Abstract
The prevalence and incidence of chronic venous leg ulcers (CVLUs) are increasing worldwide, as are the associated financial costs. Although it has long been known that their underlying etiology is venous insufficiency, the molecular aspects of healing versus nonhealing, as well as the psychoneurologic symptoms (PNS; pain, cognitive dysfunction, fatigue, depression, and anxiety) associated with CVLUs remain understudied. In this biobehaviorally focused review, we aim to elucidate the complex mechanisms that link the biological and molecular aspects of CLVUs with their PNS. Innovations in “omics” research have increased our understanding of important wound microenvironmental factors (e.g., inflammation, microbial pathogenic biofilm, epigenetic processes) that may adversely alter the wound bed’s molecular milieu so that microbes evade immune detection. Although these molecular factors are not singularly responsible for wound healing, they are major components of wound development, nonhealing, and PNS that, until now, have not been amenable to systematic study, especially over time. Further, this review explores our current understanding of the molecular mechanisms by which the immune activation that contributes to the development and persistence of CVLUs also leads to the development, persistence, and severity of wound-related PNS. We also make recommendations for future research that will expand the field of biobehavioral wound science. Biobehavioral research that focuses on the interrelated mechanisms of PNS will lead to symptom-management interventions that improve quality of life for the population burdened by CVLUs.
Keywords: chronic wounds, chronic venous ulcers, biobehavioral mechanisms, aging, symptoms
Chronic venous leg ulcers (CVLUs) are associated with the symptom burden related to a wound, including aching, wound pain, tightness, skin irritation, heaviness, muscle cramps, and other complaints attributable to venous dysfunction (Eklöf et al., 2004). Emerging evidence indicates that individuals with CVLUs, along with moderate-to-severe pain, may also have high levels of fatigue, depression (Hellström, Nilsson, Nilsson, & Fagerström, 2016), and anxiety (Do, Edwards, & Finlayson, 2016; Edwards et al., 2014), a symptom cluster collectively known as psychoneurologic symptoms (PNS). These symptoms further contribute to patients’ diminished quality of life and inability to participate in disease self-management and affect their functional status and work patterns so often that many patients must resort to early retirement (Hansen, Feuerstein, Calvio, & Olsen, 2008). Unfortunately, current treatment models tend to focus only on treating the leg ulcer in isolation instead of examining the multilevel variables that cause symptoms and nonhealing.
In this article, we provide an overview of the evolving theory of the biobehavioral basis of CVLUs. Our central hypothesis is that the interrelated cellular and molecular mechanisms whose immune activation contributes to the development and persistence of CVLUs may also lead to the development and severity of PNS. Our hope is that future biobehavioral research that focuses on the interrelated mechanisms of PNS associated with CVLUs will lead to the development of interventions for symptom management that will improve quality of life for affected patients.
Overview of Chronic Venous Leg Ulcers
Epidemiology
CVLUs, which account for 70–90% of ulcers found in the lower leg, affect 2 million persons annually, including nearly 4% of people over the age of 65 years (Margolisa, Bilkerb, & Santannab, 2002). CVLUs are a particular threat to older individuals, as peak prevalence occurs in those 60–80 years of age (Margolisa et al., 2002), and increased age is a major risk factor for impaired wound healing (Guo & Dipietro, 2010). In the United States, CVLU treatment costs are nearly $2.5 billion a year, which comprises as much as 1% of total health-care costs (Nelzen, 2000), earning CVLUs the label of a “snowballing threat to public health and the economy” (Sen et al., 2009). Unfortunately, despite their prevalence and cost, CVLUs remain an underrecognized and undertreated disease (Henke, 2010; Kolluri, 2014).
CVLUs are associated with multiple comorbidities (e.g., diabetes, obesity, cardiovascular disease) and are rarely seen in individuals who are otherwise healthy (Sen et al., 2009). Risk factors for CVLUs include older age, female sex, obesity, trauma, congestive heart failure, immobility, congenital absence of veins, deep vein thrombosis, phlebitis, family history of leg ulcers, multiple pregnancies, and Factor V Leiden mutation (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Do et al., 2016; Edwards et al., 2014; Vasudevan, 2014).
Venous Ulcer Formation
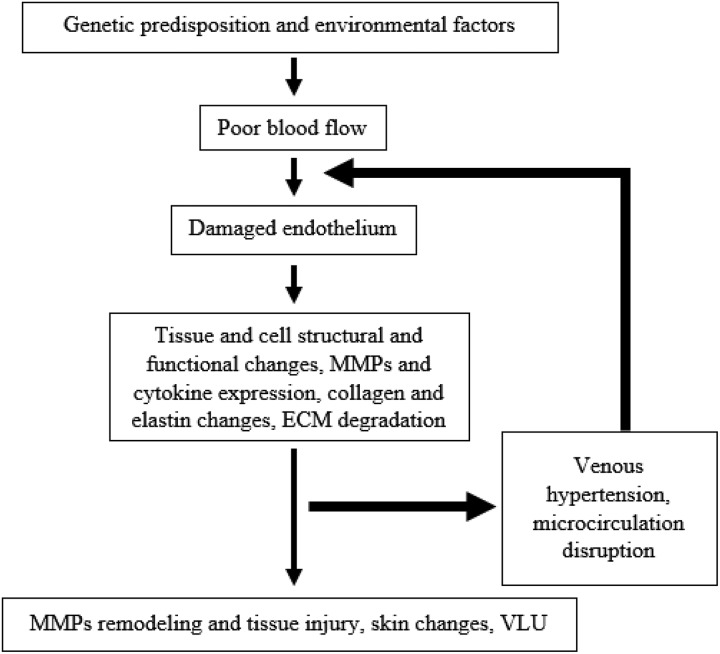
Venous reflux and obstruction contribute to the pathophysiology of chronic venous disease (CVD), which causes venous hypertension and, ultimately, venous ulcers in individuals with genetic and environmental risk factors (O’donnell et al., 2014; see Figure 1). Venous hypertension may lead to calf-pump incompetence, which results in blood stasis, capillary damage, and edema and skin hypoxia. Increased hydrostatic pressure leads to increased venous pressure and inflammation within the vein walls and valves and also moves inflammatory cells and molecules into the interstitial space. This dramatic inflammatory response activates leukocytes, primarily macrophages and monocytes, as well as T lymphocytes and mast cells (Raffetto, 2018). In addition, inflammatory modulators and chemokines, cytokines, growth factors, matrix metalloproteinases (MMPs), and regulatory pathways participate in the inflammatory response, leading to CVD and CVLUs.
Figure 1.
Schematic flow diagram of chronic venous disease pathophysiology. ECM = extracellular matrix; MMPs = matrix metalloproteinases; VLU = venous leg ulcer. Adapted from Raffetto (2018).
Characteristics of CVLUs
A venous ulcer is a full-thickness defect of the skin (confirmed via venous duplex ultrasound testing), most frequently in the ankle region, that fails to heal spontaneously, is sustained by CVD, and tends to become chronic (Lal, 2015). A CVLU is defined as an open lesion between the knee and the ankle joint that remains unhealed for at least 30 days (Baker, 2007; Tatsioni, Balk, O’Donnell, & Lau, 2007) and occurs in the presence of venous disease (Baker, 2007). A majority of patients with venous leg ulcers will have recurrences and ulcer durations of >1 year (Ruckley, 1997). CVLUs are also characterized by a prolonged inflammatory response with unbalanced cellular and molecular components within the wound microenvironment (Gould et al., 2015; Schramedei et al., 2011).
Chronic-wound microenvironment
The microenvironment of a wound is the setting for an ongoing bidirectional interaction among cells and the surrounding biochemical, biophysical, and cellular responses to injury regulating tissue regenerative responses. Interactions between cells and the extracellular matrix (ECM) guide and regulate cellular morphology, differentiation, migration, proliferation, and survival during tissue repair (Scalise et al., 2015; Schultz, Davidson, Kirsner, Bornstein, & Herman, 2011; Zenilman et al., 2013). Increased levels of inflammatory molecules, including pro-inflammatory cytokines and C-reactive protein (CRP), have been identified as components of chronic-wound environments (T. Liu, Yang, Li, Yi, & Bai, 2014), and researchers have identified significantly elevated levels of both CRP and interleukin-6 (IL-6) in wound fluid (Ligi, Mosti, Croce, Raffetto, & Mannello, 2016). MMPs, or extracellular proteases, can also thwart or support wound healing. While low levels of MMPs are necessary for healing, high levels contribute to a molecular mechanism that prevents wounds from healing (Simpson et al., 2013). In one study, higher MMP levels were also associated with more severe pain (Raffetto, Mosti, Santi, Ligi, & Mannello, 2015; see Table 1). Most studies, though, have not measured these components of the wound environment concurrently and/or over time (Broszczak, Sydes, Wallace, & Parker, 2017). Emerging evidence from studies using high-throughput techniques, including gene sequencing, indicates that chronic wounds are susceptible to complex bioburden, which facilitates the development of microbial biofilms in the wound microenvironment.
