Preface
There has been an explosion in the number of papers discussing the hypothesis of ‘pathogenic spread’ in neurodegenerative disease — the idea that abnormal forms of disease-associated proteins, such as tau or α-synuclein, physically move from neuron to neuron to induce disease progression. However, whether inter-neuronal spread of protein aggregates actually occurs in humans and, if so, whether it causes symptom onset remains uncertain. Even if pathogenic spread is proven in humans, it is unclear how much this would alter the specific therapeutic approaches that are in development. A critical appraisal of this popular hypothesis thus appears both important and timely.
Introduction
Progressive accumulation of aggregates of specific proteins in the brain is a defining feature of many common neurodegenerative diseases, including Alzheimer disease (AD), Parkinson disease (PD), and fronto-temporal dementia (FTD)1, 2. Certain rare infectious neurological diseases known as transmissible spongiform encephalopathies (TSEs) are associated with abnormal folding and aggregation of the prion protein (PrP)3, and the steady accumulation of PrP aggregates is a necessary prequel to neurodegeneration in most TSEs (Box 1). It has been speculated that the protein deposits present in other neurodegenerative diseases may form and spread from region to region in a manner analogous to that of misfolded PrP in TSEs4, 5. A recent comprehensive review from scientists who support this hypothesis concluded that “the paradigm of pathological protein propagation in neurodegenerative disease is now firmly established”6. However, important gaps remain in our understanding of whether neuron–to–neuron physical spread of protein aggregates actually occurs in humans with neurodegenerative diseases and, if it does, whether it is required for pathogenesis. Moreover, the emphasis on terms such as “prion-like” as a mechanistic explanation for common neurodegenerative diseases seems premature. In terms of protein aggregation, “prion-like templating” is very similar to the long-standing concept of the seeded polymerization of amyloid-prone proteins7, 8, but the molecular mechanisms of PrP proliferation and neurotoxicity in classical prion diseases are not fully understood, and therefore referring to a pathogenic process as prion-like does not provide mechanistic precision.
Box 1: Prion disease.
Current understanding of prion diseases is rooted in the study of scrapie, an infectious disease that can develop in healthy sheep housed with diseased animals or pastured on land previously occupied by diseased animals101. In the late 1930s, it was established that inoculation of healthy sheep with CNS material from diseased sheep caused scrapie after an incubation period of more than 1 year102. Subsequently, it was shown that injection of scrapie sheep brain into mice caused encephalopathy103. In 1959, the striking neuropathological similarity between scrapie and a human epidemic, Kuru, which afflicted an isolated Polynesian population that practiced ritualistic cannibalism, was noted104. Intracerebral inoculation of chimpanzees with brain suspensions from Kuru patients precipitated a Kuru-like syndrome105. Contemporaneously, it was noted that Creutzfeld-Jakob disease (CJD) had many similarities to Kuru106, and inoculation of monkeys with human CJD brain induced a CJD-like disorder107.
In a landmark opinion piece, Prusiner drew on ideas from Griffith108 and others to detail a “protein only” hypothesis of transmissible spongiform encephalopathies (TSEs), and introduced the term prion – a “proteinaceous infectious particle which is resistant to most procedures that modify nucleic acids”3. His prion hypothesis was strongly supported by purification of infectious activity from diseased hamster brain, which resulted in the enrichment of a single major (protease-resistant) protein from the diseased hamster brain designated PrP27–30109. Amino acid analysis revealed that PrP27–30 was derived from a slightly larger normal protein -- the cellular prion protein (PrP)110, 111. The identification of the gene encoding PrP (PRN) and the discovery that all familial prion diseases are linked to mutations in PRN112, 113 and that expression of PrP is necessary for disease114 revolutionized the study of TSEs.
The central tenet of the prion hypothesis remains that infectivity is mediated by a change in the structure of cellular PrP leading to the formation of a conformer that can bind and convert other PrP molecules into aberrant multimers. In sporadic and familial prion diseases, aberrant PrP arises spontaneously, whereas infectious TSEs result from the introduction of exogenous aberrant PrP, which can corrupt endogenous normal PrP. Classical nucleation-dependent protein polymerization models are consistent with this sort of prion proliferation115–117, but it is important to note that although all TSEs are associated with the PrP aggregation and PrP can form amyloid fibrils, not all PrP aggregates are amyloid.
It is now recognized that there are multiple infectious forms of PrP, that they span a large mass range118, and that some are protease-sensitive119, 120. Similarly, evolving data suggest that the infectious agent and the toxic species may be partially distinct71, 73, 121. A major challenge in prion disease research is to identify the forms of the aberrant PrP protein that mediate neurotoxicity and determine how they do so.
In this Perspective, we review and discuss the strengths and weaknesses of the current evidence underpinning the hypothesis of pathogenic spread in neurodegenerative disease. Here we define the pathogenic spread hypothesis as the theory that abnormal forms of a protein implicated in human neurodegeneration, for example, tau or α-synuclein, move from neuron to neuron to induce disease progression. In doing so, we conclude that pathogenic spread of protein aggregates could contribute to non-PrP neurodegenerative diseases but that selective neuronal vulnerability is also likely to play a major part. We also list a number of currently unanswered scientific questions about the pathogenic spread hypothesis and suggest experimental approaches to rigorously test its fundamental tenets. Specifically, we advocate for an experimental focus on the measurement of changes in neuronal function, rather than merely measuring the progressive accrual of protein aggregates in the nervous system. We address the possibility, suggested by the pathogenic spread hypothesis, of iatrogenic transmission of major neurodegenerative diseases and advocate for the prioritization of studies on the potential for horizontal transmission of common neurodegenerative diseases. Finally, we consider whether proving that this hypothesis is correct would materially change current drug development efforts9.
Spread or selective vulnerability?
