Short abstract
Background
Eosinophilic cholecystitis (EC) is a rare condition that presents in a manner comparable to acute cholecystitis. The diagnosis is based on classical symptoms of cholecystitis with excessive eosinophilic infiltration within the gallbladder. EC has been reported alone or in combination with manifestations, such as eosinophilic gastrointestinal tract inflammation. However, association with airway inflammation in patients with EC is rare.
Case Presentation: We report the case of a 65-year-old man who had refractory eosinophilic chronic rhinosinusitis with bronchial asthma. A second endoscopic sinus surgery (ESS) was performed as treatment for recurrent nasal polyps. EC occurred while inhaled corticosteroids were reduced after ESS. Pathologic examination of the excised gallbladder demonstrated submucosal infiltration with a number of eosinophils. Furthermore, immunohistostaining revealed many galectin-10-positive cells in both the gallbladder mucosa and the paranasal sinus mucosa. Galectin-10 is a major constituent of human eosinophils, also known as the Charcot–Leyden crystal protein, which has been linked with eosinophilic inflammation. Interestingly, nasal polyps were reduced without any additional treatments 1 month after the cholecystectomy.
Conclusions
We experienced a rare case wherein EC onset occurred in a patient with refractory eosinophilic airway inflammation during inhaled corticosteroid tapering. Galectin-10 might help diagnose rare cases of eosinophilic inflammation in multiple organs.
Keywords: asthma, eosinophilic cholecystitis, eosinophilic chronic rhinosinusitis, galectin-10, eosinophilic inflammation
Background
Eosinophilic cholecystitis (EC) is an uncommon condition that was first described in 1949.1 The diagnosis of EC is based on classical symptoms of cholecystitis with the presence of >90% eosinophilic infiltration within the gallbladder. Only 64 reports of EC were found based on an online search using PubMed from 1950 to 2017 and Japana Centra Revuo Medicina Web from 1983 to 2017. Most cases of EC are accidentally found due to the pathological diagnosis after cholecystectomy. In a previous report of 625 cases of surgically removed gallbladders, 16 (2.6%) had eosinophilic infiltration, and only 3 (0.05%) fell into EC criteria.2 It has been reported that EC is not limited to the bladder and can be involved in eosinophilic gastrointestinal tract inflammation, such as eosinophilic cholangiopathy, eosinophilic gastroenteritis, eosinophilic granulomatous hepatitis, and eosinophilic ascites.3 In addition, EC can be complicated by eosinophilic granulomatosis with polyangiitis (EGPA) closely associated with airway inflammation. However, EC concomitant with only airway inflammation, such as asthma and eosinophilic chronic rhinosinusitis (ECRS), but not EGPA is unusual. ECRS is known as a refractory eosinophilic airway inflammatory disease closely related to bronchial asthma.4 We experienced a case wherein EC may have been associated with ECRS with asthma, and the existence of eosinophilic inflammation could be confirmed both in the nasal polyp mucosa and in the gallbladder wall mucosa.
Case Presentation
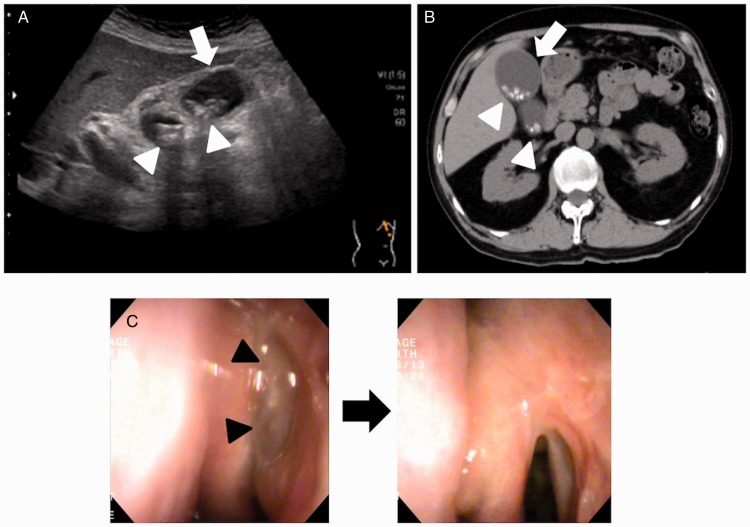
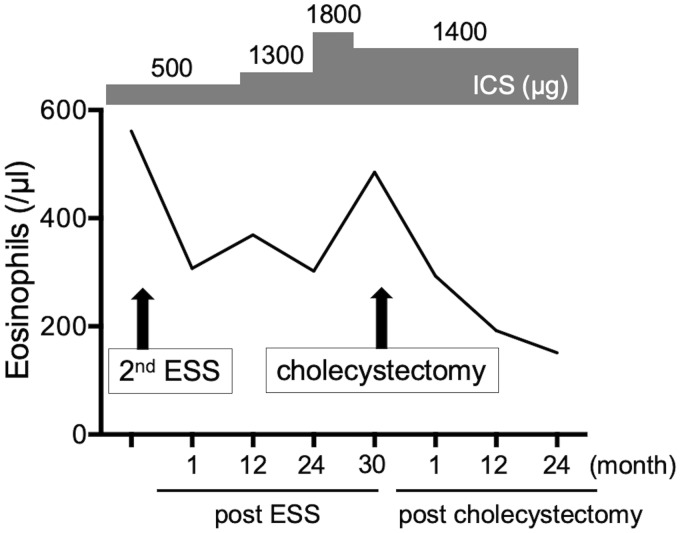
