Abstract
Background
Thrombophilia testing is frequently ordered in the inpatient setting despite its limited impact on clinical decision-making and unreliable results in the setting of acute thrombosis or ongoing anticoagulation. We sought to determine the effect of an educational intervention in reducing inappropriate thrombophilia testing for hospitalized patients.
Methods
During the 2014 academic year, we implemented an educational intervention with a phase implementation design for Internal Medicine interns at Stanford University Hospital. The educational session covering epidemiology, appropriate thrombophilia evaluation and clinical rationale behind these recommendations. Their ordering behavior was compared with a contemporaneous control (non-medicine and private services) and a historical control (interns from prior academic year). From the analyzed data, we determined the proportion of inappropriate thrombophilia testing of each group. Logistic generalized estimating equations were used to estimate odds ratios for inappropriate thrombophilia testing associated with the intervention.
Results
Of 2151 orders included, 934 were deemed inappropriate (43.4%). The two intervention groups placed 147 orders.
A pooled analysis of ordering practices by intervention groups revealed a trend toward reduction of inappropriate ordering (p = 0.053). By the end of the study, the intervention groups had significantly lower rates of inappropriate testing compared to historical or contemporaneous controls.
Conclusion
A brief educational intervention was associated with a trend toward reduction in inappropriate thrombophilia testing. These findings suggest that focused education on thrombophilia testing can positively impact inpatient ordering practices.
Electronic supplementary material
The online version of this article (10.1186/s12911-019-0889-6) contains supplementary material, which is available to authorized users.
Keywords: Thrombophilia, High value care, Education, Inpatient
Background
Venous thromboembolism (VTE) has an incidence of 1–2 per 1000 patients per year, and it is a leading cause of morbidity and mortality [1–4]. The causes of VTE are often multifactorial, with common major risk factors including surgery, trauma, immobilization, malignancy, and pregnancy [5]. A number of other risk factors, including genetic and acquired thrombophilias, can also contribute to this risk [6].
Patients with VTE often undergo laboratory evaluation for genetic or acquired thrombophilias [7]. However, recent data suggest such evaluation is often conducted inappropriately [8]. For this reason, the American Society of Hematology (ASH) “Choosing Wisely” task force recently recommended against testing for thrombophilia in adult patients with VTE occurring in the setting of transient risk factors [9].
Testing for thrombophilic tendencies may be of limited value for several reasons. First, inherited or acquired thrombophilias are very common, with detectable thrombophilia defects identified in over half of patients with known VTE [10]. Second, the presence of an abnormal test result does not necessarily predict the risk of VTE recurrence and is not helpful for risk stratification [11]. Once treatment with anticoagulation is initiated, the outcome of these patients is no different than in those without thrombophilia [10, 12–15]. Third, there have been no prospective trials evaluating anticoagulation strategies for primary prevention in patients with inherited thrombophilias and current society guidelines recommend against this [16–18]. Lastly, with the exception of the DNA-based tests, the results of thrombophilia assays can be affected by acute thrombosis or the concurrent anticoagulation regimen, leading to invalid results [12, 19].
Given the limited value of thrombophilia evaluation, hospitals and professional societies have created initiatives to lower the rate of inappropriate inpatient thrombophilia ordering. The University of Texas Southwestern Medical Center created a system in which the transfusion medicine hemostasis service would communicate with ordering physicians if thrombophilia testing did not meet established guidelines. This initiative lowered inpatient thrombophilia test ordering by 84% [20]. The University of British Columbia instituted a hard stop with a required printed request form before inpatient hereditary thrombophilia testing could be performed, and this also reduced inpatient ordering [21].
In a recently published study, we reviewed all inpatient thrombophilia ordering at Stanford Hospital over a 2-year period and found 42.7% of ordering inappropriate [6]. To address this issue, we designed an interactive educational intervention to teach house staff the reasoning behind deferring inpatient thrombophilia testing and studied whether this changed their ordering habits. As others have encouraged [22], our goal was for our educational intervention to promote long-term behavioral changes independent of practice environment and additionally encourage trainees to disseminate what they learned. In this paper, we study the impact of our educational intervention on thrombophilia test ordering practices.
