Abstract
Dopamine (DA) affects GABA neuronal function in striatum and together these neurotransmitters play a large role in locomotor function. We recently reported that unilateral striatal administration of GDNF, a growth factor that has neurotrophic effects on DA neurons and enhances DA release, bilaterally increased striatal neuron activity related to locomotion in aged rats. We hypothesized that the GDNF enhancement of DA function and resulting bilateral enhancement of striatal neuronal activity was due to prolonged bilateral changes in DA- and GABA-regulating proteins. Therefore in these studies we assessed dopamine- and GABA-regulating proteins in striatum and substantia nigra (SN) of 24 month old Fischer 344 rats, 30 days after a single unilateral striatal delivery of GDNF. The nigrostriatal proteins investigated were the DA transporter (DAT), tyrosine hydroxylase (TH), and TH phosphorylation and were examined by blot-immunolabeling. The striatal GABA neuron-related proteins were examined by assay of the DA D1 receptor, DARPP-32, DARPP-32 Thr34 phosphorylation, and glutamic acid decarboxylase (GAD). Bilateral effects of GDNF on TH and DAT occurred only in SN, as 30 µg GDNF increased ser19 phosphorylation, and 100 µg GDNF decreased DAT and TH protein levels. GDNF also produced bilateral changes in GAD protein in striatum. A decrease in DARPP-32 occurred in the ipsilateral striatum, while increased D1 receptor and DARPP-32 phosphorylation occurred in the contralateral striatum. The 30 µg GDNF infusion into lateral striatum was confined to ipsilateral striatum and substantia nigra. Thus, long-lasting bilateral effects of GDNF on proteins regulating DA and GABA neuronal function likely alter physiological properties in neurons, some with bilateral projections, associated with locomotion. Enhanced nigrostriatal excitability and DA release by GDNF may trigger these bilateral effects.
Keywords: nigrostriatal, striatonigral, trophic factor, dopamine transporter, DARPP-32, glutamic acid decarboxylase (GAD), tyrosine hydroxylase, GDNF, bilateral
Glial cell line-derived neurotrophic factor (GDNF) improves locomotor function in animal models of parkinsonism and may produce effects in patients with advanced Parkinson’s disease (Gash et al., 1996; Gill et al., 2003; Grondin et al., 2002, 2003; Slevin et al., 2005). The site and method of delivery into the brain are critical for the success of GDNF in this regard (Nutt et al., 2003; Lang et al., 2006) and for its distribution in the basal ganglia (Salvatore et al., 2006). Increased dopamine (DA) or DA metabolite levels, in particular in the substantia nigra (SN), is a common feature of GDNF improvement of motor function in animal models of parkinsonism or aging (Gash et al., 1996; Hebert and Gerhardt, 1997; Gerhardt et al., 1999; Grondin et al., 2002; Grondin et al., 2003). A unique property of GDNF treatment is a documented bilateral improvement in locomotion following unilateral delivery (Grondin et al., 2003; Slevin et al., 2005). We recently reported that unilateral striatal delivery of GDNF increases striatal neuron firing rates that are related to locomotor activity in a bilateral manner (Stanford et al., 2007). These effects suggest a potential mechanism for the influence of GDNF on locomotor activity that involves altering neuronal activity in the striatum or, more generally, throughout the basal ganglia.
Increased neuronal activity implies increased neurotransmitter release. Growth factors like GDNF can increase electrical activity of nigrostriatal DA neurons (Shen et al., 1994; Bourque and Trudeau, 2000; Yang et al., 2001). Improved locomotion could also involve extrastriatal loci, particularly the SN (Bjorklund and Dunnet, 2007). Striatal GDNF administration increases tyrosine hydroxylase (TH) phosphorylation at serine 31 in the SN (Salvatore et al., 2004). Increased phosphorylation of TH at ser31 or ser40 has been shown to increase DA biosynthesis, depending upon the level of phosphorylation achieved (Salvatore et al., 2001). The use of GDNF, particularly in aging studies and regardless of the site of administration, increases DA tissue or synaptic levels in the SN or striatum, and such increases are associated with improved motor function (Gash et al., 1996; Gerhardt et al., 1999; Grondin et al., 2003; Salvatore et al., 2004), which may be via increased TH phosphorylation at ser31 or ser40. Furthermore, enhanced bilateral striatal neuronal activity and DA function in the SN appear to be important for locomotor improvement following GDNF. The molecular basis for bilateral effects following unilateral delivery is not known. Since the vast majority of neurons in striatum are GABAergic, it follows that the ability of GDNF to alter their activities may have origins in altering function of proteins related to GABA neuronal function, possibly through GFRα−1 receptors which are expressed on GABA neurons (Sarabi and et al., 2001) and DA neurons (Sarabi et al., 2001; Smith et al., 2003). The GFRα−1 receptors signal via the transmembrane receptor tyrosine kinase, RET, and also via NCAM (Coulpier et al., 2002; Paratcha et al., 2003). The effects of GDNF on DA neurons may not be limited to TH. The dopamine transporter (DAT) is known to be a critical player in locomotor function (Hebert and Gerhardt, 1998; Spielewoy et al., 2000; Uhl 2003; Nutt et al., 2004) and any effect of GDNF on this protein has yet to be reported.
Experimental findings make a case for hemispheric interdependency in basal ganglia function. One possibility for bilateral effects may involve GDNF transport to areas outside of striatum. The extrastriatal and bilateral expression of GDNF in the nigrostriatal pathway following unilateral striatal delivery of GDNF has been previously shown (Lapchak et al., 1998; Yi et al., 2003; Eslamboli et al., 2005; Salvatore et al., 2006), and the extent of GDNF distribution is dosage-dependent. Bilateral molecular compensation in the SN and other basal ganglia structures does occur following DA loss from a unilateral lesion to the nigrostriatal pathway (Kozlowski et al., 2004; Yang et al., 2007; Breit et al., 2008). These studies highlight the critical importance of DA in maintaining function of the other components of the basal ganglia in both hemispheres. Possible pathways of bilateral interactions in the basal ganglia include the corticostriatal neurons (Dunnett et al., 2005) and nigral efferents from the pedunculopontine nuclei (Breit et al., 2008). Bilateral changes in the functional properties of proteins associated with striatal neuronal activity could be a source of bilateral locomotor effects of GDNF.
The clinical and animal study results that demonstrate bilateral effects of GDNF on physiological and motoric activity prompted this study to investigate the molecular basis for the long term effects on the striatal GABAergic and dopaminergic nerve terminals and nigral dopaminergic cell bodies following a unilateral delivery of GDNF. In the current study we examined tissue from the same rats that showed bilateral effects in striatal neuron function (Stanford et al., 2007) in order to determine the bilateral effects of a single, unilateral dose (30 µg) of GDNF on proteins related to post-synaptic neurons (DA D1 receptor, DARPP-32, pDARPP-32 (Thr34), and GAD 65/67) in the striatum. As effects of GDNF have been reported to be dosage-dependent (Aoi et al., 2000; Eslamboli et al., 2005), we also examined the effects of two unilateral doses (30 & 100 µg) of GDNF on DA-regulating proteins (TH and DAT) in striatum and SN.
MATERIALS & METHODS:
Animals:
Aged (24–25 months-old) male Fischer (F344) rats were obtained from NIA colonies. Animals were housed with ad libitum access to food and water. Protocols for animal handling were approved by the local Institutional Animal Care and Use Committee and procedures adhered to the Guide for the Care and Use of Laboratory Animals. The rats that were injected with the 30 µg dose of GDNF were also implanted with head stages and multi-wire stainless steel Teflon-coated microelectrode arrays (50 µm diameter per wire) immediately after GDNF or vehicle infusion. Electrophysiological recordings of these particular rats were conducted 30 days afterward (Stanford et al., 2007). Within one week after electrophysiological recordings, the rats were euthanized and the striatum and SN were dissected and frozen at −70 C until processing for western blot. Rats receiving the 100 µg dose were euthanized ~30 days following GDNF or vehicle.
