Abstract
Objective:
Postprandial fluxes in oxidative stress, inflammation, glucose, and lipids, particularly after a high-fat meal (HFM), have been implicated in the development of cardiovascular disease (CVD). The aim of this study is to determine whether acute freeze-dried mango consumption modulates the postprandial response to an HFM. We hypothesized that the addition of mango, which is a rich source of many bioactive components, to an HFM would lower postprandial triglycerides, glucose, and inflammation, and increase antioxidant enzymes, compared to a standard HFM alone.
Methods:
In a randomized cross-over study, 24 healthy adult males (18-25 years old) consumed a typical American breakfast (670 kcal; 58% fat) with or without the freeze-dried mango pulp (50 g). Lipids, glucose, antioxidant enzymes, and inflammatory markers were assessed at baseline/fasting and 1, 2, and 4 hours after the HFM.
Results:
Addition of mango resulted in lower glucose (95.8 ± 4.4 mg/dL; P = .002) and higher high-density lipoprotein cholesterol (HDL-C; 58.4 ± 2.7 mg/dL; P = .01) 1 hour post-HFM compared to control (glucose: 104.8 ± 5.4 mg/dL; HDL-C: 55.2 ± 2.3 mg/dL), although no differences were observed in triglycerides (P = .88 for interaction). No significant meal × time interactions were detected in markers of inflammation (C-reactive protein, P = .17; interleukin-6, P = .30) or antioxidant enzymes (superoxide dismutase, P = .77; glutathione peroxidase, P = .36; catalase, P = .32) in the postprandial period.
Conclusions:
When added to an HFM, acute mango consumption had modest beneficial effects on postprandial glucose and HDL-C responses, but did not alter triglyceride, inflammatory, or antioxidant enzymes.
Keywords: Mango, postprandial, glucose, inflammation, antioxidant enzymes
Introduction
The importance of the postprandial state in the development of chronic diseases is increasingly being recognized.1 Approximately 16 hours per day are spent in the postprandial phase and changes that occur during this state, particularly after consuming a high-fat meal (HFM), can have a significant impact on the development of chronic diseases, such as diabetes and cardiovascular disease (CVD).1,2 Following an HFM, there is a transient increase in circulating triglycerides (TG) that lasts for several hours.3 This postprandial increase in TG is involved in atherogenesis by increasing cholesterol-dense chylomicron remnants (CMRs) that are produced when TG are removed from chylomicrons and exchanged with cholesterol from high-density lipoprotein (HDL). High-density lipoprotein cholesterol (HDL-C) is reduced as a result of this lipid exchange. This cholesterol-dense CMR is more easily oxidized and can form foam cells that contribute to the formation of atherosclerotic plaque.3
An HFM is also accompanied by an increase in oxidative stress and reactive oxygen species (ROS).4-8 Reactive oxygen species is a normal product of metabolic processes during the postprandial response such as the oxidation of carbohydrate, fats, and proteins. Reactive oxygen species circulate throughout the body and interact with lipids, proteins, and nucleic acids, and can damage cellular components and increase risk for many chronic diseases, including CVD. Reactive oxygen species are tightly regulated under normal conditions by antioxidants in the body such as enzymes, vitamins, and phenolic compounds.9 Endogenous as well as dietary antioxidants scavenge the extra electrons in ROS and stabilize the molecule, thus preventing damage to the cells. However, in diseases such as CVD, the production and function of these antioxidant enzymes decreases while ROS continue to increase, resulting in an overall redox state that favors chronic oxidative stress.
In addition to oxidative stress, inflammation also plays a role in the pathogenesis of CVD.10 Interestingly, it has been demonstrated that markers of inflammation can increase in the acute period following HFM consumption.11 Specifically, interleukin (IL)-6 has been found to increase in >70% of postprandial studies, approximately doubling in concentration in response to an HFM.12 The continuous release of IL-6, and the acute phase protein, C-reactive protein (CRP), produced in the liver, can result in increased production of adhesion molecules and ultimately lead to endothelial dysfunction and increased risk of CVD.13,14
It is well known that lipid levels, endothelial dysfunction, oxidative stress, and inflammation are linked to the dietary pattern.2,3 Functional foods are foods which have the ability to perform tasks in the body beyond the basic functions of a nutrient. They contain phytochemicals, antioxidants, and other nutrients which have been shown to modulate lipid profile, decrease inflammatory markers, increase antioxidant enzymes, regulate the immune system, and much more.15-18 The effects of these functional foods, particularly fruits and vegetables, on the glycemic response and postprandial oxidative stress and endothelial dysfunction have also been investigated.19-22 One fruit of interest is mango, grown and popular to sunny, warm climates. Mangos are packed with many nutrients (beta-carotene, vitamin C, and folate) as well as phenolic compounds (chlorogenic, gallic, vanillic, and protocatechuic acids) which confer health-promoting properties.23,24 Extracts from the stem, skin, and pulp of mango have all been tested in cell culture and animal models and have generally been shown to possess anti-inflammatory, antidiabetic, and immune-regulatory properties.15,16,18,25,26
Although a few human studies have been conducted to investigate the health benefits of mango consumption, improvements in lipids, glucose, and antioxidant capacity have been reported.25,27 There are even fewer studies that investigated the effects of mango on the postprandial response. Two pilot human studies compared the glycemic response of 25 g of mango and other tropical fruits in 10 diabetic subjects.19,20 Roongpisuthipong et al19 found mango and banana had significantly lower glucose response curves compared to pineapple, rambutan, and durian in female diabetics. Contractor et al20 found mango and sapota had a lower area under the curve (AUC) for glucose than banana in diabetic subjects. These two studies demonstrate that mango pulp, despite its sweet taste, does not cause a rapid increase in blood glucose. In addition, mango has been reported to have a moderate glycemic index (approximately 41-60).28 However, the effects of mango fruit in combination with a high-fat meal on postprandial responses have not been explored.
Therefore, the purpose of this study is to investigate the effects of acute consumption of freeze-dried mango together with an HFM on postprandial metabolic, antioxidant enzymes, and inflammatory responses. We hypothesize that the addition of mango, which is a rich source of many bioactive components, to an HFM will modulate the postprandial response, resulting in lower TG, glucose, and inflammation, and greater antioxidant status, compared to a standard HFM alone.
