Abstract
The treatment of metastatic lesions in the brain represents a serious unmet medical need in the field of neurooncology. Even though many effective compounds have demonstrated success in treating peripheral (non-CNS) tumors with targeted agents, one aspect of this lack of success in the brain may be related to poor delivery of otherwise effective compounds. Many factors can influence the brain delivery of these agents, but one key barrier is a heterogeneously “leaky” BBB that expresses efflux transporters that limit the BBB permeability for many targeted agents. Future success in therapeutics for brain metastases must take into account the adequate delivery of “active, free drug” to the target, and may include combinations of targeted drugs that are appropriate to address each individual patient’s tumor type. This review discusses some issues that are pertinent to precision medicine for brain metastases, using specific examples of tumor types that have a high incidence of brain metastases.
Keywords: blood-brain barrier, brain metastases, drug delivery, efflux transporters, metastasize, molecularly-targeted anti-cancer agents
INTRODUCTION
Metastatic spread of tumor cells from primary lesions to distant organs is a significant concern in the management of patients suffering from cancer (1,2). The formation of tumor metastases in vital organs, particularly the brain, can lead to a dismal quality of life and ultimately organ failure and death. Brain metastases are difficult to detect and diagnose, especially early in the disease course (3). Even after diagnosis, metastases to the brain are difficult to effectively treat due to various challenges associated with their treatment. While limited numbers of discrete metastases can be effectively treated with focal radiation and/or surgery, these patients have a high risk of subsequent metastases developing from pre-existing sub-clinical ‘micrometastases’ that are not detectable at the time of focal therapy. Patients with advanced brain disease (>10 metastases), or otherwise at high risk for micrometastases, are typically treated with whole brain irradiation, which is associated with an adverse effect on neuro-cognitive function (4,5). Thus, in light of the significant morbidity associated with radiation, efficacious small molecules that could effectively replace whole brain radiation therapy could have a significant positive impact on patients requiring treatment for brain metastases.
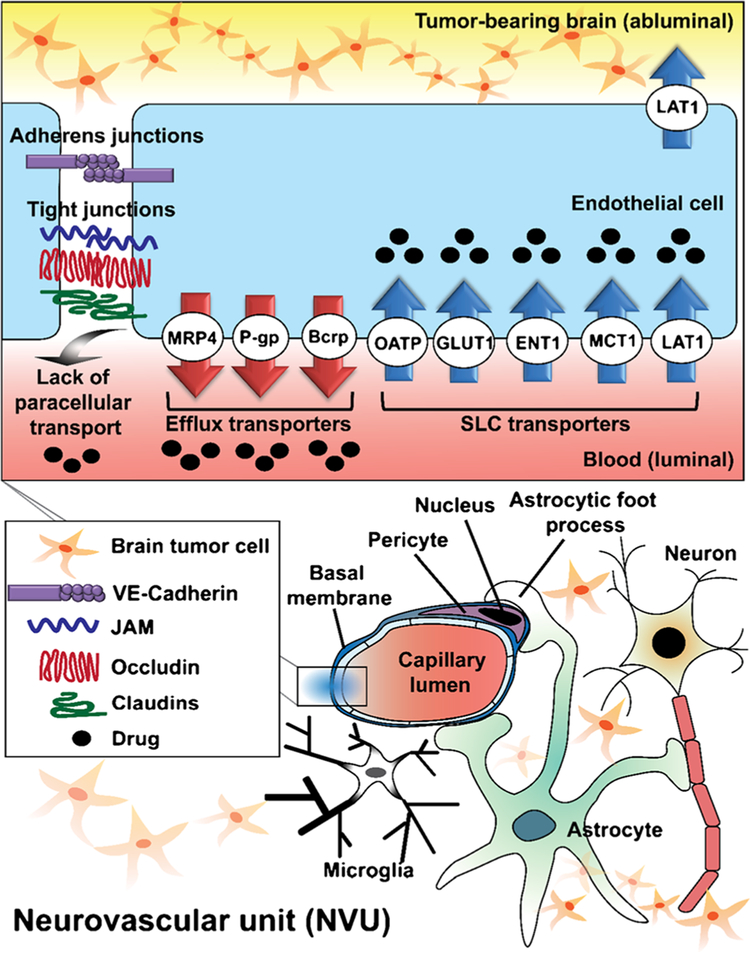
There has been important progress in the development of anti-cancer therapeutics, including molecularly-targeted agents (6–9) and novel immunotherapies (10). Both of these modalities are clearly efficacious towards tumors at the primary site (6–10), however, it remains a struggle to deliver these therapeutics across an intact BBB to many metastatic sites in the brain (11). The capillaries and associated cellular components in the brain have a highly specialized structure called the blood-brain-barrier (BBB) or neurovascular unit (NVU) that keeps many solutes, especially water-soluble solutes and large molecules, out of the brain (12). The tight junctions between the endothelial cells in the BBB in conjunction with multiple transport systems, both influx and efflux, regulate selective movement of molecules across the BBB into the brain (12) (Fig. 1 - a depiction of the NVU with transporters). Such mechanisms prevent the entry of various drugs, intended for the treatment of CNS diseases, into the brain. Specifically, many small molecule anti-cancer targeted agents have been shown to be substrates of active efflux transporters at the BBB, resulting in limited brain penetration of such therapies (Tables II, III, and IV) (13,14).
Fig. 1.
The expression and localization of transporters in the brain endothelial cell in the context of the overall composition and structure of the neurovascular unit (NVU). Important drug transporters include: SLC, solute carrier; MRP, multidrug resistance protein; LAT, L-type amino acid transporter; OATP, organic anion transporting polypeptide; MCT, monocarboxylate transporter; ENT, equilibrative nucleoside transporter.
Table II.
Brain and Transporter Related Features of Molecularly Targeted Therapy for Lung Cancer
| Compound | Molecular target | Dose in patients (mg/day) | Brain penetration (% of CSF to plasma levels) in patient* | Brain penetration (% of brain to plasma ratio) in pre-clinical model* | Response rate in BM patients (%) | Transporter effect | Reference |
|---|---|---|---|---|---|---|---|
| Gefitinib | EGFR-TKI | 750–1000 | 1.07–3.58 | 27 | 27% | P-gp substrate | (109,192) |
| Erlotinib | EGFR-TKI | 150 | 2.77–5.1 | 13.7 | 82.4% (EGFR mutation) | P-gp and Bcrp substrate | (93,108,193) |
| Afatinib | EGFR-TKI | 50 | 0.7 | ND | 35% | P-gp substrate | (114,194) |
| Osimertinib | EGFR-TKI | 80 | NA | 180 | ND | P-gp and Bcrp substrate | (115) |
| AZD3759 | EGFR-TKI | 100–1000 | 1 1 1 | 282 | 83% | ND | (117) |
| Crizotinib | ALK-TKI | 500 | 0.26 | 23 | 18–33% | P-gp substrate but not Bcrp | (100) |
| Alectinib | ALK-TKI | 200 | 0.3 | 63–94 | 52% | Not a P-gp substrate | (127–129) |
| Ceritinib | ALK-TKI | 400 | ND | 15 | 34.5–58.8% | P-gp and Bcrp substrate | (132,195,196) |
| Brigatinib | ALK and EGFR TKI | 300 | ND | ND | 53% | ND | (197) |
| Lorlatmib (PF-06463922) | ALK-TKI | 100 | ND | 64 | ND | Not a P-gp substrate | (133) |
| Entrectinib | ALK-TKI | ND | ND | 43 | ND | ND | (134,198) |
(ND, not determined; NA, not available)
Total drug concentrations are reported
Table III.
Brain and Transporter Related Features of Molecularly Targeted Therapy for Melanoma
| Compound | Molecular target | Dose in patients (mg/day) | Brain penetration (% of CSF to plasma levels) in patient* | Brain penetration (% of brain to plasma ratio) in pre-clinical model* | Response rate in BM patients (%) | Transporter effect | Reference |
|---|---|---|---|---|---|---|---|
| Vemurafenib | BPAF inhibitor | 960 (b.i.d.) | 0.98 | 0.012 | NA | P-gp and Bcrp substrate | (144,199) |
| Dabrafenib | BPAF inhibitor | 150×2 (b.i.d.) | ND | 4.4 | 71–78 | P-gp and Bcrp substrate | (70,149) |
| Cobimetinib | MEK inhibitor | 60 | ND | 8 | Under investigation () | P-gp substrate (Not Bcrp) | (154) |
| Trametinib | MEK inhibitor | 2 | ND | 0.28 | NA | P-gp substrate, but not Bcrp | (71) |
| E620I | MEK inhibitor | ND | 270 | NA | Minimal effect with P-gp and Bcrp | (200) |
(ND, not determined; NA, not available)
Total drug concentrations are reported
Table IV.
Brain and Transporter Related Features of Molecularly Targeted Therapy for Breast Cancer and Renal Cancer Cell
| Compound | Molecular target | Dose in patients (mg/day) | Brain penetration (% of CSF to plasma levels) in patient* | Brain penetration (% of brain to plasma ratio) in pre-clinical model* | Response rate in BM patients (%) | Transporter effect | Reference |
|---|---|---|---|---|---|---|---|
| Lapatinib | EGFR and HER2 | 1250 | 0.11 | 3 | 6% | P-gp and Bcrp substrate | (99,167,171) |
| Trastuzumab | HER2 | NA | 0.24 | ND | 0.5 | NA | (201) |
| Rucaparib | PARP inhibitor | 40 | ND | ND | P-gp and Bcrp substrate | (97) | |
| Olaparib | PARP inhibitor | 400 × 2 (b.i.d.) | ND | 1.1# | ND | P-gp substrate | (202) |
| Veliparib (ABT-888) | PARP inhibitor | 400 × 2 (b.i.d.) | ND | less than 5% | ND | P-gp and Bcrp substrate | (95) |
| Talazoparib (BMN-673) | PARP inhibitor | 1 | ND | 2 | ND | P-gp substrate, but not Bcrp | (203) |
| Niraparib | PARP inhibitor | 300 | 10–52+ | 85–99 | ND | NA | (173) |
| Vorinostat | HDAC inhibitor | 360 | ND | 4 | ND | P-gp and Bcrp substrate | (176) |
| Sunitinib | TKI | 50 | ND | 42 | 12 | P-gp and Bcrp substrate | (96,182) |
| Sorafenib | Multi-kinase inhibitor | 400×2 (b.i.d.) | 0.02–3.4+ | 9.4 | ND | P-gp and Bcrp substrate | (204) |
| Axitinib | VEGFR inhibitor | 5 mg × 2 (b.i.d.) | ND | Less than 10% | ND | P-gp and Bcrp substrate | (205) |
(ND, not determined; NA, not available)
Total drug concentrations are reported
Unpublished data
in non-human primate
Another key issue for the treatment of brain metastases is the difference in gene expression profiles in tumor cells growing in the brain microenvironment compared to the peripheral (non-brain) lesions (15). The local tumor microenvironment between the brain and peripheral tumor lesions can potentially dictate that such differences will ultimately result in the development of resistance to therapies. This is a critical issue that needs to be tackled along with brain drug delivery to effectively treat tumors in the brain. While we recognize the importance of microenvironment in the context of resistance, the main focus of this review is to discuss aspects related to the delivery of targeted agents to the brain. We will give a brief overview on the clinical presentation of brain metastases as well as the currently available therapeutic options, before describing the difficulties encountered in the treatment of brain metastases.
Clinical Presentation of Brain Metastases
Brain metastases, a devastating complication of systemic malignancies, substantially raise the burden of cancer morbidity and mortality (1,2). They typically stem from hematogenous spread that seed at the distal fields of the main cerebral arteries (the “anatomic watershed areas”) (16). Consequently, approximately 80% of brain metastases are localized in the cerebral hemispheres (16). Initial symptoms at diagnosis range from headaches and seizures to focal neurological deficits and cognitive dysfunction; however asymptomatic brain metastases are also commonly found during initial staging exams. The symptoms presented by the patients often depend on the location of lesions and extent of metastatic disease burden (16).
