Abstract
Adeno-associated virus (AAV) has emerged as the vector of choice for delivering genes to the retina. Indeed, the first gene therapy to receive FDA approval in the United States is an AAV-based treatment for the inherited retinal disease, Leber congenital amaurosis-2. Voretigene neparvovec (Luxturna™) is delivered to patients via subretinal (SR) injection, an invasive surgical procedure that requires detachment of photoreceptors (PRs) from the retinal pigment epithelium (RPE). It has been reported that subretinal administration of vector under the cone-exclusive fovea leads to a loss of central retinal structure and visual acuity in some patients. Due to its technical difficulty and potential risks, alternatives to SR injection have been explored in primates. Intravitreally (Ivt) delivered AAV transduces inner retina and foveal cones, but with low efficiency. Novel AAV capsid variants identified via rational design or directed evolution have offered only incremental improvements, and have failed to promote pan-inner retinal transduction or significant outer retinal transduction beyond the fovea. Problems with retinal transduction by Ivt-delivered AAV include dilution in the vitreous, potential antibody-mediated neutralization of capsid in this nonimmune privileged space, and the presence of the inner limiting membrane (ILM), a basement membrane separating the vitreous from the neural retina. We have developed an alternative “subILM” injection method that overcomes all three hurdles. Specifically, vector is placed in a surgically induced, hydrodissected space between the ILM and neural retina. We have shown that subILM injection promotes more efficient retinal transduction by AAV than Ivt injection, and results in uniform and extensive transduction of retinal ganglion cells (RGCs) beneath the subILM bleb. We have also demonstrated transduction of Muller glia, ON bipolar cells, and photoreceptors by subILM injection. Our results confirm that the ILM is a major barrier to transduction by AAV in primate retina and that, when it is circumvented, the efficiency and depth to which AAV2 promotes transduction of multiple retinal cell classes is greatly enhanced. Here we describe in detail methods for vector preparation, vector dilution, and subILM injection as performed in macaque (Macaca sp.)
Keywords: Inner limiting membrane, AAV, Gene delivery, Gene replacement, Retinal ganglion cells, Bipolar cells, Photoreceptors, Novel surgical technique
1. Introduction
Inherited retinal diseases are caused by mutations in genes that encode for proteins expressed in various cells of the retina. In the vast majority of cases, the cells responsible for disease are retinal ganglion cells (RGCs) in the inner retina, and photoreceptors and retinal pigment epithelium (RPE), both of which are in the outer retina. The ideal strategy for treating such diseases is one that ensures efficient delivery of therapy to these target cells. Subretinal (SR) injection of adeno-associated virus (AAV) vectors is the current standard method for delivering therapeutic genes to PRs and RPE, as vector is deposited directly between these two cell layers. Subretinally delivered AAV2-based vectors are being used to treat patients with RPE65 Leber congenital amaurosis (LCA2), Choroideremia, retinitis pigmentosa (RP), and the CNGB3 and CNGA3 forms of Achromatopsia. Alternative AAV capsids that promote more efficient transduction of PRs in nonhuman primate have been identified [1–3]. These capsids, AAV5 and AAV8, are being applied subretinally to treat LCA2, RP, and Achromatopsia. However, targeting of foveal cones by subretinally injected AAV is problematic in patients with retinal degeneration, and may be contraindicated in diseases where underlying pathology complicates delivery or retinal detachment is expected to exacerbate pathology [4–6].
Intravitreal (Ivt) injection has been used to target RGC-mediated diseases such as Leber’s hereditary optic neuropathy (LHON), and is now being pursued as an alternative delivery approach to target outer retinal cells for the reasons mentioned above. However, recent studies in nonhuman primates revealed the formidable hurdles associated with this approach. While some transduction of RGCs is accomplished by Ivt injection of AAV2-based vectors in NHPs, the area of transduction is restricted to a “ring” of RGCs around the fovea and scattered foci in the peripheral retina, some foveal cones, and a mixture of retinal cells proximal to large retinal blood vessels [7, 8]. Newer AAV capsid variants identified via rational design or directed evolution are improved in the magnitude of transduction relative to AAV2, but the extent of transduction is essentially limited to the same areas [8, 9]. Transduction of foveal cones is possible, but is efficient only at high vector doses that promote inflammation [8]. Ivt injection is less invasive than SR injection, and may be well suited for treating patients with advanced degenerative disease that are prone to further damage upon surgically induced retinal detachment. However, there has not yet been an AAV capsid identified which promotes efficient transduction of PRs/RPE across the entire retina.