Materials and Methods
Participants
Twenty-four healthy males (age 18-25 years) were recruited from the campus of Oklahoma State University to participate in this study. Interested individuals were interviewed over the phone to determine if they met the inclusion and exclusion criteria. Any healthy males within the specified age range and not violating any of the exclusion criteria were included in the study. Exclusion criteria included having taken (within the last month) or currently taking any kind of dietary supplements, diagnosed with a medical condition, smoking, or consuming alcohol or illicit drugs. This study was conducted according to the guidelines in the Declaration of Helsinki and all procedures were approved by the Institutional Review Board at the Oklahoma State University.
Overall study design
In this randomized cross-over study, participants consumed an HFM alone (Control condition) and an HFM with the addition of a mango shake (Mango condition) in random order. For each trial, participants came to the study site following a 10-hour overnight fast. First, participants completed questionnaires, and anthropometric measurements were taken (described below). Next, a baseline blood draw was performed by the study phlebotomist. After the first blood draw in the first session, subjects were randomized to either the Control or Mango condition. They were given 20 minutes to consume the respective meal. The HFM was a typical high-fat American breakfast consisting of a sausage and egg biscuit with hash browns from McDonald’s (675 kcal; 19 g protein, 53 g carbohydrate, 43 g fat [16 g saturated fat]; 11% protein, 31% carbohydrate, 58% fat). For the Mango condition, a mango shake (containing 50 g freeze-dried mango powder, a cup of water, and a cup of ice blended together) was added to the meal. The 50 g serving of freeze-dried mango powder utilized in the present study contains 45.4 g carbohydrate (181.6 kcal), 2.2 g fiber, 1.8 g protein (7.2 kcal), and 0.6 g fat (5.4 kcal) yielding 194 kcal per serving (Nestle Purina Analytical Laboratories; St. Louis, MO). The dose of 50 g of freeze-dried mango is approximately 500 g or three cups of fresh mango.18 The mango pulp is a rich source of vitamin C, β-carotene, and phenolic compounds.23,24 The HFM and mango shake were consumed simultaneously in the Mango condition. In the Control condition, participants were given water to drink during the meal. After consumption of the meal, participants were allowed to drink water ad libitum throughout the visit. Repeated blood draws were taken at 1, 2, and 4 hours after the meal was complete. After the first visit, participants completed the other condition following a 3- to 4-week washout period, where the same procedure was performed. The washout period of 3 to 4 weeks has been used in other cross-over studies29,30 and was chosen to ensure that the participants were back to their normal routine in terms of diet and physical activity as well as to remove any residual effects of mango and the stress of blood draws. Participants were asked to follow their normal diet and physical activity patterns between the two visits.
Anthropometrics, questionnaires, and blood collection
Anthropometric measurements (height, weight, waist and hip circumference, and blood pressure), health and physical activity questionnaires, and a 24-hour dietary recall were recorded at both visits. Height was measured to the nearest centimeter using a wall-mounted stadiometer (Shorr Productions; Olney, MD), while weight was acquired to the nearest 10th of a pound using a digital scale (SECA; Chino, CA). Waist and hip measurements were taken using a flexible tape measure (to the nearest ½ inch). An automatic blood pressure monitor (Omron Healthcare; Bannockburn, IL) was used to measure blood pressure.
The Physical Activity Scale 2 (PAS 2) questionnaire31 was used to determine whether the participants altered their exercise habits while participating in the study. This questionnaire assessed the number of hours of light, moderate, and heavy physical activity performed for work or leisure time during a week.31 Dietary intake was assessed using 24-hour dietary recall, which was analyzed using the Food Processor Nutrition Analysis and Fitness software (ESHA Research; Salem, OR).
Approximately 5-mL blood samples were collected via single venipuncture in ethylenediaminetetraacetic acid (EDTA) tubes at 0, 1, 2, and 4 hours after the meal in both trials. The samples were placed on a Labquake shaker (Barnstead Thermolyne; Dubuque, IA) and allowed to mix for 5 to 10 minutes. Blood was centrifuged at 3500 r/min to obtain plasma, which was aliquoted, and stored at −80°C for later analyses.
Clinical chemistry analyses
A Biolis 24i automated clinical chemistry analyzer (Carolina Chemistry; Winston-Salem, NC) was used for the determination of plasma concentrations of alanine aminotransferase (ALT), low-density lipoprotein cholesterol (LDL-C), HDL-C, total cholesterol (Total-C), TG, high-sensitivity CRP, and glucose (all from Carolina Liquid Chemistries Corp; Brea, CA), as well as nonesterified fatty acids (NEFAs; Wako; Richmond, VA) per manufacturer’s instructions. All measurements were performed in duplicate and the inter-assay coefficient of variation were 2.7%, 2.5%, 1.8%, 2.2%, 3.0%, 3.7%, 2.7%, and 2.8% for ALT, LDL-C, HDL-C, Total-C, TG, high-sensitivity CRP, glucose, and NEFA, respectively.
Antioxidant enzymes and inflammatory marker analyses
Colorimetric kits from Cayman Chemical (Ann Arbor, MI) were used to analyze total superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activity. Interleukin-6 activity was determined using the sandwich enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN). All measurements were done in duplicate and the inter-assay coefficient of variation were 3.7%, 9.9%, 7.2%, and 4.5% for SOD, CAT, GPx, and IL-6, respectively.
Statistical analyses
Statistical analyses, power calculation, and figure generation were performed using SAS software version 9.4 (SAS Institute; Cary, NC) and GraphPad Prism software version 7.03 (GraphPad Software; La Jolla, CA). Powers for detecting differences were calculated based on a range of possible decreases in plasma glucose with mango supplementation.25 The mean value was assumed to be 95 mg/dL with a standard deviation of 7.5 mg/dL. These values were arrived at under the assumption that the study group contains only starting glucose values ranging from 80 to 110 mg/dL. To calculate power, or the probability of detecting a specified difference, a two-tailed significance level of .05 was used. A noncentral t distribution with the analysis of variance (ANOVA) error degrees of freedom and the noncentrality parameter of the percent decrease expressed as a fraction of the standard error of a paired comparison were used. The power for detecting a 6 unit difference in blood glucose (6.3% change) was calculated to be 80%.
Data were checked for normality via Shapiro-Wilk normality test. Potential pretrial differences in baseline participant characteristics, fasting metabolic levels, and sleep activity data leading up to each trial were assessed via paired t test. A two-way (trial × time) repeated measures ANOVA with Tukey’s adjustment for multiple comparisons was used to test for postprandial interactions or meal/group effects on markers of metabolism, inflammation, and antioxidant enzymes. A P value of less than .05 was considered statistically significant.