Table 1.
Biological and Chemical Factors Involved in the Inflammation and Healing of a Venous Ulcer.
| Biological or Chemical Factor | Role in Inflammation and Healing | Activity in Chronic Venous Ulcers | Outcome of Activity |
|---|---|---|---|
| C-reactive protein | Blood marker for inflammation in the body | Increased during inflammation | Signal of inflammation |
| Cytokines | Small secreted proteins that can be either pro- or anti-inflammatory | During inflammation, pro-inflammatory cytokines are increased, while anti-inflammatory cytokines are decreased | Increased pro-inflammatory cytokines enhance the inflammatory process |
| Interleukins | A group of glycoproteins (cytokines) produced by leukocytes that regulate immune responses | Increased during inflammation | Promotion of the inflammatory process |
| IL-1β | Pro-inflammatory cytokine that initiates inflammatory mediators (increases MMPs) | Increased during inflammation | Damage to ECM and vascular endothelium |
| TNF-α | Pro-inflammatory cytokine that initiates inflammatory mediators (increase MMPs) | Increased during inflammation | Damage to ECM and vascular endothelium |
| MMPs | Proteases that degrade collagen and collagen fragments | Increased during inflammation | Damage to ECM and vascular endothelium |
| TIMP | Protein regulator that inhibits the activity of MMPs | Reduced during inflammation | Reduced concentration of TIMPs leads to increased concentration of MMPs and prolonged inflammation |
| Proteases | Enzymes that break down proteins and peptides | Increased during inflammation | Prolonging of inflammation and damage to ECM |
| Neutrophils | Neutrophilic white blood cells generate free radicals, debride ulcer via secretion of proteolytic enzymes, and phagocytose the dead bacteria and matrix debris | Increased during inflammation | Production of excess proteases and increased tissue damage |
| Fibroblasts | Biological cells that synthesize ECM and collagen | Increased during healing | Formation of granulation tissue, which leads to healing |
| Macrophages | White blood cells that secrete growth factors, chemokines, and cytokines and also clean up nonfunctional host cells, bacteria-filled neutrophils, damaged matrix, foreign debris, and remaining bacteria | Increased during inflammation | Secretion of pro-inflammatory cytokines increases MMP production, reduces TIMPs, and reduces collagen synthesis, which promotes inflammation. However, growth-factor secretion should resolve inflammation and promote healing. |
| Eosinophils | White blood cells that are activated during allergic reactions, autoimmune disease, and parasitic infections | Increased during inflammation | Increase in tissue damage |
| Reactive oxygen species | Oxygen-containing chemically reactive species, overproduction of which can lead to oxidative damage and increased production of serine proteases, MMPs, and inflammatory cytokines | Increased during inflammation | Increased damage to ECM and cell membrane and premature cell deterioration |
| Hypochlorous acid | Chemical inhibitor of TIMPs | Increased during inflammation | Wound-tissue degradation |
| N-chloramines | Chemical inhibitor of TIMPs | Increased during inflammation | Wound-tissue degradation |
Note. ECM = extracellular matrix; IL = interleukin; ROS = reactive oxygen species; TIMP = tissue inhibitor of metalloproteinases; TNF = tumor necrosis factor; MMPs = matrix metalloproteinase.
Biofilms
Biofilms are permanently aggregated microbial cells on a variety of surfaces (Donlan, 2002). Once accumulated, these cells are hard to remove because they are protected by a tough enclosure made predominately of polysaccharide casing and other nearby material. A biofilm’s morphology depends not only on the surrounding environment but also on the type of wound. Compared to chronic wounds, acute wounds are significantly less likely to have biofilms, and the biofilms that do form on acute wounds are morphologically different from those that form on chronic wounds (James et al., 2008).
There is no general agreement as to how biofilms affect chronic wounds and prevent healing (Bjarnsholt et al., 2008; James et al., 2008; Percival, Hill, Malic, Thomas, & Williams, 2011; Wolcott, Rhoads, & Dowd, 2008). Conclusions have been limited by small sample sizes and different treatment regimens across studies (G. S. Lazarus et al., 2016; Y. C. Liu, Margolis, & Isseroff, 2011). In addition, there are no relevant animal models that reflect the chronic-wound environment in humans (Ansell, Holden, & Hardman, 2012). Exploration of the functional complexity of a wound’s microenvironment would lead to an increased understanding of the associations of host factors, comorbidities, and systemic inflammation with the symptom clusters that accompany the impaired wound-healing process (Scalise et al., 2015).
Symptom Clusters Associated With CVLUs
Researchers have determined that a number of concurrent symptoms are highly prevalent in individuals with CVLUs (Edwards et al., 2014; Kelechi, Mueller, & Dooley, 2017). In fact, the majority of patients experience four or more concurrent symptoms, which frequently include pain, fatigue, depression, leg swelling, and sleep disturbances. Thus, researchers have begun considering the interrelationship between PNS and chronic wounds, questioning whether some or all of the PNS might share biological mechanisms (Cleeland et al., 2003) and hypothesizing that these mechanisms might also underlie the chronicity of the wounds (Dodd et al., 2001). One well-validated, integrative biobehavioral paradigm links the adverse effects of inflammation to multiple health outcomes, including high levels of PNS (Do et al., 2016; Reuben et al., 2002), excess morbidity and mortality in multiple chronic conditions (Cryan & Dinan, 2012; Miller et al., 2000), and aging (Reuben et al., 2002). With recent technological advances in the use of biomarkers, researchers are becoming increasingly interested in understanding how the interactions among multiple systems contribute to the immune system’s inflammatory response and PNS.
Pain
Pain commonly affects individuals with CVLUs and greatly complicates their disease self-management (Alföldi, Wiklund, & Gerdle, 2014) by causing delays in treatment and reducing these patients’ overall functioning and quality of life (Althaus et al., 2012; Edwards et al., 2014; Haythornthwaite, Menefee, Heinberg, & Clark, 1998; Kelechi et al., 2017; Poobalan et al., 2003; Saxe, Smith, & McNerney, 2013). Pain associated with CVLUs is often not adequately assessed or managed and is related to diminished quality of life and delays in wound healing (Edwards et al., 2014; Lal, 2015; Raffetto, 2018).
Cognitive Dysfunction, Fatigue, Depression, and Anxiety
In addition to condition-specific symptoms, many individuals with chronic diseases such as CVLUs experience cognitive dysfunction, fatigue, depression, and anxiety that may co-occur with pain (Kelechi et al., 2017). These symptoms go hand in hand with pain because of cognitive processes that may influence pain perception and bias nociceptive processing in the brain (Wiech, Ploner, & Tracey, 2008) and because chronic pain, anxiety, and depressive symptoms may impair cognitive abilities (Seminowicz & Davis, 2007).
In a recent study, 42% of depressed and anxious participants reported difficulties with memory and concentration, while less than 8% of medical controls experienced the same symptoms (Saffer, Lanting, Koehle, Klonsky, & Iverson, 2015). For individuals and families affected by serious comorbidities, PNS may be particularly overwhelming because they affect the mental and emotional processing that is crucial for disease self-management. Furthermore, there is increasing evidence that individuals with chronic wounds experience both event-related pain and somatic pain (Rosique, Rosique, & Farina Junior, 2015) and that inflammation and pain can delay healing (Soon & Acton, 2006). Depression has also been associated with delayed wound healing (Cole-King & Harding, 2001; Kiecolt-Glaser & Glaser, 2002). Although research has demonstrated that pain and cognitive/affective symptoms contribute to outcomes in CVLUs, to date there has been little research focusing on symptoms in individuals with CVLUs outside of studies of wound-related pain.
