Abstract
Background
Gonorrhea and chlamydia testing rates are poor among Chinese men who have sex with men (MSM). A quasi-experimental study suggested that a pay-it-forward strategy increased dual gonorrhea/chlamydia testing among MSM. Pay-it-forward offers an individual a gift (e.g., a free test) and then asks the same person if they would like to give a gift to another person. This article reports the protocol of a randomized controlled trial to evaluate dual gonorrhea/chlamydia test uptake and other outcomes among MSM in three arms – a pay-it-forward arm, a pay-what-you-want arm, and a standard of care arm.
Methods
Three hundred MSM will be recruited at three HIV testing sites in Guangzhou and Beijing. Testing sites include two hospital-based MSM sexually transmitted diseases clinics and one MSM community-based organization. Eligible participants will be born biologically male, aged 16 years or older, reporting previous anal sex with another man, having never participated in the pay-it-forward program, without previous gonorrhea and chlamydia testing in the past 12 months, and residing in China. Following a cluster randomized design, every cluster of ten participants will be randomly allocated into one of three arms: (1) a pay-it-forward arm in which men are offered free gonorrhea and chlamydia testing and then asked whether they would like to donate (“pay it forward”) toward testing for future testers; (2) a pay-what-you-want arm in which men are offered free testing and told to decide how much to pay after receiving the test; (3) a standard of care arm in which men can pay the full price for dual gonorrhoea and chlamydia testing. The primary outcome is dual gonorrhoea/chlamydia testing as verified by administrative records. Secondary outcomes include incremental cost per test, incremental cost per diagnosis, community connectedness, and social cohesion. Primary outcome will be calculated for each arm using intention-to-treat and compared using one-sided 95% confidence intervals with a margin of 20% increase defined as superiority.
Discussion
This study will examine the pay-it-forward strategy in comparison to the standard of care in improving test uptake for gonorrhea and chlamydia. We will leverage the cluster randomized controlled trial to provide scientific evidence on the potential effect of pay-it-forward. Findings from this study will shed light on novel intervention methods for increasing preventive health service utilization and innovate ways to finance it among communities.
Trial registration
ClinicalTrials.gov, NCT03741725. Registered on 12 November 2018.
Electronic supplementary material
The online version of this article (10.1186/s40249-019-0581-1) contains supplementary material, which is available to authorized users.
Keywords: Men who have sex with men, Gonorrhoea and chlamydia testing, Pay-it-forward, Integrated HIV testing, China, Randomized controlled trial
Multilingual abstracts
Please see Additional file 1 for translations of the abstract into the five official working languages of the United Nations.
Background
Gonorrhea and chlamydia are among the most prevalent sexually transmitted infections (STIs) in China [1, 2]. Men who have sex with men (MSM) is a recognized high risk group for STIs and is estimated to have a population of 2–10 million in China [1, 3]. One study conducted from 2015 to 2017 among MSM in Guangzhou reported an overall gonorrhea prevalence of 12.5% and a chlamydia prevalence of 18.1% [2]. Gonorrhea and chlamydia infections are also associated with increased risk for HIV transmission and acquisition [4, 5]. Yet, infections are commonly asymptomatic and easily missed without screening, especially those at extragenital sites [6–8]. For MSM, the World Health Organization recommends periodic testing for asymptomatic urethral and rectal gonorrhea and chlamydia using nucleic acid amplification tests (NAAT) [9]. US Centers for Disease Control and Prevention (CDC) also suggests annual NAAT testing for gonorrhea and chlamydia in asymptomatic MSM who are at high risk [10].
Yet, gonorrhea and chlamydia testing rate remains low among MSM in China [11], where studies show that less than 50% of MSM have ever been tested [12, 13]. Factors that contribute to poor gonorrhea and chlamydia testing among Chinese MSM include poor government funding for testing programs [14], inadequate linking of STI testing to existing HIV testing systems [15, 16], and insufficient MSM community involvement in STI efforts other than HIV. First, few government incentives exist to support the additional training and laboratory technology associated with regular gonorrhea and chlamydia testing [14]. Second, while many HIV testing programs for key populations have began to offer syphilis testing, most are still not set up to facilitate comprehensive STI testing [15, 16]. Third, MSM community organizations have had an increasing yet still limited role in China’s HIV/STI response [17–19]. Most community-based outreach programs prioritize HIV, with few focused on gonorrhea or chlamydia [20].
In light of this need, several community organizations collaborated with public health staff to organize a gonorrhea and chlamydia testing pilot program using a pay-it-forward strategy [21]. In pay-it-forward, one person is offered a gift (e.g., a free test), then they are asked whether they would like to give a gift to another person [22, 23]. Pay-it-forward has been studied in behavioral economics as a form of consumer elective pricing, defined as an economic transaction that allows individuals to purchase goods or services for any price (including zero) [24]. Pay-it-forward chains of giving is a type of cooperative behavior that can take place between known or anonymous individuals [25]. In social science experiments, pay-it-forward has been shown to promote generosity and engage group cohesiveness [22, 24].
One pilot study found that pay-it-forward increased gonorrhea/chlamydia testing 19-fold, with gonorrhea/chlamydia testing received by 53.7% (109/203) in the pay-it-forward group and 5.9% (12/205) in the standard of care group [21]. However, this was not a randomized controlled trial (RCT) and the study only compared two groups of individuals. We will evaluate the pay-it-forward model using a three-arm cluster randomized controlled trial. The purpose of this trial is to evaluate a pay-it-forward intervention for increasing gonorrhea and chlamydia testing among Chinese MSM.