Methods
Intervention
This was an educational intervention to improve high-value care among residents at an academic internal medicine program.
The Stanford Internal Medicine Residency program and the Division of Hospital Medicine designed an educational series to teach principles of high-value care [22]. One of these sessions during the curriculum addressed appropriate thrombophilia evaluation, epidemiology, and the clinical rationale behind the ASH recommendations.
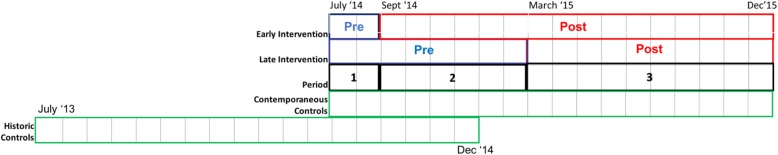
Internal Medicine intern physicians were randomized into two groups to receive the lecture at different times in the academic year (e.g. early-intervention group and late-intervention group). Twenty-four interns were placed in the early intervention group that received their lecture at the start of September 2014 and 25 interns were placed in the late intervention group received that their lecture at the start of March 2015 (Fig. 1). Their ordering practices were recorded from July 2014 through December 2015, thereby including the entire first year and the first 6 months of their second year. The intervention groups were not made aware that their ordering practices would be monitored. The lecture was not provided to other classes of the Internal Medicine residency, Internal Medicine attending physicians, other residency specialties, or non-teaching services.
Fig. 1.
Duration and distribution of each group and interval
We compared the ordering habits of the two intervention groups as a whole against two control groups. The first control was the first-year class from the prior academic year (2013), which served as a historical control. The historical control’s ordering habits were examined from their first year up to the first 6 months of their second residency year. The second control group was a contemporaneous control which included all non-intervention services during the interventional year, including third year internal medicine residents, all other residency specialties, and non-teaching private medical services. Second year internal medicine residents during the interventional observation period were excluded from the contemporaneous control group as their orders overlapped from their inclusion as the historical control in the year prior.
Outcomes
We reviewed the following commonly ordered thrombophilia tests: factor V Leiden mutation, prothrombin G20210A mutation, antithrombin III, protein C or S activity/level, lupus anticoagulant, beta-2 glycoprotein 1 IgM/IgG antibody, anticardiolipin IgM/IgG, dilute Russell viper venom time, and JAK2 V167F mutation. We defined criteria for test appropriateness using current society guidelines and current published evidence [9, 13, 23–25]. The criteria were reviewed by a senior hematologist at our institution (CB) with specific expertise in thrombophilia and thrombosis (Tables 1 and 2).
Table 1.
Timing and context used to determine appropriateness of testing
| Assay | Acute Thrombosis | UFH | LMWH | Warfarin | DOAC | Within 2w of stopping VKA |
|---|---|---|---|---|---|---|
| Factor V Leiden | √ | √ | √ | √ | √ | |
| Prothrombin gene mutation | √ | √ | √ | √ | √ | |
| Protein C | ? | ? | ? | X | X | X |
| Protein S | ? | ? | ? | X | X | X |
| Antithrombin III | X | X | X | ? | X | |
| Lupus anticoagulant | √ | X | X | ? | Xa | |
| dRVVT | √ | √ | √ | ? | X | |
| Beta-2 glycoprotein 1 Ab | √ | √ | √ | √ | √ | |
| Anti-cardiolipin Ab | √ | √ | √ | √ | √ | |
| JAK2 mutation | √ | √ | √ | √ | √ |
a False Positive
Table 2.
