance of early phagocyte responses is thus clear when it is possible to measure these responses non-invasively and longitudinally follow the consequences of differential efficiency in early pathogen containment.
As a consequence of enhanced early containment and blockade of hyphal growth, the survival of larvae infected with opsonized Candida is significantly enhanced compared to that of mock-opsonized Candida infected larvae. Antibody-enhanced containment of fungi may be generally relevant to vaccine-induced protection against pathogens in other fish, as others have shown that vaccine-induced antibodies mediate complement deposition that enhances phagocytosis of ciliates and microsporidian fungi in turbot (Leiro et al., 1996; Leiro et al., 2008). In addition to enhanced phagocytosis, complement deposition can also activate fish immune cells (Rotllant et al., 2004) and mammalian adaptive immune responses (Li et al., 2008; Peng et al., 2008). Similar pathways may play an analogous role in antibody-mediated protection in humans.
The single anti-β-mannan specific mouse IgM monoclonal antibody tested is ineffective in enhancing survival in this zebrafish model of C. albicans infection. Monoclonal anti-Candida antibodies are generally not very effective in the mouse candidemia model, and some are even anti-protective (Bromuro et al., 2002; Casadevall et al., 1998; Polonelli and Cassone, 1999). It is not known why some are more protective than others. The IgM we chose has been well characterized to be protective in the mouse model by targeting a mannan epitope (Han and Cutler, 1995). The lack of efficacy in this zebrafish model may be because this IgM provokes a species-or mammalian-specific response through its constant region. Mouse IgMs may not as efficiently interact with the zebrafish complement system, a known mechanism by which the anti-β-mannan IgM provides protection in mice (Han et al., 2001). Furthermore, it is not known if this IgM antibody stimulates phagocytosis in vivo, which may be a required mechanism-of-action for enhanced protection in this infection model where the fungi are delivered through injection into the hindbrain ventricle.
Here we have described cross-species enhancement of fungal phagocytosis by co-injection of polyclonal rabbit anti-Candida antibodies in the zebrafish larva. The enhanced fungal containment improves survival of infection, likely by the previously described mechanism of blocking invasive hyphal growth (Brothers et al., 2013). We also found that coating with human IgG can enhance phagocytosis of polystyrene beads, in line with previously described effects of human serum for phagocytosis by fish macrophages (Johnson and Smith, 1984). Our results with the polyclonal anti-Candida antibody demonstrate the potential power of the zebrafish model for assaying the cellular effects of potential biological therapeutics in vivo. As zebrafish are not a natural host for C. albicans, this model holds more promise in elucidation of well-conserved immune mechanisms and has limited utility for discovery of human-or mammalian-specific immune responses. While more data will be necessary to test its broader utility as a screening model, this first set of results suggest the zebrafish larva is worth pursuing as a new pre-clinical model for testing the efficacy of opsonizing reagents.
Supplementary Material
C. albicans CAF2-dTomato was grown overnight, washed, and stained with 0, 10, 20, 50 or 100 μg/ml anti-Candida or anti-GFP polyclonal antibodies. The cells were washed twice and stained with goat anti-rabbit IgG-FITC at 10 μg/ml, washed twice and analyzed by flow cytometry. Histograms illustrate (A) rabbit anti-Candida IgG and (B) rabbit anti-GFP IgG staining results.
(A-B) Zebrafish of the mpx:EGFP line at the prim25 stage were infected in the hindbrain with 10–15 cells of CAF2-dTomato C. albicans, with screening within 30 minutes for confirming dose. (A) Initial inoculum of C. albicans Caf2-dTomato cells in zebrafish larvae hindbrain ventricle. No significant difference among inoculae in treatment groups p > 0.05 (One-way ANOVA). (B) Average Survival Kaplan-Meier curves of zebrafish infected with C. albicans with either 10 μg/ml rabbit anti-GFP IgG or 10 μg/ml monoclonal anti-Candida IgM. There is no significant difference in survival (Log-Rank test on Kaplain-Meier). Data shown are pooled from six independent experiments.
Highlights.
-
>
Opsonization of beads with human IgG stimulates zebrafish phagocytosis.
-
>
Polyclonal anti-Candida antibody opsonization stimulates fungal containment in vivo.
-
>
Efficient early fungal engulfment is prognostic for infection survival.