A 65-year-old man with poorly controlled ECRS with bronchial asthma had recurrent paranasal sinus polyps, although he underwent endoscopic sinus surgery (ESS) at the age of 58. A second ESS was performed for his refractory ECRS. In addition, inhaled corticosteroid (ICS) exhalation through the nose (ETN) treatment, which provides better control for both asthma and nasal symptoms,5,6 was initiated instead of conventional ICS exhalation through the mouth treatment. To prevent recurrence of ECRS and reduce asthma symptoms, the doses of ICS were increased to up to 1800 µg. Thirty months after the second ESS, cholecystitis occurred concomitantly with the recurrence of nasal polyps while the doses of ICS were decreased to 1400 µg, 2 months before the onset of biliary colic. After confirmation of the diagnosis with an abdominal ultrasound and a computed tomography scan (Figure 1(A) and (B)), a cholecystectomy was performed. Pathologic examination of the excised gallbladder demonstrated submucosal infiltration with a number of eosinophils, consistent with EC criteria. There were no histological findings indicating angiitis. The laboratory tests at the onset of biliary colic showed a white blood cell count of 6300 cells per µL with mild eosinophilia (7.7%, 485 per µL). Serum total bilirubin was 0.7 mg/dL (normal, 0.2–1.1 mg/dL), aspartate aminotransferase was 32 U/L (normal, 10–40 U/L), alanine aminotransferase was 28 U/L (normal, 5–45 U/L), and alkaline phosphatase was 216 U/L (normal, 110–360 U/L). Thus, no elevation of hepatic functional enzymes was observed when cholecystitis occurred without any signs of inflammation. In addition, serum IgE was 135 IU/mL (normal, 0–320 IU/mL). We confirmed that nasal polyps were concomitantly reduced (from 6 to 4 in polyp score)7 with decreased blood eosinophils without any additional treatments 1 month after the cholecystectomy (Figure 1(C)) and were recurrence-free with no increase in blood eosinophils for at least 24 months (Figure 2).
Figure 1.
Abdominal ultrasound (A) and computed tomography (B) at the onset of eosinophilic cholecystitis, demonstrating thickening of the gallbladder wall (white arrow) and cholecystolith (white arrowheads). Endoscopic findings of the left nasal cavity before (left panel) and 1 month after (right panel) cholecystectomy (C). Black arrowheads indicate nasal polyps in the superior meatus.
Figure 2.
Dynamics of eosinophil counts. Values indicate peripheral blood eosinophil counts at each point as follows; before second ESS; 1, 12, and 24 months after second ESS; onset of eosinophilic cholecystitis; and 1, 12, and 24 months after cholecystectomy. Values of inhaled corticosteroid indicate fluticasone propionate equivalent daily doses (µg). ESS, endoscopic sinus surgery; ICS, inhaled corticosteroid.
Discussion and Conclusions
In the present study, EC is a part of eosinophilic inflammation that occurred in the same patient. Till date, there are no reports that describe the relationship between EC and ECRS. The reduction of chemokines and activators against eosinophils via the control of local eosinophilic inflammation (cholecystectomy) could be one of reasons why the nasal polyps shrunk after cholecystectomy. We previously showed that activated eosinophils release eosinophil chemokines, such as CCL4, and enhance their accumulation into local sites.8 As we can confirm the reduction of blood eosinophils after removal of nasal polyps which include plenty of activated eosinophils, blood eosinophils were reduced after cholecystectomy also in this case (Figure 2).