This is due, at least in part, to the following hurdles. First, Ivt-delivered AAV is immediately diluted upon mixing with the vitreous humor (0.05–0.10 mL of vector into ~2.5–4 mL of vitreous). Second, the vitreous is not an immune privileged space, and thus Ivt-delivered AAV can be neutralized by preexisting antibodies [10], and can potentially generate inflammatory responses. Finally, Ivt-delivered AAV must bypass the inner limiting membrane (ILM). The ILM is a typical basement membrane that forms the vitreoretinal junction and poses a biological barrier to Ivt-delivered capsids [11, 12]. Primates have a more impenetrable ILM than rodents, except in and around the fovea, immediately above the optic nerve head, and close to large blood vessels [13]. Enzymatic digestion of the ILM has been shown to improve retinal transduction by Ivt-delivered AAV, but the long-term effects of this approach on retinal structure and function have not been investigated [7, 14].
We recently developed a “subILM” injection method wherein AAV is placed in a surgically induced space between the ILM and neural retina [15]. This overcomes the dilution effect, the potential for immune neutralization and inflammatory responses, and the ILM barrier faced by Ivt-injected AAV. SubILM injection promotes more efficient retinal transduction by AAV than Ivt injection, and may provide a safe and efficient alternative to surgeons administering retinal gene therapies to patients with fragile retinas prone to further damage by subretinal surgery.
2. Materials
2.1. Vector Production
- AAV plasmids (see Note 1). Plasmids required for the triple-transfection method of AAV production include:
- Vector Plasmid containing AAV2 inverted terminal repeats (ITRs) flanking the transgene of interest. This will typically contain a promoter, splicing signal, reporter gene, and poly adenylation signal, such as pTR-UF11, containing the chimeric CMV-chicken beta actin (CAG) promoter driving “humanized” green fluorescent protein (GFP) [20], or pAAV-GFP, containing the CMV promoter driving GFP [21].
Balanced Salt Solution (BSS).
Tween (polysorbate) 20.
2.2. Preparation of Vector Solution
Micropipettes.
Sterile micropipette tips.
Wide bore 1 mL pipette tip.
Balanced Salt Solution (BSS).
BSS/Tween Vector Diluent Buffer: BSS with 0.014% Tween (polysorbate) 20.
10 mg/mL sodium hyaluronate: Healon (McKesson).
Sterile individually wrapped microcentrifuge tubes.
High-speed microcentrifuge.
Vortex mixer.
Laminar flow hood.
2.3. Animal Preparation and Postoperative Care
100 mg/mL Ketamine.
Glycopyrrolate.
10 mg/mL Cerenia.
1 g Cefazolin.
2 mg/mL Dexamethasone.
IV Catheter.
Endotracheal tubing.
Ventilator.
Sustained Release Buprenorphine.
Sustained Release Meloxicam.
1% Tropicamide Ophthalmic Solution.
2.5% Phenylephrine Hydrochloride Ophthalmic Solution.
1% Cyclopentolate Ophthalmic Solution.
Isoflurane.
0.9% Sodium Chloride.
10% Povidone-Iodine Topical Solution.
Benzoin Tincture Swab Stick.
4 mg/mL Dexamethasone.
Neomycin/Polymyxin B Sulfates and Dexamethasone Ophthalmic Suspension.
2.4. SubILM Injection
BSS Plus Irrigating Solution (Alcon Labs).
BSS Solution.
Sterile surgical drape (medium).
Interlink T-Connector extension set (Baxter).
18G × 1 1/2″ BLUNT fill needles.
1 mL syringe with luer-lock tip (Fisher #309628 is preferred).
0.5 mL syringe.
Total Plus 25G or 23G Vitrectomy Pak (Alcon Labs).
25G Valved Trocars (Alcon Labs).
Machemer magnifying vitrectomy contact lens (Ocular Instruments).
20G V-Lance Knife (Alcon Labs).
36–42G retinal needle.
Syringe pump.
8–0 and 9–0 Vicryl Suture.
Bishop-Harmon Irrigating Cannula.
