Abstract
The peripheral hearing alterations and central auditory processing disorder (CAPD) associated with age-related hearing loss (ARHL), may impact cognitive disorders in older age. In older age, ARHL is also a significant marker for frailty, another age-related multidimensional clinical condition with a nonspecific state of vulnerability, reduced multisystem physiological reserve, and decreased resistance to different stressors (i.e. sensorial impairments, psychosocial stress, diseases, injuries). The multidimensional nature of frailty required an approach based on different pathogeneses because this clinical condition may include sensorial, physical, social, nutritional, cognitive, and psychological phenotypes. In the present narrative review, the cumulative epidemiological evidence coming from several longitudinal population-based studies, suggested convincing links between peripheral ARHL and incident cognitive decline and dementia. Moreover, a few longitudinal case-control and population-based studies also suggested that age-related CAPD in ARHL, may be central in determining an increased risk of incident cognitive decline, dementia, and Alzheimer’s disease (AD). Cumulative meta-analytic evidence confirmed cross-sectional and longitudinal association of both peripheral ARHL and age-related CAPD with different domains of cognitive functions, mild cognitive impairment, and dementia, while the association with dementia subtypes such as AD and vascular dementia remained unclear. However, ARHL may represent a modifiable condition and a possible target for secondary prevention of cognitive impairment in older age, social isolation, late-life depression, and frailty. Further research is required to determine whether broader hearing rehabilitative interventions including coordinated counseling and environmental accommodations could delay or halt cognitive and global decline in the oldest old with both ARHL and dementia.
Keywords: age-related hearing loss, Alzheimer’s disease, auditory system, cognitive functions, dementia, frailty, lifestyle, presbycusis, vascular dementia
Introduction
Age-related hearing loss (ARHL), also known as presbycusis, is a progressive, bilateral, and symmetrical multifactorial disorder of hearing sensitivity primarily observed in the high-frequency region, occurring as the consequence of lifetime insults to the auditory system.1 ARHL is common among older individuals and its prevalence is estimated to be 25–30% in individuals aged 65–74 years,2 affecting 63.1% of people over 70 years,3 and 80.6% of people over 85 years,3,4 and is the third most common chronic health condition in older age.5 Sensory changes, particularly hearing and vision impairments, are dementia-modifiable risk factors as suggested by an exploratory workshop of the National Institute on Aging, concluding that sensory and motor changes may anticipate the cognitive symptoms of Alzheimer’s disease (AD) by several years, significantly increasing AD risk.6 In particular, among sensory changes, the United States (US) National Institutes of Health and the United Kingdom (UK) National Institute of Health and Care Excellence identified peripheral ARHL and social isolation as potentially modifiable dementia risk factors.7 In fact, increasing epidemiological evidence suggested a strong link between ARHL and late-life cognition and that midlife ARHL was a risk factor for the development of AD and dementia in older age.7,8
At the early stages of ARHL, hearing difficulties may be not related only to the peripheral auditory system impairment but also to the central auditory processing disorder (CAPD), with problems in understanding speech in a background noise.8 In fact, physiological alterations of both central and peripheral auditory systems may contribute to ARHL, that is characterized by high-frequency impairment (6000 and 8000 Hz), compromised localization of sound sources, and slowed central processing of acoustic information.1 ARHL can be caused by several mechanisms involving not only the inner ear leading to a decreased hearing threshold, but also the central neural auditory pathways, with impaired discrimination, especially in noisy environments.9 In particular, CAPD is defined as a specific deficit in the processing of auditory information along the central auditory nervous system, including bottom-up and top-down neural connectivity and one or more areas of auditory discrimination, temporal processing, and binaural processing, clinically characterized in older age by the difficulty of understanding words in background noise.10–12 Moreover, CAPD has a heterogeneous clinical presentation and it is very hard to distinguish from a peripheral auditory deficit. Therefore, to better understand the impact of ARHL on late-life cognitive disorders, the assessment of both peripheral and central auditory dysfunctions may be useful.
ARHL is also a significant marker for frailty in older age,13 another age-related multidimensional clinical condition characterized by a nonspecific state of vulnerability, reduced multisystem physiological reserve, and decreased resistance to stressors that identified older persons with an increased risk of falls, institutionalization, hospitalization, disability, and death.14 In fact, multiple subclinical and age-related comorbidities and different stressors (i.e. sensorial impairments, psychosocial stress, diseases, injuries) may exacerbate the functional physiological decline of several systems in older age, resulting in a homeostatic imbalance or frailty.14 However, the multidimensional nature of frailty requires an approach based on different pathogeneses because this heterogeneous clinical condition may include physical, social, nutritional, cognitive, and psychological components,15 greatly influencing its definition and treatment. Of these domains, cognition appears to be strictly linked to frailty and an increased risk of adverse outcomes.15 In fact, in cognitively impaired older patients, there is often a rapid cognitive decline when they have reduced everyday activities due to physical limitations, suggesting a strong connection of cognitive function with physical fitness or physical activities, and therefore with the concept of a physical frailty phenotype, which is probably the most popular model of this condition.14 This phenotype or biological model offers an operational definition based on an assumed state of negative energy balance, sarcopenia, diminished strength, and low tolerance for exertion.14 However, other different frailty models, including the deficit accumulation model16 and the biopsychosocial/multidimensional model,17 incorporating a large number of candidate factors ranging from disease states, symptoms, signs, to abnormal laboratory values, have been associated with cognition in older age.18 A recent and growing body of epidemiological evidence suggested that frailty may increase the risk of future cognitive decline and dementia and that cognitive impairment may increase the risk of frailty, suggesting that cognition and frailty may interact in advancing aging.19 Therefore, through frailty prevention, it may be also possible to prevent cognitive-related adverse outcomes.12 The present narrative review investigates the epidemiological evidence of age-related changes in peripheral ARHL and CAPD in modulating the risk of dementia, AD, mild cognitive impairment (MCI), and other late-life cognitive disorders, discussing also the possible underlying mechanisms and factors mediating the proposed associations between ARHL and cognition, particularly in different frailty models.
Peripheral ARHL and late-life cognitive disorders
Although the first report of a possible association between ARHL and cognitive dysfunction appeared over 50 years ago,20 it was almost three decades ago that a review article including original findings summarized the first 25 years of research on possible associations between ARHL and cognitive disorders.21 This first series of studies suggested a strong association between peripheral ARHL and cognitive impairment or decline in demented or institutionalized patients, while there was no link in nondemented older adults.21
In the last 10 years, the cumulative evidence coming from several longitudinal population-based studies suggested a robust link between peripheral ARHL and late-life cognitive disorders,22–33 except for the negative findings coming from a 2-year follow up of the Australian Longitudinal Study of Ageing.34 However, in another report from the same sample with a longer follow up of 8 years there was a weak association between ARHL and memory decline.35 Recent findings from the Australian cohort of the Blue Mountains Eye Study suggested that higher proportions of older participants with visual, hearing or dual sensory impairment (hearing and vision) had greater global cognitive decline after 5 and 10 years than participants without sensory impairment, after adjusting for age and sex and other potential confounders.36 Furthermore, in a population-based study conducted in Taiwan of about 5000 adults aged over 50 years who were followed up for 11 years, self-reported ARHL increased the age-related differences in the intercept of the memory function by over 25%, with the association significantly greater in older people than in younger people, but without significant difference in the slope of memory decline over time based on hearing impairment status.37 The slightly discrepant findings among these studies may be partly explained by different operational definitions of ARHL and late-life cognitive disorders and the lack of adjustment for multiple potential confounders.38 For example, among population-based studies establishing a link between peripheral ARHL and cognition, only two reports used a self-reported measure of hearing loss,24,37 while pure tone audiometry (PTA) remains the gold standard for clinical assessment of peripheral auditory dysfunction.38 There was also a great variability in cognitive measurement tools across different studies, with a wide range of neuropsychological tests coming from global cognitive screening tests to tools assessing different domains such as memory, language or executive functioning. In cognitively healthy older adults, global cognitive screening tools captured only limited variability, with ceiling effects potentially leading to an underestimation of the ARHL-cognition link.38 Moreover, although the ARHL-cognition link persisted also when auditory items were removed from cognitive screening tests,8,39,40 and when nonauditory cognitive tests were used,28,41 these confounders may overestimate the strength of the association.
