Abstract
Rationale: Symptom subtypes have been described in clinical and population samples of patients with obstructive sleep apnea (OSA). It is unclear whether these subtypes have different cardiovascular consequences.
Objectives: To characterize OSA symptom subtypes and assess their association with prevalent and incident cardiovascular disease in the Sleep Heart Health Study.
Methods: Data from 1,207 patients with OSA (apnea–hypopnea index ≥ 15 events/h) were used to evaluate the existence of symptom subtypes using latent class analysis. Associations between subtypes and prevalence of overall cardiovascular disease and its components (coronary heart disease, heart failure, and stroke) were assessed using logistic regression. Kaplan-Meier survival analysis and Cox proportional hazards models were used to evaluate whether subtypes were associated with incident events, including cardiovascular mortality.
Measurements and Main Results: Four symptom subtypes were identified (disturbed sleep [12.2%], minimally symptomatic [32.6%], excessively sleepy [16.7%], and moderately sleepy [38.5%]), similar to prior studies. In adjusted models, although no significant associations with prevalent cardiovascular disease were found, the excessively sleepy subtype was associated with more than threefold increased risk of prevalent heart failure compared with each of the other subtypes. Symptom subtype was also associated with incident cardiovascular disease (P < 0.001), coronary heart disease (P = 0.015), and heart failure (P = 0.018), with the excessively sleepy again demonstrating increased risk (hazard ratios, 1.7–2.4) compared with other subtypes. When compared with individuals without OSA (apnea–hypopnea index < 5), significantly increased risk for prevalent and incident cardiovascular events was observed mostly for patients in the excessively sleepy subtype.
Conclusions: OSA symptom subtypes are reproducible and associated with cardiovascular risk, providing important evidence of their clinical relevance.
Keywords: sleep apnea, symptom subtypes, cardiovascular disease, sleepiness, cluster analysis
At a Glance Commentary
Scientific Knowledge on the Subject
Patients with obstructive sleep apnea can be classified into different symptom-based subtypes. This study describes how a clinical subtype characterized primarily by excessive sleepiness has increased prevalence of adverse cardiovascular outcomes and is at a higher risk of incident cardiovascular events in the Sleep Heart Health Study.
What This Study Adds to the Field
This study provides evidence that clinical symptoms are informative to identify subtypes of patients with moderate–severe obstructive sleep apnea. In addition, these symptom-based subtypes can inform the risk of prevalent and incident adverse cardiovascular consequences. These results suggest that obstructive sleep apnea symptom subtypes represent true underlying disease characteristics with clinical relevance.
Obstructive sleep apnea (OSA) is a common, chronic condition associated with multiple adverse outcomes (1), with increased prevalence concomitant with increasing obesity rates (2). Currently, OSA severity is primarily characterized by the apnea-hypopnea index (AHI), which measures the number of cessations (apneas) or reductions (hypopnea) in breathing per hour of sleep (3). Using the AHI, mild sleep apnea is defined as between 5 and 15 events/h, moderate OSA as between 15 and 30 events/h, and severe disease as AHI greater than or equal to 30 events/h. However, these severity definitions are somewhat arbitrary, because they are based on consensus rather than using data about specific clinical outcomes (3). Moreover, this characterization only captures one aspect of disease heterogeneity among patients (4).
To better characterize individual patients with OSA, recent studies have been undertaken to evaluate disease subtypes (4–8). In particular, our group has focused on the identification, replication, and validation of subtypes based on clinical symptoms at diagnosis among patients with moderate–severe OSA within both clinical (5, 6) and population-based samples (7). Through these efforts, we have consistently identified three primary subtypes characterized by 1) disturbed sleep (i.e., insomnia) symptoms, 2) a relative lack of traditional OSA symptoms, or 3) marked excessive daytime sleepiness. Beyond these, analyses in the Sleep Apnea Global Interdisciplinary Consortium (SAGIC), a worldwide ethnically diverse sample of patients with OSA from sleep clinics, identified two additional subtypes characterized by either upper airway symptoms or moderate sleepiness (6). Ultimately, the consistency of these results provides strong evidence that clinical symptom subtypes represent true underlying disease characteristics.
To understand the clinical relevance of OSA symptom subtypes, it is crucial to verify their association with relevant outcomes. Toward this end, recent work within the Icelandic Sleep Apnea Cohort (ISAC) found that symptom subtypes benefit in different ways with regard to symptom changes after 2 years of treatment with continuous positive airway pressure (CPAP) (9). Currently, however, it is unknown whether these symptom subtypes have different long-term health consequences, particularly with respect to cardiovascular disease (CVD).
To address this question, the present study uses data from the Sleep Heart Health Study (SHHS). This highly successful community-based study has established the association between sleep apnea and several different cardiovascular outcomes (10–21). Using this resource, we first leverage information on baseline symptoms to determine whether the previously described clinical subtypes exist in patients with OSA from the SHHS. After validating the existence of similar subtypes, we next assess whether different subtypes are associated with prevalence of CVD at baseline and risk of incident cardiovascular outcomes during the follow-up period, when compared with other subtypes and with individuals without OSA (AHI < 5).
Methods
Study Participants
The SHHS is a multicenter prospective community‐based cohort study of participants aged greater than 40 years from ongoing epidemiologic studies, assessing the cardiovascular consequences of OSA (see online supplement for details) (22, 23). Participants had a baseline examination in 1995–1998 and the median period of observation was 11.8 years. Data on 5,804 individuals were available through the National Sleep Research Resource (24, 25). To assess symptom subtypes, 1,207 (21%) individuals with moderate–severe OSA (AHI ≥ 15) and questionnaire data were included in clustering analysis. Individuals with mild OSA (5 ≤ AHI < 15) were excluded given the goal of evaluating the impact of symptom subtypes currently defined exclusively in moderate–severe OSA; this restriction also ensures significant disease burden within the study sample. To understand the cardiovascular risk among OSA subtypes compared with individuals without OSA, we included data from 2,830 (49%) individuals in SHHS with AHI less than five.
Cardiovascular Outcomes and Covariates
Our primary outcome was CVD, defined as one or more event of coronary heart disease (CHD), heart failure (HF), stroke (13; 26–28), or cardiovascular mortality (incident analysis only). Individual components were evaluated separately as secondary outcomes. CHD was defined as one or more event of myocardial infarction or coronary revascularization procedure (14). Stroke was defined according to previously reported protocols (15). Cardiovascular mortality included death from CHD, sudden death, or stroke (19). Prevalent or incident disease was defined as the occurrence of one or more event before baseline or between baseline and the end of the follow-up, respectively. Time to incident events was calculated based on the first occurrence after baseline; participants with no incident events were censored at their last follow-up. Figure E1 in the online supplement represents the number of individuals with each outcome, including the overlap of individuals with multiple outcomes. Covariates included age, sex, body mass index (BMI), AHI, presence of diabetes (29) and hypertension (30), high-density lipoprotein cholesterol, total cholesterol, triglycerides, smoking status, alcohol usage, race, ethnicity, and lipid-lowering medication use.
Statistical Analysis
A latent class analysis was performed among patients with moderate–severe OSA (AHI ≥ 15) using 14 symptom questions plus the Epworth Sleepiness Scale (ESS) (31), reflecting questions similar to prior publications on symptom clusters (see Table E1) (5–7; 9). The number of clusters with the lowest Bayesian information criterion value was considered optimal and evaluated for clinical interpretations and follow-up analyses. Associations between OSA subtypes and prevalent outcomes were assessed using logistic regression. Kaplan-Meier survival analysis and Cox proportional hazards models were used to evaluate associations with incident outcomes, excluding participants with the corresponding disease at baseline. We performed sensitivity analyses excluding individuals with central apnea index greater than or equal to 2.5 events/h (see Table E3) or individuals with any prevalent CVD (see Table E4). Associations were evaluated unadjusted (see Table E5) and adjusted for covariates described previously. We also evaluated associations between subtypes and either incident or recurrent events, including all available individuals and adjusting for prevalent disease.
