Abstract
Polymer-based electronics with low bending stiffnesses and high flexibility, including recently reported macroporous syringe-injectable mesh electronics, have shown substantial promise for chronic studies of neural circuitry in the brains of live animals. A central challenge for exploiting these highly flexible materials for in-vivo studies has centered on the development of efficient input/output (I/O) connections to an external interface with high yield, low bonding resistance and long-term stability. Here we report a new paradigm applied to the challenging case of injectable mesh electronics that exploits high flexibility of nanoscale thickness two-sided metal I/O pads that can deform and contact standard interface cables in high yield with long-term electrical stability. First, we describe the design and facile fabrication of two-sided metal I/O pads that allow for contact without regard to probe orientation. Second, systematic studies of the contact resistance as function I/O pad design and mechanical properties demonstrate the key role of I/O pad bending stiffness in achieving low resistance stable contacts. Additionally, computational studies provide design rules for achieving high-yield multiplexed contact interfacing in the case of angular misalignment such that adjacent channels are not shorted. Third, in-vitro measurement of 32-channel mesh electronics probes bonded to interface cables using the direct contact method shows a reproducibly high yield of electrical connectivity. Last, in-vivo experiments with 32-channel mesh electronics probes implanted in live mice demonstrate chronic stability of the direct contact interface, enabling consistent tracking of single-unit neural activity over at least 2 months without loss of channel recording. The direct contact interfacing methodology paves the way for scalable long-term connections of multiplexed mesh electronics neural probes for neural recording and modulation, and moreover, could be used to facilitate scalable interconnection of other flexible electronics in biological studies and therapeutic applications.
Keywords: double-sided metal input/output, flexible input/output, multiplexed electrophysiology, biocompatible neural probes, chronic neural interface, flexible electronics
Graphical Abstract
Unraveling the complexity of the brain requires the development of tools capable of bridging a wide range of spatial and temporal scales, from tens of nanometers of individual synapses to centimeters of interconnected regions of the brain, and from the millisecond duration of single action potentials to long-term changes associated with development, learning, memory, and disease over months to years,1–3 respectively. Implantable electrophysiology probes have been widely explored in this context,4–6 with current silicon-based electronics4,7–12 and metallic microwire electrodes13–16 demonstrating single-neuron spatial and temporal resolutions with recent high-electrode-density Si-probes7–9 further showing these capabilities in recording from 100s to 1000s of neurons simultaneously. Nevertheless, these rigid neural recording technologies have exhibited limited chronic stability due to chronic immune response and relative shear motion at the probe-tissue interface resulting from mechanical mismatch with soft neural tissue.17–19 Thus, tracking the evolution of circuity relevant to understanding many critical neural functions requires new implantable probe technologies with substantial improvements in the duration of stable neural recording.4
Recently, we introduced a new paradigm for implantable neural probes termed mesh electronics that are designed to ‘look’ and ‘feel’ like the neural tissue they are designed to probe.20–27 Mesh electronics probe design features include three-dimensional (3D) open macroporous structure, low bending stiffness comparable to neural tissue, and feature sizes similar to neuron somata and axons.4,20,21,23,26 These design features have been shown to yield unique biocompatibility5,18,28,29 as characterized by minimal long-term chronic immune response and seamless integration between the electronic and neural networks, thereby enabling stable long-term recording and tracking of the same single-neurons on at least a year time scale. In addition, there has been considerable effort by other research groups developing flexible electronics for recording from neural and other tissues.4,5,18,30–42
In the case of the implanted mesh electronics probes, it has been especially challenging to obtain multiplexed recording given challenges in interfacing the mesh electronics probe input/output (I/O) connections to recording and stimulation instrumentation since these I/O connections are made after the injection process used to implant electronics.19,43,44 Specifically, studies to date20–22,25,26 have injected ultraflexible mesh electronics probes through small capillary needles to targeted brain regions, leaving the top end of the I/O pads exposed for electrical connection to an external recording interface after the entire mesh is ejected from the needle. Conventional I/O bonding techniques such as soldering,45 wire bonding,46 and anisotropic conductive film47 are, however, incompatible with intraoperative connection due to the sub-millimeter scale I/O pads and high temperature and pressure conditions necessary for these methods. Approaches used to overcome these obstacles for facile implementation of mesh electronics probes have included computerized but serial conductive ink printing21,22,25,26 and a parallel plug-and-play I/O interface.43 Despite the practical success of these approaches, limitations, including (i) long processing time depending on number of connection channels, (ii) the risk of electrical shorts between neighboring contacts, (iii) structural constraints to achieve multiplexed connection without breaking the metal interconnects during clamping with the zero insertion force (ZIF) connectors, and (iv) the need for specific orientation of the I/O pads, have created a barrier to implementation of the attractive properties of mesh electronics by other laboratories.