Criteria Used to Determine Appropriateness of Test Ordering
| Inappropriate Ordering | |
| Patient characteristics | |
| Provoked VTE occurring in the setting of major transient risk factor | |
| Women with pregnancy-related VTE | |
| Patients with advanced liver disease and abnormal function | |
| Ordering factor V Leiden or prothrombin gene mutation in patients who have had a liver transplant (no correlation between patient’s DNA and factor status produced by the new liver) | |
| Testing for the MTHFR polymorphism (obsolete test, does not correlate with risk of VTE) | |
| Timing issues | |
| Ordering antithrombin III, protein C or S level during the first 3 months of anticoagulant therapy | |
| Ordering protein C or S levels during warfarin therapy, or within 2 weeks of stopping warfarin | |
| Ordering lupus anticoagulant, antithrombin III, protein C or S levels in patients on novel oral anticoagulants | |
| Duplicate ordering of heritable thrombophilias: factor V Leiden, prothrombin gene mutation, JAK2 V167F mutation). | |
| Ordering of heritable thrombophilia workups (FV Leiden, Prothrombin gene mutation, and protein C/S levels) in the inpatient setting, if fails to impact clinical management decisions. | |
| Appropriate Ordering | |
| Patients with unprovoked VTE in whom test results may impact duration or choice of anticoagulant (e.g. positive antiphospholipid antibody screening) | |
| Patients with VTE and multiple family members with history of VTE (higher risk of thrombophilia such as AT3 deficiency) | |
| Thrombosis at unusual sites (e.g. splanchnic, Budd-Chiari, renal, or cerebral venous thrombosis) | |
| Recurrent provoked VTE | |
| Screen for antiphospholipid antibody in the setting of recurrent pregnancy loss (we can define further) | |
| Unexplained arterial thromboses | |
| Ordering antiphospholipid antibody testing in the setting of arterial thrombosis (e.g. peripheral arterial events, CVA) | |
| Unclear Ordering | |
| Female patients who develop VTE on hormonal therapy | |
| Patients with upper extremity DVT | |
| Testing ordered at the patient’s request | |
| VTE with minor risk factor (e.g. travel-related or flight < 6-8 h, minor surgery, prolonged sitting, etc.) | |
| VTE in young patients with stroke and PFO |
Two independent reviewers (HK, EM) then performed manual review of electronic medical records of all patients included in the data set to determine the clinical history, timing of testing, and concurrent anti-coagulant use. The reviewers determined the appropriateness of each test ordered based on the the patient’s presentation and scored each entry as appropriate, inappropriate, or unclear based on defined criteria. Any test that was initially felt unclear by a single rater was then reviewed jointly by the study authors and only left as unclear if a conclusion still could not be made. The inter-rater correlation concordance was 0.88 for identical data sets of the first 100 entries [6].
Using the manually reviewed data set of all inpatient thrombophilia ordering, we analyzed the change in ordering behavior of the two intervention groups before and after their respective intervention over an 18 month period. This 18 month period constituted the entirety of the intervention group’s intern year as well as the first 6 months of their second residency year.
To examine the overall effect of the intervention on thrombophilia testing and to account for the potential that an educational intervention could not only decrease inappropriate ordering but also inadvertently decrease appropriate thrombophilia testing, we also recorded the total number of patients admitted during the study period to account for fluctuations in patient population. The rate of appropriate and inappropriate testing was determined per 1000 patients admitted with the assumption of a constant rate of VTE cases per 1000 patients admitted.
Data
We examined all inpatient thrombophilia testing from June 2013 through December 2015, extracted from our hospital’s electronic medical records. The extracted data included the patient’s age, sex, medical record number, admission date, thrombophilia testing ordered, ordering provider and service, and admission diagnosis. Depending on the time of test order and the provider who ordered the test, the tests were categorized as pre or post-intervention, early or late intervention, historical or contemporaneous controls.
Statistical analysis
To estimate the impact of the intervention on rates of inappropriate thrombophilia test ordering, three comparisons were utilized. The first was a pre-post comparison in each of the two groups of internal medicine residents that received the intervention. The second comparison looked at the pre-post change in the intervention groups compared to attendings and residents of other services, during the same time period, who did not receive the intervention (contemporaneous controls). The third comparison looked at the pre-post change in the intervention groups compared to the internal medicine residents from the prior academic year who did not get the intervention (historical controls). In all three cases, estimates of the intervention’s impact were obtained for the early and late groups combined (overall) as well as separately through stratified analysis. As patients may be seen by multiple providers and providers may see multiple patients, we considered the test ordering data to be clustered by patient and provider in a cross-classified manner. Binary logistic Generalized Estimating Equations were used to estimate the association (Odds ratio, 95% confidence interval and Wald p-value) between the intervention and inappropriate testing, with robust standard errors to account for the cross-classified clustering of tests by patient and provider according to the methodology of Miglioretti and Heagerty [26]. For the purposes of our study, p < 0.05 is considered statistically significant. Full details of the model specifications used can be found in Additional file 1.