Methods/design
Specific aims
Aim 1: To compare the effectiveness of a pay-it-forward program to standard of care in promoting gonorrhea/chlamydia testing among Chinese MSM. Hypothesis: Pay-it-forward is superior to the standard of care in promoting gonorrhea/chlamydia testing.
Aim 2: To compare the effectiveness of a pay-what-you-want program to standard of care in promoting gonorrhea/chlamydia testing among Chinese MSM. Hypothesis: Pay-what-you-want is superior to the standard of care in promoting gonorrhea/chlamydia testing.
Aim 3: To determine the gonorrhea/chlamydia cost per test and the cost per case diagnosed within the three trial arms. Hypothesis: Pay-it-forward is associated with lower cost per test and lower cost per case diagnosed.
Methods
Trial design and timeline
This study will be a three-arm cluster randomized controlled superiority trial, with the main comparisons being pay-it-forward vs standard of care and pay-what-you-want vs standard of care. Men will be randomly assigned in a 1:1:1 ratio into three arms. The three arms of the study will include: (1) a pay-it-forward arm in which men are offered free gonorrhea and chlamydia testing and then given the option to donate (“pay it forward”) toward testing for future testers; (2) a pay-what-you-want arm in which men are offered free testing and told to decide how much to pay after receiving the test; and (3) a standard of care arm in which men can pay the full price for dual gonorrhoea and chlamydia testing (RMB 150, or USD 22). Men will be recruited at three sites: two hospital-based MSM sexually transmitted disease (STD) clinics in Guangzhou and one MSM community-based organization in Beijing.
Following a cluster randomized design, every cluster of ten participants in each of the three study sites will be randomly allocated into one of the three arms independently: pay-it-forward arm, pay-what-you-want arm, or the standard of care arm. The rationale for choosing ten participants per cluster as the unit of randomization rather than individual participants is to minimize contamination of intervention across individuals and to facilitate cooperation with clinic staff. Gonorrhoea/chlamydia testing is performed immediately following each trial arm. Gonorrhoea/chlamydia test uptake will be ascertained by assessing administrative records. Eligible men will also complete a single survey (see Additional file 2). No follow-up surveys will be conducted in this study.
This study will span approximately 3 months. The first month will consist of RCT preparations and a pilot for the RCT. The following period will consist of RCT recruitment and implementation. Recruitment will continue at all sites following a cluster randomization process until the pre-determined sample size is reached - a process that is expected to take approximately 1.5 months. The study will be managed by the Social Entrepreneurship to Spur Health (SESH) group, a multi-sectoral implementation research collaboration led by the Southern Medical University Dermatology Hospital and the University of North Carolina Project-China.
Study setting and recruitment
Guangzhou and Beijing are large metropolitan areas with high burdens of STDs and HIV among MSM. The RCT will enroll from an STD clinic and a local MSM community-based organization (CBO) sites that provide free HIV testing for MSM. The STD clinic site operates within an ongoing hospital-based program; the CBO testing site operates within an ongoing community-based program. All sites already provide free HIV testing to MSM, and are staffed by MSM volunteers, nurses, and public health staff. There are no clinical doctors at either site. The staff at each site is responsible for handling blood draws, testing, results reporting, and follow-up of HIV tests. All sites will follow the same study procedures.
Eligibility criteria
Potential participants must first agree to a print informed consent in order to participate. Inclusion criteria are: born biologically male, age 16 years or older, report previous anal sex with another man, never participated in the pay-it-forward program, and report one of the following with regards to gonorrhoea/chlamydia testing history: not tested for both gonorrhoea and chlamydia in the past 12 months, report testing for both gonorrhoea and chlamydia in the past 12 months with high-risk sexual behaviour following testing, report previous testing for either or both gonorrhoea and chlamydia but does not recall whether tested within the past 12 months. All eligible participants must provide a working unique mobile phone number or WeChat account identification to be enrolled. These will be used only for results notification.
Interventions
Pay-it-forward arm
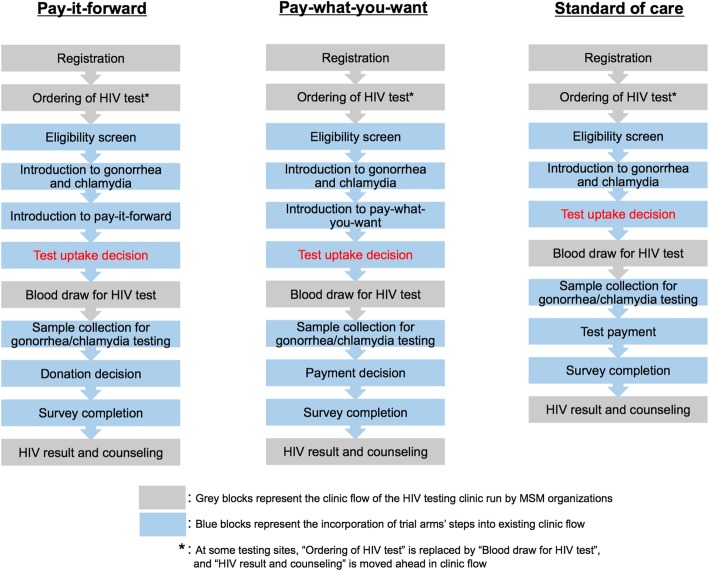
The pay-it-forward (PIF) program is developed using crowdsourcing. An open challenge contest [26] developed the name and format of the program materials. Hand-written postcards from previous participants in the program will encourage testing among future participants. Community volunteers will provide inputs to the program. At all program sites, MSM will be offered dual gonorrhea/chlamydia testing during their appointments for HIV testing (Fig. 1). First, all men will be provided a brief (5 min) introduction to gonorrhea and chlamydia testing using a pamphlet (see Additional file 3). Program organizers will then explain the pay-it-forward program to each man using a pamphlet (see Additional file 4). Briefly, men will be told that the hospital list price of gonorrhea and chlamydia testing is RMB 150 (USD 22), and that previous men attending the clinic had donated money to cover the cost of this test. Thus, each man could receive gonorrhea and chlamydia testing for free, then decide whether to donate money (pay-it-forward) for future men to have the same option. Men will be assured that donating is voluntary and told to pay any amount as they desire. A combination of initial research funding and donations from previous participants will be used to cover each man’s gonorrhea and chlamydia testing fees. Men will also be shown a postcard with a message written in simplified Chinese characters by a previous pay-it-forward contributor and told that they could also write a postcard message for a future participant. Men will then decide whether or not to receive combined gonorrhea and chlamydia testing. Regardless of their decision to test, men will be asked to fill out a brief survey about their sexual history, testing history, attitudes toward the testing program, and psychosocial conditions.