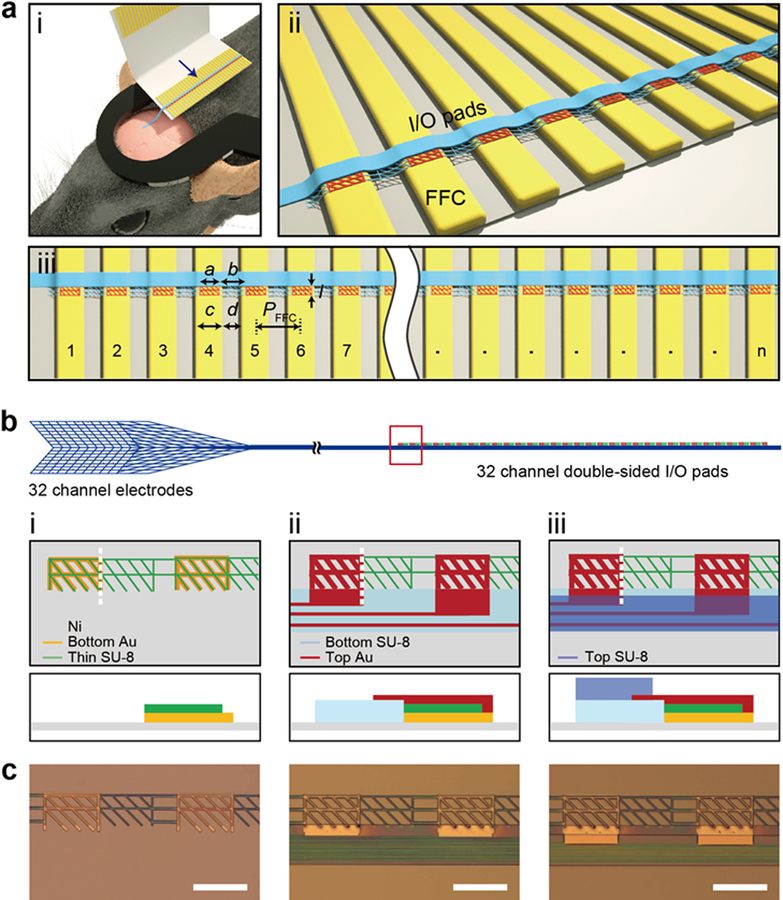
To address these limitations we asked whether it would be possible to design and implement a new facile approach for forming highly multiplexed and misalignment-tolerant electrical connections between the mesh electronics neural probe I/O and the external interface for large-scale and long-term brain mapping at the single-neuron level. Our overall concept addressing this question in Figure 1a illustrates schematically the connection of the I/O portion of the mesh electronics to a standard flat-flexible cable (FFC) instrument interface following syringe-based implantation in the brain and highlights several key design points. First, to facilitate the overall surgery the FFC or other interface is fixed to a head-stage that is glued to the animal skull prior to implantation (Figure 1a, (i)). When the I/O portion of the mesh probe is ejected over FFC metal contacts, we reasoned that a critical advance would be integrating ‘two-sided’ connection pads so that the orientation they exit the capillary needle does not affect making metal-to-metal contacts. Indeed, the necessity of orienting single-sided I/O pads in the plug-and-play methodology43 represents one significant weakness of this previously reported connection scheme. Second, the bending stiffness or flexibility of the individual I/O pads should be critical to their deformation and formation of low resistance connections to FFC metal leads (Figure 1a, (ii)) without any additional heat or pressure. Third, the design of the size and pitch of the double-sided I/O pads with respect to width and pitch of the corresponding metal leads on an FFC or related instrument interface (Figure 1a, (iii)) allows for high-yield scalable connections that are tolerant of misalignment. We set a = PFFC – c where a is the width of the I/O pads, PFFC is the pitch of metal leads of the FFC, and c is the width of metal leads in the FFC, by specifically considering the ideal case where the array of mesh I/O pads is perfectly aligned with the FFC metal leads as shown in Figure 1a. This choice of parameters prevents electrical shorting for this ideal case without any rotational misalignment (Materials and Methods, Supporting Information). In addition, by controlling the length, l, of the I/O pad, it is also possible to design a tolerance for rotational misalignment (i.e., the stem with I/O pads are oriented at an angle with respect to the metal leads which differs from the ideal perpendicular configuration) without shorting of adjacent channels (Materials and Methods, Supporting Information).
Figure 1. Overview of direct contact input/output (I/O) interface for syringe-injectable mesh electronics.
(a) Schematics illustrating the concept of direct contact I/O interface. (i) Mesh electronics (blue) implanted into the mouse brain (pink) with the I/O portion of the mesh ejected over and electrically-connected to the metal leads (gold) of a FFC, where the FFC is bonded to a head stage (black) glued to the mouse skull. (ii) Close-up view of the I/O interface indicated by the blue arrow in (i), where double-sided metal I/O pads (red) directly contact the FFC metal leads (gold), and electrically isolated parallel interconnect lines are indicated by the blue linear structure. (iii) Scalability of direct contact interface, where n indicates the number of I/O pads on the mesh probe that are contacted simultaneously to n metal leads on the FFC or other instrument interface. Here we focus on n = 32. The design parameters a, b, c, d, l and PFFC correspond to the width of I/O pad, the gap between neighboring I/O pads, the width of metal FFC leads, gap between neighboring metal FFC leads, the length of the I/O pad, and the pitch of the FFC, which is defined as PFFC = c + d = a + b, respectively. (b) Schematic of the 32-channel mesh electronics neural probe, highlighting (left) mesh structure with neural recording electrodes, (middle) parallel interconnects, and (right) corresponding double-sided I/O pads. Key fabrication steps of I/O pads are shown in close-up views from the red box: (i) Bottom Au pads (orange) are fabricated on Ni sacrificial layer (gray), with the thin SU-8 layer (green) patterned on top of and connecting between the bottom Au pads; (ii) bottom passivation layer of SU-8 (light blue) is patterned. Parallel metal interconnects are fabricated on this layer and connect to the top Au pads (dark red), which are fabricated on top of the bottom Au/thin SU-8; (iii) top passivation layer of SU-8 (darker blue) is fabricated to encapsulate the metal interconnects but leave the bottom/top (double-sided) I/O pads exposed. The bottom row of diagrams show corresponding side-view structures along the white vertical dashed lines in the top row. (c) Bright-field microscopy images of the fabrication steps corresponding to the schematics in (b) for design III discussed in text and Figure 2. Scale bars, 200 μm.
To explore the critical design features described above, we have investigated 32-channel mesh, where our previous studies have focused primarily on 16-channel designs given challenges in reliable I/O interfacing.21,25,26 The overall mesh electronics probes are designed with three distinct functional regions (Figure 1b; Figure S1a; Figure S2, top): the mesh device region that comprises exposed Pt recording electrodes (Figure 1b, left; Figure S1b; Figure S2, i), a stem region which contains parallel metal interconnects between the electrodes and the I/O pads (Figure 1b, middle; Figure S1c; Figure S2, ii), and the I/O region, which provides an electrical interface to the external recording instrument (Figure 1b, right; Figure S1d; Figure S2, iii). This latter region represents the focus and unique enabling advance in the present work.