Results
Ordering habits pre and post intervention
We reviewed 2584 inpatient thrombophila orders and excluded 433 (16.8%) based on pre-defined exclusion criteria (Additional file 1: Table S1). Of the 2151 qualifying orders, 934 (43.4%) were found to be inappropriate based on pre-defined inappropriateness criteria.
The two intervention group participants placed 147 orders within the 18 month observation period from July 2014 to December 2015. After their intervention in the first half of the year, the early intervention group’s ordering habits changed from 40.0% inappropriate (2/5 orders) to 24.5% inappropriate (13/53 orders) (p = 0.60). Similarly after their intervention in the second half of the year, the late intervention group of 25 interns changed their inappropriate ordering from 39.1% inappropriate (18/46 orders) to 23.3% inappropriate (10/43 orders) (p = 0.11). Individually, the two intervention groups did not demonstrate statistically significant improvement after their respective interventions. Together, the two intervention groups decreased ordering from 39.2% inappropriate (20/51 orders) to 23.0% inappropriate (23/96 orders), a finding that trended toward statistical significance (p = 0.053) (Table 3).
Table 3.
Frequency and percent of inappropriate thrombophilia tests among interventional groups - pre and post intervention
| Group | Pre-Intervention | Post-Intervention | P Value |
|---|---|---|---|
| Early Intervention | 2/5 (40.0%) | 13/53 (24.5%) | 0.60a |
| Late Intervention | 18/46 (39.1%) | 10/43 (23.3%) | 0.11 |
| Combined Intervention | 20/51 (39.2%) | 23/96 (24.0%) | 0.053 |
P values from Chi square test except where indicated
a Fisher Exact test
Overall in the intervention groups, the odds of inappropriate testing post intervention were 53% less than those prior to the intervention (OR = 0.47; [95% CI: 0.14–1.61]; p = 0.23). When comparing the pre- and post- change for the intervention groups to that of a contemporary control group, the overall reduction in inappropriate testing was 54% (OR = 0.46; [95% CI: 0.12–1.71]; p = 0.25). The overall impact of the intervention was again similar when compared to the historical control from the prior year (OR = 0.42; [95% CI: 0.09, 2.06]; p = 0.29). While no estimates of the interventions impact achieved statistical significance (p < 0.05), all estimates across early and late periods intervention groups and the different control groups were consistent (Table 4).
Table 4.
Estimated impact of intervention by intervention cohort and comparison groupa
| Intervention group | Comparison | ||
|---|---|---|---|
| Within intervention | Contemporary controls | Historical controls | |
| Odds Ratio (95% CI); p | Odds Ratio (95% CI); p | Odds Ratio (95% CI); p | |
| Overall | 0.47 (0.14, 1.61); 0.23 | 0.46 (0.12, 1.71); 0.25 | 0.42 (0.09, 2.06); 0.29 |
| Early intervention | 0.49 (0.08, 2.98); 0.44 | 0.46 (0.09, 2.40); 0.35 | 0.47 (0.08, 2.91); 0.42 |
| Late intervention | 0.47 (0.11, 2.04); 0.32 | 0.43 (0.10, 1.77); 0.24 | 0.37 (0.06, 2.35); 0.29 |
CI Confidence Interval. p = Wald p-value
a All models include clustering for patient and provider
Ordering behavior by discrete intervals
We further evaluated the chronological correlation between the intervention and ordering habits by analyzing the intervention period during 3 discrete time intervals. Interval One occured before either group received their educational sessions and spanned from July 2014 through August 2014. Interval Two ranged from September 2014 through February 2015, encompassing the time period between early and late interventions. Interval Three ranged from March 2015 through December 2015, covering the time period after both intervention groups had received education (Fig. 1).
