Abstract
Rationale: Needle-free intranasal vaccines offer major potential advantages, especially against pathogens entering via mucosal surfaces. As yet, there is no effective vaccine against respiratory syncytial virus (RSV), a ubiquitous pathogen of global importance that preferentially infects respiratory epithelial cells; new strategies are urgently required.
Objectives: Here, we report the safety and immunogenicity of a novel mucosal RSV F protein vaccine linked to an immunostimulatory bacterium-like particle (BLP).
Methods: In this phase I, randomized, double-blind, placebo-controlled trial, 48 healthy volunteers, aged 18–49 years, were randomly assigned to receive placebo or SynGEM (low or high dose) intranasally by prime-boost administration. The primary outcome was safety and tolerability, with secondary objectives assessing virus-specific immunogenicity.
Measurements and Main Results: There were no significant differences in adverse events between placebo and vaccinated groups. SynGEM induced systemic plasmablast responses and significant, durable increases in RSV-specific serum antibody in healthy, seropositive adults. Volunteers given low-dose SynGEM (140 μg F, 2 mg BLP) required a boost at Day 28 to achieve plateau responses with a maximum fold change of 2.4, whereas high-dose recipients (350 μg F, 5 mg BLP) achieved plateau responses with a fold change of 1.5 after first vaccination that remained elevated up to 180 days after vaccination, irrespective of further boosting. Palivizumab-like antibodies were consistently induced, but F protein site ∅-specific antibodies were not detected, and virus-specific nasal IgA responses were heterogeneous, with the strongest responses in individuals with lower pre-existing antibody levels.
Conclusions: SynGEM is thus the first nonreplicating intranasal RSV subunit vaccine to induce persistent antibody responses in human volunteers.
Clinical trials registered with www.clinicaltrials.gov (NCT02958540).
Keywords: mucosal, respiratory, virus, clinical trial, immunology
At a Glance Commentary
Scientific Knowledge on the Subject
Respiratory syncytial virus (RSV) is a major global pathogen, especially affecting young children and older adults. Studies of natural and experimental infection indicate that mucosal antibodies are associated with protection from infection, but after RSV infection these are short lived, likely owing to viral immunomodulation. Despite the clear advantages of needle-free vaccines, the only currently available intranasal vaccine (live attenuated influenza vaccine) is known to be ineffective in adults with pre-existing immunity.
What This Study Adds to the Field
In this first-in-human phase I randomized, controlled trial, SynGEM (a novel subunit intranasal vaccine comprising empty bacterium-like particles [BLPs] linked with the surface glycoprotein F from RSV) is shown to be safe and immunogenic in healthy adults despite high pre-existing antibody levels. F protein BLP rapidly induces RSV-specific systemic and local nasal immune responses that are more long lasting than those that occur after natural infection, although antibodies unique to prefusion F protein were undetected and fold changes were modest. SynGEM is therefore the first nonreplicating intranasal RSV vaccine to induce persistent local and systemic antibodies, and the BLP platform has wide potential applications where mucosal immunity is desired.
Needle-free intranasal vaccines have major advantages over parenteral preparations, including public acceptability, reduced risk of complications, and, importantly, inducing local immune responses directed to the primary site of pathogen entry. However, existing intranasal vaccines are exclusively live attenuated agents that have poor efficacy in adults and must be balanced between immunogenicity and overattenuation (1, 2). Subunit intranasal vaccines might offer an effective alternative, but none has yet found a place on the market.
Respiratory syncytial virus (RSV) is a major global pathogen especially important in infancy and old age. In children under 5 years, it causes around 3 million severe cases, mostly in the developing world (3, 4). It is also responsible for approximately 10% of pneumonia admissions in older adults, with attributable mortality up to 5% (5, 6). Despite the clinical need, no effective RSV vaccine yet exists (7). Inadequate understanding of protective immunity against RSV means that vaccine development continues to carry major risks, as demonstrated by recent negative phase III clinical trials (8). Novel vaccination strategies are therefore urgently required.
Studies in experimentally infected volunteers indicate that reduced infection risk with RSV is most closely associated with mucosal secretory IgA (s-IgA) (9). IgA is actively transported across the respiratory epithelium, and is therefore found at high levels in both upper and lower airways, mediating immune exclusion and sterilizing protection (10). Mucosal vaccine delivery may preferentially induce these antibodies. SynGEM is a novel subunit vaccine designed for intranasal administration, comprising RSV F protein linked to a peptidoglycan bacterium-like particle (BLP) derived from Lactococcus lactis (11). F protein is highly conserved across RSV strains (12), and is the target of the licensed protective monoclonal antibody palivizumab, making it the preferred antigenic target. Its prefusion conformation (pre-F), which predominates on infectious virions, displays a distinct antigenic site (site Ø) preferentially targeted by the most potent neutralizing antibodies (13). SynGEM therefore incorporates an F protein with mutations to maintain a prefusion conformation, whereas BLP conjugation enhances mucosal stimulation via TLR2-dependent adjuvantation (11).
Preclinical studies using SynGEM showed induction of high levels of both systemic and mucosal antibodies (11). We now present the findings of the first-in-human, placebo-controlled, phase I clinical trial of SynGEM (clinicaltrials.gov identifier NCT02958540), the aim of which was to assess the safety and tolerability of the vaccine in humans and analyze the levels of serum anti-RSV IgG, nasal IgA, and B cell responses.