Results
Participant characteristics
Twenty-four healthy males participated in this cross-over study. At each visit, height, body mass, waist and hip circumference, and systolic and diastolic blood pressure were measured and no statistical differences were found (Table 1). The only significant difference in fasting metabolic markers was observed in Total-C, which was higher in the Control trial than the Mango trial (Table 1). There were no significant differences in nutrient intake (i.e. total calories, macronutrients, and fiber), sleep duration or physical activity levels before each visit (Table 2).
Table 1.
Participant characteristics and fasting metabolic levels at baseline of each trial.
| Parameters | Mango (n = 24) | Control (n = 24) | P value |
|---|---|---|---|
| Age (years) | 21.0 ± 1.9 | 21.0 ± 1.9 | .91 |
| Height (in.) | 70.7 ± 0.6 | 70.6 ± 0.6 | .26 |
| Weight (kg) | 79.3 ± 3.0 | 78.3 ± 3.0 | .82 |
| BMI (kg/m2) | 24.6 ± 0.9 | 24.3 ± 0.9 | .81 |
| Waist circumference (in.) | 34.6 ± 0.9 | 34.4 ± 0.9 | .41 |
| Hip circumference (in.) | 37.2 ± 0.9 | 37.0 ± 0.7 | .50 |
| Systolic BP (mm Hg) | 122.8 ± 1.7 | 121.0 ± 2.4 | .26 |
| Diastolic BP (mm Hg) | 70.1 ± 1.5 | 69.6 ± 1.5 | .68 |
| Triglycerides (mg/dL) | 75.0 ± 11.5 | 77.1 ± 16.2 | .75 |
| Glucose (mg/dL) | 93.4 ± 1.8 | 96.0 ± 2.5 | .17 |
| NEFA (mEq/L) | 0.44 ± 0.05 | 0.42 ± 0.03 | .72 |
| HDL-C (mg/dL) | 58.2 ± 2.5 | 57.2 ± 2.3 | .56 |
| LDL-C (mg/dL) | 84.9 ± 4.0 | 86.9 ± 5.0 | .62 |
| Total-C (mg/dL) | 148.4 ± 6.2 | 159.3 ± 6.3 | .03 |
Data represent mean ± SEM.
Abbreviations: BMI, body mass index; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NEFA, nonesterified fatty acids; Total-C, total cholesterol.
Table 2.
Nutrient intake, sleep, and physical activity (PA) data leading up to each trial.
| Parameters | Mango (n = 24) | Control (n = 24) | P value |
|---|---|---|---|
| Select nutrient intake | |||
| Calories | 2158 ± 148 | 2240 ± 161 | .69 |
| Protein (g) | 89.4 ± 8.7 | 89.5 ± 7.8 | .99 |
| Fat (g) | 85.3 ± 8.2 | 86.8 ± 7.2 | .90 |
| Carbohydrates (g) | 261.8 ± 21.4 | 282.5 ± 23.7 | .37 |
| Monosaccharides (g) | 3.1 ± 0.9 | 4.7 ± 1.5 | .34 |
| Disaccharides (g) | 12.6 ± 3.5 | 13.2 ± 4.3 | .93 |
| Dietary fiber (g) | 14.4 ± 1.5 | 14.8 ± 1.4 | .84 |
| Sleep and physical activity | |||
| Sleep (min/night) | 431.3 ± 12.9 | 428.8 ± 10.9 | .78 |
| Sedentary activity (min/day) | 337.9 ± 44.0 | 291.3 ± 42.7 | .47 |
| Standing/walking (min/days) | 89.2 ± 25.3 | 106.0 ± 25.0 | .39 |
| Heavy work (min/day) | 29.6 ± 9.0 | 34.6 ± 9.8 | .65 |
| Leisure—no PA (min/day) | 210.0 ± 42.2 | 173.8 ± 28.1 | .29 |
| Leisure—light PA (min/week) | 283.1 ± 48.6 | 213.5 ± 45.1 | .29 |
| Leisure—moderate PA (min/week) | 88.8 ± 21.2 | 113.8 ± 25.2 | .42 |
| Leisure—strenuous PA (min/week) | 174.0 ± 36.7 | 146.3 ± 31.5 | .35 |
Data represent Mean ± SEM.
Leisure (no PA)—min/day watching TV, sitting quietly, reading, and listening to music or the like. Leisure (light PA)—min/week engage in light physical activity such as walking, light cleaning, raking lawn, or lightly strenuous exercise such as yoga, bowling, or similar activities. Leisure (moderate PA)—min/week engaged in gardening, carrying, loads upstairs, or moderately strenuous sport such as gymnastics, swimming, bicycling, strengths conditioning, or similar activities. Leisure (strenuous PA)—min/week engaged in strenuous sport and conditioning exercise such as running, jogging, soccer, tennis, aerobics, or similar activities.
Lipids and glucose
Fasting and postprandial metabolic values are displayed in Figure 1. Acute mango consumption in conjunction with an HFM resulted in significantly lower (P = .002) 1-hour postprandial plasma glucose (95.8 ± 4.4 mg/dL) compared to no supplementation (104.8 ± 5.4 mg/dL). However, no difference was observed in plasma glucose at any other time points after the meal. Plasma TG was elevated at every time point in the postprandial period in both groups, but there were no differences between groups. Similarly, NEFA were lower at 1 and 2 hours post-meal, but there were no group differences.
Figure 1.
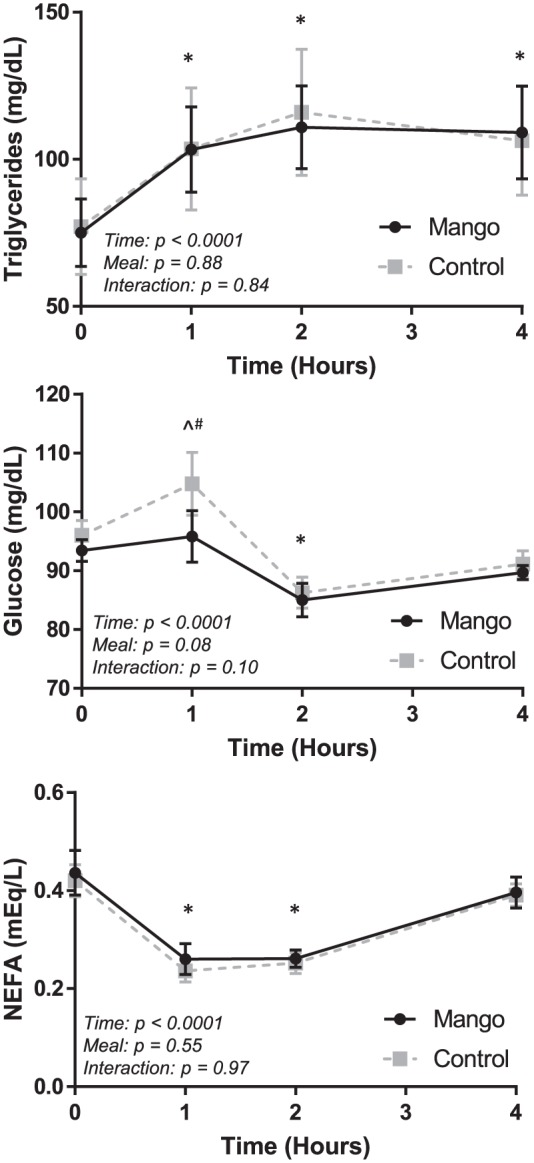
Fasting and postprandial metabolic values in the Mango and Control trials. Triglycerides (top panel), glucose (middle panel), and NEFA (bottom panel) were assessed at baseline/fasting (time 0) and 1, 2, and 4 hours after the high-fat meal. Significant differences (P < .05) at specific time points reflect the results of a two-way (trial × time) repeated measures ANOVA. Error bars reflect SEM.
