Abstract
Introduction:
Sugammadex is used for the reversal of neuromuscular blockade caused by rocuronium bromide and vecuronium bromide. As part of the post licensing phase of drug development, adverse events related to the use of sugammadex are still being uncovered and being reported. The potential association between sugammadex and adverse events bronchospasm and coronary arteriospasm using a retrospective pharmacovigilance signal analysis was carried out.
Methods:
Food and Drug Administration’s Adverse Event Reporting System database was used to run disproportionality analyses to investigate the potential association of sugammadex with bronchospasm or coronary arteriospasm. In this analysis we report the adverse event signal using frequentist methods of Relative reporting ratio (RRR), proportional reporting ratio (PRR), reporting odds ratio (ROR) and the Bayesian based Information Component metric.
Results:
A statistically significant disproportionality signal is found between sugammadex and bronchospasm (n = 44; chi-squared = 2993.87; PRR = 71.95 [95% CI: 54.00–95.85]) and sugammadex and coronary arteriospasm (n = 6; chi-squared = 209.39; PRR = 43.82 [95% CI: 19.73–97.33]) as per Evans criteria. Both statistically significant disproportionality signals persisted when stratified by gender. Based upon dynamic cumulative PRR graph, the PRR value has steadily increased and the 95% CI narrowed since December 2012.
Conclusion:
The results of the pharmacovigilance analysis highlight a statistically significant disproportionality signal between sugammadex usage and bronchospasm and coronary arteriospasm adverse events. Physicians need to be aware of these adverse events when using sugammadex. The results of the pharmacovigilance signal analysis highlight a statistically significant disproportionality signal between sugammadex usage and bronchospasm and coronary arteriospasm adverse events. Physicians need to be aware of these adverse events when using sugammadex.
Keywords: Sugammadex, Bronchospasm, Coronary Arteriospasm
Introduction
Neuromuscular blocking agents (NMBA) are often given in conjunction with anesthesia during surgical procedures to facilitate endotracheal intubation and inhibit spontaneous ventilation. Use of NMBA during intubation can decrease the chances of damage to the vocal cords and facilitate mechanical ventilation in patients with decreased lung compliance.1,2 Sometimes, during or after procedures, there is a need to reverse the neuromuscular blocking agent. Specifically, the risk of residual neuromuscular blockade can necessitate the need for a NMBA reversal drug.3 One of the oldest and most commonly used NMBA reversal drugs is neostigmine. Neostigmine was patented in 1931 and the World Health Organization includes it in the list of medications considered to be the most efficacious and safe: “WHO Model List of Essential Medicines”.4 Until late 2015, neostigmine and other acetylcholinesterase inhibitors were the primary treatment option for NBMA reversal in the U.S. On December 15, 2015, sugammadex was approved by the Food and Drug Administration (FDA) for the reversal of neuromuscular blockade caused by rocuronium bromide and vecuronium bromide.5 However, worldwide, sugammadex has been clinically used for several years, including in the European Union from July 2008 and subsequently in Japan from April 2010.
As part of the post licensing phase of drug development, adverse events related to the use of sugammadex are still being uncovered and being reported. As of September 3, 2018, the FDA was evaluating the need for regulatory action regarding a link between sugammadex use and bronchospasm and between sugammadex use and laryngospasm.6 Bronchospasm is a condition in which there is a sudden involuntary contraction of muscle in the bronchiole. It can result in difficulty in breathing, desaturation and, in some cases, death.7,8 In addition, in 2014, the Pharmaceuticals and Medical Devices Agency of Japan recommended adding “coronary arteriospasm” in the clinically significant adverse reactions section of sugammadex’s drug label in Japan.9,10 Coronary arteriospasm results in decreased blood flow to the muscle of the heart and can result in myocardial infarction.