25 mg Indocyanine Green (ICG).
Scanning Laser Ophthalmoscope, such as Heidelberg Spectralis®.
Fundus Camera, such as Topcon TRC-50EX.
Ophthalmic Surgical Microscope with video, such as Zeiss Visu 200.
Vitrectomy Surgery System, such as Accurus 800CS with Xenon light source.
3. Methods
Conduct the subILM injection technique in macaque monkeys in a sterile surgical suite with the assistance of an appropriately qualified vitreoretinal surgeon and their staff. An overview of the procedure is provided in Fig. 1.
Fig. 1.
SubILM injection of AAV and Healon. A schematic of an intact retina (left) is magnified (right) to reveal the location of the subILM injection bleb. A needle is placed posterior to the ILM and anterior to NFL and GCL. Approximately 10 μL of a 1:1 solution of AAV:healon is injected into this space. RPE retinal pigment epithelium, PR OS photoreceptor outer segments, PR IS photoreceptor inner segments, ONL outer nuclear layer, OPL outer plexiform layer, IN L inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, NFL nerve fiber layer, ILM inner limiting membrane
3.1. Vector Production
AAV vectors should be manufactured and purified using methods established to result in highly pure AAV particles free of contamination by helper virus (e.g., Adenovirus, Herpes simplex virus, and baculovirus) and host cell DNA (see Note 2). Protocols for AAV production can be found in Chapters 3, 7, 19, 21, 22, and 23.
Use an AAV storage buffer compatible with intraocular use. Appropriate buffers have physiologic osmolarity (approximately 300 mOsm) and a pH range of 7.0–8.0. Buffers that contain small amounts (2 mM) of divalent cations Mg2+ and Ca2+ are preferred, as this facilitates AAV capsid stability. Of the commercially available buffers, Balanced Salt Solution (BSS) is the most widely used buffer for storage and dilution of AAV vectors to be injected into the eye.
The AAV storage and dilution buffer should also contain a small amount of nonionic surfactant to reduce nonspecific binding of virus to the tubing and syringes. For intraocular delivery, supplement AAV with 0.014% tween (polysorbate) 20 or 0.001% pluronic F68 [22, 23]. BSS with 0.014% tween has previously been shown to be compatible with the addition of Healon ophthalmic viscoelastic, which may be required for subILM injection [15].
Ensure that adequate amounts of the storage buffer are manufactured for later use as diluent buffer.
AAV should be titrated relative to a well-characterized standard. Acceptable methods for AAV titration include dot blot, quantitative/real-time polymerase chain reaction (qPCR), and digital PCR (see Chapter 4) [24–26].
AAV vectors should be confirmed to be free of contaminating protein by visualization via SDS PAGE gel stained with coomassie blue or silver stain. A quantification of the bands conforming to VP1, VP2, and VP3 should represent >95% of the protein visible [24].
The final AAV vector should be tested for endotoxin and should contain no more than 5 units of endotoxin per mL.
AAV vectors should be stored at −80 °C until shortly before use. Thawing and preparation of dosing solution is detailed in Subheading 2.4.
3.2. Preparation of Vector Solution
Calculate vector dilution based on the AAV stock concentration, the number of injections to be performed, the desired dose for each injection, and either 1:1 or 2:1 (v/v) dilution with Healon (1% sodium hyaluronate cohesive solution).
A 1:1 AAV:Healon mixture will be more difficult to prepare than a 2:1 AAV:Healon mixture, but may improve bleb formation when surgeons are not experienced in the technique.
Depending on the number of injections to be performed, a minimum of 250 μL of vector solution should be prepared prior to mixing with Healon. It is important to note that that the final solution will be very viscous, and it will not be possible to remove all of it from the 1.5 mL centrifuge tube. Prepare a total volume of 500 μL per eye.
Follow sterile technique when preparing AAV solutions, buffers, cannulas, and syringes. Prepare vector in a laminar flow hood, if available.
Thaw AAV on ice. Once thawed, mix AAV by inverting and flicking the tube. Quickly spin in a microcentrifuge at low speed (≤1000 rpm) to collect AAV solution at the bottom of the tube.
Open the AAV vector tube just enough to unseal the cap, and allow the solution to degas for at least 15 min at room temperature. This step is critical to reduce bubble formation in the final injection solution.