GDNF Infusion
Two anesthetics were used in the two sets of studies involving the two different doses of GDNF infusion; sodium pentobarbital (38 mg/kg, i.p., for 30 µg dose) or chloral hydrate (350 mg/kg, for 100 ug dose). Access to striatum for GDNF or vehicle (citrate solution) delivery was made by drilling small holes in the skull. GDNF (human recombinant form expressed in E. coli) and vehicle were both from Amgen (Thousand Oaks, CA). To administer GDNF or vehicle, a 26 gauge needle was used to infuse 30 µg GDNF (6 µg/µl, 5 µl total) at a rate of 0.25 µl/min at coordinates, relative to bregma (+1.0 mm AP, +4.0 ML, 5.0 mm from the dura; Paxinos and Watson, 1986) and to also infuse 100 µg GDNF (5 µg/µl, 20 µl total) at a rate of 1.0 µl/min at coordinates +1.0 mm AP, 2.5 mm ML, 5.0 mm from the dura.
Blot-immunolabeling experiments
Frozen tissue samples were sonicated in 300 µl of 1% sodium dodecyl sulfate solution (pH ~8; 5 mM Tris, 1 mM EDTA) using a Fisher 60 sonic Dismembrator. Protein concentration was determined for each sample using the bicinchoninic acid method. Samples were subjected to SDS-PAGE electrophoresis, and sample proteins were transferred electrophorectically (25–30V / 10 cm) overnight in Tris/glycine/methanol buffer onto nitrocellulose membranes (0.45 µm, Bio-Rad Laboratories, Hercules, CA, USA). Ponceau S staining of the blots was done after the transfer to ascertain relative sample-to-sample protein transfer. The membranes were then immersed in quenching buffer containing 1% polyvinylpyrrolidone and 0.05% Tween 20 for a minimum of 2 h before incubation with primary antibodies for 1–2 h, followed by 1 h incubation with an appropriate secondary antibody [swine anti-rabbit IgG (0.8 µg/ml) or rabbit anti-goat IgG (1 µg/ml); Dako, Glostriatumup, Denmark], then a 1 h incubation with [I125] protein A (~30 µCi/µg); Amersham, Piscataway, NJ). Due to limited amounts of tissue available for all analyses, some were conducted with less than eight samples for both treatment groups. Analyses were performed by assaying samples from vehicle-treated and GDNF-treated rats from the same hemisphere; thus tissues obtained from ipsilateral and contralateral sides to the site of GDNF administration were analyzed separately.
Antibodies
Blot-immunolabeling assays were conducted within the dynamic working range of each antibody. These were established using tissue standard curves of striatum and SN harvested from rats independent from this study. The levels for each protein of interest were normalized to total protein. Protein phosphorylation levels were normalized to total specific protein concentration in each sample. Total TH levels (tTH) and site-specific phospho-TH values were determined in separate blots for each primary antibody by quantifying gamma radioactivity per sample and interpolating against the standard curve generated for each assay, as previously described (Salvatore et al., 2000). The TH phosphorylation stoichiometries (PS) for serine 19, 31, and 40 were obtained by dividing ng of phospho-TH by ng of tTH loaded for phosphorylation analysis. Antibodies were obtained from the following sources: all TH antibodies were a gift from Dr. John Haycock (total, p19, p31, and p40), DAT (Santa Cruz, #sc-1433), DA D1 receptor (Santa Cruz, #sc-14001), DARPP-32 (Chemicon, #AB1656), phospho-DARPP-32Thr34 (Phosphosolutions, #p1025–34), and GAD 65/67 (Chemicon, #AB1511).
GDNF immunostaining
After infusion with 30 µg GDNF, as described in GDNF Infusion, anesthetized animals were perfused with phosphate-buffered saline (PBS), followed by cold 4% paraformaldehyde in PBS. The brain was removed and immersed in fixative overnight at 4ºC. Brains were equilibrated in a cryoprotectant solution of 30% sucrose/PBS at 4ºC. Coronal sections (50 µm thick) were cut on a sliding microtome with a freezing stage. Antigen detection was conducted on free-floating sections by a peroxidase method. Endogenous peroxidase activity was quenched with 0.1% H2O2/PBS for 10 min. The sections were washed in PBS and incubated for 5 min in 0.3% Triton X-100/PBS, and washed before applying the primary anti-GDNF antibody (R & D Systems, Minneapolis, MN; 1:500) and incubating overnight at 4ºC on a shaking platform. The biotinylated secondary antibody was from DAKO Cytomation (Carpinteria, CA; 1:2000), incubated for 1 hr at room temperature. The sections were washed with PBS and labeled with horseradish peroxidase-conjugated Extravidin (Sigma, St. Louis, MO; 1:2000) for 30 min at room temperature. The chromogen was diaminobenzidine (0.67 mg; Sigma) in 0.3% H202, 80 mM sodium acetate buffer containing 8 mM imidazole and 2% NiSO4. After mounting on slides, the sections were dehydrated in a series of alcohol and xylene and coverslipped with Eukitt (Electron Microscopy Sciences, Hatfield, PA).
Statistics
Dissection of striatum and SN was done alternating the treatment group in order. For each assay of specific protein levels or phosphorylation, samples were analyzed by region (striatum or SN) and by side (ipsilateral or contralateral). Analysis in this manner allowed for both treatments to be assayed from all subjects on the same blot. An alternative method for this analysis would have been to compare ipsilateral and contralateral tissues from the same rat by running them on the same gel. However, this approach would have precluded the analysis of all rats in the study on the same gel. We chose the former method to operationally-match the side (ipsilateral or contralateral) with treatment. There were nine different assays conducted in this study to measure the levels and/or phosphorylation state of six proteins (tyrosine hydroxylase (total, p19, p31, and p40), DA transporter, DA D1 receptor, DARPP-32, phospho-DARPP-32Thr34, and GAD 65/67). Statistical significance was determined using an unpaired (if unequal n between the groups) t-test, or paired (if equal n from both treatment groups could be assayed). In paired analyses, results from each assay were ranked highest to lowest for both treatment groups. A two-tailed t-test was used for all measures of significance. In the case of total TH assessment, we employed the use of an unpaired ANOVA with Bonferroni post-hoc test. In spite of assay of the ipsilateral and contralateral tissues on separate gels/blots, the normalization to the total TH standard justifies its use. In the case of analyses without such standard but with equal numbers, the use of a paired t-test is justified, given equal n, since the tissues were obtained from an inbred rat species of identical age (24 months), their handling, housing, and experimental treatment (each was implanted with an electrode) was uniform, the samples for each data point are independent (that is, only one analysis for each dependent measure per sample is used for statistical analysis), and tissue analysis was operationally-matched (subject to same blot transfer, primary, secondary, and protein A exposures). Statistical significance was defined as p<0.05.
RESULTS:
GDNF distribution
Infusion of 30 µg GDNF at the infusion rate and volume described resulted in a widespread diffusion in the ipsilateral, but not contralateral, dorsolateral striatum one day following the infusion (Fig. 1 A top panel). GDNF was also detected in the ipsilateral, but not contralateral dorsolateral striatum one week following infusion (Fig. 1 B top panel). GDNF was also detected in the ipsilateral, but not contralateral, substantia nigra one day following infusion (Fig 1 A, B bottom panel). One week following infusion, there was much less GDNF detected (Fig 1 C bottom panel). No GDNF was detected in sections caudal to SN where the pedunculopontine nucleus is located (data not shown).
Figure 1.