Biobehavioral Mechanisms of CVLU Symptom Clusters
Innovations in “omics” research have increased our understanding of important wound microenvironmental factors (e.g., local inflammation, microbial pathogenic biofilm, epigenetic processes through microRNAs) that adversely alter the wound bed’s molecular milieu so that microbes evade immune detection. Although these molecular factors are not singularly responsible for wound healing, they are now recognized as major components of wound development and nonhealing as well as associated PNS that, until now, have not been amenable to systematic study, especially over time.
Meanwhile, innovations in genomics have also advanced our understanding of debilitating chronic symptoms such as pain, fatigue, anxiety, and sleep disturbance. For individuals living with CVLUs, both the immune and central nervous systems may contribute to the development and progression of this symptom burden. Research has demonstrated that the immune system plays an important role in brain function, bidirectionally communicating with the brain via cytokines, resulting in effects on symptoms (Kipnis, 2018). In fact, studies have shown that inflammation and cytokine levels correlated with symptoms (e.g., stress, pain, decreased appetite, increased sleep, social isolation).
Immune System
Inflammation and wound proteome
Hundreds of proteins, including cytokines, interleukins, and MMPs (Broszczak et al., 2017; Edsberg, Wyffels, Brogan, & Fries, 2012), make up the wound-fluid proteome of both healing and inflamed, nonhealing wounds (Broszczak et al., 2017; Edsberg et al., 2012; Eming et al., 2010; Ganesh et al., 2012; Grieb et al., 2012; Krisp et al., 2013; Ruzehaji et al., 2012; Shah, Omar, Pai, & Sood, 2012; Steinsträßer et al., 2010; Wyffels et al., 2010). Chronic wounds are characterized by increased protease activity, which is primarily caused by fibroblasts, macrophages, eosinophils, and neutrophils releasing elevated levels of MMPs, which eventually degrade the ECM (Broszczak et al., 2017). The activity of the MMPs becomes uncontrollable as their inhibitors, the tissue inhibitors of metalloproteinases (TIMPs), become unable to provide equilibrium, which then results in a wound that gets “stuck” in a prolonged inflammatory state. Bacterial proteases further contribute to the protease activities, which hinders the normal healing processes.
Pro-inflammatory cytokines also play a major role in delayed wound healing and a chronic wound state. IL-1β and tumor necrosis factor-α (TNF-α) are the two leading cytokines that initiate the release of inflammatory mediators, which damage the endothelium. Neutrophils attached to the endothelium are then able to invade the wound and produce reactive oxygen species, which further contribute to tissue damage within the wound bed. Neutrophils also produce hypochlorous acid and N-chloramines, which can effectively inhibit the TIMPs’ actions, further contributing to wound-tissue degradation. It is thus important to continue studying the differential expression and activity of proteins in wounds during the phases of inflammation and wound healing and to also understand how they affect wound healing (Broszczak et al., 2017).
Inflammation and wound metabolomics
Metabolomics is the identification and quantification of metabolites generated as a result of cellular physiology at a fixed point in time (Broszczak et al., 2017; Idle & Gonzalez, 2007). It can provide a snapshot of cell and tissue metabolism in an inflamed wound. Examples of metabolites include vitamins, carbohydrates, lipids, steroids, amino acids, nucleic acids, and peptides (Arakaki, Skolnick, & McDonald, 2008; Broszczak et al., 2017; Zhou, Xiao, Tuli, & Ressom, 2012). These biomolecules are ideal for use as biomarkers when diagnosing clinical states including inflammation. Given the recent adoption of high-throughput methods for metabolomics inquiry, we are still early in our metabolomics investigations of CVLUs. Metabolites currently of interest in this field of study include l-arginine, nitric oxide, oxidative free radicals, and iron, each of which has been found to be significantly elevated within the chronic-wound environment and directly associated with inflammation. Future research is necessary to more fully explore the chronic-wound metabolome.
Central Nervous System
Although still in its early stages, the emerging field of psychoneuroimmunology provides a mechanism for studying the brain’s response to immunological information; the resulting knowledge may advance our understanding of PNS (Kipnis, 2018). New findings indicate that the immune system and the brain interact constantly during health and illness. Kipnis (2018) showed that, by actively detecting microorganisms in the body and informing the brain about their presence, the immune system assists the brain in dealing with stress and in developing sickness behaviors. One of the most characteristic of these behaviors is fatigue (Abbas, Jorgensen, & Lindor, 2010; Menzies et al., 2015; Newton, 2010), often accompanied by increased sedentarism and sleep disturbance (Newton & Jones, 2012). Sickness behaviors are also associated with cognitive changes (Elliott, Frith, Pairman, Jones, & Newton, 2011; Lee, Otgonsuren, Younoszai, Mir, & Younossi, 2013; Newton, Pairman, Wilton, Jones, & Day, 2009; Weinstein, 2011). Intensive study to better understand the linkages among inflammatory mediators and behavioral outcomes is ongoing across multiple acute and chronic medical conditions.
Perceived psychological stress leads to an inflammatory response and activation of the hypothalamic–pituitary–adrenocortical (HPA) axis through increased release of cortisol and catecholamines (Menzies et al., 2015). Increased psychological stress can also induce elevations in the levels of pro-inflammatory cytokines, which suggests a direct link between stress and inflammation (Menzies et al., 2015) and is associated with delayed wound healing (Kiecolt-Glaser, Marucha, Mercado, Malarkey, & Glaser, 1995).
Implications for Biobehavioral Research
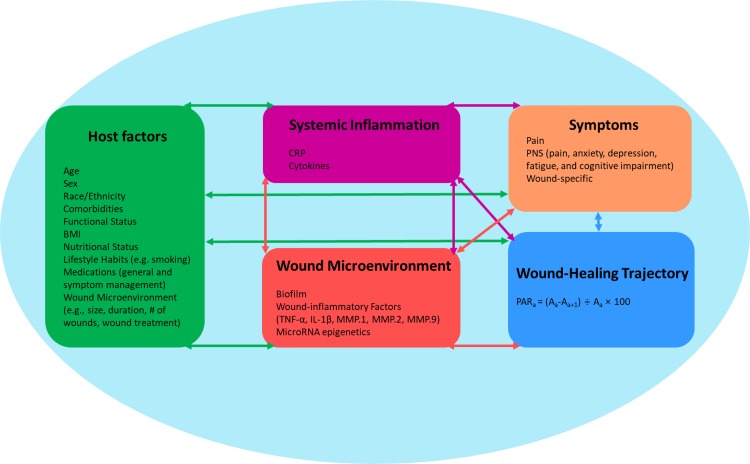
Given the shared symptoms among many individuals with CVLUs, researchers agree that common, related mechanisms may be at work in chronic wounds (Menzies et al., 2015). Extensive findings suggest bidirectional relationships among host factors that affect the immune system, the microenvironment of the wound, inflammation, PNS and other symptoms, and the healing trajectory of CVLUs (Dantzer et al., 2008; Do et al., 2016; Edwards et al., 2014; Gould et al., 2015; Guo & Dipietro, 2010; Han et al., 2011; Hareendran et al., 2005; Hellström et al., 2016; Lai & Siu, 2014; Lal, 2015; G. S. Lazarus et al., 2016; Ligi et al., 2016; Y. C. Liu et al., 2011; Mann & Mann, 2013; Margolis, Bilker, Santanna, & Baumgarten, 2002; Raffetto et al., 2015; Ruckley, 1997; Sarkar & Fisher, 2006; Sisco et al., 2008; Wang et al., 2012; Watters et al., 2013; Zhao et al., 2013). Based on the existing research, we propose that a wound’s microenvironmental components, which include local inflammation and biofilm, trigger both a local and a systemic host inflammatory reaction (Han et al., 2011; Zhao et al., 2013) that may lead to further biological perturbations such as epigenetic alterations and symptoms (Bavan & Midwood, 2011; Das, Ganesh, Khanna, Sen, & Roy, 2014; Lai & Siu, 2014; Madhyastha, Madhyastha, Nakajima, Omura, & Maruyama, 2012; Mann & Mann, 2013; Robinson et al., 2013; Sisco et al., 2008; Wang et al., 2012; Watters et al., 2013). Figure 2 depicts a conceptual framework describing the potential mechanisms underlying the common symptoms experienced by older adults with CVLUs.
Figure 2.