Methods
Study Design
Healthy volunteers were recruited to take part in the double-blind, placebo-controlled study, MUC-SynGEM-001, according to the inclusion and exclusion criteria in Table E1 in the online supplement. A total of 48 volunteers received either placebo (PBS + 2.5% glycerol) or SynGEM vaccine administered intranasally (125 μl per nostril) at a ratio of 1:3. Heat and acid were used to treat nonrecombinant gram-positive L. lactis, degrading internal proteins and other bacterial components to leave particles with bacteria-like shape and size made up of peptidoglycan alone. Addition of a Protan tag to the recombinant RSV F protein allowed covalent binding to the peptidoglycan shell on mixing to form SynGEM. The vaccine was given at two dose levels; a low dose, containing 140 μg of F protein and 2 mg of BLP, and a high dose, containing 350 μg of F protein and 5 mg of BLP. These were administered according to a prime-boost schedule with the boost vaccination at 28 days after prime. The sample size of 18 vaccinees at each dose level was computed from the binomial distribution to result in a 98% probability of one or more adverse events (AEs) being observed with a true AE incidence of 20%, and 84% probability with a true incidence of 10%. Blood and nasal lavage were collected at study visits up to 180 days after first dosing. Nasal lavage was performed, as previously described (9), by introducing 5 ml of normal saline into each nostril using a syringe attached to a nasal olive attachment and washing by alternately withdrawing and advancing the plunger of the syringe 10 times while maintaining a tight seal with the nostril. The study was overseen by an independent data safety monitoring committee.
Randomization and Masking
Dose cohort allocation was sequential to the low- and then the high-dose group. Participants were assigned to receive either SynGEM vaccine or an identical placebo by block randomization; at each dose level, sentinel cohorts were randomized in the ratio of 1:1 in two blocks of two, and remaining participants were randomized in the ratio of 4:1 in eight blocks of five, via a randomly generated sequence using an integer seed in the range 21–2,147,483,649. An unmasked research nurse (with no subsequent involvement in participant follow-up) prepared and administered the vaccine in a masked syringe with a Vaxinator device. Investigators and participants were masked to vaccine allocation until 28 days after boost vaccination. Laboratory staff was blinded for all time points (including Days 120 and 180). Sealed, opaque envelopes were provided for emergency code break, but none was used.
Antibody Assays
Anti-RSV IgG and IgA antibodies were measured using stabilized pre-F or unstabilized F protein or Ga (from RSV A) or Gb (from RSV B) protein in ELISA assays, as previously described (9). Serum plaque reduction neutralization titer assays were performed at Viroclinics Biosciences, as previously described (14). Palivizumab- and D25-competing antibodies were quantified in serum by competition ELISA. See the online supplement for additional details.
Antibody-Secreting Cell Quantification
Flow cytometry analysis was performed using heparinized whole blood with anti-CD19 fluorescein isothiocyanate, anti-CD27 APC, anti-CD38 phycoerythrin (PE), and anti-CD3/anti-CD20 both on PE-CF594 (BD Biosciences) run on a Fortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software. Antibody-secreting cells (ASCs) were quantified using enzyme-linked immunospot (ELISpot) assays as previously described (15). Spots were counted using an automated ELISpot reader (AID), and results are expressed as spot-forming cells per million peripheral blood mononuclear cells.
Measurement of Antibodies and Cytokines in Adenotonsillar Cell Culture Supernatants
Adenotonsillar tissues were obtained from a separate cohort of nonvaccinated patients undergoing elective tonsillectomy from whom informed consent was obtained (ethics reference 14/SS/1058). Mononuclear cells (MNCs) were isolated from adenotonsillar tissues and cultured, as described previously (16). Adenotonsillar MNCs were cocultured with SynGEM BLP-F with F protein concentration at 1 μg/ml or 5 μg/ml, BLP alone (25 μg/ml) and F protein alone (1 μg/ml), or medium. Cell culture supernatants were harvested at Day 12 and F protein–specific antibodies were measured by ELISA, as described previously (17).
After stimulation of adenotonsillar MNCs for 3 days with the SynGEM BLP-F (5 μg/ml), culture supernatants were analyzed using cytometric bead array for cytokines (LEGENDplexTM; Biolegend) following the manufacturer’s instructions. T cell responses in adenotonsillar MNCs were analyzed by carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) labeling as previously described (16, 18).
Statistical Analysis
Data analyses and graphs were produced using the software R and Graphpad Prism. Additional details on the statistical analysis are provided in the online supplement.
Results
SynGEM Vaccination Is Generally Safe and Well Tolerated
A total of 79 individuals were potentially eligible after prescreening (Figure E1). Of these, 48 were recruited according to protocol-defined inclusion and exclusion criteria (Table E1).
No significant differences were found in demographics, baseline medical history, or physical examination among the vaccinated or placebo groups (Table 1). At each dose level, two sequential sentinel cohorts of two subjects each were recruited initially, “prime”-vaccinated and followed up for 3 days after dosing. No predefined pausing rules (Table 2) were met, and recruitment was subsequently extended to the remaining 20 subjects in each dose-level group. All predefined study endpoints were adhered to (Table E2). Over the course of the study, no significant differences were seen in routine hematology and biochemistry blood tests between the vaccinated and placebo groups (Table E3).
Table 1.