Overall CVD was considered our primary outcome, with statistical significance based on a P less than 0.05. In secondary analyses, we evaluated each component separately, with statistical significance based on Bonferroni-corrected thresholds of P less than 0.0167 for three prevalent outcomes (CHD, HF, or stroke) and P less than 0.0125 for four incident/recurrent outcomes (CHD, HF, stroke, or cardiovascular mortality). Results with uncorrected P less than 0.05 were considered nominal evidence of an association.
Results
Sample Characteristics
A total of 1,207 subjects with moderate–severe OSA (AHI ≥ 15) and available symptom questionnaire data at baseline, as well as 2,830 subjects with AHI less than five, were included from the SHHS in the present study. Individuals with OSA had a mean (SD) age of 66.0 (10.5) years, BMI of 30.4 (5.7) kg/m2, and most were men (67.3%). Participants had severe OSA on average, with an AHI of 30.7 (16.9) events/h. The average ESS score was 8.7 (4.7). Individuals without OSA were younger (60.9 [11.3] yr; P < 0.0001), had lower BMI (26.8 [4.5] kg/m2; P < 0.0001), and a lower proportion of men (35.4%; P < 0.0001) compared with individuals with OSA (see Table E2).
A total of 1,048 (86.8%) individuals with OSA and 2,448 (86.5%) individuals without OSA had follow-up information on incident CVD, CHD, HF, and stroke. Of these 3,496 individuals, 3,089 (88.4%), 3,205 (91.7%), 3,411 (97.6%), and 3,396 (97.1%) did not have prevalent disease at baseline, respectively. Among individuals with OSA, there were a total of 227 (26.3%), 170 (18.7%), 145 (14.5%), 60 (6.0%), and 91 (8.7%) incident cases of CVD, CHD, HF, stroke, and cardiovascular mortality over the follow-up period, respectively. Incident events were significantly less common (P ≤ 0.002) among individuals without OSA, with 392 (17.6%) incident cases of CVD, 266 (11.6%) of CHD, 203 (8.4%) of HF, 86 (3.6%) of stroke, and 137 (5.6%) of death from CVD (see Table E2).
Similar OSA Symptom Subtypes in Patients with Moderate–Severe OSA in the SHHS
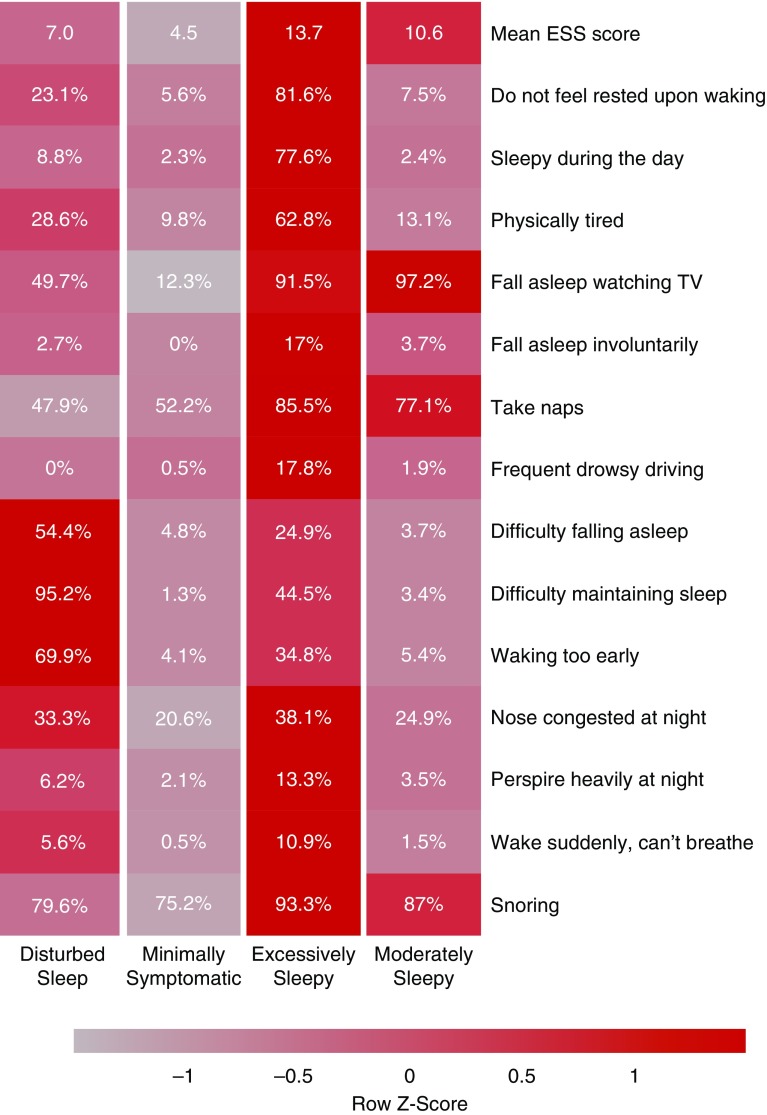
Clustering analysis identified four optimal clinical subtypes based on symptoms in individuals with moderate–severe OSA (see Figure E2). Figure 1 shows the relative proportion of each symptom, and average ESS scores, across the symptom subtypes. Based on the distribution of observed symptoms, the subtypes were labeled as disturbed sleep (n = 147; 12.2%), minimally symptomatic (n = 394; 32.6%), excessively sleepy (n = 201; 16.7%), and moderately sleepy (n = 465; 38.5%). These definitions are similar to those found in our previous studies (5–7), thereby demonstrating the existence of symptom subtypes within subjects with OSA from the SHHS.
Figure 1.
Symptom profile of the identified obstructive sleep apnea symptom subtypes in the Sleep Heart Health Study. The relative differences in symptom burden among subtypes are shown by the color scale, which represents the standardized (z-score) symptom proportion or mean Epworth Sleepiness Scale across groups. Brighter red indicates higher relative symptom burden. ESS = Epworth Sleepiness Scale.
Table 1 summarizes the clinical characteristics of these symptom subtypes. The excessively sleepy subtype was significantly younger and had higher BMI and AHI compared with the other subtypes. Moreover, the disturbed sleep subtype had a higher proportion of women and lower AHI when compared with the moderately sleepy subtype. Although statistically significant, these differences are relatively small from a clinical standpoint, underscoring the fact that patients with clinically similar disease severity and demographic characteristics present with distinct OSA subtypes.
Table 1.