Putative mechanisms underlying the symptom experience in chronic venous leg ulcers (CVLUs). Several simultaneously occurring biological mechanisms likely contribute to the development of the symptom cluster. Inflammation and biofilm contribute to the development and progression of CVLUs. The central and autonomic nervous systems respond to stress, whether psychological or physiological (i.e., inflammation). With this response comes with further immune-system activation with the potential for delayed wound healing. The co-occurring symptoms of CVLUs have a potential bidirectional relationship with the immune system within the microenvironment of the wound and also systemically. BMI = body mass index; CRP = C-reactive protein; IL-1β = interleukin-1β; MMP = matrix metalloproteinase; PAR = percent area reduction (in the equation, A = area of the wound at a given time); PNS = psychoneurologic symptoms; TNF-α = tumor necrosis factor-α. Adapted from Raffetto (2018).
Host Factors
We propose that patient host factors (e.g., age, disease burden, sex, race/ethnicity, body mass index, nutritional status, lifestyle habits including smoking) affect all other factors in the model. Demographic characteristics and age are related to increased incidence and prevalence of CVLUs. In addition, multiple studies have shown sex-related differences in wound healing (Ligi et al., 2016) and outcomes.
Sex
In a recent study, researchers showed that symptom clusters differed between women and men with VLUs: The top three symptoms for women, in order of frequency, were achy legs, swelling, and pain, while for men, they were swelling, achy legs, and heavy legs. Women described their symptoms as being primarily hurting and annoying, while men described theirs as nagging and irritating (T. Liu et al., 2014).
Aging
Older aged people experience an increased abundance of pro-inflammatory cytokines and a lower count of growth factors. Pro-inflammatory cytokines (e.g., IL-1, IL-6, TNF-α) tend to sustain inflammation, while growth factors (e.g., endothelial growth factor, fibroblast growth factor-2, transforming growth factor-β, platelet-derived growth factor, and vascular endothelial growth factor) combat inflammation via reepithelialization (Kurosaka et al., 2009). Unlike relatively healthy patients with acute wounds in which growth factors and cytokines are increased, patients with chronic wounds experience decreased levels of growth factors and increased levels of pro-inflammatory cytokines (Barrientos, Stojadinovic, Golinko, Brem, & Tomic-Canic, 2008). Further, patients with CVLUs experience even greater reductions in cell growth and healing than might be expected based solely on their reduced levels of growth factors because their fibroblasts are not as reactive to growth factors’ stimuli as are those of healthier patients (Ågren, Steenfos, Dabelsteen, Hansen, & Dabelsteen, 1999; Falanga, Zhou, & Yufit, 2002; Kim et al., 2003).
Overexpression and increased activity of MMPs in older adults can also delay healing. MMPs, which increasingly degrade collagen and collagen fragments, can be upregulated by certain hormonal changes that are characteristic in older adults. For example, higher levels of norepinephrine in older patients (Veith, Featherstone, Linares, & Halter, 1986; Yang et al., 2006) and lower levels of estrogen in postmenopausal women (Ashcroft, Greenwell-Wild, Horan, Wahl, & Ferguson, 1999) can elevate levels of MMPs, leading to sustained inflammation.
Similar to MMPs, neutrophil elastase breaks proteins and peptide bonds and tends to be more abundant in chronic wounds and older patients (Enoch & Price, 2004). By degrading proteins (e.g., fibronectin, vitronectin, tenascin) that aid in the reformation of the ECM, elastase delays healing and enables inflammation. Furthermore, the response to the work of proteases is slower in older adults due to their bodies’ weakened signal transduction, which also contributes to delays in ECM reconstruction and healing (Ashcroft, Mills, & Ashworth, 2002).
Older patients with CVLUs are at additional risk of delayed healing due to the characteristically high iron content of their wounds, which feeds macrophages and stimulates prolonged inflammation (Sindrilaru et al., 2011). These conditions, along with other effects of advanced age, can put a tremendous burden on a body that is attempting to heal.
Comorbidities
It is well established that multiple factors may contribute to inflammatory dysfunction and higher symptom burden across comorbid conditions (Sarkar & Fisher, 2006). However, the underlying mechanisms associated with the trajectory of wound healing in the growing population of older individuals with CVLUs, as well as the ways comorbidities and other factors affect healing outcomes, are virtually unknown (Gould et al., 2015).
Comorbidities are associated with multiple biological perturbations including heightened pro-inflammatory activation and reduced microbial clearance. The host immune system is governed by comorbidities that may affect the wound-healing process, such as diabetes, venous insufficiency, congestive heart failure, abnormal white blood cell function, and obesity (Wolcott et al., 2008). Additionally, healing in patients with chronic wounds can be influenced by medications, antibiotic resistance, additional infected areas, pro-inflammatory wound condition, compromised immunity, poor circulation, poor hydration, and other nutritional deficiencies (Moreo, 2005).
Medications and antibiotic resistance
Chemotherapy medications such as antineoplastic agents (e.g., Eloxatin for metastatic colon cancer) can interfere with wound healing by disrupting cellular replication (Pollack, 1982). Meanwhile, other medications, such as antibiotics (e.g., penicillin), which are still prescribed by some practitioners for CVLUs, may simply be ineffective in treating chronic leg wounds, especially in patients with antibiotic resistance (Howell-Jones et al., 2005).
Additional infected area
When a patient has an additional area of infection (whether viral, bacterial, fungal, or protozoal), the host monocytes are transferred to peripheral tissues including the area of the leg wound. The presence of monocytes and their eventual differentiation into macrophages can further reinforce inflammation and delay healing (Shi & Pamer, 2011).
Pro-inflammatory conditions
Pro-inflammatory conditions, such as rheumatoid arthritis, result in the release of pro-inflammatory cytokines (e.g., IL-1, IL-6, and TNF-α), which, in turn, promote and prolong the inflammatory process (Choy & Panayi, 2001).
Compromised immunity
Healing can also be delayed due to a body’s lack of response to pathogens. For example, patients who are HIV-positive with AIDS experience compromised immunity because they have low (<100) CD4+ T-cell counts (Njunda, Nsagha, Assob, Kamga, & Teyim, 2012; Weledji, Kamga, Assob, & Nsagha, 2012). Low counts of these white blood cells means limited protection from infection and, thus, prolonged wound healing (Weledji et al., 2012).
Poor circulation
Patients with circulatory problems (such as in diabetes mellitus) have trouble with tissue repair because their reduced blood flow leads to limited oxygen supply for the wound (Greenhalgh, 2003). Since oxygen is imperative for collagen synthesis by fibroblasts and for tissue regeneration, a limited blood supply can further delay wound healing (Trabold et al., 2003).
Nutritional and hydration deficiencies
While changes in oxygen supply to the wound are not always obvious, the effects of a patient’s nutritional intake and digestive process can be more apparent. Patients who have frequent diarrhea from medications for their comorbidities (e.g., omeprazole for heartburn or stomach ulcers, nonsteroidal anti-inflammatories for arthritis, metformin for diabetes) can experience nutrient and hydration deficiencies. Limited hydration and nutrient supply to the wound can hinder many of the processes of healing including epithelialization and collagen synthesis (Stechmiller, 2010).
Obesity
Patients who are obese experience low-grade inflammation, which includes many components of the traditional inflammatory response to pathogens, such as local wounding and systemic increases in cytokines.
Inflammation
Host immune factors (systemic inflammation) and the wound microenvironment affect each other as well as symptoms and wound healing. CRP level, a clinically validated marker of inflammation, is a useful tool for determining the degree of systemic inflammation as well as the effectiveness of treatments. Measuring systemic cytokines can also provide much needed information about the extent of systemic inflammation in older individuals with CVLUs.
CVLU treatments
Studies on treatments for CVLUs have mainly focused on the correction of hemodynamic alterations and compression therapy. Meanwhile, there has been little examination of biological intervention targets (Ligi et al., 2016) such as the presence of biofilm and its impact on inflammation in the microenvironment and the entire system (Ligi et al., 2016; T. Liu et al., 2014). Consequently, patients with CVLUs are often treated with sequential treatments without an evidence-based rationale for the order or timing of treatment strategies.
Further knowledge regarding the biological characteristics associated with healing and nonhealing are thus especially important for determining the best use of medications, surgery, and other treatments for CVLUs (G. Lazarus et al., 2014). Similarly, the relationship of treatment variables with symptoms has been understudied in individuals with CVLUs, though their excessive symptom burden is well documented (Hareendran et al., 2005; Kelechi & Johnson, 2012).