Thaw BSS/Tween diluent if needed for diluting AAV stock, as described in steps 5 and 6.
Allow Healon solution to warm to room temperature within the unopened sterile packaging.
9. Pre-dispense Healon into the microcentrifuge tube that will hold the final vector solution. Use an empty sterile 1.5 mL or 2 mL microcentrifuge tube (see Note 3).
Recap the tube containing the AAV stock, briefly vortex for 1–2 s (medium speed), and spin at ~5000 rpm for 2 min in a microcentrifuge. Do the same for the tube containing BSS/Tween diluent (if needed). If the AAV stock does not need to be diluted prior to adding to Healon, proceed to step 12.
If the AAV stock solution needs to be diluted with BSS/Tween solution before mixing with the Healon, dispense the appropriate amount of BSS/Tween solution into a fresh microcentrifuge tube, then pipette the appropriate volume of AAV stock solution directly into the BSS/Tween. Pipette the mixture up and down ~5 times. Vortex for 1–2 s (medium speed) and pulse spin at 1000 rpm in a microcentrifuge.
Pipette the predetermined volume of AAV solution into the microcentrifuge tube containing the Healon (step 9). The two solutions will not mix easily; do not attempt to pipette up and down. Use the pipette tip to briefly stir the AAV/Healon mixture together (the solutions will still not appear to mix well together).
Vortex the tube on the lowest setting for 30–60 s. Bubbles will form. Spin at max speed in a microcentrifuge for at least 2 min to remove air bubbles. Repeat this step until mixed.
The vector solution is now ready to be loaded into the injection device (step 7 in Subheading 3.4). Use either a wide bore 1 mL pipette tip or a 1 mL syringe with an 18G blunt fill needle. The solution will be very viscous if a 1:1 AAV:Healon mixture is used.
3.3. Animal Preparation
Collect serum approximately 6 weeks prior to planned subILM injection.
Test serum for the presence of neutralizing antibodies (NAb) to the AAV capsid serotype that will be utilized in the subILM injections, as previously described [1, 27, 28]. Animals with high titers of NAb should be avoided.
Obtain reference fundus optical coherence tomography (OCT) and fluorescence images (see Note 4).
A prophylactic steroid and antibiotic regimen is strongly recommended for this procedure. The day before surgery, administer 0.25 mg/kg of intramuscular (IM) Dexamethasone. The day of surgery, administer 0.25 mg/kg of IM Dexamethasone and 25 mg/kg of IM Cefazolin. Additionally, after surgery inject subconjunctivally 0.5 mL of 4 mg/mL Dexamethasone and 0.5 mL of 330 mg/mL Cefazolin. If any postsurgical inflammation is observed without sign of infection, administer 0.25 mg/kg of IM Dexamethasone for 2 days, followed by a 3–5 day taper.
Prior to surgery, sedate animals using 20 mg/kg of IM Ketamine, 0.005 mg/kg of subcutaneous Glycopyrrolate, and 1 mg/kg of subcutaneous Cerenia.
Place an IV catheter and start a saline drip, then intubate the animal.
Once sedated, dilate the eyes using 2.5% phenylephrine, 1% tropicamide, and 1% cyclopentalate.
Place the animal on a ventilator and maintain general anesthesia using isoflurane (1.5–2.5%) with continuous monitoring of vital signs.
Postsurgically, administer sustained release Buprenorphine and Meloxicam.
3.4. SubILM Injection
Prepare eyes with povidone-iodine topical solution plus a Benzoin Tincture swab stick and drape in standard sterile fashion.
Carry out the surgical procedure under sterile conditions in a dedicated veterinary ophthalmic surgical suite equipped with anesthesia, ophthalmic surgical microscope with video, and vitrectomy surgical system. We use an Accurus 800CS surgical system with Xenon light source (see Note 5), Total Plus gauge 23 or 25 Vitrectomy Pak with valved trocars (see Note 6), and Zeiss VISU 200 ophthalmic surgical microscope equipped with digital video.
Visualize the posterior segment of retina using an irrigating Machemer magnifying vitrectomy contact lens.
Perform a standard three-port pars plana vitrectomy using an inferior infusion irrigating cannula and BSS Plus irrigating solution (maintain a pressure of 10–30 mm/Hg).
Enlarge the superior-temporal sclerotomy with a 20–25G V-Lance Knife to provide access for the injection cannula.