The design constraints for the I/O pads discussed above were explored in studies of direct contact interfacing to a standard FFC (Figure 1a), where the FFC leads have a width, c, and pitch, PFFC, of 300 and 500 μm, respectively, with gap between adjacent metal leads, d, being 200 μm. For these interface parameters, the width and gap of the mesh I/O pads (a and b, Figure 1a, (iii)) were designed to match those of the FFC leads with the following relationships: a = d = 200 μm and b = c = 300 μm (Figure 1a, (iii)). For an I/O pad length (l) one half the gap between FFC leads, 100 μm, we carried out analyses to determine the maximum angular displacement of the mesh stem relative to the ideal perpendicular alignment with respect to direction of the FFC interface leads before electrical shorts can occur between adjacent channels (Materials and Methods; Figure S3). These results summarized in Figure S3 show several key points. First, by setting the width of I/O pads, a, equal to the gap of the FFC metal leads, d, with same pitch, there are no electrical shorts at 0° rotational misalignment regardless of where along the horizontal axis the I/O pads are centered. Second, decreasing l from c to d/2 reduces the propensity for shorting between adjacent leads. For rotational misalignments as large as 15°, no more than two I/O pads cause shorting for any angle, while for the longer I/O pads, where l = c, as many as five consecutive I/O pads can be shorted at once. Third, by positioning the first I/O pad at the center of the first FFC lead in both dimensions, there is no shorting for any angular misalignment value for which all of the pads are on the FFC, i.e. for all angles between ±7°. Therefore, by choosing the I/O pad design parameters as described above, our method is capable of forming a reliable electrical interface with FFC leads that is tolerant to misalignment over a relatively wide range of angles without producing shorting between adjacent channels.
Critical to facile connection in these studies is the implementation of flexible I/O pads consisting of a mesh structure for flexibility with exposed gold contact surfaces on both sides – termed “double-sided” I/O pads such that contacts to the instrument interface (FFC leads in our studies) can be carried out with either orientation of the I/O pad side (i.e., top or bottom) facing the interface following ejection from the capillary needle (Figure S4). The key steps involved in fabrication of flexible double-sided Au mesh I/O pads are as follows (Figure 1b, (i)–(iii); Materials and Methods). First, 100-nm-thick Au mesh pads with 10-μm-wide elements were patterned on a Ni sacrificial layer as the bottom I/O electrical contacts. Second, a 200-nm-thick SU-8 layer was patterned with a mesh structure consisting of 6-μm-wide elements that overlap with but are smaller than the bottom Au mesh pads and provide structural elements between these pads (Figure 1b, (i)). This thin SU-8 layer also is important in matching the heights of I/O pad and the bottom SU-8 layer of the stem region, which is patterned in a subsequent step defining the bottom layer of the overall mesh probe. Third, the Au interconnects and top I/O pads are patterned such that the top and bottom I/O pads are electrically-connected to each other and their corresponding interconnect and electrode channels of the mesh probe (Figure 1b, (ii)). Last, a top SU-8 passivation layer is fabricated to insulate the Au interconnects, leaving the recording electrodes and both sides of the I/O pads exposed, thereby enabling formation of electrical contacts on both sides of the I/O pads (Figure 1b, (iii)). Optical microscopy images (Figure 1c) show these three key steps of fabrication corresponding to the schematics in Figure 1b. We note that the flexible double-sided Au I/O pads for the direct contact interface can be easily fabricated in this manner by conventional 2D photolithography, thus enabling rapid testing of key parameters relevant to robust interface connections and allowing for straightforward scaling of the number and density of I/O pads to match the instrumentation interface leads.
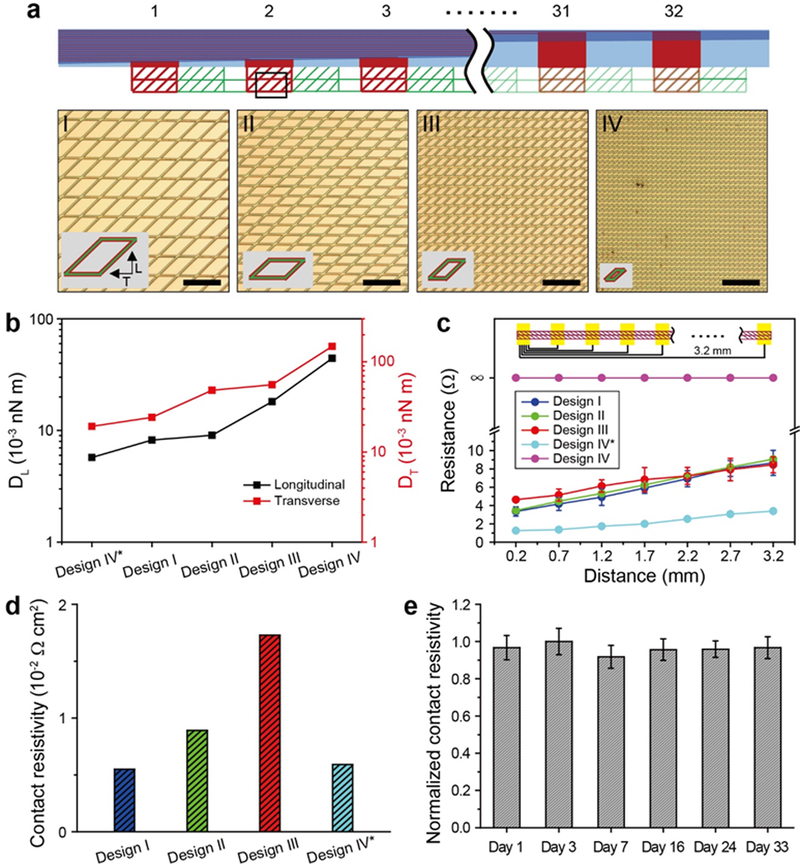
To determine optimal structural parameters for I/O pads (Figure 2a, top schematic), we first investigated the correlation between the bending stiffness and contact resistivity for two-sided mesh I/O pads following direct connection to interface metal leads. The contact resistivity was measured after ejecting a continuous mesh I/O pad onto the leads of a 32 channel FFC interface, allowing the aqueous solution to evaporate, and then measuring the resistance between pairs of FFC leads separated by distances ranging from 0.2 to 3.2 mm. Four distinct unit-cell sizes were examined (Figure 2a, I – IV; Figure S5a), where the mesh structures in these designs have Au/SU-8/Au layers with thicknesses of 100/100/100 nm, respectively. In addition, a fifth design, IV*, was considered with the same unit cell as IV but reduced Au/SU-8/Au layer thicknesses of 50/50/50 nm, respectively (Figure S5b).