Fig. 1.
Schematic of three trial arms. The block diagrams describe the implementation steps for each arm
MSM who decide to test will be asked about their sexual practices and will be advised to consider receiving urine, anal, or both urine and anal dual gonorrhea/chlamydia testing. All participants will be advised that their information will be kept confidential, and that the results will be sent to them after approximately 1 week via WeChat, a phone- and computer-based instant messaging application. Men who agree to participate will choose to collect the sample on their own or have a staff member assist with sample collection [27]. After sample collection, men will decide whether or not to donate money to the next group of participants. Men can choose to leave donations for future participants with the program organizers via either cash or WeChat’s monetary transfer functionality. Each man will then be invited to write a message on a postcard for future pay-it-forward participants.
Program organizers will inform participants of their test results via WeChat. Samples collected in Guangzhou will be stored at room temperature overnight and then transported to Southern Medical University Dermatology Hospital in Guangzhou for laboratory testing within 1 week. Samples collected in Beijing will be shipped in insulated shipping container to Southern Medical University Dermatology Hospital for laboratory testing within 1 week. Patients who test positive will be counseled and directed to the WeChat page of the designated partnering hospital in each city, where they are able to make an appointment to receive treatment and follow-up.
Pay-what-you-want arm
Pay-what-you-want (PWYW) is a well-documented pricing strategy where the buyers decide and pay their desired amount for a given product or service, which is usually consumed by themselves [24]. Financially, pay-what-you-want is identical to pay-it-forward pricing: the customers choose the price they want to pay for a certain good or service, and the seller receives the payments. Socially, pay-what-you-want is different in that the customer is paying for him- or herself, while in pay-it-forward the customer is paying on behalf of someone else. Just as the pay-it-forward arm, MSM will be offered dual gonorrhea/chlamydia testing during their appointments for HIV testing. First, all men will be provided a brief (5 min) introduction to gonorrhea and chlamydia testing using a pamphlet. Program organizer will then explain the pay-what-you-want program to each man separately using verbal descriptions. Briefly, men will be told that the hospital list price of gonorrhea and chlamydia testing was RMB 150 (USD 22). Then they will be told that if they were to test today, they can participate in the pay-what-you-want program, in which they receive the test first and then decide how much they would like to pay. Men will be assured that payment is completely optional and advised to pay any amount that is desired by them. Men will then decide whether or not to receive urine, anal, or both urine and anal dual gonorrhea/chlamydia testing. Each man’s gonorrhea and chlamydia test fees will be covered by a combination of the initial research funding from the program organizers and payments from participants.
All participants will be ensured that their information will be kept confidential. Procedures for sample collection, sample processing, payment method, results reporting, and treatment linkage will be identical to those of the pay-it-forward arm.
Standard of care arm
Among men assigned to the standard of care arm, gonorrhea and chlamydia testing will be offered at the usual hospital list price (RMB 150, USD 22). All men will be provided a brief introduction about gonorrhea and chlamydia testing using the same pamphlet, but will not be introduced to other content of the above arms. Procedures for sample collection, sample processing, payment method, results reporting, and treatment linkage will be identical to those of the pay-it-forward arm.
Randomization and allocation
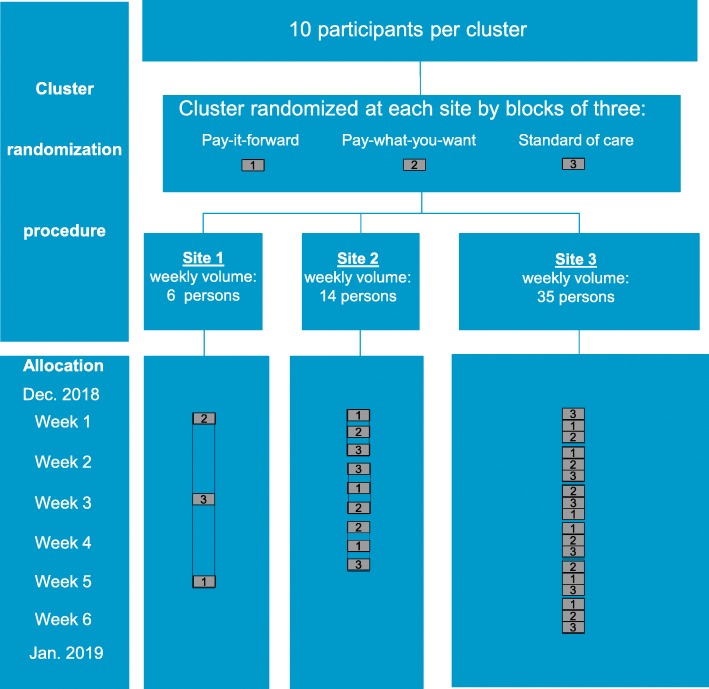
At each site, men will be assigned to one of the three arms through a cluster randomization procedure with ten participants per cluster (Fig. 2). To ensure equal number of clusters in each of the three arms, the clusters will be randomized in a block of three at each study site. The schedule will be determined prior to start of the study and then provided to each of the sites. STATA 15.1 software (StataCorp, StataCorp LLC, College Station, Texas) will be used to create the allocation sequence [28]. Ten blocks will be generated in total. Each block has three clusters consisting of pay-it-forward, pay-what-you-want, and standard of care, randomly created from all possible permutations.