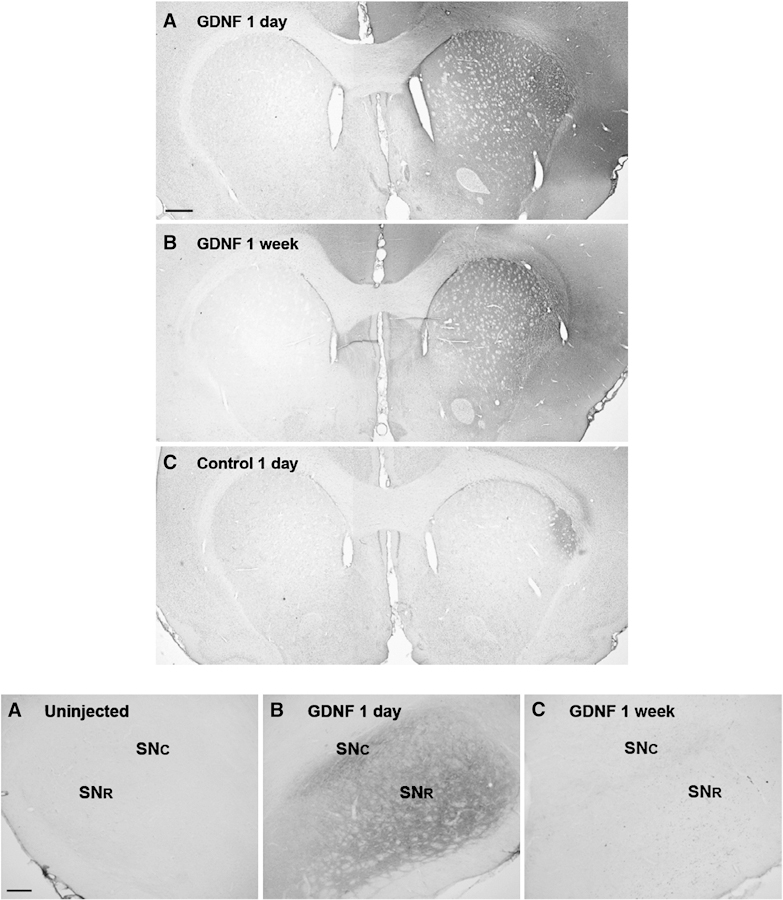
Diffusion and distribution of 30 µg GDNF in the striatum (top panel) and substantia nigra (bottom panel) at one day and one week following infusion. Top panel: One day following infusion (A), GDNF staining is visible throughout dorsolateral striatum and most dense at the injection location (M/L 3.5 mm, D/V 5.0 mm) and is not detectable in the contralateral striatum. One week following infusion (B), GDNF staining is not as intense after one day, but is still easily detectable in the dorsolateral striatum. GDNF is not detectable in the contralateral striatum. (C) Negative control, staining protocol performed on adjacent section without GDNF primary antibody. Scale bar=536 µm. Bottom panel: One day following GDNF infusion (A), GDNF is not detectable in substantia nigra (SN) on the uninjected side, but is detected throughout the ipsilateral SN (B) in pars compacta (SNc) and pars reticulata (SNr). One week following infusion (C), detection of GDNF in SN is far less than that seen after one day. Scale bar=134 µm.
DA-regulating proteins
tyrosine hydroxylase
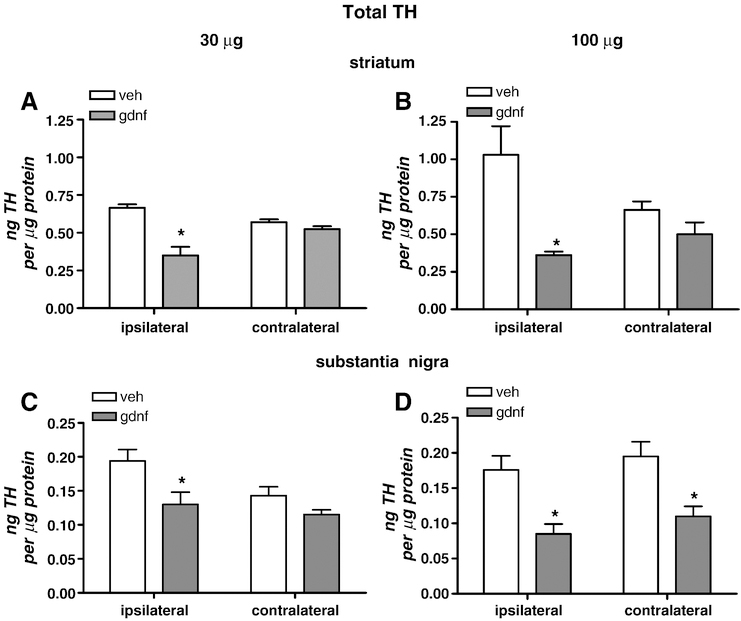
The 30 and 100 µg doses of GDNF reduced levels of total TH by 50 to 60 % in the ipsilateral, but not contralateral, striatum (Fig. 2 A, B). However, whereas 30 µg GDNF reduced total TH levels in the ipsilateral SN, the higher 100 µg dose reduced total TH levels in both ipsilateral and contralateral SN (Fig 2 C, D). It is noted that the levels of TH in the SN contralateral to the vehicle injection in the 30 µg group rats were less than that in the ipsilateral SN, which may have masked an effect of GDNF at this dose, since the TH values as ng/µg were around 0.10 for both doses in both hemispheres. These findings show that GDNF can modify the TH levels away from its site of administration and bilaterally in the SN in a dose-dependent manner.
Figure 2.
Tyrosine hydroxylase protein levels in striatum and SN at 30 days following vehicle or GDNF administration into right (ipsilateral) striatum. Values are normalized to total protein and quantified against total TH protein standard to attain ng TH per µg protein values (A, 30 µg, B, 100 µg, striatum; C, 30 µg, D, 100 µg SN). Total TH levels normalized to total protein [n=4–6, vehicle; 6–8, GDNF]. *p<0.05
Tyrosine hydroxylase phosphorylation
Ser19
In striatum, a dose of 30 µg GDNF did not significantly increase ser19 phosphorylation in either the ipsilateral or contralateral side (Fig 3 A). However, in the ipsilateral striatum, a trend toward an increase was observed and a significant difference in variance in the GDNF-treated group contributed to the lack of statistical significance. As shown in previous studies, 100 µg of GDNF did increase ser19 phosphorylation in the ipsilateral striatum (Salvatore et al, 2004). However, no effect on phosphorylation was seen in the contralateral striatum (Fig 3 B).
Figure 3.
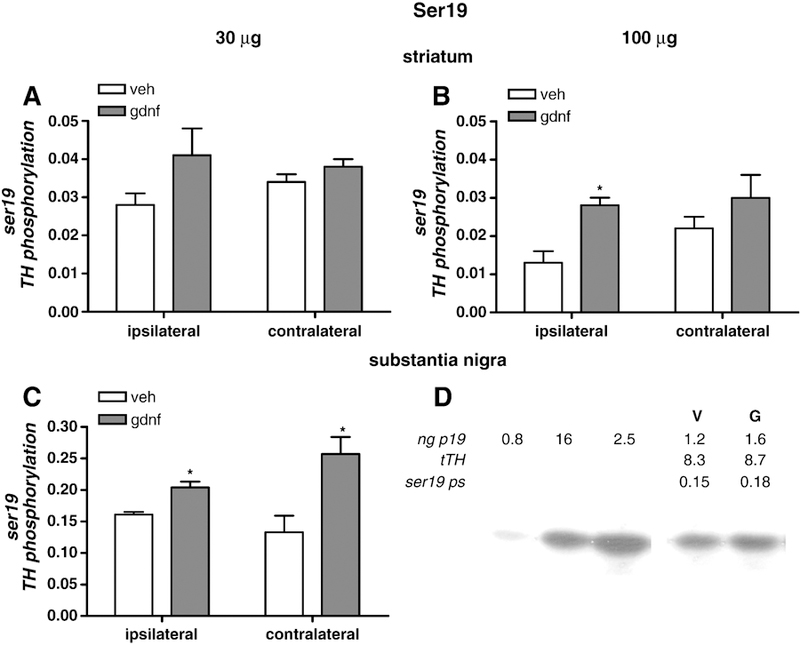
Serine 19 TH phosphorylation. Phosphorylation of TH at ser19 in striatum as a result of GDNF delivery at 30 µg (A) and 100 µg (B) normalized to total TH levels. In C, ser19 phosphorylation in the SN is shown following 30 µg GDNF. Results are presented as phosphorylation stoichiometry, derived from assessment of site-specific phosphorylation with phospho-specific antibody to ser19 for each sample against a calibrated phospho-TH standard for ser19, and normalized to the load of TH previously determined for each sample. [n=4–5, vehicle; 5–8, GDNF]. *p<0.05. D. Representative quantitative western blot to show TH phosphorylation standard curve, as ng of phosphorylated ser19 standard, the resulting ng ser19 from the vehicle (V)- and 30 µg GDNF (G)-treated rats, the ng of total TH protein (tTH) loaded for this result, and the stoichiometry of phosphorylation at ser19 (ng p19/ng tTH).
In surprising contrast to the lack of effect seen in striatum, the 30 µg dose of GDNF increased ser19 phosphorylation in both ipsilateral and contralateral SN (Fig. 3 C). A lack of sample availability prevented analysis of the effects of 100 µg GDNF on ser19 in SN. This finding supports the contention that GDNF can increase DA neuron function by increasing DA neuron excitability, as this phosphorylation site, ser19, shows increased phosphorylation with depolarizing stimulation in vivo (Haycock and Haycock, 1991).