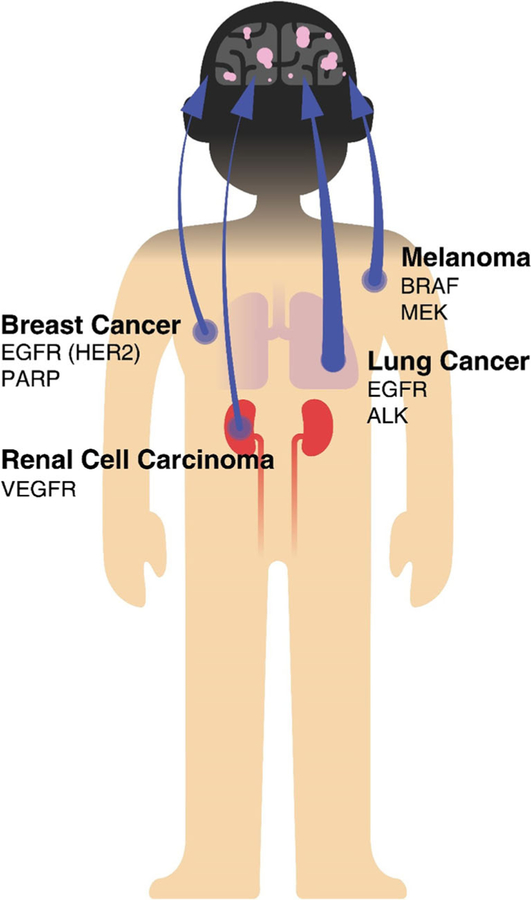
Population studies underestimate the true incidence rates of brain metastases because of issues related to diagnosis and underreporting. The incidence of brain metastases, observed in 8.5–9.6% of cancer patients, is estimated to be approximately ten times higher compared to primary brain tumors that represent 1.4% of cancer patients (17–20). Lung cancer, breast cancer, melanoma and renal cancer have a high propensity to metastasize to the brain and account for up to 80% of brain metastases (Table I and Fig. 2) (21). Patients with lung cancer are likely to develop brain metastases during the course of the disease (reported to be 16.3–19.9% of lung cancer patients (17,18)). This incidence can be as much as 50–60% in other reports, depending on individual study methods and analyses (22,23). Small cell lung cancer (SCLC) is known to be associated with a slightly higher occurrence of brain metastases than non-small cell lung cancer (NSCLC) at 5 years after the diagnosis, but the incidence rates of brain metastases for both subtypes tend increase over the course of the disease (22,23). The treatment of SCLC patients with prophylactic intracranial radiation following completion of definitive radio/chemotherapy markedly reduces subsequent risk of brain metastases and is associated with a survival benefit (24,25). Incidence of brain metastases from breast cancer is second to that of lung, even though only 5% breast cancer patients develop brain metastases, due to the high overall incidence of breast cancer (21,26,27). Autopsy series reveal brain metastases in about 30% of patients dying from breast cancer (28). Melanoma accounts for 6–11% of all metastatic brain lesions, and is the third most frequent cause of brain metastases. The observations from clinical and autopsy series estimate that the incidence of brain metastases in patients with malignant melanoma ranges from 10 to 70% (29–32). While lung cancer is reported to have the largest proportion of incidence of brain metastases, melanoma has the highest predilection to metastasize to the brain (21).
Table I.
Incidence Rate of Brain Metastases
Fig. 2.
Primary tumors that preferentially metastasize to the brain and the occurrence of brain metastases from each of these primary tumors. Examples of key tumor driving oncogenes are represented for each tumor type.
Several reasons contribute to the rising incidence of brain metastases. Advanced imaging techniques have improved the detection of occult brain metastases. Preliminary MRI screening for brain lesions is now routinely practiced for patients with newly diagnosis advanced lung cancer even in the absence of neurologic symptoms (21,24). Another possible reason for the increased incidence of brain metastases is that the median survival of patients with peripheral (non-brain) tumors is prolonged due to the novel therapeutic agents, that introduces the time it can take for the peripheral tumor cells to spread to the brain and thus, cause metastatic lesions in the brain (20,33). The restricted entry of systemically active therapeutic agents into the brain is another contributor to the increasing incidence of brain metastases because it creates pharmacological sanctuary that nurtures and protects the tumor cells to thrive in the brain (13,14). Thus, to advance drug therapies that are effective for controlling brain metastases, the field needs to consider that the BBB is likely intact in some regions of the tumor, especially in early non-contrast enhancing micrometastases, and can compromise the penetration of anti-cancer agents across the BBB (14,34,35).
Current Therapy for Brain Metastases
The initial treatment of brain metastases involves the management of acute symptoms. For instance, the use of glucocorticoids to alleviate symptoms secondary to brain edema, and anti-epileptic drugs (AEDs) to treat seizures. However, coadministration of AEDs and anti-cancer agents can increase the potential risk of clinically significant drug-drug interactions because of the shared metabolic pathways or transport systems across these different drug regimen (36,37). Some AEDs can modulate the hepatic cytochrome (P450) enzymes and/or expression of drug transporters, causing an increase or decrease of systemic drug exposures, each of which can have consequences on resulting efficacy or toxicity. Such a complex interplay between transporters and drug-metabolizing enzymes (38,39), along with reduced drug delivery to the brain, can result in undesirable therapeutic outcomes in patients with brain metastases.
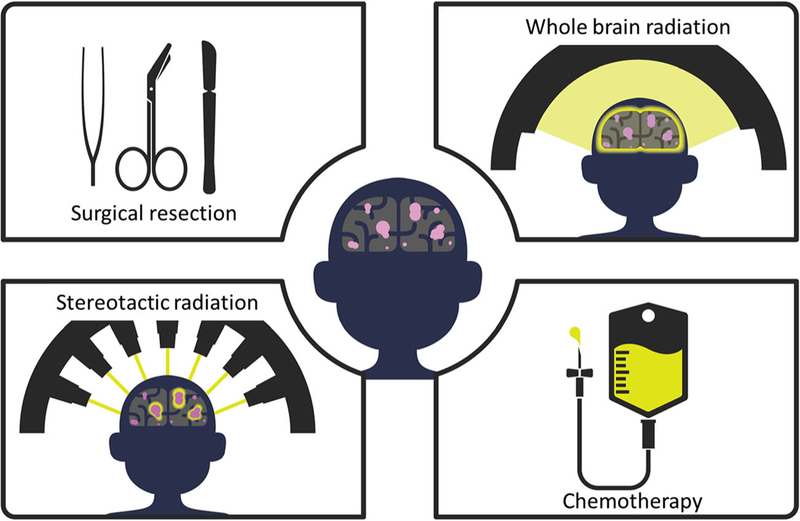
Surgical resection is the preferred treatment option, in part because as yet only surgery can drastically reduce the tumor mass in the brain (40) and improve survival outcome (Fig. 3. Treatment options). In cases where surgical resection is not feasible due to multiple low-volume metastases or inaccessible or eloquent locations where tumor cannot be surgically resected, alternative approaches such as stereotactic radiosurgery (SRS) and whole brain radiation therapy (WBRT) are considered. SRS delivers high-dose of radiation to a specific region, whereas WBRT delivers to the whole brain. While surgery or SRS can provide a high rate of durable local tumor control, both of these strategies do not reduce the significant risk of developing additional brain metastases. In contrast, WBRT can reduce the risk of intracranial relapses, but the lower dose applied to the entire brain associated with this treatment failed to improve overall survival and is associated with a risk of neuro-cognitive impairment (41).
Fig. 3.
Depiction of the most common treatment options for brain metastases.
Systemic therapies have historically been considered ineffective against brain metastases. However, examples of limited success with systemic therapies have been reported in patients with brain metastases. For example, in patients with chemotherapy-naive brain metastases, the combination of cisplatin and etoposide achieved an overall objective response rate of 38% in breast cancer and 30% in NSCLC (42). Also, treatment with a combination of carboplatin and pemetrexed exhibited an overall response rate of 40% in patients with chemotherapy-naive brain metastases from NSCLC (43). In addition to these studies, several clinical trials also report limited efficacy from using a combination therapeutic approach in order to treat various types of cancer. The phase II LANDSCAPE trial, a combination therapy with lapatinib and capecitabine resulted in an objective partial response rate of 66% in patients with brain metastases from HER2-positive breast cancer (44). The phase II BREAK-MB study showed that in patients with V600E BRAF mutant melanoma meta-static to the brain, dabrafenib had an overall objective response rate of 39% in treatment naive patients, and 31% in patients that were previously treated (45). An open-label pilot study with vemurafenib reported an objective response rate of 42% in patients with non-resectable, symptomatic brain metastases from BRAFV600 mutation-positive melanoma that were previously treated (46). However, there were no overall survial benefit with these therapeutics, even though there have been partial responses. Lapatinib, dabrafenib and vemurafenib, are all substrates for both P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), and the observed limited therapeutic responses might be in part due to transporter-mediated efflux and consequential restricted drug delivery to the brain metastases (Tables II, III, and IV). Transporter-mediated efflux and the consequential poor brain penetration across an intact BBB can severely limit the effectiveness of targeted therapies used in the treatment of brain tumors (13,14). Also, a heterogeneous brain-tumor barrier permeability can lead to non-uniform drug distribution in various sites of brain metastases adding another level of complexity in the treatment of brain metastases (11,47,48).
Barriers to Effective Treatment
Limitations in diagnostic imaging for early brain metastases are an obstacle for early detection and diagnosis of the disease. Magnetic resonance imaging (MRI), the most commonly used imaging method, utilizes a hydrophilic contrast dye, gadolinium diethylene-triamine-pentaacetate (Gd-DTPA). However, Gd-DTPA was reported to fail to visualize early-stage brain metastases that have little angiogenesis and BBB disruption, which will be problematic for early diagnosis of brain metastases (49), due to its poor distribution to the brain across an intact BBB. Moreover, a recent study has shown that repeated exposure to gadolinium-based contrast agents can lead to an increase in gadolinium deposition in the endothelium and neuropil even in the absence of intracranial abnormalities, that can significantly interfere with the MR signal (50,51). Therefore, it is difficult to rely on Gd-DTPA contrast enhancement to identify brain metastases, especially small-volume lesions, or “micro-metastases” (52).
The exclusion of brain tumor patients from clinical trials is yet another barrier to develop effective treatments for brain metastases. Since metastatic brain lesions may be difficult to control, associated with significant morbidity, and are associated with a dire prognosis, clinical trials often specifically exclude patients with known brain metastases. In a meta-analysis of 413 NSCLC clinical trials, only 31% of the industrysponsored and 16% of university/investigator-sponsored trials allowed inclusion of patients with diagnosed brain metastases (53). For cancer other than NSCLC, the majority of oncology clinical trials have excluded patients with brain metastases. Even those trials that enrolled this patient population, the outcome criteria have not always been clinically relevant. The recent review article by the Neuro-Oncology Brain Metastases (RANO-BM) working group reported in Lancet Oncology that many clinical trials for brain metastases utilized inconsistent endpoint criteria across trials that can limit interpretation across these studies (54). As a result, patients with brain metastases are often left with limited novel treatment options and are unable to participate in clinical trials testing potentially useful therapeutic treatments. The question that arises is: why are drugs that prove to be effective against peripheral metastases not as effective against brain metastases?
An important problem with regard to the pharmacological treatment of brain metastases is that many chemotherapeutic agents do not efficiently cross the blood-brain barrier (BBB) and blood-tumor barrier (BTB), and are unable to mount a pharmacodynamic impact on their target of interest (47,55). The structure of BTB is basically the BBB where the tumor exists, and can have “normal” BBB structure and function, especially in case of brain metastases that grow from small lesion at multiple locations, although the integrity of the barrier, i.e., the BTB, can be variable depending on the size and characteristics of tumor (11,47,56). The desired pharmacodynamic impact (efficacy) cannot be achieved without appropriate pharmacokinetic considerations (exposure). Inaccessibility of potentially effective anti-cancer agents to the parenchymal brain metastases can create a pharmacologic sanctuary where drugs that are otherwise effective against peripheral metastases fail to control brain metastases. Both the BBB and BTB in brain metastases have a unique anatomical barrier comprised of endothelial cells that establish robust tight junction between cells and express a variety of efflux transporters, which are absent in the peripheral microvasculature. P-glycoprotein (P-gp) and Breast cancer resistant protein (BCRP), the two most highly expressed efflux transporters at the BBB, can efflux a wide range of drug molecules and restrict drug delivery to the brain. Numerous studies have shown that many compounds tested in clinical trials are substrates of P-gp and/or BCRP that have often failed to show clinical efficacy against brain metastases. As this discussion indicates, a thorough knowledge of the BBB and BTB in brain metastases, and how selected agents interact with these barriers, is critical in understanding the success or failure of pharmacological treatments that employ one or more of these targeted therapies.