-
>
Polyclonal anti-Candida antibody treatment enhances overall survival of candidemia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akula S, Mohammadamin S, Hellman L, 2014. Fc receptors for immunoglobulins and their appearance during vertebrate evolution. PLoS One 9, e96903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengten E, Wilson M, 2015. Antibody Repertoires in Fish. Results Probl Cell Differ 57, 193–234. [DOI] [PubMed] [Google Scholar]
- Bojarczuk A, Miller KA, Hotham R, Lewis A, Ogryzko NV, Kamuyango AA, Frost H, Gibson RH, Stillman E, May RC, Renshaw SA, Johnston SA, 2016. Cryptococcus neoformans Intracellular Proliferation and Capsule Size Determines Early Macrophage Control of Infection. Sci Rep 6, 21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromuro C, Torosantucci A, Chiani P, Conti S, Polonelli L, Cassone A, 2002. Interplay between protective and inhibitory antibodies dictates the outcome of experimentally disseminated Candidiasis in recipients of a Candida albicans vaccine. Infect Immun 70, 5462–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers KM, Gratacap RL, Barker SE, Newman ZR, Norum A, Wheeler RT, 2013. NADPH oxidase-driven phagocyte recruitment controls Candida albicans filamentous growth and prevents mortality. PLoS Pathog 9, e1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers KM, Newman ZR, Wheeler RT, 2011. Live imaging of disseminated candidiasis in zebrafish reveals role of phagocyte oxidase in limiting filamentous growth. Eukaryot Cell 10, 932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers KM, Wheeler RT, 2012. Non-invasive imaging of disseminated candidiasis in zebrafish larvae. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC, 2012. Hidden killers: human fungal infections. Sci Transl Med 4, 165rv113. [DOI] [PubMed] [Google Scholar]
- Carradice D, Lieschke GJ, 2008. Zebrafish in hematology: sushi or science? Blood 111, 3331–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Cassone A, Bistoni F, Cutler JE, Magliani W, Murphy JW, Polonelli L, Romani L, 1998. Antibody and/or cell-mediated immunity, protective mechanisms in fungal disease: an ongoing dilemma or an unnecessary dispute? Med Mycol 36 Suppl 1, 95–105. [PubMed] [Google Scholar]
- Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, Ramakrishnan L, 2007. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe 2, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci-Guyon E, Tinevez JY, Renshaw SA, Herbomel P, 2011. Strategies of professional phagocytes in vivo: unlike macrophages, neutrophils engulf only surface-associated microbes. J Cell Sci 124, 3053–3059. [DOI] [PubMed] [Google Scholar]
- Detrich HW 3rd, Westerfield M, Zon LI, 2010. The zebrafish: cellular and developmental biology, part A. Preface. Methods Cell Biol 100, xiii. [DOI] [PubMed] [Google Scholar]
- Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ, 2011. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117, e49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch DA, Ludlam HA, Brown NM, 2006. Invasive fungal infections: a review of epidemiology and management options. J Med Microbiol 55, 809–818. [DOI] [PubMed] [Google Scholar]
- Freeman SA, Grinstein S, 2014. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev 262, 193–215. [DOI] [PubMed] [Google Scholar]
- Froystad MK, Rode M, Berg T, Gjoen T, 1998. A role for scavenger receptors in phagocytosis of protein-coated particles in rainbow trout head kidney macrophages. Dev Comp Immunol 22, 533–549. [DOI] [PubMed] [Google Scholar]
- Golay J, Introna M, 2012. Mechanism of action of therapeutic monoclonal antibodies: promises and pitfalls of in vitro and in vivo assays. Arch Biochem Biophys 526, 146–153. [DOI] [PubMed] [Google Scholar]
- Gratacap RL, Rawls JF, Wheeler RT, 2013. Mucosal candidiasis elicits NF-kappaB activation, proinflammatory gene expression and localized neutrophilia in zebrafish. Dis Model Mech 6, 1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C, Loynes CA, Whyte MK, Crossman DC, Renshaw SA, Chico TJ, 2011. Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb Haemost 105, 811–819. [DOI] [PubMed] [Google Scholar]
- Han Y, Cutler JE, 1995. Antibody response that protects against disseminated candidiasis. Infect Immun 63, 2714–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kozel TR, Zhang MX, MacGill RS, Carroll MC, Cutler JE, 2001. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J Immunol 167, 1550–1557. [DOI] [PubMed] [Google Scholar]
- Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM, 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48, 1695–1703. [DOI] [PubMed] [Google Scholar]
- Ichiki S, Kato-Unoki Y, Somamoto T, Nakao M, 2012. The binding spectra of carp C3 isotypes against natural targets independent of the binding specificity of their thioester. Dev Comp Immunol 38, 10–16. [DOI] [PubMed] [Google Scholar]