Ser31
In striatum, 30 µg GDNF significantly increased ser31 phosphorylation in the ipsilateral, but not contralateral side (Fig. 4 A). The stoichiometry was 0.144 in vehicle and 0.231 in animals injected with 30 µg GDNF. In contralateral striatum, ser31 phosphorylation stoichiometry was 0.211 in the vehicle and 0.230 in the rats injected with 30 µg GDNF. 100 µg GDNF also significantly increased ser31 phosphorylation in the ipsilateral, but not contralateral, striatum (Fig. 4 B).
Figure 4.
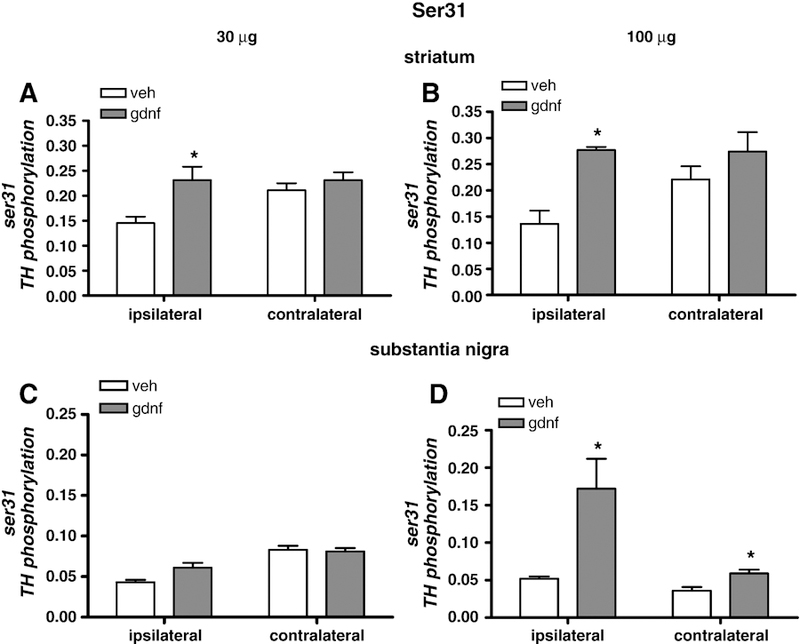
Serine 31 TH phosphorylation. Phosphorylation of TH at ser31 in striatum as a result of GDNF delivery at 30 µg (A) and 100 µg (B) normalized to total TH levels. Phosphorylation at ser31 in SN 30 days following 30 µg GDNF (C) and 100 µg (D) normalized to total TH levels. Results are presented as phosphorylation stoichiometry, derived from assessment of site-specific phosphorylation with phospho-specific antibody to ser31 for each sample against a calibrated phospho-TH standard for ser31, and normalized to the load of TH previously determined for each sample. [n=4–5, vehicle; 5–8, GDNF]. *p<0.05.
It was noted that the mean ser31 phosphorylation in the contralateral striatum of the vehicle-injected rats of both groups was higher (0.211) than in the ipsilateral side (.144). For this reason, we analyzed both sides from the vehicle-injected rats together on the same assay and found that this relationship held; that is, in vehicle-injected rats, ser31 phosphorylation in the contralateral striatum was significantly higher than that in the side ipsilateral to the vehicle injection. Total TH was subsequently analyzed in the same manner, yet there was no significant difference between the two sides of the vehicle-injected rat (data not shown).
In the SN, there was a trend toward an increase in ser31 phosphorylation in the ipsilateral side with 30 µg GDNF, but not in the contralateral side (Fig 4 C). However, the 100 µg GDNF dose increased phosphorylation at ser31 in both ipsilateral and contralateral sides (Fig. 4 D). It was also noted that the ser31 phosphorylation stoichiometry in the contralateral side of vehicle injected animals was not increased compared to the value from the ipsilateral side as it was in the rat group in the 30 µg study.
Ser40
In striatum, 30 µg GDNF had no effect on ser40 TH phosphorylation in either the ipsilateral or contralateral side (Fig. 5 A). However, there was evidence of a trend toward an increase in ser40 phosphorylation associated with the 100 µg dose on the ipsilateral side. Previous work has shown that 100 µg GDNF can significantly increase ser40 phosphorylation in the striatum without effect in the ipsilateral SN (Salvatore et al., 2004). The 100 µg GDNF dose had no significant effect on ser40 phosphorylation in the contralateral SN (data not shown). Given these findings and results from previous work, it was unexpected that there was an increase in ser40 phosphorylation associated with the 30 µg GDNF dose in contralateral SN (Fig 5 C). Increased ser40 phosphorylation can also occur with depolarizing stimulation (Haycock and Haycock, 1991). The differences in ser40 phosphorylation changes seen from the two doses point out the possibility that the GDNF engages different signaling pathways, depending upon the dose.
Figure 5.
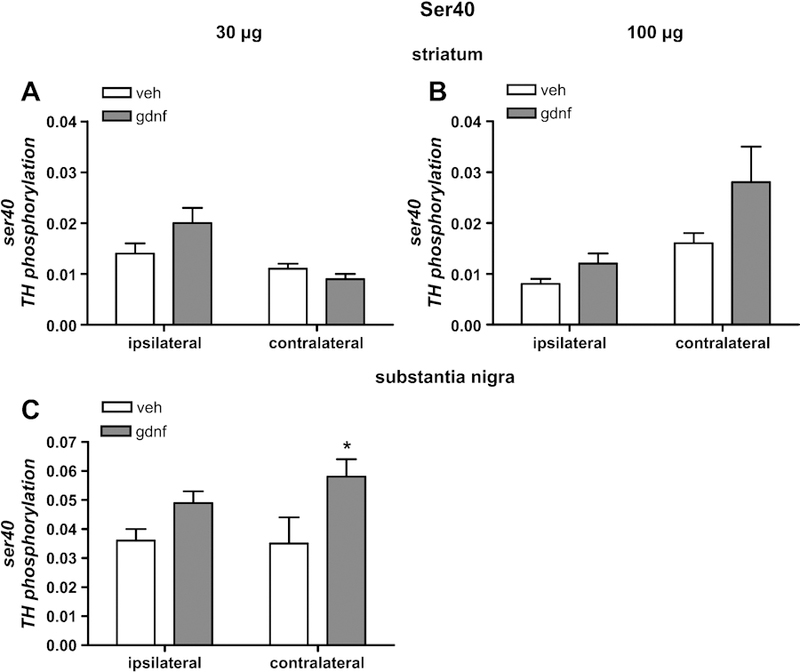
Serine 40 TH phosphorylation. Phosphorylation of TH at ser40 in striatum as a result of GDNF delivery at 30 µg (A) and 100 µg (B) normalized to total TH levels. Phosphorylation at ser40 in SN, 30 days following striatal delivery of 30 µg (C) normalized to total TH levels. Results are presented as phosphorylation stoichiometry, derived from assessment of site-specific phosphorylation with phospho-specific antibody to ser40 for each sample against a calibrated phospho-TH standard for ser40, and normalized to the load of TH previously determined for each sample. [n=4–5, vehicle; 5–8, GDNF]. *p<0.05.
DA transporter (DAT)
At the 30 µg dose of GDNF, there was no significant effect on DAT protein levels in either striatum or SN for either side (data not shown). However, the 100 µg dose of GDNF decreased DAT protein levels in ipsilateral striatum, but not contralateral striatum (Fig 6A). In the SN, there was a bilateral decrease in DAT protein from the 100 µg dose (Fig 6 B,C). This dose-dependence implies GDNF may have mechanisms of effect in addition to increasing nigrostriatal neuron excitability.
Figure 6.