Age-related CAPD and late-life cognition
There are four possible interactions between the peripheral and central auditory components that could help us in the understanding of pathophysiology of ARHL. In fact, the central component of ARHL could be a consequence of peripheral auditory deficit, otherwise central changes may be independent of peripheral ARHL a combination of both auditory components, or, finally, a result of cognitive dysfunction. There are two basic hypotheses that may explain the concept of age-related CAPD, that is, a direct and an indirect hypothesis or a combination of both.42 The direct hypothesis proposes that pure central changes occurr without concomitant peripheral deficit, referred to as a ‘central effect of biological aging’.42,43 This hypothesis was supported by animal studies with evidence that some auditory structures in the central nervous system, such as the inferior colliculus, demonstrated age-related anatomical or physiological deficits without concomitant peripheral deficits.44,45 The indirect hypothesis proposes that a central effect of the peripheral dysfunction is exhibited as an increase in the auditory threshold, supposing that central auditory changes can be induced, from the cochlear nucleus through the auditory portions of the cortex, by the presence of a peripheral hearing loss.42 The indirect hypothesis proposes this as a ‘central effect of peripheral pathology’.43 In fact, degraded peripheral stimulation46 or a long sensory deprivation42,47 may cause cognitive dysfunction. Therefore, age-related CAPD is not an isolated entity, but a multifactorial condition linked to age- or disease-related auditory and brain changes.1 In fact, CAPD increases with age,1 but the concomitant increased incidence of both peripheral ARHL and cognitive impairment in older age made the interpretation of central auditory tests difficult. In particular, at an early stage of cognitive decline, the differential impact of the two types of auditory deficits on late-life cognitive dysfunction may be not easy to determine.13
A few studies investigated the link between age-related CAPD and MCI or AD.48–57 Several cross-sectional case-control sudies48,51,54,57 and two population-based studies52,56 suggested a convincing involvement of age-related CAPD in MCI,54,56,57 dementia,52,56 and AD.48,51,54,56 In particular, the Adult Changes in Thought (ACT) study found that age-related CAPD was related to executive dysfunction in older people with and without memory impairment and dementia.52 Therefore, the executive control function appeared to be a central factor in the speech-based behavioral tasks evaluating CAPD,48 given that central auditory pathway lesions were infrequent in early AD. In fact, a seminal neuropathological study showed that brain β-amyloid deposition (senile plaques), believed to be the initial event in AD, were uncommon in central auditory pathways early in the clinical course of the disease, while there was early formation of neurofibrillary tangles, mainly constituted of hyperphosphorylated tau protein, suggesting that neurodegeneration in the auditory system may be an ongoing process throughout the AD course.58
A few longitudinal case-control54 and population-based studies49,50,53 suggested that age-related CAPD in ARHL may increase the risk of cognitive decline and incident dementia,49 and AD.50,53 Therefore, age-related CAPD could be a precocious marker of MCI or AD, with a ‘gradient’ existing in CAPD among patients with subjective memory complaints, MCI, and early AD.55 These population-based studies suggested that age-related CAPD was associated with a significantly increased risk for incident dementia with hazard ratios (HRs) ranging from 9.9 [95% confidence interval (CI): 3.6–26.7] to 23.3 (95% CI: 6.6–82.7).49,50,59 In particular, findings from the Framingham Heart study showed that the relative risk (RR) of developing cognitive decline or dementia was twice as high for older adults with bilateral CAPD assessed with the Synthetic Identification-Ipsilateral Competing Message (SSI-ICM) scores in comparison with those with unilateral poor SSI-ICM scores.49 Furthermore, the cumulative evidence coming from these population-based studies suggested that impaired central auditory functions preceded AD diagnoses by 5–10 years.60
Hearing loss-cognition link: recent meta-analyses
In the last 5 years, a series of systematic reviews and meta-analyses investigated associations of ARHL with late-life cognitive disorders (Table 1),7,33,61–66 with contrasting findings partly explained the different operational definitions of ARHL and late-life cognitive disorders and adjustment for different potential confounders.67 The first of these systematic reviews and meta-analyses investigated 33 studies, suggesting that peripheral ARHL impacted all cognitive domains examined, including executive functioning, semantic processing and word knowledge, attention and processing speed, short-term/working, and long-term memory, although the effects found were small.61 The degree of cognitive impairment appeared to be associated with the severity of both untreated and treated peripheral ARHL. However, different designs and different measures of ARHL of the included studies may be a potential bias for the meta-analysis of Taljaard and colleagues.61
Table 1.
Principal systematic reviews and meta-analyses of peripheral ARHL and age-related CAPD in relation to late-life cognitive decline, MCI, dementia, and AD.
| Reference | Studies/patient age | Type of auditory function | Cognitive-related outcomes | Principal findings |
|---|---|---|---|---|
| Taljaard and colleagues61 | 33 studies 5735 participants. Mean age 57.7 years | Treated or untreated peripheral ARHL | General cognitive function, attention and processing speed, semantic processing and word knowledge, short-term and working memory, long-term memory, and executive functioning | Cognition was significantly poorer in individuals with untreated ARHL and remains poorer in treated ARHL compared with normal hearers. Hearing intervention significantly improves cognition. Better hearing is associated with better performance across all cognitive domains examined, including attention and processing speed, short-term/working, and long-term memory, executive functioning, and semantic processing and word knowledge, although the effects were all small |
| Thomson and colleagues62 | 17 studies (12 prospective studies). Systematic review only | Peripheral ARHL and age-related CAPD | Dementia and cognitive decline | ARHL was associated with higher incidence of dementia or cognitive decline |
| Zheng and colleagues63 | 4 prospective studies 7461 participants. Mean age 76.8 years | Peripheral ARHL and age-related CAPD | Incident AD and incident cognitive decline | The overall combined RR of people with hearing impairment to develop AD was 4.87 (95% CI: 0.90–26.35), compared with the control group, while the overall combined RR of AD and incident cognitive decline was 2.82 (95% CI: 1.47–5.42) |
| Livingston and colleagues7 | 3 prospective studies 3585 participants. Mean age 67.7 years | Peripheral ARHL | Incident dementia | Peripheral ARHL was a significant risk factor for incident dementia, calculating a pooled risk ratio of 1.94 (95% CI: 1.38–2.73) |
| Wei and colleagues64 | 10 prospective studies 15,521 participants. Mean age ranged from 56.1 to 77.4 years | Peripheral ARHL and age-related CAPD | Incident dementia and MCI | ARHL was associated with a greater risk of MCI (RR = 1.30; 95% CI: 1.12–1.51) and dementia (RR = 2.39; 95% CI: 1.58–3.61) |
| Loughrey and colleagues65 | 36 studies 20,264 participants. Mean age ranged from 51.4 to 85.0 years | Peripheral ARHL (pure tone audiometry) | Cognitive impairment and decline, dementia, AD, and vascular dementia | Among cross-sectional studies, a significant association was found for cognitive impairment (OR = 2.00; 95% CI: 1.39–2.89) and dementia (OR = 2.42; 95% CI: 1.24–4.72). Among prospective cohort studies, a significant association was found for cognitive impairment (OR = 1.22; 95% CI: 1.09–1.36) and dementia (OR = 1.28; 95% CI: 1.02–1.59) but not for AD (OR = 1.69; 95% CI: 0.72–4.00) |
| Yuan and colleagues66 | 11 prospective studies 176,893 participants. Mean age ranged from 63.6 to 79.6 years | Peripheral ARHL and age-related CAPD | Incident cognitive impairment | Peripheral ARHL and age-related CAPD had a higher risk of cognitive impairment (for moderate/severe peripheral ARHL: RR = 1.29; 95% CI: 1.04–1.59 during a follow up ⩽ 6 years. RR = 1.57; 95% CI: 1.13–2.20 during a follow up > 6 years; for severe age-related CAPD, RR = 3.21; 95% CI: 1.19–8.69) compared with those with normal hearing function |
| Ford and colleagues33 | 14 prospective studies 72,831 participants. Mean age ranged from 56.1 to 79.6 years | Peripheral ARHL and age-related CAPD | Incident dementia | A pooled HR of 1.49 (95% CI: 1.30–1.67) was found for all-cause dementia |
AD, Alzheimer’s disease; ARHL, age-related hearing loss; CAPD, central auditory processing dysfunction; CI, confidence interval; HR, hazard ratio; MCI, mild cognitive impairment; OR, odds ratio; RR, relative risk.