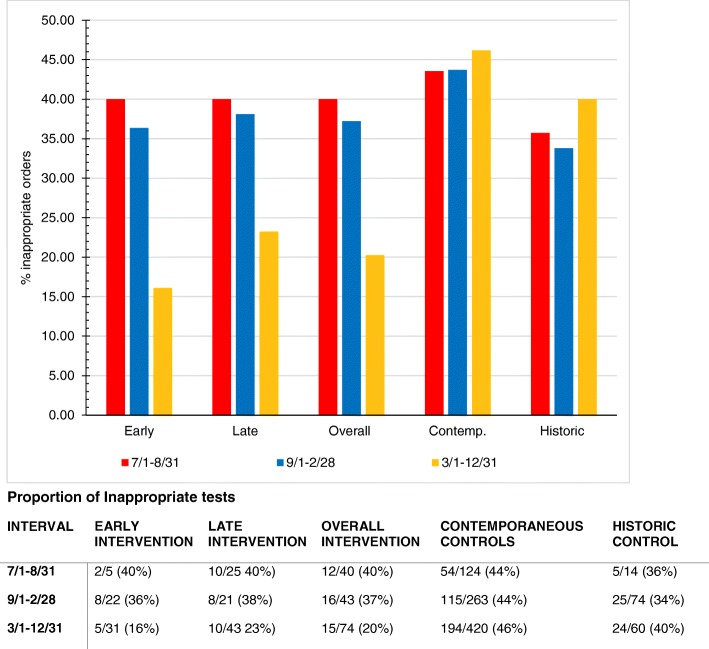
Over the course of these three intervals, the rate of inappropriate orders in the early intervention group changed from 40.0% (Interval One) to 36.4% (Interval Two) to 16.1% (Interval Three). Despite already receiving the intervention, there was only a 3.6% improvement in ordering from 40.0 to 36.4% between the first and second interval. The rate of inappropriate orders in the late intervention group changed from 40.0% (Interval One) to 38.1% (Interval Two) to 23.3% (Interval Three) inappropriate orders. There was no significant difference in ordering habits between the two intervention groups within each interval.
Combined, the two intervention groups improved from 40.0 to 37.1% to 20.3% across each interval. We also monitored ordering habits of the contemporaneous control group during these time intervals and of the historical control group during the same intervals from the previous year. Neither the historical control nor the contemporaneous control showed improvement in inappropriate ordering, with the former ordering 35.7, 33.8, 40.0% and the latter ordering 43.5, 43.7, and 46.2% over their respective time intervals. When combining our observations for the two intervention groups over the study period, the combined intervention group showed statistically significant improvement compared to both the Historical Control (P = 0.001) and the Contemporaneous Control groups (P < 0.001) (Fig. 2).
Fig. 2.
Percentage of inappropriate tests in each time interval
Standardization for census
Total University Medicine patient census was utilized to further determine whether the decrease in inappropriate orders over time in the intervention groups could be explained by fluctuations in patient census. Because of the potential that an educational intervention would also inadvertently decrease appropriate thrombophilia testing, we also explored standardized appropriate testing over time. With the assumption of a constant rate of VTE per 1000 patients, we calculated the appropriate and inappropriate testing per 1000 patients admitted. There was no significant change in appropriate testing before or after intervention in either intervention group. There was no significant change in inappropriate testing in the early intervention group while the late intervention group demonstrated a statistically significant decrease in inappropriate orders per 1000 patients (p = 0.043) (Table 5).
Table 5.
Tests and Confidence Intervala per 1000 patients admitted
| Pre-Intervention | Post-Intervention | P value | |
|---|---|---|---|
| Early Interventiona | |||
| Inappropriate tests | 3.68 (0–8.76) | 3.13 (1.43–4.83) | 0.832 |
| Appropriate tests | 5.51 (0–11.74) | 9.63 (6.63–12.61) | 0.343 |
| Late intervention | |||
| Inappropriate tests | 8.47 (4.57–12.36) | 3.89 (1.48–6.30) | 0.043 |
| Appropriate tests | 13.17 (8.32–18.02) | 12.84 (8.49–17.19) | 0.92 |
a Lower confidence limit restricted to 0
Discussion
We previously performed a retrospective analysis of inpatient thrombophilia testing practices at Stanford Hospital and Clinics and demonstrated a 42.7% inappropriate ordering rate over a two-year period [6]. Thrombophilia testing is relatively costly, with the Canadian Agency for Drugs and Technologies and Health determining that routine testing for certain inherited thrombophilias after first time VTE does not have clinical utility and eliminating or reducing such testing would lead to significant cost savings [27].