NEFA indicates nonesterified fatty acids.
*Both groups different from baseline, P < .05.
^Control different from baseline, P < .05.
+Mango different from baseline, P < .05.
#Mango different than Control, P < .05.
Cholesterol responses are displayed in Figure 2. Significant meal × time interactions were detected for HDL-C (P = .04) and Total-C (P = .03). At 1 hour post-meal, HDL-C was significantly higher (P = .01) in the Mango trial (58.4 ± 2.7 mg/dL) than in the Control trial (55.2 ± 2.3 mg/dL). Total-C was lower at baseline (P < .0001) and 2 (P = .0009) and 4 hours (P = .0007) post-meal in the Mango trial than in the Control trial.
Figure 2.
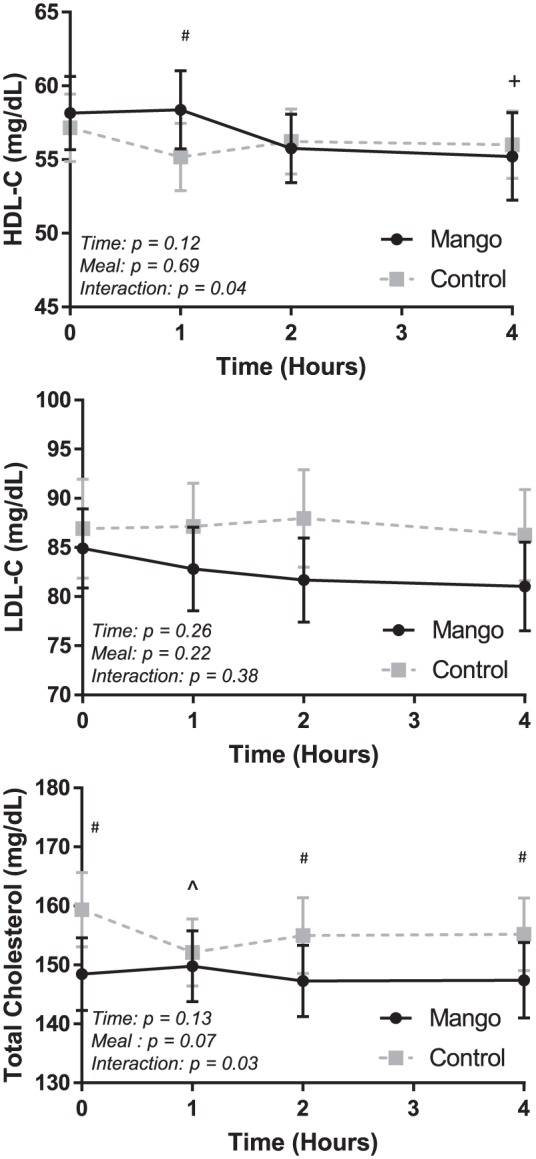
Fasting and postprandial cholesterol values in the Mango and Control trials.
HDL-C (top panel), LDL-C (middle panel), and total cholesterol (bottom panel) were assessed at baseline/fasting (time 0) and 1, 2, and 4 hours after the high-fat meal. Significant differences (P < .05) at specific time points reflect the results of a two-way (trial × time) repeated measures ANOVA. Error bars reflect SEM.
HDL-C indicates high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
*Both groups different from baseline, P < .05.
^Control different from baseline, P < .05.
+Mango different from baseline, P < .05.
#Mango different than Control, P < .05.
Liver and inflammatory markers
Alanine aminotransferase and inflammation responses are exhibited in Figure 3. A significant meal × time interaction was determined for ALT (P = .02), but not for CRP (P = .17) or IL-6 (P = .30). Alanine aminotransferase decreased significantly in the Control trial (P = .0005) from baseline (23.8 ± 2.4 IU/L) to 1 hour post-meal (20.4 ± 1.8 IU/L), and was lower than the Mango trial (23.1 ± 2.2; P = .007). No trial- or time-based differences were detected in CRP or IL-6.
Figure 3.