Figure 2. Designs and properties of direct contact I/O pads.
(a) Top, schematic of 32-channel direct contact I/O pads. Bottom, optical microscopy images of four I/O pad designs (designs I to IV) with different unit-cell sizes. Two different I/O pads are made for IV, where design IV* has the same in-plane geometry as design IV but the total thickness is halved (ca. 150 nm for design IV* vs. 300 nm for design IV). Scale bars, 200 μm. The insets show schematics of the corresponding unit cells. (b) Simulated bending stiffness for the longitudinal (DL, left y-axis) and transverse directions (DT, right y-axis) for the five I/O pad designs, I, II, III, IV and IV*. (c) Measured pairwise resistance values for the five I/O pad designs as a function of distance between two FFC leads, on which a continuous mesh I/O pad is mounted (top schematic). Error bars denote ±1-standard deviation (SD). (d) Contact resistivities are defined as contact resistance, Rc (1.16, 1.27, 2.02, and 0.34 Ω) derived from the extrapolated y-intercepts in (c), multiplied by ideal contact areas, Ac (0.004, 0.006, 0.008 and 0.012 cm2) based on the unit cells of designs I, II, III, and IV*. (e) Measured contact resistivity of design III over one month, normalized against the maximum value from the chronic measurements. A single-factor ANOVA test was used to determine if there was a significant change in the mean contact resistivity over time, which yields a p-value of 0.9142, suggesting that there is no statistically significant change in contact resistivity over at least one month. Error bars denote ±1 SD.
First, we asked how the bending stiffness of the I/O pads would be expected to vary for these different designs. To answer this question, we carried out finite element analyses to calculate the longitudinal (DL) and transverse (DT) bending stiffness values for the five mesh designs with the different in-plane geometries, designs I to IV, and thicknesses, design IV* (see Materials and Methods in Supporting Information). Notably, the simulation results (Figure 2b) show systematic increases in the longitudinal and transverse bending stiffness values from 0.57 – 4.4 × 10−2 and 1.9 – 15 × 10−2 nN·m, respectively. With respect to our five distinct structural designs, the smallest DL and DT values correspond to design IV* due to the reduced layer thicknesses, while designs I to IV show progressive increases in these values due to their decreasing unit-cell sizes.
Second, we asked how these systematic changes in mechanical stiffness affect the quality of the electrical interface between I/O pads and the FFC leads. We measured the resistance as a function of distance between pairs of FFC leads for the different pad designs to obtain the contact resistance (Rc), where only the intrinsic capillary force, which arises during solution evaporation, is used to form the electrical contacts in all cases. Significantly, these data (Figure 2c) show small resistances of <10 Ω for designs I, II, III and IV*, with a ca. linear increase in the measured resistance with contact separation (from 0.2 to 3.2 mm). In contrast, no electrical connection was observed for the stiffest I/O pad, design IV, showing that flexibility plays a critical role in forming low-resistance contacts during aqueous solution evaporation.
To better define the electrical characteristics of the successful contacts, we calculated the contact resistivity, which is defined as the contact resistance normalized by the conductor area since the contact area varies for the different unit cell sizes of the designs. A summary of these results (Figure 2c,d) demonstrates that there is a direct relationship between I/O pad bending stiffness and contact resistance with values of 0.34, 1.2, 1.3 and 2.0 Ω for designs IV*, I, II and III, respectively, and corresponding contact resistivities of 0.59 × 10−2, 0.55 × 10−2, 0.89 × 10−2 and 1.73 × 10−2 Ω·cm2, respectively, where the smallest bending stiffness designs, I and IV*, yield the smallest contact resistivities. All four of these designs have reasonably low resistivity values of < 2 × 10−2 Ω·cm2, although we selected design III as the mesh I/O pad for subsequent experiments as it is somewhat more robust mechanically. Comparing these results to previous mesh electronics interfacing studies20,43,44 shows that the highest baseline contact resistance of the four designs, 2.0 Ω, is still comparable to the recent plug-and-play contact resistance, ca. 3 Ω,43 and 10 – 1000 times smaller than values reported for anisotropic conductive film20 and conductive ink,44 34 and 4200 Ω, respectively.
Third, to make an initial assessment of the potential of the direct contact I/O interface for long-term multiplexed brain mapping in live animals, we evaluated the stability of the mesh I/O interface of design III with the mesh and FFC mounted on the head-stage of a live mouse but without brain implantation. The mesh I/O pads were connected to the FFC leads, passivated with epoxy and the contact resistivity was evaluated over the course of one month (Materials and Methods, Supporting Information). Comparison of the normalized contact resistivity over 33 days (Figure 2e) demonstrates that the I/O pad-to-FFC lead interface is stable without any statistically significant changes over this 1-month period. We address the chronic stability of mesh electronics probes with direct contact I/O interfaces that are implanted in the brains of mice below.