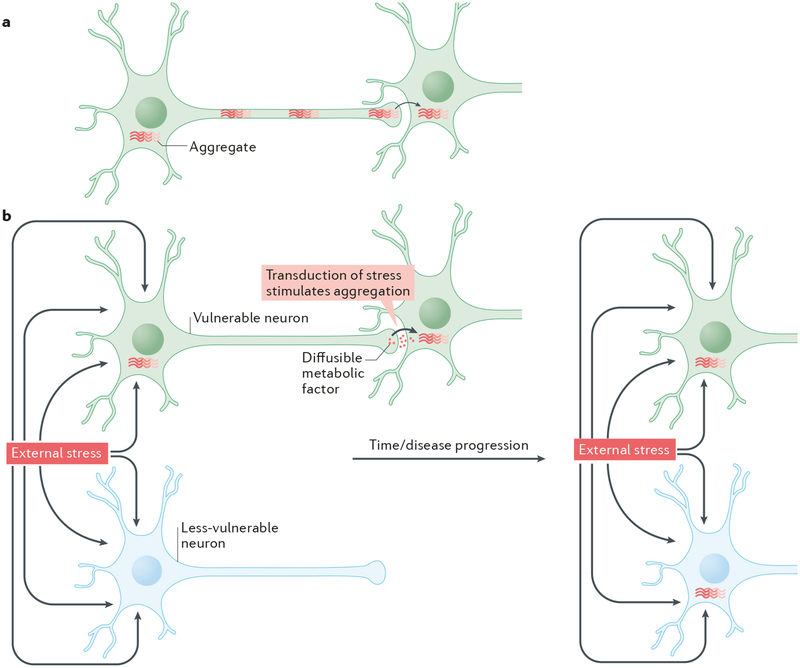
The pathogenic spread hypothesis suggests that the progressive accumulation of protein aggregates across neuronal populations and brain regions that is observed in common neurodegenerative diseases10, 11 is analogous to the accumulation of PrP3 within the brain in TSEs (Fig 1a), both in terms of its mechanisms and its contribution to symptom progression. Proponents of this hypothesis therefore characterize the spreading mechanism in diseases such as AD, PD or FTD as ‘prion disease’4, ‘prion-like’5, 12, 13 or ‘prionoid’14, 15. The principal alternative hypothesis for the progressive involvement of regional populations of neurons in protein misfolding diseases is the concept of selective neuronal vulnerability. According to this older concept, certain neurons are intrinsically more vulnerable to the underlying pathogenic process of a disease (such as those that cause the misfolding and aggregation of a certain protein) than others16, 17, perhaps based on their genetic expression profiles, and the former neurons thus become dysfunctional and structurally abnormal earlier than the latter (Fig 1b). The pathogenic spread hypothesis tends to emphasize a non-cell-autonomous mechanism of disease, whereas the selective neuronal vulnerability hypothesis tends to emphasize a more cell-autonomous mechanism. However, as we will discuss, neither model appears to fit perfectly with the pathogenic processes in human and experimental disease.
Figure 1. The pathogenic spread and selective vulnerability hypotheses.
There are at least two possible explanations for how the localization of aggregates in the brain might change as neurodegenerative diseases progress. a| According to the pathogenic spread hypothesis aggregates generated in one brain region physically move from neuron to neuron and thus spread into connected brain regions. b| The selective vulnerability hypothesis suggests that, in response to certain adverse conditions (such as external stress), protein aggregation is initiated in a subset of neurons that are particularly vulnerable to the adverse stimuli. Protein aggregates first appear in the cells most susceptible to the adverse conditions, and with time emerge in less susceptible cells. This hypothesis also supports the idea that disease pathogenesis may spread trans-synaptically: however, it suggests that this is mediated by the spread of diffusible metabolic factors that result in the transduction of the effects of the adverse conditions to a neighbouring neuron, rather than a direct physical transfer of protein aggregates. It is important to note that these 2 possibilities are not mutually exclusive and that a combination of both hypotheses may occur.
Even in the case of ‘classical’ prion diseases, it is not always clear whether the spread of PrP across brain regions can be better explained by non-cell-autonomous or cell-autonomous mechanisms. This is illustrated by a single mutation in PrP, the D178N mutation, which can cause two distinct diseases that target different brain regions. Which disease occurs depends on the identity of a polymorphic codon in PrP. The D178N mutation causes fatal familial insomnia (FFI) when the carrier expresses PrP with a methionine at position 129, whereas a familial form of Creutzfeldt-Jakob disease (fCJD) results when codon 129 is valine18. fCJD is primarily a cognitive disorder targeting the cortex and is characterized by severe neuronal loss, spongiosis and amorphous PrP aggregates19. In striking contrast, FFI causes neuronal loss in the thalamus, with little evidence of PrP deposits or spongiosis19. The fact that two different non-pathogenic PrP variants that are present in all neurons can dictate the specific populations of neurons that are affected by a mutant PrP molecule that is also present in all neurons suggests that the site of origin and initial form of misfolded PrP is mediated by factors present in certain neurons but not others that make the former neurons more vulnerable to misfolding of particular PrP structures. Thereafter, the regional progression of disease may be dictated in part by neuronal connectivity. However, although there is experimental evidence of neural spread of infection to the brain following injection of the scrapie agent (PrPsc) into the sciatic or optic nerves20, 21, little is known about whether actual trans-synaptic movement of aberrant PrP between neurons occurs in human prion disease (Dr. S. Brandner, personal communication). Thus in classical prion diseases, the apparent ‘spread’ of aberrant PrP across brain regions may be determined by both cell-autonomous and non-cell-autonomous factors, and the same could be true of common neurodegenerative diseases.
Diseases proposed to involve inter-neuronal spread
Much of the interest in the pathogenic spread hypothesis has focused on three diseases: AD, PD and FTD. In each of these, specific proteins that are normally expressed by all neurons throughout life in soluble, physiological forms can accumulate as abnormally folded and increasingly insoluble forms and become deposited inside or outside neurons. In AD, abnormal forms of amyloid β-protein (Aβ) accumulate over time as soluble oligomers and insoluble extracellular amyloid fibrils, and the microtubule-associated protein, tau, accumulates in abnormal oligomers and insoluble filaments (neurofibrillary tangles) inside select cortical and subcortical neurons. In a substantial portion of patients with FTD, tau accumulates as soluble oligomers and insoluble tangles that are biochemically similar but not identical to those seen in AD, and some familial cases of FTD are caused by missense or splicing mutations in the gene encoding tau (MAPT)22. In the case of PD, most familial and sporadic (“idiopathic”) cases are characterized by the accumulation of insoluble deposits of the ubiquitous neuronal protein, α-synuclein, in select perikarya (known as Lewy bodies) and neurites (called Lewy neurites). Such Lewy aggregates can also occur in some or sometimes many cortical and subcortical neurons in AD23.
Human data and the spread hypothesis
Many papers which promote the pathogenic spread hypothesis begin by citing the elegant neuropathological staging of neurofibrillary tangles in AD24 and Lewy inclusions in PD10 that was reported by Braak and colleagues. Their staging scales were developed by examining postmortem brains from many unrelated humans that had died at various ages and from various causes. The Braak staging suggested that certain sets of neurons and brain regions are affected by neurofibrillary tangles or Lewy bodies well before others and that there is an approximate temporal sequence of regional lesion accrual over decades. This has been widely interpreted as providing support for the idea that there is a physical spread of protein aggregates from one neuron to the next5, 6. However, assembling the brains of many different individuals dying at different ages into a unified temporal continuum of AD is problematic, especially because tangles of tau protein occur in more than a dozen human diseases of diverse etiologies, not just in AD25. Therefore, in our Opinion, the Braak staging system is neither proof of nor an argument against the spreading hypothesis; it does not preclude the alternate concept of a temporally selective regional vulnerability of neurons to lesion formation.