Objective
This research paper quantifies the signal between sugammadex and adverse events bronchospasm and coronary arteriospasm using a retrospective pharmacovigilance signal analysis. Specifically, a disproportionality analysis is conducted to determine if the signal score is significant. A disproportionality analysis can be used to identify potential statistical associations between a product and an event based on safety/case reports. The analysis is performed on the FDA Adverse Event Reporting System (FAERS),11 a passive reporting system, which does not provide information about the prior medical history of the patient. Further, the pharmacovigilance signal analysis, by itself, does not demonstrate causal associations. However, FAERS has advantages in identifying drug-safety signals in real-world situations. In addition, case reports from literature are summarized and used to support or contradict the analysis.
Methods
Approval by institutional review board or human subjects’ committee was not required as the analysis was of retrospective public domain safety data that was without any personal identifiers.
The FDA maintains and manages the FDA Adverse Event Reporting System. Case reports of adverse events for FAERS are submitted by healthcare professionals, consumers, and manufacturers. FAERS allows users to explore reports of adverse events that may occur in potential association with use of drug products, singularly or in combination with other drugs. FAERS is updated quarterly.11 FAERS data from January 1, 2004 to June 30, 2018 was accessed and used for this analysis. The FAERS data was searched for adverse event case reports in which sugammadex was listed as a drug administered and bronchospasm or coronary arteriospasm was reported as an adverse event, respectively for each of the two adverse event disproportionality analyses. Efforts were made to remove duplicate case reports prior to running the analysis.
Disproportionality analysis in drug safety research can be used to identify signals and potential statistical associations between a product and an event based on databases of safety/case reports.12,13 The analysis compares the reported expected count for a product-event combination with the reported observed count. High reporting ratios indicate that disproportionality exists and that there may be a potential statistical association between the product and the adverse event.14,15 Relative reporting ratio (RRR), proportional reporting ratio (PRR), reporting odds ratio (ROR) and chi squared with Yates’ correction are calculated in the disproportionality analysis. The PRR is the rate of reporting of one specific event among all events for a given drug, the comparator being this reporting rate for all drugs present in the database, including the drug of interest. The ROR is the ratio of the odds of reporting of one specific event versus all other events for a given drug compared to this reporting odds for all other drugs present in the database.16 The RRR compares the probability of reporting one specific event by a given drug to the probability of reporting the same specific event by all drugs.
Other disproportionality measures which can be used to decipher a signal are information component (IC) and the empirical Bayes geometric mean (EBGM). These algorithms, however, differ from the above disproportionality algorithms in that the PRR, RRR and ROR utilize a frequentist approach, whereas the IC and EBGM utilize a Bayesian approach.17,18 The PRR is currently used by the UK Medicines and Healthcare products Regulatory Agency (MHRA), the ROR by the Netherlands Pharmacovigilance Centre, the IC by the World Health Organization (WHO), and the EBGM by the FDA.18 For large sample sizes, as in FAERS database, the score that each of these statistics produces for any given drug-event combination is likely to be similar. FDA implemented Multi-item Gamma Poisson Shrinker (MGPS) data mining algorithm to calculate EBGM values, which are the ratios of the observed to the expected number of drug-event pairs (reporting ratios). MGPS allows the use of stratification for elimination of some confounding effects, so it will typically provide lower scores if there is a confounding variable involved, such as age or gender, compared to a statistic that does not involve stratification. However, randomized trials are the only way of being sure there is no confounding in a dataset. Moreover, Bayesian Confidence Propagation Neural Network (BCPNN) analysis was proposed based on Bayesian logic where the relation between the prior and posterior probability was expressed as the information component. The IC given by the BCPNN is applied by the WHO Uppsala Monitoring Center (UMC).