Although there are certain clinical scenarios where it is appropriate to order thrombophilia testing (e.g., if antiphospholipid antibody syndrome or paroxysmal noctural hemoglubinuria is suspected), thrombophilia testing should often be avoided in hospitalized patients with unprovoked VTE because inaccuracies with testing results, cost, patient anxiety, and inappropriate prolongation of anticoagulation [28]. Furthermore, thrombophilia testing takes time and may not be available until after the patient is discharged, which could lead to unnecessary repeat testing by outpatient providers if the status of these pending tests are not communicated. Initiatives to reduce such testing have increasingly become a topic of interest across multiple heathcare systems, with efforts mostly focused on developing system-level interventions to prevent inappropriate ordering. To the best of our knowledge, our study is the first published attempt at showing how a targeted educational intervention directy impacted thrombophilia testing. Such educational interventions are important as they can promote ongoing, long-term practice pattern improvement by targeting trainees at a formative time in their careers.
The combined intervention groups showed a decrease in inappropriate thrombophilia ordering after their interventions, a result that trended toward statistical significance. Individually, the two educational intervention groups also demonstrated an overall decrease in inappropriate ordering, but this result did not reach approach statistical significance, likely due to the small sample size. Reassuringly, we determined that the rate of appropriate thrombophilia testing did not decrease during the intervention, suggesting that our efforts at discouraging inappropriate ordering did not result in excessively cautious ordering behaviors.
Notably, during Interval Two spanning from September 2014 through February 2015, the early intervention group had already received their intervention but the late intervention group had not yet received it. Despite this, both intervention groups ordered inappropriate thrombophilia tests at a similar rate that did not differ from the control arms, making it difficult to definitively show a direct impact from our intervention. It is possible that the small sample sizes analyzed for the early intervention group, especially during Interval One, are the reason we did not see a change until the third interval. With small sample sizes when dividing into time intervals, chance could play a role in these findings. Alternatively, these results could reflect the time needed for a significant culture change amongst the housestaff or attendings. Indeed, all intervention participants were second year residents who have more autonomy with regard to patient orders by Interval Three. Perhaps in this more supervisory role the participants felt more empowered to practice high-value ordering habits.
The lack of improvement in ordering habits in the contemporaneous control group suggests that institution-wide ordering practices did not change over the study period. Similarly, the historical control group did not improve ordering habits, suggesting that intern resident physicians not exposed to the education intervention did not simply improve as they progressed through their training. This is important because our intervention groups outperformed the historical and contempraneous controls at the end of the study period, suggesting a positive impact of the intervention.
Study strengths
The educational intervention session was designed by a multidisciplinary team that included multiple specialties, and the timing and content of sessions was reviewed by our residency program leadership. Interns were randomized into their intervention groups and were not aware that their ordering patterns would be analyzed, in order to reduce the Hawthorne effect.
We performed a comprehensive review of institution-wide inpatient thrombophilia testing over 30 months to create a large sample size of thousands of entries to which we would be able to compare with the intervention groups over time. Through manual review of every patient medical record and utilization of pre-defined criteria for assessment, we thoroughly substantiated the appropriateness of each test.
Study limitations
As we conducted a single-center study with 49 residents in the Internal Medicine intern class, we had a small intervention group sample size. Additionally, the follow-up duration was limited. We had limited power to detect small to moderately-sized effects, particularly given that thrombophilia testing is relatively uncommon. We were unable to analyze thrombophilia testing of patients with non-thrombotic conditions as there are no established guidelines.