Furthermore, the detection by Braak et al. of minor amounts of neurofibrillary change in the locus coeruleus of children that died at ages 6 and 14 of other causes does not imply, as those authors suggested26, that such individuals would necessarily have developed AD had they lived to a late age. Nevertheless, a recent review of pathogenic protein spreading cited this study to conclude that6 “a stereotypical pathology pattern was first established for AD, in which tau aggregates are found first in the locus coeruleus”6. We believe that the fact that tau aggregates do not occur solely in AD makes this simple deduction from the study of postmortem brains hazardous.
With regard to using the pattern of Lewy bodies to “stage” PD, some studies have reported significant patient heterogeneity and suggested that the Braak system does not agree with the pattern observed in almost half of cases27, 28. This large degree of heterogeneity in PD suggests that the spatiotemporal route of spreading proposed by Braak is not the only one. Although these variations do not preclude cell-to-cell transfer of α−synuclein, they do exclude the notion of a stereotyped progression from a single start site. Moreover, PD-causing mutations can produce diverse neuropathological changes, even within a single family: for example, in families carrying mutations in Parkin, some affected individuals can lack α−synuclein deposits whereas their affected siblings can have many Lewy bodies29–31. Thus, the presence and inter-neuron spread of Lewy-type aggregates is not required for dysfunction and loss of dopaminergic neurons and ensuing motor symptoms of parkinsonism.
Another clinical observation that helped provide a conceptual basis for the pathogenic spread hypothesis was the discovery of the development over many years of scattered Lewy bodies in fetal neurons that were therapeutically implanted into the striata of a small number of advanced PD patients32, 33. Several postmortem studiesdetected Lewy bodies in ~5–10% of the grafted cells in patients that had survived 9 or more years32–34. Overall, grafted cells remain viable and apparently functional for long periods of time35, 36, and the appearance of Lewy bodies in a small minority of cells appears to have had little functional consequence37. Several factors could be responsible for the development of Lewy bodies and loss of tyrosine hydroxylase-positive neurons within grafts. One possibility is that occasional Lewy bodies arise in grafted neurons because of the surrounding PD pathogenic process (including astrocytosis, microgliosis and neuronal death), which facilitates the misfolding of a portion of the abundant endogenous α−synuclein in the grafted cells – a prospect supported by the finding that microglial activation within grafts is associated with the local development of Lewy bodies33–35. By contrast, the pathogenic spread hypothesis proposes that misfolded α−synuclein is physically transferred from diseased host cells into grafted cells and that the aberrant host α−synuclein “corrupts” the normal α−synuclein of graft cells. These two possibilities are often thought of as mutually exclusive, but they may be synergistic. For example, the patchy activation of microglia observed in transplant tissue may explain why LBs are not distributed in a manner confined to synaptic pathways formed by the integration of the graft34. Whatever the explanation for how occasional Lewy bodies arise in grafted tissue, it cannot be resolved by histological study of the end-stage tissue. Thus, the data from fetal graft studies can be viewed as potentially consistent with the pathogenic spread hypothesis but not proof that such a mechanism occurs in humans.
Some mouse studies suggest inter-neuron spread
Compelling evidence of the physical transfer of tau within the perforant pathway comes from transgenic mouse models that express intraneuronal human tau selectively in the entorhinal cortex and then develop human tau cytopathology in downstream neurons (dentate granule cells)38–40. In such studies, it has been noted that “in many [recipient] cells, mouse tau aggregates were morphologically more robust than the human tau nidus”41, suggesting a templating of endogenous mouse tau within the dentate granule neurons by human tau aggregates that were presumably transported trans-synaptically from the entorhinal cortex neurons. A technical caveat to the interpretation of these studies has been raised: whether the firing of the promoter of the tau transgene is entirely restricted to the entorhinal neurons40. Further mechanistic analyses of such cell-selective transmission models of intraneuronally expressed tau or αSyn represent a particularly attractive way to strengthen the evidence for the pathogenic spread hypothesis.
As has been shown for Aβ inoculation41, the acceleration of tau lesion formation in tau transgenic mice by intracerebral inoculation of brain extracts from tangle-bearing tau transgenic mice42 or brain extracts from human tauopathy cases43 or recombinant tau44 is now well established. However, tau transgenic mice often exhibit progressive motor phenotypes that make it difficult to assess AD- or FTD-like effects on cognition, and only recently have investigators begun to provide evidence connecting such accelerated tau deposition to dysfunction in neural circuits that mediate memory and learning44. As yet, there is no evidence that inoculation of primates with tau-containing AD brain extracts induces tangle formation45; therefore, it remains uncertain whether seeded aggregation of tau can occur in humans that express endogenous levels of normal tau. On the other hand, there are data indicating that intracerebral inoculation of wild-type rodents and primates with “pre-formed fibrils” (PFFs) of recombinant αSyn46 or Lewy bodies isolated from human brain47 can induce Lewy body formation in the recipients. Although these experiments used extracellular application of artificially high concentrations of “preformed fibrils” or highly enriched Lewy body preparations and examined only small numbers of injected monkeys, they are important because they showed induction of disease-relevant histological lesions in wild-type animals. Nonetheless, further experiments using more substantial numbers of primates and searching for functional consequences are now required.
A concept that is often under-emphasized by proponents of the pathogenic spread theory is the need to link the progressive development of histological lesions to actual functional effects48, 49. Longitudinal studies in transgenic mice suggest that the existence of neurofibrillary tangles per se may not necessarily disrupt neural function50, 51. Similarly, a very recent mouse study suggests formation of tau tangles alone may be insufficient to induce toxicity52. The accumulation of protein aggregates certainly indicates that a disease-associated alteration in the homeostasis of that protein has occurred; however, it is plausible that neurofibrillary tangles and Lewy bodies are temporary storage lesions that may alter neuronal architecture, whereas soluble aggregates (oligomers) that are in equilibrium with them may be the principal mediators of active neural dysfunction9, 53. Furthermore, not all aggregates that form from a particular protein are toxic; only some may have significant bioactivity. Whether we are speaking of selective neuronal vulnerability to protein aggregation or a cell-to-cell spread of the aggregates, it is important to link the process of aggregation of a causative protein and disposal of the aggregates to the cytotoxicity that gives patients (or animals) their symptoms.