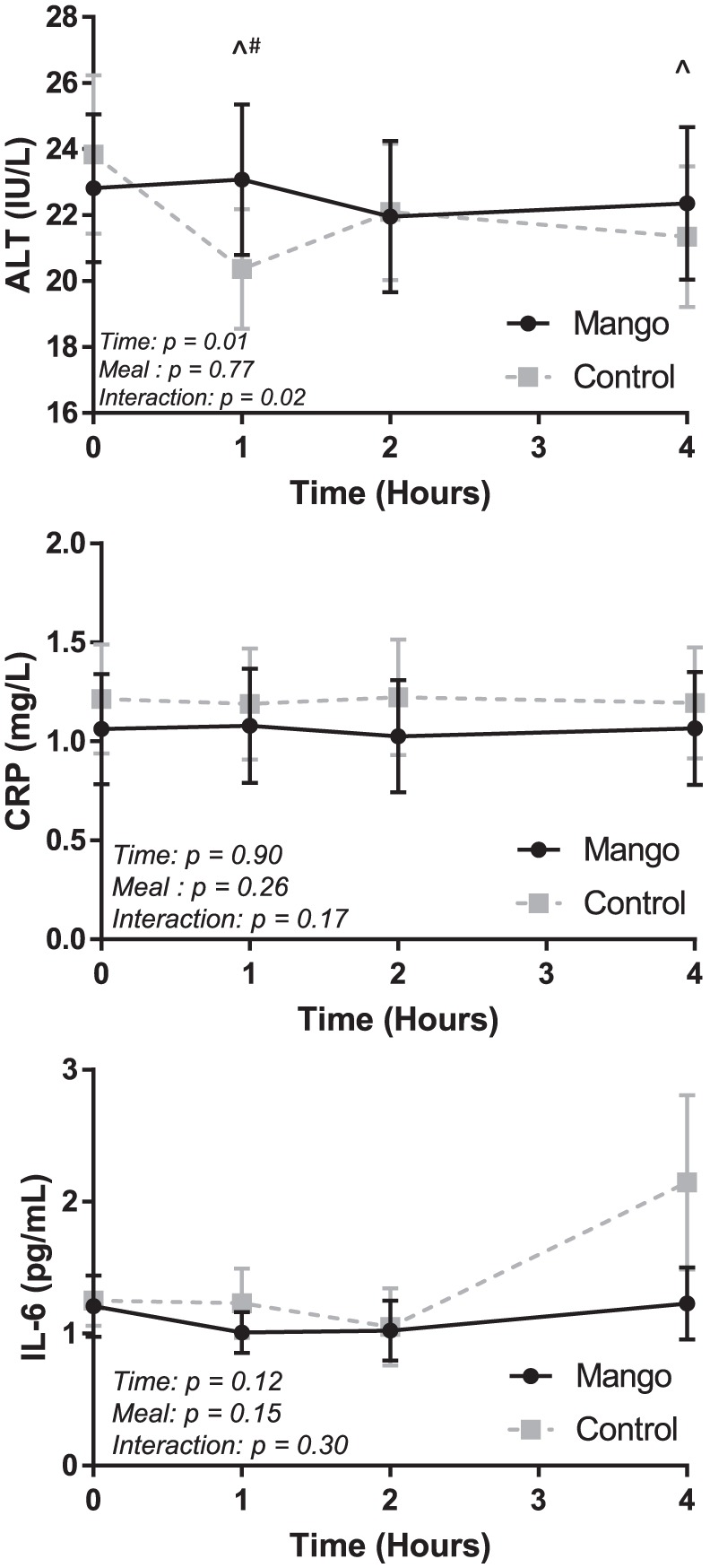
Fasting and postprandial ALT and inflammation values in the Mango and Control trials.
ALT (top panel), CRP (middle panel), and IL-6 (bottom panel) were assessed at baseline/fasting (time 0) and 1, 2, and 4 hours after the high-fat meal. Significant differences (P < .05) at specific time points reflect the results of a two-way (trial × time) repeated measures ANOVA. Error bars reflect SEM.
ALT indicates alanine aminotransferase; CRP, C-reactive protein; IL, interleukin.
*Both groups different from baseline, P < .05.
^Control different from baseline, P < .05.
+Mango different from baseline, P < .05.
#Mango different than Control, P < .05.
Antioxidant enzymes
With regard to antioxidant enzymes (Figure 4), there were no statistically significant time × trial interactions for SOD (P = .77), GPx (P = .36), or CAT (P = .32). A time effect (P = .03) was detected for SOD, but post hoc analyses of specific time points revealed no significant differences (P > .05).
Figure 4.
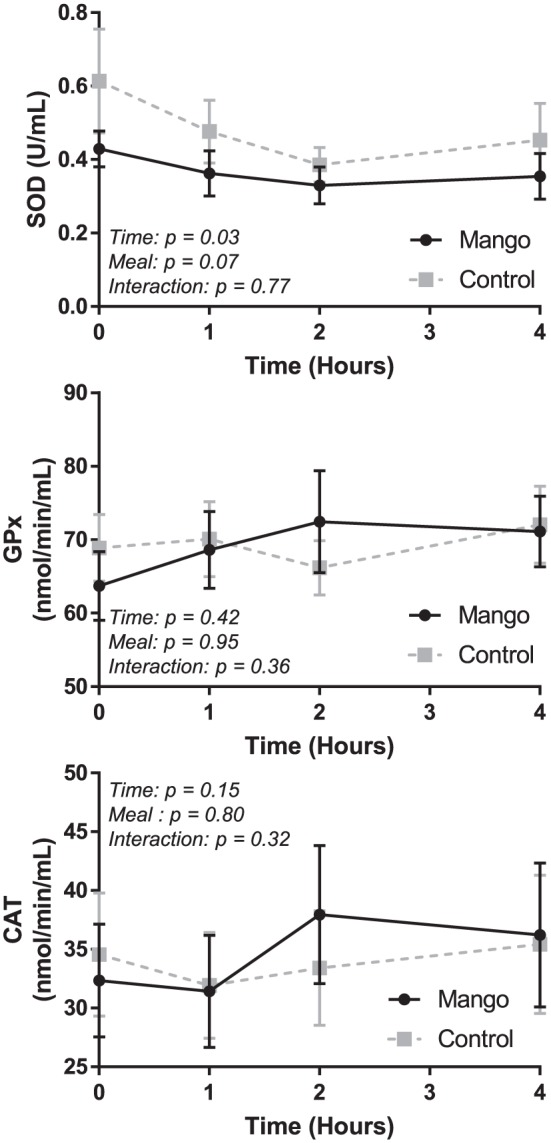
Fasting and postprandial antioxidant enzymes in the Mango and Control trials.
SOD (top panel), GPx (middle panel), and CAT (bottom panel) were assessed at baseline/fasting (time 0) and 1, 2, and 4 hours after the high-fat meal. Significant analyses were conducted using a two-way (trial × time) repeated measures ANOVA. Error bars reflect standard error. No significant differences.
SOD indicates superoxide dismutase; GPx, glutathione peroxidase; CAT, catalase.
Discussion
The aim of this study is to investigate whether acute consumption of freeze-dried mango pulp together with an HFM would modulate postprandial responses in healthy young men. This study demonstrated that acute mango consumption together with an HFM had modest effect on postprandial lipids and glucose, but no effect on antioxidant enzymes and inflammatory markers, compared to the HFM without mango fruit.
Glucose, lipids, and liver markers
In this study, plasma glucose was significantly decreased 1 hour postmeal after the mango supplemented HFM compared to the nonsupplemented meal. Two studies previously looked at the glucose AUC of mango compared to other fruits high in carbohydrate and found mango’s response to be significantly lower.19,20 The lower peak in blood glucose in this study could have been due to decreased or delayed absorption from the fiber content in the mango. Kay and Holub21 also reported a significant decrease in plasma glucose 3 and 4 hours following 100 g blueberry supplementation after an HFM consumed by healthy adults. However, unlike this study, significant decreases in LDL-C were also observed at 3 and 4 hours after meal.21 Violi et al32 also found similar results in the glycemic and lipid response to that of Kay et al after extra virgin olive oil supplementation during a Mediterranean style meal. Similar to this study design and findings, Esposito et al33 compared an HFM alone to an HFM supplemented with antioxidants and did not find any significant differences in blood lipids or glucose parameters in healthy participants. Overall, the finding in our study of lower glucose levels 1 hour after meal intake in the Mango trial points to a potentially noteworthy health benefit of freeze-dried mango pulp consumption.