Subject Baseline Physical and Demographic Characteristics
| Characteristic | Low Dose (n = 18) | High Dose (n = 18) | Placebo (n = 12) |
|---|---|---|---|
| Age, yr | |||
| Mean (SD) | 28.6 (7.99) | 27.3 (8.37) | 28.3 (8.55) |
| Median | 27.0 | 23.0 | 26.5 |
| Minimum–maximum | 20–49 | 19–46 | 20–46 |
| Sex, n (%) | |||
| M | 11 (61.1) | 8 (44.4) | 5 (41.7) |
| F | 7 (38.9) | 10 (55.6) | 7 (58.3) |
| Race, n (%) | |||
| White | 12 (66.66) | 15 (83.33) | 10 (83.33) |
| Black British/black other | 3 (16.66) | 1 (5.55) | 1 (8.33) |
| Asian British/Indian/Asian other | 3 (16.66) | 2 (11.11) | 1 (8.33) |
| Mixed | 0 | 0 | 0 |
| Other | 0 | 0 | 0 |
| Height, cm | |||
| Mean (SD) | 175.24 (8.96) | 172.94 (10.09) | 171.67 (9.54) |
| Median | 174.0 | 170.5 | 170.0 |
| Weight, kg | |||
| Mean (SD) | 72.40 (11.33) | 69.48 (11.76) | 69.51 (12.38) |
| Median | 71.8 | 71.57 | 68.8 |
| Body mass index, kg/m2 | |||
| Mean | 23.50 (2.68) | 23.12 (2.62) | 23.54 (3.31) |
| Median | 23.65 | 23.2 | 23.56 |
Table 2.
Pausing Rules
| Applicable to sentinel cohorts |
| 1. Occurrence of any death |
| 2. Occurrence of any serious adverse event, defined as life threatening, requiring hospitalization, resulting in a persistent or significant disability/incapacity, a congenital anomaly or birth defect in the offspring of a study participant, or a medically important condition that may have jeopardized the subject, and may have required medical or surgical intervention to prevent a serious outcome |
| 3. Occurrence of any case of severe allergic reaction, such as anaphylaxis, generalized urticaria, laryngospasm, or bronchospasm |
| 4. One or more subjects experience a severe (nonserious) adverse event, including local, febrile or systemic reactions |
| Applicable to post-sentinel cohorts |
| 1. Occurrence of any death |
| 2. Occurrence of any serious adverse event other than the result from trauma or accident, regardless of relatedness to study product |
| 3. Occurrence of any case of severe allergic reaction, such as anaphylaxis, generalized urticaria, laryngospasm, or bronchospasm |
| 4. Two or more subjects recruited up to that point experience a severe (nonserious) adverse event, defined as causing inability to perform usual social and functional activities, including local, febrile, or systemic reactions, considered at least possibly related to the investigational product |
| 5. Three or more subjects recruited up to that point experience a severe (nonserious) adverse event, irrespective of the relationship with the investigational product |
Five participants presented with respiratory tract symptoms at their vaccination or follow-up visits. On the basis of PCR-confirmed rhinovirus infection, boost vaccination was delayed by 3 days in one participant and withheld in another. Two additional participants described upper respiratory tract symptoms leading to delayed boost vaccination by 3 days. One subject was diagnosed with influenza A infection shortly after boosting. The severe sore throat reported as a serious AE (SAE) in this participant was temporally associated with the PCR-confirmed infection and therefore considered unrelated to the study vaccine.
One other SAE was noted, with a participant describing moderate pulsatile tinnitus and mild unilateral hearing loss manifesting 16 days after prime vaccination. This was not reported by the participant until after having undergone boost vaccination. The participant was assessed by an ear, nose, and throat specialist, but no clear etiology was determined. Owing to the timing of onset, the SAE was considered possibly related to the vaccine, but symptoms persisted unchanged to the end of follow-up.
Other AEs were most commonly local site reactions typical of intranasal administration (Tables E5 and E6). These events were all self-limiting and mild to moderate in severity. Four subjects in the low-dose group (22.2%), one in the high-dose group (5.5%), and four in the placebo group (33.3%) reported AEs within 1 hour of dosing, with no significant differences between groups (relative risk [RR] compared with placebo RR [95% confidence interval (CI)]: low-dose group, 1.0 [0.29–3.39]; high-dose group, 0.25 [0.03–2.02]; chi-square test for trend P = 0.051; Table E5). During the follow-up period, most participants reported at least one solicited local AE (15 [83.3%] in the low-dose group; 15 [83.3%] in the high-dose group; and 10 [83.3%] in the placebo group). Again, there were no significant differences between the groups (low-dose RR = 1.0 [0.72–1.39]; high-dose RR = 1.0 [0.72–1.39]; Chi-square test for trend P > 0.99; Table E6). Moderate AEs were recorded for the three participants who had concurrent rhinovirus or influenza infection. The median duration of after-vaccination symptoms was 1.25 days (range, 1–7 d).
Solicited systemic AEs were reported by 16 (88.9%) subjects in the low-dose group (RR = 1.19 [0.82–1.71]), 13 (72.2%) subjects in the high-dose group (RR = 0.96 [0.62–1.49]), and 9 (75.0%) subjects in the placebo group (Table E5). Again, there were no significant differences between groups and no increase in AEs after boost compared with prime (chi-square test for trend P = 0.73). Severe solicited systemic AEs were reported only in those with concomitant viral infections. Therefore, with the caveat of a single SAE of uncertain etiology, SynGEM was generally safe and well tolerated.
SynGEM Significantly Boosts F Protein–Specific Serum IgG
Because the study period overlapped with the local RSV season, natural RSV infection was assessed by measuring seroconversion of RSV G–specific IgG levels as well as multiplex respiratory viral PCR of nasal lavage if participants attended with suggestive symptoms. No RSV infections were detected by PCR, but a total of seven participants seroconverted with G protein–specific responses during the study period, suggestive of natural infection (Figure E2). Measurements from these individuals at time points after G protein seroconversion were excluded from subsequent analysis to avoid overestimation of antibody titers by infection-induced immune responses.
F-specific serum IgG titers over the course of the study were measured by ELISA with unstabilized F protein as coating antigen. Before vaccination, all individuals already had moderate-to-high levels of anti–F IgG (low-dose geometric mean titer [GMT; 95% confidence interval (CI)] = 8.1 [7.5–8.7]; high-dose GMT = 8.5 [8.1–8.9]; placebo GMT = 8.1 [7.6–8.7]) (Figure 1). There was a trend toward average baseline anti–F IgG being higher in the high-dose group (Mann Whitney test, placebo versus high P = 0.2804; placebo versus low = 0.8841; high versus low = 0.2173) (Figures 1A and 1B).