Signal detection using IC is done using the IC025 metric, a criterion indicating the lower bound of the 95% two-sided confidence interval of the IC, and a signal is detected if the IC025 value exceeds zero.19 Evans et al. define a criterion by which to evaluate whether the disproportionality analysis indicates a statistically significant signal or not: a PRR greater than or equal to 2, chi-squared greater than or equal to 4 and number of events greater than or equal to 3.15 In addition, according to Van Puijenbroek et al., a lower 95% CI of ROR greater than 1 indicates a statistically significant signal between a drug and an event.20 In this analysis we report the adverse event signal using frequentist methods of RRR, ROR and PRR and the Bayesian based IC025 metric.
Results
The FAERS database (January 1, 2004 to June 30, 2018) contained over 9 million worldwide adverse event reports as associated with any drug. In the database, an adverse event was reported with sugammadex use in 698 case reports. 44 and 6 cases of bronchospasm and coronary arteriospasm, respectively, were reported as associated with sugammadex as the suspect drug.
2 × 2 contingency tables were constructed to calculate the PRR, RRR and ROR along with their 95% confidence intervals, the chi-squared with Yates correction and the information component metrics. All of these disproportionality values are shown in Tables 1 and 2. When using the information component criteria (IC lower 95% CI > 0), a signal is detected for sugammadex with bronchospasm (IC lower 95% CI = 4.82) and sugammadex with coronary arteriospasm (IC lower 95% CI = 1.94) (Table 1). When comparing the results of the disproportionality analysis of sugammadex associated bronchospasm (n = 44; chi-squared = 2993.87; PRR = 71.95 [95% CI: 54.00–95.85]) to Evans criteria (n >= 3; chi-squared >= 4; PRR >= 2), sugammadex is found to contain a statistically significantly disproportionally signal with bronchospasm. Similarly, the disproportionality results of sugammadex associated coronary arteriospasm (n = 6; chi-squared = 209.39; PRR = 43.82 [95% CI: 19.73–97.33]) are statistically significant as per Evans criteria. When using Van Puijenbroek’s criteria (ROR Lower 95% CI > 1), sugammadex associated bronchospasm (ROR lower 95% CI = 56.49), and sugammadex associated coronary arteriospasm (ROR lower 95% CI = 19.76) are found to be statistically significant (Table 2).
Table 1.
Information component value and information component lower 95% CI for the potential association between bronchospasm and coronary arteriospasm adverse events and sugammadex between 1 January 2004 and 30 June 2018 as a total and stratified by gender and by age group.
| Stratification | Information component value | IC025 | |
|---|---|---|---|
| Bronchospasm | Total | 5.32 | 4.82 |
| Male | 4.36 | 3.49 | |
| Female | 4.95 | 4.25 | |
| 0–40 years old | 3.31 | 1.90 | |
| 41–60 years old | 3.90 | 2.76 | |
| 61–80 years old | 3.50 | 2.20 | |
| Coronary Arteriospasm | Total | 3.35 | 1.94 |
| Male | 2.62 | 0.55 | |
| Female | 2.67 | 0.60 |
Table 2.
Proportional reporting ratio (PRR), reporting odds ratio (ROR), relative reporting ratio (RRR) and chi-squared with Yates correction for the potential association between bronchospasm and coronary arteriospasm adverse events and sugammadex between 1 January 2004 and 30 June 2018 as a total and stratified by gender and by age group.