Elevations in TG during the postprandial period have been proposed as an independent CVD risk factor due to the production TG-rich lipoproteins (TRLs) which are readily acted upon by lipase enzymes forming small, dense easily oxidizable lipoproteins.34 This study showed that acute mango consumption did not contribute to any significant differences in postprandial TG. Chronic (3 month) intakes of mango and mangiferin supplements have been shown to significantly improve glycemic and lipid profiles in animal and human studies, respectively.26,35 For effects on lipid profile, it was proposed that mangiferin, the main phenolic compound in mango, increased transcription of genes involved in fatty acid oxidation.35 The short duration of a postprandial study is likely insufficient to detect transcriptional changes in enzymes involved in lipid metabolism. Interestingly, we did find higher HDL-C in the Mango trial compared to the Control trial 1 hour postmeal. High-density lipoprotein cholesterol is known to generally decrease in the postprandial period following HFM consumption and is typically inversely associated with TG.36 The explanation for the beneficial effect of Mango on HDL-C without differences in TG is unclear, but suggests a modest protective effect of mango when consumed in conjunction with an HFM. In terms of Total-C, it is hard to determine the exact effects of mango supplementation, since there was a significant difference in baseline concentration between the Control and Mango trial. We cannot explain the difference in baseline concentrations of Total-C between the Control and Mango trial. Nonetheless, consumption of mango shake did not alter Total-C concentration during the postprandial state.
Markers of inflammation
Elevations in inflammatory markers have been reported as risk factors for CVD.14,37 In this study, there were no significant differences in inflammatory markers after either meal. Interleukin-6 is an inflammatory marker that appears to be upregulated following a variety of stressors to the human body, including exercise and HFM intake.37-39 Interleukin-6 stimulates the acute phase response, resulting in increased secretion of CRP from the liver, an independent risk factor for CVD.14,40 Other studies have demonstrated IL-6 increases in response to an HFM.7,41 Specifically, a recent systematic review found that IL-6 increased in >70% of studies assessing postprandial inflammation.12 This review also found that the peak IL-6 response occurs ~6 hours post-HFM. In our study, it appears that IL-6 may be increasing at 4 hours postmeal, although not statistically significant. Therefore, it is possible that our postprandial period was simply not long enough in duration to sufficiently detect a change in IL-6.
Mixed results have been seen for CRP during the postprandial response.33 The previously mentioned systematic review found that CRP did not change in 79% of previous studies.12 Given that CRP is a downstream marker, primarily increasing in response to IL-6, it is understandable why CRP may not increase in the acute postprandial period. Previous work in an endotoxin model suggests that CRP increases ~24 hours poststimulus.11 Therefore, the lack of meal-induced changes and group differences in this study are not surprising. Overall, in our study, mango did not have a noteworthy effect on the postprandial inflammatory process, as measured by IL-6 and CRP.
Antioxidant enzymes
Oxidative stress is a significant factor in the development of CVD by damaging the endothelium and oxidizing LDL.9 In this study, endogenous antioxidant enzymes were measured as an indirect marker of oxidative stress. However, other than a significant main effect of time (with no significant pairwise comparisons) in SOD, we did not find any postprandial changes or group differences in antioxidant enzymes. Our findings differ from the results of Cardona et al,42 who observed a 30% increase in SOD and significant reductions in GPx in healthy adults following an HFM. The discrepancies between results could be due to the (1) type of meal consumed; a lipid load elicited a much higher oxidative stress response when compared to a meal of all three macronutrients, (2) physical fitness; physically active individuals exhibited significant increases in SOD compared to inactive participants, and (3) prior meals of the participants; previous HFMs can cause an increased postprandial response.8,41,43,44 The HFM used by Cardona et al is a commercial liquid preparation (Supracalw; SHS International, Liverpool, UK) containing 60 g of fat (12 g are saturated) while we used a typical HFM American breakfast consisting of a sausage and egg biscuit with hash browns (43 g fat and 16 g are saturated fat). In addition, Cardona et al did not provide information on physical activity or nutrient intake. These factors might explain the differences in SOD and GPx between the two studies.
Instead of antioxidant enzymes, other investigators have used total antioxidant capacity as a marker of oxidative stress response. Kay and Holub21 showed significant increases in oxygen radical absorbance capacity (ORAC) and total antioxidant status (TAS) after blueberry supplementation with an HFM. In a study investigating the response from vitamin C, strawberry, spinach, or red wine consumption in elderly females, Cao et al22 observed significant increases in ORAC and ferric-reducing ability of plasma (FRAP) after all supplements compared to a control. There was also a significant increase in trolox equivalent antioxidant capacity (TEAC) following the spinach supplement. Thirty-day mango supplementation in healthy adults increased antioxidant capacity which was proposed to be through absorption of antioxidant polyphenols or increasing endogenous antioxidant production.25 In this study, looking at different markers of oxidative stress (malondialdehyde (MDA), oxidized LDL, and H2O2 and antioxidant status (ORAC, TAS, and TEAC), may have developed a more complete picture.
Strengths and experimental considerations
This study has several limitations. The first limitation is the absence of a true control group. We did not account for the extra kcal, carbohydrates, and other nutrients in the mango shake, as the mango supplement was simply added to the HFM. Moreover, the amount and specific types of carbohydrates that can influence the postprandial response (i.e. dietary fiber, monosaccharides, disaccharides, amylase, or amylopectin) found in the freeze-dried mango were also not accounted for. Nonetheless. Masibo and He23 reported that the amount of insoluble, soluble, and total dietary fiber found in mango pulp are 13.80%, 14.25%, and 28.05% on dry matter basis, respectively. Similar concentrations of glucose, fructose, and sucrose are reported to be the major sugars in ripe mango.45 Knowing the types of the carbohydrates in our freeze-dried mango and matching the control for the different carbohydrates, fiber, and caloric content may have provided a better picture of the effects of the mango fruit. However, the question of our study was to determine whether the addition of mango modulates the postprandial response to an HFM. In this context, our findings of a blunted glucose response and a greater HDL-C response are potentially meaningful, considering that the participants consumed additional carbohydrate and kcal in the mango trial and still exhibited improved outcomes.