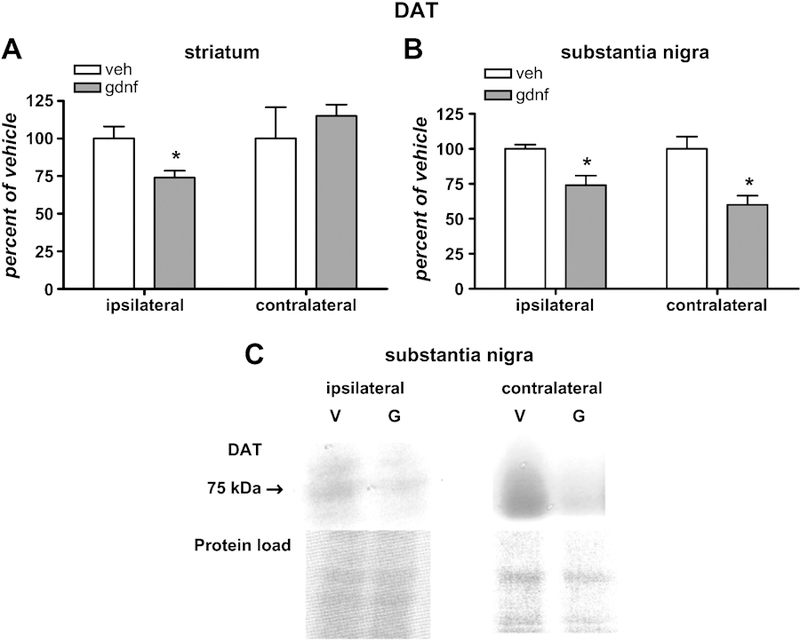
DAT immunoreactivity in striatum and SN following 100 µg GDNF. A. Changes in DAT, relative to vehicle, in striatum [n=5, vehicle; 6, GDNF] and, B. DAT, relative to vehicle, in SN [n=5, vehicle; 4–5, GDNF]. Nominal protein loads were 10–15 µg from striatum and 20–30 µg from SN. *p<0.05. C. top panels: Representative immunoblots of nigral DAT immunoreactivity from ipsilateral and contralateral SN (V, vehicle-treated side; G, 100 µg GDNF-treated side). Bottom panels: Image of Ponceau stains depicting relative protein loads for these samples.
GABA Regulation
Glutamic acid decarboxylase (GAD 65/67) is the rate-limiting enzyme for the biosynthesis of GABA. The medium spiny neurons that are targeted by both the DA and glutamatergic corticospinal neurons synthesize and release GABA. The changes in locomotor-activated or non-locomotor attenuation in activity shown following GDNF might have origin in altered GABA regulation in the striatum and we therefore first examined the possibility that GABA production might be affected through altered levels of the rate-limiting enzyme for its synthesis. There were modest, yet significant and curiously opposing changes in GAD 65/67 protein levels following 30 µg GDNF. Levels of GAD 65/67 immunoreactivity were significantly less in GDNF-treated rats on the ipsilateral side whereas, in contrast, GAD 65/67 immunoreactivity was significantly greater in striatum contralateral to the site of GDNF injection (Fig. 7).
Figure 7.
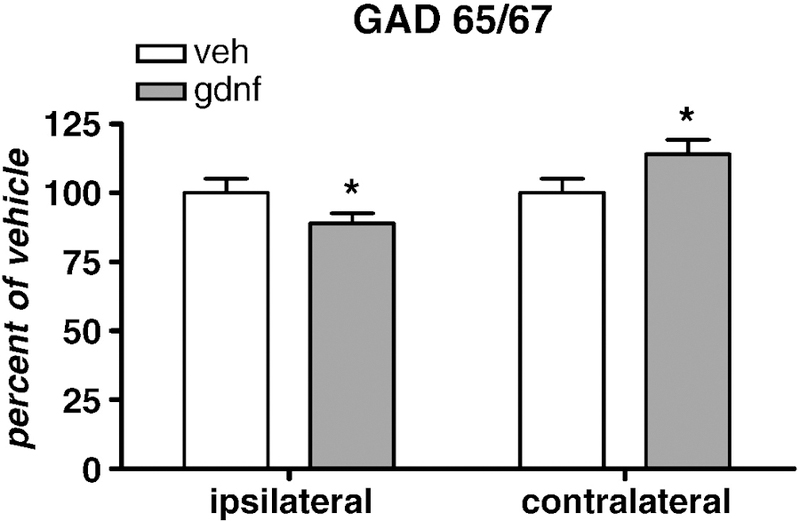
Glutamic acid decarboxylase (GAD 65/67) immunoreactivity in striatum 30 days following vehicle or 30 µg GNDF administration in striatum (n=8 for each group, both sides). Nominal protein loads were 30–40 µg [n=8 both groups, paired t-test]. *p<0.05
D1 receptor
The DA D1 receptor is expressed on the GABAergic medium spiny neurons which are associated with the direct pathway of the basal ganglia. It is not expressed on DA neuropil (Caille et al., 1996). The 30 µg dose of GDNF significantly affected striatal neuronal activity associated with both locomotor and non-locomotor activity (Stanford et al., 2007). The effect of this dose of GDNF on those neurons associated with locomotor activity would be those expressing D1 receptors, since this population of neurons is part of the direct pathway in the basal ganglia, which is active during periods of locomotion (Wichmann and DeLong, 1996). There was a trend toward an increase in D1 receptor immunoreactivity in the ipsilateral striatum and a significant increase in the contralateral striatum from 30 µg GDNF (Fig 8). The increase in D1 receptor protein levels caused by this quantity could promote DA-mediated effects in the direct pathway. Increased expression in contralateral striatum could be a compensatory mechanism to balanced enhanced DA-mediated signaling in the striatum that received GDNF.
Figure 8.
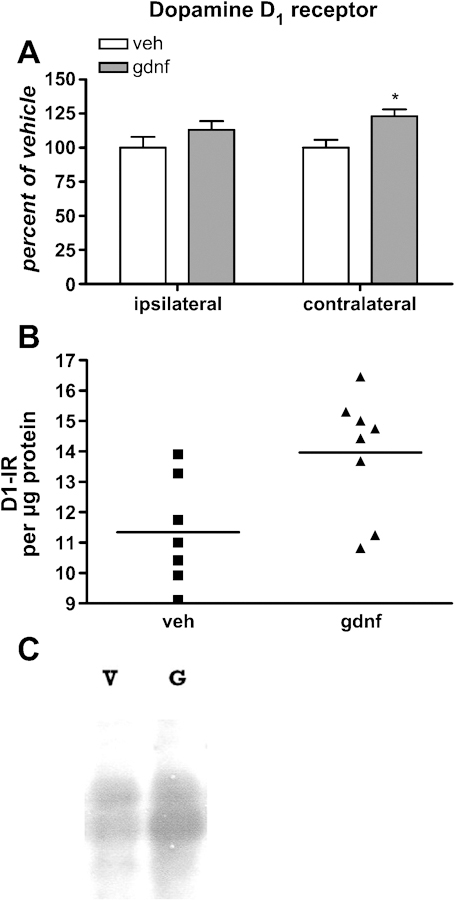
A. D1 receptor immunoreactivity in striatum 30 days following vehicle or 30 µg GDNF administration in striatum [n=5, 7, vehicle; 5, 8, GDNF]. Nominal protein loads were 80 µg for the assay. *p<0.05. B. D1 receptor immunoreactivity normalized to total protein in contralateral striatum showing data points from the test subjects in relation to the grouop mean. C. Representative immunoblot of D1 receptor-immunoreactivity, 80 µg nominal protein load.
DARPP-32 and phospho (Thr34)-DARPP-32
DARPP-32 is expressed in the GABAergic medium spiny neurons expressing DA receptors. Phosphorylation of DARPP-32 at thr34 is indicative of elevated synaptic DA in the striatum. Phosphorylation of thr34 is mediated by PKA and PKA is activated by DA binding to DA D1 receptors (Nishi et al., 1997). This site-specific increase in phosphorylation may be associated with regulation of motor activity (Hakannson et al., 2004). Thus, we examined phosphorylation of DARPP-32 to determine if physiological effects previously observed with locomotor and nonlocomotor neuron activities (Stanford et al., 2007) could have been associated with specific DA-mediated molecular events in postsynaptic DA receptor-expressing GABA neurons, wherein DARPP-32 is exclusively expressed. GDNF (30 µg) decreased total DARPP-32 protein in the ipsilateral, but not contralateral striatum (Fig 9A). There was a significant difference in the variance in immunoreactivity between the two groups, with more variance in the GDNF group (Fig 9B). DARPP-32 phosphorylation at Thr34 increased modestly (~10%), but significantly, in the contralateral striatum, while no effect on phosphorylation was seen in the ipsilateral striatum (Fig 10). Like the effect on D1 receptors in contralateral, but not ipsilateral striatum, the down regulation of the DARPP-32 protein in ipsilateral striatum could be a compensatory mechanism in an enhanced DA signaling environment, whereas upregulated DA D1 receptors could elevate PKA signaling in GABA neurons with normal DA release in the contralateral striatum, thereby increasing DARPP-32 phosphorylation at the PKA-targeted site, Thr34.
Figure 9.