Heterogeneous BBB and/or BTB integrity is a drug-delivery related challenge in terms of optimizing efficacy of targeted agents for the treatment of brain metastases. Several studies report variable intra-tumoral BBB permeability that results in non-uniform drug distribution and compromised drug efficacy (55). The study by Lockman et al. evaluated the pharmacodynamic effect resulting from heterogeneous intratumoral drug distribution of doxorubicin or paclitaxel in an experimental brain metastases of breast cancer (11,56). Each of these drugs reached a cytotoxic concentration (i.e., cleaved caspase-3 staining) in the “leaky” BTB regions, but significantly sub-therapeutic concentrations in the areas of intact BTB. In these models, there was no hindrance of drug delivery to the peripheral (non-brain) metastases with drug concentration more than 10-fold higher than the brain metastases. The overall outcome of non-uniform intratumoral distribution in the brain metastases was that each of these drugs failed to reduce tumor burden in in vivo animal studies (11).
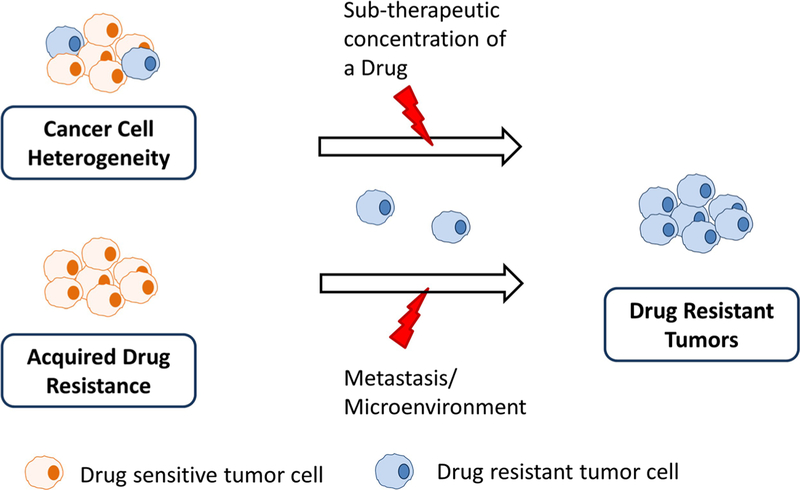
Besides an anatomical barrier that is unique to the brain, treatment of brain metastases may be further complicated by the oncogenic footprints that are distinctive to the brain. Brain metastases, sharing a common ancestor with the primary tumor of origin, have evolved independently after reaching the brain, in part, because the brain has a different microenvironment than the rest of the body (15). A whole exome sequencing study by Brastianos et al. identified mutational signatures that are distinctive to brain metastases compared to their corresponding primary tumors, and 53% of the examined cases displayed such a phenomenon (15). In this context, analysis of peripheral tumor tissue biopsies could misguide the choice of molecularly-targeted therapies for the treatment of brain metastases. Moreover, a suboptimal drug concentration in the brain metastases could promote emergence of drug resistance, adding to the challenge of treating brain metastases. (Fig. 4).
Fig. 4.
Mechanisms of resistance development against drug in brain metastases due to inherent tumor heterogeneity or acquired resistance.
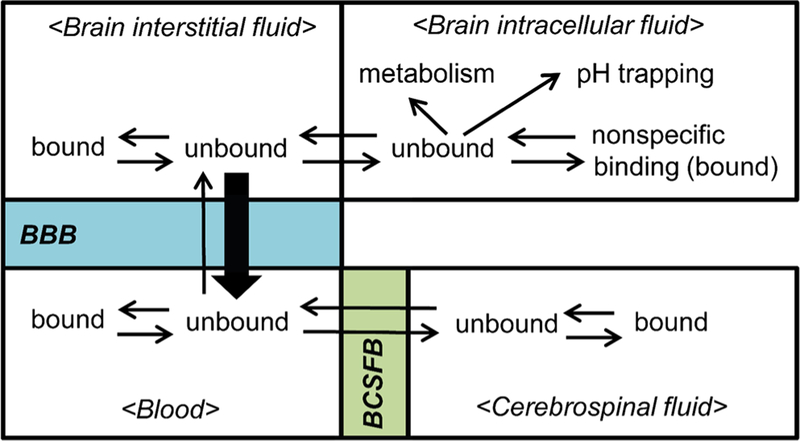
Determination of brain drug exposure, especially in patients, is also challenging when choosing proper therapeutics for the treatment of brain metastases. Cerebral spinal fluid (CSF) is frequently thought to be surrogate of a concentration in the brain drug exposure. However, CSF concentration may not represent brain concentration for many molecularlytargeted agents that are substrates of efflux transporters, such as P-gp and/or BCRP. The CSF concentration is influenced by CSF turnover, extracellular fluid (ECF) bulk flow, and drug transporters at the blood-CSF barrier (BCSFB) (Fig. 5) (57–60). Specifically in contrast to the BBB, drug delivery across the BCSFB is unhindered by P-gp and BCRP, because the blood side of choroid epithelial cells lack P-gp and BCRP expression (61,62). For lipophilic compounds that readily diffuse across the choroid epithelial cells, the CSF concentration can overestimate the actual brain exposure (61). The study by Kodaira et al. showed that unbound CSF-to-brain concentration ratios of many compounds that are substrates of P-gp and BCRP were lower in Mdr1a/1b(−/−)Bcrp (−/−) mice compared to the wild-type, indicating that there can be discrepancies between the unbound CSF concentration and the unbound brain concentration (Cu,brain) for P-gp and Bcrp substrates (61). Considering the structural and functional differences in the blood/brain versus the blood/CSF interface, CSF concentrations are often a poor predictor of brain drug exposure.
Fig. 5.
Multiple equilibria in different compartments in the brain, blood, and cerebrospinal fluid.
Microdialysis, a standard method to directly measure unbound brain interstitial fluid concentration (Cu,brain), currently has limited applications in patients (59,63–65). Many anticancer agents are often lipophilic, so that practical problems can rise with using microdialysis due to high nonspecific binding (59) and poor drug recovery (59). In an attempt to bypass such challenges, the brain homogenate and brain slice techniques have been developed to estimate the fraction unbound of drug in the brain. Each of these methods, used in combination with total brain concentrations, has demonstrated to be useful in obtaining surrogates of microdialysis Cu,brain. The study by Friden et al. showed that brain homogenate method predicted the Cu,brain from microdialysis measurements within a 3-fold error for 10 out of 15 compounds (66). In the same study, the brain slice method predicted Cu,brain for 14 out of 15 com pounds within a 3-fold error. These findings indicate that, for some compounds, either the brain slice or homogenate method can reasonably estimate the Cu,brain. Recently there was a development in expanding the analysis of the homogenate equilibrium dialysis method to determine unbound fraction of compounds, that might be useful for drug compounds with high protein binding (67). These techniques can allow determination of “active” free concentrations in different regions of the brain, including important regions of a growing brain tumor, such as the core of the tumor and the growing edge (68).
The application of the brain homogenate method has been readily employed in order to evaluate brain drug distribution in the CNS drug development. Traditionally, the brain-to-plasma ratio (Kp), which is the ratio of total brain concentration to total plasma concentration, has been considered to represent the degree of brain drug distribution. However, Kp,uu, the ratio of free brain concentration to free plasma concentration, has recently been introduced as a measurement of brain drug delivery that allows assessment of BBB transport mechanisms, separate from the influence of nonspecific binding in the brain (69). According to free drug hypothesis, only unbound drug concentration is considered to be a “pharmacologically active” concentration. The studies that examined brain penetration of molecularly-targeted agents have utilized both Kp and Kp,uu parameters (70,71), and are useful in assessing the effect of efflux transporters in brain drug delivery, but the latter allows differentiation of bound vs. unbound drug and may correlate better with efficacy. This has been recently discussed in the context of brain tumors by Heffron (72,73).
The evaluation of brain drug penetration has no single Kp or Kp,uu value that represents “optimal” brain penetration (69). The articles by Doran et al (2005) reported that a set of 32 commonly prescribed CNS drugs have a wide range of Kp (0.1 to 24), indicative that a compound having Kp of 0.1 can be still efficacious (69). This 240-fold difference in Kp indicates that Kp alone is inadequate to characterize brain drug delivery (69). On other hand, for the same set of CNS drugs, there is a 34-fold difference for Kp,uu (74), indicating that nonspecific tissue binding can affect assessment of Kp and brain drug penetration (69). Even though there are limited studies that explore a direct relationship between Kp,uu and pharmacodynamic effect for the treatment of brain metastases, higher values of Kp and/or Kpuu often serve as parameters to indicate more advantageous delivery of drug across the blood-brain barrier (72,73).
Mechanisms that Limit Drug Delivery across the BBB
The blood-brain barrier (BBB) is a specialized structure of the cerebral microvasculature (Fig. 1), comprised of a complex network of cells, extracellular matrix, and proteins that regulate solute and xenobiotic transport into and out of the brain. Given this complexity, and the fact that the BBB is dependent on signaling from the neuronal environment, the BBB is now frequently referred to as the neurovascular unit (Fig. 1). The NVU contains numerous cell types, including vascular endothelial cells, pericytes, glial cells, and neurons (75,76). Pericytes mainly provide both structural and regulatory support of BBB and also contribute to the regulation of angiogenesis, neuroinflammation and stem cell activity (75). Astrocytes are cells that play an important role in regulation of BBB tight junctions, expression and localization of transporters and formation of specialized enzymes (77,78). Unlike the endothelium of many peripheral organs, the endothelial cells forming the capillaries of the brain are held together by tight junctions and adherens junctions that prevent the paracellular transport of most blood-borne substances to the brain (79). Adherens junctions are formed between vascular endothelial cadherins and initiate the contact between adjacent endothelial cells (79). Tight junctions are consisted of transmembrane proteins such as occludin, claudin, and junctional adhesion molecule (JAM) that interact with cytoskeletal proteins and recruit membrane-associated cytoplasmic proteins (12). Transporters at the BBB are important in maintaining the brain homeostasis and providing the brain with the necessary nutrients for normal function, while protecting the brain from toxic substances that may be in the systemic circulation (80). Solute carriers (SLCs) and ATP-binding cassette transporters (ABC transporters) are two major families of transporters at the BBB (81). These transporters play a crucial role in selectively moving specific substances (substrates) into or out of the brain depending on their physicochemical and structural properties, and often coordinate (80) with each other to efflux potentially toxic compounds out of the brain (82–84).
The SLC transporters are known to transport many polar compounds, including glucose and amino acids, that are essential nutrients for cell survival. However, this group of transporters is also involved in transporting organic anions (85). Organic anion transporters (OAT) and organic anion transporting polypeptides (OATP) are some members of this family, and are known to be present at the BBB (81). Transporters in this group can be unidirectional or bidirectional and are located in abluminal or luminal side of endothelial cells. Most of them have broad substrate specificity and some of substrates overlap with those of ABC transporters, which may indicate their coordinate function (86–88).
ABC transporters play a major role as active efflux pumps, consuming ATP, to limit the distribution of toxic substances in the CNS (83,84,89). P-gp and BCRP are the examples of efflux pumps which have broad substrate specificity and are able to actively transport substrates back into systemic circulation (80). In addition to P-gp and BCRP, multidrug resistance-associate proteins (MRPs) can also be involved in actively limiting the brain delivery of certain drugs (90,91). In the treatment of brain tumors, the BBB often limits the brain distribution of therapeutic agents through the efflux activity of ATP binding cassette (ABC) transport proteins (70,80,92–100).