Another major limitation of the study is the lack of assessment of insulin levels. This would have given us a more complete picture of the insulin response to the two meals and a more complete picture of the postprandial metabolic response. Next, our study sample was not very representative of the general population, since our cohort was comprised of only young males with generally healthy BMIs. Another limitation of the study is the markers of oxidative stress used. As previously mentioned, other markers of oxidation, such as H2O2, MDA, or oxidized LDL, would have provided information on whether or not mango reduced oxidative stress by free radical scavenging, instead of assessing antioxidant enzymes, which may require more time to see changes. Similarly, total antioxidant capacity was not evaluated, which would have provided a better picture of the overall response. As mentioned previously, some markers of inflammation, such as CRP, may take longer to change and would not be observed immediately within the 4 hours after a meal. Finally, the PAS 2 questionnaire provided the physical activity in a course of a single week and not whether the person exercise the day prior to the postprandial tests that can alter the postprandial response.
Despite the study limitations, the study did possess several strengths. To the best of our knowledge, this is the first study to look at postprandial clinical parameters, inflammation, and antioxidant enzymes, after acute mango consumption together with an HFM. The cross-over design allowed for participants to be their own control, reducing between-subject variability. The participants also consumed a traditional HFM providing a real-world application.
Conclusion
Acute mango consumption in conjunction with an HFM had modest effects on the postprandial response in young healthy males, including beneficial effects in glucose and HDL-C. However, there were no improvements in antioxidant enzymes or inflammation following the HFM supplemented with mango. Future studies should look at the effect of mango supplementation on the postprandial response in more at-risk populations, such as individuals who are older, over-weight, obese, diabetic, or have metabolic syndrome. The postprandial response has been shown to be exaggerated in these population46-50 and may benefit more from dietary intervention. In addition, the effects of chronic mango supplementation on postprandial response should also be explored.
Acknowledgments
The authors would like to thank all the study participants, the staff at the Stillwater Medical Center, and the Oklahoma State University Department of Nutritional Sciences for support with this research.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Oklahoma State University College of Human Sciences and through a grant from the National Mango Board. The National Mango Board had no involvement in the study design, data collection, data analysis, manuscript preparation, or decision in manuscript submission. Supported, in part, by a grant from the National Mango Board.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Experimental design: EL, PP, MP, JH, and BS. Preparation of the freeze-dried mango powder: PP. Statistical analyses: MP. Research procedures: CO, BO, SE, AS, SP, PP, JH, BS, and EL. First draft of the manuscript: SE and CO. Revisions and final approved version of paper: SE, BS, and EL. All authors reviewed and approved of the final manuscript
ORCID iD: Edralin A Lucas  https://orcid.org/0000-0002-4983-1193
https://orcid.org/0000-0002-4983-1193
References
- 1. Schmid A, Petry N, Walther Bet al. Inflammatory and metabolic responses to high-fat meals with and without dairy products in men. Br J Nutr. 2015;113:1853-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karupaiah T, Sundram K. Modulation of human postprandial lipemia by changing ratios of polyunsaturated to saturated (P/S) fatty acid content of blended dietary fats: a cross-over design with repeated measures. Nutr J. 2013;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kolovou G, Ooi TC. Postprandial lipaemia and vascular disease. Curr Opin Cardiol. 2013;28:446-451. [DOI] [PubMed] [Google Scholar]
- 4. Tsai WC, Li YH, Lin CC, Chao TH, Chen JH. Effects of oxidative stress on endothelial function after a high-fat meal. Clin Sci. 2004;106:315-319. [DOI] [PubMed] [Google Scholar]
- 5. McCarthy CG, Farney TM, Canale RE, Dessoulavy ME, Bloomer RJ. High-fat feeding, but not strenuous exercise, increases blood oxidative stress in trained men. Appl Physiol Nutr Metab. 2013;38:33-41. [DOI] [PubMed] [Google Scholar]
- 6. Devaraj S, Wang-Polagruto J, Polagruto J, Keen CL, Jialal I. High-fat, energy-dense, fast-food–style breakfast results in an increase in oxidative stress in metabolic syndrome. Metabolism. 2008;57:867-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Derosa G, Ferrari I, D’Angelo Aet al. Oral fat load effects on inflammation and endothelial stress markers in healthy subjects. Heart Vessels. 2009;24:204-210. [DOI] [PubMed] [Google Scholar]
- 8. Bloomer RJ, Kabir MM, Marshall KE, Canale RE, Farney TM. Postprandial oxidative stress in response to dextrose and lipid meals of differing size. Lipids Health Dis. 2010;9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le N-A. Lipoprotein-associated oxidative stress: a new twist to the postprandial hypothesis. Int J Mol Sci. 2014;16:401-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. New Engl J Med. 2005;352:1685-1695. [DOI] [PubMed] [Google Scholar]
- 11. Herieka M, Erridge C. High-fat meal induced postprandial inflammation. Mol Nutr Food Res. 2014;58:136-146. [DOI] [PubMed] [Google Scholar]
- 12. Emerson SR, Kurti SP, Harms CAet al. Magnitude and timing of the postprandial inflammatory response to a high-fat meal in healthy adults: a systematic review. Adv Nutr. 2017;8:213-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nappo F, Esposito K, Cioffi Met al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39:1145-1150. [DOI] [PubMed] [Google Scholar]
- 14. Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439-493. [PubMed] [Google Scholar]
- 15. Saleh S, El-Maraghy N, Reda E, Barakat W. Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: role of adiponectin and TNF-α. An Acad Bras Cienc. 2014;86:1935-1948. [DOI] [PubMed] [Google Scholar]
- 16. Garrido G, Gonzalez D, Lemus Yet al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. Pharmacol Res. 2004;50:143-149. [DOI] [PubMed] [Google Scholar]
- 17. Reverri EJ, Randolph JM, Steinberg FM, Kappagoda CT, Edirisinghe I, Burton-Freeman BM. Black beans, fiber, and antioxidant capacity pilot study: examination of whole foods vs. functional components on postprandial metabolic, oxidative stress, and inflammation in adults with metabolic syndrome. Nutrients. 2015;7:6139-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lucas EA, Li W, Peterson SKet al. Mango modulates body fat and plasma glucose and lipids in mice fed a high-fat diet. Br J Nutr. 2011;106:1495-1505. [DOI] [PubMed] [Google Scholar]