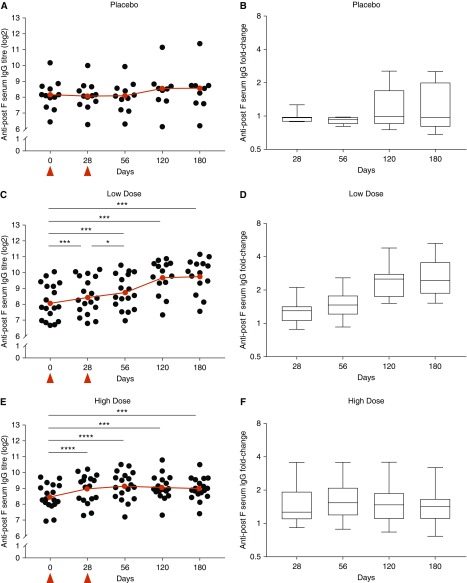
Figure 1.
Intranasal SynGEM induces significant increases in F-specific serum IgG. Volunteers were given SynGEM or placebo and serum IgG was measured by ELISA using unstabilized F protein as coating antigen at time points up to 180 days after “prime.” Titers (scatter plots) and fold changes (box-and-whisker plots) compared with baseline are shown after (A and B) placebo, (C and D) low dose, and (E and F) high dose. Geometric means are shown in red. Wilcoxon signed-rank test was used to test statistically significant rises compared with prevaccination; *P < 0.05, ***P < 0.001, and ****P < 0.0001. For box-and-whisker plots, boxes indicate the median and interquartile range and whiskers the range of the fold changes for each time point after vaccination. Vaccinations are indicated by red triangles.
After vaccination, anti–F IgG titres of both dosing groups increased significantly after the first dose, from a GMT of 8.1 to a GMT of 8.5 (low dose, P = 0.0005) (Figures 1C and 1D) and from a GMT of 8.5 to a GMT of 9.0 (high dose, P < 0.0001) (Figures 1E and 1F). In the low-dose group, this incremented further on boost vaccination (GMT = 8.5 at Day 28 to GMT = 8.8 on Day 56, P = 0.0108). Interestingly, anti–F IgG levels continued to increment to a GMT of 9.8 (P = 0.0001) at Day 180 (Figure 1E) after low-dose vaccination. In the high-dose group, peak anti–F IgG levels were achieved after a single vaccination with no further statistically significant increase. In both dosing groups, serum anti–F IgG titers remained significantly elevated through to the end of the follow-up period (6 mo after prime). Despite the significant increases in virus-specific serum IgG, maximal fold changes after vaccination were modest (2.43 at Day 120 in the low-dose group and 1.54 at Day 56 in the high-dose group), given the high prevaccination titers (Figures 1D and 1F). Nevertheless, F-specific antibodies were boosted in both vaccinated groups, with serum antibody levels persisting up to 6 months.
A second ELISA assay using a stabilized pre-F protein (DS-Cav1) was used to test whether additional prefusion-specific antibodies could be detected. Surprisingly, anti–F IgG titers measured using the pre-F antigen showed less statistically significant responses (Figure E3). The associated fold changes were also less marked than detection by the post-F assay. Thus, measurement of serum anti–F IgG titers by stabilized F and unstabilized F protein ELISAs did not give fully concordant results.
SynGEM Preferentially Induces Nonneutralizing Palivizumab-Competing Antibodies
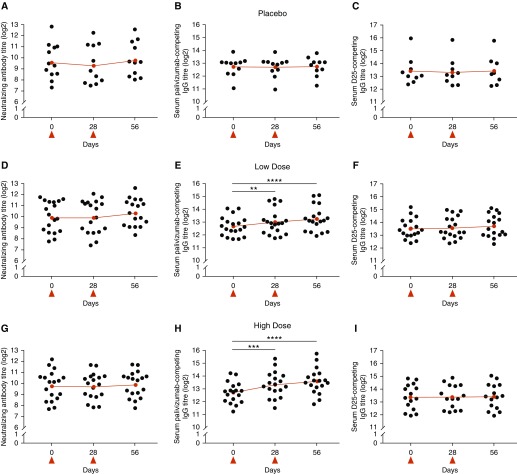
To further investigate the quality and functionality of the induced antibodies, serum neutralizing antibodies against RSV were measured by plaque-reduction neutralization titer assay (Figures 2A, 2D, and 2G). In contrast to the highly significant increases in post-F ELISA-binding antibodies, no increment in neutralizing antibodies was detectable. This implied that the systemic antibodies induced by SynGEM were preferentially nonneutralizing. To investigate this in more detail, competition ELISAs were performed to estimate the contribution of palivizumab-competing antibodies, which recognize the site II epitope present on both before- and after-fusion conformations of F (Figures 2B, 2E, and 2H), and D25-competing antibodies, which bind the site Ø unique to pre-F (Figures 2C, 2F, and 2I). As with the total F protein ELISA, significant increments in palivizumab-competing antibodies were shown after prime and boost (low-dose GMT = 12.7 [12.3–13.1] prevaccination to GMT = 13.3 [12.8–13.8] at Day 56, P < 0.0001; high-dose GMT = 12.7 [12.3–13.1] to GMT = 13.6 [13.1–14.1] at Day 56, P < 0.0001). In contrast, no rises were seen in D25-competing antibodies. Thus, SynGEM primarily induced anti–F IgG directed against epitopes common to both before- and after-fusion F, but little site Ø or neutralizing antibody.