Use a 36–42G retinal needle to deliver vector into the subILM space beneath the macular region (see Note 7). Couple the needle to a 0.5 mL syringe using a 6″ T-Connector extension set. The injection is made by placing the tip of the cannula almost parallel to the surface of the ILM (see Note 8), and advancing just enough to penetrate the ILM (Fig. 1). Once subILM access is observed, introduce the vector solution by either manual injection or using a syringe pump (see Note 9). After a bleb of sufficient size and location is observed, retract the needle slowly (see Note 10). A typical injection volume is approximately 20 μL. Estimate the amount of introduced vector by manually inspecting the change in volume in the syringe.
Close the vitrectomy: remove ports and suture sclera/conjunctiva with 8–0 or 9–0 vicryl.
We recommend performing OCT (see Note 4) immediately after surgery to document the location and extent of the subILM injections. If OCT is not available, document the bleb (s) using the surgical microscope video recording device as well as a surgical dictation describing the bleb locations with respect to anatomical landmarks within the retina (see above).
Apply 2–3 drops of Neomycin/Polymyxin B Sulfates and Dexamethasone Ophthalmic Suspension to each treated eye.
The length of animal survival following this procedure will depend on the experiment. Perform a clinical examination at 3 days after surgery, and at regular intervals thereafter, to ensure that there are no inflammatory responses.
In vivo imaging should be performed at regular intervals to confirm that the bleb has resolved without incident, and to monitor expression of the fluorescent reporter (if used).
At the end of the experiment, euthanize the animals (see Note 11) and prepare eyes for histology (see Note 12).
4. Notes
Rep-cap and Helper plasmids can be propagated in a variety of bacterial cells that do not already contain the antibiotic resistance encoded on the plasmids. AAV vector plasmids should be propagated in recombination (recB, recJ) deficient bacteria such as Sure cells to avoid recombination of the AAV ITRs. Bacteria can be grown in terrific broth supplemented with the appropriate antibiotic. Vector plasmid- and Rep-cap plasmid-containing cells are best purified using a Qiagen EndoFree plasmid purification kit of the appropriate scale to meet AAV packaging requirements, typically Maxi or Giga size. Recovery of large Helper plasmids using commercially available plasmid purification kits is inefficient, and requires density gradient purification such as a CsCl gradient. However, this results in higher levels of endotoxin in the purified plasmid DNA, which may carry through to the final AAV prep. Several Contract Research Organizations (CROs) such as Aldevron offer plasmid purification in which most endotoxin is removed, and also sell stock AAV helper plasmids such as pXX6.
AAV vectors of suitable purity and sterility for use in NHP studies can be obtained from several academic Vector Cores, such as the Powell Gene Therapy Center (University of Florida), the University of Pennsylvania Vector Core, and the University of North Carolina Gene Therapy Center Vector Core.
Full-strength Healon is very difficult to pipette. To avoid pipetting, fill the microcentrifuge tube with an identical volume of sterile BSS. Mark the microcentrifuge at the height of the BSS volume, then remove and discard the BSS. Open the Healon packaging and assemble the dispenser. Slowly dispense Healon into the microcentrifuge tube until it reaches the previously marked volume line (step 10). A brief spin in a microcentrifuge may be necessary to collect Healon in the bottom of the tube and evaluate the volume.