Impact of Transporters on the Treatment of Brain Metastases
The transport of a drug from the systemic circulation to the brain parenchyma is often depicted as a multi-step process. Initially, cerebral blood flow carries a drug compound to the brain and the drug is considered to be in equilibrium between the bound and unbound state in plasma, and the unbound drug is available to penetrate across the plasma-tissue barriers. Drug distribution to the brain at the blood-brain interface is regulated by the blood-brain barrier (BBB), blood-cerebrospinal fluid barrier (BCSFB), and other physiological systems in the brain (63,101). Upon successful drug delivery across the relevant anatomical barrier, the unbound (free) drug in the brain interacts with the target site and elicits pharmacological activities (59,61,63). Such unbound drug concentration in the brain interstitial fluid (Cu,brain) is assumed to represent a pharmacologically active concentration. However, if a drug compound has an affinity for efflux transporters, the drug can be “pumped” back into the vasculature before interacting with its intended target (Fig. 5) (63).
Many molecularly-targeted agents examined in clinical trials for brain cancer have been reported to be substrates of P-gp and/or BCRP (see Table II) (70,80,96–98,102,103). Even though there has been some discussion that class I compounds in the Biopharmaceutics Drug Disposition Classification System (BDDCS) that have high solubility and high permeability may be less affected by efflux transporters (104). These active efflux transporters at the BBB are known to prevent anticancer therapeutics from reaching parenchymal tumor cells, especially those from micro-metastases that may reside behind an intact BBB (72). Genetic knockout mice lacking transporters and transfected cell lines overexpressing transporters (MDCK-II cell line, or Madin-Darby Canine Kidney Epithelial cell line) are widely utilized in vivo and in vitro models to evaluate the substrate status of an investigational compound for P-gp and BCRP in the preclinical setting (70,96–98).
COMMON TYPES OF BRAIN METASTASES
Lung Cancer
Lung cancer, a prevalent tumor with about 222,500 new cases in 2017 in US, according to the American Cancer society’s estimate, is the leading cause of brain metastases. Approximately 20% of lung cancer patients will eventually develop brain metastases (17). The epidermal growth factor receptor (EGFR) and the anaplastic lymphoma kinase (ALK) are the most well-known and well-developed drug targets to treat lung cancer, especially in non-small cell lung cancer (NSCLC) patients (105,106). Over 45% of the patients with EGFR+ or ALK+ have brain metastases at some stage of disease (107). The three most commonly-used and studied EGFR tyrosine kinase inhibitors (TKIs) are gefitinib, erlotinib, and afatinib. Gefitinib and erlotinib are first-generation EGFR TKIs and their CNS delivery is reported to be very limited (1.07–3.58% for gefitinib and 2.77% for erlotinib, see Table II) (108), mainly due to the efflux transporters, P-gp and BCRP (93,109–111). However, despite this low brain penetration, these first-line TKIs seem to modestly reduce the risk of CNS progression when compared to standard chemotherapy (112). Therefore, erlotinib and gefitinib may have a prophylactic effect on brain metastases. Afatinib is a second generation inhibitor, which irreversibly blocks signaling from EGFR, HER2, ErbB3, and ErbB4 (113). While the CSF to plasma ratio of afatinib was reported to be extremely low in patients, less than 1%, due to active efflux by P-gp (114), a study showed that response rate to afatinib in the patients with or without CNS metastases were similarly efficacious, possibly due to the high potency of the compound (114). Osimertinib (AZD9291) is a recently approved drug to treat patients with T790 M resistant mutant NSCLC showed pre-clinical evidence that it can penetrate the BBB (115). Moreover, in a clinical trial, osimertinib has shown efficacy in patients with EGFR-driven NSCLC lung metastases in the CNS (116). Another EGFR inhibitor, AZD3759, has been specifically designed to penetrate the BBB (117), and efficacy in patients with NSCLC with brain metastases is under current clinical investigation (118).
ALK is another potent target that is constitutively active due to gene rearrangements in approximately 2 to 7% of lung cancer patients (119–121). Crizotinib, the first ALK targeted TKI, has been reported to have poor brain penetration based on a low CSF-to-plasma ratio (0.26%) in humans (122), mainly due to P-gp (100). It has been reported that the efficacy of crizotinib in the CNS seems to be limited, even though the overall objective response rate and the median duration of response were significantly improved with crizotinib treatment when compared to standard chemotherapy (123–125).
Alectinib and ceritinib are the second generation of ALK inhibitors that demonstrate their activity in crizotinib-resistant patients. Alectinib also inhibits RET, an oncogene involved in the development of several human cancers, including NSCLC (126). Several clinical studies that include patients with brain metastases at baseline have shown that alectinib has some efficacy in CNS tumors (127,128), possibly due to the fact that it is not a substrate of major efflux transporters (129). These efficacy results are in line with its high brain penetration in pre-clinical rat model (63–94%, Table II). However, the mean ratio of CSF concentration to the plasma concentration in patients was approximately 0.3% (128), which again suggests that concentration measured in CSF may not represent the concentration in brain or at the target site. (Fig. 5).
Ceritinib is another potent ALK inhibitor that showed some efficacy with intracranial metastases in a preclinical rat model with a brain to plasma ratio of 15% (130,131). This brain penetration may seem higher than expected considering ceritinib is a substrate of both P-gp and Bcrp. Nevertheless, the ASCEND study for advanced ALK-positive NSCLC has shown that ceritinib has efficacy against brain metastases, based on both the response and disease control rates (132).
Several other therapeutic agents are under clinical investigation to examine their efficacy in lung cancer patients with CNS metastases, including brigatinib, lorlatinib, entrectinib, cabozantinib, and tesevatinib. Brigatinib, a second generation ALK inhibitor, potently inhibits both ALK and EGFR.
According to a recently reported phase I/II clinical trial that included patients with CNS metastases, brigatinib has shown efficacy against brain disease (response rate 53% and PFS of 97 weeks).
Recently developed as third generation ALK inhibitors, lorlatinib and entrectinib are specifically designed to improve brain penetration, and a clinical trial is currently recruiting lung cancer patients for their first efficacy evaluation in patients. Lorlatinib is structurally designed to have a low affinity for P-gp (133). Based on an analysis to examine the influence of physicochemical properties on p-glycoprotein affinity, lorlatinib was intentionally designed to have logD range of 2–3 and minimal introduction of hydrogen bond donors (133). To minimize the hydrogen bond donors, intramolecular hydrogen bonds are introduced in the adjacent ether oxygen in the molecule. This chemical design aimed to have enhanced intrinsic permeability and to avoid efflux transporter liability, allowing lorlatinib to target brain metastases (133). Entrectinib has also been shown to have high brain penetration in nude mice (43% brain concentration to plasma concentration ratio) (134). While we await results from the clinical trials regarding improved efficacy, the fact that intentional structure-delivery efforts are being made bodes well for the development of compounds that will be efficacious against brain tumor cells that are behind an intact BBB.
Melanoma
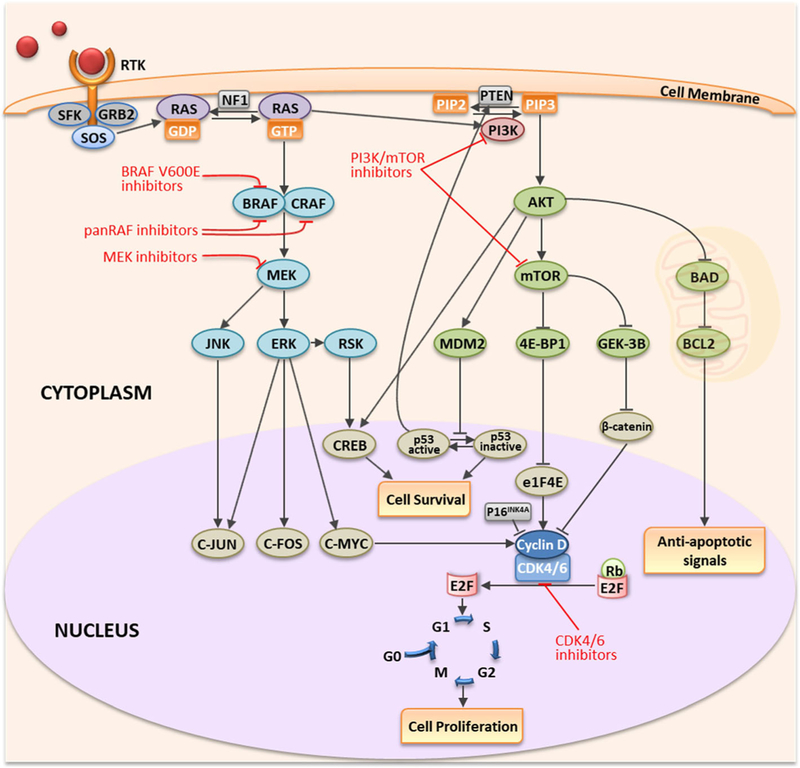
Melanoma is a lethal form of skin cancer with a projected diagnosis of 87,110 patients in the United States for the year 2017, with close to 9730 deaths expected in the US (American Cancer Society 2017). The most common peripheral sites of melanoma metastases are lung, liver, bones and brain. Melanoma has the second highest prevalence of brain metastasis followed by lung cancer with an overall incidence of 5%–8% (135). Metastases in the brain have been identified in 55–75% of melanoma patients at autopsy, indicating a high tropism of melanoma to metastasize to the brain (31,32). Melanoma patients with metastatic disease that has spread to the brain are associated with a poor median overall survival of less than 6 months (136,137). Approximately 40–60% of melanoma patients have a mutation in the serine threonine kinase v-RAF murine sarcoma viral oncogene homolog B1 (BRAF-mu) (138). This oncogenic mutation results in sustained activation of the mitogen-activated protein kinase (MAPK) oncogenic signaling pathway (Fig. 6) (139–141). Therefore, the development of inhibitors specifically targeting BRAF-mu isoform was of significant interest in the treatment of melanoma. Vemurafenib, a FDA approved BRAF-mu inhibitor for the treatment of late-stage melanoma, showed an improvement in the 6-month overall survival rate by 20% when compared to dacarbazine chemotherapy with a response rate of approximately 50% (142,143). However, the results of preclinical studies show that the brain penetration of vemurafenib is limited due to active efflux by P-glycoprotein and Bcrp (141,144). A few patient case studies show that vemurafenib may have a potential efficacy to induce remission of brain metastases (46). This partial efficacy may be related to factors such as the size of the tumor and the degree of disruption of the BBB (145,146). A review of the clinical studies and case reports related to vemurafenib suggests that the effectiveness of vemurafenib seems to be limited in intracranial tumors, but it may provide successful therapeutic outcomes in a subset of patients depending on the tumor characteristics, the stage of tumor progression and other unknown factors (46,147) (, ).
Fig. 6.
Signaling pathways and oncogenic targets of molecularly-targeted therapeutics for melanoma.
Another BRAF-mu inhibitor, dabrafenib, showed notable benefits in a phase 1 dose-escalation study in V600E BRAF-melanoma patients with untreated brain metastases (148). Nine out of 26 patients with brain metastases showed intracranial tumor size reduction, and four of them had complete tumor regression (148). Another study performed with a total of 23 patients from a single institution has reported that the response rate in intracranial disease was 78% and that in extracranial disease was 90% in BRAF-mutant melanoma patients with brain metastases (149). According to the study results, both intracranial and extracranial disease appear to respond similarly to dabrafenib. However, importantly, dabrafenib is known to be a substrate of both P-gp and Bcrp, with a brain to plasma ratio in normal mouse brain of 2%. (70). Currently, several clinical studies are ongoing to examine the efficacy of dabrafenib in combination with stereotactic radiosurgery and other therapeutics (www.clinicaltrials.gov). As data from these trials become available, it will be critical to establish if the responses are durable and to examine reasons for relapse. Insufficient drug distribution to areas of brain metastases that have an intact BBB replete with efflux transporters, may be related to disease progression.