Conclusion
Thrombophilia work up findings obtained in the inpatient setting are often unreliable and generally do not alter clinical management. We looked at the impact of an educational intervention to reduce unnecessary thrombophilia testing among residents at an academic internal medicine program. Our findings suggest that a brief educational intervention can have a positive and sustained impact on ordering of thrombophilia testing. Prior investigators have examined a variety of promising online educational resources for trainee engagement and for continuing medical education, including social media venues such as Facebook or Twitter [29, 30]. Accordingly, future educational efforts at our institution may involve interactive, innovative online approaches. Currently, we are studying the combination of educational interventions and best practice alerts to further reduce the number of inappropriate thrombophilia tests.
Additional file
Compilation of criteria used for determining appropriateness and statistical models used for analysis. (DOCX 59 kb)
Acknowledgements
The authors would like to acknowledge contributions from Dr. Jason Bentley of the Stanford Quantitive Science Unit for his help with statistical analysis and Mehran Teymourtash of the Financial Department for his aid obtaining data sets of electronic medical record information for our analysis.
Abbreviations
- ASH
American Society of Hematology
- VTE
Venous thromboembolism
Authors’ contributions
EM, IR, AK, CB, NA, SH, TJ, NS, RW, RH, RK, LS and JH participated in curricular and study conception, design and implementation. All authors participated in data analysis. HK was responsible for drafting of the initial manuscript. All authors provided critical revision of the manuscript for important intellectual content. RW, NA, SH and LS facilitated administrative and material support. All authors provided final approval of the submitted manuscript. HK and JH are responsible for the overall content as guarantors.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to inclusion of protected health information but are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study received exempt status from the Stanford University Institutional Review Board (IRB) and informed consent was deemed unnecessary, given minimal risks to patients or providers.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Henry Kwang, Email: henrykwang@gmail.com.
Eric Mou, Email: emou@stanford.edu.
Ilana Richman, Email: ilana.richman@yale.edu.
Andre Kumar, Email: akumar3@stanford.edu.
Caroline Berube, Email: cberube@stanford.edu.
Rajani Kaimal, Email: rkaimal@stanford.edu.
Neera Ahuja, Email: nkahuja@stanford.edu.
Stephanie Harman, Email: smharman@stanford.edu.
Tyler Johnson, Email: tpjmd@stanford.edu.
Neil Shah, Email: NeiShah@stanfordhealthcare.org.
Ronald Witteles, Email: witteles@stanford.edu.
Robert Harrington, Email: robert.harrington@stanford.edu.
Lisa Shieh, Email: lshieh@stanford.edu.
Jason Hom, Phone: (650) 725-5071, Email: jasonhom@stanford.edu.
References
- 1.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28(3):370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delluc Aurélien, Tromeur Cécile, Le Ven Florent, Gouillou Maelenn, Paleiron Nicolas, Bressollette Luc, Nonent Michel, Salaun Pierre-Yves, Lacut Karine, Leroyer Christophe, Le Gal Grégoire, Couturaud Francis, Mottier Dominique. Current incidence of venous thromboembolism and comparison with 1998: a community-based study in Western France. Thrombosis and Haemostasis. 2016;116(11):967–974. doi: 10.1160/TH16-03-0205. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 4.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 6.Mou E, Kwang H, Hom J, et al. Magnitude of potentially inappropriate thrombophilia testing in the inpatient hospital setting. J Hosp Med. 2017;12(9):735–738. doi: 10.12788/jhm.2819. [DOI] [PubMed] [Google Scholar]
- 7.Rambaldi MP, Mecacci F, Guaschino S, Paidas MJ. Inherited and acquired thrombophilias. Reprod Sci. 2014;21(2):167–182. doi: 10.1177/1933719113497282. [DOI] [PubMed] [Google Scholar]
- 8.Cox N, Johnson SA, Vazquez S, et al. Patterns and appropriateness of thrombophilia testing in an Academic Medical Center. J Hosp Med. 2017;12(9):705–709. doi: 10.12788/jhm.2804. [DOI] [PubMed] [Google Scholar]