Trajectory of Chronic-Wound Healing
Healing in a chronic wound is challenging, requiring a complex process of sequential stages including inflammation, reepitheliazation, formation of granulation tissue, local revascularization, and remodeling (Järbrink et al., 2016). During epithelialization, cellular mechanisms are responsible for keratinocyte migration and proliferation, which are essential for successful wound closure. If keratinocytes fail to close the wound and maintain a barrier, chronicity and wound recurrence are likely to occur. Thus, unsuccessful reepithelization characterizes a nonhealing chronic wound (Rousselle, Braye, & Dayan, 2018).
Multiple pathophysiologic factors prevent a chronic wound from reepithelializing and maintaining closure. Those associated with the chronicity of VLUs include venous hypertension, infection, biofilms, malnutrition, age, diabetes and other comorbidities, tissue hypoxia, exudates, excessive levels of inflammatory cytokines and proteases, reactive oxygen species, and senescent cells (Rousselle et al., 2018). Studies have shown that older adults burdened with CVLUs have low keratinocyte-cell activity, which contributes to delayed reepithelialization (Grove & Kligman, 1983).
To accurately monitor the wound-healing trajectory in clinical research, investigators must precisely document the wound size (i.e., perform assessment of the wound area; Bowling, Paterson, & Ndip, 2013). Recent innovations in three-dimensional wound imaging have enhanced the ability to compute the curvature of the wound through analysis of laser-beam traces (Foltynski, Wojcicki, Ladyzynski, & Sabalinska, 2014). Specifically, the Silhouette, a handheld device that minimizes variability between wound measurements, provides laser-enhanced accuracy in the measurement of a wound’s length, width, and depth, while advanced software makes other calculations including volume (Casas, Castaneda, & Treuillet, 2011; Nixon & Moore, 2016). The Silhouette has demonstrated a high level of reliability, validity, and reproducibility across studies (Casas et al., 2011; Foltynski et al., 2014; Nixon & Moore, 2016).
Future Interventional Research Involving Biobehavioral Mechanisms and PNS
Future research exploring shared biobehavioral mechanisms of wound chronicity and PNS would not only expand our understanding of these mechanisms and associated symptoms but would also lead to the development of nursing and other targeted interventions that could improve symptom management and quality of life (Menzies et al., 2015). Psychosocial and behavioral nursing interventions that aim to reduce chronically ill patients’ stress, strengthen their self-management skills, and enhance their coping skills have the potential to reduce CNS overload and cytokine dysregulation and, subsequently, to improve patients’ quality of life. For example, studies of strategies such as mindfulness-based stress reduction meditation programs have demonstrated decreased levels of stress, reduction in Th1 (pro-inflammatory cytokines, and enhanced quality of life in other diseases; Carlson, Speca, Faris, & Patel, 2007; Menzies et al., 2015). To date, however, no studies have examined the influence of such interventions on the symptom experience in individuals with CVLUs. Behavioral interventions such as physical activity or exercise, weight loss, smoking cessation, and diet modification could also improve the physiological aspects of CVLUs and patients’ level of functioning (Bergasa, Mehlman, & Bir, 2004; Menzies et al., 2015; Promrat et al., 2010). However, whether or not these interventions affect the symptom experience has yet to be explored (Menzies et al., 2015).
Conclusion
The similarity in symptom experiences across patients with CVLUs suggests that there may be pathophysiological processes in common between these nonhealing wounds and symptoms. In other words, these findings suggest the presence of symptom clusters associated with CVLUs. With the growing number of individuals living long term with CVLUs, it is crucial that we develop a better understanding of the biological underpinnings of these symptom clusters so that we may begin to develop more targeted and effective symptom-management strategies. Recent gains in knowledge regarding communication between the brain and the immune system open new opportunities for nurse-researchers to examine interdependent and possibly synergistic interactions among biological and behavioral factors, symptom clusters, and outcomes in CVLUs.
Acknowledgments
We would like to acknowledge our clinical staff at the University of Florida Health Wound Care and Hyperbaric Center for helping us in this study.
Footnotes
Author Contributions: Joyce K. Stechmiller contributed to conception, design, acquisition, analysis, and interpretation; drafted manuscript critically; revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Debra Lyon contributed to conception, design, acquisition, analysis, and interpretation; drafted manuscript critically; revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Gregory Schultz contributed to conception, design, acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Daniel J. Gibson contributed to design, acquisition, analysis, and interpretation; drafted manuscript critically; revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Michael T. Weaver contributed to conception, design, analysis, and interpretation; drafted manuscript critically; revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Diana Wilkie contributed to conception, acquisition, analysis, and interpretation; drafted manuscript critically; revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Anastasiya V. Ferrell contributed to conception, acquisition; drafted manuscript critically; revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Joanne Whitney contributed to conception, acquisition; drafted manuscript critically; revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Junglyun Kim contributed to conception, design, and acquisition; drafted manuscript critically; revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Susan B. Millan contributed to design and acquisition; drafted manuscript critically; revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institutes of Health (NIH), National Institute of Nursing Research (NINR), Grant No. 1RO1NR016986-01A1.
References
- Abbas G., Jorgensen R. A., Lindor K. D. (2010). Fatigue in primary biliary cirrhosis. Nature Reviews Gastroenterology & Hepatology, 7, 313. [DOI] [PubMed] [Google Scholar]
- Ågren M. S., Steenfos H. H., Dabelsteen S., Hansen J. B., Dabelsteen E. (1999). Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is Ulcer-Age Dependent. Journal of Investigative Dermatology, 112, 463–469. [DOI] [PubMed] [Google Scholar]
- Alföldi P., Wiklund T., Gerdle B. (2014). Comorbid insomnia in patients with chronic pain: A study based on the Swedish quality registry for pain rehabilitation (SQRP). Disability and Rehabilitation, 36, 1661–1669. [DOI] [PubMed] [Google Scholar]
- Althaus A., Hinrichs-Rocker A., Chapman R., Becker O. A., Lefering R., Simanski C.…Trojan S. (2012). Development of a risk index for the prediction of chronic post-surgical pain. European Journal of Pain, 16, 901–910. [DOI] [PubMed] [Google Scholar]
- Ansell D. M., Holden K. A., Hardman M. J. (2012). Animal models of wound repair: Are they cutting it? Experimental Dermatology, 21, 581–585. [DOI] [PubMed] [Google Scholar]
- Arakaki A. K., Skolnick J., McDonald J. F. (2008). Marker metabolites can be therapeutic targets as well. Nature, 456, 443. [DOI] [PubMed] [Google Scholar]
- Ashcroft G. S., Greenwell-Wild T., Horan M. A., Wahl S. M., Ferguson M. W. (1999). Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. American Journal of Pathology, 155, 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft G. S., Mills S. J., Ashworth J. J. (2002). Ageing and wound healing. Biogerontology, 3, 337–345. [DOI] [PubMed] [Google Scholar]
- Baker M. (2007). Re: National coverage analysis (NCA) for autologous blood derived products for chronic non-healing wounds (CAG-00190R2) [Public comment].
