The clinical consequences of pulmonary hypertension (PH) in patients with chronic obstructive pulmonary disease (COPD) and the effect of right ventricular (RV) failure on prognosis has long been recognized. Mild to moderate PH is common in patients with severe COPD; however, severe PH (mean pulmonary arterial pressure [mPAP] ≥35 mm Hg or a mPAP ≥25 mm Hg with a cardiac index <2.0 L · min−1 · m−2) is less frequent and, when present, is often associated with comorbid conditions such as left heart disease or chronic thromboembolic disease (1–3). More recently, however, it has been recognized that the pulmonary vasculature may be significantly compromised in some patients with mild to moderate COPD and minimal emphysema, the so-called “pulmonary vascular phenotype” (4). Often referred to as out-of-proportion PH in the past, these patients have severe PH in the setting of mild to moderate airflow obstruction, normal or low PaCO2, low DlCO, and circulatory, rather than ventilatory, limitation during exercise (4, 5).
In this issue of the Journal, Washko and colleagues (pp. 454–461) elegantly tease out supportive evidence for a pulmonary vascular phenotype in COPD by examining the relationships among distal pulmonary arterial pruning, emphysema, and RV size with exercise capacity and survival (6). Using volumetric computed tomography (CT) scans, they measured the percentage of emphysematous lung tissue, and performed morphologic assessment of distal pulmonary blood vessel volume (those with a cross-sectional area <5 mm2 [BV5]) and RV epicardial volume (RVEV) in >3,500 ever-smokers enrolled in the COPDGene study. The researchers found that RVEV was smaller with progressively worsening airflow obstruction; that is, from Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 to GOLD stage 4, the RV size actually decreased. The extent of emphysematous lung and arterial pruning, defined as lower arterial BV5, were both independently associated with larger RVs, such that for any degree of emphysema, a 10-ml decrease in arterial BV5 gave a 1-ml increase in RV size. In stratified multivariable models, however, arterial vascular pruning was associated with RV enlargement only in patients in the mildest quartile of airflow obstruction (FEV1 > 73.6% predicted) and in patients with <9.13% emphysema. Lending further support to the pulmonary vascular phenotype hypothesis was their finding that patients with milder COPD may have had pulmonary vascular and circulatory exercise limitation, as the RVEV independently predicted 6-minute-walk distance only in the quartile of patients in the least amount of emphysema. RV enlargement was also associated with higher mortality, but this effect was modified by arterial BV5, such that mortality was 63% higher in patients with RV enlargement and arterial pruning, but there was no significant increased risk for death without pruning.
The results reported by Washko and colleagues illustrate a subgroup of patients with mild to moderate COPD and lesser degrees of emphysema who suffer from disproportionate arterial drop-out and have RV dilation and a worse prognosis. These patients with mild COPD with arterial vascular pruning are reminiscent of the “vanishing capillary syndrome,” a recently proposed explanation for smoking-related pulmonary vasculopathy in patients otherwise diagnosed with idiopathic pulmonary arterial hypertension (IPAH) (7). In 2013, Trip and colleagues described a well-characterized group of patients with IPAH with a low DlCO (<45% predicted) who were older and more often male, with a significant smoking history, and with 68% demonstrating mild to moderate emphysema on CT scan (8). Despite having similar hemodynamic severity as those with a higher DlCO, 5-year survival was much lower in the low-DlCO group (38% vs. 80%). More recently, Olsson and colleagues described a small group of patients with IPAH with a low DlCO and no parenchymal lung disease on CT (9). PAH therapies were rather ineffective in these patients, with a mean decline in the 6-minute-walk distance of 10 m, and with only 1/22 patients improving functional class.
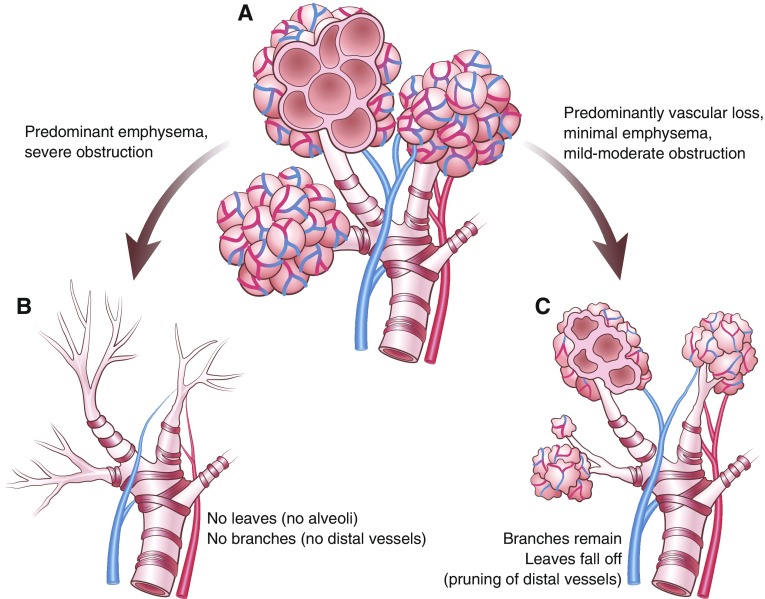
In the pulmonary vascular tree, distal arterial and capillary loss could be attributed to destruction of the “branches and leaves” from emphysematous changes, or to direct smoking-induced injury to the small arteries and capillaries (pruning of the leaves; Figure 1). Indeed, endothelial injury and pulmonary vascular remodelling may occur in smokers before the development of airflow obstruction or emphysema (10–12). The mechanisms underlying preferential vascular injury over airway-predominant and emphysematous changes are incompletely understood, but may be related to inducible nitric oxide synthase from bone marrow–derived cells and are independent of hypoxia (10). Identification of the genetic and cellular mechanisms associated with arterial pruning could help identify those susceptible to smoking-induced pulmonary vasculopathy and development of severe PH.