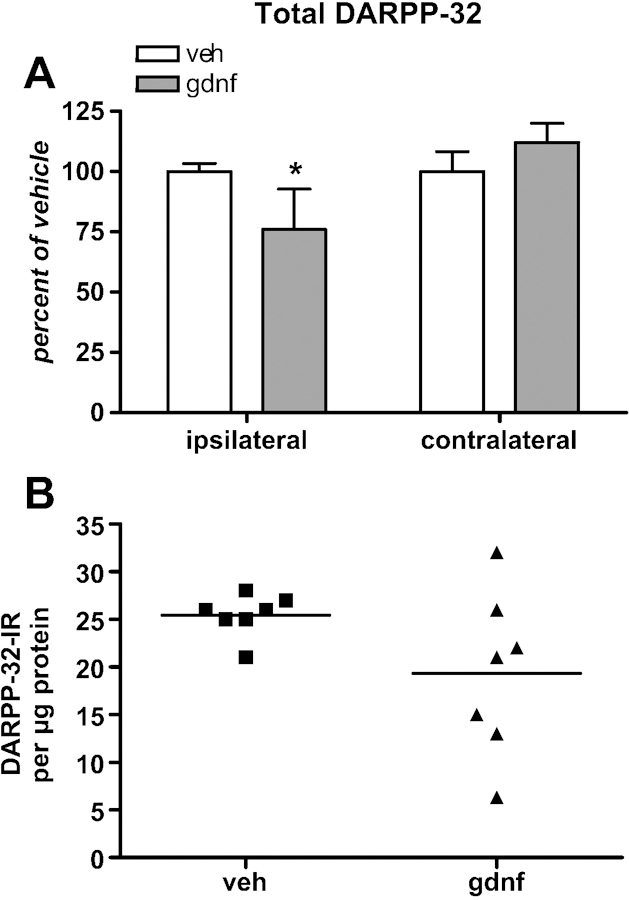
A. Total DARPP-32 in striatum 30 days following vehicle or 30 µg GNDF administration. [n=7 both groups, paired t-test]. Nominal protein loads were 30 µg for the assay. *p<0.05. B. Graph depicting mean and a significant difference in variance between both groups due to variance in the GDNF group.
Figure 10. DARPP-32 phosphorylation at Thr34.
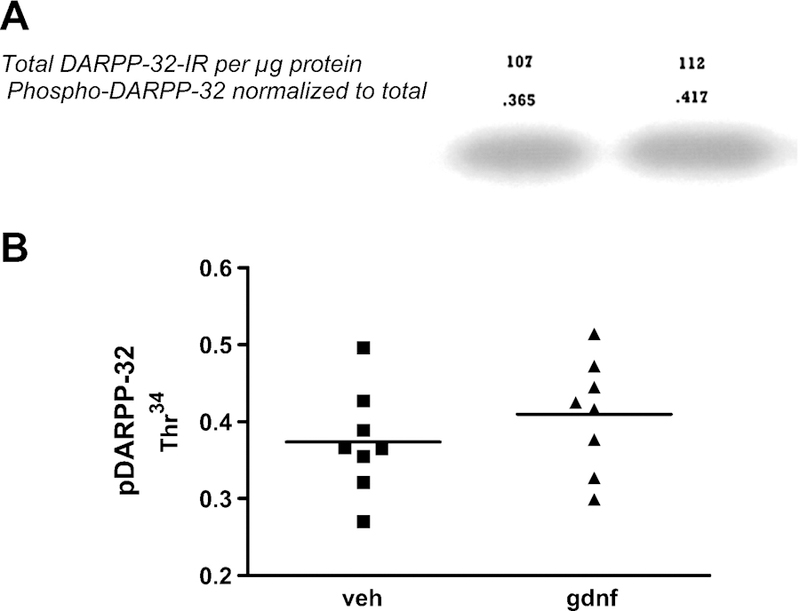
A. A representative western blot showing the modest increase in DARPP-32 phosphorylation at the PKA-targeted site (Thr34). The numbers directly above the band are the unitless values for phospho-DARPP32 immunoreactivity normalized to the total DARPP-32 protein (which are the values above the phosph-DARPP32 numbers) for those samples. B. Graph depicting the mean and variance in individuals between both groups. Paired t-test showed significant increase (p<0.05) in phosphorylation of DARPP32 in striatum contralateral to GDNF administered side.
DISCUSSION:
We sought to determine molecular correlates to the enhanced bilateral striatal neuron activity associated with GDNF, using the same rats producing this physiological data (Stanford et al., 2007). Thus far, molecular and physiological mechanisms associated with the effects of GDNF on motor function have pinpointed elevated DA function as a critical starting event (Cass and Manning, 1999; Aoi et al., 2000; Bourque and Trudeau, 2000; Yang et al., 2001; Salvatore et al., 2004; Boger et al., 2006; Stanford et al., 2007). Such improvements may have a neuroanatomical locus in the substantia nigra (Gash et al., 1996; Gerhardt et al., 1999), but clearly the bilateral modulation of striatal neuron function associated with locomotion (Stanford et al., 2007) implies that proteins associated with GABA regulation may be involved in enhanced neuronal activity caused by GDNF. A compelling observation from GDNF delivery in animal models and in a Phase I open label clinical trial is that its unilateral administration into the striatum may bilaterally improve neurochemical markers of neuronal function and motor function (Gash et al., 1996; Grondin et al., 2003; Slevin et al., 2005). This may be due to a yet unexplained bilateral transport mechanism that is dependent upon the GDNF dosage or expression levels (Eslamboli, et al., 2005). It may also be due to enhanced excitability of the DA neuron that would affect GABA function via enhanced DA actions on DA receptors. Our delivery method of 30 µg GDNF into the striatum did not produce GDNF diffusion to the contralateral striatum nor SN (Fig 1). Although it is possible that our detection method of GDNF may have not been sensitive enough to detect the presence of GDNF on the contralateral side, the data still show an overwhelming level of GDNF confined to the ipsilateral side. Therefore it appears that the bilateral effects on DA- and GABA-regulating proteins are likely mediated through bilateral projections of the basal ganglia circuitry.
Dosage-dependent bilateral effects from GDNF upon TH and DAT were seen in the SN, with a novel observation that bilateral effects on DA-regulating proteins, including DAT, were seen only in SN and not striatum. Given reports of bilateral effects of GDNF in the lab and clinic (Gash et al., 1996; Grondin et al., 2003; Slevin et al., 2005), this bilateral effect implies that restoring DA function in the SN, rather than striatum, may be critical for treating Parkinson’s disease locomotor dysfunction and may be dosage-dependent. It also suggests that locomotor effects from GDNF are dose-dependent. Given the importance of DA in regulating motor function, the bilateral motoric improvements seen with GDNF could be the result of a bilateral increase in DA availability in the SN, caused by 1) an increased capacity for DA biosynthesis, via increased TH phosphorylation (Figs 3–5), or 2) a prolonged synaptic life, via reduced DAT protein levels, given a sufficient quantity of GDNF (Fig 6). Effects on TH and DAT were not bilateral in striatum and also ser31 was the only TH phosphorylation site affected in the 30 µg dose, implying that striatal effects may be related to activation of GFR receptors whereas in SN, the increase in ser19 at this dose certainly implies a depolarization-mediated mechanism, as depolarizing stimuli increase phosphorylation at all three sites in vivo (Haycock and Haycock, 1991).
We point out that the effects of GDNF in both striatal and nigral tissues in our study are from tissue that may not have been exposed to GDNF as opposed to areas closer to the point of infusion. The volume of distribution of GDNF is dependent upon quantity, frequency, and type of infusion (Lapchak et al., 1998; Eslamboli et al., 2005; Salvatore et al., 2006). Therefore, our results may be more conservative compared to what GDNF may do at the actual site of infusion, particularly since ours was a one-time infusion. Nonetheless, we found that 30 µg GDNF affected both DA D1 receptors and DARPP-32 protein levels or its phosphorylation. These results imply that DA release capacity was modified at this dose and that a change in GABA neuron function could arise from increased D1-mediated GABA synaptic currents in striatum (Radnikow et al., 1998). DARPP-32 protein levels decreased on the ipsilateral side and an increase in Thr34 phosphorylation was seen on the contralateral side. Dopaminergic stimulation of D1 receptors on these GABA neurons would increase PKA activity and phosphorylation of DARPP-32 at the Thr34 residue. Therefore, increased DA release could trigger altered bilateral striatal neuron activities related to locomotion from these molecular changes (Fig. 10) (Stanford et al., 2007). Given that DARPP-32 is inherent to GABA neurons and translational or post-translational changes were seen with it, we also expected translational changes in GAD. We found reduced GAD levels in ipsilateral striatum but increased levels in contralateral striatum. The functional parameters of GAD are influenced by changes in dopaminergic signaling (Segovia et al., 1990; Lindefors, 1993; Schwarting and Huston, 1996). Thus, GDNF-altered neuronal activities may be based upon its effects on proteins intrinsic to the GABAergic neurons caused by increased DA release.