A systematic review summarized 17 studies on ARHL and dementia or cognitive decline, including studies investigating both peripheral ARHL and age-related CAPD, showing that ARHL was linked with a higher incidence of dementia or cognitive decline in older adults; however there was great variability in the assessment of ARHL and cognition among the different studies.62 Moreover, Zheng and colleagues’ pooling of data of four prospective population-based studies found that the overall combined RR of patients with both peripheral ARHL and age-related CAPD to develop AD was 4.87 (95% CI: 0.90–26.35), compared with the control group, while the overall combined RR of AD and incident cognitive decline was 2.82 (95% CI: 1.47–5.42).63 In another meta-analysis on only three population-based studies with follow-up periods ranging from 9 to 17 years, Livingstone and colleagues found that peripheral ARHL was a significant risk factor for incident dementia with a pooled risk ratio of 1.94 (95% CI: 1.38–2.73).7 Moreover, Wei and colleagues performed a meta-analysis identifying 10 longitudinal population-based studies that investigated ARHL as a predictor of MCI and dementia in older age, including studies investigating both peripheral ARHL and age-related CAPD.64 In particular, all studies on ARHL and dementia indicated positive associations despite variations in ARHL assessment tools and outcome measures. The pooled analysis confirmed a convincing association between ARHL and MCI (RR = 1.30; 95% CI: 1.12–1.51) and ARHL and dementia (RR = 2.39; 95% CI: 1.58–3.61) among older adults.64
On this basis, Loughrey and colleagues explored the associations among ARHL and cognitive function, cognitive impairment, and dementia, using a series of 36 observational studies on 20,264 participants from 12 countries assessing ARHL using PTA, and therefore investigating peripheral ARHL.65 In this meta-analysis, among cross-sectional studies, ARHL showed significant associations with all of the 10 cognitive domains investigated, including global cognition, episodic memory, executive functions, semantic memory, processing speed, and visuospatial ability, and with cognitive impairment, and dementia, excluding AD.65 Among prospective cohort studies, there was a significant association for cognitive function in seven of eight domains, excluding fluency, and with cognitive impairment and dementia, but not for AD, while the association for vascular dementia (VaD) only approached significance.65 In this meta-analysis, only studies using PTA to reduce conceptual heterogeneity were included, and while PTA remained the gold standard for clinical assessment of peripheral auditory dysfunction, CAPD was common in MCI and AD8 and assessment of CAPD in people with ARHL might be useful for identifying older patients at increased risk of developing MCI and AD.67 Furthermore, the full inclusion of hearing loss samples without a PTA cutoff may be the source of some heterogeneity.
In fact, more recently, Yuan and colleagues perfumed a meta-analysis on 11 selected studies including both peripheral ARHL and age-related CAPD, showing that both hearing dysfunctions appeared to contribute to the risk of cognitive impairment in older age.66 The overall risk of cognitive impairment increased 29% (follow up ⩽ 6 years, RR = 1.29, 95% CI: 1.04–1.59) or 57% (follow up > 6 years, RR = 1.57, 95% CI: 1.13–2.20) in older participants who had disabled peripheral ARHL compared with people with normal hearing function.66 This meta-analysis showed also that the association between age-related CAPD and cognitive impairment was stronger (RR = 3.21; 95% CI: 1.19–8.69 for SSI-ICM < 50% correct; RR = 2.42; 95% CI: 1.14–5.11 for SSI-ICM < 80% correct). A dose–response trend was found between peripheral ARHL and cognition.66
Finally, Ford and colleagues explored the association of self-reported ARHL and dementia through the Health in Men Study (HIMS), an Australian longitudinal population-based study of 37,898 older men (mean follow up: 11.1 years), and through a systematic review and meta-analysis of 14 longitudinal studies examining both peripheral ARHL and age-related CAPD and dementia risk.33 In the HIMS cohort, the hazard of dementia increased by 69% (95% CI: 54–85%) in men with self-reported ARHL compared with those with normal hearing adjusting also for age and other medical conditions common in older age. This finding confirmed those from previous prospective studies with an HR of 1.49 (95% CI: 1.30–1.67) in the pooled analysis of 14 studies. The duration of ARHL and dementia subtype did not have a significant impact on the findings.33
Cumulative meta-analytic evidence confirmed the cross-sectional and longitudinal association of both peripheral ARHL and age-related CAPD with different domains of cognitive functions, MCI, and dementia,7,33,61–66 while the association with dementia subtypes such as AD and VaD was unclear.63,66 Different operational definitions of ARHL, cognitive impairment, dementia and its principal subtypes and adjustment for different potential confounders could partly explain these discrepant findings among these recent systematic reviews and meta-analyses,67 although Loughrey and colleagues’ meta-analysis also performed exploratory subgroup and meta-regression analyses to investigate the possible explanations for heterogeneity due to study and patient characteristics, audiometric factors, and cognitive tools.65
Neurobiological mechanisms underlying the link between ARHL and late-life cognitive disorders
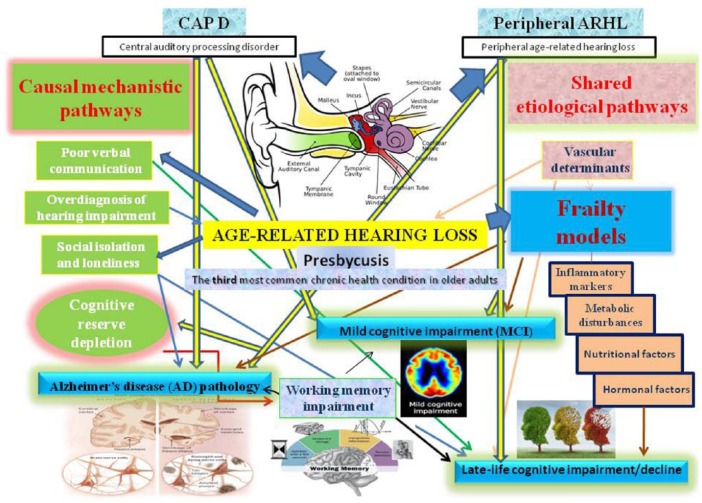
Current epidemiological evidence did not confirm a causal relationship between ARHL and cognition in older age. However, several factors may be involved in causal mechanistic pathways linking ARHL and cognition, with intermediate steps known as mediators (Figure 1).8 On the other hand, other factors could constitute common pathological processes or shared etiological (not causal) pathways affecting both ARHL and late-life cognitive disorders.8 Both peripheral and central auditory dysfunction may be implicated in the continuum of cognitive dysfunction from prodromal AD to the advanced stages of the disease (Figure 1).
Figure 1.
Overview of the principal causal mechanistic and shared pathways linking the different components of ARHL, peripheral ARHL and CAPD, with frailty models, mild cognitive impairment, Alzheimer’s disease, and late-life cognitive impairment or decline.
ARHL, age-related hearing loss; CAPD, central auditory processing disorder.