Sample Characteristics according to Obstructive Sleep Apnea Symptom Subtype
| Variable | Disturbed Sleep (n = 147) | Minimally Symptomatic (n = 394) | Excessively Sleepy (n = 201) | Moderately Sleepy (n = 465) | P Value*† |
|---|---|---|---|---|---|
| Age, yr | 67.5 (10.1) | 66.3 (11.0) | 63.2 (11.3) | 66.3 (9.7) | <0.001‡§‖ |
| Sex, n (%) | |||||
| Men | 80 (54.4) | 259 (65.7) | 130 (64.7) | 343 (73.8) | <0.001¶ |
| Women | 67 (45.6) | 135 (34.3) | 71 (35.3) | 122 (26.2) | |
| BMI, kg/m2 | 29.7 (5.7) | 29.9 (5.4) | 32.2 (6.6) | 30.2 (5.3) | <0.001‡§‖ |
| Race, n (%) | |||||
| European American | 127 (86.4) | 339 (86.0) | 170 (84.6) | 403 (86.7) | 0.963 |
| African American | 13 (8.8) | 33 (8.4) | 21 (10.4) | 42 (9.0) | |
| Other | 7 (4.8) | 22 (5.6) | 10 (5.0) | 20 (4.3) | |
| Ethnicity, n (%) | |||||
| Not Hispanic or Latino | 140 (95.2) | 375 (95.2) | 192 (95.5) | 450 (96.8) | 0.650 |
| Hispanic or Latino | 7 (4.8) | 19 (4.8) | 9 (4.5) | 15 (3.2) | |
| Alcohol use, drinks/d | 3.5 (10.0) | 3.8 (7.9) | 2.6 (5.3) | 3.3 (6.3) | 0.311 |
| Smoking status, n (%) | |||||
| Never | 60 (41.1) | 183 (46.7) | 90 (45.0) | 211 (45.5) | 0.886 |
| Current | 12 (8.2) | 25 (6.4) | 17 (8.5) | 31 (6.7) | |
| Former | 74 (50.7) | 184 (46.9) | 93 (46.5) | 222 (47.8) | |
| AHI, events/h | 26.8 (12.2) | 28.7 (15.2) | 36.0 (20.5) | 31.3 (17.1) | <0.001‡§‖¶ |
| OAI, events/h | 9.9 (0–53.1) | 10.2 (0–82.7) | 11.4 (0–106) | 10.7 (0–89.9) | 0.002‡§ |
| CAI, events/h | 0.2 (0–32.8) | 0.3 (0–54.4) | 0.2 (0–27.1) | 0.3 (0–40.1) | 0.523 |
| ESS score | 7.0 (3.6) | 4.5 (2.2) | 13.7 (4.3) | 10.6 (3.3) | <0.001** |
| HDL, mg/dl | 48.7 (14.8) | 47.9 (15.5) | 45.5 (13.9) | 45.9 (14.1) | 0.071 |
| Total cholesterol, mg/dl | 209.9 (36.2) | 208.2 (35.8) | 207.9 (34.5) | 206.2 (34.9) | 0.714 |
| Triglycerides, mg/dl | 172.1 (123.1) | 159.3 (105.0) | 172.6 (112.4) | 161.3 (105.2) | 0.411 |
| Type 2 diabetes, n (%) | 16 (11.2) | 45 (11.7) | 26 (13.6) | 44 (9.9) | 0.585 |
| Hypertension, n (%) | 81 (55.1) | 197 (50.0) | 112 (55.7) | 246 (52.9) | 0.525 |
| Prevalent CVD, n (%) | 22 (16.5) | 55 (15.8) | 36 (22.5) | 73 (17.9) | 0.319 |
| Prevalent CHD, n (%) | 17 (12.8) | 41 (11.8) | 22 (13.7) | 57 (14.0) | 0.826 |
| Prevalent HF, n (%) | 5 (3.8) | 14 (4.0) | 16 (10.0) | 15 (3.7) | 0.010‡‖ |
| Prevalent stroke, n (%) | 5 (3.8) | 12 (3.4) | 10 (6.2) | 16 (3.9) | 0.508 |
| Follow-up time, yr | 10.3 (3.7) | 10.5 (3.5) | 10.5 (3.8) | 10.9 (3.1) | 0.170 |
| CVD incidence rate | 3.37 (2.47–4.59) | 3.48 (2.88–4.20) | 5.10 (4.01–6.48) | 3.37 (2.83–4.01) | 0.033 |
| CHD incidence rate | 2.11 (1.44–3.10) | 2.10 (1.66–2.66) | 3.15 (2.34–4.23) | 2.11 (1.71–2.62) | 0.129 |
| HF incidence rate | 1.33 (0.83–2.14) | 1.58 (1.21–2.07) | 2.66 (1.94–3.64) | 1.63 (1.28–2.07) | 0.029 |
| Stroke incidence rate | 0.45 (0.20–1.01) | 0.78 (0.54–1.14) | 0.58 (0.30–1.11) | 0.80 (0.57–1.12) | 0.520 |
| CVD mortality rate | 0.66 (0.36–1.23) | 0.82 (0.59–1.15) | 0.62 (0.36–1.07) | 0.67 (0.48–0.94) | 0.839 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; CAI = central apnea index; CHD = coronary heart disease; CVD = cardiovascular disease; ESS = Epworth Sleepiness Scale; HDL = high-density lipoprotein; HF = heart failure; OAI = obstructive apnea index.
P value from ANOVA, chi-square test, or Poisson regression comparing variable across subtypes.
Significant differences in pairwise comparisons (P < 0.05, Bonferroni adjusted).
Minimally symptomatic vs. excessively sleepy.
Excessively sleepy vs. disturbed sleep.
Excessively sleepy vs. moderately sleepy.
Disturbed sleep vs. moderately sleepy.
All pairwise comparisons. Quantitative variables are represented by mean (SD), except for OAI and CAI, which are represented by median (range). Incidence and mortality rates are represented per 100 person-years (95% confidence interval).
Symptom Subtypes Are Associated with Differences in Prevalent Cardiovascular Outcomes among Patients with Moderate–Severe OSA
We investigated whether the different symptom subtypes were associated with prevalent CVD and its components (CHD, HF, and stroke) in individuals with OSA, controlling for conventional cardiovascular risk factors. We found significant associations (Table 1) between symptom subtypes and prevalent HF (P = 0.010), with a higher proportion of cases with HF at baseline in the excessively sleepy subtype compared with both the minimally symptomatic (P = 0.020) and moderately sleepy (P = 0.010).
Results of the logistic regression models are shown in Table 2. In adjusted models, no significant associations with CVD were found. Among secondary outcomes, we observed an association between symptom subtype and prevalent HF (P = 0.015), which was significant at a Bonferroni-corrected threshold (P < 0.0167). In between group comparisons (Table 2), the excessively sleepy subtype was associated with increased risk of prevalent HF compared with the minimally symptomatic (odds ratio [OR], 3.07; 95% confidence interval [CI], 1.26–7.46; P = 0.013), disturbed sleep (OR, 3.67; 95% CI, 1.03–13.1; P = 0.045), and moderately sleepy (OR, 3.62; 95% CI, 1.56–8.41; P = 0.003) subtypes. Thus, results indicate that symptom subtypes are independent predictors of prevalent HF among patients with moderate–severe OSA.
Table 2.