Cell biology of pathogenic spread
The signaling pathways by which the accumulation of soluble oligomers and insoluble fibrils of Aβ on the neuronal surface (Box 2) leads to hyperphosphorylation and insolublization of intraneuronal tau are ill-defined. Likewise, why a portion of the abundant wild-type αSyn protein present in all neurons begins to misfold and aggregate in a small subset of neurons, principally on one side of the brain, early in sporadic PD is unclear. It is acknowledged that the pathogenic spread hypothesis does not explain the initiation of the first misfolding or aggregation events in previously healthy humans6. Instead, it hypothesizes that at least four subsequent steps occur. First, some tau or some α−synuclein proteins are released, by unknown mechanisms, into the extracellular space (interstitial fluid, ISF) after (and perhaps even before) their self-aggregation into “seeds”. Second, the seeds (aggregates) travel in the ISF to nearby and/or distant neurons (perhaps in part by a trans-synaptic process). Third, the aggregates are selectively internalized by some neurons (but not by many other adjacent neurons) through unknown mechanisms. Fourth, they then induce (“template”) normal cytoplasmic tau or α−synuclein to misfold and aggregate in the recipient cells. This cycle would be repeated a great many times over years to produce the large number of neuronal and neuritic protein aggregates found in the diseased human brain.
Box 2: Propagation of extracellular Aβ aggregates.
Aβ is normally present in the interstitial fluid and can aggregate outside cells. It has been relatively straightforward to induce progressive Aβ aggregation by extracellular injection in rodents122–125 and primates45, 96 predisposed to develop amyloid deposits. These findings demonstrate an acceleration of protein aggregation by seeds and are in line with the seeded fibrilization of Aβ reported in vitro more than a decade earlier8. Using synthetic Aβ, it was demonstrated that addition of preformed fibrils to a monomeric solution accelerated aggregation of the monomer. This seeding effect was interpreted to mean that amyloid formation is a nucleation-dependent process: that is, formation of a kinetically stable nucleus is rate limiting, but once a stable nucleus is formed, further polymerization and fibril growth become exponential126. More recently, the mechanistic understanding of amyloid formation has been expanded to incorporate secondary nucleation, a process by which fibril surfaces catalyze the rapid conversion of monomers into aggregation-competent nuclei117, 127. Thus, for Aβ, the occurrence of progressive, seeded extracellular polymerization is well-supported and poses no unusual cell biological requirements.
As far as we are aware, the in vivo Aβ seeding studies reported to date have focused on the formation of amyloid deposits without determining whether the accelerated formation of deposits has AD-relevant functional consequences. In our opinion, this is a serious flaw, given that amyloid plaque number per se correlates poorly with memory impairment in humans128 and that plaques isolated from AD brain have low synaptotoxic activity compared to the diffusible oligomers with which they are in equilibrium129. In contrast to the in vivo Aβ seeding studies, analogous experiments in prion diseases use changes in neurological function and time to death as outcomes. It should also be noted that there are disorders in which fibrous amyloid deposits formed by other proteins appear to be relatively inert130, 131. Insoluble amyloid plaques in AD are space-occupying lesions that can probably disrupt local neuronal connections132 and induce an unhelpful inflammatory response, but they appear not to be the principal toxic form that triggers neuronal dysfunction. Indeed, plaques may often possess a halo of synaptotoxic oligomers133 or may be relatively inert. Given that different Aβ-rich inocula can induce different types of aggregated Aβ deposits125, it becomes important to determine if any of the latter also mediate neuronal dysfunction.
To make the elegant and detailed in vivo injection studies of Aβ preparations122–125, 134, 135 truly relevant to AD in humans, one needs to demonstrate functional consequences of the accelerated extracellular aggregation. Similarly, a recent observational study reported that individuals who had been treated with human cadaver-derived growth hormone and subsequently developed iCJD also had detectable levels of vascular and parenchymal β-amyloid136, 137. Importantly, their brains did not contain neurofibrillary tangles, indicating that these individuals did not have AD, and even if they had lived longer, it is uncertain that they would have developed AD. In terms of clinical relevance, the observed vascular Aβ deposits (congophilic amyloid angiopathy, CAA) may be of more concern than the parenchymal Aβ deposits, because the physical build up of CAA can sometimes lead to micro-hemorrhages and ultimately stroke, whereas it is less clear that Aβ deposits in the parenchyma alone have significant functional consequences.
Several fundamental tenets of cell biology as currently understood have to be overcome or at least modified to enable these steps to occur. Tau and α−synuclein are both largely cytosolic, and their disease-associated aggregates also occur overwhelmingly in the cytoplasm. Small amounts of tau54–60 and α−synuclein61–63 are detected in the medium of cultured neural cells, in cerebrospinal fluid and in plasma, and treatment of transgenic mice with antibodies (which should largely act extracellularly) has been shown to attenuate α−synuclein and tau deposition (e.g.64, 65). But the mechanism for the release of cytoplasmic α−synuclein and tau into the extracellular space needs to be established (Fig. 2). The proteins must then stay aggregated in the ISF, where they are likely to be in a more dilute solution than they were intracellularly, and they must potentially diffuse long distances between cells, even though their exposed amphipathic amino acids predict that they would adsorb in large part to the lipid surfaces of myriad local cells and their processes. Next, the aggregates must be selectively internalized by just certain neurons (Fig. 2). If this occurs by some form of pinocytosis or receptor-mediated endocytosis, as has been suggested66,66, they would enter the cell in vesicles, where they could not contact the large cytosolic pools of endogenous tau or α−synuclein (Fig. 2). Thus, a specialized mechanism for their transport from the vesicle lumen into the cytosol needs to be invoked before the fourth step proposed by the pathogenic spread hypothesis can occur (Fig. 2).
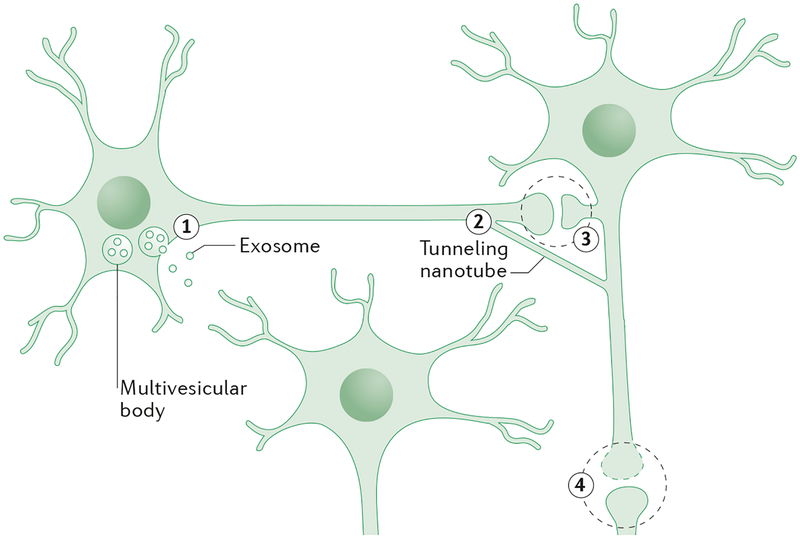
Figure 2. Possible mechanisms for inter-neuronal transfer of proteins.