| Stratification | Analysis | Value | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|---|
| Bronchospasm | Total | PRR | 71.95 | 54.00 | 95.85 |
| ROR | 76.72 | 56.49 | 104.19 | ||
| RRR | 71.56 | 53.71 | 95.33 | ||
| Chi-squared with Yates correction | 2993.87 | – | – | ||
| Male | PRR | 59.33 | 36.21 | 97.21 | |
| ROR | 62.62 | 37.18 | 105.49 | ||
| RRR | 59.03 | 36.03 | 96.72 | ||
| Chi-squared with Yates correction | 799.45 | – | – | ||
| Female | PRR | 89.17 | 60.19 | 132.12 | |
| ROR | 96.83 | 63.17 | 148.41 | ||
| RRR | 88.73 | 59.89 | 131.46 | ||
| Chi-squared with Yates correction | 1910.11 | – | – | ||
| 0–40 years old | PRR | 38.95 | 17.85 | 85.00 | |
| ROR | 41.08 | 18.02 | 93.64 | ||
| RRR | 38.79 | 17.77 | 84.65 | ||
| Chi-squared with Yates correction | 184.98 | – | – | ||
| 41–60 years old | PRR | 66.17 | 35.23 | 124.27 | |
| ROR | 71.45 | 36.16 | 141.19 | ||
| RRR | 65.80 | 35.04 | 123.58 | ||
| Chi-squared with Yates correction | 512.02 | – | – | ||
| 61–80 years old | PRR | 43.42 | 21.03 | 89.63 | |
| ROR | 45.51 | 21.27 | 97.35 | ||
| RRR | 43.23 | 20.94 | 89.25 | ||
| Chi-squared with Yates correction | 248.40 | – | – | ||
| Coronary Arteriospasm | Total | PRR | 43.82 | 19.73 | 97.33 |
| ROR | 44.19 | 19.76 | 98.83 | ||
| RRR | 43.67 | 19.66 | 97.01 | ||
| Chi-squared with Yates correction | 209.39 | – | – | ||
| Male | PRR | 43.83 | 14.19 | 135.39 | |
| ROR | 44.30 | 14.17 | 138.49 | ||
| RRR | 43.67 | 14.14 | 134.88 | ||
| Chi-squared with Yates correction | 86.07 | – | – | ||
| Female | PRR | 59.20 | 19.17 | 182.83 | |
| ROR | 59.81 | 19.14 | 186.92 | ||
| RRR | 59.01 | 19.11 | 182.23 | ||
| Chi-squared with Yates correction | 118.01 | – | – |
The data was also stratified by gender and by age for case reports with sugammadex and bronchospasm. The disproportionality analysis results are also displayed in Tables 1 and 2. The results show that the signal of bronchospasm was statistically significantly associated with sugammadex use for males and for females and for the age groups using Evan’s criteria and Van Puijenbroek’s criteria. Coronary arteriospasm was statistically significantly associated with sugammadex use for males and for females using Evan’s criteria and Van Puijenbroek’s criteria. Coronary arteriospasm associated with sugammadex case reports was not stratified by age group due to the limited number of case reports.
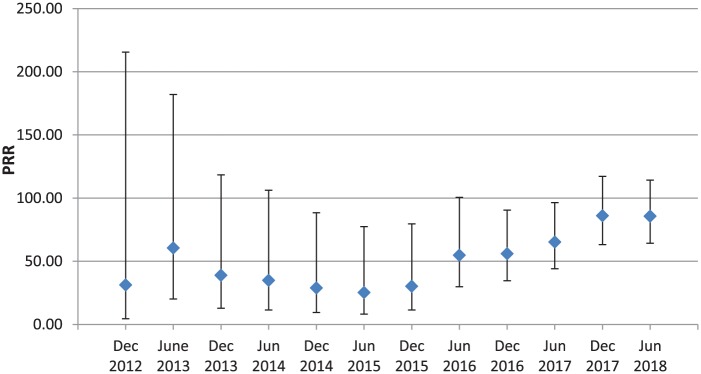
To investigate how the PRR has changed over time, a dynamic cumulative PRR graph (December 2012 to June 2018) was constructed for sugammadex and bronchospasm adverse events (Figure 1). December 2012 was chosen as the initial date for the dynamic cumulative PRR because the first case report showing a bronchospasm adverse event after administering sugammadex occurred in the fourth quarter of 2012. Generally, the PRR value has steadily increased and the 95% CI narrowed since December 2012. The dynamic graph for coronary arteriospasm was not generated as the number of cases was low.
Figure 1.
Dynamic cumulative proportional reporting ratio (PRR) of sugammadex and bronchospasm events since December 2012.