Summary of the Results of the Adjusted Logistic Regression and Cox Proportional Hazards Models Assessing the Association between Obstructive Sleep Apnea Symptom Subtypes and Cardiovascular Outcomes
| Outcome | Prevalent* |
Incident† |
Incident + Recurrent‡ |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Cardiovascular disease | ||||||
| Overall adjusted comparison§ | — | 0.141 | — | <0.001 | — | 0.016 |
| ES vs. MinS | 1.62 (0.91–2.91) | 0.1032 | 2.28 (1.53–3.40) | 0.0001 | 1.50 (1.08–2.09) | 0.016 |
| ES vs. DS | 2.28 (1.05–4.95) | 0.0374 | 2.37 (1.40–4.01) | 0.0013 | 1.81 (1.15–2.85) | 0.0105 |
| ES vs. ModS | 1.75 (1.01–3.04) | 0.0475 | 2.23 (1.52–3.27) | <0.0001 | 1.59 (1.16–2.18) | 0.0037 |
| ModS vs. MinS | 0.93 (0.59–1.46) | 0.7498 | 1.02 (0.73–1.43) | 0.8949 | 0.94 (0.72–1.23) | 0.6652 |
| ModS vs. DS | 1.30 (0.66–2.58) | 0.4462 | 1.06 (0.66–1.71) | 0.7989 | 1.13 (0.75–1.70) | 0.5443 |
| DS vs. MinS | 0.71 (0.36–1.43) | 0.3386 | 0.96 (0.60–1.55) | 0.8723 | 0.83 (0.55–1.25) | 0.377 |
| Coronary heart disease | ||||||
| Overall adjusted comparison§ | — | 0.784 | — | 0.015 | — | 0.068 |
| ES vs. MinS | 1.23 (0.62–2.42) | 0.5592 | 1.85 (1.17–2.93) | 0.0086 | 1.54 (1.02–2.32) | 0.0383 |
| ES vs. DS | 1.51 (0.63–3.62) | 0.3568 | 1.99 (1.07–3.72) | 0.0302 | 1.84 (1.03–3.27) | 0.0385 |
| ES vs. ModS | 1.09 (0.57–2.06) | 0.8022 | 1.99 (1.28–3.10) | 0.0022 | 1.61 (1.09–2.37) | 0.0161 |
| ModS vs. MinS | 1.13 (0.68–1.88) | 0.6394 | 0.93 (0.63–1.37) | 0.7089 | 0.96 (0.68–1.35) | 0.8088 |
| ModS vs. DS | 1.39 (0.66–2.93) | 0.3865 | 1.00 (0.57–1.77) | 0.9959 | 1.14 (0.68–1.93) | 0.6169 |
| DS vs. MinS | 0.81 (0.38–1.75) | 0.5956 | 0.93 (0.52–1.64) | 0.797 | 0.84 (0.49–1.43) | 0.5187 |
| Heart failure | ||||||
| Overall adjusted comparison§ | — | 0.015 | — | 0.018 | — | 0.279 |
| ES vs. MinS | 3.07 (1.26–7.46) | 0.0134 | 2.22 (1.34–3.68) | 0.0021 | 1.52 (0.95–2.41) | 0.0782 |
| ES vs. DS | 3.67 (1.03–13.1) | 0.0447 | 2.04 (1.04–4.02) | 0.0389 | 1.60 (0.86–3.01) | 0.141 |
| ES vs. ModS | 3.62 (1.56–8.41) | 0.0028 | 1.71 (1.08–2.72) | 0.0228 | 1.43 (0.93–2.21) | 0.1035 |
| ModS vs. MinS | 0.85 (0.37–1.96) | 0.6976 | 1.29 (0.85–1.97) | 0.2274 | 1.06 (0.72–1.55) | 0.7752 |
| ModS vs. DS | 1.01 (0.30–3.43) | 0.9834 | 1.19 (0.65–2.17) | 0.5668 | 1.12 (0.64–1.97) | 0.6961 |
| DS vs. MinS | 0.84 (0.24–2.86) | 0.7756 | 1.09 (0.58–2.02) | 0.7936 | 0.94 (0.53–1.68) | 0.8456 |
| Stroke | ||||||
| Overall adjusted comparison§ | — | 0.601 | — | 0.427 | — | 0.043 |
| ES vs. MinS | 1.58 (0.58–4.32) | 0.3681 | 1.42 (0.63–3.21) | 0.4041 | 1.13 (0.51–2.50) | 0.7714 |
| ES vs. DS | 2.18 (0.51–9.34) | 0.2947 | 2.83 (0.81–9.91) | 0.1048 | 4.33 (1.19–15.7) | 0.0259 |
| ES vs. ModS | 1.84 (0.70–4.84) | 0.2154 | 1.26 (0.58–2.73) | 0.5659 | 0.83 (0.38–1.78) | 0.6311 |
| ModS vs. MinS | 0.86 (0.36–2.04) | 0.7325 | 1.13 (0.61–2.09) | 0.7026 | 1.36 (0.78–2.35) | 0.2759 |
| ModS vs. DS | 1.18 (0.30–4.61) | 0.8089 | 2.25 (0.74–6.88) | 0.1551 | 5.22 (1.67–16.4) | 0.0046 |
| DS vs. MinS | 0.73 (0.19–2.84) | 0.6472 | 0.50 (0.16–1.57) | 0.2359 | 0.26 (0.08–0.81) | 0.0197 |
| Cardiovascular mortality | ||||||
| Overall adjusted comparison§ | — | — | — | 0.768 | — | — |
| ES vs. MinS | — | — | 0.91 (0.45–1.81) | 0.7786 | — | — |
| ES vs. DS | — | — | 1.33 (0.52–3.40) | 0.5545 | — | — |
| ES vs. ModS | — | — | 1.10 (0.56–2.18) | 0.7828 | — | — |
| ModS vs. MinS | — | — | 0.82 (0.50–1.36) | 0.449 | — | — |
| ModS vs. DS | — | — | 1.21 (0.54–2.68) | 0.6454 | — | — |
| DS vs. MinS | — | — | 0.68 (0.31–1.51) | 0.3451 | — | — |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; DS = disturbed sleepy; ES = excessively sleepy; HDL = high-density lipoprotein; HR = hazard ratio; MinS = minimally symptomatic; ModS = moderately sleepy; OR = odds ratio.
Values in bold represent significant differences in pairwise comparisons.
Logistic regression model adjusted for age, sex, BMI, type 2 diabetes, hypertension, HDL, total cholesterol, triglycerides, apnea–hypopnea index, alcohol use, smoking status, race, ethnicity, and use of lipid-lowering medication.
Cox proportional hazards regression model adjusted for age, sex, BMI, type 2 diabetes, hypertension, HDL, total cholesterol, triglycerides, apnea–hypopnea index, alcohol use, smoking status, race, ethnicity, and use of lipid-lowering medication, excluding individuals with the corresponding disease at baseline.
Cox proportional hazards regression model adjusted for age, sex, BMI, type 2 diabetes, hypertension, HDL, total cholesterol, triglycerides, apnea–hypopnea index, alcohol use, smoking status, race, ethnicity, use of lipid-lowering medication, and status of corresponding disease at baseline. The reference category for all comparisons is always the second category presented in the column “Pairwise Comparison.” Results of the tests of proportional-hazards assumption are presented in Table E7.
P values evaluating null hypothesis of no differences in risk among subtypes.
Given the known relationship between HF and central sleep apnea (32), we also performed a sensitivity analysis excluding individuals presenting with a central apnea index greater than or equal to 2.5 events/h. Results were similar, with potentially stronger effects of the association between the excessively sleepy subtype and prevalent HF based on OR estimates (see Table E3).
Symptom Subtypes Are Independent Predictors of Future Cardiovascular Events in Patients with Moderate–Severe OSA
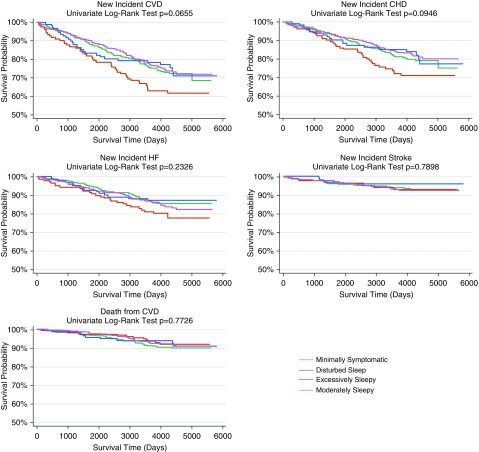
We next assessed whether the symptom subtypes were predictive of future occurrence of cardiovascular events, including cardiovascular mortality, among patients with moderate–severe OSA. No differences in follow-up time were found among the four symptom subtypes (P = 0.170) (Table 1). Results from unadjusted Kaplan-Meier survival analyses (Figure 2) showed suggestive differences in survival curves among symptom subtypes for CVD (P = 0.066), with the excessively sleepy subtype demonstrating worse survival than other symptom subtypes.