Figure 2.
SynGEM induces palivizumab-like, but not prefusion F-specific (pre-F), antibodies. Participants were given SynGEM or placebo. (A, D, and G) Serum neutralizing antibody titers were measured by classical plaque-reduction neutralization assay up to 28 days after boost. (B, C, E, F, H, and I) Palivizumab-like (B, E, and H) and D25-like (C, F, and I) antibodies were measured by competition ELISA up to 28 days after boost. Geometric means are shown in red. Wilcoxon signed-rank test was used to test statistically significant rises compared with prevaccination; **P < 0.01, ***P < 0.001, and ****P < 0.0001. Vaccinations are indicated by red triangles.
Nasal Anti-RSV IgA Responses Demonstrate Marked Variance after SynGEM Vaccination
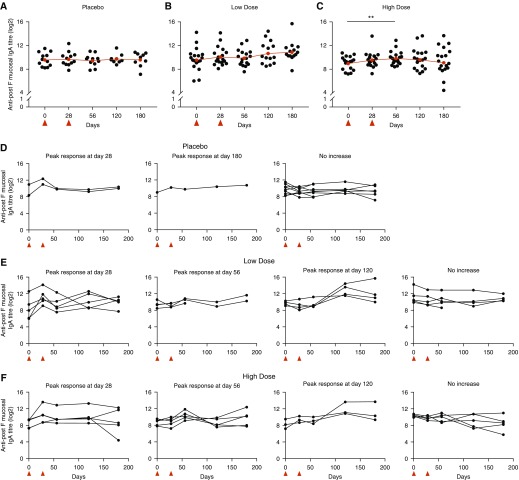
We hypothesized that intranasal delivery of RSV F protein BLPs could preferentially induce nasal s-IgA and therefore enhance local protection. Using a validated IgA ELISA, we therefore analyzed the induction of anti–F s-IgA in nasal lavage samples. Compared with serum anti–F IgG, there was greater interindividual variability in nasal IgA titers and response to vaccination. Before vaccination, nasal IgA endpoint titers showed a wide range, with the greatest variance seen in the low-dose group, within which the log2 titer ranged from a minimum of 6.0 to a maximum of 14.2. (Figures 3A–3C). After vaccination, analysis of each group in totality showed a significant titer rise in the high-dose group at Day 56 after vaccination (GMT = 9.9 at Day 56 compared with 9.0 prevaccination, P = 0.009). However, examining the individual participant-level data (Figures 3D–3F), it was evident that this masked the wide differences between individuals, both in magnitude and kinetics. We therefore performed cluster analysis of vaccinees according to the timing of their maximal nasal IgA fold change to further explore the diversity of responses.
Figure 3.
Intranasal SynGEM protein induces heterogeneous mucosal IgA responses. Subjects were given SynGEM or placebo and nasal wash IgA was measured by ELISA using unstabilized F protein as coating antigen at time points up to 180 days after “prime.” (A–C) Individuals in the placebo (A), low-dose (B), and high-dose (C) groups were clustered if they displayed a greater than twofold rise in nasal IgA titers according to the time point of maximal increase. (D–F) Cluster analysis of individual participant-level data was carried out by Fisher's exact test on placebo (D), low-dose (E), and high-dose (F) groups at the indicated time points after vaccination. **P < 0.01. Vaccinations are indicated by red triangles.
By Fisher’s exact test, a significantly higher proportion of individuals in the vaccinated cohorts underwent a twofold or greater rise at any time after vaccination than in the placebo group (P = 0.0236). Although some changes were seen in the placebo group, these were few and of low magnitude (Figure 3D). In contrast, in the low-dose group, 13/18 (72%) demonstrated a greater than twofold rise (five showing maximal change compared with prevaccination at Day 28, four at Day 56, and four at Day 120; Figure 3E). In the high-dose group, 13/18 (72%) also showed an increment (five changing maximally compared with prevaccination at Day 28, five at Day 56, and three at Day 120; Figure 3F). Furthermore, although maximal fold change in the placebo group was only 8.6, individuals in the vaccinated groups displayed strikingly large fold changes of up to a 98-fold increase. The size of vaccine responses correlated with lower pre-existing antibody titers (Figure E4), suggesting either that pre-existing anti–F IgA impaired the vaccine response or a ceiling of antibody production was being reached. Thus, although the small sample size and heterogeneity of responses limit interpretation, these data imply that SynGEM does induce RSV-specific mucosal IgA in most individuals, sometimes with highly dynamic responses, particularly in those with low pre-existing F-specific IgA titers.
Intranasal SynGEM Vaccination Induces Dose-Dependent Divergence in Systemic Plasmablast Responses
Systemic vaccines, including inactivated influenza and live yellow fever vaccines, have been consistently shown to stimulate short-lived plasmablasts that correlate with seroconversion (19). In contrast, live attenuated intranasal and replication-incompetent viral-vectored vaccines in adults rarely induce plasmablasts in the blood (20, 21). The plasmablast response to SynGEM was therefore investigated using flow cytometry, but, owing to high background and variance, no significant changes were seen (Figure E5).