Figure 1.
(A) A normal, healthy bronchovascular tree. Imagine the airways and alveoli are like branches on a tree with pulmonary arterial–capillary networks reflecting the individual leaves. (B) In most patients with chronic obstructive pulmonary disease, smoking-induced damage to the distal airways and alveolar destruction are the main mechanisms for loss of the pulmonary vascular bed and the consequent pulmonary hypertension. Dropout of the distal arteries and capillaries (leaves) is congruent with the degree of damage to the branches (i.e., emphysema and severe airflow obstruction). (C) In patients with chronic obstructive pulmonary disease with a pulmonary vascular phenotype, smoking-related vascular injury predominates. There may be primarily a loss of the distal arterial–capillary networks (pruning of the leaves) with relative preservation of the airways and alveoli (branches). Illustration by Patricia Ferrer Beals.
It is important to note some limitations when interpreting the results of Washko and colleagues, as there were no right heart catheterization data to confirm the presence or mechanism of PH (i.e., precapillary vs postcapillary PH) in those with arterial pruning or an enlarged RV; thus, further invasive hemodynamic data to support these findings are necessary (6). Second, as acknowledged by the authors, noncardiac gated CT image acquisition is a limitation that precludes measurement of the RV wall thickness and may inaccurately reflect true RV volume. Furthermore, chronic thromboembolic disease and CT features of left heart disease, such as left atrial enlargement, were not systematically excluded in the patients with arterial pruning and large RV size. These are confounding causes of PH independent of emphysema or airflow obstruction; thus, their potential effects on CT measurements of RVEV, vascular pruning, and prognosis are not clear. Last, DlCO measurements were not available in this study, so any potential link between the phenotype of IPAH with a low DlCO and patients with mild COPD with arterial pruning remains speculative.
We must properly define this phenotype with hemodynamic and imaging criteria before embarking further down the rabbit hole of PAH-targeted therapy trials in patients with arterial pruning and mild to moderate COPD. To date, trials of PAH therapies in COPD have shown mixed, but overall disappointing, results (5). Therefore, properly designed and adequately powered studies with meaningful clinical endpoints could be justified if enriched with patients with mild to moderate COPD with markers of pulmonary vascular disease, such as arterial pruning. Washko and colleagues take us one step forward in understanding the characteristics and importance of a pulmonary vascular phenotype in COPD. Although there are poor outcomes associated with this phenotype and there are currently limited specific treatment options, expectations for PAH therapies in this group should be cautious, in light of the disconcerting experience with patients with vanishing capillary syndrome IPAH (7, 9). A tree without leaves may not easily be unpruned.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201901-0248ED on March 25, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Andersen KH, Iversen M, Kjaergaard J, Mortensen J, Nielsen-Kudsk JE, Bendstrup E, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant. 2012;31:373–380. doi: 10.1016/j.healun.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Chaouat A, Bugnet A-S, Kadaoui N, Schott R, Enache I, Ducoloné A, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–194. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs G, Agusti A, Barberà JA, Celli B, Criner G, Humbert M, et al. Pulmonary vascular involvement in chronic obstructive pulmonary disease: is there a pulmonary vascular phenotype? Am J Respir Crit Care Med. 2018;198:1000–1011. doi: 10.1164/rccm.201801-0095PP. [DOI] [PubMed] [Google Scholar]
- 5.Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53:1801914. doi: 10.1183/13993003.01914-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Washko GR, Nardelli P, Ash SY, Vegas Sanchez-Ferrero G, Rahaghi FN, Come CE, et al. COPDGene Investigators. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease: a longitudinal observational study. Am J Respir Crit Care Med. 2019;200:454–461. doi: 10.1164/rccm.201811-2063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeper MM, Vonk-Noordegraaf A. Is there a vanishing pulmonary capillary syndrome? Lancet Respir Med. 2017;5:676–678. doi: 10.1016/S2213-2600(17)30291-6. [DOI] [PubMed] [Google Scholar]
- 8.Trip P, Nossent EJ, de Man FS, van den Berk IAH, Boonstra A, Groepenhoff H, et al. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J. 2013;42:1575–1585. doi: 10.1183/09031936.00184412. [DOI] [PubMed] [Google Scholar]
- 9.Olsson KM, Fuge J, Meyer K, Welte T, Hoeper MM. More on idiopathic pulmonary arterial hypertension with a low diffusing capacity. Eur Respir J. 2017;50:1700354. doi: 10.1183/13993003.00354-2017. [DOI] [PubMed] [Google Scholar]
- 10.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey B-G, Strulovici-Barel Y, et al. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med. 2011;184:224–232. doi: 10.1164/rccm.201012-2061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos S, Peinado VI, Ramírez J, Melgosa T, Roca J, Rodriguez-Roisin R, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–638. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.