Models of Parkinson’s disease have focused primarily on the consequences of DA loss in the striatum. However, a growing body of evidence, including the results reported here, indicates DA in the SN can influence locomotion (Robertson and Robertson, 1989; Trevitt et al., 2004; Gash et al., 1996; Hoffer et al., 1994; Bjorklund and Dunnett, 2007). Regardless of the brain delivery site, GDNF increases DA release and tissue levels of DA and/or metabolites, notably in the SN (Gash et al., 1996; Hebert and Gerhardt, 1997; Hoffman et al., 1997; Gerhardt et al., 1999; Grondin et al., 2002) and bilateral nigral increases in DA occur with unilateral delivery (Grondin et al., 2003). Therefore the bioavailability of DA in the SN may be a critical factor for enhanced locomotor activity. Multiple lines of evidence implicate DAT function in regulating locomotor functions. Blockade of the DAT by i.p. injection of the DAT-binding ligand, nomifensine, increases locomotor activity in rats of all ages (Hebert and Gerhardt, 1998). The DAT −/− genotype in mice confers a behavioral phenotype that is marked by a three-fold increase in spontaneous locomotion (Spielewoy et al., 2000; Beaulieu et al., 2006)). In humans, methylphenidate, a DAT ligand, improves parameters of locomotion, including walking speed, when given in conjunction with L-DOPA (Nutt et al., 2004). The bilateral reduction of DAT protein in the SN caused by GDNF could prolong the life of DA in the SN and improve locomotion. The molecular mechanisms involved in the dose-dependent and bilateral effects of GDNF could be due to GDNF-related changes in ERK signal transduction, as GDNF can decrease total TH levels in the aged or intact nigrostriatal system with altered ERK signaling reported (Georgievska et al., 2004; Salvatore et al., 2004).
Whether or not GDNF acts as a trophic factor to engage tyrosine kinase signaling via GFR receptors or increases membrane excitability was not tested in this study. However, our observations here and in Stanford et al. (2007) suggest that GDNF probably increased membrane excitability. Enhanced membrane excitability increases TH phosphorylation at all sites (Haycock and Haycock, 1991; Salvatore et al., 2001). A single dose of striatal GDNF can increase ERK activity in the SN for at least seven days (Lindgren et al., 2008) and the fact that increased ser31 TH phosphorylation is seen after 30 days here and in previous work (Salvatore et al., 2004) suggests that ERK activity is still upregulated, in spite of the apparent absence or major decrease of GDNF protein in SN after one week (Figure 1). Elevated nigrostriatal neuron excitability is suggested by the bilateral increases in ser19 phosphorylation, a target of CAMKII. An inherent capacity for GDNF transport exists in vivo, wherein endogenous GDNF in the DA cell bodies of the SN originate in striatum (Barroso-Chinea et al., 2005). However, no evidence for GDNF crossing the hemispheres exists. Recovery of GDNF in ipsilateral, but not contralateral SN, occurs following unilateral striatal GDNF delivery (Yi et al., 2003; Salvatore et al., 2006). Thus the bilateral increase in TH phosphorylation suggests excitability-based increases in DA function must occur for bilateral effects. The rats used in the current study showed evidence of altered neuronal activity and post-translational modifications in striatum and SN. Therefore it is possible that GDNF, while restricted to the ipsilateral side, increases DA release in the striatum and SN and causes bilateral changes in neuronal excitability via bilateral corticostriatal and pedunculopontine afferents targeting the contralateral striatum and SN, respectively. With a higher dose of GDNF of 100 µg, it is possible that DA release could be further enhanced and cause the bilateral decrease in TH and DAT protein seen in the SN; a decrease arising in intact dopaminergic neuropil as perhaps a compensation for increased GDNF-induced activation of DA biosynthesis via increased TH phosphorylation. It seems apparent that from our study and others, in the intact DA neuropil, decreased TH protein from GDNF (Carl et al., 2003; Georgievska et al., 2004; Salvatore et al., 2004) may be a compensation for modulating DA levels due to enhanced phosphorylation of TH, whereas in DA neuropil with decreased TH, GDNF increases TH wherein there is deficient DA (Gash et al., 1996; Aoi et al., 2000). Such differences revealed by GDNF treatment indicate that the DA neuropil may have an inherent mechanism to regulate DA bioavailability, but such regulation fails in degenerating DA neuropil possibly due to deficient signaling that is reactivated by trophic factors like GDNF.
In summary, this study examined the potential involvement of DA- and GABA-regulating proteins underlying the effect of unilateral GDNF upon striatal neuron activities associated with locomotion. Future work must clarify the mechanism by which GDNF causes the bilateral changes in proteins seen here, but our data in conjunction with known bilateral pathways associated with striatum and SN indicate that GDNF may increase DA release to effect such changes. The 30 µg dose, which produced the bilateral effect on striatal neuron activities, was associated with significant increases in TH phosphorylation, but bilateral increases were seen only in the SN. We speculate an increase in DA function in ipsilateral SN may ultimately affect bilateral excitatory projections from the pendunculopontine nuclei to the contralateral SN, which produced the increase in TH phosphorylation seen therein. Bilateral changes in GABA-regulating proteins that are associated with DA function occurred in striatum, signifying that basal ganglia functions altered by GDNF may have their origins in altered protein functions related to altered DA release capabilities. The larger dose of GDNF produced a bilateral decrease in DAT and TH protein only in the SN, which suggests that the changes in transcriptional or translational capacities to DA- and GABA-regulating proteins are responsive in varying degrees. Our study illustrates the scope of influence that GDNF can have over basal ganglia function and may have its origins in altering neuronal activity via translational and post-translational changes to neurotransmitter-regulating proteins. This study, in conjunction with our previously published work in these rats, shows a definite relationship between striatal neuron functions and DA and GABA-regulating proteins.
TABLE 1:
Summary of GDNF effects in vivo
| 30 µg | 100 µg | ||||
|---|---|---|---|---|---|
| DA-regulating proteins | ipsilat. | contralat | ipsilat. | contralat | |
| Striatum | TH, total protein | ↓ 37% | ns | ↓ 65% | ns |
| TH, ser19p | ns | ns | ↑ 115% | ns | |
| TH, ser31p | ↑ 59% | ns | ↑ 104% | ns | |
| TH, ser40p | ns | ns | ns | ns | |
| DAT | ns | ns | ↓ 26% | ns | |
| Substantia nigra | ipsilat. | contralat | ipsilat. | contralat | |
| TH, total protein | ↓ 33% | ns | ↓ 52% | ↓ 44% | |
| TH, ser19p | ↑ 27% | ↑ 93% | not assayed | ||
| TH, ser31p | ns | ns | ↑ 231% | ↑ 64% | |
| TH, ser40p | ns | ↑ 66% | not assayed | ||
| DAT | ns | ns | ↓ 24% | ↓ 40% | |
| 30 µg | |||||
| GABA-regulating proteins (striatum only) | ipsilat. | contralat | |||
| GAD 65/67 | ↓ 11% | ↑ 14% | |||
| DA D1 receptor | ns | ↑ 23% | |||
| DARPP-32, total protein | ↓ 24% | ↑ 12% | |||
| DARPP-32, Thr34p | ns | ↑ 10% | |||
Legend: A designation of ns signifies that data were not significant.