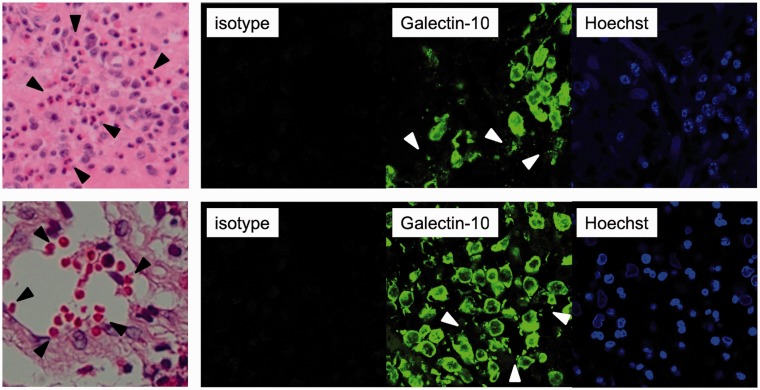
To further confirm the association between EC and ECRS as eosinophilic inflammation, we focused on a protein called galectin-10, which is a major constituent of human eosinophils. Galectin-10 is also known as the Charcot–Leyden crystal protein and characteristically forms bipyramidal hexagonal crystals. This glycan-binding protein is associated with eosinophilic inflammation.9–12 We found many galectin-10-positive cells in both the gallbladder mucosa and in the paranasal sinus mucosa (Figure 3). Although the function of galectin-10 remains largely unknown and it is unclear whether galectin-10 causes eosinophilic inflammation, several studies have demonstrated its association with eosinophilic inflammatory diseases, such as asthma and allergic rhinitis,13,14 and also EC and ECRS,10,15 suggesting that at least galectin-10 might be a proof for eosinophilic inflammatory disease.
Figure 3.
Histological findings of nasal mucosa (upper panels) and gallbladder mucosa (lower panels), showing infiltration of eosinophils (hematoxylin and eosin staining; 400× magnification, black arrowheads in left panels) and galectin-10-positive cells (immunohistostaining; 630× magnification, right panels). White arrowheads indicate extracellular granular structures. Galectin-10 was stained as previously described.10
Galectin-10 positively correlates the percentages of eosinophils or eosinophil extracellular trap cell death (EETosis) which promotes inflammation via the release of free extracellular granules and the development of filamentous chromatin structures.11 In addition, recent studies have revealed that EETosis mediates Charcot–Leyden crystal formation10 and that galectin-10 crystal enhances Th2 immune responses.11 Interestingly, extracellular granular structures were also stained by galecin-10, indicating that galectin-10 could induce activated eosinophilic inflammation at local sites.10 Taken together, the reduction of activated eosinophils could cause less release of eosinophilic chemokines and galectin-10, resulting in decreased eosinophil accumulation into nasal inflammatory sites and nasal polyps.
We have reported that treatment with ICS-ETN is effective for ECRS with bronchial asthma.5,6 Furthermore, in this case, ICS-ETN treatment provided good control for ECRS with asthma. When the doses of ICS are increased, slight systemic effect of corticosteroid could be induced. Therefore, the reduction of ICS dose might cause EC concomitantly with the recurrence of nasal polyps. In contrast, the increase of ICS dose or addition of systemic corticosteroid might protect against the onset of EC. We kept eosinophilic inflammation in control with high doses of ICS-ETN after cholecystectomy.
When EC is suspected, it is necessary to confirm the existence of eosinophilic inflammation and the spread of inflammation to the gastrointestinal tract and/or airway. Treatment with corticosteroids can be effective in cases wherein EC is not limited within the bladder and affects the gastrointestinal tract, including the bile duct.16 In this study, eosinophilic inflammation in gastrointestinal tract was not observed; however, eosinophilic inflammation with galectin-10-positive extracellular granular structures was associated with the airway over the bladder, indicating that treatment with corticosteroids or biological drugs against IL-5 may be preferred over surgical management. Although it is difficult to detect eosinophils at the inflammatory site in some cases, the detection of galectin-10 may provide us proof of spatiotemporally spread of eosinophilic inflammation. Galectin-10 is also detectable in biological fluids from the inflammatory sites, such as sputum and nasal secretion.14,17,18 Recent studies have revealed that galectin-10 levels in the sputum were significantly increased in asthmatics with strong correlation to the levels in sputum eosinophils.14,18 We also found that galetin-10 levels in nasal mucin from ECRS patients were much higher than those in serum (data not shown), suggesting that galectin-10 in the biological fluids obtained from local sites could be a useful marker of activated eosinophilic inflammation.
We present a rare case wherein EC spatiotemporally occurred in a patient with refractory eosinophilic airway inflammation during inhaled corticosteroid tapering. Galectin-10 may help the diagnosis of rare cases with eosinophilic inflammation in multiple organs.
Acknowledgments
The authors are grateful to Noriko Tan for her technical assistance and support.