- Barrientos S., Stojadinovic O., Golinko M. S., Brem H., Tomic-Canic M. (2008). Growth factors and cytokines in wound healing. Wound Repair and Regeneration, 16, 585–601. [DOI] [PubMed] [Google Scholar]
- Bavan L., Midwood K. (2011). MicroRNA epigenetics. BioDrugs, 25, 27–41. [DOI] [PubMed] [Google Scholar]
- Bergasa N. V., Mehlman J., Bir K. (2004). Aerobic exercise: A potential therapeutic intervention for patients with liver disease. Medical Hypotheses, 62, 935–941. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T., Kirketerp-Møller K., Jensen P. Ø., Madsen K. G., Phipps R., Krogfelt K.…Givskov M. (2008). Why chronic wounds will not heal: A novel hypothesis. Wound Repair and Regeneration, 16, 2–10. [DOI] [PubMed] [Google Scholar]
- Bowling F. L., Paterson J., Ndip A. (2013). Applying 21st century imaging technology to wound healing: An Avant-Gardist approach. Los Angeles, CA: Sage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broszczak D. A., Sydes E. R., Wallace D., Parker T. J. (2017). Molecular aspects of wound healing and the rise of venous leg ulceration: Omics approaches to enhance knowledge and aid diagnostic discovery. The Clinical Biochemist Reviews, 38, 35. [PMC free article] [PubMed] [Google Scholar]
- Carlson L. E., Speca M., Faris P., Patel K. D. (2007). One year pre–post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain, Behavior, and Immunity, 21, 1038–1049. [DOI] [PubMed] [Google Scholar]
- Casas L., Castaneda B., Treuillet S. (2011). Imaging technologies applied to chronic wounds: A survey. Paper presented at the Proceedings of the 4th International Symposium on Applied Sciences in Biomedical and Communication Technologies, Barcelona, Spain. [Google Scholar]
- Choy E. H., Panayi G. S. (2001). Cytokine pathways and joint inflammation in rheumatoid arthritis. New England Journal of Medicine, 344, 907–916. [DOI] [PubMed] [Google Scholar]
- Cleeland C. S., Bennett G. J., Dantzer R., Dougherty P. M., Dunn A. J., Meyers C. A.…Wang X. S. (2003). Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? Cancer, 97, 2919–2925. [DOI] [PubMed] [Google Scholar]
- Cole-King A., Harding K. G. (2001). Psychological factors and delayed healing in chronic wounds. Psychosomatic Medicine, 63, 216–220. [DOI] [PubMed] [Google Scholar]
- Cryan J. F., Dinan T. G. (2012). Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience, 13, 701. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J. C., Freund G. G., Johnson R. W., Kelley K. W. (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Ganesh K., Khanna S., Sen C. K., Roy S. (2014). Engulfment of apoptotic cells by macrophages: A role of microRNA-21 in the resolution of wound inflammation. The Journal of Immunology, 192, 1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do H. T. T., Edwards H., Finlayson K. (2016). Identifying relationships between symptom clusters and quality of life in adults with chronic mixed venous and arterial leg ulcers. International Wound Journal, 13, 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd M., Janson S., Facione N., Faucett J., Froelicher E. S., Humphreys J.…Rankin S. (2001). Advancing the science of symptom management. Journal of advanced nursing, 33, 668–676. [DOI] [PubMed] [Google Scholar]
- Donlan R. M. (2002). Biofilms: Microbial life on surfaces. Emerging Infectious Diseases, 8, 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsberg L. E., Wyffels J. T., Brogan M. S., Fries K. M. (2012). Analysis of the proteomic profile of chronic pressure ulcers. Wound Repair and Regeneration, 20, 378–401. [DOI] [PubMed] [Google Scholar]
- Edwards H., Finlayson K., Skerman H., Alexander K., Miaskowski C., Aouizerat B., Gibb M. (2014). Identification of symptom clusters in patients with chronic venous leg ulcers. Journal of Pain and Symptom Management, 47, 867–875. doi:10.1016/j.jpainsymman.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Eklöf B., Rutherford R. B., Bergan J. J., Carpentier P. H., Gloviczki P., Kistner R. L.…Padberg F. T. (2004). Revision of the CEAP classification for chronic venous disorders: Consensus statement. Journal of Vascular Surgery, 40, 1248–1252. [DOI] [PubMed]
- Elliott C., Frith J., Pairman J., Jones D. E., Newton J. L. (2011). Reduction in functional ability is significant postliver transplantation compared with matched liver disease and community dwelling controls. Transplant International, 24, 588–595. [DOI] [PubMed] [Google Scholar]
- Eming S. A., Koch M., Krieger A., Brachvogel B., Kreft S., Bruckner-Tuderman L.…Fox J. W. (2010). Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. Journal of Proteome Research, 9, 4758–4766. [DOI] [PubMed] [Google Scholar]
- Enoch S., Price P. (2004). Cellular, molecular and biochemical differences in the pathophysiology of healing between acute wounds, chronic wounds and wounds in the aged. World Wide Wounds, 13, 1–17. [Google Scholar]
- Falanga V., Zhou L., Yufit T. (2002). Low oxygen tension stimulates collagen synthesis and COL1A1 transcription through the action of TGF-β1. Journal of Cellular Physiology, 191, 42–50. [DOI] [PubMed] [Google Scholar]
- Foltynski P., Wojcicki J., Ladyzynski P., Sabalinska S. (2014). A comparison of three techniques for wound area measurement. Paper presented at the XIII Mediterranean Conference on Medical and Biological Engineering and Computing 2013, pp. 1071–1074. Cham, Switzerland: Springer. [Google Scholar]
- Ganesh K., Das A., Dickerson R., Khanna S., Parinandi N. L., Gordillo G. M.…Roy S. (2012). Prostaglandin E2 induces oncostatin M expression in human chronic wound macrophages through Axl receptor tyrosine kinase pathway. The Journal of Immunology, 189, 2563–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould L., Abadir P., Brem H., Carter M., Conner-Kerr T., Davidson J.…Schmader K. (2015). Chronic wound repair and healing in older adults: Current status and future research. Wound Repair and Regeneration. doi:10.1111/wrr.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh D. G. (2003). Wound healing and diabetes mellitus. Clinics in Plastic Surgery, 30, 37–45. [DOI] [PubMed] [Google Scholar]
- Grieb G., Simons D., Eckert L., Hemmrich M., Steffens G., Bernhagen J., Pallua N. (2012). Levels of macrophage migration inhibitory factor and glucocorticoids in chronic wound patients and their potential interactions with impaired wound endothelial progenitor cell migration. Wound Repair and Regeneration, 20, 707–714. [DOI] [PubMed] [Google Scholar]
- Grove G. L., Kligman A. M. (1983). Age-associated changes in human epidermal cell renewal. Journal of Gerontology, 38, 137–142. [DOI] [PubMed] [Google Scholar]
- Guo S., Dipietro L. A. (2010). Factors affecting wound healing. Journal of Dental Research, 89, 219–229. doi:10.1177/0022034509359125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A., Zenilman J. M., Melendez J. H., Shirtliff M. E., Agostinho A., James G.…Rickard A. H. (2011). The importance of a multifaceted approach to characterizing the microbial flora of chronic wounds. Wound Repair and Regeneration, 19, 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. A., Feuerstein M., Calvio L. C., Olsen C. H. (2008). Breast cancer survivors at work. Journal of Occupational and Environmental Medicine, 50, 777–784. [DOI] [PubMed] [Google Scholar]
- Hareendran A., Bradbury A., Budd J., Geroulakos G., Hobbs R., Kenkre J., Symonds T. (2005). Measuring the impact of venous leg ulcers on quality of life. Journal of Wound Care, 14, 53–57. [DOI] [PubMed] [Google Scholar]
- Haythornthwaite J. A., Menefee L. A., Heinberg L. J., Clark M. R. (1998). Pain coping strategies predict perceived control over pain. Pain, 77, 33–39. [DOI] [PubMed] [Google Scholar]
- Hellström A., Nilsson C., Nilsson A., Fagerström C. (2016). Leg ulcers in older people: A national study addressing variation in diagnosis, pain and sleep disturbance. BMC Geriatrics, 16, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke P. (2010). The pacific vascular symposium 6: The venous ulcer summit in perspective. Journal of Vascular Surgery, 52, 1S–2S. doi:10.1016/j.jvs.2010.05.066 [DOI] [PubMed] [Google Scholar]
- Howell-Jones R., Wilson M., Hill K., Howard A., Price P., Thomas D. (2005). A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. Journal of Antimicrobial Chemotherapy, 55, 143–149. [DOI] [PubMed] [Google Scholar]