Figure 2.
Unadjusted Kaplan-Meier survival curves indicating the time to incidence of cardiovascular disease (CVD), coronary heart disease (CHD), heart failure, stroke, and death from CVD grouped by obstructive sleep apnea symptom subtype. The log-rank test was used to compare the survival distribution across subtypes. There were suggestive differences in CVD and trending differences in CHD survival curves among symptom subtypes. HF = heart failure.
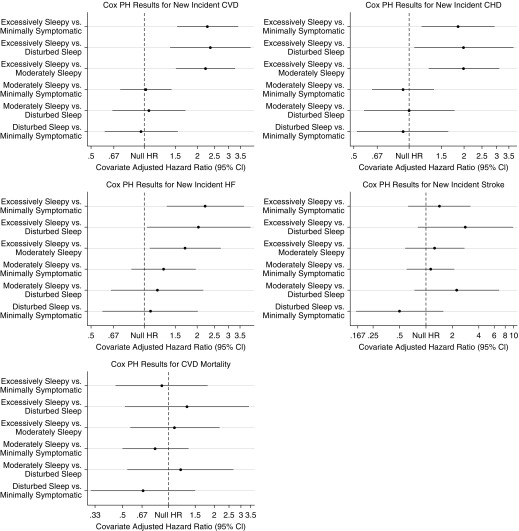
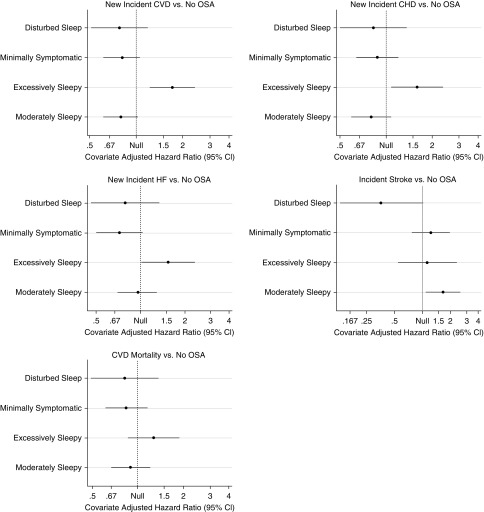
Results from adjusted Cox proportional hazards are summarized in Figure 3 and Table 2, and demonstrate significant associations between symptom subtypes and CVD (P = 0.0001). Individuals in the excessively sleepy subtype were at increased risk of new-onset CVD when compared with each of the three other symptom subtypes (Table 2). In particular, the excessively sleepy subtype demonstrated hazard ratios (HR) of 2.28 (95% CI, 1.53–3.40), when compared with the minimally symptomatic subtype (P = 0.0001), 2.37 (95% CI, 1.40–4.01), compared with the disturbed sleep (P = 0.0013), and 2.23 (95% CI, 1.52–3.27), compared with the moderately sleepy (P < 0.0001).
Figure 3.
Results of the Cox proportional hazards regression models to evaluate the association between obstructive sleep apnea symptom subtypes and incident cardiovascular disease (CVD), coronary heart disease (CHD), heart failure (HF), stroke, and death from CVD. The sample consisted of individuals without the corresponding outcome at the Sleep Heart Health Study baseline visit. Adjusted models included age, sex, body mass index, type 2 diabetes, hypertension, high-density lipoprotein, total cholesterol, triglycerides, apnea–hypopnea index, alcohol use, smoking status, race, ethnicity, and use of lipid-lowering medication as covariates. Pairwise comparisons are performed using each subtype as the reference group. The hazard ratio represented in the x-axis is shown in the log scale. More detailed results are presented in Table 2. The excessively sleepy subtype is the only subtype at increased risk for incident CVD, CHD, and HF. CI = confidence interval; Cox PH = Cox proportional hazards; HR = hazard ratio.
When evaluating components of CVD, nominally significant associations between symptom subtypes and incident CHD (P = 0.015) and HF (P = 0.018) were found, although results did not reach statistical significance based on a Bonferroni-corrected threshold (P < 0.0125). Individuals in the excessively sleepy subtype were at increased risk of all new-onset outcomes when compared with each of the three other symptom subtypes (Table 2). In particular, the excessively sleepy subtype demonstrated HR for CHD and HF of 1.85 (95% CI, 1.17–2.93) and 2.22 (95% CI, 1.34–3.68) compared with the minimally symptomatic subtype (all P < 0.009), 1.99 (95% CI, 1.07–3.72) and 2.04 (95% CI, 1.04–4.02) compared with the disturbed sleep (all P < 0.038), and 1.99 (95% CI, 1.28–3.10) and 1.71 (95% CI, 1.08–2.72) compared with the moderately sleepy (all P < 0.023).
We also evaluated the associations between OSA symptom subtypes and risk of either incident or recurrent cardiovascular events, including individuals with the corresponding cardiovascular outcome at baseline (Table 2). In adjusted analyses, we found a significant association between symptom subtype and incidence or recurrence of CVD (P = 0.016). The excessively sleepy subtype had a greater risk for incident or recurrent CVD compared with each of the other subtypes, with HR of 1.50 (95% CI, 1.08–2.09; P = 0.016) compared with the minimally symptomatic, 1.81 (95% CI, 1.15–2.85; P = 0.011) compared with the disturbed sleep, and 1.59 (95% CI, 1.16–2.18; P = 0.004) compared with the moderately sleepy. Thus, the excessively sleepy subtype is at increased risk for incident or recurrent CVD, independent of other cardiovascular risk factors.
For incidence or recurrence of individual components, we also found a nominal association between symptom subtype and incidence or recurrence of stroke (P = 0.043), although it did not reach significance at a Bonferroni-corrected threshold (P < 0.0125). When assessing between subtype associations, interestingly, the disturbed sleep subtype had evidence for decreased risk compared with the minimally symptomatic (HR, 0.26; 95% CI, 0.08–0.81; P = 0.020), excessively sleepy (HR, 0.23; 95% CI, 0.06–0.84; P = 0.026), and moderately sleepy (HR, 0.19; 95% CI, 0.06–0.60; P = 0.005) subtypes.
Sensitivity analyses performed excluding individuals with central apnea index greater than or equal to 2.5 (see Table E3) or with any prevalent CVD (see Table E4) showed similar, and at times stronger, results based on HR; thus, results in the full population seem robust.
Excessively Sleepy Subtype Is Associated with Increased Prevalence and Incidence of New Cardiovascular Outcomes Compared with Individuals without OSA
Having demonstrated that symptom subtype is associated with differential risk for cardiovascular outcomes among patients with moderate–severe OSA, we evaluated whether specific subtypes were associated with increased cardiovascular risk relative to individuals without OSA (AHI < 5). In adjusted analyses, we found that the excessively sleepy was the only subtype at increased risk for prevalent CVD (OR, 2.00; 95% CI, 1.21–3.31; P = 0.007) when compared with individuals without OSA. Analyses of secondary outcomes found that the excessively sleepy was also the only subtype at increased risk for prevalent HF (OR, 4.64; 95% CI, 2.17–9.92; P = 0.0001). No other subtypes demonstrated increased risk of prevalent disease relative to individuals with AHI less than five (see Table E6).