Limitations
FAERS is a passive reporting system. Not every adverse event is reported to the database, and therefore, the incidence of an adverse event cannot be calculated.21 The limitations of FAERS have been documented earlier by FDA.11 FAERS does not provide information about the prior medical history of the patient nor any risk factors they have. These data and the analysis do not, by themselves, demonstrate causal associations. The event could occur as a result of the underlying disease or diseases and the concomitant drugs or their interaction thereof. Randomized controlled studies are needed in order to establish causality. However, FAERS has advantages in that it can and has been used to identify drug-safety signals, drug-drug interaction and idiosyncratic adverse drug-reactions.22
FAERS has advantages in identifying signals in real-world situations, which is near impossible with the limited number of subjects used in the randomized clinical trials. Further, FAERS can help in identifying global differences in occurrence of adverse events.
Discussion
Overview
Sugammadex works by binding to rocuronium or vecuronium in the plasma. This binding leads to a lower concentration of rocuronium/vecuronium in the plasma and, as such, rocuronium/vecuronium exit the neuromuscular junction due to the concentration gradient and enter the plasma. The new rocuronium/vecuronium in the plasma binds to the available sugammadex leading to a decrease in free rocuronium/vecuronium and a subsequent decrease in neuromuscular blocking.23,24
19 cases of sugammadex induced bronchospasm or laryngospasm were found in 8 unique case reports when searching PubMed for published case reports that contained the terms sugammadex and bronchospasm or laryngospasm published between January 2010 and August 2018. Two cases had patients with a prior history of asthma while four cases stated that the patients had not been diagnosed with any pulmonary disease. In addition, the other 13 cases did not mention any prior respiratory issues in the patients.
Airway obstruction
A phase III, randomized, 9 hospital site, parallel-group, comparative, safety-assessor blinded study was sponsored/performed by the manufacturer of sugammadex to examine a link between sugammadex and bronchoconstriction.25 Seventy seven patients with preexisting pulmonary disease who had to undergo surgery and required neuromuscular blockade were included in the study. Two patients, who had history of asthma, received 4 mg/kg sugammadex and were given desflurane for maintenance of anesthesia, developed bronchospasms. However, Eskander et al. reported three patients, who did not have prior pulmonary diseases, developed bronchospasm after sugammadex administration.26 In addition, Lee et al. reported a patient who had good cardio-respiratory functional capacity and developed laryngospasm after sugammadex administration.27 These cases indicate that a positive pulmonary history may not be the sole cause of the bronchospasm/laryngospasm after sugammadex administration.
Mcguire and Dalton reported eight cases in which upper airway obstruction occurred after administration of sugammadex.28 After seeing a similar airway obstruction in the first three cases, they made variations in the anesthetic drugs administered to the patients to account for a confounding drug. For example, fentanyl was given instead of remifentanil in one patient and sevoflurane was used for maintenance of anesthesia in one patient instead of desflurane. However, all patients who received sugammadex had a subsequent upper airway obstruction. One patient was given neostigmine instead of sugammadex and this patient did not have upper airway obstruction. There are ethical questions regarding the protocol followed by the authors and whether a sufficient maintenance anesthesia depth was used.29
Rocuronium-sugammadex complex
In the 77 patient study described above25 one of the two patients who developed bronchospasms had bronchospasms occur about 1 h after sugammadex administration. The delayed adverse event may be due to the chelation of rocuronium or the sugammadex-rocuronium complex rather than directly due to sugammadex. Okuno et al. suggest in a case report that coronary vasospasm induced by anaphylactic shock in a 46 year old may be caused by the rocuronium-sugammadex complex.30
Coronary arteriospasm
During the time period 2011–2014, four cases were reported in Japan of sugammadex associated coronary arteriospasm. In all four cases, a causal relationship could not be ruled out and no fatalities were reported.10 A 76 year old man, with no notable medical history, had repetitive cardiac arrests after administration of sugammadex after prostatectomy.31 The authors identified the administration of sugammadex as a more probable cause of the coronary spasm. Hoshino et al. also reported repetitive cardiac arrests due to coronary vasospasm after sugammadex administration in a 58 year old man and recommended that clinicians should consider sugammadex as one of the causative agents of cardiac arrest in the operating room.32
The mechanism by which the spasms are occurring after sugammadex administration is still being understood. However, case reports and the clinical trial suggest that there is a signal between sugammadex usage and bronchospasm and coronary arteriospasm/vasospasm.