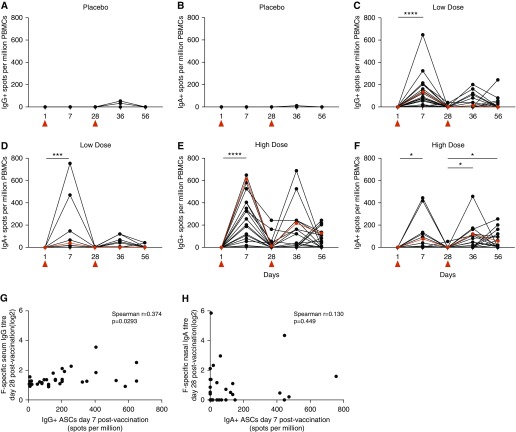
To better assess the antigen-specific B cell response to vaccination, B cell ELISpots were then performed to quantify IgG- and IgA-producing ASCs recognizing unstabilized recombinant F protein (Figure 4). Both low- and high-dose SynGEM induced IgG-producing ASCs in all vaccinated individuals 7 days after prime (low-dose median = 135 spots/million peripheral blood mononuclear cells, interquartile range [IQR] = 139; high-dose median = 230 spots/million, IQR = 330). The higher dose showed a trend toward larger plasmablast responses. In most individuals, ASCs had disappeared by the time of boost vaccination. However, the high-dose group had a significantly higher frequency of IgG+ ASCs remaining at the later time-point (high-dose median = 5 spots/million, IQR = 41; low-dose median = 0 spots/million; P = 0.0076), suggesting a more protracted response. Similar frequencies were seen after boost vaccination (Figure 4G). Thus, SynGEM induced systemic IgG+ ASC responses in all vaccinated individuals, with significantly increased duration and a trend toward greater frequency responses with the higher dose.
Figure 4.
Intranasal SynGEM protein stimulates IgG+ and IgA+ antibody-secreting cells (ASCs) in peripheral blood from volunteers administered low or high doses of SynGEM. (A–F) ASCs from peripheral blood at time points up to 28 days after boost were enumerated by B cell ELISpot. (G and H) There was no correlation between IgG+ ASC frequencies and F-specific serum IgG titers (G) or IgA+ ASC frequencies and F-specific nasal IgA titers (H). Median values are shown in red. *P < 0.05, ***P < 0.001, and ****P < 0.0001. PBMCs = peripheral blood mononuclear cells. Vaccinations are indicated by red triangles.
At Day 7 after prime, IgA+ ASCs were less frequent than IgG+ ASCs after low-dose (median = 41, IQR = 57; P = 0.0045) and high-dose vaccination (median = 28, IQR = 135; P = 0.0091) (Figure 4). Again, the response to high-dose boost vaccination persisted for longer than that to the low dose, with significantly higher frequencies of IgA+ ASCs at Day 56 in that group (high-dose median = 20 spots/million, IQR = 122; low-dose median = 0 spots/million, IQR = 54; P = 0.0048). There was no correlation between IgA+ ASC frequencies in blood and changes in nasal F-specific IgA titers (Figure 4H). Thus, although the overall IgA+ ASC response in blood was lower than IgG+ ASCs, there was some discordance in the different isotypes, suggesting that higher doses are more efficacious in boosting prolonged IgA-producing ASC responses.
SynGem Induces F-Specific Antibody Production by Tonsillar Cells In Vitro in a Dose-Dependent Manner
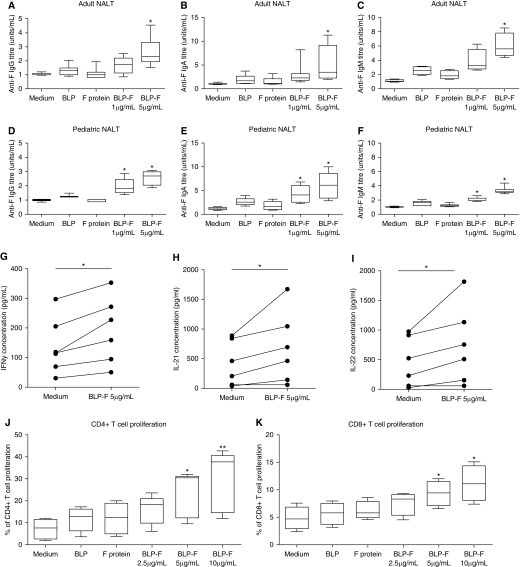
To investigate these dose-dependent effects, we tested the capacity of SynGEM to induce responses in human nasopharynx-associated lymphoid tissues cultured as previously described (16, 18). Recombinant F protein alone or BLP alone did not stimulate significant production of F-specific antibodies (Figures 5A–5F). However, in adults, culture with SynGEM induced F-specific IgG (Figure 5A), IgA (Figure 5B), and IgM (Figure 5C), with the higher SynGEM concentration inducing significantly higher titers. As expected in these upper respiratory tissues, substantially higher concentrations of IgA were produced at both dose levels (P < 0.05). Similar results were seen in pediatric samples, suggesting that comparable responses might be induced by SynGEM in children (Figures 5D–5F). In addition, antibody induction was associated with the significant production of IFN-γ, IL-22, and IL-21, suggesting the stimulation of type 1, type 17/22, and T follicular helper cell responses, respectively (Figures 5G–5I). Trends toward increased IL-2 production, TNF, IL-17A, and IL-10 were also seen, but not type 2 cytokines (IL-4, IL-5, and IL-13; Figure E6). Production of these cytokines was associated with proliferation of CD4+ and (to a lesser extent) CD8+ T cells in a dose-dependent manner (Figures 5J and 5K), suggesting that CD4+ T cells were major contributors to cytokine production.
Figure 5.
SynGEM (bacterium-like particle [BLP]-F) stimulation of adenotonsillar cells provokes dose-dependent antibody and T cell responses. Tonsil cells from (A–C) adult and (D–F) pediatric donors were cultured with SynGEM (BLP-F) containing 5 μg/ml and 1 μg/ml F protein, BLP alone (25 μg/ml), F protein alone (1 μg/ml), and medium only. F-specific IgG, IgA, and IgM in resulting supernatant were measured by ELISA. (G–I) Cytokines were measured in culture supernatant by cytometric bead array. Tonsil cells were cultured with SynGEM (BLP-F), BLP alone (25 μg/ml), or F protein alone (1 μg/ml). (J) CD4+ and (K) CD8+ T cell proliferation was then measured by analysis of CFSE dilution and expressed as a percentage of dividing cells in the CD3+ CD4+ or CD3+ CD8+ populations. Mann-Whitney U test and Wilcoxon signed-rank test were used to test significant differences. *P < 0.05 and **P < 0.005. NALT = nasal-associated lymphoid tissue.