In the figure, two neurons are synaptically connected, with illustrative synapses shown in magnified views. A third neuron (lower center) is near to the other neurons but not synaptically connected. Neither α−synuclein nor tau contain the signal sequences necessary for conventional secretion, so their release must occur by a non-classical mechanism. Over the past decade, two non-classical mechanisms for inter-cellular communication have emerged: the secretion of small vesicles called exosomes138 (1), and the formation of thin membranous bridges termed tunneling nanotubes (TNTs)139 (2). Exosomes could potentially travel across synapses or longer distances and facilitate transfer of proteins to other cell types. Similarly, TNTs facilitate communication between neural and non-neural cells, often over long distances. Interestingly, both exosomes and TNTs have been implicated in the movement of infectious PrP in experimental models140. If cell-to-cell transfer of non-PrP neurodegenerative disease-related proteins occurs via exosomes or TNTs, then the proteins inside these structures are unlikely to be fully accessible to antibodies used in immunotherapy (see Figure. 3). A third possible mechanism (3) involves the release and uptake of the naked protein. α−synuclein is present in pre-synaptic endings76, 141, and tau is present in post-synaptic elements142, 143. During neurotransmission, it is possible that small amounts of either protein could leak between the pre- and post-synapse. The more abundant a neuronal protein is, the more likely this is to occur (both α−synuclein and tau are highly abundant)59, 141. Finally, an obvious but little discussed possibility (4) is that proteins are released as a secondary effect of synaptic or cellular compromise144. Little is known about the cell biological mechanisms by which pathogenic proteins that are released are taken up by neurons and how they encounter the cognate endogenous protein that they are proposed to template.
The pathogenic spread field has so far obtained few insights into how these salient cell biological issues can be addressed to validate the biological plausibility of inter-neuronal seeding as responsible for disease propagation in patients. Rather, studies in this field have applied often supra-physiological concentrations of various in vitro aggregated, sonicated forms of recombinant tau or α−synunclein (such as PFF) extracellularly and then analyzed their cytopathological consequences, focusing principally on the induction of new aggregates and rarely on their potential functional toxicity.
As regards pathogenic spread in TSEs, the four requirements detailed above for cytosolic proteins like tau and α-synuclein do not apply. PrPc is anchored extracellularly on the plasma membrane, where it undergoes recycling endocytosis67. In addition, PrP is secreted under normal circumstances68–70, so there are plausible mechanisms through which PrP could be released from a donor cell and taken into a recipient cell. Stating that the disease mechanisms of AD, PD and FTD are similar to that of Creutzfeld-Jacob disease and other prion disorders misses the concern that the mechanism of regional vulnerability and selective neurotoxicity in the latter diseases is not established17. It should be emphasized that even after >30 years of intensive investigation, the sites of conversion of cellular PrP into infectious and neurotoxic forms of PrP are unknown67, 71, 72. While there is no doubt that prion diseases are potentially infectious and can under special circumstances be transmitted between humans (Box 1), the actual neuropathology of the classical prion diseases (TSEs) is varied and widespread, and it is not necessarily restricted to well-defined anatomical pathways. Precisely how prions induce neuronal dysfunction and cell death (and thus rapidly progressive encephalopathies) is not settled73–75. While there is value comparing and contrasting prion diseases with AD and PD, likening AD and PD to disorders whose mechanism of clinical dysfunction remains to be delineated does not provide mechanistic clarity. In terms of understanding the biological mechanisms of protein release and uptake, referring to tau and αSyn as “prions” or “prion-like” tells us little about the actual cellular mechanisms involved..
Additional challenges
The complexity and heterogeneity of brain region involvement among different humans with AD, PD or FTD suggest that establishing a stepwise regional hierarchy of disease progression is not straightforward. For example, in studies of PD, a “dual-hit” corollary of the pathogenic spread hypothesis has been put forward56 in light of evidence that early α−synuclein aggregates can be detected in both the parasympathetic nervous system in the gut76, 77 and the olfactory bulb78. Moreover, there is growing evidence that scattered autonomic fibers widely distributed throughout the skin may accumulate α−synuclein in early stages of PD79. In presymptomatic AD (for example, in Down’s syndrome subjects <20 years old), diffuse extracellular Aβ deposits can be very widespread in the telencephalon80, making it challenging to deduce a specific temporal hierarchy of affected brain regions. The incredible complexity of neuronal connectivity in the human nervous system suggests that almost any regional pattern of lesions that one might observe could be said to follow some neuroanatomical pathways.
Even if specific neuroanatomical pathways are implicated in lesion formation, this does not necessarily indicate the physical spread of protein aggregates from neuron to neuron. Application of preformed aggregates of tau or α−synuclein to neurons in vitro has shown uptake and axonal movement of some aggregates81–86, but such experiments involve the extracellular application of supra-physiological concentrations of otherwise overwhelmingly intraneuronal proteins, in a way that may not occur in humans. It could instead be that accrual of protein aggregates in a neuron causes its dysfunction (and ultimately death) and thereby produces abnormal inter-cellular signaling to downstream neurons, which promotes their own abundant intracellular tau or α−synuclein molecules to misfold and aggregate (Fig. 1b). This would convey a kind of trans-synaptic metabolic insult without necessitating a physical spread of the aggregates17.
Those who favor the pathogenic spread mechanism acknowledge that it does not explain how protein misfolding is originally initiated in a few neurons, thereby enabling subsequent inter-neuron spread. In this regard, let us consider the case of inherited mutations in tau or α−synuclein that cause aggressive early-onset forms of otherwise rather typical FTD and PD, respectively. In these individuals, 50% of the very abundant tau and α−synuclein molecules present in every neuron is mutant and thus prone to misfolding. It is not biologically parsimonious to stipulate that the involvement of many neurons over time in such familial patients requires a cell-to-cell physical delivery of misfolded aggregates rather than a (relatively) cell-autonomous misfolding due to the lifelong abundance of endogenous seeds in each neuron which could enable intracellular templating. Thus, cell-to-cell transport of tau or α−synuclein aggregates may not be required for the occurrence of clinical disease. Further, it seems unlikely that such familial cases operate by an entirely different cell biological mechanism than the common “idiopathic” cases; in AD, the familial and “sporadic” forms are largely indistinguishable as to clinicopathological patterns, save for their different ages of onset.