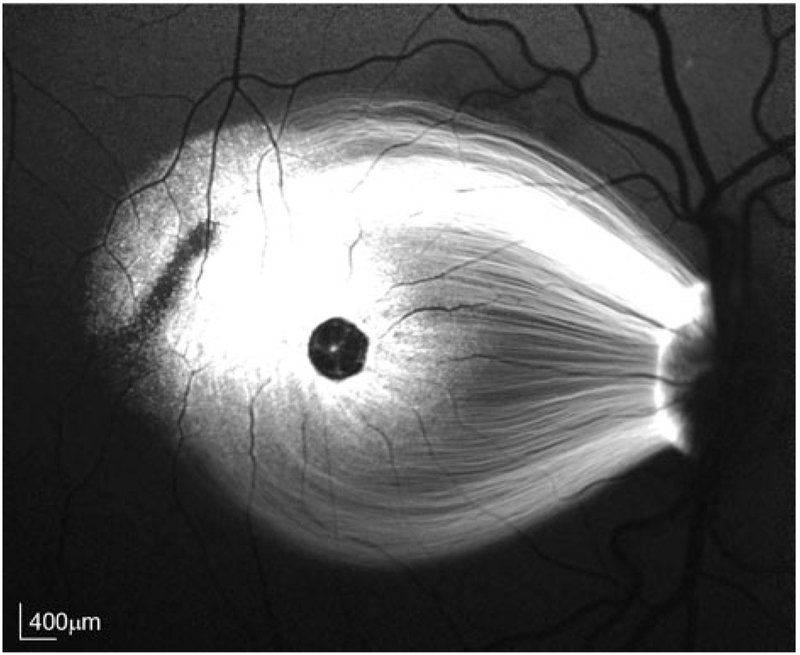
Normal and fluorescent fundus images should be obtained both prior to surgery and after surgery. In studies using GFP as a reporter, the 488 laser of the Heidelberg Spectralis® confocal scanning laser ophthalmoscope with fluorescence detection capability should be used (see below). However, for reporters excited at other wavelengths, a fundus camera (Topcon TRC-50EX) equipped with custom excitation and barrier filters is required. For most in vivo retinal imaging, we recommend a Heidelberg Spectralis® confocal scanning laser ophthalmoscope, with spectral-domain optical coherence tomography (SD-OCT) and fluorescence detection capability. The signal-to-noise ratio of GFP fluorescence is excellent with this system (Fig. 2). Immediate postsurgical OCT images can confirm that the injection is subILM and does not penetrate the retina. Subsequent imaging can confirm expression of GFP and document that the injection bleb has resolved.
The Alcon Accurus 800CS has reached end-of-life and has now been replaced with the Alcon CONSTELLATION® Vision System.
The original procedure was performed with 23G ports. We now prefer 25G self-sealing ports.
The design of the original cannula was not optimal, and the design of an optimal cannula for subILM injections is ongoing.
ICG Indocyanine green may be used to stain and better visualize the ILM prior to subILM injection. Inject a diluted solution of ICG (~0.1–0.3 mL) over the planned injection site and allow it to stain the ILM to effect prior to injection. To prepare the ICG solution, dissolve 25 mg of sterile ICG powder in0.5–1.0 mL of sterile water. Dilute this in 4.0–4.5 mL of BSS, resulting in a 0.5% ICG solution with an osmolarity of 270 mOsm.
We originally used a 500 μL Hamilton syringe operated manually or by a syringe pump to inject the vector. We currently recommend use of a MicroDose™ Injection Kit (Katalyst Surgical) or similar.
Clear visualization of the bleb with the surgical camera is strongly recommended to provide video stills and photos for postsurgical analysis.
In this and all other cases of euthanasia, the investigator should follow the AVMA Guidelines for the Euthanasia of Animals [29].
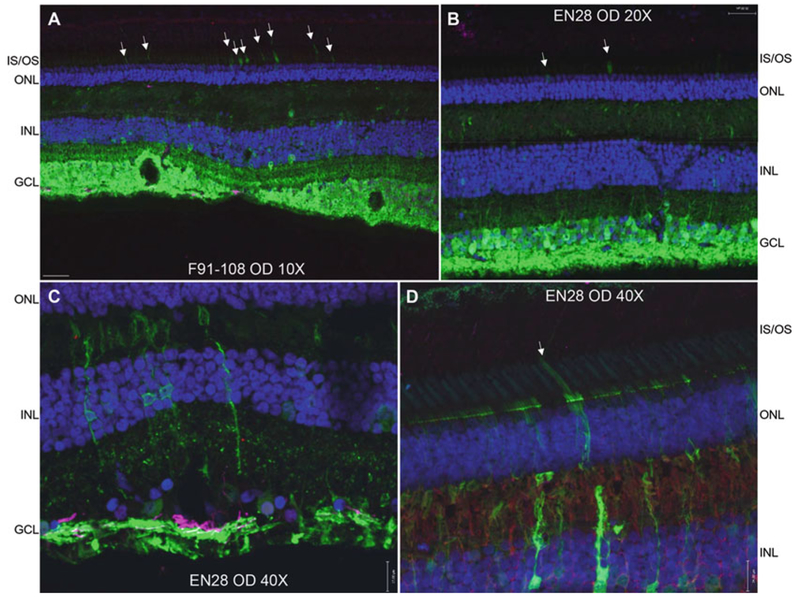
Expected histology results are shown in Fig. 3. Protocols are outlined in our previously published work [1, 15].