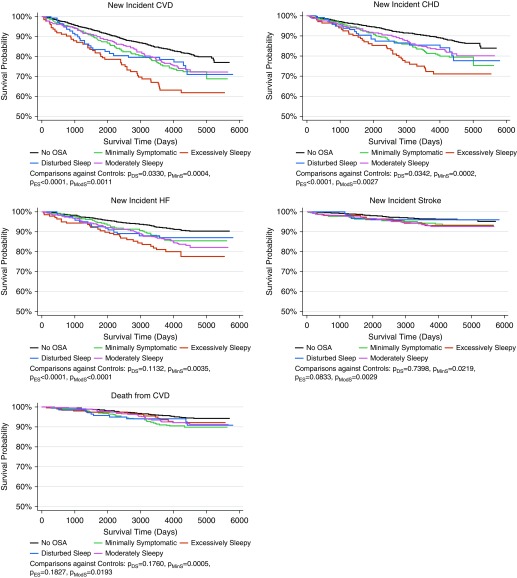
Unadjusted Kaplan-Meier analyses among OSA symptom subtypes and individuals without OSA are shown in Figure 4. Significant differences in log-rank tests comparing all curves were observed for CVD (P < 0.0001), and individual components of CHD (P < 0.0001),HF (P < 0.0001), stroke (P = 0.011), and cardiovascular mortality (P = 0.003). Pairwise comparisons in survival curves between each subtype and control subjects are also represented in Figure 4. For the primary outcome of CVD incidence, and CHD and HF, the excessively sleepy subtype demonstrated the worst survival, whereas individuals without OSA showed the best. For stroke and cardiovascular mortality, the minimally symptomatic and the moderately sleepy subtypes demonstrated worst survival compared with individuals without OSA.
Figure 4.
Unadjusted Kaplan-Meier survival curves indicating the time to incidence of cardiovascular disease (CVD), coronary heart disease (CHD), heart failure (HF), stroke, and death from CVD grouped by obstructive sleep apnea symptom subtype, including the sample of individuals without obstructive sleep apnea (OSA) in the Sleep Heart Health Study. Results of pairwise log-rank tests between each subtype and individuals without OSA are shown below each curve. There were significant differences in survival curves for incident CVD, CHD, and HF, when symptom subtypes were compared with individuals without OSA. In all cases, the excessively sleepy subtype demonstrated the worst survival. For stroke and cardiovascular mortality, the minimally symptomatic and the moderately sleepy subtypes demonstrated worse survival than individuals without OSA.
Results from adjusted Cox proportional hazards survival models comparing symptom clusters with individuals without OSA are summarized in Figure 5 and Table E6. We found significant associations in the comparisons between symptom subtypes and individuals without OSA for incident CVD (P = 0.004). Results demonstrated significantly greater risk for incident CVD (HR, 2.01; 95% CI, 1.25–3.23; P = 0.004) in the excessively sleepy subtype compared with individuals without OSA. We also found nominal associations between symptom subtypes and individuals without OSA for incident HF (P = 0.046), although this was not significant after Bonferroni correction (P < 0.0125). Results again suggest greater risk for incident HF (HR, 1.71; 95% CI, 1.00–2.92; P = 0.048) in the excessively sleepy subtype compared with individuals without OSA.
Figure 5.
Results of the adjusted Cox proportional hazards regression models to evaluate the association between each obstructive sleep apnea symptom subtype and incident cardiovascular disease (CVD), coronary heart disease (CHD), heart failure (HF), stroke, and death from CVD compared with individuals without obstructive sleep apnea (no OSA). The sample consisted of individuals without the corresponding outcome at the Sleep Heart Health Study baseline visit. Models were adjusted for age, sex, body mass index, type 2 diabetes, hypertension, high-density lipoprotein, total cholesterol, triglycerides, alcohol use, smoking status, race, ethnicity, and use of lipid-lowering medication as covariates. Individuals without OSA were used as the reference group. The hazard ratio represented in the x-axis is shown in the log scale. More detailed results are presented in Table E6. The excessively sleepy subtype is at increased risk for incident CVD, CHD, and HF when compared with individuals without OSA. CI = confidence interval.
When examining incident or recurrent events, we observed significant associations with incident or recurrent CVD (P = 0.017). We found a significantly increased risk of incident or recurrent CVD (HR, 1.69; 95% CI, 1.10–2.57; P = 0.016) in individuals of the excessively sleepy subtype. Despite the low incidence rate of stroke (see Table E2), we observed nominal associations with incident or recurrent stroke (P = 0.045), but results did not achieve significance after Bonferroni-correction (P < 0.0125). An increased risk of incident or recurrent stroke (HR, 1.78; 95% CI, 1.10–2.88; P = 0.019) was suggested in individuals of the moderately sleepy subtype when compared with individuals without OSA (see Table E6).
Discussion
This study provides further evidence of the existence of clinical symptom subtypes of OSA described within a number of previous studies, encompassing both clinical and population-based samples (5–7). Beyond this, we provide new evidence on their clinical relevance, demonstrating that clinical subtypes are associated with differential risk for prevalent and incident CVD among patients with moderate–severe OSA. In particular, the excessively sleepy subtype has consistently increased prevalence of CVD at baseline and a higher risk of incident or recurrent cardiovascular events compared with the other symptom subtypes. Analyses compared with patients without OSA demonstrate that the significant increased overall cardiovascular risk related to OSA is driven by patients with the excessively sleepy subtype. Altogether, our results provide important insights into the clinical impact of OSA symptom subtypes and the importance of considering them in clinical care and when performing clinical trials of the cardiovascular benefits of OSA treatment.
Several studies to date provide convincing evidence that similar symptom-based subtypes of moderate–severe OSA are found within patients of different ethnicities, identified either from sleep clinics or in the population (5–7). The three original subtypes of disturbed sleep, minimally symptomatic, and excessive sleepiness are observed in all studies, including the present analysis. Similar to recent analyses in the SAGIC cohort (6), the current study found an additional subgroup defined primarily by moderate sleepiness when we looked at the optimal cluster solution. Thus, results are consistent with prior studies.
Although similar symptom subtypes were found, there are differences in subtype frequency across samples, reflecting known differences in symptom burden. A prior population-based cohort study in Iceland found a greater than 15% prevalence of moderate–severe OSA (based on sleep studies), but a much lower symptom burden compared with patients who present to sleep centers (33). Supporting this observation, a higher percentage of asymptomatic patients was found in a population-based Korean cohort (55.7%) (7) than in clinical cohorts from ISAC (24.7%) (5) and SAGIC (40.4%) (6). Similarly, we found a higher percentage of the subtypes with lower symptom burden (i.e., minimally symptomatic and moderately sleepy, 71.1% combined) in the SHHS.
The SHHS has made important contributions to the understanding of OSA-related cardiovascular risk (10–21). The present results add to these contributions, indicating that the increased cardiovascular risk among patients with OSA is mainly driven by the excessively sleepy subtype. The concept that subjects with excessive sleepiness have increased cardiovascular risk is not new. Kapur and colleagues (34) showed that the odds of hypertension at higher AHI were greater in excessively sleepy participants based on the ESS in the SHHS. Moreover, Lindberg and colleagues (35) showed that those with snoring and self-reported excessive daytime sleepiness had higher rates of hypertension and diabetes than those without excessive daytime sleepiness. Also, excessive daytime sleepiness was associated with higher risk of major adverse cardiac events following a myocardial infarction among participants identified to have moderate–severe sleep‐disordered breathing (36). In contrast, a recent investigation in the SHHS did not find a combined effect of moderate–severe OSA and excessive daytime sleepiness based on an ESS greater than or equal to 11 on incident CVD, CHD, or stroke, when compared with individuals with AHI less than 15 and ESS less than 11 (10).