Conclusion
The results of the pharmacovigilance signal analysis highlight a significant disproportionality signal between sugammadex usage and bronchospasm and coronary arteriospasm adverse events. The significance of the signals persisted after stratifying the data by gender and by age. The case reports and limited controlled studies support this signal between the drug and the adverse events. Bronchospasm and coronary arteriospasm are serious adverse events that can result in mortality. Physicians need to be aware of these adverse events when using sugammadex. Further studies should be performed to better understand the mechanism by which these adverse events are occurring after sugammadex administration.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was not sponsored by any entity (department/institution) and none of the entities funded the project. There were no consultancies, equity interests, or patent licensing arrangements associated with this research paper.
Conflict of interest statement: The author declares that there is no conflict of interest.
ORCID iD: Pushkar Aggarwal  https://orcid.org/0000-0001-5271-8127
https://orcid.org/0000-0001-5271-8127
References
- 1. Renew J, Naguib M, Brull S. Clinical use of neuromuscular blocking agents in anesthesia. UpToDate, https://www.uptodate.com/contents/clinical-use-of-neuromuscular-blocking-agents-in-anesthesia (2017, accessed 5 August 2018).
- 2. Bevan DR. Neuromuscular blocking drugs: onset and intubation. J ClinAnesth 1997; 9(Suppl. 6): 36S–39S. [DOI] [PubMed] [Google Scholar]
- 3. Pani N, Dongare PA, Mishra RK. Reversal agents in anaesthesia and critical care. Indian J Anaesth 2015; 59: 664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO model list of essential medicines, http://www.who.int/medicines/publications/essentialmedicines/18th_EML.pdf (accessed 5 August 2018).
- 5. Food and Drug Administration. Bridion application summary review, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/022225Orig1s000SumR.pdf (accessed 5 August 2018).
- 6. Food and Drug Administration. Potential signals of serious risks/new safety information identified from the FDA adverse event reporting system (FAERS): January–March 2018, https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm613181.htm (accessed 3 September 2018).
- 7. Dewachter P, Mouton-Faivre C, Emala CWet al. Case scenario: bronchospasm during anesthetic induction. Anesthesiology 2011; 114: 1200–1210. [DOI] [PubMed] [Google Scholar]
- 8. Monsó A, Riudeubàs J, Palanques Fet al. A new application for superior laryngeal nerve block: treatment or prevention of laryngospasm and stridor. Reg Anesth Pain Med 1999; 24: 186–187. [DOI] [PubMed] [Google Scholar]
- 9. Pharmaceuticals and Medical Devices Agency. Bridion review report, https://www.pmda.go.jp/files/000153538.pdf (accessed 5 August 2018).
- 10. Pharmaceuticals and Medical Devices Agency. Summary of investigation results sugammadex sodium, https://www.pmda.go.jp/files/000207758.pdf (accessed 5 August 2018).
- 11. FDA’s Adverse Event Reporting System (FAERS). Food and drug administration, https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm (accessed 5 August 2018).