Thus, in vitro data support the clinical observations that SynGEM induces a dose-dependent antibody response associated with appropriate T helper cytokine signaling. Although antibodies targeting site ∅, that are believed to be the most potent for virus neutralization, were not significantly induced, further enhancement of the prolonged vaccine-induced responses seen may be achieved by additional alterations in antigen and/or dose.
Discussion
Previously, we showed that antibodies induced after RSV infection were short-lived, and hypothesized that viral immunomodulatory mechanisms impaired anti-RSV humoral memory responses (9). Here, we have shown that delivering F protein using a nonliving subunit mucosal vaccine not only induced bursts of plasmablast activity and mucosal IgA, but also boosted systemic RSV-specific antibodies for at least 6 months. These data suggest that the BLP vaccine platform may permit more potent induction of both systemic and mucosal responses than existing intranasal vaccines, including live attenuated influenza vaccine (22) and live attenuated RSV vaccine candidates (2, 23). Indeed, adults do not respond to these vaccines, presumably due to pre-existing immunity that prevents attenuated virus replication. Futhermore, an adenovirus vector expressing F protein recently tested via intranasal administration (20) led to minimal boosting compared with intramuscular injection.
However, immunogenicity data are complicated where pre-existing immunity against vaccine antigens exists, such as with RSV in older children and adults (24, 25). After SynGEM vaccination, serum antibody titers increased significantly despite the background of moderate-to-high levels of pre-existing F-specific antibodies. However, the seropositivity of the volunteers limited the size of vaccine responses, with serum IgG only reaching a maximum fold increase of 2.43 (in the low-dose group late after boost vaccination). Furthermore, nasal IgA only significantly increased in the high-dose group with a fold change of 1.87, although low pre-existing F-specific IgA titers were more predictive of the strongest response to vaccination, with, in some cases, a rise of greater than 90-fold. Previous studies of intranasal subunit candidates (against diphtheria, tuberculosis, HIV, and influenza) suggest that immunogenicity could be greater in the absence of high levels of strain-specific immunity. Indeed, an influenza vaccine using a similar BLP platform as SynGEM (26) induced significantly higher IgG and IgA levels than the inactivated vaccine comparator. Against pathogens where there is no prior immunity, the BLP platform may therefore have broad potential.
Although intranasal diphtheria toxin adjuvanted with chitosan boosted both serum IgG and IgA with no safety issues (27), intranasal vaccines containing an inactive Escherichia coli heat-labile toxin have been implicated in causing facial nerve (Bell’s) palsy (28, 29). In our study, a single participant complained of pulsatile tinnitus and hearing impairment after vaccination. These symptoms were sufficiently mild that the participant did not declare them on direct questioning, and only reported them after boost vaccination, owing to their prolonged nature. No cause could be found, and they did not worsen after the boost, but an association with the vaccine could not be excluded owing to the timing of onset. Sudden sensorineural hearing loss is common, affecting 2–20 per 100,000 individuals each year (30). In most cases, as here, no cause is definitively identified, and it therefore remains unclear whether the vaccine was related.
One further unexplained observation is the lack of significant boosting of serum virus neutralization despite induction of F protein–binding antibodies. The F protein in SynGEM was engineered with stabilizing mutations to maintain the pre-F conformation and preclinical studies had shown stimulation of neutralizing antibodies in animal models (11). As part of product release testing, extensive stability tests were performed that showed stable D25 binding. It was therefore surprising to find no detectable neutralizing or D25-competing antibody responses. This may have been due to the limited overall size of antibody responses or suboptimal presentation of the F protein in vivo resulting in relatively little generation of the most potent neutralizing antibodies. Although this does not preclude a protective role for the nonneutralizing and palivizumab-like antibodies that were induced, further iterations of SynGEM should overcome this limitation. In particular, we anticipate that better induction of site ∅ antibodies will enhance virus-neutralizing responses both systemically and locally, with likely concomitant increase in efficacy.
Nevertheless, the intranasal BLP platform used here did lead to prolonged increases in virus-specific antibodies in blood and mucosa of antigen-experienced adults. Testing of other BLP-conjugated antigens should further progress this novel and broadly applicable strategy for mucosal vaccination.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Cmed for their assistance with regulatory applications, project management, clinical trial monitoring, and statistical analysis.
Footnotes
Supported by Wellcome Trust Translation Award WT108516; additional support was provided by a National Institute for Health Research Senior Investigator Award (P.J.O.).
This article presents independent research funded by the Wellcome Trust. Infrastructure and other support were provided by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre and the NIHR Imperial Clinical Research Facility. The views expressed are those of the author(s), and not necessarily those of the Welcome Trust, the National Health Service, the NIHR, or the Department of Health and Social Care.