- 19. Roongpisuthipong C, Banphotkasem S, Komindr S, Tanphaichitr V. Postprandial glucose and insulin responses to various tropical fruits of equivalent carbohydrate content in non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1991;14:123-131. [DOI] [PubMed] [Google Scholar]
- 20. Contractor Z, Hussain F, Jabbar A. Postprandial glucose response to mango, banana and sapota. J Pak Med Assoc. 1999;49:215-216. [PubMed] [Google Scholar]
- 21. Kay CD, Holub BJ. The effect of wild blueberry (Vaccinium angustifolium) consumption on postprandial serum antioxidant status in human subjects. Br J Nutr. 2002;88:389-398. [DOI] [PubMed] [Google Scholar]
- 22. Cao G, Russell RM, Lischner N, Prior RL. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J Nutr. 1998;128:2383-2390. [DOI] [PubMed] [Google Scholar]
- 23. Masibo M, He Q. Mango bioactive compounds and related nutraceutical properties—a review. Food Rev Int. 2009;25:346-370. [Google Scholar]
- 24. Palafox-Carlos H, Yahia EM, González-Aguilar a GA. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC–DAD–MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012;135:105-111. [Google Scholar]
- 25. Evans SF, Meister M, Mahmood Met al. Mango supplementation improves blood glucose in obese individuals. Nutr Metab Insights. 2014;7:77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lucas EA, Brown A, Li Wet al. Mango modulates blood glucose similar to rosiglitazone without compromising bone parameters in mice fed high fat diet. JPANS. 2012;2:115-126. [Google Scholar]
- 27. Robles- Sánchez M, Astiazarán-García H, Martín-Belloso Oet al. Influence of whole and fresh-cut mango intake on plasma lipids and antioxidant capacity of healthy adults. Food Res Int. 2011;44:1386-1391. [Google Scholar]
- 28. Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31:2281-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim Y, Keogh JB, Clifton PM. Differential effects of red meat/refined grain diet and dairy/chicken/nuts/whole grain diet on glucose, insulin and triglyceride in a randomized crossover study. Nutrients. 2016;8:E687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eelderink C, Rietsema S, van Vliet IMYet al. The effect of high compared with low dairy consumption on glucose metabolism, insulin sensitivity, and metabolic flexibility in overweight adults: a randomized crossover trial. Am J Clin Nutr. 2019;109:1555-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersen LG, Groenvold M, Jorgensen T, Aadahl M. Construct validity of a revised Physical Activity Scale and testing by cognitive interviewing. Scand J Public Health. 2010;38:707-714. [DOI] [PubMed] [Google Scholar]
- 32. Violi F, Loffredo L, Pignatelli Pet al. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr Diabetes. 2015;5:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Esposito K, Nappo F, Giugliano F, Giugliano G, Marfella R, Giugliano D. Effect of dietary antioxidants on postprandial endothelial dysfunction induced by a high-fat meal in healthy subjects. Am J Clin Nutr. 2003;77:139-143. [DOI] [PubMed] [Google Scholar]
- 34. Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis. 2012;220:22-33. [DOI] [PubMed] [Google Scholar]
- 35. Na L, Zhang Q, Jiang Set al. Mangiferin supplementation improves serum lipid profiles in overweight patients with hyperlipidemia: a double-blind randomized controlled trial. Sci Rep. 2015;5:10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patsch JR, Karlin JB, Scott LW, Smith LC, Gotto AM., Jr. Inverse relationship between blood levels of high density lipoprotein subfraction 2 and magnitude of postprandial lipemia. Proc Natl Acad Sci U S A. 1983;80:1449-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poppitt SD, Keogh GF, Lithander FEet al. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-α, and C-reactive protein to a high-fat dietary load. Nutrition. 2008;24:322-329. [DOI] [PubMed] [Google Scholar]
- 38. Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36:379-386. [DOI] [PubMed] [Google Scholar]
- 39. Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563-1569. [DOI] [PubMed] [Google Scholar]
- 40. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1 moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118:145-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dekker MJ, Wright AJ, Mazurak VCet al. Fasting triacylglycerol status, but not polyunsaturated/saturated fatty acid ratio, influences the postprandial response to a series of oral fat tolerance tests. J Nutr Biochem. 2009;20:694-704. [DOI] [PubMed] [Google Scholar]
- 42. Cardona F, Tunez I, Tasset I, Garrido-Sanchez L, Collantes E, Tinahones FJ. Circulating antioxidant defences are decreased in healthy people after a high-fat meal. Br J Nutr. 2008;100:312-316. [DOI] [PubMed] [Google Scholar]
- 43. Tushuizen ME, Nieuwland R, Scheffer PG, Sturk A, Heine RJ, Diamant M. Two consecutive high-fat meals affect endothelial-dependent vasodilation, oxidative stress and cellular microparticles in healthy men. J Thromb Haemost. 2006;4:1003-1010. [DOI] [PubMed] [Google Scholar]
- 44. Johnson BD, Padilla J, Harris RA, Wallace JP. Vascular consequences of a high-fat meal in physically active and inactive adults. Appl Physiol Nutr Metab. 2011;36:368-375. [DOI] [PubMed] [Google Scholar]
- 45. Medlicott AP, Thompson AK. Analysis of sugars and organic acids in ripening mango fruits (Mangifera indica L. Var Keitt) by high performance liquid chromatography. J Sci Food Agric. 1985;36:561-566. [Google Scholar]
- 46. Schauren B, Portal V, Beltrami F, dos Santos TJ, Pellanda LC. Postprandial metabolism and inflammatory markers in overweight adolescents. J Dev Orig Health Dis. 2014;5:299-306. [DOI] [PubMed] [Google Scholar]
- 47. Anderson RA, Evans ML, Ellis GRet al. The relationships between post-prandial lipaemia, endothelial function and oxidative stress in healthy individuals and patients with type 2 diabetes. Atherosclerosis. 2001;154:475-483. [DOI] [PubMed] [Google Scholar]
- 48. Burton-Freeman B, Linares A, Hyson D, Kappagoda T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J Am Coll Nutr. 2010;29:46-54. [DOI] [PubMed] [Google Scholar]
- 49. Cruz-Teno C, Perez-Martinez P, Delgado-Lista Jet al. Dietary fat modifies the postprandial inflammatory state in subjects with metabolic syndrome: the LIPGENE study. Mol Nutr Food Res. 2012;56:854-865. [DOI] [PubMed] [Google Scholar]
- 50. Emerson SR, Kurti SP, Emerson EMet al. Postprandial metabolic responses differ by age group and physical activity level. J Nutr Health Aging. 2018;22:145. [DOI] [PubMed] [Google Scholar]