Another target in the MAPK pathway that is important in metastatic melanoma is MEK (150). Since MEK is a signaling molecule that is downstream of BRAF (Fig. 6), MEK inhibitors can overcome the resistance developed against BRAF inhibitors (151,152). Trametinib and cobimetinib are FDA approved MEK inhibitors for the treatment of melanoma. Combinations of dabrafenib/trametinib and vemurafenib/cobimetinib have also received FDA approval. Dabrafenib/trametinib combination therapy showed an improvement in progression free survival compared to monotherapy in melanoma patients (151). Cobimetinib/vemurafenib combination also showed a significant improvement in progression free survival in patients with BRAF V600-mutant metastatic melanoma (153). However, both trametinib and cobimetinib are substrates of p-glycoprotein (71,154). There are reports that suggest that MEK inhibitors in combination with BRAF inhibitors or RT may improve survival rates in patients with brain metastases, despite the fact that the brain delivery of both trametinib and cobimetinib can be limited due to active drug efflux in areas of metastases with an intact BBB (155,156). Currently, clinical trials recruiting patients with brain metastases to examine their clinical efficacy in combination with BRAF inhibitor or RT are underway (, , ). Preliminary data from the study in patients with melanoma brain metastases implies that a combination of dabrafenib and trametinib with SRS may show improvements in survival compared to treatment with dabrafenib alone (157). As stated above for dabrafenib, durable responses may be limited by progression of tumor in areas with an intact BBB, and if disease progression occurs, it will be important to investigate why.
Immune modulation using monoclonal antibodies (mAbs) directed towards cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) (e.g, ipilimumab) and programmed cell death receptor (PD-1) (e.g., nivolumab) is an emerging strategy to manage tumors, especially in melanoma. Immune checkpoint inhibitors can cause activation and proliferation of T lymphocytes, which can then attack tumor cells. The rationale for using immune checkpoint inhibitors for the treatment of brain tumors is that the activated T cells are able to gain access to the CNS and show responses (158,159). Their efficacy in the treatment of melanoma that has metastasized to the brain is critical to investigate, but there is limited evidence available to prove it in patients with brain metastases, due to the exclusion of patients with brain metastases in many clinical trials. In an open-label phase 2 clinical trial with melanoma and brain metastases study, ipilimumab proved its potency against small and asymptomatic brain metastases (160). In a different clinical study with asymptomatic brain metastases patients, ipilimumab has shown benefits in patients, who had failed or did not tolerate previous treatments (161). Lately, there are ongoing clinical trials using immune modulation as a single agent or in combination with RT or radiosurgery against brain metastases (, , ).
As we noted in the therapeutic agents for other tumors, many treatments for melanoma have limited access to the brain across the BBB due to efflux transporters. (Table III).
Breast Cancer
The incidence rate of brain metastases from breast cancer is different depending on the subtype. It is about 20% in triple-negative tumors, i.e., those that do not express hormone receptors, including estrogen and progesterone, and HER2, and increases to 25%–50% in HER2 positive tumors (162). Molecularly targeted therapeutics for HER2 positive breast cancer patients have been significantly improved over the last 2 decades, but the efficacy of these agents in patients with breast cancer brain metastases seems to be limited.
The most frequently prescribed therapeutic agent for HER2 positive breast cancer patients is trastuzumab, an anti-HER2 antibody (163). Trastuzumab has shown to be efficacious for systemic HER2 positive breast cancer, however patients receiving trastuzumab tend to have higher incidence of brain metastases (164). The brain penetration of trastuzumab across an intact BBB is very low, as expected for an antibody drug due to its large molecular weight (165). Retrospective research has shown that 50% of patients with CNS metastases responded to the trastuzumab, even though it is difficult to distinguish the systemic efficacy from CNS metastases specific efficacy, due to the limitations in a retrospective study that simply reports the overall objective response rates (166).
Lapatinib is a small molecule dual tyrosine kinase inhibitor of EGFR and HER2, and interestingly, its percentage brain penetration across the BBB is as low as trastuzumab. The ratio between the patient drug concentration in CSF and plasma averaging 0.11% (see Table IV) (167). Preclinical studies in mice have shown that P-gp and Bcrp coordinate with each other to limit the delivery of lapatinib across the BBB (99). Consistent with the heterogeneous integrity of the BBB in breast cancer brain metastases, the distribution of lapatinib is highly variable in breast cancer brain metastases (56,168). The efficacy of lapatinib as a single agent in brain metastases is modest (overall response rate: 21.4%) (169,170), but lapatinib in combination with capecitabine showed increased response rate toward CNS metastases (29.2%) (171).
The triple negative subtype is also prone to develop brain metastases and there are not many treatment options available for this subtype. Few molecularly targeted drugs are currently being tested for their efficacy in patients with triple negative tumors (172). One possible druggable target for this subtype is polyadenosine diphosphate ribose polymerase (PARP). There are several PARP inhibitors already approved and additional compounds are being tested in clinical trials, including niraparib, rucaparib, olaparib, veliparib (ABT-888), and talazoparib (BMN 673). Of these, niraparib showed good brain penetration in an in vivo rat model with brain to plasma ratio of 0.85–0.99 (173). On the other hand, the distribution of rucaparib to the brain is low, due to the effect of the major efflux transporters, P-gp and Bcrp (97). Veliparib showed significantly improved overall median survival in a phase I study when it was used in conjunction with WBRT (174), even though its brain penetration is reported to be low (less than 5%), due to both P-gp and Bcrp (see Table IV) (95).
Vorinostat is the first targeted drug specifically approved to inhibit histone deacetylase (HDAC), a novel target to treat breast cancer, and this compound showed some efficacy in preventing brain metastases in a mouse model with triple negative breast cancer (175). However the brain delivery of Vorinostat seems to be limited by efflux transport (176).
Because of the limited success in treating breast cancer brain metastases with molecularly targeted agents that have limited delivery across the BBB, there has been an emphasis on developing new brain-penetrant therapies. TPI-287 is a new brain permeable taxane that stabilizes microtubules (177) that is under clinical investigation as a treatment of breast cancer brain metastases and primary brain tumor (, , ). There are also several clinical trials evaluating novel compounds for b rain m etastases in cluding 4-dem ethyl-4-cholesteryloxycarbonylpenclome (DM-CHOC-PEN) (), eribulin mesylate (), cabozantinib (), abemaciclib (, , ). Neratinib, a recently approved tyrosine kinase inhibitor, has shown some efficacy against breast cancer brain metastases (178).
Even though there were several successful developments of molecularly targeted agents to treat breast cancer, the incidence of patients with brain metastases has increased and have a poor prognosis (179), mainly due to resistance development and poor brain penetration of therapeutic agents. Several brain penetrant therapeutics have shown to have promising efficacy on brain metastases in preclinical studies.
Renal Cell Carcinoma
The incidence rate of renal cell carcinoma (RCC) in adults is 2–3%, and metastases occur in about 50% of these patients. About 8–10% of metastatic renal cell carcinoma (mRCC) patients are known to develop brain metastases (180). The median overall survival is reported to be less than 13.3 months in the presence of brain metastases when using whole-brain radiotherapy (181). The treatment regimen with molecularly targeted therapeutics has not been established or evaluated for brain metastases, and standard therapy for mRCC has been applied to patients with brain metastases. Several tyro-sine kinase inhibitors are approved by FDA for treatment of mRCC, including sorafenib, sunitinib, and axitinib, and have improved survival (Table IV). Sunitinib has some clinical benefit towards brain metastases as a single agent by stabilizing the disease by more than 3 months, but objective response rate was only 12% among 213 patients enrolled (182,183). Brain distribution of sunitinib is reported to be high (42%) in a preclinical study, even though sunitinib is found to be substrates of both P-gp and BCRP (96). According to a few case reports, a newer generation TKI, pazopanib, seems to have good response rate or increase overall survival as a single agent (184). The efficacy of sorafenib has been studied in advanced metastatic renal cell carcinoma, but most of these studies excluded the patients with diagnosed bran metastases. Brain penetration of sorafenib is reported to be modest (9.4%) in mice, probably due to the efflux of both P-gp and BCRP. Recently, novel tyrosine kinase inhibitors, including cabozantinib and lenvatinib, received FDA approval for mRCC. However, the brain delivery of these agents across the BBB as well as their efficacy against brain metastases have not been reported. Since brain metastases are distinct from other organ metastases, especially in drug delivery, more clinical studies that examine the efficacy of a treatment specifically against brain metastases should be performed.
CONCLUSION
Several instructive observations can be made from the experience with the use of targeted agents in the context of brain metastases (Box 1). There is an overall correlation of brain penetration and substrate status of efflux transporters at the BBB, especially P-gp and BCRP (see Tables II, III, and IV). Many molecularly targeted agents are substrates of these efflux transporters, and their accessibility to the brain has been shown to be extremely low when the BBB is intact. This could be one reason why the incidence rate of metastases to the brain has been rising, despite of development of numerous molecularly targeted drugs. Tumors that reside in the brain can be protected from exposure to anticancer therapeutics by the BBB, resulting in limited delivery (Fig.7). Therefore, delivery of therapeutic agents to the brain is critical concern in treating metastatic tumors.
Box 1. Important factors for successful treatment of brain metastases.
Knowledge of the biology of metastatic growth in the brain
- Knowledge of the condition of the blood-brain barrier in brain metastases
- Heterogeneity of the blood-brain barrier within and around the brain metastases
The influence of radiation on drug delivery
- Free drug concentration at the sites of micro-metastases and the areas behind intact blood-brain barrier
- Pharmacologically active drug concentration at the target
- Design the drugs to be brain penetrant
- High permeability across the intact blood-brain barrier
- Structural modification to avoid efflux transporters
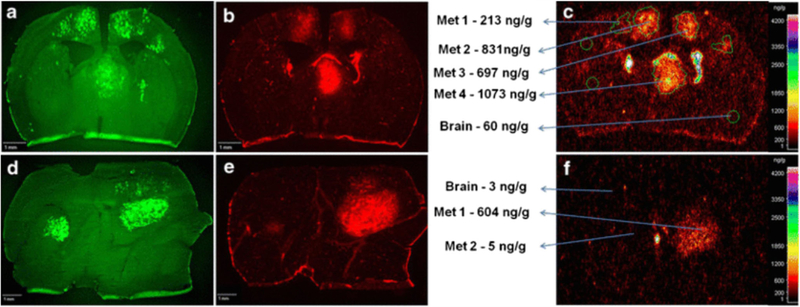
Fig. 7.
Heterogeneity of blood-brain barrier disruption and drug concentration in the experimental model (mice) of breast cancer brain metastases (from reference (56)). 14C-lapatinib was measured in normal brain and different brain metastases by quantitative autoradiography at 2 h (a-c) and 12 h (d-f) following the oral administration of 100 mg/kg 14C-lapatinib. Signal from metastatic cells labelled with EGFP (a,d), Texas Red 3kD dextran (b,e), and 14C-lapatinib (c,f) indicate the location of tumor cells, the integrity of the BBB, and the concentration of drug, respectively. The concentrations of lapatinib, as well as disruption of BBB, were highly variable within and between the metastatic breast cancer lesions in the brain.
There have been many previous reviews on various methods to improve drug delivery to the brain for the treatment of brain tumors by enhancing the brain penetration of drugs that are systemically available. These methods include changing formulations of existing compounds by using nanoparticles (185,186) and using concomitant therapy that inhibits efflux transporters at the BBB to improve brain delivery of substrates (187–189). However, it will be even more critical to assess the efflux transporter liability when developing and designing therapeutics, and consider a brain delivery as a key factor in the early phase of discovery and development, especially for the anticancer therapeutics that are often subject to efflux transporters at the BBB. There are examples of using in silico-guided drug design to make a brain penetrant anticancer drug by reducing efflux liability, including GNE-317 and lorlatinib (133,190,191).