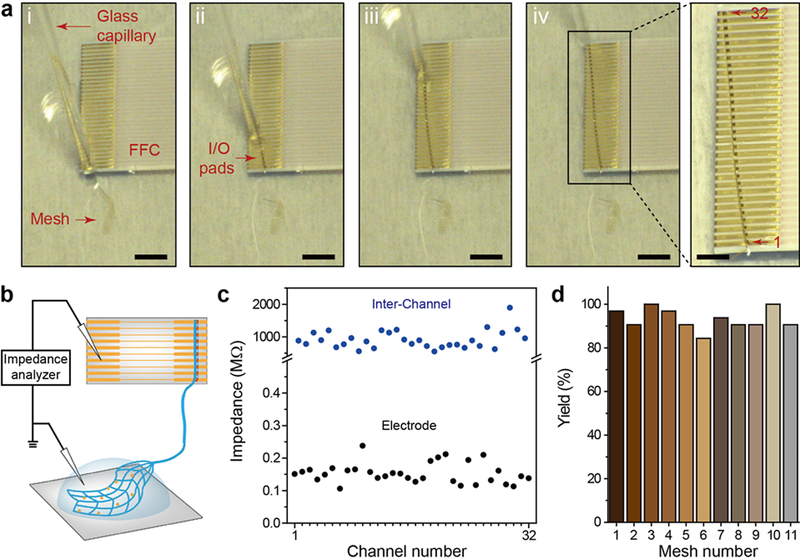
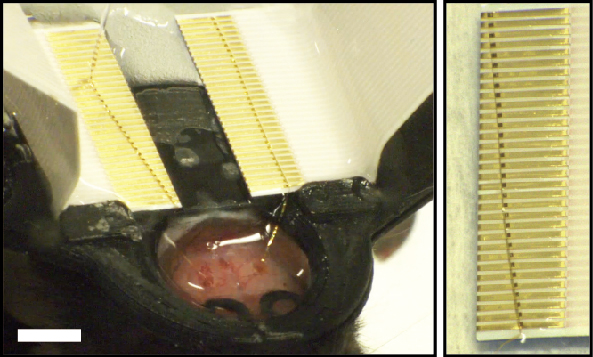
To quantify the functional connection yield of multi-channel direct contact I/O interfaces, we performed in-vitro impedance measurement with 32-channel mesh electronics probes, where the I/O pads of the probes are connected to FFC interface leads and the mesh electrodes are immersed in phosphate buffered saline (PBS; Materials and Methods, Supporting Information). The impedance of the Pt-recording electrodes, which based on previous studies should have values in the range of 200 – 600 kΩ,21,26 can serve as a good measure of I/O contacts since high-resistance contact or shorted contacts would lead to anomalously large or low, respectively, impedance values. Sequential images (Figure 3a) and Supplementary Video 1 highlight the process of ejecting the mesh electronics I/O pads onto the leads of the FFC and formation of the direct contact interface with key steps as follows. First, the FFC was fixed on a glass slide with dental cement and oxygen plasma treatment was carried out to render the FFC surface hydrophilic (Figure 3a, (i)). After loading the entire mesh probe into a 1.1 mm inner diameter (ID) glass pipette from deionized (DI) water (Materials and Methods, Supporting Information), the device region with recording electrodes was first ejected onto the glass slide followed by the I/O pad region, which was carefully positioned on the FFC leads (Figure 3a, (ii) and (iii)). If the I/O pads were misaligned with respect to FFC leads (i.e., alignment angle >10°; Figure S3), the I/O pads were pulled back into the capillary by applying negative pressure, and ejected onto the FFC metal leads until an alignment angle of <10° was achieved. Last, the remaining DI water was removed by spear sponges and the bonded I/O interface was left to dry naturally for 5–10 min (Materials and Methods, Supporting Information). A representative example showing a good alignment with a 100% connection yield of 32-channel I/O pads to the underlying 32 FFC leads, despite the presence of small angular misalignment between the rows of I/O pads and FFC leads, is shown in Figure 3a, (iv).
Figure 3. In-vitro demonstration and characterization of direct contact I/O interface.
(a) A series of optical microscopy images ((i) to (iv)) showing electrical connection of mesh I/O pads to the FFC via the direct contact I/O interface. Scale bars, 4 mm. Right, zoomed-in image of the black box in (iv), which shows 100% alignment of I/O pads with FFC metal leads (32 out of 32 channels). Scale bar, 2 mm. (b) Schematic of impedance measurement, where 1× phosphate-buffered saline (PBS) solution covers the recording electrode region of the mesh electronics to complete the circuit for impedance measurement. (c) Measured in-vitro electrode (black dots) and inter-channel (blue dots) impedance values at 1 kHz of a 32-channel mesh. (d) Yields of electrical connection of eleven 32-channel meshes with the direct contact I/O interfaces.
The reproducibility of the direct contact interface for functional measurements was tested by characterizing the impedance of the mesh probe recording electrodes in 1× PBS as shown in Figure 3b, where each of the 32 recording electrodes was tested independently through the FFC output connections (Materials and Methods, Supporting Information). A representative interfacial impedance measurement at 1 kHz for a 32-channel mesh probe (Figure 3c, lower black points) shows a 100% yield of electrical connection with the impedance values of all channels less than ca. 200 kΩ. We have also addressed the functional reproducibility of the direct contact interface by carrying out measurements on 10 additional 32-channel meshes (11 in total). These data, which are summarized in Figure 3d, show that 328 out of 352 channels (ca. 93%) have successful electrical connection to the FFC in the in-vitro impedance measurement. Additionally, to investigate potential electrical cross-talk of the direct contact interface, inter-channel impedances between adjacent Au interconnects were characterized (Figure 3c, upper blue points) with values of ca. 1 GΩ consistent with an absence of shorting or partial shorting of the direct contact I/O connections. Last, inter-channel impedance measurements made in the same manner from 3 mesh with 96 channels in total yield an average impedance of ca. 1 GΩ ± 0.4 GΩ (±1 SD), thus confirming the absence of shorting or partial shorting and the overall reliability for the direct contact interface.