Author Contributions
Y. K., A. K., and H. I. were involved in the conception and design of this case report. Y. K., Y. Y., and M. A. were involved in collection of the clinical data. S. U. performed the histological examination. T. K. and Y. K. were involved in drafting the manuscript for important intellectual content. All authors contributed to revisions and approved the final draft.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
This study was approved by our institutional review board (KanIRin1313).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Grants-in-Aid for Scientific Research (19K07949 [Y. K.], 16K08926 [S. U.]) from Ministry of Education, Culture, Sports, Science and Technology of Japan and in part by the fund of academic society for research in Otolaryngology, Kansai Medical University, and by Japanese Society of Laboratory Medicine Fund for Promotion of Scientific Research.
Statement of Human and Animal Rights
This article does not contain any studies with human or animal subjects.
Statement of Informed Consent
There are no human subjects in this article and informed consent is not applicable.
References
- 1.Shakov R, Simoni G, Villacin A, Baddoura W. Eosinophilic cholecystitis, with a review of the literature. Ann Clin Lab Sci. 2007; 37(2):182–185. [PubMed] [Google Scholar]
- 2.Fox H, Mainwaring AR. Eosinophilic infiltration of the gallbladder. Gastroenterology. 1972; 63(6):1049–1052. [PubMed] [Google Scholar]
- 3.Butler TW, Feintuch TA, Caine WP. Eosinophilic cholangitis, lymphadenopathy, and peripheral eosinophilia: a case report. Am J Gastroenterol. 1985; 80(7):572–574. [PubMed] [Google Scholar]
- 4.Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015; 70(8):995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi Y, Yasuba H, Asako M, et al. HFA-BDP metered-dose inhaler exhaled through the nose improves eosinophilic chronic rhinosinusitis with bronchial asthma: a blinded, placebo-controlled study. Front Immunol. 2018; 9:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi Y, Asako M, Kanda A, Tomoda K, Yasuba H. A novel therapeutic use of HFA-BDP metereddose inhaler for asthmatic patients with rhinosinusitis: case series. Int J Clin Pharmacol Ther. 2014; 52(10):914–919. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol. 2006; 118(5 Suppl):S17–S61. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi Y, Konno Y, Kanda A, et al. Critical role of CCL4 in eosinophil recruitment into the airway. Clin Exp Allergy. 2019; 49(6):853–860. [DOI] [PubMed] [Google Scholar]
- 9.Ackerman SJ, Liu L, Kwatia MA, et al. Charcot-Leyden crystal protein (galectin-10) is not a dual function galectin with lysophospholipase activity but binds a lysophospholipase inhibitor in a novel structural fashion. J Biol Chem. 2002; 277(17):14859–14868. [DOI] [PubMed] [Google Scholar]
- 10.Ueki S, Tokunaga T, Melo RCN, et al. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood. 2018; 132(20):2183–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persson EK, Verstraete K, Heyndrickx I, et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science (New York, N.Y.). 2019; 364(6442):eaaw4295. [DOI] [PubMed] [Google Scholar]
- 12.Ueki S, Miyabe Y, Yohei Y, et al. Charcot-Leyden crystals in eosinophilic inflammation: active cytolysis leads to crystal formation. Curr Allergy Asthma Rep. 2019; 19(8):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryborn M, Hallden C, Sall T, Cardell LO. CLC—a novel susceptibility gene for allergic rhinitis? Allergy. 2010; 65(2):220–228. [DOI] [PubMed] [Google Scholar]
- 14.Chua JC, Douglass JA, Gillman A, O’Hehir RE, Meeusen EN. Galectin-10, a potential biomarker of eosinophilic airway inflammation. PLoS One. 2012; 7(8):e42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujibayashi M, Aiba M, Suga M, Shiozawa S, Ogawa K. Mast cell degranulation in erosive eosinophilic cholecystitis with Charcot-Leyden Crystal: evaluation by mast cell tryptase/CD117 ratio: report of a case and comparative study with chronic cholecystitis. J Tokyo Wom Med Univ. 2010; 80(3):77–82. [Google Scholar]
- 16.del-Moral-Martinez M, Barrientos-Delgado A, Crespo-Lora V, Cervilla-Saez-de-Tejada ME, Salmeron-Escobar J. Eosinophilic cholecystitis: an infrequent cause of acute cholecystitis. Rev Esp Enferm Dig. 2015; 107(1):45–47. [PubMed] [Google Scholar]
- 17.Negrete-Garcia MC, Jimenez-Torres CY, Alvarado-Vasquez N, et al. Galectin-10 is released in the nasal lavage fluid of patients with aspirin-sensitive respiratory disease. ScientificWorldJournal. 2012; 2012:474020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyenhuis SM, Alumkal P, Du J, Maybruck BT, Vinicky M, Ackerman SJ. Charcot-Leyden crystal protein/galectin-10 is a surrogate biomarker of eosinophilic airway inflammation in asthma. Biomark Med. 2019; 13(9):715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]