Fig. 2.
Schematic of cluster randomization procedure and sample allocation. This schematic diagram illustrates the cluster randomization procedure, average volume of testers who visit the study site for HIV tests during a typical week, and allocation. There are 30 clusters and 300 total participants, which satisfy the sample size. The sequence shown is for illustration and is not the actual allocation sequence
Each eligible individual who presents to the site during a given 10-person cluster will be provided the allocated program. This RCT is not blinded. Participants will be able to determine their assignment based on whether they received intervention materials during the study period. Study organizers will be aware of randomization assignment because intervention materials provided to participants.
Laboratory testing
All urine and anal samples will be analyzed using Cobas 4800 CT/NG DNA detection kits (Roche Diagnostics, Basel, Switzerland) at Southern Medical University Dermatology Hospital. At the two MSM STD clinic sites, HIV testing will be performed using the Diagnostic Kit for Antibody to HIV ½ (Abon Pharmaceuticals, Northvale, New Jersey, USA) and syphilis testing, for those that choose to test, will be performed using the Treponema Pallidum Antibodies Rapid Test (Abon Pharmaceuticals, Northvale, New Jersey, USA). At the CBO site, HIV testing will be performed using the Third Generation Diagnostic Kit for Antibody to HIV rapid test (InTec, Xiamen, China) and syphilis testing, for those choosing to test, will be performed using the Syphilis Antibody (anti-Treponema Pallidum) rapid test (InTec, Xiamen, China).
Sample size
Sample size calculation (Table 1) is informed by a quasi-experimental study conducted in 2017–2018 at two similar study sites [21] and by a pilot study for the RCT conducted from October to November 2018. In the quasi-experimental study, gonorrhea/chlamydia testing was 53.7% in the pay-it-forward group and 5.9% in the standard of care group. In the pilot study for the RCT (Table 2), gonorrhea/chlamydia testing was 88% in the pay-it-forward group and 42% in the pay-what-you-want group. Based on these results and pilot data for the RCT, we assume that in the RCT we will have 54% test uptake in the pay-it-forward group, 42% test uptake in the pay-what-you-want group, and 6% in the standard of care. For the pay-it-forward uptake estimate, we chose to use the quasi-experimental study results (54%) instead of the pilot results (88%) because the quasi-experimental study was considered more similar in nature to our RCT, given that the pilot focused primarily on implementation optimization.
Table 1.
Sample size calculations
| Pay-it-forward and standard of care | ICC | 0.01 | 0.05 |
| Proportion in control group | 0.06 | 0.06 | |
| Superiority margin | 0.2 | 0.2 | |
| Proportion in intervention group assuming H0 | 0.26 | 0.26 | |
| Proportion in intervention group assuming H1 | 0.54 | 0.54 | |
| Difference at which power is calculated | 0.48 | 0.48 | |
| Number of people per cluster | 10 | 10 | |
| Alpha | 0.05 | 0.05 | |
| Power | 0.8 | 0.8 | |
| Total sample size per arm | 30 | 40 | |
| Number of clusters per arm | 3 | 4 | |
| Pay-what-you-want and standard of care | ICC | 0.01 | 0.05 |
| Proportion in control group | 0.06 | 0.06 | |
| Superiority margin | 0.2 | 0.2 | |
| Proportion in intervention group assuming H0 | 0.26 | 0.26 | |
| Proportion in intervention group assuming H1 | 0.42 | 0.42 | |
| Difference at which power is calculated | 0.36 | 0.36 | |
| Number of people per cluster | 10 | 10 | |
| Alpha | 0.05 | 0.05 | |
| Power | 0.8 | 0.8 | |
| Total sample size per arm | 80 | 110 | |
| Number of clusters per arm | 8 | 11 |
ICC Intra-class correlation
Table 2.
Two piloted arms and the historical standard of care
| Arms | Standard of carea | Pay-it-forward | Pay-what-you-want |
|---|---|---|---|
| Days piloted | 52 | 4 | 5 |
| Number of MSM offered intervention | 205 | 17 | 26 |
| GC/CT test uptake | 12 (6%) | 15 (88%) | 11 (42%) |
| Number of MSM who contributed any amount | NA | 15 (100%) | 10 (91%) |
| Total contribution amount | NA | RMB 833.88 (USD 125.08) | RMB 465.99 (USD 69.90) |
| Average contribution Amount per tester | NA | RMB 55.59 (USD 8.34) | RMB 42.36 (USD 6.35) |
| Gonorrhea positive | NA | 0 | 0 |
| Chlamydia positive | NA | 2 | 1 |
MSM Men who have sex with men
GC/CT Gonorrhea and chlamydia
NA Not applicable
aHistorical standard of care data from a quasi-experimental study between March 11th and May 1st, 2018
We will have three arms -- pay-it-forward, pay-what-you-want, standard of care, with numbers of clusters at a ratio of 1:1:1. The trial between pay-it-forward and standard of care will be testing superiority in promoting gonorrhea/chlamydia test uptake at a superiority margin of 0.2 (20% difference in percentage agreeing to test in those two arms). This margin was chosen as a clinically significant difference in test uptake based on results from a modeling study [29]. Similarly, the trial between pay-what-you-want and standard of care will be also testing superiority with the same margin of 0.2. Assuming we will have three equal sized arms, with cluster size of 10 per cluster, alpha of 0.05, power of 0.8, inter class correlation of 0.01 or 005, we will at most need eight or 11 clusters per arm, respectively (Table 1). To integrate the sample size calculations and to consider practicality, the final sample size would be ten clusters in pay-it-forward, ten clusters in pay-what-you-want, and ten clusters in standard of care, with a total cluster size of 30 (300 participants).