Fig. 2.
Fluorescence fundus image of AAV-mediated GFP expression in NHP subject EN-28 taken 4 months postinjection. Healon and AAV (1.7 × 1012 vector genomes per mL) were subILM-injected at a ratio of 1:1. Total bleb volume was 7.5 μL and contained a total of 6.2 × 109 vector genomes. Scale bars = 400 μm
Fig. 3.
AAV2-CBA-mediated GFP expression in subjects EN-28 and F91–108. Raw GFP (green), DAPI (blue), and Glial fibrillary acidic protein (purple) are shown in all panels. Glutamine synthetase (red) is shown in panel D. The vast majority of retinal ganglion cells within the subILM injection blebs are GFP positive (a, b). Transduction of photoreceptors (white arrows in a, b, d) and Muller glia (c, d) is also observed. IS/OS inner segments/outer segments, ONL outer nuclear layer, INL inner nuclear layer, GCL ganglion cell layer. Scale bars = 70 μm (a), 35 μm (b), 17 μm (c, d)
References
- 1.Boye SE, Alexander JJ, Boye SL, Witherspoon CD, Sandefer KJ, Conlon TJ, Erger K, Sun J, Ryals R, Chiodo VA, Clark ME, Girkin CA, Hauswirth WW, Gamlin PD (2012) The human rhodopsin kinase promoter in an AAV5 vector confers rod- and cone-specific expression in the primate retina. Hum Gene Ther 23(10):1101–1115. 10.1089/hum.2012.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, Wang L, Castle MJ, Maguire AC, Grant R, Wolfe JH, Wilson JM, Bennett J (2011) Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med 3(88):88ra54 10.1126/scitranslmed.3002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenberghe LH, Bell P, Maguire AM, Xiao R, Hopkins TB, Grant R, Bennett J, Wilson JM (2013) AAV9 targets cone photoreceptors in the nonhuman primate retina. PLoS One 8(1):e53463 10.1371/journal.pone.0053463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, Peden MC, Aleman TS, Boye SL, Sumaroka A, Conlon TJ, Calcedo R, Pang JJ, Erger KE, Olivares MB, Mullins CL, Swider M, Kaushal S, Feuer WJ, Iannaccone A, Fishman GA, Stone EM, Byrne BJ, Hauswirth WW (2012) Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol 130(1):9–24. 10.1001/archophthalmol.2011.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanoff M, Kertesz Rahn E, Zimmerman LE (1968) Histopathology of juvenile retinoschisis. Arch Ophthalmol 79(1):49–53 [DOI] [PubMed] [Google Scholar]
- 6.Condon GP, Brownstein S, Wang NS, Kearns JA, Ewing CC (1986) Congenital hereditary (juvenile X-linked) retinoschisis. Histopathologic and ultrastructural findings in three eyes. Arch Ophthalmol 104(4):576–583 [DOI] [PubMed] [Google Scholar]
- 7.Yin L, Greenberg K, Hunter JJ, Dalkara D, Kolstad KD, Masella BD, Wolfe R, Visel M, Stone D, Libby RT, Diloreto D Jr, Schaffer D, Flannery J, Williams DR, Merigan WH (2011) Intravitreal injection of AAV2 transduces macaque inner retina. Invest Ophthalmol Vis Sci 52(5):2775–2783. 10.1167/iovs.10-6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV (2013) In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 5(189):189ra176 10.1126/scitranslmed.3005708 [DOI] [PubMed] [Google Scholar]
- 9.Ye GJ, Budzynski E, Sonnentag P, Miller PE, Sharma AK, Ver Hoeve JN, Howard K, Knop DR, Chulay JD (2015) Safety and biodistribution evaluation in cynomolgus macaques of rAAV2tYF-CB-hRS1, a recombinant adeno-associated virus vector expressing retinoschisin. Hum Gene Ther Clin Dev 26(3):165–176. 10.1089/humc.2015.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotterman MA, Yin L, Strazzeri JM, Flannery JG, Merigan WH, Schaffer DV (2015) Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther 22(2):116–126. 10.1038/gt.2014.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalkara D, Kolstad KD, Caporale N, Visel M, Klimczak RR, Schaffer DV, Flannery JG (2009) Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther 17(12):2096–2102. 10.1038/mt.2009.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boye SL, Bennett A, Scalabrino ML, McCullough KT, Van Vliet K, Choudhury S, Ruan Q, Peterson J, Agbandje-McKenna M, Boye SE (2016) Impact of heparan sulfate binding on transduction of retina by recombinant adeno-associated virus vectors. J Virol 90(8):4215–4231. 10.1128/JVI.00200-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto B, Blanks JC, Ryan SJ (1984) Topographic variations in the rabbit and primate internal limiting membrane. Invest Ophthalmol Vis Sci 25(1):71–82 [PubMed] [Google Scholar]