- 9.Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely(R) campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879–3883. doi: 10.1182/blood-2013-07-518423. [DOI] [PubMed] [Google Scholar]
- 10.Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis. 2011;31(3):275–281. doi: 10.1007/s11239-011-0572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352–2361. doi: 10.1001/jama.293.19.2352. [DOI] [PubMed] [Google Scholar]
- 12.Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35(7):695–710. doi: 10.1055/s-0029-1242723. [DOI] [PubMed] [Google Scholar]
- 13.Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209–220. doi: 10.1111/j.1365-2141.2009.08022.x. [DOI] [PubMed] [Google Scholar]
- 14.Kearon C. Influence of hereditary or acquired thrombophilias on the treatment of venous thromboembolism. Curr Opin Hematol. 2012;19(5):363–370. doi: 10.1097/MOH.0b013e328356745b. [DOI] [PubMed] [Google Scholar]
- 15.Gupta Arjun, Sarode Ravi, Nagalla Srikanth. Thrombophilia Testing in Provoked Venous Thromboembolism. JAMA Internal Medicine. 2017;177(8):1195. doi: 10.1001/jamainternmed.2017.1815. [DOI] [PubMed] [Google Scholar]
- 16.Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Stefano V, Rossi E. Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the guidelines from scientific societies and working groups. Thromb Haemost. 2013;110(4):697–705. doi: 10.1160/TH13-01-0011. [DOI] [PubMed] [Google Scholar]
- 18.Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e691S–e736S. doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackie I, Cooper P, Kitchen S. Quality assurance issues and interpretation of assays. Semin Hematol. 2007;44(2):114–125. doi: 10.1053/j.seminhematol.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Shen YM, Tsai J, Taiwo E, et al. Analysis of thrombophilia test ordering practices at an academic center: a proposal for appropriate testing to reduce harm and cost. PLoS One. 2016;11(5):e0155326. doi: 10.1371/journal.pone.0155326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith TW, Pi D, Hudoba M, Lee AY. Reducing inpatient heritable thrombophilia testing using a clinical decision-making tool. J Clin Pathol. 2014;67(4):345–349. doi: 10.1136/jclinpath-2013-201840. [DOI] [PubMed] [Google Scholar]
- 22.Hom Jason, Kumar Andre, Evans Kambria H, Svec David, Richman Ilana, Fang Daniel, Smeraglio Andrea, Holubar Marisa, Johnson Tyler, Shah Neil, Renault Cybele, Ahuja Neera, Witteles Ronald, Harman Stephanie, Shieh Lisa. A high value care curriculum for interns: a description of curricular design, implementation and housestaff feedback. Postgraduate Medical Journal. 2017;93(1106):725–729. doi: 10.1136/postgradmedj-2016-134617. [DOI] [PubMed] [Google Scholar]
- 23.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154–164. doi: 10.1007/s11239-015-1316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis. 2015;39(3):367–378. doi: 10.1007/s11239-015-1197-3. [DOI] [PubMed] [Google Scholar]
- 26.Miglioretti DL, Heagerty PJ. Marginal modeling of nonnested multilevel data using standard software. Am J Epidemiol. 2007;165(4):453–463. doi: 10.1093/aje/kwk020. [DOI] [PubMed] [Google Scholar]
- 27.Effectiveness of Factor V Leiden and Prothrombin Mutation Testing in Patients Presenting With a First Unprovoked Venous Thromboembolic Episode: A Systematic Review and Economic Analysis [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015 Mar. (CADTH Optimal Use Report, No. 4.1A.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK361450/. [PubMed]
- 28.Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801–804. doi: 10.1002/jhm.2616. [DOI] [PubMed] [Google Scholar]
- 29.MacWalter G, McKay J, Bowie P. Utilisation of internet resources for continuing professional development: a cross-sectional survey of general practitioners in Scotland. BMC Med Educ. 2016;16:24. doi: 10.1186/s12909-016-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talaei-Khoei T, Talaei-Khoei A. Americas Conference on Information Systems. 2015. Peer learning in the class or on Facebook? A correlational experiment on learning outcomes. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Compilation of criteria used for determining appropriateness and statistical models used for analysis. (DOCX 59 kb)
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to inclusion of protected health information but are available from the corresponding author on reasonable request.