All of these arguments by no means obviate the pathogenic spread hypothesis, but they suggest that selective neuronal vulnerability in a partially cell-autonomous form may contribute importantly to the development and progression of human neurodegenerative disease. As often occurs in natural systems, these two mechanisms (and more) may operate simultaneously and synergistically to yield the complex phenotypes of these slowly evolving disorders.
Potential for iatrogenic infection
Proponents of the pathogenic spread hypothesis often state that there is no evidence for iatrogenic transmission of non-PrP neurodegenerative diseases. Thus, while emphasizing the similarities of PrP, tau, Aβ and α−synuclein and highlighting the evidence that exogenously delivered protein can induce aggregation of the host protein, they suggest that PrP and TSEs are special and distinct from the common neurodegenerative diseases. Human prion diseases initiated by actual PrP infection are believed to account for only a small percentage of human TSEs and to require direct exposure of the host to prion-infected tissue or consumption of contaminated meat. Human TSEs are not contagious in a manner analogous to diseases mediated by microbes. Rather iatrogenic human CJD can occur following treatment with pituitary hormones derived from human cadavers, implantation of dura mater grafts, corneal transplantation, the use of prion-contaminated surgical instruments, and blood transfusions37, 87, 88. Kuru (Box 1) and variant-CJD (vCJD) are the only examples of non-iatrogenic acquired human prion disease and are even more rare than iatrogenic CJD89, 90.
AD and PD are assumed not to be transmissible in humans because there is a lack of epidemiological evidence to support such a mechanism91, 92, and inoculation of non-human primates with material from AD and PD brains did not induce the respective diseases93–95. However, both these assurances are worth careful re-examination. In laboratory experiments and epidemiological studies, the potentially infectious nature of AD and PD has been judged by comparison to iatrogenic CJD. Such comparisons are complicated by the dramatically different temporal courses of clinical AD and PD on the one hand and clinical CJD on the other, and by the fact that CJD is extremely rare, whereas AD and PD are much more prevalent.
The two best known efforts to investigate the potentially infectious nature of AD came from the Laboratory for CNS Studies at the NIH93–95 and from the MRC Comparative Cognition Team at Cambridge University45, 96, 97. The work of both groups spanned decades and largely focused on TSEs but included some AD and PD cases. The NIH group used brains from 115 pathologically confirmed AD patients to inoculate 240 monkeys, with a mean time before culling of >9 years post-inoculation. Four out of 21 animals supposedly injected with tissue from 2 patients with familial AD died with CJD-like neuropathology, but these cases were later attributed to an experimental error: that is, it was likely that the inocula were accidentally contaminated with CJD tissue93, 98. All other AD inoculations and all 71 monkeys injected with PD brain extracts failed to induce disease. However, it is important to consider the crude outcomes that were used to measure the presence of disease: obvious neurological syndromes or death. AD is a chronic disorder that is often restricted initially to impairment of memory, but the memory capabilities of the inoculated primates were never assessed. Moreover, only rudimentary neuropathology was undertaken, and, although amyloid plaques were detected in the brains of some inoculated monkeys, it was unclear if these were induced by inoculation or arose from natural aging (Dr. C Masters, personal communication).
The Cambridge group was somewhat more systematic, and they reported compelling evidence that inoculation of marmosets with AD brain extracts induced modest cerebral β-amyloidosis. Aβ immunoreactive deposits were detected in 16 of 18 animals aged <10 years and 8 of 9 animals aged >10 years. All AD-inoculated animals (regardless of age) with incubation times longer than 3.5 years showed Aβ-immunoreactive deposits, whereas 3 marmosets euthanized after 11–14 months evinced no Aβ deposits. By contrast, spontaneous cerebral amyloid deposition was found in 0 of 11 uninjected marmosets <10 years and 5 of 29 >10 years45. The only confounding finding was that 3 of 3 marmosets inoculated with brain material from a 40 year old non-AD subject also showed Aβ immunoreactivity – a concern mitigated by the fact none of 5 marmosets inoculated with brain extract from a 20 year old subject had Aβ immunoreactivity. Neurofibrillary tangles were not detected in any animals. It seems, therefore, that inoculation with AD (and potentially prodromal AD) brain accelerated Aβ (but not tau) deposition in these primates. Beyond the fact that the animals did not develop overt neurological disease, any functional consequences of the induced Aβ deposition were not investigated (Dr. R. Ridley, personal communication). The importance of including functional assessments in the absence of overt clinical disease is demonstrated by the provocative recent finding that intrastriatal injection of PD brain-derived Lewy bodies into rhesus monkeys reduced nigrostriatal dopaminergic innervation (by PET scanning) just 9 months post-inoculation. Five months later, a similar reduction of striatal TH immunoreactivity was observed postmortem47. This report is highly preliminary, as it involved only 4 injected monkeys, and it is also artificial in terms of modeling possible iatrogenic transmission of PD, since it used semi-purified Lewy bodies. Nonetheless, the study provides the first indication of the possibility of a seeded aggregation event that can lead to neuronal dysfunction in man’s closest evolutionary relatives. Clearly, further investigation employing inoculation of primates with PD or AD brain extracts should now be undertaken.
Testing the spread hypothesis
It will be difficult to prove definitively in humans that inter-neuronal spread of misfolded protein seeds is necessary for the development of clinical symptoms in AD, PD and FTD. Nonetheless, the effort to achieve proof must be made. Here, we prioritize four broad types of experiments.
As reviewed above, attempts were made years ago to transmit AD by injecting extracts of AD brain into certain primate species, but these experiments used limited readouts. The field should return to primate injections of human brain extracts, using sensitive immunohistochemical and biochemical assays to search for the initiation of the AD or PD process and then cell-to-cell and region-to-region spread in the recipients. For AD, any spread could be searched for longitudinally by amyloid and tau PET scanning. Furthermore, by combining these scans with fluordeoxyglucose (FDG) PET scanning and cognitive assessment, it may be possible to determine whether there is a relationship between protein deposition and functional change in the injected primates
Second, endogenous aggregates of Aβ, tau and α−synuclein should be purified from affected human postmortem cortices, bioassayed for disease-relevant spreading potency in culture, and the most active preparations labeled with fluorescent or radioisotope tracers. The labeled bioactive preps can then be microinjected into various brain regions of (preferably knock-in) rodents expressing the corresponding human protein (Aβ, tau, or α−synuclein), and any movement from the site of inoculation be tracked by harvesting brains over increasing time intervals. Such quantitative experiments that begin with bona fide human brain-derived seeds could further delineate both the kinetics and the cytological consequences of protein spreading under more pathophysiologically relevant conditions. Later, rodents engineered to over-express or lack certain proteins required for endocytosis and vesicular trafficking could be used to dissect the cell biological mechanisms of neuronal release and uptake of seeds. Misfolded protein seeds might also be present in human CSF (and rodent ISF), and it may be possible to isolate these seeds and use them to induce aggregation in animals.