In the first set of pathways, peripheral ARHL may cause or increase the risk of cognitive impairment. For example, peripheral ARHL may result in a change of brain structure which may, in turn, increase the risk of cognitive impairment and dementia.68,69 Furthermore, although AD-related pathology was not found in the peripheral auditory pathways,58,70 peripheral ARHL was related to reduced cortical volumes in the primary auditory cortex,68 accelerated rates of decline in whole brain volume, and regional brain volume in the right temporal lobe,71 in the secondary association cortices, and the auditory thalamus, which is visualized in functional neuroimaging tasks as decreased neural activations to auditory stimuli.68
Although there is plenty of evidence that peripheral ARHL may lead to changes in the central auditory system,72–74 the audiological deficits observed in AD patients were not directly associated with maladaptive plastic changes driven by peripheral ARHL. This finding would be consistent with the anatomical data showing a greater presence of AD-related pathology in higher central auditory structures.58,75 Other interesting and emerging hallmarks of AD-related pathology were loss of top-down modulation of incoming auditory stimuli and loss of sensory gating ability.58,75 Another interpretation of the audiological data involved cochlear synaptopathy, manifesting in AD patients with ‘hidden hearing loss’, preserving PTA values, but with an impact on speech-in-noise performance.76
ARHL may also unmask or hasten cognitive decline by depleting cognitive reserve, which represents the adaptability of cognitive processes that helps to explain differential susceptibility of cognitive abilities or day-to-day function to brain aging, pathology or insult.77 Cognitive reserve may act as a modulator of the interplay between neuropathology and cognitive-related outcomes.78 In older patients with peripheral ARHL, the amount of cognitive resources dedicated to auditory perceptual processing are increased. This reallocation may be detrimental for other cognitive functions, such as working memory, one of the first domains to be affected in individuals with ARHL. The ‘information-degradation hypothesis’79–82 proposes that age-related cognitive decline manifested as a consequence of compensating for impaired auditory input,81,82 owing either to pathological or normal aging. Higher-level cognitive processing is impaired because mental resources have been reallocated to perception.81 Significant evidence supported the hypothesis that the compensation for degraded speech may reduce resources for other downstream cognitive processes crucial for ongoing speech communication. Verbal long-term memory perceived in degraded form tended to be poorer than memory for clear ones.83–90
A third theoretical causal mechanism hypothesized that ARHL may reduce cognitively stimulating input to the brain. The ‘sensory deprivation’ hypothesis proposes that perceptual decline may cause more permanent cognitive decline,79–82,91,92 probably through neuroplastic changes disadvantaging global cognition for processes increasing speech perception. The sensory deprivation hypothesis emphasizes that this chronic reallocation of cognitive resources may cause permanent age-related cognitive changes. The sensory deprivation hypothesis may cause de-afferentation and atrophy in the auditory system as well as subsequent reorganization, owing to long-term sensory deprivation. ARHL or noise-induced hearing loss may also result in cortical changes,93–98 which could also affect cognitive functions localized in the affected brain regions. These included changes in excitatory, inhibitory and neuromodulatory networks, consistent with theories of homeostatic plasticity,93–97 functional alterations in gene expression and in protein levels,93,98 and broader network processing effects with cognitive and behavioral implications.93 Sensory loss may be compensated also triggering neurovascular and neurophysiological changes, including cerebral blood flow dysregulation and disruption of the neurovascular unit and the blood–brain barrier, similar to those of dementia and leading to cognitive decline. Moreover, a compelling negative relationship also exists between gray matter density in primary auditory areas and peripheral hearing ability, probably reflecting cortical reorganization following reduced sensory input.58,68,99 Recently, in a 6-month follow up, moderate hearing loss caused decreases in cognition in young mice associated with spatial working and recognition memories, suggesting that hearing loss can be an independent causal factor for developing cognitive impairment, irrespective of age,100 providing further evidence for a causal relationship between ARHL and cognitive impairment, and supporting the sensory deprivation hypothesis.
Alternatively, social isolation, loneliness and depression may result from the communication impairments caused by ARHL. Communication difficulties can severely limit the social integration of patients with ARHL or cognitive impairment. Reduced social interaction may be linked with depression and withdrawal,101,102 and indeed ARHL and cognitive impairment were associated with late-life depression.103–108 ARHL might add to the cognitive load of a vulnerable brain leading to cognitive changes, or it may cause social disengagement or depression all of which contributing to accelerated cognitive decline.7 Meta-analytic findings suggested that ARHL was among the most common chronic conditions associated with late-life depression, and self-reported and PTA-based ARHL were significantly associated with depression, particularly in women.109 However, there was no direct demonstration that depression may mediate or moderate the ARHL-cognition link.105,110 Very recent findings from a cross-sectional analysis of the English Longitudinal Study of Ageing showed that social isolation appeared to be a mediating factor in the link between ARHL and cognition for those who have untreated hearing loss,111 with social dysfunction also associated with cognition in older age.112 Finally, another possibility may be that sensory decline may cause cognitive decline. ARHL may increase cognitive load, meaning that so much attentional effort is expended decoding sounds into words that little brain capacity remains to process the actual meaning of the message.79,80
In the second set of mechanistic pathways, ARHL did not cause cognitive impairment. Age-related cognitive decline tended to affect multiple cognitive domains of cognitive supporting the ‘common-cause’ hypothesis.79,80,113 Cognitive aging, given the widespread age-related neural degeneration characterized by reductions in volume and length of dendritic spine densities in multiple cortical regions, particularly pre-frontal regions, implied compromising ability to ignore irrelevant information during cognitive processing.114 A general slowing of motor task processing and performance may be the central deficit associated with aging,79,115 affecting performance across multiple cognitive and sensory domains. The extent to which processing speed may explain the ARHL-cognition link remained unclear.
Different frailty models and phenotypes, sensorial changes, and late-life cognitive disorders
Among factors that could constitute common pathological processes or shared etiological pathways affecting both ARHL and late-life cognitive disorders, older adults with significant ARHL reported lower physical activity levels and slowed gait speeds.116 Physical frailty characteristics such as slowed gait speed exhibited significant reciprocal relationships with cognition117 and thus may represent an intermediate step in the progression of some older adults from ARHL to cognitive decline. The presence of frailty increased the risk of adverse health-related outcomes such as falls, disability, hospitalization, institutionalization, and death, while ARHL may be a strong marker for the most widely diffused models of frailty.8,118 In fact, not only may the physical/biological model14 act as a possible modulator of the link between sensorial changes and cognition in older age, but also the deficit accumulation model16 and biopsychosocial/multidimensional model (Figure 2).17 In fact, the multidimensional nature of these two models prompted an approach to frailty based on different pathogeneses, and this heterogeneous clinical syndrome may include physical, cognitive, social, and psychological domains. Therefore, the principal phenotypes are physical frailty,14 cognitive frailty,119,120 social frailty,121 and psychological frailty,122 this last encompassing motivational and mood components, that is, the recently proposed depressive frail phenotype.123 Given that hearing and visual loss in older age are frequent conditions included in many models of frailty and related diagnostic tools,8 a novel phenotype called ‘sensorial frailty’ may be proposed. In fact, among the nontraditional risk factors for dementia and AD there was hearing and vision impairment.124 In particular, for age-related vision loss, changes in visual acuity in older adults predicted a change in cognitive functioning in longitudinal population-based studies.34,125,126 Furthermore, visual disturbance is often an early complaint of AD patients, with studies reporting reduced visual performance on tests of visual field, color vision, pupil reaction, and contrast sensitivity.127 Finally, also dual sensory loss (DSL; combined vision and hearing loss) is a condition highly prevalent in the older adult population, occurring in up to 69% of adults aged over 65 years.128 DSL has been correlated with decreased levels of wellbeing and late-life depression, with a significant relationship between DSL and decreased mental health with those with DSL either displaying depressive symptoms or being at risk for developing depression.129 A recent epidemiological study indicated that higher levels of depressive symptoms were associated with ARHL and DSL but not with vision loss.107 Although this growing body of evidence suggested the possible introduction of a sensorial frailty phenotype associated to cognition and depression in older age, further studies are needed to define and operationalize this novel construct in clinical ad epidemiological settings.
Figure 2.
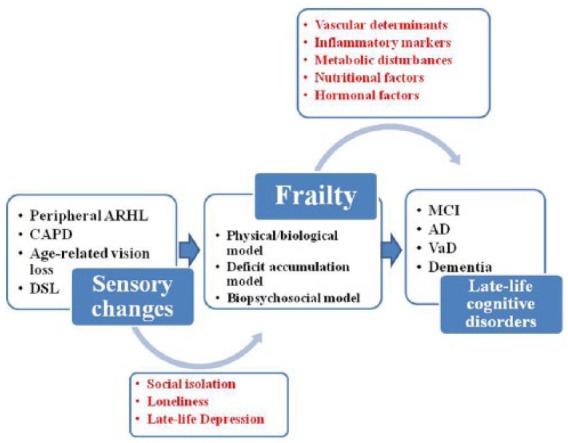
Different models of frailty (physical/ biological model, deficit accumulation model, and biopsychosocial/multidimensional model), acting as possible modulators of the link sensorial changes cognition in older age.
AD, Alzheimer’s disease; ARHL, age-related hearing loss; CAPD, central auditory processing disorder; DSL, dual sensory loss; MCI, mild cognitive impairment; VaD, vascular dementia.