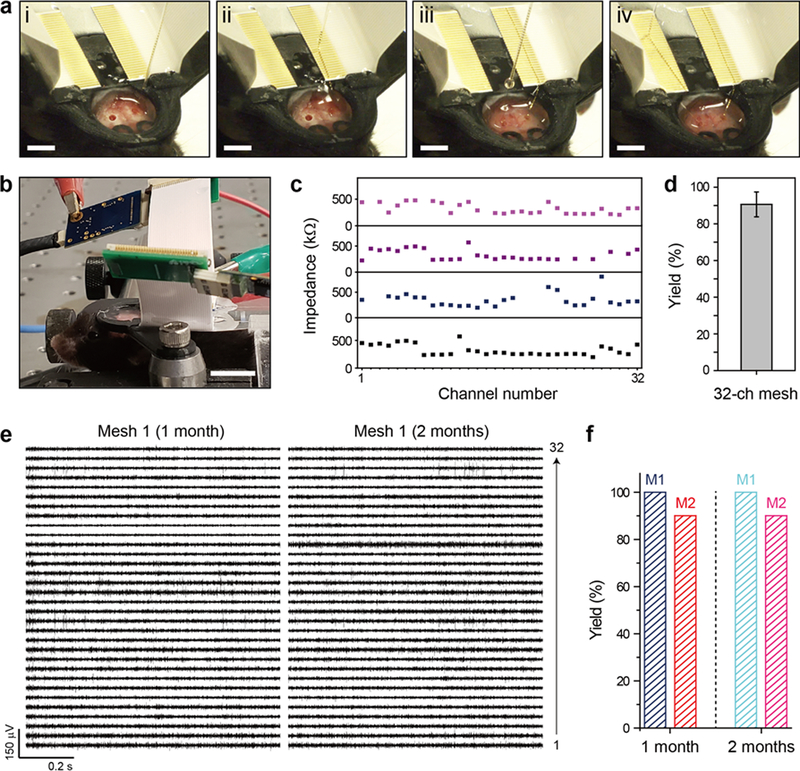
Next we asked whether it would be possible to use the direct contact I/O interfacing paradigm for facile and robust chronic electrophysiological recording from the brains of live mice. To this end we carried out chronic in-vivo experiments testing the implantation and connection of two 32-channel mesh electronics probes in the left and right hippocampal regions of the same mouse brain (Materials and Methods, Supporting Information). The procedure for direct contact I/O interfacing in live animals is a straightforward adaptation of our in-vitro methods described above and shown in Figure 4a (see also Supplementary Video 2 and Supporting Information) with several key points. First, the head-stage with two FFCs affixed, where the 32 exposed metal leads are oriented anterior-posterior (AP) on the two sides adjacent to the central opening of the stage, was fixed to the exposed skull with dental cement (Figure 4a, (i)). The FFC surface was wet with sterile DI water to maintain hydrophilicity. Second, a mesh electronics probe was injected into the hippocampus using the field of view (FoV) method while the needle was withdrawn during the injection,44 keeping the I/O pads inside the needle. Using the motorized stereotaxic frame to drive the needle, the two-sided I/O pads were roughly positioned over the FFC leads and ejected, and the I/O pads were then aligned with the row of FFC leads (Figure 4a, (ii)). The above steps were repeated for injection of the second mesh probe in the opposite hemisphere of the brain (Figure 4a, (iii) and (iv)). The entire process is also shown in Supplementary Video 2. The time required for I/O connection during surgery is only 5–10 minutes for each 32-channel interface connection, which represents a significant time savings compared to previous printing methods,21,22,26 and the I/O interface is very compact due to elimination of connectors30 otherwise needed for the instrument interface.
Figure 4. In-vivo demonstration of direct contact I/O interface.
(a) Images showing the alignment and direct contact connection of I/O pads to the two FFCs fixed on the mouse head-stage. The mesh electronics probes were injected into the hippocampus of both cerebral hemispheres. (b) Image of a head-fixed mouse while recording from both implanted mesh probes, which were connected to the FFCs by the direct contact method. Instrument amplifiers are attached to each of the FFC interfaces for the recording session and are visible in the upper portion of the image. Scale bar, 4 mm. (c) Measured in-vivo impedance values at 1 kHz for four 32-channel implanted mesh probes. Channels for which no impedance value is shown were disconnected, with impedance values above 2 MΩ. (d) Yield of the electrical connection of the 32-channel meshes (N = 4). Error bar denotes ±1 SD. (e) 32-channel neural recordings from Mesh 1 at 1 month (left) and 2 months (right) post-injection. (f) Yields for electrical connection of the two 32-channel mesh probes (Mesh 1 in Figure 4e and Mesh 2 in Figure S6) at 1 month and 2 months post-injection.
To assess the stability of the direct contact I/O interface, we carried out multiplexed electrophysiological recording in a head-fixed configuration over a two-month period. In brief, for each recording session the mouse was brought from the animal facility, the head-stage was screwed to a stage to fix the animal’s head position and the free end of the FFC was connected to the amplifier/digitizer of the recording instrument through as standard PCB interface (Figure 4b; Materials and Methods, Supporting Information). Initially, we characterized the yield of functional electrical connections post-implantation by measurement of the impedance of each of the recording electrodes at 1 kHz 2 h after implantation. Measured in-vivo impedance values and yield of two 32-channel probes implanted in two mice (Figure 4c and 4d) show several important points. First, the direct contact interface can be applied to in-vivo electrophysiological recording with facile interfacing. Second, the direct contact interface exhibits a mean in-vivo impedance value of ca. 320 kΩ, on the same order of magnitude as in-vitro impedance value of ca. 200 kΩ (Materials and Methods, Supporting Information). Last, this method shows a connection yield of ca. 90% between the external interface and recording electrodes from four meshes implanted into the brain (116 out of 128 channels).
With this basic in-vivo connection information in hand, we asked about the ability to record multiplexed single neuron activity over extended periods of time, where chronic single unit stability has been a unique advantage of mesh electronics demonstrated primarily for 16-channel probes previously.21,25,26 Representative 32-channel data from 2 probes implanted in the right/left hippocampal regions of a mouse at 1 and 2 months (Figure 4e; Figure S6a) highlight several key points. First, multiplexed electrophysiology traces show stable recording of characteristic extracellular action potentials from neurons over 2 months using the direct contact interface. Second, 32 and 8 representative channels with sorted spikes from mesh 1 and mesh 2, respectively, show similar waveforms at one and two months post-injection, which confirms the chronic stability of not only the interface between the mesh probe and the brain tissue, but also the direct contact interface between the mesh probe and external recording instrumentation (Figure S6b; Figure S7). Third, the majority of channels recorded neural activity from two or three neurons on average, with a total number of 110 single units recorded at 1 month post-injection and 115 single units at 2 months post-injection from the 40 representative channels in mesh 1 and mesh 2. No channels exhibited a decrease in the number of recorded single units during this time period. Last, no disconnection of channels from 1 month to 2 months post-injection in either mesh was observed, which confirms the chronic stability of the electrical interface between the I/O pads and FFC produced through the direct contact method (Figure 4f).