Quality control
We will examine data collected from all participants. We will deduplicate participants by examining cell phone number and WeChat handle. The primary outcome for this study is uptake of dual gonorrhea/chlamydia testing by administrative record as a single outcome. For the pay-it-forward arm, we will record the amount that each participant donates to future participants. For the pay-what-you-want arm, we will record the amount that participants pay for their own test.
Information on socio-demographics, sexual behaviors, HIV/STI testing history, and psychosocial conditions will be collected using a standardized survey instrument. Socio-demographic characteristics include participants’ age, ethnicity, marital status, highest level of education completed, place of residency (urban or rural), and annual income level. Sexual behaviors and HIV/STI testing history include previous anal sex with men, role during anal sex, number of sex partners, condom use, sexual orientation disclosure, endorsement of STI symptoms, and testing history for HIV, gonorrhea, and chlamydia. Psychosocial variables include adapted measures for community engagement [30], community connectedness [31], identify fusion [32], and social cohesion [33, 34]. For all participants, we will collect HIV test results, as well as gonorrhea and chlamydia results for those who tested. We will also collect information on reasons for accepting or declining gonorrhea and chlamydia tests. For men assigned to the pay-it-forward group, we will also ask about perceived benefits and barriers of the pay-it-forward program. Upon completion of the study survey, participants will receive a pen valued approximately one USD.
Outcomes
The main outcome of this study will be gonorrhea and chlamydia testing by PCR as assessed by administrative records. The choice of diagnostics is based on World Health Organization and US CDC guidelines recommending regular gonorrhea/chlamydia testing among MSM with risks [9, 10]. Secondary outcomes will include incremental cost per test, incremental cost per diagnosis, community connectedness, and social cohesion. Effect modification analysis will include community engagement. Existing literature on community engagement [30], community connectedness [31, 32], and social cohesion [33, 34], informed the development of adapted scales in this study. Community engagement is measured using an adapted six-item yes/no scale used in a previous study on Chinese MSM [30]. A six-item community connectedness scale [31] and a seven-item social cohesion scale [33, 34] ask participants to rank their level of disagreement and agreement with each item using answer choices “Strongly Agree,” “Agree,” “Disagree” and “Strongly Disagree”. All three community scales were adapted to the local context and piloted among 43 Chinese MSM before RCT recruitment. Survey items are detailed in Additional file 2.
Confidentiality
Survey data will be collected partially from online-based questionnaire and then stored on a secure online survey platform (Wenjuanxing), and partially from paper-based questionnaire. Electronic data will be transmitted using 128-bit encryption across the Internet and mobile data networks. Paper-based data will be entered by research staff members on the same day as data collection. Responses to surveys, including participant mobile phone numbers and WeChat account numbers, will be stored on a secure server (electronic) and in a locked cabinet (offline), which can be accessed with login information known only to the research team.
Monitoring
A data monitoring committee will not be formed for this RCT because potential for harm to participants is minimal. If any person feels they have experienced an adverse event or unwanted effect from participating in this RCT, they can withdraw at any time. A telephone number and WeChat account will be provided to participants to contact the primary investigator with questions or concerns.
Data analysis
Primary analysis
Socio-demographic characteristics will be summarized using descriptive statistics. The gonorrhoea/chlamydia test uptake rate will be calculated for each of the three arms. Generalized estimating equations will be used to account for potential correlation in outcomes due to clustering. The primary analysis will evaluate the hypotheses that the pay-it-forward intervention is superior to standard of care and that the pay-what-you-want intervention is superior to standard of care. The trial between pay-it-forward and standard of care will be testing superiority in promoting test uptake at a superiority margin of 20% in percentage agreeing to test in those two arms [29]. The trial between pay-what-you-want and standard of care will be testing with the same 20% margin. One-sided 95% confidence interval will be computed for pay-it-forward vs standard of care and for pay-what-you-want vs standard of care, and then compared to the superiority margin.
Subgroup analysis
Effect modification analyses will evaluate whether the effect of the pay-it-forward intervention on gonorrhoea/chlamydia test uptake varied in relation to the following factors: (1) younger age (less than 28 years old, 28 or older); (2) community-level constructs: community engagement (high or low level), community connectedness (high or low level), social cohesion (high or low level). For each factor of interest, three measures of association will be calculated: A) a crude measure of association between treatment group and test uptake, B1) a measure of association between treatment group and test uptake among all participants who report the factor of interest (younger age, or high level of a community-level construct), B2) a measure of association between treatment group and test uptake among all participants who deny a history of having the factor of interest (younger age, or high level of a community-level construct). Effect modification will be deemed present when B1 and B2 are different from one another, and at least one (B1 or B2) is different from A.
Missing data plan
Men will only be involved at a single time point and so there is no loss to follow up. However, there may be missing data in the primary and secondary outcomes. If the primary outcome is missing for < 15% of participants, analyses will use a complete-case approach. If an outcome is missing for ≥15% of participants, missingness mechanism will be investigated and multiple imputation will be used if suitable.