Inflammatory markers, hormones, nutritional factors, metabolic disturbances, and especially diabetes mellitus, congestive heart failure and stroke are all factors and diseases linked to frailty,18,118 and also associated with cognitive impairment, suggesting that these two age-related conditions may share an underlying pathogenesis, probably linked to vascular determinants (Figure 2). Moreover, some recent population-based studies showed that ARHL was associated with an increased risk of falling over time and developing frailty130 and poorer objective physical functioning and incident disability in older adults.131 Therefore, ARHL may be an important frailty indicator and frailty itself can be a determinant of cognitive decline directly or indirectly through concomitant factors such as limited communication, consequent social isolation and loneliness, and late-life depression (Figure 2).8,13
Among different frailty phenotypes, the term ‘cognitive frailty’ was proposed to indicate a particular state of cognitive vulnerability to MCI and other similar predementia syndromes, as well as an increased risk of progression to overt dementia in individuals exposed to vascular risk.119 Physical frailty was proposed by prospective population-based studies as a prodromal stage of VaD.15 Vascular disease can cause both ARHL and dementia, and therefore VaD could be a common pathway resulting in both clinical conditions. Moreover, at a molecular level, the SIRT1-PGC1α and LKB1 (or CaMKKβ)-AMPK pathways may play a role in the preservation of brain function regulating mitochondrial function.132 Then, the vascular endothelial growth factor signal pathway may be activated to promote vascular angiogenesis and maintenance of the blood–brain barrier integrity. Moreover, studies on ARHL and AD mouse models suggested that these enzymes and protein expression levels may be altered in both age-related clinical conditions.132
Discussion
Over the last half-century, the cumulative epidemiological evidence coming from a large number of longitudinal population-based studies suggested convincing links between peripheral ARHL and late-life cognitive disorders, including incident cognitive decline and dementia. Many cross-sectional case-control and population-based studies suggested also a strong involvement of age-related CAPD in MCI, dementia, and AD. Moreover, sparse longitudinal case-control and population-based studies suggested that age-related CAPD in ARHL may be central in determining an increased risk of incident cognitive decline, dementia, and AD. In the same direction, in the last 5 years, cumulative meta-analytic evidence confirmed the cross-sectional and longitudinal association of both peripheral ARHL and age-related CAPD with different domains of cognitive functions, MCI, and dementia, while the association with dementia subtypes such as AD and VaD remained unclear.
Nonetheless, with this increasing body of epidemiological evidence, ARHL appeared to be influenced by different determinants including genetic, lifestyle, and environmental factors.59,133 Therefore, the pathophysiological consequences of ARHL should not be studied as isolated factors, and many of the existing epidemiological studies did not take in account different determinants linked to both ARHL and cognition. Furthermore, we do not know whether ARHL and AD-related biomarkers may be associated prior to the clinical onset of dementia.59 Another open issue is to determine whether the relationship of ARHL and late-life cognition is causal or not. Therefore, it is mandatory to continue to investigate plausible mechanistic links, including more sophisticated brain imaging modalities.134 Given that ARHL represents a distinct condition from earlier onset hearing loss and that the effect of ARHL on cognition was greater among patients older than 75 years,37 suggesting a strong link with another age-related condition such as frailty, future studies should focus specifically on the older age group.
Early intervention, lifestyle changes, and utilizing available public health strategies such as hearing loss management and correction could delay or prevent a third of AD diagnoses worldwide.7 The strong epidemiological evidence suggesting the ARHL-cognition link supported the potential for correcting hearing loss with possible cognitive improvement in older patients with subsequent improved social involvement and quality of life. However, there were very few randomized controlled trials (RCTs) showing cognitive, social, or global functioning improvements in hearing aid users not cognitively impaired135 or with dementia,136 and determining whether treating ARHL could delay or halt cognitive decline and dementia remained an unsolved issue. Very recently, the Alzheimer Disease, Presbycusis and Hearing Aids (ADPHA) study group showed that two RCTs did not provide evidence of a cognitive benefit137 or improvement in neuropsychiatric symptoms, global functional measures, or quality of life of hearing-impaired AD patients and their caregivers after 6 months of hearing aid use.138 For observational studies, a cross-sectional study on a large sample of 164,770 UK adults, hearing aid use was correlated with better cognition.139 In two longitudinal population-based studies with a 6-year follow-up period, there was no impact of sensory intervention (cataract surgery or hearing aids) on cognitive measures126 and cognitive decline rates were not significantly attenuated in individuals using hearing aids than those without hearing aids.28 However, a recent population-based study with a 25-year follow up suggested that the treatment of ARHL with hearing aids may attenuate cognitive decline,140 suggesting some long-term protective effects of hearing aids against cognitive decline.
Further longitudinal studies with larger sample sizes, clearly defined sensory impairment duration, and the use of appropriate testing tools to disentangle cognitive impairment from sensory impairments are needed to confirm or refute the hypothesis that sensory impairment, particularly ARHL, may lead to an increased risk of cognitive decline in older age. In the future, inter-professional teamwork should be organized so that hearing information can guide cognitive healthcare and information about cognitive status could guide hearing healthcare. For intervention, further larger RCTs with longer follow ups, and more representative technology are needed (i.e. digital hearing aids or cochlear implants). In a pilot study on 40 older patients randomized to a best practice hearing or successful aging intervention, the Aging and Cognitive Health Evaluation in Elders Pilot (ACHIEVE-P) study showed efficacy signals of the hearing intervention in perceived hearing handicap and memory,141 setting the stage for the full-scale ACHIEVE trial (N = 850, recruitment beginning November 2017, 3 years follow up). The results of this ongoing large RCT will help to determine the efficacy of best practice hearing interventions on reducing cognitive decline in older adults with ARHL. Preliminary findings on small case-series or pilot studies demonstrated some improvements in cognitive function and auditory perception following cochlear implantation,142–144 although hearing aids alone were not enough to manage ARHL and cognitive impairment. It is also mandatory to investigate also how ARHL may influence the caregiver burden, with the development of a related program to demonstrate the benefits of interventions in reducing caregiver burden for family members of demented patients with ARHL.
Acknowledgments
Francesco Panza and Madia Lozupone contributed equally to this work.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Francesco Panza  https://orcid.org/0000-0002-7220-0656
https://orcid.org/0000-0002-7220-0656
Contributor Information
Francesco Panza, Department of Basic Medical Sciences, Neurosciences, and Sense Organs, Neurodegenerative Disease Unit, University of Bari ‘Aldo Moro’, Piazza Giulio Cesare 11, 70100, Bari, Italy.
Madia Lozupone, Neurodegenerative Disease Unit, Department of Basic Medicine, Neuroscience, and Sense Organs, University of Bari Aldo Moro, Bari, Italy.
Rodolfo Sardone, National Institute of Gastroenterology ‘Saverio de Bellis’, Research Hospital, Castellana Grotte Bari, Italy.
Petronilla Battista, Neurodegenerative Disease Unit, Department of Basic Medicine, Neuroscience, and Sense Organs, University of Bari Aldo Moro, Bari, Italy; Istituti Clinici Scientifici Maugeri SPA SB, IRCCS, Institute of Cassano Murge, Bari, Italy.
Marco Piccininni, Neurodegenerative Disease Unit, Department of Basic Medicine, Neuroscience, and Sense Organs, University of Bari Aldo Moro, Bari, Italy.
Vittorio Dibello, National Institute of Gastroenterology ‘Saverio de Bellis’, Research Hospital, Castellana Grotte Bari, Italy; Interdisciplinary Department of Medicine (DIM), Section of Dentistry, University of Bari Aldo Moro, Bari, Italy.
Maddalena La Montagna, Psychiatric Unit, Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy.
Roberta Stallone, Neurodegenerative Disease Unit, Department of Basic Medicine, Neuroscience, and Sense Organs, University of Bari Aldo Moro, Bari, Italy; National Institute of Gastroenterology ‘Saverio de Bellis’, Research Hospital, Castellana Grotte Bari, Italy.
Pietro Venezia, Department of Prosthodontics, Section of Dentistry, University of Catania, Catania, Italy.
Angelo Liguori, Neurodegenerative Disease Unit, Department of Basic Medicine, Neuroscience, and Sense Organs, University of Bari Aldo Moro, Bari, Italy.
Gianluigi Giannelli, National Institute of Gastroenterology ‘Saverio de Bellis’, Research Hospital, Castellana Grotte Bari, Italy.
Antonello Bellomo, Psychiatric Unit, Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy.
Antonio Greco, Geriatric Unit, Fondazione IRCCS ‘Casa Sollievo della Sofferenza’, San Giovanni Rotondo, Foggia, Italy.
Antonio Daniele, Institute of Neurology, Catholic University of Sacred Heart, Rome, Italy; Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Davide Seripa, Geriatric Unit, Fondazione IRCCS ‘Casa Sollievo della Sofferenza’, San Giovanni Rotondo, Foggia, Italy.
Nicola Quaranta, Otolaryngology Unit, University of Bari Aldo Moro, Bari, Italy.
Giancarlo Logroscino, Neurodegenerative Disease Unit, Department of Basic Medicine, Neuroscience, and Sense Organs, University of Bari Aldo Moro, Bari, Italy; Neurodegenerative Disease Unit, Department of Clinical Research in Neurology, University of Bari Aldo Moro, ‘Pia Fondazione Cardinale G. Panico’, Tricase, Lecce, Italy.