Another important consideration is that the integrity of BBB in and around the tumor in the brain is heterogeneous (11). The BBB around the tumor core can be relatively permeable to therapeutics, since its structure tends to be disrupted. However, the tumor rim and micro-metastases may have an intact BBB, replete with efflux transporter systems, making these regions more likely to be resistant to therapeutics. Previously, several papers have shown that concentration of drugs in tumor is high enough to result in desirable efficacy in the tumor cells, based on the concentration measured in tumors resected from patients, due to disrupted BBB around tumor (Fig 7). However, since the integrity of BBB is heterogeneous depending on the region, and even within a region, the concentration of drug measured in a resected tumor specimen does represent the concentration throughout the entirety of the tumor. Given this, it is feasible that variable efficacy of many agents between patients or even amongst metastatic sites is related to inconsistent delivery. There are some drugs that have been shown to have some efficacy against brain metastases, despite of their limited delivery to the brain. In case of erlotinib for lung cancer, even though it has low brain penetration due to P-gp and BCRP, its efficacy against brain metastases patients carrying a specific EGFR mutation was demonstrated (response rate of 82.4%, Table II). This can be explained by not only improved delivery of a drug through perturbed BBB around tumor core, but also the selectivity and potency of a drug for a particular tumor. Drug may be able to induce desirable efficacy in the brain, if it has sufficient selectivity and potency against its target so that the concentration of a drug required at the site of action can be achieved. This observation indicates that both potency and delivery need to be considered hand-in-hand when evaluating and deciding upon a course of therapy for brain metastases. The potency of a drug against its target is often represented by an inhibitory concentration or efficacious concentration when measured using in vitro experiments. However, that in vitro concentration may not be the same as the efficacious concentration needed at the site of action in patients. Therefore, it is important to consider the concentration of a drug needed at the target, because potency can help overcome limited delivery, when potency is high enough. Since a lack of efficacy of systemic anticancer agents in CNS disease can lead to higher incidence rates of brain metastases (85), the BBB penetration, subsequent distribution into the brain metastatic site, and the potency against a particular CNS target, of molecularly-targeted agents need to be considered in the early phases of drug development.
REFERENCES
- 1.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–51. [DOI] [PubMed] [Google Scholar]
- 2.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruzzone MG, D’Incerti L, Farina LL, Cuccarini V, Finocchiaro G. CT and MRI of brain tumors. Q J Nucl Med Mol Imaging. 2012;56(2):112–37. [PubMed] [Google Scholar]
- 4.Brown PD, Buckner JC, Uhm JH, Shaw EG. The neurocognitive effects of radiation in adult low-grade glioma patients. Neuro-Oncology. 2003;5(3):161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro-Oncology. 2013;15(10): 1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reungwetwattana T, Weroha SJ, Molina JR. Oncogenic pathways, molecularly targeted therapies, and highlighted clinical trials in non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 2012;13(4):252–66. [DOI] [PubMed] [Google Scholar]
- 7.Bayraktar S, Gluck S. Molecularly targeted therapies for metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2013;138(1):21–35. [DOI] [PubMed] [Google Scholar]
- 8.Druker BJ. Perspectives on the development of a molecularly targeted agent. Cancer Cell. 2002;1(1):31–6. [DOI] [PubMed] [Google Scholar]
- 9.Becker JC, Kirkwood JM, Agarwala SS, Dummer R, Schrama D, Hauschild A. Molecularly targeted therapy for melanoma: current reality and future options. Cancer. 2006;107(10):2317–27. [DOI] [PubMed] [Google Scholar]
- 10.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–87. [DOI] [PubMed] [Google Scholar]
- 11.Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36(3):437–49. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011;13:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gampa G, Vaidhyanathan S, Sarkaria JN, Elmquist WF. Drug delivery to melanoma brain metastases: can current challenges lead to new opportunities? Pharmacol Res. 2017;123:10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45(7):741–4. [DOI] [PubMed] [Google Scholar]
- 17.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan Detroit Cancer surveillance system. J Clin Oncol. 2004;22(14):2865–72. [DOI] [PubMed] [Google Scholar]
- 18.Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–705. [DOI] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 20.Kromer C, Xu J, Ostrom QT, Gittleman H, Kruchko C, Sawaya R, et al. Estimating the annual frequency of synchronous brain metastasis in the United States 2010–2013: a population-based study. J Neuro-Oncol. 2017;134(1):55–64. [DOI] [PubMed] [Google Scholar]
- 21.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 22.Newman SJ, Hansen HH. Proceedings: frequency, diagnosis, and treatment of brain metastases in 247 consecutive patients with bronchogenic carcinoma. Cancer. 1974;33(2):492–6. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain MC, Baik CS, Gadi VK, Bhatia S, Chow LQ. Systemic therapy of brain metastases: non-small cell lung cancer, breast cancer, and melanoma. Neuro-Oncology. 2017;19(1):i1–i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. N Engl J Med. 1999;341(7):476–84. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Jiang W, Luan L, Wang L, Zheng X, Wang G. Prophylactic cranial irradiation for patients with small-cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2014;14:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167(4):913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–17. [DOI] [PubMed] [Google Scholar]
- 28.Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B. Brain metastasis in breast cancer: a comprehensive literature review. J Neuro-Oncol. 2016;127(3):407–14. [DOI] [PubMed] [Google Scholar]
- 29.Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1): 11–20. [DOI] [PubMed] [Google Scholar]
- 30.Zakrzewski J, Geraghty LN, Rose AE, Christos PJ, Mazumdar M, Polsky D, et al. Clinical variables and primary tumor characteristics predictive of the development of melanoma brain metastases and post-brain metastases survival. Cancer. 2011;117(8):1711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McWilliams RR, Brown PD, Buckner JC, Link MJ, Markovic SN. Treatment of brain metastases from melanoma. Mayo Clin Proc. 2003;78(12):1529–36. [DOI] [PubMed] [Google Scholar]
- 32.Gorantla V, Kirkwood JM, Tawbi HA. Melanoma brain metastases: an unmet challenge in the era of active therapy. Curr Oncol Rep. 2013;15(5):483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro-Oncology. 2012;14(9):1171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Essig M, Weber MA, von Tengg-Kobligk H, Knopp MV, Yuh WT, Giesel FL. Contrast-enhanced magnetic resonance imaging of central nervous system tumors: agents, mechanisms, and applications. Top Magn Reson Imaging. 2006;17(2):89–106. [DOI] [PubMed] [Google Scholar]
- 35.Osswald M, Blaes J, Liao Y, Solecki G, Gommel M, Berghoff AS, et al. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res. 2016;22(24):6078–87. [DOI] [PubMed] [Google Scholar]
- 36.Yap KY, Chui WK, Chan A. Drug interactions between chemo-therapeutic regimens and antiepileptics. Clin Ther. 2008;30(8): 1385–407. [DOI] [PubMed] [Google Scholar]
- 37.Cheung YT, Yap KY, Chui WK, Chan A. Drug-drug interactions between oral antiepileptics and oral anticancer drugs: implications to clinicians. Eur Neurol. 2010;64(2):88–94. [DOI] [PubMed] [Google Scholar]
- 38.Shi S, Li Y. Interplay of drug-metabolizing enzymes and transporters in drug absorption and disposition. Curr Drug Metab. 2014;15(10):915–41. [DOI] [PubMed] [Google Scholar]
- 39.Pang KS, Maeng HJ, Fan J. Interplay of transporters and enzymes in drug and metabolite processing. Mol Pharm. 2009;6(6):1734–55. [DOI] [PubMed] [Google Scholar]
- 40.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500. [DOI] [PubMed] [Google Scholar]
- 41.Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franciosi V, Cocconi G, Michiara M, Di Costanzo F, Fosser V, Tonato M, et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 1999;85(7):1599–605. [PubMed] [Google Scholar]
- 43.Bailon O, Chouahnia K, Augier A, Bouillet T, Billot S, Coman I, et al. Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma. Neuro-Oncology. 2012;14(4):491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 45.Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–95. [DOI] [PubMed] [Google Scholar]
- 46.Dummer R, Goldinger SM, Turtschi CP, Eggmann NB, Michielin O, Mitchell L, et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer. 2014;50(3):611–21. [DOI] [PubMed] [Google Scholar]
- 47.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3(1):53–7. [DOI] [PubMed] [Google Scholar]
- 48.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16(1): 116–22. [DOI] [PubMed] [Google Scholar]
- 49.On NH, Mitchell R, Savant SD, Bachmeier CJ, Hatch GM, Miller DW. Examination of blood-brain barrier (BBB) integrity in a mouse brain tumor model. J Neuro-Oncol. 2013;111(2):133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275(3):772–82. [DOI] [PubMed] [Google Scholar]
- 51.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Paolini MA, Murray DL, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546–54. [DOI] [PubMed] [Google Scholar]
- 52.Preusser M, Capper D, Ilhan-Mutlu A, Berghoff AS, Birner P, Bartsch R, et al. Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathol. 2012;123(2):205–22. [DOI] [PubMed] [Google Scholar]
- 53.McCoach CE, Berge EM, Lu X, Baron AE, Camidge DR. A brief report of the status of central nervous system metastasis enrollment criteria for advanced non-small cell lung Cancer clinical trials: a review of the ClinicalTrials.gov trial registry. J Thorac Oncol. 2016;11(3):407–13. [DOI] [PubMed] [Google Scholar]
- 54.Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–8. [DOI] [PubMed] [Google Scholar]
- 55.Pafundi DH, Laack NN, Youland RS, Parney IF, Lowe VJ, Giannini C, et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radio-therapy target delineation: results of a prospective pilot study. Neuro-Oncology. 2013;15(8):1058–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taskar KS, Rudraraju V, Mittapalli RK, Samala R, Thorsheim HR, Lockman J, et al. Lapatinib distribution in HER2 overex-pressing experimental brain metastases of breast cancer. Pharm Res. 2012;29(3):770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Lange EC, Danhof M. Considerations in the use of cerebro-spinal fluid pharmacokinetics to predict brain target concentrations in the clinical setting: implications of the barriers between blood and brain. Clin Pharmacokinet. 2002;41(10):691–703. [DOI] [PubMed] [Google Scholar]
- 58.Shen DD, Artru AA, Adkison KK. Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics. Adv Drug Deliv Rev. 2004;56(12):1825–57. [DOI] [PubMed] [Google Scholar]
- 59.Liu X, Van Natta K, Yeo H, Vilenski O, Weller PE, Worboys PD, et al. Unbound drug concentration in brain homogenate and cerebral spinal fluid at steady state as a surrogate for unbound concentration in brain interstitial fluid. Drug Metab Dispos. 2009;37(4):787–93. [DOI] [PubMed] [Google Scholar]