Author Contributions: B.-J.H., M.v.R., R.G., Q.Z., K.L., P.J.O., and C.C. designed and conceived the study; I.V. and C.C. supervised and performed the clinical study; S.A., M.K., S.W.-W, M.D.-T., M.S.A., and M.v.R. performed the laboratory experiments; S.A., I.V., P.J.O., and C.C. wrote the manuscript; all authors reviewed the manuscript prior to submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201810-1921OC on February 12, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lin Y-H, Deatly AM, Chen W, Miller LZ, Lerch R, Sidhu MS, et al. Genetic stability determinants of temperature sensitive, live attenuated respiratory syncytial virus vaccine candidates. Virus Res. 2006;115:9–15. doi: 10.1016/j.virusres.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Buchholz UJ, Cunningham CK, Muresan P, Gnanashanmugam D, Sato P, Siberry GK, et al. International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1114 Study Team. Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. J Infect Dis. 2018;217:1338–1346. doi: 10.1093/infdis/jiy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheltema NM, Gentile A, Lucion F, Nokes DJ, Munywoki PK, Madhi SA, et al. PERCH Study Group. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. 2017;5:e984–e991. doi: 10.1016/S2214-109X(17)30344-3. [Published erratum appears in Lancet Glob Health 5:e1190.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13:371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colosia AD, Yang J, Hillson E, Mauskopf J, Copley-Merriman C, Shinde V, et al. The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: a systematic review. PLoS One. 2017;12:e0182321. doi: 10.1371/journal.pone.0182321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guvenel AK, Chiu C, Openshaw PJ. Current concepts and progress in RSV vaccine development. Expert Rev Vaccines. 2014;13:333–344. doi: 10.1586/14760584.2014.878653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falloon J, Yu J, Esser MT, Villafana T, Yu L, Dubovsky F, et al. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis. 2017;216:1362–1370. doi: 10.1093/infdis/jix503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, et al. Mechanisms of Severe Acute Influenza Consortium Investigators. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med. 2015;191:1040–1049. doi: 10.1164/rccm.201412-2256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki T, Ainai A, Hasegawa H. Functional and structural characteristics of secretory IgA antibodies elicited by mucosal vaccines against influenza virus. Vaccine. 2017;35:5297–5302. doi: 10.1016/j.vaccine.2017.07.093. [DOI] [PubMed] [Google Scholar]
- 11.Rigter A, Widjaja I, Versantvoort H, Coenjaerts FEJ, van Roosmalen M, Leenhouts K, et al. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. PLoS One. 2013;8:e71072. doi: 10.1371/journal.pone.0071072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol. 2013;372:83–104. doi: 10.1007/978-3-642-38919-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med. 2015;7:309ra162. doi: 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sande CJ, Mutunga MN, Medley GF, Cane PA, Nokes DJ. Group- and genotype-specific neutralizing antibody responses against respiratory syncytial virus in infants and young children with severe pneumonia. J Infect Dis. 2013;207:489–492. doi: 10.1093/infdis/jis700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saletti G, Çuburu N, Yang JS, Dey A, Czerkinsky C. Enzyme-linked immunospot assays for direct ex vivo measurement of vaccine-induced human humoral immune responses in blood. Nat Protoc. 2013;8:1073–1087. doi: 10.1038/nprot.2013.058. [DOI] [PubMed] [Google Scholar]
- 16.Aljurayyan A, Puksuriwong S, Ahmed M, Sharma R, Krishnan M, Sood S, et al. Activation and induction of antigen-specific T follicular helper cells play a critical role in live-attenuated influenza vaccine–induced human mucosal anti-influenza antibody response. J Virol. 2018;92:e00114–e00118. doi: 10.1128/JVI.00114-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullin J, Ahmed MS, Sharma R, Upile N, Beer H, Achar P, et al. Activation of cross-reactive mucosal T and B cell responses in human nasopharynx-associated lymphoid tissue in vitro by modified Vaccinia ankara–vectored influenza vaccines. Vaccine. 2016;34:1688–1695. doi: 10.1016/j.vaccine.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Gray C, Ahmed MS, Mubarak A, Kasbekar AV, Derbyshire S, McCormick MS, et al. Activation of memory Th17 cells by domain 4 pneumolysin in human nasopharynx-associated lymphoid tissue and its association with pneumococcal carriage. Mucosal Immunol. 2014;7:705–717. doi: 10.1038/mi.2013.89. [DOI] [PubMed] [Google Scholar]
- 19.Li G-M, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng N-Y, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci USA. 2012;109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green CA, Scarselli E, Sande CJ, Thompson AJ, de Lara CM, Taylor KS, et al. Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci Transl Med. 2015;7:300ra126. doi: 10.1126/scitranslmed.aac5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki S, Jaimes MC, Holmes TH, Dekker CL, Mahmood K, Kemble GW, et al. Comparison of the influenza virus–specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81:215–228. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 23.Karron RA, Luongo C, Thumar B, Loehr KM, Englund JA, Collins PL, et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med. 2015;7:312ra175. doi: 10.1126/scitranslmed.aac8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jounai N, Yoshioka M, Tozuka M, Inoue K, Oka T, Miyaji K, et al. Age-specific profiles of antibody responses against respiratory syncytial virus infection. EBioMedicine. 2017;16:124–135. doi: 10.1016/j.ebiom.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falsey AR, Walsh EE. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis. 1998;177:463–466. doi: 10.1086/517376. [DOI] [PubMed] [Google Scholar]
- 26.Van Braeckel-Budimir N, Haijema BJ, Leenhouts K. Bacterium-like particles for efficient immune stimulation of existing vaccines and new subunit vaccines in mucosal applications. Front Immunol. 2013;4:282. doi: 10.3389/fimmu.2013.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills KHG, Cosgrove C, McNeela EA, Sexton A, Giemza R, Jabbal-Gill I, et al. Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin A. Infect Immun. 2003;71:726–732. doi: 10.1128/IAI.71.2.726-732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 29.Lewis DJM, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 2009;4:e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence R, Thevasagayam R. Controversies in the management of sudden sensorineural hearing loss: an evidence-based review. Clin Otolaryngol. 2015;40:176–182. doi: 10.1111/coa.12363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.