References
- 1. Gates GA, Mills JH. Presbycusis. Lancet 2005; 366: 1111–1120. [DOI] [PubMed] [Google Scholar]
- 2. Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med 2008; 168: 1522–1530. [DOI] [PubMed] [Google Scholar]
- 3. Lin FR, Thorpe R, Gordon-Salant S, et al. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci 2011; 66: 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quaranta N, Coppola F, Casulli M, et al. Epidemiology of age related hearing loss: a review. Hearing Balance Commun 2015; 17: 77–81. [Google Scholar]
- 5. Collins JG. Prevalence of selected chronic conditions: United States, 1990–1992. Vital Health Stat 1997; 10:1–89. [PubMed] [Google Scholar]
- 6. Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement 2015; 11: 70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017; 390: 2673–2734. [DOI] [PubMed] [Google Scholar]
- 8. Panza F, Solfrizzi V, Logroscino G. Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nat Rev Neurol 2015; 11: 166–175. [DOI] [PubMed] [Google Scholar]
- 9. Otto WC, McCandless GA. Aging and auditory site of lesion. Ear Hear 1982; 3: 110–117. [DOI] [PubMed] [Google Scholar]
- 10. American Speech-Language-Hearing Association. Central auditory processing disorders, http://www.asha.org/policy/TR2005–00043/ (2005) (accessed 25 October 2018).
- 11. American Academy of Audiology. Diagnosis, treatment and management of children and adults with central auditory processing disorder, http://audiology-web.s3.amazonaws.com/migrated/CAPD%20Guidelines%2082010.pdf_539952af956c79.7389761.pdf (2010) (accessed 25 October 2018).
- 12. Iliadou VV, Ptok M, Grech H, et al. A European perspective on auditory processing disorder-current knowledge and future research focus. Front Neurol 2017; 8: 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panza F, Solfrizzi V, Seripa D, et al. Age-related hearing impairment and frailty in Alzheimer’s disease: interconnected associations and mechanisms. Front Aging Neurosci 2015; 7: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 15. Panza F, Lozupone M, Solfrizzi V, et al. Different cognitive frailty models and health- and cognitive-related outcomes in older age: from epidemiology to prevention. J Alzheimers Dis 2018; 62: 993–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World Journal 2001; 1: 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, et al. In search of an integral conceptual definition of frailty: opinions of experts. J Am Med Dir Assoc 2010; 11: 338–343. [DOI] [PubMed] [Google Scholar]
- 18. Panza F, Solfrizzi V, Frisardi V, et al. Different models of frailty in predementia and dementia syndromes. J Nutr Health Aging 2011; 15: 711–719 [DOI] [PubMed] [Google Scholar]
- 19. Malmstrom TK, Morley JE. Frailty and cognition: linking two common syndromes in older persons. J Nutr Health Aging 2013; 17: 723–725. [DOI] [PubMed] [Google Scholar]
- 20. Kay DWK, Beamish P, Roth M. Old age mental disorders in Newcastle upon Tyne, II: a study of possible social and medical causes. Br J Psychiatry 1964; 110: 668–682. [DOI] [PubMed] [Google Scholar]
- 21. Gennis V, Garry PJ, Haaland KY, et al. Hearing and cognition in the elderly. New findings and a review of the literature. Arch Intern Med 1991; 151: 2259–2264. [PubMed] [Google Scholar]
- 22. Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 2004; 52: 1996–2002. [DOI] [PubMed] [Google Scholar]
- 23. Valentijn SA, van Boxtel MP, van Hooren SA, et al. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the Maastricht aging study. J Am Geriatr Soc 2005; 53: 374–380. [DOI] [PubMed] [Google Scholar]
- 24. Wallhagen MI, Strawbridge WJ, Shema SJ. The relationship between hearing impairment and cognitive function: a 5-year longitudinal study. Res Gerontol Nurs 2008; 1: 80–86. [DOI] [PubMed] [Google Scholar]
- 25. Lin FR, Metter EJ, O’Brien RJ, et al. Hearing loss and incident dementia. Arch Neurol 2011; 68: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gallacher J, Ilubaera V, Ben-Shlomo Y, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology 2012; 79: 1583–1590. [DOI] [PubMed] [Google Scholar]
- 27. Kiely KM, Gopinath B, Mitchell P, et al. Cognitive, health, and sociodemographic predictors of longitudinal decline in hearing acuity among older adults. J Gerontol A Biol Sci Med Sci 2012; 67: 997–1003. [DOI] [PubMed] [Google Scholar]
- 28. Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med 2013; 173: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harrison Bush AL, Lister JJ, Lin FR, et al. Peripheral hearing and cognition: evidence from the staying keen in later life (SKILL) study. Ear Hear 2015; 36: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fischer ME, Cruickshanks KJ, Schubert CR, et al. Age-related sensory impairments and risk of cognitive impairment. J Am Geriatr Soc 2016; 64: 1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heywood R, Gao Q, Nyunt MSZ, et al. Hearing loss and risk of mild cognitive impairment and dementia: findings from the Singapore longitudinal ageing study. Dement Geriatr Cogn Disord 2017; 43: 259–268. [DOI] [PubMed] [Google Scholar]
- 32. Deal JA, Betz J, Yaffe K, et al. Hearing impairment and incident dementia and cognitive decline in older adults: the health ABC study. J Gerontol A Biol Sci Med Sci 2017; 72: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ford AH, Hankey GJ, Yeap BB, et al. Hearing loss and the risk of dementia in later life. Maturitas 2018; 112: 1–11. [DOI] [PubMed] [Google Scholar]
- 34. Anstey KJ, Luszcz MA, Sanchez L. Two-year decline in vision but not hearing is associated with memory decline in very old adults in a population-based sample. Gerontology 2001; 47: 289–293. [DOI] [PubMed] [Google Scholar]
- 35. Anstey KJ, Hofer SM, Luszcz MA. A latent growth curve analysis of late-life sensory and cognitive function over 8 years: evidence for specific and common factors underlying change. Psychol Aging 2003; 18: 714–726. [DOI] [PubMed] [Google Scholar]
- 36. Hong T, Mitchell P, Burlutsky G, et al. Visual impairment, hearing loss and cognitive function in an older population: longitudinal findings from the Blue Mountains Eye Study. PLoS One 2016; 11: e0147646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu ST, Chiu CJ. Age-related trajectories of memory function in middle-aged and older adults with and without hearing impairment. Neuroepidemiology 2016; 46: 282–289. [DOI] [PubMed] [Google Scholar]
- 38. Logroscino G, Panza F. The role of hearing impairment in cognitive decline: need for the special sense assessment in evaluating cognition in older age. Neuroepidemiology 2016; 46: 290–291. [DOI] [PubMed] [Google Scholar]
- 39. Dupuis K, Pichora-Fuller MK, Chasteen AL, et al. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Aging Neuropsychol Cogn 2015; 22: 413–437. [DOI] [PubMed] [Google Scholar]
- 40. Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev 2015; 23: 154–166. [DOI] [PubMed] [Google Scholar]
- 41. Jayakody DMP, Friedland PL, Eikelboom RH, et al. A novel study on association between untreated hearing loss and cognitive functions of older adults: baseline non-verbal cognitive assessment results. Clin Otolaryngol 2018; 43: 182–191. [DOI] [PubMed] [Google Scholar]
- 42. Humes LE, Dubno JR, Gordon-Salant S, et al. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol 2012; 8: 635–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Willott JF. Anatomic and physiologic aging: a behavioral neuroscience perspective. J Am Acad Audiol 1996; 7: 141–151. [PubMed] [Google Scholar]
- 44. Walton JP, Frisina RD, O’Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of CBA mouse. J Neurosci 1998; 18: 2764–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willott JF, Chisolm TH, Lister JJ. Modulation of presbycusis: current status and future directions. Audiol Neurootol 2001; 5: 231–249. [DOI] [PubMed] [Google Scholar]
- 46. Wingfield A, Tun PA, Mccoy SL. Hearing loss in older adulthood—what it is and how it interacts with cognitive performance. Curr Dir Psychol Sci 2005; 3: 144–148. [Google Scholar]
- 47. Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging 1997; 1: 12–21. [DOI] [PubMed] [Google Scholar]
- 48. Kurylo DD, Corkin S, Allard T, et al. Auditory function in Alzheimer’s disease. Neurology 1993; 43: 1893–1899. [DOI] [PubMed] [Google Scholar]
- 49. Gates GA, Cobb JL, Linn RT, et al. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch Otolaryngol Head Neck Surg 1996; 122: 161–167. [DOI] [PubMed] [Google Scholar]
- 50. Gates GA, Beiser A, Rees TS, et al. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J Am Geriatr Soc 2002; 50: 482–488. [DOI] [PubMed] [Google Scholar]