- 60.de Lange EC. Utility of CSF in translational neuroscience. J Pharmacokinet Pharmacodyn. 2013;40(3):315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kodaira H, Kusuhara H, Fujita T, Ushiki J, Fuse E, Sugiyama Y. Quantitative evaluation of the impact of active efflux by pglycoprotein and breast cancer resistance protein at the blood-brain barrier on the predictability of the unbound concentrations of drugs in the brain using cerebrospinal fluid concentration as a surrogate. J Pharmacol Exp Ther. 2011;339(3):935–44. [DOI] [PubMed] [Google Scholar]
- 62.Zhuang Y, Fraga CH, Hubbard KE, Hagedorn N, Panetta JC, Waters CM, et al. Topotecan central nervous system penetration is altered by a tyrosine kinase inhibitor. Cancer Res. 2006;66(23): 11305–13. [DOI] [PubMed] [Google Scholar]
- 63.de Lange EC. The mastermind approach to CNS drug therapy: translational prediction of human brain distribution, target site kinetics, and therapeutic effects. Fluids Barriers CNS. 2013;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hillered L, Persson L, Nilsson P, Ronne-Engstrom E, Enblad P. Continuous monitoring of cerebral metabolism in traumatic brain injury: a focus on cerebral microdialysis. Curr Opin Crit Care. 2006;12(2):112–8. [DOI] [PubMed] [Google Scholar]
- 65.Ederoth P, Tunblad K, Bouw R, Lundberg CJ, Ungerstedt U, Nordstrom CH, et al. Blood-brain barrier transport of morphine in patients with severe brain trauma. Br J Clin Pharmacol. 2004;57(4):427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friden M, Gupta A, Antonsson M, Bredberg U, Hammarlund-Udenaes M. In vitro methods for estimating unbound drug concentrations in the brain interstitial and intracellular fluids. Drug Metab Dispos. 2007;35(9):1711–9. [DOI] [PubMed] [Google Scholar]
- 67.Kalvass JC, Phipps C, Jenkins GJ, Stuart P, Zhang X, Heinle L, et al. Mathematical and experimental validation of flux Dialysis method: an improved approach to measure unbound fraction for compounds with high protein binding and other challenging properties. Drug Metab Dispos. 2018;46(4):458–69. [DOI] [PubMed] [Google Scholar]
- 68.Laramy JK, Kim M, Gupta SK, Parrish KE, Zhang S, Bakken KK, et al. Heterogeneous binding and central nervous system distribution of the multitargeted kinase inhibitor Ponatinib restrict Orthotopic efficacy in a patient-derived xenograft model of glioblastoma. J Pharmacol Exp Ther. 2017;363(2):136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X, Chen C, Smith BJ. Progress in brain penetration evaluation in drug discovery and development. J Pharmacol Exp Ther. 2008;325(2):349–56. [DOI] [PubMed] [Google Scholar]
- 70.Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF. Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2013;344(3):655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaidhyanathan S, Mittapalli RK, Sarkaria JN, Elmquist WF. Factors influencing the CNS distribution of a novel MEK-1/2 inhibitor: implications for combination therapy for melanoma brain metastases. Drug Metab Dispos. 2014;42(8):1292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heffron TP. Small molecule kinase inhibitors for the treatment of brain Cancer. J Med Chem. 2016;59(22):10030–66. [DOI] [PubMed] [Google Scholar]
- 73.Heffron TP. Challenges of developing small-molecule kinase inhibitors for brain tumors and the need for emphasis on free drug levels. Neuro-Oncology. 2018;20(3):307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maurer TS, Debartolo DB, Tess DA, Scott DO. Relationship between exposure and nonspecific binding of thirty-three central nervous system drugs in mice. Drug Metab Dispos. 2005;33(1): 175–81. [DOI] [PubMed] [Google Scholar]
- 75.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19(6):771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. [DOI] [PubMed] [Google Scholar]
- 77.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1): 41–53. [DOI] [PubMed] [Google Scholar]
- 78.Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25(1):25–39. [DOI] [PubMed] [Google Scholar]
- 79.Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol. 2015;209(4): 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agarwal S, Hartz AM, Elmquist WF, Bauer B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gate-keepers team up. Curr Pharm Des. 2011;17(26):2793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117(2):333–45. [DOI] [PubMed] [Google Scholar]
- 82.Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77(4):491–502. [DOI] [PubMed] [Google Scholar]
- 83.Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97(11):2517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10(12):1295–312. [DOI] [PubMed] [Google Scholar]
- 85.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11(5):352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kis O, Robillard K, Chan GN, Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2010;31(1):22–35. [DOI] [PubMed] [Google Scholar]
- 87.Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol. 2000;58(6): 1357–67. [DOI] [PubMed] [Google Scholar]
- 88.Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res. 2004;64(9):3296–301. [DOI] [PubMed] [Google Scholar]
- 89.de Lange EC. Potential role of ABC transporters as a detoxification system at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56(12):1793–809. [DOI] [PubMed] [Google Scholar]
- 90.Eilers M, Roy U, Mondal D. MRP (ABCC) transporters-mediated efflux of anti-HIV drugs, saquinavir and zidovudine, from human endothelial cells. Exp Biol Med (Maywood). 2008;233(9):1149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lingineni K, Belekar V, Tangadpalliwar SR, Garg P. The role of multidrug resistance protein (MRP-1) as an active efflux transporter on blood-brain barrier (BBB) permeability. Mol Divers. 2017; [DOI] [PubMed] [Google Scholar]
- 92.de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13(21):6440–9. [DOI] [PubMed] [Google Scholar]
- 93.de Vries NA, Buckle T, Zhao J, Beijnen JH, Schellens JH, van Tellingen O. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Investig New Drugs. 2012;30(2):443–9. [DOI] [PubMed] [Google Scholar]
- 94.Elmeliegy MA, Carcaboso AM, Tagen M, Bai F, Stewart CF. Role of ATP-binding cassette and solute carrier transporters in erlotinib CNS penetration and intracellular accumulation. Clin Cancer Res. 2011;17(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin F, de Gooijer MC, Roig EM, Buil LC, Christner SM, Beumer JH, et al. ABCB1, ABCG2, and PTEN determine the response of glioblastoma to temozolomide and ABT-888 therapy. Clin Cancer Res. 2014;20(10):2703–13. [DOI] [PubMed] [Google Scholar]
- 96.Oberoi RK, Mittapalli RK, Elmquist WF. Pharmacokinetic assessment of efflux transport in sunitinib distribution to the brain. J Pharmacol Exp Ther. 2013;347(3):755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parrish KE, Cen L, Murray J, Calligaris D, Kizilbash S, Mittapalli RK, et al. Efficacy of PARP inhibitor Rucaparib in Orthotopic glioblastoma xenografts is limited by ineffective drug penetration into the central nervous system. Mol Cancer Ther. 2015;14(12): 2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parrish KE, Pokorny J, Mittapalli RK, Bakken K, Sarkaria JN, Elmquist WF. Efflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor model. J Pharmacol Exp Ther. 2015;355(2):264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Polli JW, Humphreys JE, Harmon KA, Castellino S, O’Mara MJ, Olson KL, et al. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}−6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos 2008;36(4):695–701. [DOI] [PubMed] [Google Scholar]
- 100.Tang SC, Nguyen LN, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer. 2014;134(6):1484–94. [DOI] [PubMed] [Google Scholar]
- 101.Westerhout J, Smeets J, Danhof M, de Lange EC. The impact of P-gp functionality on non-steady state relationships between CSF and brain extracellular fluid. J Pharmacokinet Pharmacodyn. 2013;40(3):327–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mittapalli RK, Chung AH, Parrish KE, Crabtree D, Halvorson KG, Hu G, et al. ABCG2 and ABCB1 limit the efficacy of Dasatinib in a PDGF-B-driven brainstem glioma model. Mol Cancer Ther. 2016;15(5):819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Becker CM, Oberoi RK, McFarren SJ, Muldoon DM, Pafundi DH, Pokorny JL, et al. Decreased affinity for efflux transporters increases brain penetrance and molecular targeting of a PI3K/mTOR inhibitor in a mouse model of glioblastoma. Neuro-Oncology. 2015;17(9):1210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Broccatelli F, Larregieu CA, Cruciani G, Oprea TI, Benet LZ. Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012;64(1):95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Doroshow JH. Targeting EGFR in non-small-cell lung cancer. N Engl J Med. 2005;353(2):200–2. [DOI] [PubMed] [Google Scholar]
- 107.Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Togashi Y, Masago K, Fukudo M, Terada T, Fujita S, Irisa K, et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol. 2010;5(7): 950–5. [DOI] [PubMed] [Google Scholar]
- 109.Chen Y, Wang M, Zhong W, Zhao J. Pharmacokinetic and pharmacodynamic study of Gefitinib in a mouse model of non-small-cell lung carcinoma with brain metastasis. Lung Cancer. 2013;82(2):313–8. [DOI] [PubMed] [Google Scholar]
- 110.Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther. 2010;334(1):147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Agarwal S, Manchanda P, Vogelbaum MA, Ohlfest JR, Elmquist WF. Function of the blood-brain barrier and restriction of drug delivery to invasive glioma cells: findings in an orthotopic rat xenograft model of glioma. Drug Metab Dispos. 2013;41(1):33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heon S, Yeap BY, Lindeman NI, Joshi VA, Butaney M, Britt GJ, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18(16):4406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343(2):342–50. [DOI] [PubMed] [Google Scholar]
- 114.Hoffknecht P, Tufman A, Wehler T, Pelzer T, Wiewrodt R, Schutz M, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol. 2015;10(1):156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of Osimertinib with other EGFRTKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–40. [DOI] [PubMed] [Google Scholar]
- 116.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-Pemetrexed in EGFR T790M-positive lung Cancer. N Engl J Med. 2017;376(7):629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeng Q, Wang J, Cheng Z, Chen K, Johnstrom P, Varnas K, et al. Discovery and evaluation of clinical candidate AZD3759, a potent, oral active, central nervous system-penetrant, epidermal growth factor receptor tyrosine kinase inhibitor. J Med Chem. 2015;58(20):8200–15. [DOI] [PubMed] [Google Scholar]
- 118.Yang Z, Guo Q, Wang Y, Chen K, Zhang L, Cheng Z, et al. AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med. 2016;8(368):368ra172. [DOI] [PubMed] [Google Scholar]
- 119.Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wong DW, Leung EL, So KK, Tam IY, Sihoe AD, Cheng LC, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115(8):1723–33. [DOI] [PubMed] [Google Scholar]
- 121.Rodig SJ, Mino-Kenudson M, Dacic S, Yeap BY, Shaw A, Barletta JA, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15(16):5216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–5. [DOI] [PubMed] [Google Scholar]
- 123.Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, et al. Clinical experience with Crizotinib in patients with advanced ALK-rearranged non-small-cell lung Cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77. [DOI] [PubMed] [Google Scholar]
- 125.A Clinical Trial Testing The Efficacy Of Crizotinib Versus Standard Chemotherapy Pemetrexed Plus Cisplatin Or Carboplatin In Patients With ALK Positive Non Squamous Cancer Of The Lung (PROFILE 1014) [Internet]. 2014.