In conclusion, our results demonstrate the ability of double-sided I/O pads to form a chronically stable, high-yield electrical interface between syringe-injectable mesh electronics and external recording instrumentation interface in a facile and reproducible manner. In contrast to conventional I/O bonding techniques45,46 and previous I/O interfacing with mesh electronics,21,43 our direct contact method makes electrical connections to the external interface by the capillary force-induced deformation of I/O pads without any additional pressure or heating. We have described the design and facile fabrication of two-sided metal I/O pads that allow for contact without regard to probe orientation, carried out systematic studies demonstrating that the contact resistance is determined directly by I/O pad design and mechanical properties, and provided design rules for achieving high-yield multiplexed contact interfacing in the case of angular misalignment without shorting of adjacent channels. In particular, optimal design nanoscale thickness double-sided metal I/O pads yielded < 2 × 10−2 Ω cm2 contact resistivity regardless of the side of the pad in contact with a standard FFC instrument interface, and functional connection yields of at least 90% in in-vitro and in-vivo studies. In addition, multiplexed in-vivo electrophysiological recording data show clear single-unit action potentials, which were confirmed by spike sorting of the data, and demonstrate chronic stability of the electrical interface between the I/O pads and FFC using the direct contact interface over a period of at least 2 months. These results thus suggest that leveraging the chronically stable facile direct contact I/O interface with the ability of mesh electronics to seamlessly integrate with surrounding neural tissue and track single-neuron activity up to at least a year21,25,26 could pave the way for reliable multiplexed recording that can uncover complex circuit evolution underlying processes such as learning, memory, and age-dependent neurodegeneration. Last, the direct contact interface provides a pathway for significantly increasing the multiplexity of mesh electronics electrodes while maintaining high electrical connection yield and ease of interface formation. This potential arises from the generality of our I/O pad design concept, which can be adapted to the needs of the target external interface using conventional 2D lithography processes. Furthermore, the direct contact I/O interfacing provides a new paradigm for electrical connection in other flexible electronics platforms30–42 and thus could impact areas beyond the specific application of stable single-neuron tracking in neuroscience.
Supplementary Material
ACKNOWLEDGMENTS
C.M.L. acknowledges support of this work by the Air Force Office of Scientific Research (FA9550-18-1-0469) and a NIH Director’s Pioneer Award (5DP1EB025835–02). This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE1144152 and DGE1745303 (R.D.V.). H.-G.P. acknowledges support of this work by the National Research Foundation of Korea (NRF) Grant funded by the Korean government (MSIP) (No. 2018R1A3A3000666). G.H. acknowledges support of this work by the American Heart Association Postdoctoral Fellowship 16POST27250219 and National Institutes of Health Pathway to Independence Award from NIA 5R00AG056636-04. T.G.S. acknowledges support by the Department of Defense (DoD) through the National Defense Science & Engineering Graduate Fellowship (NDSEG) program. This work was performed in part at the Harvard University Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation.
Footnotes
The authors declare no competing financial interest.
Supporting Information Available: Materials and methods; Overall design of mesh electronics neural probes (Figure S1); 32-channel mesh with double-sided I/O pads for direct contact (Figure S2); Design of I/O pad geometry to prevent shorting of adjacent channels (Figure S3); Double-sided I/O pad geometry for facile alignment on FFC (Figure S4); I/O pad unit cell designs for optimizing contact resistivity (Figure S5); Chronically stable recordings of a second implanted mesh (Figure S6); Spike sorting of recorded electrical traces to identify single units (Figure S7); Supplementary Videos 1 and 2; Supplementary References.
REFERENCES
- 1.Herry C; Johansen JP Nat. Neurosci 2014, 17, 1644–1667. [DOI] [PubMed] [Google Scholar]
- 2.Geva-Sagiv M; Las L; Yovel Y; Ulanovsky N Nat. Rev. Neurosci 2015, 16, 94–108. [DOI] [PubMed] [Google Scholar]
- 3.Moser EI; Moser M-B; McNaughton BL Nat. Neurosci 2017, 20, 1448–1464. [DOI] [PubMed] [Google Scholar]
- 4.Hong G; Lieber CM Nat. Rev. Neurosci 2019, 20, 330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feiner R; Dvir T Nat. Rev. Mater 2018, 3, 17076. [Google Scholar]
- 6.Lacour SP; Courtine G; Guck J Nat. Rev. Mater 2016, 1, 16063. [Google Scholar]
- 7.Jun JJ; Steinmetz NA; Siegle JH; Denman DJ; Bauza M; Barbarits B; Lee AK; Anastassiou CA; Andrei A; Aydın Ç Nature 2017, 551, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinmetz NA; Koch C; Harris KD; Carandini M Curr Opin Neurobiol 2018, 50, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raducanu BC; Yazicioglu RF; Lopez CM; Ballini M; Putzeys J; Wang S; Andrei A; Rochus V; Welkenhuysen M; Helleputte NV; Musa V; Puers R; Kloosterman F; Hoof CV; Fiáth R; Ulbert I; Mitra S Sensors 2017, 17, 2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rios G; Lubenov EV; Chi D; Roukes ML; Siapas AG Nano Lett 2016, 16, 6857–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiáth R; Beregszászi P; Horváth D; Wittner L; Aarts AA; Ruther P; Neves HP; Bokor H; Acsády L; Ulbert IJ Neurophysiol 2016, 116, 2312–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochberg LR; Serruya MD; Friehs GM; Mukand JA; Saleh M; Caplan AH; Branner A; Chen D; Penn RD; Donoghue JP Nature 2006, 442, 164–171. [DOI] [PubMed] [Google Scholar]