- 51. Gates GA, Anderson ML, Feeney MP, et al. Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Head Neck Surg 2008; 134: 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gates GA, Gibbons LE, McCurry SM, et al. Executive dysfunction and presbycusis in older persons with and without memory loss and dementia. Cogn Behav Neurol 2010; 23: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gates GA, Anderson ML, McCurry SM, et al. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol Head Neck Surg 2011; 137: 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Idrizbegovic E, Hederstierna C, Dahlquist M, et al. Central auditory function in early Alzheimer’s disease and in mild cognitive impairment. Age Ageing 2011; 40: 249–254. [DOI] [PubMed] [Google Scholar]
- 55. Idrizbegovic E, Hederstierna C, Dahlquist M, et al. Short-term longitudinal study of central auditory function in Alzheimer’s disease and mild cognitive impairment. Dement Geriatr Cogn Dis Extra 2013; 3: 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Quaranta N, Coppola F, Casulli M, et al. The prevalence of peripheral and central hearing impairment and its relation to cognition in older adults. Audiol Neurootol 2014; 19(Suppl. 1): 10–14. [DOI] [PubMed] [Google Scholar]
- 57. Edwards JD, Lister JJ, Elias MN, et al. Auditory processing of older adults with probable mild cognitive impairment. J Speech Lang Hear Res 2017; 60: 1427–1435. [DOI] [PubMed] [Google Scholar]
- 58. Sinha UK, Hollen KM, Rodriguez R, et al. Auditory system degeneration in Alzheimer’s disease. Neurology 1993; 43: 779–785. [DOI] [PubMed] [Google Scholar]
- 59. Jayakody DMP, Friedland PL, Martins RN, et al. Impact of aging on the auditory system and related cognitive functions: a narrative review. Front Neurosci 2018; 12: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iliadou V, Kaprinis S. Clinical psychoacoustics in Alzheimer’s disease central auditory processing disorders and speech deterioration. Ann Gen Hosp Psychiatry 2003; 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taljaard DS, Olaithe M, Brennan-Jones CG, et al. The relationship between hearing impairment and cognitive function: a meta-analysis in adults. Clin Otolaryngol 2016; 41: 718–729. [DOI] [PubMed] [Google Scholar]
- 62. Thomson RS, Auduong P, Miller AT, et al. Hearing loss as a risk factor for dementia: a systematic review. Laryngoscope Investig Otolaryngol 2017; 2: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zheng Y, Fan S, Liao W, et al. Hearing impairment and risk of Alzheimer’s disease: a meta-analysis of prospective cohort studies. Neurol Sci 2017; 38: 233–239. [DOI] [PubMed] [Google Scholar]
- 64. Wei J, Hu Y, Zhang L, et al. Hearing impairment, mild cognitive impairment, and dementia: a meta-analysis of cohort studies. Dement Geriatr Cogn Dis Extra 2017; 7: 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loughrey DG, Kelly ME, Kelley GA, et al. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2018; 144: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yuan J, Sun Y, Sang S, et al. The risk of cognitive impairment associated with hearing function in older adults: a pooled analysis of data from eleven studies. Sci Rep 2018; 8: 2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Panza F, Quaranta N, Logroscino G. Sensory changes and the hearing loss-cognition link: the cognitive ear. JAMA Otolaryngol Head Neck Surg 2018; 144: 127–128. [DOI] [PubMed] [Google Scholar]
- 68. Peelle JE, Troiani V, Grossman M, et al. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci 2011; 31: 12638–12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peelle JE, Wingfield A. The neural consequences of age-related hearing loss. Trends Neurosci 2016; 39: 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Grimes AM, Grady CL, Pikus A. Auditory evoked potentials in patients with dementia of the Alzheimer type. Ear Hear 1987; 8: 157–161. [DOI] [PubMed] [Google Scholar]
- 71. Lin FR, Ferrucci L, An Y, et al. Association of hearing impairment with brain volume changes in older adults. Neuroimage 2014; 90: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Eggermont JJ. Acquired hearing loss and brain plasticity. Hear Res 2017; 343: 176–190. [DOI] [PubMed] [Google Scholar]
- 73. Lesicko AM, Llano DA. Impact of peripheral hearing loss on top-down auditory processing. Hear Res 2017; 343: 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev 2002; 82: 601–636. [DOI] [PubMed] [Google Scholar]
- 75. Sinha UK, Saadat D, Linthicum FH, et al. Temporal bone findings in Alzheimer’s disease. Laryngoscope 1996; 106: 1–5. [DOI] [PubMed] [Google Scholar]
- 76. Plack CJ, Barker D, Prendergast G. Perceptual consequences of “hidden” hearing loss. Trends Hearing 2014; 18: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. ; Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. Epub ahead of print 14 September 2018. DOI: 10.1016/j.jalz.2018.07.219. [DOI] [Google Scholar]
- 78. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012; 11: 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. CHABA. Speech understanding and aging. Working group on speech understanding and aging. Committee on hearing, bioacoustics, and biomechanics, commission on behavioral and social sciences and education, national research council. J Acoust Soc Am 1988; 83: 859–895. [PubMed] [Google Scholar]
- 80. Lindenberger U, Baltes PB. Sensory Functioning and intelligence in old-age- a strong connection. Psycho Aging 1994; 9: 339–355. [DOI] [PubMed] [Google Scholar]
- 81. Pichora-Fuller MK. Cognitive aging and auditory information processing. Int J Audiol 2003; 42 (Suppl. 2): 2S26–2S32. [PubMed] [Google Scholar]
- 82. Schneider BA, Pichora-Fuller K. Implications of perceptual deterioration for cognitive aging research. In: Craik FIM, Salthouse TA. (eds) Handbook of aging and cognition. Mahwah, NJ: Erlbaum, 2000. [Google Scholar]
- 83. Burkholder RA, Pisoni DB, Svirsky MA. Effects of a cochlear implant simulation on immediate memory in normal-hearing adults. Int J Audiol 2005; 44: 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J Acoust Soc Am 1995; 97: 593–608. [DOI] [PubMed] [Google Scholar]
- 85. Pichora-Fuller MK, Singh G. Effects of age on auditory and cognitiveprocessing: implications for hearing aid fitting and audiologic rehabilitation. Trends Amplification 2000; 10: 29–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Piquado T, Cousins KAQ, Wingfield A, et al. Effects of degraded sensory input on memory for speech: behavioral data and a test of biologically constrained computational models. Brain Res 2010; 1365: 48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rabbitt PM. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Otolaryngol Suppl 1990; 476: 167-75; discussion 176. [DOI] [PubMed] [Google Scholar]
- 88. Wingfield A, Tun PA, McCoy SL. Hearing loss in older adulthood - what itis and how it interacts with cognitive performance. Curr Dir Psychol Sci 2005; 14: 144–148. [Google Scholar]
- 89. McCoy SL, Tun PA, Cox LC, et al. Hearing loss and perceptual effort: downstream effects on older adults’memory for speech. Q J Exp Psychol A 2005; 58: 22–33. [DOI] [PubMed] [Google Scholar]
- 90. Murphy DR, Craik FI, Li KZ, et al. Comparing the effects of aging and background noise on short-term memory performance. Psychol Aging 2000; 15: 323–334. [DOI] [PubMed] [Google Scholar]
- 91. Humes LE, Kidd GR, Lentz JJ. Auditory and cognitive factors underlying individual differences in aided speech-understanding among older adults. Front Syst Neurosci 2013; 7: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Uhlmann RF, Larson EB, Rees TS, et al. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA 1989; 261: 1916–1919. [PubMed] [Google Scholar]
- 93. Gold JR, Bajo VM. Insult-induced adaptive plasticity of the auditory system. Front Neurosci 2014; 8: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Meredith MA, Keniston LP, Allman BL. Multisensory dysfunction accompanies crossmodal plasticity following adult hearing impairment. Neuroscience 2012; 214: 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pienkowski M, Eggermont JJ. Cortical tonotopic map plasticity and behavior. Neurosci Biobehav Rev 2011; 35: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 96. Dietrich V, Nieschalk M, Stoll W, et al. Cortical reorganization in patients with high frequency cochlear hearing loss. Hearing Res 2001; 158: 95–101. [DOI] [PubMed] [Google Scholar]
- 97. Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev 2002; 82: 601–636. [DOI] [PubMed] [Google Scholar]