Secondary analyses
Within each arm of the study, we will calculate the incremental cost per test and the incremental cost per diagnosis. Cost will be collected from the perspective of the program provider. To estimate the program cost, we will identify the cost of each program element, such as personnel, facilities, equipment, and materials, and categorize costs into start-up costs, fixed costs, and variable costs. Start-up costs are incurred in the period between the decision to implement this trial and the start of trial recruitment (e.g. personnel training, testing sites coordination, pilot study). Fixed costs remain constant during the trial period regardless of the number of people served (e.g. administration, durable goods, and equipment). Variable costs are those that vary with the number of participants served (e.g. recruitment, counselling and testing, and nondurable goods and supplies). Personnel costs will be calculated by multiplying the staff time associated with each program activity by the compensation received by the staff who perform these activities. We will first calculate the total cost for each group, then divide these costs by the number of men tested and by the number of cases diagnosed with gonorrhea or chlamydia.
Pilot study results summary
To inform the development of this randomized controlled trial, the pay-it-forward and pay-what-you-want arms were evaluated through a pilot study conducted from October 2018 to November 2018 (Table 2).
A total of 43 eligible MSM were recruited at the selected study sites for the RCT. Recruitment took place on 9 days interspersed between 13 October and 15 November All men on a given day were assigned to either pay-it-forward or pay-what-you-want arm. In total, 17 men were recruited for the pay-it-forward arm over 4 days; 26 men were recruited for the pay-what-you-want arm over 5 days. Of these, 88% (15/17) of men received gonorrhea/chlamydia testing in the pay-it-forward arm and 42% (11/26) of men received gonorrhea/chlamydia testing in the pay-what-you-want arm. All men (100%, 15/15) in the pay-it-forward arm donated some amount, with average donation per participant of RMB 56. 91% (10/11) of men in the pay-what-you-want paid some amount, with average payment per participant of RMB 42. None were diagnosed with gonorrhea and three men were diagnosed with chlamydia.
These pilot results, along with results of a quasi-experimental study among 408 men [21], were used as the basis to inform sample size calculation. Through this pilot period, implementation steps and survey instrument were also optimized through an iterative process consisting of feedback from MSM participants, organizers, and community partners.
Discussion
This trial will assess the effectiveness of pay-it-forward intervention in promoting gonorrhoea and chlamydia testing among Chinese MSM, a marginalized population where sustainable strategies for integrated HIV/STD testing programs are urgently needed. Few studies have evaluated the use of pay-it-forward interventions focused on improving health, and none to our knowledge have used a RCT design. This three-arm cluster RCT will demonstrate pay-it-forward’s ability to be effective in the complex context of local settings. The pay-it-forward model includes both free or subsidized testing and a sense of social obligation to contribute to the health of other gay men. With comparison to the pay-what-you-want arm, a model that is financially similar to pay-it-forward but socially contrasting, there is an opportunity to explore the extent to which social and financial factors contribute to pay-it-forward health programs.
Although this trial will yield important insights for STD testing programming in China, some limitations exist. The RCT will be conducted at sites of HIV/syphilis testing for MSM. This limits our sample to MSM who are undergoing HIV/syphilis testing and are connected with community-based organizations. The level of trust that MSM hold toward sites in which pay-it-forward programs are embedded may influence program effectiveness. The testing sites in this study are long-standing programs and trusted by the MSM community. Findings may be limited in generalizability to settings that lack existing HIV testing services or trusted sites for MSM.
Our study will generate important research and policy implications regarding the use of pay-it-forward to preventative health services. The study outcomes will help guide policy and intervention practice of governmental departments and community-based organizations regarding the expansion of key population STD testing strategies. Moreover, practical knowledge gained from developing and implementing an innovative, pay-it-forward intervention may be applicable for future efforts to build self-sustaining STD testing programs.
Trial status
At the time of writing this article, RCT recruitment is ongoing. Data analysis for the RCT has not begun. The study is registered in the ClinicalTrials.gov database (NCT03741725). The database will also be used for documenting protocol modifications. The trial protocol conforms to the Standard Protocol Items: Recommendation for Interventional Trials (SPIRIT) 2013 statement.
Additional files
Multilingual abstracts in the five official working languages of the United Nations. (PDF 541 kb)
Survey instrument (English version). This is the English version of the survey for our study. (DOCX 74 kb)
Introduction to Gonorrhea and Chlamydia pamphlet (English version). This is the English version of the pamphlet used by study organizers to introduce Gonorrhea and Chlamydia to eligible men. (PPTX 779 kb)
Introduction to Pay-It-Forward pamphlet (English version). This is the English version of the pamphlet used by study organizers to introduce pay-it-forward to eligible men. (PPTX 311 kb)
Informed consent form (English version). This is the English version of the informed consent form for our study. (DOCX 47 kb)
Acknowledgements
We thank all study participants, CBO volunteers, and staff members at Social Entrepreneurship to Spur Health (SESH) Global, Zhitong Guangzhou LGBT Center, BlueD, and Southern Medical University Guangdong Provincial Center for Skin Diseases and STI Control who contributed.
Abbreviations
- CBO
Community-based organization
- CDC
US Center for Disease Control and Prevention
- H0
Null hypothesis
- H1
Alternative hypothesis
- LMIC
Low- and middle-income countries
- MSM
Men who have sex with men
- RCT
Randomized controlled trial
- SESH
Social Entrepreneurship to Spur Health Group
Authors’ contributions
JT, MA, LF, KL, TZ, FY, and WT conceived the study. TZ and FY led pilot and implementation optimization. TZ, FY, MA, LF, and NK participated in survey development. FY, FZ, and WT provided statistical expertise. LY, GM, YW, WH, AL, WZ, and DW assisted with recruitment. PV advised modeling approaches. FY, YW, AL, WZ, LY, and WT are leading implementation in Guangzhou. TZ, WH, and GM are leading implementation in Beijing. JT, NC, BY, and WT provided oversight. JT, MA, and LF wrote initial drafts of the paper. TZ redrafted and finalized the paper with inputs from FY, WT, NK, DW, and JT. All authors read and authorized the final version.