- Idle J. R., Gonzalez F. J. (2007). Metabolomics. Cell Metabolism, 6, 348–351. doi:10.1016/j.cmet.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G. A., Swogger E., Wolcott R., Secor P., Sestrich J., Costerton J. W., Stewart P. S. (2008). Biofilms in chronic wounds. Wound Repair and Regeneration, 16, 37–44. [DOI] [PubMed] [Google Scholar]
- Järbrink K., Ni G., Sönnergren H., Schmidtchen A., Pang C., Bajpai R., Car J. (2016). Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Systematic Reviews, 5, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelechi T. J., Johnson J. J. (2012). Guideline for the management of wounds in patients with lower-extremity venous disease: An executive summary. Journal of Wound Ostomy & Continence Nursing, 39, 598–606. doi:10.1097/WON.0b013e31827179e9 [DOI] [PubMed] [Google Scholar]
- Kelechi T. J., Mueller M., Dooley M. (2017). Sex differences in symptom severity and clusters in patients with stage C4 and stage C5 chronic venous disease. European Journal of Cardiovascular Nursing, 16, 28–36. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Glaser R. (2002). Depression and immune function: Central pathways to morbidity and mortality. Journal of Psychosomatic Research, 53, 873–876. doi:10.1016/S0022-3999(02)00309-4 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Marucha P. T., Mercado A., Malarkey W. B., Glaser R. (1995). Slowing of wound healing by psychological stress. The Lancet, 346, 1194–1196. [DOI] [PubMed] [Google Scholar]
- Kim B. C., Kim H. T., Park S. H., Cha J. S., Yufit T., Kim S. J., Falanga V. (2003). Fibroblasts from chronic wounds show altered TGF-β-signaling and decreased TGF-β Type II receptor expression. Journal of Cellular Physiology, 195, 331–336. [DOI] [PubMed] [Google Scholar]
- Kipnis J. (2018). Immune system: The “seventh sense”. Journal of Experimental Medicine, 215, 397–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri R. (2014). Management of venous ulcers. Techniques in Vascular and Interventional Radiology, 17, 132–138. doi10.1053/j.tvir.2014.02.012 [DOI] [PubMed] [Google Scholar]
- Krisp C., Jacobsen F., McKay M. J., Molloy M. P., Steinstraesser L., Wolters D. A. (2013). Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics, 13, 2670–2681. [DOI] [PubMed] [Google Scholar]
- Kurosaka M., Suzuki T., Hosono K., Kamata Y., Fukamizu A., Kitasato H.…Majima M. (2009). Reduced angiogenesis and delay in wound healing in angiotensin II type 1a receptor-deficient mice. Biomedicine & Pharmacotherapy, 63, 627–634. [DOI] [PubMed] [Google Scholar]
- Lai W. F., Siu P. M. (2014). MicroRNAs as regulators of cutaneous wound healing. Journal of Biosciences, 39, 519–524. [DOI] [PubMed] [Google Scholar]
- Lal B. K. (March, 2015). Venous ulcers of the lower extremity: Definition, epidemiology, and economic and social burdens. Seminars in Vascular Surgery, 28(1), 3–5. [DOI] [PubMed] [Google Scholar]
- Lazarus G., Valle M. F., Malas M., Qazi U., Maruthur N. M., Doggett D.…Zenilman J. (2014). Chronic venous leg ulcer treatment: Future research needs. Wound Repair and Regeneration, 22, 34–42. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Kirsner R. S., Zenilman J., Valle M. F., Margolis D. J., Cullum N.…Tunis S. (2016). Clinical interventions for venous leg ulcers: Proposals to improve the quality of clinical leg ulcer research. Wound Repair and Regeneration, 24, 767–774. [DOI] [PubMed] [Google Scholar]
- Lee K., Otgonsuren M., Younoszai Z., Mir H. M., Younossi Z. M. (2013). Association of chronic liver disease with depression: A population-based study. Psychosomatics, 54, 52–59. [DOI] [PubMed] [Google Scholar]
- Ligi D., Mosti G., Croce L., Raffetto J. D., Mannello F. (2016). Chronic venous disease–part I: Inflammatory biomarkers in wound healing. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1862, 1964–1974. [DOI] [PubMed] [Google Scholar]
- Liu T., Yang F., Li Z., Yi C., Bai X. (2014). A prospective pilot study to evaluate wound outcomes and levels of serum C-reactive protein and interleukin-6 in the wound fluid of patients with trauma-related chronic wounds. Ostomy Wound Manage, 60, 30–37. [PubMed] [Google Scholar]
- Liu Y. C., Margolis D. J., Isseroff R. R. (2011). Does inflammation have a role in the pathogenesis of venous ulcers? A critical review of the evidence. Journal of Investigative Dermatology, 131, 818–827. [DOI] [PubMed] [Google Scholar]
- Madhyastha R., Madhyastha H., Nakajima Y., Omura S., Maruyama M. (2012). MicroRNA signature in diabetic wound healing: Promotive role of miR-21 in fibroblast migration. International Wound Journal, 9, 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J., Mann D. A. (2013). Epigenetic regulation of wound healing and fibrosis. Current Opinion in Rheumatology, 25, 101–107. [DOI] [PubMed] [Google Scholar]
- Margolis D. J., Bilker W., Santanna J., Baumgarten M. (2002). Venous leg ulcer: Incidence and prevalence in the elderly. Journal of the American Academy of Dermatology, 46, 381–386. [DOI] [PubMed] [Google Scholar]
- Margolisa D. J., Bilkerb W., Santannab J. (2002). Venous leg ulcer: Incidence and prevalence in the elderly. Journal of the American Academy of Dermatology, 46, 381–386. [DOI] [PubMed] [Google Scholar]
- Menzies V., Jallo N., Kinser P., Robins J. W., An K., Driscoll C.…Lyon D. (2015). Shared symptoms and putative biological mechanisms in chronic liver disease: Implications for biobehavioral research. Biological Research for Nursing, 17, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. T., Sleeper L. A., Pegelow C. H., Enos L. E., Wang W. C., Weiner S. J.…Kinney T. R. (2000). Prediction of adverse outcomes in children with sickle cell disease. New England Journal of Medicine, 342, 83–89. [DOI] [PubMed] [Google Scholar]
- Moreo K. (2005). Understanding and overcoming the challenges of effective case management for patients with chronic wounds. The Case Manager, 16, 62–67. [DOI] [PubMed] [Google Scholar]
- Nelzen O. (2000). Leg ulcers: Economic aspects. Phlebology, 15, 110–114. [Google Scholar]
- Newton J. L. (2010). Systemic symptoms in non-alcoholic fatty liver disease. Digestive Diseases, 28, 214–219. [DOI] [PubMed] [Google Scholar]
- Newton J. L., Jones D. E. (2012). Managing systemic symptoms in chronic liver disease. Journal of Hepatology, 56, S46–S55. [DOI] [PubMed] [Google Scholar]
- Newton J. L., Pairman J., Wilton K., Jones D. E., Day C. (2009). Fatigue and autonomic dysfunction in non-alcoholic fatty liver disease. Clinical Autonomic Research, 19, 319. [DOI] [PubMed] [Google Scholar]
- Nixon M., Moore C. (2016). Evidence-based wound surveillance. Retrieved from http://www.aranzmedical.com/wp-content/uploads/ARANZ-Medical_WP_Evidence-Based-Wound-Surveillance_201608_A4.pdf
- Njunda A. L., Nsagha D. S., Assob J. C., Kamga H. L., Teyim P. (2012). In vitro antifungal susceptibility patterns of Candida Albicans from HIV and AIDS patients attending the Nylon Health District Hospital in Douala, Cameroon. Journal of Public Health in Africa, 3, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’donnell T. F., Passman M. A., Marston W. A., Ennis W. J., Dalsing M., Kistner R. L.…Eklöf B. G. (2014). Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. Journal of Vascular Surgery, 60, 3S–59S. [DOI] [PubMed] [Google Scholar]
- Percival S. L., Hill K. E., Malic S., Thomas D. W., Williams D. W. (2011). Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair and Regeneration, 19, 1–9. [DOI] [PubMed] [Google Scholar]
- Pollack S. V. (1982). Wound healing: A review. IV. Systemic medications affecting wound healing. The Journal of dermatologic surgery and oncology, 8, 667–672. [DOI] [PubMed] [Google Scholar]
- Poobalan A. S., Bruce J., Smith W. C. S., King P. M., Krukowski Z. H., Chambers W. A. (2003). A review of chronic pain after inguinal herniorrhaphy. The Clinical Journal of Pain, 19, 48–54. [DOI] [PubMed] [Google Scholar]
- Promrat K., Kleiner D. E., Niemeier H. M., Jackvony E., Kearns M., Wands J. R.…Wing R. R. (2010). Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology, 51, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffetto J. D. (2018). Pathophysiology of chronic venous disease and venous ulcers. Surgical Clinics of North America, 98, 337–347. doi:10.1016/j.suc.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Raffetto J. D., Mosti G., Santi M., Ligi D., Mannello F. (2015). Matrix metalloproteinase profiles in chronic venous ulcer wound fluid of inflammatory and granulating venous leg ulcers. Journal of Vascular Surgery: Venous and Lymphatic Disorders, 3, 119–120. [DOI] [PubMed] [Google Scholar]