Third, current efforts should be accelerated to identify PET imaging ligands specific for pre-fibrillar aggregates99, because such ligands could potentially provide dynamic imaging evidence of region-to-region spread of these intermediate species in rodent models of AD or PD and perhaps later in patients. There are, of course, many challenging steps to achieve this goal, but the project to produce these ligands for clinical diagnostic purposes is already underway.
Fourth, in the case of PD, one should undertake even more rigorous efforts to document in humans the common initiation of the α−synuclein aggregation process in enteric neurons or in other peripheral sites prior to their appearance in various nuclei of the brain stem and then the basal ganglia and then the cerebral cortex. Although finding such a temporal association would not exclude the alternate possibility of selective neuronal vulnerability, pinning down a stereotyped initiation of α−synuclein aggregates in a spatial and temporal hierarchy of interconnected neuronal pathways could provide further support in humans for the pathogenic spread hypothesis.
Conclusions
Taken together, the extant data in the field at this writing suggest that some form of pathogenic spread of protein aggregates may contribute to non-PrP neurodegenerative diseases, but many details remain to be determined. We suggest that the prioritization of the experiments outlined above should be guided first by approaches that could have an immediate impact on human health, and second by studies that might enable identification of novel targets for development of therapeutics. Since we do not yet know whether there is a real potential for iatrogenic or incidental transmission of non-PrP neurodegenerative diseases, we suggest that exhaustive and sophisticated epidemiological studies, for example in corneal transplant recipients, be quickly initiated to examine this important and sensitive issue. The implementation of rigorous lab practices for scientists conducting experiments with relevant human neurodegenerative disease tissues and recombinant proteins should also be considered. Simultaneously, studies should focus not just on end-stage pathology of protein aggregates but also on brain imaging in vivo and behavioral assessments in primates. These approaches are essential to demonstrate human disease-relevant neural dysfunction and may also expedite read-outs in the primate studies, which are inherently long-term.
The terms “prion-like” or “prionoid” have been applied to many kinds of progressive protein templating by seeds, but this general idea has long been known as the seeded polymerization of proteins in various amyloidoses and preceded the specific knowledge of prions8. Thus, we suggest that the terms prion and prion-like should be reserved for diseases involving PrP and should only be applied to other proteinopathies if they are shown to require PrP per se or to mediate neuronal dysfunction by the same mechanism as prion diseases.
With regard to drug development for AD and PD, we previously summarized five targetable steps that are applicable to most human proteinopathies (Fig. 3)9. In terms of cell-to-cell transmission and the involvement of discrete anatomical pathways, the inter-neuron protein spread hypothesis, assuming it is ultimately proven in man, does not materially change the targets previously suggested for Aβ-directed therapies and currently being evaluated in humans. An exception would be avoiding implantation of tissues from subjects with these diseases if future work indicates that this is a indeed a cause of the diseases in some humans. However, for diseases involving intracellular proteins such as α−synuclein and tau, the validation of pathogenic spread in humans would elevate two notable targets: preventing release of the misfolded protein from donor cells, and preventing the uptake of the protein into recipient cells. Since these are the least understood aspects of the cell biology of pathogenic spread, priority should be given to identifying the mechanisms by which tau and α−synuclein are released from and then taken into neurons (Fig. 3). Of course, from a therapeutic perspective, preventing cellular release and uptake is a only an actionable target when release and uptake occur in a regulated manner. It is much more challenging when these processes happen in a constitutive manner or as a result of cell death. Moreover, there remains an urgent need to identify the actual neurotoxic agents in both prion diseases and non-PrP neurodegenerative diseases. This point cannot be overstated, since agents that prevent physical spread may not prevent neurotoxicity, and it may not be necessary to prevent spread to prevent neurotoxicity50, 100.
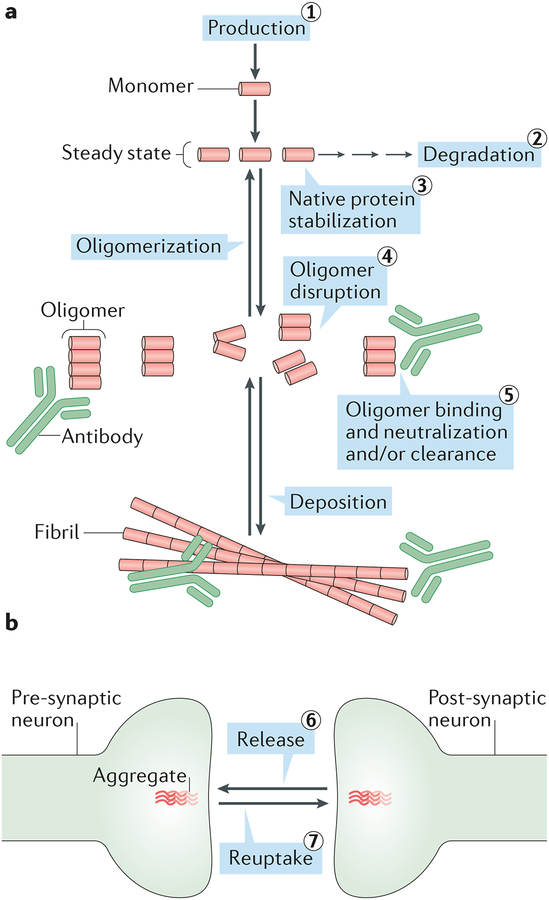
Figure 3. Strategies for targeting disease-associated neural protein aggregates.