Our present study supports the concept that ESS alone may be insufficient to characterize the excessive sleepiness phenotype within patients with moderate–severe OSA at increased cardiovascular risk. Although patients in our sample with the excessively sleepy subtype, based on reporting multiple symptoms related to excessive sleepiness (including a high mean ESS of 13.7), were at increased risk, those in the moderately sleepy subtype (mean ESS of 10.6) were not. Thus, a more comprehensive symptom profile characterization seems necessary. Relatedly, when we used the available questionnaires to categorize a subset of individuals without OSA into subgroups with similar clinical symptom profiles as in apneics, we observed no differences in cardiovascular risk among subgroups (data not shown). This indicates that the increased relative risk observed within the excessively sleepy subtype may be specific to those with moderate–severe OSA. Thus, the excessive sleepiness phenotype may be a surrogate marker of underlying cardiovascular risk pathways influenced by OSA, rather than an independent risk factor in the absence of elevated AHI.
The prevalence of excessive daytime sleepiness has also been associated with increased mortality in individuals with OSA, in both the Cardiovascular Health Study (37) and research from our group in older adults (38). However, in the current study, we did not find significant associations between the excessively sleepy subtype and cardiovascular mortality. This is possibly caused by the limited number of cardiovascular mortality events among individuals with OSA in our study (n = 90). Nevertheless, a recent study, also in the SHHS, found that short respiratory event duration, rather than AHI, independently predicted all-cause mortality in both men and woman (39). This suggests that other definitions of OSA severity, not based on the AHI, might be more specific to inform associations with mortality. The effect of specific OSA symptom subtypes in patients with shorter respiratory event duration remains to be investigated.
Complementary to our findings on the excessively sleepy subtype, our results suggest that the disturbed sleep subtype could be at reduced risk for incident or recurrent stroke when compared with other subtypes. In a previous study in the SHHS, a significantly increased risk of stroke with increasing quartiles of obstructive AHI was found among men, but not women (15). Also, women with higher arousal indexes had reduced incidence of stroke (15). In the present study, the disturbed sleep subtype had the highest proportion of women, which may help explain this result. Although intriguing, the associations with stroke should be interpreted with some caution, given the relatively low incidence (60 events among individuals with OSA), which results in wide 95% CI, and, thus, less reliable effect estimates.
The present study highlights the importance of considering different symptom-based OSA subtypes when designing future studies assessing the cardiovascular benefits of CPAP treatment. For example, the RICCADSA study, a randomized trial in individuals with severe OSA who were not excessively sleepy, found no cardiovascular benefit of CPAP (40, 41). A much larger study in patients with known CVD, the SAVE trial, also found no difference in the rates of future cardiovascular events between patients randomized to CPAP or no treatment for OSA (42). The SAVE trial found the same negative results within subgroups with different degrees of daytime sleepiness. However, because of ethical concerns associated with not treating sleepy patients as a result of increased crash risk (43), subjects with high ESS scores (>15) were excluded. Most clustering studies (5–7) show an average ESS of nearly 15 within the excessively sleepy subtype and in the present study, the average ESS in this subtype was 13.7 and 39.3% of patients had ESS greater than or equal to 15. Thus, many of the patients at greatest OSA-related cardiovascular risk may have been excluded from previous randomized trials. Given their higher risk of OSA-related cardiovascular events, excluding excessively sleepy patients from randomized trials limit the ability to detect beneficial treatment effects, and may be one explanation for previous negative studies.
Ultimately, the increased cardiovascular risk among the excessively sleepy subtype in our study suggests that future trials of the cardiovascular benefit of CPAP should not exclude subjects with excessive sleepiness. Rather, studies should focus on these patients, who are likely to show the largest benefit. There are, however, both practical and ethical concerns regarding randomization of excessively sleepy individuals to receive no specific OSA treatment, given the impact of sleepiness on both quality of life and motor vehicle crash risk (43). A possible alternative is to use a pragmatic design using such techniques as propensity score matching to allow causal inferences within the context of observational studies that include measures of CPAP treatment adherence (44). Although this type of design can effectively overcome the ethical concerns of randomization, it is not without its own challenges, because it requires a robust set of relevant covariates for matching, and assumes that these covariates adequately explain the known association of adherence with cardiovascular outcomes that is independent of the effects of treatment per se (45–47).
The finding of increased cardiovascular risk among only certain OSA symptom subtypes complements a recent study on “physiologic subtypes” (4). In particular, Zinchuk and colleagues (4) have identified seven subgroups based on standard physiologic data from the overnight sleep study. Only two of the subtypes, one with mild OSA but a high rate of period limb movements in sleep and one with severe OSA (hypopnea and hypoxia), had evidence of cardiovascular benefits from CPAP (4). Notably, the hypopnea and hypoxia subtype had significant OSA and the highest average ESS (4), and thus may share underlying pathways to CVD with the excessively sleepy subtype described here. Understanding the physiologic basis for the different clinical symptom subtypes remains an area for future investigation.
Our study has limitations. Analyses included individuals with AHI greater than or equal to 15, and thus results may not generalize to individuals with less severe disease. This AHI threshold was chosen because prior research in symptom subtypes has focused exclusively on moderate–severe OSA; it also ensures significant disease-related burden within the population being studied. The existence and cardiovascular relevance of symptom subtypes in mild OSA should be addressed in future investigations. Similarly, the SHHS is a relatively older-aged cohort, and thus results may not generalize to younger individuals, in whom the OSA-related cardiovascular risk might be greater (14). Adjustment for more refined covariate measurements, such as fat distribution and other cardiovascular risk factors (e.g., diet, exercise), would have provided more robust estimates of underlying risk. Given differences in the inclusion/exclusion criteria and in outcome adjudication methods of the SHHS parent cohorts, it is plausible to expect cohort effects on the reported associations. However, in primary analyses assessing the relationship between sleep apnea and incident CHD and HF, no significant cohort effects were found (14). Moreover, the inclusion of race and ethnicity as covariates is expected to indirectly account for site differences, as noted in a previous publication (14). The lack of accurate CPAP therapy data is a limitation. However, only approximately 2% of the SHHS sample reported CPAP treatment (14, 15), limiting its potential to influence results. Moreover, recent analyses suggest a marginally increased rate of PAP adherence among the excessively sleepy subtype, which is likely to bias our results toward the null hypothesis (9).
Strengths of this study include the large sample with data on symptoms and long-term follow-up on cardiovascular events, the application of robust statistical methods to identify symptom subtypes, and adjustment for many established cardiovascular risk factors. Results have identified several future directions, some of which have been discussed previously. Beyond these, associations between subtypes and cardiovascular outcomes should be replicated within independent samples, potentially leveraging resources available through electronic health records. To improve their clinical utility, it is essential to develop an efficient tool for accurately classifying new patients into their respective subtype, as has recently been done in chronic obstructive pulmonary disease (48). Although this analysis is best performed across multiple samples, particularly in patients presenting to sleep clinics, our results (see Figure 1) suggest that complaints of feeling sleepy during the day, not feeling rested on waking up, often feeling physically tired, and a high ESS score likely distinguish patients with the excessively sleepy subtype.