- Reuben D. B., Cheh A. I., Harris T. B., Ferrucci L., Rowe J. W., Tracy R. P., Seeman T. E. (2002). Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. Journal of the American Geriatrics Society, 50, 638–644. [DOI] [PubMed] [Google Scholar]
- Robinson P. M., Chuang T. D., Sriram S., Pi L., Luo X. P., Petersen B. E., Schultz G. S. (2013). MicroRNA Signature in wound healing following excimer laser ablation: Role of miR-133b on TGFβ1, CTGF, SMA, and COL1A1 expression levels in rabbit corneal fibroblasts. Investigative Ophthalmology & Visual Science, 54, 6944–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosique R. G., Rosique M. J., Farina Junior J. A. (2015). Curbing inflammation in skin wound healing: A review. International Journal of Inflammation, 2015, 316235 doi:10.1155/2015/316235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P., Braye F., Dayan G. (2018). Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Advanced Drug Delivery Reviews, S0169-409X(18)30158–3. [DOI] [PubMed] [Google Scholar]
- Ruckley C. (1997). Socioeconomic impact of chronic venous insufficiency and leg ulcers. Angiology, 48, 67–69. [DOI] [PubMed] [Google Scholar]
- Ruzehaji N., Grose R., Krumbiegel D., Zola H., Dasari P., Wallace H.…Cowin A. J. (2012). Cytoskeletal protein Flightless (Flii) is elevated in chronic and acute human wounds and wound fluid: Neutralizing its activity in chronic but not acute wound fluid improves cellular proliferation. European Journal of Dermatology, 22, 740–750. [DOI] [PubMed] [Google Scholar]
- Saffer B. Y., Lanting S. C., Koehle M. S., Klonsky E. D., Iverson G. L. (2015). Assessing cognitive impairment using PROMIS(®) applied cognition-abilities scales in a medical outpatient sample. Psychiatry Research, 226, 169–172. doi:10.1016/j.psychres.2014.12.043 [DOI] [PubMed] [Google Scholar]
- Sarkar D., Fisher P. B. (2006). Molecular mechanisms of aging-associated inflammation. Cancer Letters, 236, 13–23. [DOI] [PubMed] [Google Scholar]
- Saxe J. M., Smith V., McNerney K. (2013). A blueprint to managing multiple chronic conditions and pain. Journal of Family Practice, 62, S4–S11. [PubMed] [Google Scholar]
- Scalise A., Bianchi A., Tartaglione C., Bolletta E., Pierangeli M., Torresetti M.…Di Benedetto G. (2015). Microenvironment and microbiology of skin wounds: The role of bacterial biofilms and related factors. Seminars in Vascular Surgery, 28, 3. [DOI] [PubMed] [Google Scholar]
- Schramedei K., Mörbt N., Pfeifer G., Läuter J., Rosolowski M., Tomm J. M.…Brocke-Heidrich K. (2011). MicroRNA-21 targets tumor suppressor genes ANP32A and SMARCA4. Oncogene, 30, 2975–2985. doi:10.1038/onc.2011.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G. S., Davidson J. M., Kirsner R. S., Bornstein P., Herman I. M. (2011). Dynamic reciprocity in the wound microenvironment. Wound Repair and Regeneration, 19, 134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz D. A., Davis K. D. (2007). A re-examination of pain-cognition interactions: Implications for neuroimaging. Pain, 130, 8–13. doi:10.1016/j.pain.2007.03.036 [DOI] [PubMed] [Google Scholar]
- Sen C. K., Gordillo G. M., Roy S., Kirsner R., Lambert L., Hunt T. K.…Longaker M. T. (2009). Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair and Regeneration, 17, 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. M. Y., Omar E., Pai D. R., Sood S. (2012). Cellular events and biomarkers of wound healing. Indian Journal of Plastic Surgery: Official Publication of the Association of Plastic Surgeons of India, 45, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Pamer E. G. (2011). Monocyte recruitment during infection and inflammation. Nature Reviews Immunology, 11, 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J. L., McDonald V. M., Baines K. J., Oreo K. M., Wang F., Hansbro P. M., Gibson P. G. (2013). Influence of age, past smoking, and disease severity on TLR2, neutrophilic inflammation, and MMP-9 levels in COPD. Mediators of Inflammation, 2013, 462934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H.…Schlecht A. (2011). An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. The Journal of Clinical Investigation, 121, 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisco M., Kryger Z. B., O’Shaughnessy K. D., Kim P. S., Schultz G. S., Ding X. Z.…Mustoe T. A. (2008). Antisense inhibition of connective tissue growth factor (CTGF/CCN2) mRNA limits hypertrophic scarring without affecting wound healing in vivo. Wound Repair and Regeneration, 16, 661–673. [DOI] [PubMed] [Google Scholar]
- Soon K., Acton C. (2006). Pain-induced stress: A barrier to wound healing. Wounds UK, 2, 92–101. [Google Scholar]
- Stechmiller J. K. (2010). Understanding the role of nutrition and wound healing. Nutrition in Clinical Practice, 25, 61–68. [DOI] [PubMed] [Google Scholar]
- Steinsträßer L., Jacobsen F., Hirsch T., Kesting M., Chojnacki C., Krisp C., Wolters D. (2010). Immunodepletion of high-abundant proteins from acute and chronic wound fluids to elucidate low-abundant regulators in wound healing. BMC Research Notes, 3, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsioni A., Balk E., O’Donnell T., Lau J. (2007). Usual care in the management of chronic wounds: A review of the recent literature. Journal of the American College of Surgeons, 205, 617–624. e657. [DOI] [PubMed] [Google Scholar]
- Trabold O., Wagner S., Wicke C., Scheuenstuhl H., Hussain M. Z., Rosen N.…Hunt T. K. (2003). Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair and Regeneration, 11, 504–509. [DOI] [PubMed] [Google Scholar]
- Vasudevan B. (2014). Venous leg ulcers: Pathophysiology and classification. Indian Dermatology Online Journal, 5, 366–370. doi:10.4103/2229-5178.137819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veith R. C., Featherstone J. A., Linares O. A., Halter J. B. (1986). Age differences in plasma norepinephrine kinetics in humans. Journal of Gerontology, 41, 319–324. [DOI] [PubMed] [Google Scholar]
- Wang T., Feng Y., Sun H., Zhang L., Hao L., Shi C.…Zou Z. (2012). miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. American Journal of Pathology, 181, 1911–1920. doi:10.1016/j.ajpath.2012.08.022 [DOI] [PubMed] [Google Scholar]
- Watters C., DeLeon K., Trivedi U., Griswold J., Lyte M., Hampel K.…Rumbaugh K. (2013). Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Medical Microbiology and Immunology, 202, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. N. (2011). MicroRNAs in cancer pharmacology and therapeutics: Exploiting a natural synergy between ‘-omic’ and hypothesis-driven research. Molecular Cancer Therapeutics, 10, 2021–2021. [DOI] [PubMed] [Google Scholar]
- Weledji E., Kamga H., Assob J., Nsagha D. (2012). A critical review on HIV/AIDS and wound care. African Journal of Clinical and Experimental Microbiology, 13, 66–73. [Google Scholar]
- Wiech K., Ploner M., Tracey I. (2008). Neurocognitive aspects of pain perception. Trends in Cognitive Sciences, 12, 306–313. doi:10.1016/j.tics.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Wolcott R. D., Rhoads D. D., Dowd S. E. (2008). Biofilms and chronic wound inflammation. Journal of Wound Care, 17, 333–341. [DOI] [PubMed] [Google Scholar]
- Wyffels J. T., Fries K. M., Randall J. S., Ha D. S., Lodwig C. A., Brogan M. S.…Edsberg L. E. (2010). Analysis of pressure ulcer wound fluid using two-dimensional electrophoresis. International Wound Journal, 7, 236–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E. V., Sood A. K., Chen M., Li Y., Eubank T. D., Marsh C. B.…Yeh P. E. (2006). Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Research, 66, 10357–10364. [DOI] [PubMed] [Google Scholar]
- Zenilman J., Valle M. F., Malas M. B., Maruthur N., Qazi U., Suh Y.…Lazarus G. (2013). Chronic Venous Ulcers: A Comparative Effectiveness Review of Treatment Modalities. Retrieved from http://go.usa.gov/ZMdA [PubMed]
- Zhao G., Usui M. L., Lippman S. I., James G. A., Stewart P. S., Fleckman P., Olerud J. E. (2013). Biofilms and inflammation in chronic wounds. Advanced Wound Care, 2, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Xiao J. F., Tuli L., Ressom H. W. (2012). LC-MS-based metabolomics. Molecular BioSystems, 8, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]