- 12. Faich G, Morris J. Adverse reaction signaling and disproportionality analysis: an update. Ther Innov Regul Sci 2012; 46: 708–714. [Google Scholar]
- 13. Hou Y, Ye X, Wu Get al. A comparison of disproportionality analysis methods in national adverse drug reaction databases of China. Expert Opin Drug Saf 2014; 13: 853–857. [DOI] [PubMed] [Google Scholar]
- 14. Evans SJ. Pharmacovigilance: a science or fielding emergencies? Stat Med 2000; 19: 3199–3209. [DOI] [PubMed] [Google Scholar]
- 15. Evans SJW, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 2001; 10: 483–486. [DOI] [PubMed] [Google Scholar]
- 16. Aggarwal N. Local anesthetics systemic toxicity association with exparel (bupivacaine liposome)- a pharmacovigilance evaluation. Expert Opin Drug Saf 2018; 17: 581–587. [DOI] [PubMed] [Google Scholar]
- 17. Hauben M, Horn S, Reich Let al. Association between gastric acid suppressants and clostridium difficile colitis and community-acquired pneumonia: analysis using pharmacovigilance tools. Int J Infect Dis 2007; 11: 417–422. [DOI] [PubMed] [Google Scholar]
- 18. Tamura T, Sakaeda T, Kadoyama Ket al. Omeprazole and esomeprazole-associated hypomagnesaemia: data mining of the public version of the FDA adverse event reporting system. Int J Med Sci 2012; 9: 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bate A, Lindquist M, Edwards IRet al. A bayesian neural network method for adverse drug reaction signal generation. Eur J ClinPharmacol 1998; 54: 315–321. [DOI] [PubMed] [Google Scholar]
- 20. van Puijenbroek EP, van Grootheest K, Diemont WLet al. Determinants of signal selection in a spontaneous reporting system for adverse drug reactions. Br J Clin Pharmacol 2001; 52: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aggarwal N. Drug-induced subacute cutaneous lupus erythematosus associated with proton pump inhibitors. Drugs Real World Outcomes 2016; 3: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fang H, Su Z, Wang Yet al. Exploring the FDA adverse event reporting system (FAERS) to generate hypotheses for disease monitoring. Clin Pharmacol Ther 2014; 95: 496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nag K, Singh DR, Shetti ANet al. Sugammadex: a revolutionary drug in neuromuscular pharmacology. Anesth Essays Res 2013; 7: 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schaller SJ, Fink H. Sugammadex as a reversal agent for neuromuscular block: an evidence-based review. Core Evid 2013; 8: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amao R, Zornow MH, Cowan RMet al. Use of sugammadex in patients with a history of pulmonary disease. J Clin Anesth 2012; 24: 289–297. [DOI] [PubMed] [Google Scholar]
- 26. Eskander JP, Cornett EM, Stuker Wet al. The combination of sugammadex and desflurane may increase the risk of bronchospasm during general anesthesia. J Clin Anesth 2017; 41: 73. [DOI] [PubMed] [Google Scholar]
- 27. Lee JH, Lee JH, Lee MHet al. Postoperative negative pressure pulmonary edema following repetitive laryngospasm even after reversal of neuromuscular blockade by sugammadex: a case report. Korean J Anesthesiol 2017; 70: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGuire B, Dalton AJ. Sugammadex, airway obstruction, and drifting across the ethical divide: a personal account. Anaesthesia 2016; 71: 487–492. [DOI] [PubMed] [Google Scholar]
- 29. Chrimes N. Did sugammadex cause, or reveal, laryngospasm? Anaesthesia 2016; 71: 1112. [DOI] [PubMed] [Google Scholar]
- 30. Okuno A, Matsuki Y, Tabata Met al. A suspected case of coronary vasospasm induced by anaphylactic shock caused by rocuronium-sugammadex complex. J Clin Anesth 2018; 48: 7. [DOI] [PubMed] [Google Scholar]
- 31. Ko MJ, Kim YH, Kang Eet al. Cardiac arrest after sugammadex administration in a patient with variant angina: a case report. Korean J Anesthesiol 2016; 69: 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoshino K, Kato R, Nagasawa Set al. A case of repetitive cardiac arrest due to coronary vasospasm after sugammadex administration. Masui 2015; 64: 622–627. [PubMed] [Google Scholar]