REFERENCES
- Aoi M, Date I, Tomita S, Ohmoto T, 2000. The effect of intrastriatal single injection of GDNF on the nigrostriatal dopaminergic system in hemiparkinsonian rats: behavioral and histological studies using two different dosages. Neurosci, Res 36, 319–325. [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Cruz-Muros I, Aymerich MS, Rodriguez-Diaz M, Afonso-Oramas D, Lanciego JL, Gonzalez-Hernandez T, 2005. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur. J. Neurosci 21, 1815–1827. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG, 2006. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J. Biol. Chem 281, 32072–32080. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB, 2007. Dopamine neuron systems in the brain: an update. TiNS 30, 194–202. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Huang P, Zaman P, Smith AC, Hoffer BJ, Tomac AC, Granholm AC, 2006. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp. Neurol 202, 336–347. [DOI] [PubMed] [Google Scholar]
- Bourque M, Trudeau L, 2000. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur. J. Neurosci 12, 3172–3180. [DOI] [PubMed] [Google Scholar]
- Breit S, Martin A, Lessman L, Cerkez D, Gasser T, Schulz JB, 2008. Bilateral changes in neuronal activity of the basal ganglia in the unilateral 6-hydroxydopamine rat model. J. Neurosci. Res 86, 1388–1396. [DOI] [PubMed] [Google Scholar]
- Caille I, Dumartin B, Bloch B, 1996. Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res 730, 17–31. [DOI] [PubMed] [Google Scholar]
- Carl R, Bilijana G, Deniz K, 2003. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur. J. Neurosci 17, 260–270. [DOI] [PubMed] [Google Scholar]
- Cass WA, Manning MW, 1999. GDNF protection against 6-OHDA-induced reductions in potassium-evoked overflow of striatal dopamine. J Neurosci 19, 1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulpier M, Anders J, Ibanez CF, 2002. Coordinated Activation of Autophosphorylation Sites in the RET Receptor Tyrosine Kinase. J Biol Chem 277, 1991–1999. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Meldrum A, Muir JL, 2005. Frontal-striatal disconnection disrupts cognitive performance of the frontal-type in the rat. Neuroscience, 135, 1055–1065. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D, 2005. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson’s disease. J. Neurosci 25, 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA, 1996. Functional recovery in ÿntranigralÿ monkeys treated with GDNF. Nature 380, 252–255. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Kirik D, Bjorklund A 2004. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induced time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J. Neurosci, 24, 6437–6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Huettl P, Brock S, Zhang Z, Gash DM 1999. GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain Res 817, 163–171. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P, 2003. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nature Medicine 9, 589–595. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, Elsberry DD, Klein MC, Gerhardt GA, Gash DM, 2002. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced ÿntranigralÿ monkeys. Brain 125, 2191–2201. [DOI] [PubMed] [Google Scholar]
- Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA, 2003. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged Rhesus monkeys. J. Neurosci 23: 1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakannson K, Lindskog M, Pozzi L, Usiello A, Fisone G, 2004. DARPP-32 and modulation of cAMP signaling: involvement in motor control and levodopa-induced dyskinesia. Parkinsonism & Rel. Disorders 10, 281–286. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Haycock DA, 1991. Tyrosine Hydroxylase in Rat Brain Dopaminergic Nerve Terminals. J. Biol. Chem 266, 5650–5657. [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA, 1997. Behavioral and neurochemical effects of ÿntranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J. Pharmacol. Exp. Ther 282, 760–768. [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA, 1998. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Res 797, 42–54. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, van Horne CG, Eken S, Hoffer BJ, Gerhardt GA, 1997. In vivo microdialysis studies of somatodendritic dopamine release in the rat substantia nigra: effects of unilateral 6-OHDA lesions and GDNF. Exp. Neurol 147, 130–141. [DOI] [PubMed] [Google Scholar]
- Kozlowski DA, Miljan EA, Bremer EG, Harrod CG, Gerin C, Connor B, George D, Larson B, Bohn MC, 2004. Quantitative analysis of GFR alpha-1 and GFR alpha-2 mRNAs and tyrosine hydroxylase protein in the nigrostriatal system reveal bilateral compensatory changes following unilateral 6-OHDA lesions in the rat. Brain Res 1016,170–181. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gil l S.S., Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten GF, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M, 2006. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson’s disease. Ann. Neurol 59, 459–466. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hilt DC, Jiao S, Collin F, Miyoshi Y, Yi A, Zhang Z, and Gash DM, 1998. Topographical distribution of [125I]-glial cell line-derived neurotrophic factor in unlesioned and MPTP-lesioned rhesus monkey brain following a bolus intraventricular injection. Brain Res 789, 9–22. [DOI] [PubMed] [Google Scholar]
- Lindefors N 1993. Dopaminergic regulation of glutamic acid decarboxylase mRNA expression and GABA release in the striatum: a review. Prog. Neuro-psychopharm & Biol. Psych 17, 887–903. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Leak RK, Carlson KM, Smith AD, Zigmond MJ, 2008. Activation of the extracellular signal-regulated kinases 1 and 2 by glial cell line-derived neurotrophic factor and its relation to neuroprotection in a mouse model of Parkinson’s. J. Neurosci. Res 86, 2039–49. [DOI] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P, 1997. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J. Neurosci 17, 8147–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Lozano AM, Penn RD, Simpson RK, Stacy M, Wooten GF, ICV GDNF Study Group, 2003. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60, 69–73. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Carter JH, Sexton GJ, 2004. The dopamine transporter: Importance in Parkinson’s disease. Ann. Neurol 55, 766–773. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Ibanez CF, 2003. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 113, 867–879. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Misgeld U, 1998. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J. Neurosci 18, 2009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GS, Robertson HA, 1989. Evidence that L-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J. Neurosci 9, 3326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Garcia-Espana A, Goldstein M, Deutch AY, Haycock JW, 2000. Stoichiometry of tyrosine hydroxylase phosphorylation in the nigrostriatal and mesolimbic systems in vivo: Effects of acute haloperidol and related compounds. J. Neurochem 75, 225–232. [DOI] [PubMed] [Google Scholar]
- Salvatore M,F, Waymire JC, Haycock JW, 2001. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J. Neurochem 79, 349–360. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Zhang JL, Large DM, Wilson PE, Gash CR, Thomas TC, Haycock JW, Bing G, Stanford JA, Gash DM, Gerhardt GA, 2004. Striatal GDNF administration increased tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J. Neurochem 90, 245–254. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Ai Y, Wong S, Fisher B, Zhang A, Grondin RC, Zhang Z, Gerhardt GA, and Gash DM, 2006. Point source concentration of GDNF may explain failure of Phase II clinical trial. Exp. Neurology 202, 497–505. [DOI] [PubMed] [Google Scholar]
- Schwarting RKW, Huston JP, 1996. Unilateral 6-hydroxydopamine lesions of meso-striatal dopamine neurons and their physiological sequelae. Prog. Neurobiol 49, 215–266. [DOI] [PubMed] [Google Scholar]
- Segovia J, Tillakaratne NJK, Whelan K, Tobin AJ, Gale K, 1990. Parallel increases in striatal glutamic acid decarboxylase activity and mRNA levels in rats with lesions of the nigrostriatal pathway. Brain Res 529, 345–348. [DOI] [PubMed] [Google Scholar]
- Shen R-Y, Altar CA, Chiodo LA, 1994. Brain-derived neurotrophic factor increases the electrical activity of pars compacta dopamine neurons in vivo. Proc. Natl. Acad. Sci 91: 8920–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B, 2005. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputamenal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg 102, 216–222. [DOI] [PubMed] [Google Scholar]
- Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B, 2000. Behavioral disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav. Pharmacol 11, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JA, Salvatore MF, Joyce BM, Zhang H, Gash DM, Gerhardt GA, 2007. Bilateral effects of unilateral intrastriatal GDNF on locomotor-excited and nonlocomotor-related striatal neurons in aged F344 rats. Neurobiol. Aging 28, 156–165. [DOI] [PubMed] [Google Scholar]
- Trevitt JT, Carlson BB, Nowend K, Salamone JD, 2004. Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH23390 in the rat. Psychopharmacol 156, 32–41. [DOI] [PubMed] [Google Scholar]
- Uhl GR, 2003. Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, locomotion, and parkinsonism. Movt. Disord 18, S71–S81. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR 1996. Functional and pathophysiological models of the basal ganglia. Curr. Op. Neurobiol 6, 751–758. [DOI] [PubMed] [Google Scholar]
- Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, Wu C, Lu N, 2001. GDNF acutely modulates excitability and A-type K+ channels in midbrain dopaminergic neurons. Nature Neurosci 4, 1071–1078. [DOI] [PubMed] [Google Scholar]
- Yang J, Sadler TR, Givrad TK, Maarek JM, Holschneider DP, 2007. Changes in brain functional activation during resting and locomotor states after unilateral nigrostriatal damage in rats. Neuroimage 36, 755–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi A, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, Gash DM, 2003. Intraputamental infusion of GDNF in aged Rhesus monkeys: Distribution and dopaminergic effects. J. Comp. Neurol 461, 250–261. [DOI] [PubMed] [Google Scholar]