- 98. Wang H, Brozoski TJ, Ling L, et al. Impact of sound exposure and aging on brain-derived neurotrophic factor and tyrosine kinase Breceptors levels in dorsal cochlear nucleus 80 days following sound exposure. Neuroscience 2011; 172: 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Eckert MA, Kuchinsky SE, Vaden KI, et al. White matter hyperintensities predict low frequency hearing in older adults. J Assoc Res Otolaryngol 2013; 14: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Park SY, Kim MJ, Sikandaner H, et al. A causal relationship between hearing loss and cognitive impairment. Acta Otolaryngol 2016; 136: 480–483. [DOI] [PubMed] [Google Scholar]
- 101. Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg 2014; 150: 378–384. [DOI] [PubMed] [Google Scholar]
- 102. Mener DJ, Betz J, Genther DJ, et al. Hearing loss and depression in older adults. J Am Geriatr Soc 2013; 61: 1627–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Boi R, Racca L, Cavallero A, et al. Hearing loss and depressive symptoms in elderly patients. Geriatrics Gerontology Int 2012; 12: 440–445. [DOI] [PubMed] [Google Scholar]
- 104. Herbst KG, Humphrey C. Hearing impairment and mental state in the elderly living at home. Br Med J 1980; 281: 903–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jones DA, Victor CR, Vetter NJ. Hearing difficulty and its psychological implications for the elderly. J Epidemiol Community Health 1984; 38: 75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kalayam B, Meyers B S, Kakuma T, et al. Age at onset of geriatric depression and sensorineural hearing deficits. Biol Psychiatry 1995; 38: 649–658. [DOI] [PubMed] [Google Scholar]
- 107. Kiely KM, Anstey KJ, Luszcz M. Dual sensory loss and depressive symptoms: the importance of hearing, daily functioning, and activity engagement. Front Hum Neurosci 2013; 7: 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study cognition study part 2. Arch Neurol 2003; 60: 1394–1399. [DOI] [PubMed] [Google Scholar]
- 109. Huang CQ, Dong BR, Lu ZC, et al. Chronic diseases and risk for depression in old age: ameta-analysis of published literature. Ageing Res Rev 2010; 9: 131–141. [DOI] [PubMed] [Google Scholar]
- 110. Rutherford BR, Brewster K, Golub JS, et al. Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am J Psychiatry 2018; 175: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ray J, Popli G, Fell G. Association of cognition and age-related hearing impairment in the English Longitudinal Study of Ageing. JAMA Otolaryngol Head Neck Surg. Epub ahead of print 6 September 2018. DOI: 10.1001/jamaoto.2018.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lozupone M, Panza F, Piccininni M, et al. Social dysfunction in older age and relationships with cognition, depression, and apathy: the GreatAGE study. J Alzheimers Dis 2018; 65: 989–1000. [DOI] [PubMed] [Google Scholar]
- 113. Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging 1997; 12: 12–21. [DOI] [PubMed] [Google Scholar]
- 114. Park DC, McDonough IM. The dynamic aging mind: revelations from functional neuroimaging research. Perspect Psychol Sci 2013; 8: 62–67. [DOI] [PubMed] [Google Scholar]
- 115. Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev 1996; 103: 403–428. [DOI] [PubMed] [Google Scholar]
- 116. Chen DS, Betz J, Yaffe K, et al. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J Gerontol A Biol Sci Med Sci 2015; 70: 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Verghese J, Wang C, Lipton RB, et al. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci 2013; 68: 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res Rev 2013; 12; 840–851. [DOI] [PubMed] [Google Scholar]
- 119. Panza F, D’Introno A, Colacicco AM, et al. Cognitive frailty: predementia syndrome and vascular risk factors. Neurobiol Aging 2006; 27: 933–940. [DOI] [PubMed] [Google Scholar]
- 120. Ruan Q, Yu Z, Chen M, et al. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res Rev 2015; 20: 1–10. [DOI] [PubMed] [Google Scholar]
- 121. Bunt S, Steverink N, Olthof J, et al. Social frailty in older adults: a scoping review. Eur J Ageing 2017; 14: 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fitten LJ. Psychological frailty in the aging patient. Nestle Nutr Inst Workshop Ser 2015; 83: 45–53. [DOI] [PubMed] [Google Scholar]
- 123. Brown PJ, Rutherford BR, Yaffe K, et al. The depressed frail phenotype: the clinical manifestation of increased biological aging. Am J Geriatr Psychiatry 2016; 24: 1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Song X1, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurology 2011; 77: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lin MY, Gutierrez PR, Stone KL, et al. ; Study of Osteoporotic Fractures Research Group. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 2004; 52: 1996–2002. [DOI] [PubMed] [Google Scholar]
- 126. Valentijn SA, van Boxtel MP, van Hooren SA, et al. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. J Am Geriatr Soc 2005; 53: 374–380. [DOI] [PubMed] [Google Scholar]
- 127. Frost S, Martins RN, Kanagasingam Y. Ocular biomarkers for early detection of Alzheimer’s disease. J Alzheimers Dis 2010; 22: 1–16. [DOI] [PubMed] [Google Scholar]
- 128. Wittich W, Watanabe DH, Gagne JP. Sensory and demographic characteristics of deafblindness rehabilitation clients in Montreal, Canada. Ophthalmic Physiol Opt 2012; 32: 242–251. [DOI] [PubMed] [Google Scholar]
- 129. Heine C, Browning CJ. Mental health and dual sensory loss in older adults: a systematic review. Front Aging Neurosci 2014; 6: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kamil RJ, Betz J, Powers BB, et al. Association of hearing impairment with incident frailty and falls in older adults. J Aging Health. Epub ahead of print 5 October 2015. DOI: 10.1177/0898264315608730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chen DS, Betz J, Yaffe K, et al. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J Gerontol A Biol Sci Med Sci 2015; 70: 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shen Y, Ye B, Chen P, et al. Cognitive Decline, dementia, Alzheimer’s disease and presbycusis: examination of the possible molecular mechanism. Front Neurosci 2018; 12: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Van Eyken E, Van Camp G, Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol Neurotol 2007; 12: 345–358. [DOI] [PubMed] [Google Scholar]
- 134. Golub JS. Brain changes associated with age-related hearing loss. Curr Opin Otolaryngol Head Nec Surg 2017; 25: 347–352. [DOI] [PubMed] [Google Scholar]
- 135. Mulrow CD, Aguilar C, Endicott JE, et al. Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med 1990; 113: 188–194. [DOI] [PubMed] [Google Scholar]
- 136. Allen NH, Burns A, Newton V, et al. The effects of improving hearing in dementia. Age Ageing 2003; 32: 189–193. [DOI] [PubMed] [Google Scholar]
- 137. Nguyen MF, Bonnefoy M, et al. ; ADPHA study group. Efficacy of hearing aids on the cognitive status of patients with Alzheimer’s disease and hearing loss. A multicenter controlled randomized trial. J Alzheimers Dis 2017; 58: 123–137. [DOI] [PubMed] [Google Scholar]
- 138. Adrait A, Perrot X, Nguyen MF, et al. ; ADPHA Study Group. Do Hearing aids influence behavioral and psychological symptoms of dementia and quality of life in hearing impaired Alzheimer’s disease patients and their caregivers? J Alzheimers Dis 2017; 58: 109–121. [DOI] [PubMed] [Google Scholar]
- 139. Dawes P, Emsley R, Cruickshanks KJ, et al. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS One 2015; 10: e0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Amieva H, Ouvrard C, Giulioli C, et al. Self-reported hearing loss, hearing aids, and cognitive decline in elderly adults: a 25-year study. J Am Geriatr Soc 2015; 63: 2099–2104. [DOI] [PubMed] [Google Scholar]
- 141. Deal JA, Albert MS, Arnold M, et al. A randomized feasibility pilot trial of hearing treatment for reducing cognitive decline: results from the aging and cognitive health evaluation in elders pilot study. Alzheimers Dement (N Y) 2017; 3: 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mosnier I, Bebear JP, Marx M, et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg 2015; 141: 442–450. [DOI] [PubMed] [Google Scholar]
- 143. Cosetti MK, Pinkston JB, Flores JM, et al. Neurocognitive testing and cochlear implantation: insights into performance in older adults. Clin Interv Aging 2016; 11: 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Jayakody DMP, Friedland PL, Nel E, et al. Impact of cochlear implantation on cognitive functions of older adults: pilot test results. Otol Neurotol 2017; 38: e289–e295. [DOI] [PubMed] [Google Scholar]