Funding
This study received support from the National Key Research and Development Program of China (2017YFE0103800), the National Institutes of Health (NIAID NIAID K24AI143471; 1UG3HD096929–01, NIA P30 (P30AG034420), UNC-South China STD Research Training Center (FIC 1D43TW009532–01), UNC Center for AIDS Research (NIAID 5P30AI050410), Doris Duke Charitable Foundation (International Clinical Research Fellowship to TZ), and SESH (Social Entrepreneurship to Spur Health) Global. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. The authors thank all people who contributed to this study.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Institutional Review Board (IRB) approval has been obtained from the following institutional ethical review boards prior to study enrollment: Southern Medical University Guangdong Provincial Center for Skin Diseases and STI Control (China), Yale University (USA), and University of North Carolina at Chapel Hill (USA). All participants will provide consent prior to taking part in the study (see Additional file 5).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Tiange P. Zhang, Email: tzhang3@luc.edu
Fan Yang, Email: fan.yang@med.unc.edu.
Weiming Tang, Email: weiming_tang@med.unc.edu.
Marcus Alexander, Email: marcus.alexander@yale.edu.
Laura Forastiere, Email: laura.forastiere@yale.edu.
Navin Kumar, Email: navin.kumar@bulldogs.yale.edu.
Katherine Li, Email: ktl2002@med.cornell.edu.
Fei Zou, feizou@email.unc.edu.
Ligang Yang, Email: yanglg3@hotmail.com.
Guodong Mi, Email: miguodong@blued.com.
Yehua Wang, Email: yehuawang@seshglobal.org.
Wenting Huang, Email: wenting@seshglobal.org.
Amy Lee, Email: amylee@live.unc.edu.
Weizan Zhu, Email: zhuweizan0926@gmail.com.
Peter Vickerman, Email: Peter.Vickerman@bristol.ac.uk.
Dan Wu, Email: wudan@seshglobal.org.
Bin Yang, Email: yangbin101@hotmail.com.
Nicholas A. Christakis, Email: nicholas.christakis@yale.edu
Joseph D. Tucker, Email: jdtucker@med.unc.edu
References
- 1.Chen XS, Peeling RW, Yin YP, Mabey DC. The epidemic of sexually transmitted infections in China: implications for control and future perspectives. BMC Med. 2011;9(1):111. doi: 10.1186/1741-7015-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang LG, Zhang XH, Zhao PZ, Chen ZY, Ke WJ, Ren XQ, et al. Gonorrhea and chlamydia prevalence in different anatomical sites among men who have sex with men: a cross-sectional study in Guangzhou, China. BMC Infect Dis. 2018;18(1):675. doi: 10.1186/s12879-018-3579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong FY, Huang ZJ, Wang W, He N, Marzzurco J, Frangos S, et al. STIs and HIV among men having sex with men in China: a ticking time bomb? AIDS Educ Prev. 2009;21(5):430–446. doi: 10.1521/aeap.2009.21.5.430. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53(4):537–543. doi: 10.1097/QAI.0b013e3181c3ef29. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349(9069):1868–1873. doi: 10.1016/S0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 6.Kent CK, Chaw JK, Wong W, Liska S, Gibson S, Hubbard G, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005;41(1):67–74. doi: 10.1086/430704. [DOI] [PubMed] [Google Scholar]
- 7.Detels R, Green AM, Klausner JD, Katzenstein D, Gaydos C, Handsfield HH, et al. The incidence and correlates of symptomatic and asymptomatic chlamydia trachomatis and neisseria gonorrhoeae infections in selected populations in five countries. Sex Transm Dis. 2011;38(6):503–509. [PMC free article] [PubMed] [Google Scholar]
- 8.Cecil JA, Howell MR, Tawes JJ, Gaydos JC, McKee JKT, Quinn TC, et al. Features of chlamydia trachomatis and neisseria gonorrhoeae infection in male army recruits. J Infect Dis. 2001;184(9):1216–1219. doi: 10.1086/323662. [DOI] [PubMed] [Google Scholar]
- 9.WHO . Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people. 2011. [PubMed] [Google Scholar]
- 10.CDC . Sexually transmitted diseases treatment guidelines 2015. 2015. [Google Scholar]
- 11.Wu D, Li KT, Tang W, Ong JJ, Huang W, Fu H, Lee A, Wei C, Tucker JD. Low chlamydia and gonorrhea testing rates among men who have sex with men in Guangdong and Shandong provinces, China. Sex Transm Dis. 2019;46(4):260–265. doi: 10.1097/OLQ.0000000000000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L, Nehl EJ, Tran A, He N, Zheng T, Wong FY. Sexually transmitted infection testing practices among ‘money boys’ and general men who have sex with men in Shanghai, China: objective versus self-reported status. Sex Health. 2014;11(1):94–96. doi: 10.1071/SH13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis A, Best J, Luo J, Van Der Pol B, Dodge B, Meyerson B, et al. Risk behaviours, HIV/STI testing and HIV/STI prevalence between men who have sex with men and men who have sex with both men and women in China. Int J STD AIDS. 2016;27(10):840–849. doi: 10.1177/0956462415596302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng N, Guo Y, Padmadas S, Wang B, Wu Z. The increase of sexually transmitted infections calls for simultaneous preventive intervention for more effectively containing HIV epidemics in China. BJOG. 2014;121(s5):35–44. doi: 10.1111/1471-0528.12999. [DOI] [PubMed] [Google Scholar]