- 13.Hafting T; Fyhn M; Molden S; Moser M-B; Moser EI Nature 2005, 436, 801–806. [DOI] [PubMed] [Google Scholar]
- 14.Quiroga RQ; Reddy L; Kreiman G; Koch C; Fried I Nature 2005, 435, 1102–1107. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz DA; Lebedev MA; Hanson TL; Dimitrov DF; Lehew G; Meloy J; Rajangam S; Subramanian V; Ifft PJ; Li Z; Ramakrishnan A; Tate A; Zhuang KZ; Nicolelis MAL Nat. Methods 2014, 11, 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okubo TS; Mackevicius EL; Payne HL; Lynch GF; Fee MS Nature 2015, 528, 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salatino JW; Ludwig KA; Kozai TD; Purcell EK Nat. Biomed. Eng 2017, 1, 862–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen R; Canales A; Anikeeva P Nat. Rev. Mater 2017, 2, 16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivnay J; Wang H; Fenno L; Deisseroth K; Malliaras GG Sci. Adv 2017, 3, e1601649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J; Fu T-M; Cheng Z; Hong G; Zhou T; Jin L; Duvvuri M; Jiang Z; Kruskal P; Xie C; Suo Z; Fang Y; Lieber CM Nat. Nanotechnol 2015, 10, 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu T-M; Hong G; Zhou T; Schuhmann TG; Viveros RD; Lieber CM Nat. Methods 2016, 13, 875–882. [DOI] [PubMed] [Google Scholar]
- 22.Fu T-M; Hong G; Viveros RD; Zhou T; Lieber CM Proc. Natl. Acad. Sci. U. S. A 2017, 114, E10046–E10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong G; Yang X; Zhou T; Lieber CM Curr Opin Neurobiol 2018, 50, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong G; Viveros RD; Zwang TJ; Yang X; Lieber CM Biochemistry 2018, 57, 3995–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong G; Fu T-M; Qiao M; Viveros RD; Yang X; Zhou T; Lee JM; Park H-G; Sanes JR; Lieber CM Science 2018, 360, 1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X; Zhou T; Zwang TJ; Hong G; Zhao Y; Viveros RD; Fu T-M; Gao T; Lieber CM Nat. Materials 2019, 18, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai X; Hong G; Gao T; Lieber CM Acc. Chem. Res 2018, 51, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green JJ; Elisseeff JH Nature 2016, 540, 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadtler K; Singh A; Wolf MT; Wang X; Pardoll DM; Elisseeff JH Nat. Rev. Mater 2016, 1, 16040. [Google Scholar]
- 30.Jiang Y; Li X; Liu B; Yi J; Fang Y; Shi F; Gao X; Sudzilovsky E; Parameswaran R; Koehler K; Nair V; Yue J; Guo KH; Fang Y; Tsai H-M; Freyermuth G; Wong RCS; Kao C-M; Chen C-T; Nicholls AW; Wu XY; Shepherd GMG; Tian B Nat. Biomed. Eng 2018, 2, 508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y; Tian B Nat. Rev. Mater 2018, 3, 473–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi E; Li H; Yang L; Hou J; Li Y; Li L; Cao A; Fang Y Adv. Mater 2015, 27, 682–688. [DOI] [PubMed] [Google Scholar]
- 33.Guan S; Wang J; Gu X; Zhao Y; Hou R; Fan H; Zou L; Gao L; Du M; Li C; Fang Y Sci. Adv 2019, 5, eaav2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feiner R; Engel L; Fleischer S; Malki M; Gal I; Shapira A; Shacham-Diamand Y; Dvir T Nat. Mater 2016, 15, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin R; Xu Z; Mei M; Chen Z; Wang K; Liu Y; Tang T; Priydarshi MK; Meng X; Zhao S; Deng B; Peng H; Liu Z; Duan X Nat. Commun 2018, 9, 2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J; Liu X; Xu W; Luo W; Li M; Chu F; Xu L; Cao A; Guan J; Tang S; Duan X Nano Lett 2018, 18, 2903–2911. [DOI] [PubMed] [Google Scholar]
- 37.Kim D-H; Viventi J; Amsden JJ; Xiao J; Vigeland L; Kim Y-S; Blanco JA; Panilaitis B; Frechette ES; Contreras D; Kaplan DL; Omenetto FG; Huang Y; Hwang K-C; Zakin MR; Litt B; Rogers JA Nat. Mater 2010, 9, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y; Liu J; Chen S; Lei T; Kim Y; Niu S; Wang H; Wang X; Foudeh AM; Tok JBH; Bao Z Nat. Biomed. Eng 2019, 3, 58–68. [DOI] [PubMed] [Google Scholar]
- 39.Sekitani T; Yokota T; Kuribara K; Kaltenbrunner M; Fukushima T; Inoue Y; Sekino M; Isoyama T; Abe Y; Onodera H; Someya T Nat. Commun 2016, 7, 11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khodagholy D; Doublet T; Quilichini P; Gurfinkel M; Leleux P; Ghestem A; Ismailova E; Hervé T; Sanaur S; Bernard C; Malliaras GG Nat. Commun 2013, 4, 1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luan L; Wei X; Zhao Z; Siegel JJ; Potnis O; Tuppen CA; Lin S; Kazmi S; Fowler RA; Holloway S; Dunn AK; Chitwood RA; Xie C Sci. Adv 2017, 3, e1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Z; Li X; He F; Wei X; Lin S; Xie CJ Neural Eng 2019, 16, 035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuhmann TG Jr; Yao J; Hong G; Fu T-M; Lieber CM Nano Lett 2017, 17, 5836–5842. [DOI] [PubMed] [Google Scholar]
- 44.Hong G; Fu T-M; Zhou T; Schuhmann TG; Huang J; Lieber CM Nano Lett 2015, 15, 6979–6984. [DOI] [PubMed] [Google Scholar]
- 45.Wang J; Besnoin E; Duckham A; Spey S; Reiss M; Knio O; Powers M; Whitener M; Weihs T Appl. Phys. Lett 2003, 83, 3987–3989. [Google Scholar]
- 46.Zhong Z Microelectronics Reliability 2011, 51, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yim M-J; Paik K-W IEEE Trans. Adv. Packag 1999, 22, 166–173. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.