- Johnson E, Smith P, 1984. Attachment and phagocytosis by salmon macrophages of agarose beads coated with human C3b and C3bi. Dev Comp Immunol 8, 623–630. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF, 1995. Stages of embryonic development of the zebrafish. Dev Dyn 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Leiro J, Ortega M, Estevez J, Ubeira FM, Sanmartin ML, 1996. The role of opsonization by antibody and complement in in vitro phagocytosis of microsporidian parasites by turbot spleen cells. Vet Immunol Immunopathol 51, 201–210. [DOI] [PubMed] [Google Scholar]
- Leiro J, Piazzon MC, Budino B, Sanmartin ML, Lamas J, 2008. Complement-mediated killing of Philasterides dicentrarchi (Ciliophora) by turbot serum: relative importance of alternative and classical pathways. Parasite Immunol 30, 535–543. [DOI] [PubMed] [Google Scholar]
- Li K, Anderson KJ, Peng Q, Noble A, Lu B, Kelly AP, Wang N, Sacks SH, Zhou W, 2008. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood 112, 5084–5094. [DOI] [PubMed] [Google Scholar]
- Meeker ND, Trede NS, 2008. Immunology and zebrafish: spawning new models of human disease. Dev Comp Immunol 32, 745–757. [DOI] [PubMed] [Google Scholar]
- Nakao M, Tsujikura M, Ichiki S, Vo TK, Somamoto T, 2011. The complement system in teleost fish: progress of post-homolog-hunting researches. Dev Comp Immunol 35, 1296–1308. [DOI] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL, 2015. Immune defence against Candida fungal infections. Nat Rev Immunol 15, 630–642. [DOI] [PubMed] [Google Scholar]
- Peng Q, Li K, Anderson K, Farrar CA, Lu B, Smith RA, Sacks SH, Zhou W, 2008. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a-C3aR interaction. Blood 111, 2452–2461. [DOI] [PubMed] [Google Scholar]
- Polonelli L, Cassone A, 1999. Novel strategies for treating candidiasis. Curr Opin Infect Dis 12, 61–66. [DOI] [PubMed] [Google Scholar]
- Ramsland PA, Hutchinson AT, Carter PJ, 2015. Therapeutic antibodies: Discovery, design and deployment. Mol Immunol 67, 1–3. [DOI] [PubMed] [Google Scholar]
- Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK, 2006. A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976–3978. [DOI] [PubMed] [Google Scholar]
- Rotllant J, Parra D, Peters R, Boshra H, Sunyer JO, 2004. Generation, purification and functional characterization of three C3a anaphylatoxins in rainbow trout: role in leukocyte chemotaxis and respiratory burst. Dev Comp Immunol 28, 815–828. [DOI] [PubMed] [Google Scholar]
- Sullivan C, Kim CH, 2008. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol 25, 341–350. [DOI] [PubMed] [Google Scholar]
- Tobin DM, May RC, Wheeler RT, 2012. Zebrafish: a see-through host and a fluorescent toolbox to probe host-pathogen interaction. PLoS Pathog 8, e1002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A, 2005. A novel glycol-conjugate vaccine against fungal pathogens. J Exp Med 202, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torraca V, Masud S, Spaink HP, Meijer AH, 2014. Macrophage-pathogen interactions in infectious diseases: new therapeutic insights from the zebrafish host model. Dis Model Mech 7, 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Cutler JE, 2011. Vaccine and monoclonal antibody that enhance mouse resistance to candidiasis. Clin Vaccine Immunol 18, 1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Dziadek S, Bundle DR, Cutler JE, 2008. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc Natl Acad Sci U S A 105, 13526–13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Cui P, 2014. Complement system in zebrafish. Dev Comp Immunol 46, 3–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
C. albicans CAF2-dTomato was grown overnight, washed, and stained with 0, 10, 20, 50 or 100 μg/ml anti-Candida or anti-GFP polyclonal antibodies. The cells were washed twice and stained with goat anti-rabbit IgG-FITC at 10 μg/ml, washed twice and analyzed by flow cytometry. Histograms illustrate (A) rabbit anti-Candida IgG and (B) rabbit anti-GFP IgG staining results.
(A-B) Zebrafish of the mpx:EGFP line at the prim25 stage were infected in the hindbrain with 10–15 cells of CAF2-dTomato C. albicans, with screening within 30 minutes for confirming dose. (A) Initial inoculum of C. albicans Caf2-dTomato cells in zebrafish larvae hindbrain ventricle. No significant difference among inoculae in treatment groups p > 0.05 (One-way ANOVA). (B) Average Survival Kaplan-Meier curves of zebrafish infected with C. albicans with either 10 μg/ml rabbit anti-GFP IgG or 10 μg/ml monoclonal anti-Candida IgM. There is no significant difference in survival (Log-Rank test on Kaplain-Meier). Data shown are pooled from six independent experiments.