- 14.Cehajic-Kapetanovic J, Le Goff MM, Allen A, Lucas RJ, Bishop PN (2011) Glycosidic enzymes enhance retinal transduction following intravitreal delivery of AAV2. Mol Vis 17:1771–1783 [PMC free article] [PubMed] [Google Scholar]
- 15.Boye SE, Alexander JJ, Witherspoon CD, Boye SL, Peterson JJ, Clark ME, Sandefer KJ, Girkin CA, Hauswirth WW, Gamlin PD (2016) Highly efficient delivery of adeno-associated viral vectors to the primate retina. Hum Gene Ther 27(8):580–597. 10.1089/hum.2016.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Samulski RJ, Xiao X (1997) Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol 71(7):5236–5243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM (1999) Gene therapy vectors based on adeno-associated virus type 1. J Virol 73(5):3994–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao X, Li J, Samulski RJ (1998) Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 72(3):2224–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimm D, Kern A, Rittner K, Kleinschmidt JA (1998) Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther 9(18):2745–2760. 10.1089/hum.1998.9.18-2745 [DOI] [PubMed] [Google Scholar]
- 20.Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N (2004) Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 10(2):302–317 [DOI] [PubMed] [Google Scholar]
- 21.Gray JT, Zolotukhin S (2011) Design and construction of functional AAV vectors. Methods Mol Biol 807:25–46. 10.1007/978-1-61779-370-7_2 [DOI] [PubMed] [Google Scholar]
- 22.Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A, Chiodo VA, Fajardo DS, Roman AJ, Deng WT, Swider M, Aleman TS, Boye SL, Genini S, Swaroop A, Hauswirth WW, Jacobson SG, Aguirre GD (2012) Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci U S A 109(6):2132–2137. 10.1073/pnas.1118847109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, Mingozzi F, Hui D, Chung D, Rex TS, Wei Z, Qu G, Zhou S, Zeiss C, Arruda VR, Acland GM, Dell’Osso LF, High KA, Maguire AM, Bennett J (2008) Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther 16(3):458–465. 10.1038/sj.mt.6300389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ Jr, Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, Byrne BJ, Snyder RO (2002) Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28(2):158–167 [DOI] [PubMed] [Google Scholar]
- 25.Veldwijk MR, Topaly J, Laufs S, Hengge UR, Wenz F, Zeller WJ, Fruehauf S (2002) Development and optimization of a real-time quantitative PCR-based method for the titration of AAV-2 vector stocks. Mol Ther 6(2):272–278 [DOI] [PubMed] [Google Scholar]
- 26.Lock M, Alvira MR, Chen SJ, Wilson JM (2014) Absolute determination of single-stranded and self-complementary adeno-associated viral vector genome titers by droplet digital PCR. Hum Gene Ther Methods 25(2):115–125. 10.1089/hgtb.2013.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day TP, Byrne LC, Flannery JG, Schaffer DV (2018) Screening for neutralizing antibodies against natural and engineered AAV capsids in nonhuman primate retinas. Methods Mol Biol 1715:239–249. 10.1007/978-1-4939-7522-8_17 [DOI] [PubMed] [Google Scholar]
- 28.Desrosiers M, Dalkara D (2018) Neutralizing antibodies against adeno-associated virus (aav): measurement and influence on retinal gene delivery. Methods Mol Biol 1715:225–238. 10.1007/978-1-4939-7522-8_16 [DOI] [PubMed] [Google Scholar]
- 29.AVMA Guidelines for the Euthanasia of Animals: 2013. Edition [Google Scholar]