In conclusion, this study demonstrates that different symptom subtypes of OSA previously described in multiple cohorts (5–7) are also found in the SHHS. We show for the first time that these subtypes have different cardiovascular outcomes, demonstrating their clinical relevance. Specifically, patients with the excessively sleepy subtype are at increased risk of CVD compared not only with patients without OSA, but also relative to other patients with similar AHI in other subtypes. This concept should be introduced into routine clinical practice, by developing appropriate and validated clinical support tools and training clinicians in identifying the subtype at increased risk. At the most basic level, clinicians should recognize that patients with reports of multiple sleepiness-related symptoms and a very high ESS score are more likely to have cardiovascular consequences because of their OSA. The notion of OSA as a heterogeneous disorder is firmly established and should lead to new insights into the ways in which specific patients benefit from treatment, improving efficiency of clinical trials and facilitating personalized medicine approaches.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the participants that took part in this study, the National Sleep Research Resource and the support team behind it, and the Sleep Heart Health Study. They also thank the members of the Sleep Apnea Global Interdisciplinary Consortium for the discussion of these findings and help with interpretation of the results.
Footnotes
The Sleep Heart Health Study was supported by the NHLBI through the following cooperative agreements: U01HL53940 (University of Washington), U01HL53941 (Boston University), U01HL63463 (Case Western Reserve University), U01HL53937 (Johns Hopkins University), U01HL53938 (University of Arizona), U01HL53916 (University of California, Davis), U01HL53 934 (University of Minnesota), U01HL63429 (Missouri Breaks Research), and U01HL539 31 (New York University). The National Sleep Research Resource was supported by the NHLBI (HL114473). The development of this manuscript was also supported by the NHLBI (P01 HL094307 and R01 HL134015) and the American Academy of Sleep Medicine Foundation (194-SR-18).
Author Contributions: Study design: D.R.M., B.T.K., and A.I.P. Data analysis: D.R.M. and B.T.K. Interpretation of the results: D.R.M., B.T.K., D.C.L., D.J.G., J.K., and A.I.P. Manuscript preparation: D.R.M., B.T.K., and A.I.P. Manuscript revision, editing, and approval: D.R.M., B.T.K., D.C.L., D.J.G, J.K., and A.I.P.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201808-1509OC on February 15, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lim DC, Pack AI. Obstructive sleep apnea: update and future. Annu Rev Med. 2017;68:99–112. doi: 10.1146/annurev-med-042915-102623. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 4.Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73:472–480. doi: 10.1136/thoraxjnl-2017-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye L, Pien GW, Ratcliffe SJ, Björnsdottir E, Arnardottir ES, Pack AI, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44:1600–1607. doi: 10.1183/09031936.00032314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keenan BT, Kim J, Singh B, Bittencourt L, Chen N-H, Cistulli PA, et al. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis [abstract] Sleep 201841DOI: 10.1093/sleep/zsx214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, Keenan BT, Lim DC, Lee SK, Pack AI, Shin C. Symptom-based subgroups of Koreans with obstructive sleep apnea. J Clin Sleep Med. 2018;14:437–443. doi: 10.5664/jcsm.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch H, Schneider LD, Finn LA, Leary EB, Peppard PE, Hagen E, et al. Breathing disturbances without hypoxia are associated with objective sleepiness in sleep apnea [abstract] Sleep 201740DOI: 10.1093/sleep/zsx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pien GW, Ye L, Keenan BT, Maislin G, Björnsdóttir E, Arnardottir ES, et al. Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the Icelandic sleep apnea cohort [abstract] Sleep 201841DOI: 10.1093/sleep/zsx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32–37. doi: 10.1016/j.sleep.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aurora RN, Crainiceanu C, Gottlieb DJ, Kim JS, Punjabi NM. Obstructive sleep apnea during REM sleep and cardiovascular disease. Am J Respir Crit Care Med. 2018;197:653–660. doi: 10.1164/rccm.201706-1112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tung P, Levitzky YS, Wang R, Weng J, Quan SF, Gottlieb DJ, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc. 2017;6:e004500. doi: 10.1161/JAHA.116.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roca GQ, Redline S, Claggett B, Bello N, Ballantyne CM, Solomon SD, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities-Sleep Heart Health Study. Circulation. 2015;132:1329–1337. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179:1159–1164. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–1155. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, et al. Sleep Heart Health Study Research Group. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 20.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 21.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study JAMA 20002831829–1836.[Published erratum appears in JAMA 288:1985.] [DOI] [PubMed] [Google Scholar]
- 22.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 23.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, et al. Sleep Heart Health Research Group. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 24.Dean DA, II, Goldberger AL, Mueller R, Kim M, Rueschman M, Mobley D, et al. Scaling up scientific discovery in sleep medicine: the National Sleep Research Resource. Sleep. 2016;39:1151–1164. doi: 10.5665/sleep.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G-Q, Cui L, Mueller R, Tao S, Kim M, Rueschman M, et al. The National Sleep Research Resource: towards a sleep data commons. J Am Med Inform Assoc. 2018;25:1351–1358. doi: 10.1093/jamia/ocy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, et al. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 27.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 28.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 29.Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, et al. Sleep Heart Health Study. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–709. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 33.Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, Gislason T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 2016;47:194–202. doi: 10.1183/13993003.01148-2015. [DOI] [PubMed] [Google Scholar]
- 34.Kapur VK, Resnick HE, Gottlieb DJ Sleep Heart Health Study Group. Sleep disordered breathing and hypertension: does self-reported sleepiness modify the association? Sleep. 2008;31:1127–1132. [PMC free article] [PubMed] [Google Scholar]
- 35.Lindberg E, Berne C, Franklin KA, Svensson M, Janson C. Snoring and daytime sleepiness as risk factors for hypertension and diabetes in women--a population-based study. Respir Med. 2007;101:1283–1290. doi: 10.1016/j.rmed.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Xie J, Sert Kuniyoshi FH, Covassin N, Singh P, Gami AS, Chahal CAA, et al. Excessive daytime sleepiness independently predicts increased cardiovascular risk after myocardial infarction. J Am Heart Assoc. 2018;7:e007221. doi: 10.1161/JAHA.117.007221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, et al. The Cardiovascular Health Study Research Group. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. J Am Geriatr Soc. 2000;48:115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 38.Gooneratne NS, Richards KC, Joffe M, Lam RW, Pack F, Staley B, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep (Basel) 2011;34:435–442. doi: 10.1093/sleep/34.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler MP, Emch JT, Rueschman M, Sands SA, Shea SA, Wellman A, et al. Apnea-hypopnea event duration predicts mortality in men and women in the Sleep Heart Health Study Am J Respir Crit Care Med[online ahead of print] 19 Oct 2018; DOI: 10.1164/rccm.201804-0758OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 41.Thunström E, Glantz H, Yucel-Lindberg T, Lindberg K, Saygin M, Peker Y.CPAP does not reduce inflammatory biomarkers in patients with coronary artery disease and nonsleepy obstructive sleep apnea: a randomized controlled trial [abstract] Sleep 201740DOI: 10.1093/sleep/zsx157. [DOI] [PubMed] [Google Scholar]
- 42.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 43.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–581. [PMC free article] [PubMed] [Google Scholar]
- 44.Keenan BT, Maislin G, Sunwoo BY, Arnardottir ES, Jackson N, Olafsson I, et al. Obstructive sleep apnoea treatment and fasting lipids: a comparative effectiveness study. Eur Respir J. 2014;44:405–414. doi: 10.1183/09031936.00043614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJV, et al. CHARM Investigators. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 47.D’Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 48.Burgel P-R, Paillasseur J-L, Janssens W, Piquet J, Ter Riet G, Garcia-Aymerich J, et al. Initiatives BPCO, EABPCO, Leuven and 3CIA Study Groups. A simple algorithm for the identification of clinical COPD phenotypes. Eur Respir J. 2017;50:1701034. doi: 10.1183/13993003.01034-2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.