- 15.Tucker JD, Yang L-G, Yang B, Zheng H-P, Chang H, Wang C, et al. A twin response to twin epidemics: integrated HIV/syphilis testing at sti clinics in South China. J Acquir Immune Defic Syndr. 2011;57(5):e106–ee11. doi: 10.1097/QAI.0b013e31821d3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong JJ, Fu H, Pan S, Smith K, Wu D, Wei C, et al. Missed opportunities for HIV and syphilis testing among men who have sex with men in China: a cross-sectional study. Sex Transm Dis. 2018;45(6):382–386. doi: 10.1097/OLQ.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neilands TB, Steward WT, Choi KH. Assessment of stigma towards homosexuality in China: a study of men who have sex with men. Arch Sex Behav. 2008;37(5):838–844. doi: 10.1007/s10508-007-9305-x. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Wu Z, Detels R. Evolution of MSM community and experienced stigma among MSM in Chengdu, China. J Acquir Immune Defic Syndr. 2010;53(Suppl 1):S98–103. doi: 10.1097/QAI.0b013e3181c7df71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Kuo NT, Liu H, Korhonen C, Pond E, Guo H, et al. From spectators to implementers: civil society organizations involved in AIDS programmes in China. Int J Epidemiol. 2010;39(Suppl 2):ii65–ii71. doi: 10.1093/ije/dyq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng W, Cai Y, Tang W, Zhong F, Meng G, Gu J, et al. Providing HIV-related services in China for men who have sex with men. Bull World Health Organ. 2016;94(3):222–227. doi: 10.2471/BLT.15.156406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li KT, Tang W, Wu D, Huang W, Wu F, Lee A, et al. Pay-it-forward strategy to enhance uptake of dual gonorrhea and chlamydia testing among men who have sex with men in China: a pragmatic, quasi-experimental study. Lancet Infect Dis. 2018;19:76–82. doi: 10.1016/S1473-3099(18)30556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang YS, Takahashi N. Network homophily and the evolution of the pay-it-forward reciprocity. PLoS One. 2011;6(12):e29188. doi: 10.1371/journal.pone.0029188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyde CR. Pay it forward. New York: Simon & Schuster; 1999. [Google Scholar]
- 24.Jung MH, Nelson LD, Gneezy A, Gneezy U. Paying more when paying for others. J Pers Soc Psychol. 2014;107(3):414–431. doi: 10.1037/a0037345. [DOI] [PubMed] [Google Scholar]
- 25.Gray K, Ward AF, Norton MI. Paying it forward: generalized reciprocity and the limits of generosity. J Exp Psychol Gen. 2014;143(1):247–254. doi: 10.1037/a0031047. [DOI] [PubMed] [Google Scholar]
- 26.Tucker JD, Fenton KA. Innovation challenge contests to enhance HIV responses. Lancet HIV. 2018;5(3):e113–e1e5. doi: 10.1016/S2352-3018(18)30027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yared N, Horvath K, Fashanu O, Zhao R, Baker J, Kulasingam S. Optimizing screening for sexually transmitted infections in en using self-collected swabs - a systematic review. Sex Transm Dis. 2017;45:294-300. [DOI] [PMC free article] [PubMed]
- 28.StataCorp . Stata statistical software: release 15. College Station: StataCorp LLC; 2017. [Google Scholar]
- 29.Xiridou M, Vriend HJ, Lugner AK, Wallinga J, Fennema JS, Prins JM, et al. Modelling the impact of chlamydia screening on the transmission of HIV among men who have sex with men. BMC Infect Dis. 2013;13:436. doi: 10.1186/1471-2334-13-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang TP, Liu C, Han L, Tang W, Mao J, Wong T, et al. Community engagement in sexual health and uptake of HIV testing and syphilis testing among MSM in China: a cross-sectional online survey. J Int AIDS Soc. 2017;20(1):21372. doi: 10.7448/IAS.20.01/21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frost DM, Meyer IH. Measuring community connectedness among diverse sexual minority populations. J Sex Res. 2012;49(1):36–49. doi: 10.1080/00224499.2011.565427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swann WB, Jr, Gomez A, Seyle DC, Morales JF, Huici C. Identity fusion: the interplay of personal and social identities in extreme group behavior. J Pers Soc Psychol. 2009;96(5):995–1011. doi: 10.1037/a0013668. [DOI] [PubMed] [Google Scholar]
- 33.Grover E, Grosso A, Ketende S, Kennedy C, Fonner V, Adams D, et al. Social cohesion, social participation and HIV testing among men who have sex with men in Swaziland. AIDS Care. 2016;28(6):795–804. doi: 10.1080/09540121.2015.1131971. [DOI] [PubMed] [Google Scholar]
- 34.Lippman SA, Donini A, Diaz J, Chinaglia M, Reingold A, Kerrigan D. Social-environmental factors and protective sexual behavior among sex workers: the Encontros intervention in Brazil. Am J Public Health. 2010;100(Suppl 1):S216–S223. doi: 10.2105/AJPH.2008.147462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multilingual abstracts in the five official working languages of the United Nations. (PDF 541 kb)
Survey instrument (English version). This is the English version of the survey for our study. (DOCX 74 kb)
Introduction to Gonorrhea and Chlamydia pamphlet (English version). This is the English version of the pamphlet used by study organizers to introduce Gonorrhea and Chlamydia to eligible men. (PPTX 779 kb)
Introduction to Pay-It-Forward pamphlet (English version). This is the English version of the pamphlet used by study organizers to introduce pay-it-forward to eligible men. (PPTX 311 kb)
Informed consent form (English version). This is the English version of the informed consent form for our study. (DOCX 47 kb)
Data Availability Statement
Not applicable.