- 126.Kodama T, Tsukaguchi T, Satoh Y, Yoshida M, Watanabe Y, Kondoh O, et al. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther. 2014;13(12):2910–8. [DOI] [PubMed] [Google Scholar]
- 127.Gadgeel SM, Gandhi L, Riely GJ, Chiappori AA, West HL, Azada MC, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–28. [DOI] [PubMed] [Google Scholar]
- 128.Metro G, Lunardi G, Bennati C, Chiarini P, Sperduti I, Ricciuti B, et al. Alectinib’s activity against CNS metastases from ALK-positive non-small cell lung cancer: a single institution case series. J Neuro-Oncol. 2016;129(2):355–61. [DOI] [PubMed] [Google Scholar]
- 129.Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, Sakamoto H. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74(5):1023–8. [DOI] [PubMed] [Google Scholar]
- 130.Friboulet L, Li N, Katayama R, Lee CC, Gainor JF, Crystal AS, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4(6):662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Corporation NP. Zykadia. Product insert. [Google Scholar]
- 132.Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17(4):452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Johnson TW, Richardson PF, Bailey S, Brooun A, Burke BJ, Collins MR, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ROS oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–44. [DOI] [PubMed] [Google Scholar]
- 134.Menichincheri M, Ardini E, Magnaghi P, Avanzi N, Banfi P, Bossi R, et al. Discovery of Entrectinib: a new 3-Aminoindazole as a potent anaplastic lymphoma kinase (ALK), c-ROS oncogene 1 kinase (ROS1), and pan-tropomyosin receptor kinases (pan-TRKs) inhibitor. J Med Chem. 2016;59(7):3392–408. [DOI] [PubMed] [Google Scholar]
- 135.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neuro-Oncol. 2005;75(1):5–14. [DOI] [PubMed] [Google Scholar]
- 136.Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control. 2009;16(3):248–55. [DOI] [PubMed] [Google Scholar]
- 137.Damsky WE, Theodosakis N, Bosenberg M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene. 2014;33(19):2413–22. [DOI] [PubMed] [Google Scholar]
- 138.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54. [DOI] [PubMed] [Google Scholar]
- 139.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in meta-static melanoma. N Engl J Med. 2010;363(9):809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Capper D, Berghoff AS, Magerle M, Ilhan A, Wohrer A, Hackl M, et al. Immunohistochemical testing of BRAF V600E status in 1, 120 tumor tissue samples of patients with brain metastases. Acta Neuropathol. 2012;123(2):223–33. [DOI] [PubMed] [Google Scholar]
- 141.Durmus S, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Oral availability and brain penetration of the BRAFV600E inhibitor vemurafenib can be enhanced by the PGLYCOprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Mol Pharm. 2012;9(11):3236–45. [DOI] [PubMed] [Google Scholar]
- 142.Kim A, Cohen MS. The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma. Expert Opin Drug Discov. 2016;11(9):907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luke JJ, Hodi FS. Vemurafenib and BRAF inhibition: a new class of treatment for metastatic melanoma. Clin Cancer Res. 2012;18(1):9–14. [DOI] [PubMed] [Google Scholar]
- 144.Mittapalli RK, Vaidhyanathan S, Sane R, Elmquist WF. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032). J Pharmacol Exp Ther. 2012;342(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007;25(16):2306–12. [DOI] [PubMed] [Google Scholar]
- 146.Narayana A, Mathew M, Tam M, Kannan R, Madden KM, Golfinos JG, et al. Vemurafenib and radiation therapy in melanoma brain metastases. J Neuro-Oncol. 2013;113(3):411–6. [DOI] [PubMed] [Google Scholar]
- 147.Rochet NM, Kottschade LA, Markovic SN. Vemurafenib for melanoma metastases to the brain. N Engl J Med. 2011;365(25): 2439–41. [DOI] [PubMed] [Google Scholar]
- 148.Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Azer MW, Menzies AM, Haydu LE, Kefford RF, Long GV. Patterns of response and progression in patients with BRAF-mutant melanoma metastatic to the brain who were treated with dabrafenib. Cancer. 2014;120(4):530–6. [DOI] [PubMed] [Google Scholar]
- 150.Jiang CC, Chen LH, Gillespie S, Wang YF, Kiejda KA, Zhang XD, et al. Inhibition of MEK sensitizes human melanoma cells to endoplasmic reticulum stress-induced apoptosis. Cancer Res. 2007;67(20):9750–61. [DOI] [PubMed] [Google Scholar]
- 151.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18): 1694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–14. [DOI] [PubMed] [Google Scholar]
- 153.Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–76. [DOI] [PubMed] [Google Scholar]
- 154.Choo EF, Ly J, Chan J, Shahidi-Latham SK, Messick K, Plise E, et al. Role of P-glycoprotein on the brain penetration and brain pharmacodynamic activity of the MEK inhibitor cobimetinib. Mol Pharm. 2014;11(11):4199–207. [DOI] [PubMed] [Google Scholar]
- 155.Patel BG, Ahmed KA, Johnstone PA, Yu HH, Etame AB. Initial experience with combined BRAF and MEK inhibition with stereotactic radiosurgery for BRAF mutant melanoma brain metastases. Melanoma Res. 2016;26(4):382–6. [DOI] [PubMed] [Google Scholar]
- 156.Planchard D, Besse B, Groen HJ, Souquet PJ, Quoix E, Baik CS, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17(7): 984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Long GV, Weber JS, Infante JR, Kim KB, Daud A, Gonzalez R, et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving Dabrafenib combined with Trametinib. J Clin Oncol. 2016;34(8):871–8. [DOI] [PubMed] [Google Scholar]
- 158.Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids and Barriers of the CNS. 2011;8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Di Giacomo AM, Margolin K. Immune checkpoint blockade in patients with melanoma metastatic to the brain. Semin Oncol. 2015;42(3):459–65. [DOI] [PubMed] [Google Scholar]
- 160.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5): 459–65. [DOI] [PubMed] [Google Scholar]
- 161.Queirolo P, Spagnolo F, Ascierto PA, Simeone E, Marchetti P, Scoppola A, et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neuro-Oncol. 2014;118(1):109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S, et al. Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014;23(5):623–8. [DOI] [PubMed] [Google Scholar]
- 163.Kodack DP, Askoxylakis V, Ferraro GB, Fukumura D, Jain RK. Emerging strategies for treating brain metastases from breast cancer. Cancer Cell. 2015;27(2):163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91(4):639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leyland-Jones B Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27(31):5278–86. [DOI] [PubMed] [Google Scholar]
- 166.Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–7. [DOI] [PubMed] [Google Scholar]
- 167.Gori S, Lunardi G, Inno A, Foglietta J, Cardinali B, Del Mastro L, et al. Lapatinib concentration in cerebrospinal fluid in two patients with HER2-positive metastatic breast cancer and brain metastases. Ann Oncol. 2014;25(4):912–3. [DOI] [PubMed] [Google Scholar]
- 168.Morikawa A, Peereboom DM, Thorsheim HR, Samala R, Balyan R, Murphy CG, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro-Oncology. 2015;17(2):289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112(3):533–43. [DOI] [PubMed] [Google Scholar]
- 170.Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26(12):1993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lin NU, Dieras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–9. [DOI] [PubMed] [Google Scholar]
- 172.Dawood S, Broglio K, Esteva FJ, Yang W, Kau SW, Islam R, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol. 2009;20(4):621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mikule K, Wilcoxen K, editors. The PARP inhibitor, niraparib, crosses the blood brain barrier in rodents and is efficacious in a BRCA2-mutant intracranial tumor model. [abstract] In: Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 2015; Philadelphia (PA). [Google Scholar]
- 174.Mehta MP, Wang D, Wang F, Kleinberg L, Brade A, Robins HI, et al. Veliparib in combination with whole brain radiation therapy in patients with brain metastases: results of a phase 1 study. J Neuro-Oncol. 2015;122(2):409–17. [DOI] [PubMed] [Google Scholar]
- 175.Palmieri D, Lockman PR, Thomas FC, Hua E, Herring J, Hargrave E, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res. 2009;15(19):6148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hanson JE, La H, Plise E, Chen YH, Ding X, Hanania T, et al. SAHA enhances synaptic function and plasticity in vitro but has limited brain availability in vivo and does not impact cognition. PLoS One. 2013;8(7):e69964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fitzgerald DP, Emerson DL, Qian Y, Anwar T, Liewehr DJ, Steinberg SM, et al. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol Cancer Ther. 2012;11(9):1959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Freedman RA, Gelman RS, Melisko ME, Anders CK, Moy B, Blackwell KL, et al. TBCRC 022: Phase II trial of neratinib + capecitabine for patients (Pts) with human epidermal growth factor receptor 2 (HER2+) breast cancer brain metastases (BCBM). J Clin Oncol. 2017;35(15_suppl):1005. [Google Scholar]
- 179.Anders CK, Deal AM, Miller CR, Khorram C, Meng H, Burrows E, et al. The prognostic contribution of clinical breast cancer sub-type, age, and race among patients with breast cancer brain metastases. Cancer. 2011;117(8):1602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23(4):973–80. [DOI] [PubMed] [Google Scholar]
- 181.Bennani O, Derrey S, Langlois O, Castel H, Laquerriere A, Freger P, et al. Brain metastasis from renal cell carcinoma. Neurochirurgie. 2014;60(1–2):12–6. [DOI] [PubMed] [Google Scholar]
- 182.Gore ME, Hariharan S, Porta C, Bracarda S, Hawkins R, Bjarnason GA, et al. Sunitinib in metastatic renal cell carcinoma patients with brain metastases. Cancer. 2011;117(3):501–9. [DOI] [PubMed] [Google Scholar]
- 183.Dudek AZ, Raza A, Chi M, Singhal M, Oberoi R, Mittapalli RK, et al. Brain metastases from renal cell carcinoma in the era of tyrosine kinase inhibitors. Clin Genitourin Cancer. 2013;11(2): 155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Roberto M, Bassanelli M, Iannicelli E, Giacinti S, D’Antonio C, Aschelter AM, et al. Clinical outcome of third-line Pazopanib in a patient with metastatic renal cell carcinoma. Case Rep Oncol Med. 2015;629046:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Anders CK, Adamo B, Karginova O, Deal AM, Rawal S, Darr D, et al. Pharmacokinetics and efficacy of PEGylated liposomal doxorubicin in an intracranial model of breast cancer. PLoS One. 2013;8(5):e61359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kreuter J Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev. 2014;71:2–14. [DOI] [PubMed] [Google Scholar]
- 187.Fox E, Bates SE. Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther. 2007;7(4): 447–59. [DOI] [PubMed] [Google Scholar]
- 188.Sandler A, Gordon M, De Alwis DP, Pouliquen I, Green L, Marder P, et al. A phase I trial of a potent P-glycoprotein inhibitor, zosuquidar trihydrochloride (LY335979), administered intravenously in combination with doxorubicin in patients with advanced malignancy. Clin Cancer Res. 2004;10(10):3265–72. [DOI] [PubMed] [Google Scholar]
- 189.Morschhauser F, Zinzani PL, Burgess M, Sloots L, Bouafia F, Dumontet C. Phase I/II trial of a P-glycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride (LY335979), given orally in combination with the CHOP regimen in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2007;48(4):708–15. [DOI] [PubMed] [Google Scholar]
- 190.Salphati L, Shahidi-Latham S, Quiason C, Barck K, Nishimura M, Alicke B, et al. Distribution of the phosphatidylinositol 3-kinase inhibitors Pictilisib (GDC-0941) and GNE-317 in U87 and GS2 intracranial glioblastoma models-assessment by matrix-assisted laser desorption ionization imaging. Drug Metab Dispos. 2014;42(7):1110–6. [DOI] [PubMed] [Google Scholar]
- 191.Heffron TP, Salphati L, Alicke B, Cheong J, Dotson J, Edgar K, et al. The design and identification of brain penetrant inhibitors of phosphoinositide 3-kinase alpha. J Med Chem. 2012;55(18): 8007–20. [DOI] [PubMed] [Google Scholar]
- 192.Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crino L, VillaE. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol. 2004;15(7):1042–7. [DOI] [PubMed] [Google Scholar]
- 193.Porta R, Sanchez-Torres JM, Paz-Ares L, Massuti B, Reguart N, Mayo C, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37(3):624–31. [DOI] [PubMed] [Google Scholar]
- 194.Wind S, Giessmann T, Jungnik A, Brand T, Marzin K, Bertulis J, et al. Pharmacokinetic drug interactions of afatinib with rifampicin and ritonavir. Clin Drug Investig. 2014;34(3):173–82. [DOI] [PubMed] [Google Scholar]
- 195.Katayama R, Sakashita T, Yanagitani N, Ninomiya H, Horiike A, Friboulet L, et al. P-glycoprotein mediates Ceritinib resistance in anaplastic lymphoma kinase-rearranged non-small cell lung Cancer. EBioMedicine. 2016;3:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Crino L, Ahn MJ, De Marinis F, Groen HJ, Wakelee H, Hida T, et al. Multicenter phase II study of whole-body and intracranial activity with Ceritinib in patients with ALK-rearranged non-small-cell lung Cancer previously treated with chemotherapy and Crizotinib: results from ASCEND-2. J Clin Oncol. 2016;34(24):2866–73. [DOI] [PubMed] [Google Scholar]
- 197.Rosell R, Gettinger SN, Bazhenova LA, Langer CJ, Salgia R, Shaw AT, et al. 1330: Brigatinib efficacy and safety in patients (pts) with anaplastic lymphoma kinase (ALK)-positive (ALK+) non-small cell lung cancer (NSCLC) in a phase 1/2 trial. J Thorac Oncol. 2016;11(4 Suppl):S114. [Google Scholar]
- 198.Ardini E, Menichincheri M, Banfi P, Bosotti R, De Ponti C, Pulci R, et al. Entrectinib, a pan-TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined Cancer indications. Mol Cancer Ther. 2016;15(4):628–39. [DOI] [PubMed] [Google Scholar]
- 199.Sakji-Dupre L, Le Rhun E, Templier C, Desmedt E, Blanchet B, Mortier L. Cerebrospinal fluid concentrations of vemurafenib in patients treated for brain metastatic BRAF-V600 mutated melanoma. Melanoma Res. 2015;25(4):302–5. [DOI] [PubMed] [Google Scholar]
- 200.Gampa G, Kim M, Cook-Rostie N, Laramy JK, Sarkaria JN, Paradiso L, et al. Brain distribution of a novel MEK inhibitor E6201: implications in the treatment of melanoma brain metastases. Drug Metab Dispos. 2018;46(5):658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebro-spinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anti-Cancer Drugs. 2007;18(1):23–8. [DOI] [PubMed] [Google Scholar]
- 202.Lawlor D, Martin P, Busschots S, Thery J, O’Leary JJ, Hennessy BT, et al. PARP inhibitors as P-glyoprotein substrates. J Pharm Sci. 2014;103(6):1913–20. [DOI] [PubMed] [Google Scholar]
- 203.Kizilbash SH, Gupta SK, Chang K, Kawashima R, Parrish KE, Carlson BL, et al. Restricted delivery of Talazoparib across the blood-brain barrier limits the sensitizing effects of PARP inhibition on Temozolomide therapy in glioblastoma. Mol Cancer Ther. 2017;16(12):2735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Agarwal S, Sane R, Ohlfest JR, Elmquist WF. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther. 2011;336(1):223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Poller B, Iusuf D, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Differential impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on axitinib brain accumulation and oral plasma pharmacokinetics. Drug Metab Dispos. 2011;39(5):729–35. [DOI] [PubMed] [Google Scholar]