a| The steady state levels of all proteins are controlled by their rates of production and degradation. Above a certain critical concentration, monomers can self-associate to form abnormal dimers, trimers, larger oligomers and insoluble aggregates. Consequently, reducing the levels of monomers by inhibiting their production (1) or stimulating their degradation (2) should decrease formation of pathogenic oligomers and larger aggregates. Agents that bind to and stabilize the native protein (3) should prevent abnormal oligomerization and allow for the natural removal of the protein by the brain’s degradative machinery. In this regard, an agent which stabilizes the native structure of the transthyretin (TTR) tetramer, tafamidis, has been approved for treating TTR amyloidosis145, and an analogous approach may be feasible for the native α-synuclein tetramer146. Conversely, agents capable of disrupting abnormal oligomers (4) should reduce their concentration and may prevent formation of larger aggregates such as fibrils. Antibodies or small molecules capable of binding to various abnormal assemblies (5) could neutralize the activity of oligomers and/or facilitate the clearance of deposited aggregates. In the case of binding by antibodies, this may include uptake of the complexes by microglia and/or their transport out of the brain. Peripherally administered antibodies should also be effective in the case of potential “pathogenic spread” from the blood or lymphatic system to the CNS. For simplicity, we refer to the native assembly state of neurodegeneration-associated proteins as monomer; however, there is growing evidence that αSyn normally exists as a tetramer, in which case the first step in the pathogenic aggregation process would be tetramer disassembly to excess free monomers inside neurons147. All 5 therapeutic approaches summarized here could be applicable to both extracellular and intracellular pathogenic proteins. B| If intracellular aggregation requires direct movement of aggregates from one neuron to another, then two additional approaches would be to inhibit the release of protein seeds (6) and to inhibit their re-uptake (7). If these processes occur via exosomes or tunneling nanotubes (Fig. 1), they may not be accessible to extracellular agents such as antibodies, and therefore would require new therapeutic strategies.
Acknowledgments
We thank Drs C. Masters and R. Ridley for sharing unpublished results and Drs. S. Brandner, P. Brundin, B. Caughey, J. Collinge, D. Harris, O. Isacson, J. Kordower, V. O’Connor and H. Perry for helpful discussions.
This work was support by grants from the National Institutes of Health to DMW (AG046275 and AG047505) and DJS (AG06173 and NS083845).
Footnotes
The authors declare no competing financial interests.
References
- 1.Selkoe DJ Folding proteins in fatal ways. Nature 426, 900–4 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Soto C Unfolding the role of protein misfolding in neurodegenerative diseases. Nature Reviews Neuroscience 4, 49–60 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB Novel proteinaceous infectious particles cause scrapie. Science 216, 136–44 (1982). [DOI] [PubMed] [Google Scholar]
- 4.Prusiner SB Cell biology. A unifying role for prions in neurodegenerative diseases. Science 336, 1511–3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frost B & Diamond MI Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci 11, 155–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brettschneider J, Del Tredici K, Lee VM & Trojanowski JQ Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci 16, 109–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glenner GG Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med 302, 1283–92 (1980). [DOI] [PubMed] [Google Scholar]
- 8.Jarrett JT & Lansbury PT Jr. Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 73, 1055–8 (1993). [DOI] [PubMed] [Google Scholar]
- 9.Walsh DM & Selkoe DJ Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett 11, 213–28 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Braak H et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24, 197–211 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H & Del Tredici K Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112, 389–404 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brundin P, Melki R & Kopito R Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11, 301–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guest WC et al. Generalization of the prion hypothesis to other neurodegenerative diseases: an imperfect fit. J Toxicol Environ Health A 74, 1433–59 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Aguzzi A Cell biology: Beyond the prion principle. Nature 459, 924–5 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Ashe KH & Aguzzi A Prions, prionoids and pathogenic proteins in Alzheimer disease. Prion 7, 55–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson MP & Magnus T Ageing and neuronal vulnerability. Nat Rev Neurosci 7, 278–94 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson WS Selective vulnerability to neurodegenerative disease: the curious case of Prion Protein. Dis Model Mech 7, 21–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen RB, Parchi P, Richardson SL, Urig CB & Gambetti P Effect of the D178N mutation and the codon 129 polymorphism on the metabolism of the prion protein. J Biol Chem 271, 12661–8 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Gambetti P, Kong Q, Zou W, Parchi P & Chen SG Sporadic and familial CJD: classification and characterisation. Br Med Bull 66, 213–39 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Kimberlin RH, Hall SM & Walker CA Pathogenesis of mouse scrapie. Evidence for direct neural spread of infection to the CNS after injection of sciatic nerve. J Neurol Sci 61, 315–25 (1983). [DOI] [PubMed] [Google Scholar]
- 21.Scott JR & Fraser H Transport and targeting of scrapie infectivity and pathology in the optic nerve projections following intraocular infection. Prog Clin Biol Res 317, 645–52 (1989). [PubMed] [Google Scholar]
- 22.Yoshiyama Y, Lee VM & Trojanowski JQ Frontotemporal dementia and tauopathy. Curr Neurol Neurosci Rep 1, 413–21 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Hansen LA, Masliah E, Galasko D & Terry RD Plaque-only Alzheimer disease is usually the Lewy body variant, and vice versa. J Neuropathol Exp Neurol 52, 648–654 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Braak H & Braak E Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–59 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Avila J, Lucas JJ, Perez M & Hernandez F Role of tau protein in both physiological and pathological conditions. Physiol Rev 84, 361–84 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Braak H & Del Tredici K The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121, 171–81 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Jellinger KA A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116, 1–16 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Beach TG et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117, 613–34 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrer M et al. Lewy bodies and parkinsonism in families with parkin mutations. Ann Neurol 50, 293–300 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H et al. Familial juvenile parkinsonism: clinical and pathologic study in a family. Neurology 44, 437–41 (1994). [DOI] [PubMed] [Google Scholar]
- 31.van de Warrenburg BP et al. Clinical and pathologic abnormalities in a family with parkinsonism and parkin gene mutations. Neurology 56, 555–7 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Kordower JH, Chu Y, Hauser RA, Freeman TB & Olanow CW Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14, 504–6 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Brundin P & Kordower JH Neuropathology in transplants in Parkinson’s disease: implications for disease pathogenesis and the future of cell therapy. Prog Brain Res 200, 221–41 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Ahn TB, Langston JW, Aachi VR & Dickson DW Relationship of neighboring tissue and gliosis to alpha-synuclein pathology in a fetal transplant for Parkinson’s disease. Am J Neurodegener Dis 1, 49–59 (2012). [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper O et al. Lack of functional relevance of isolated cell damage in transplants of Parkinson’s disease patients. J Neurol 256 Suppl 3, 310–6 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Hallett PJ et al. Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Rep 7, 1755–61 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown P, Preece MA & Will RG “Friendly fire” in medicine: hormones, homografts, and Creutzfeldt-Jakob disease. Lancet 340, 24–7 (1992). [DOI] [PubMed] [Google Scholar]
- 38.de Calignon A et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73, 685–97 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L et al. Trans-synaptic spread of tau pathology in vivo. PLoS One 7, e31302 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yetman MJ, Lillehaug S, Bjaalie JG, Leergaard TB & Jankowsky JL Transgene expression in the Nop-tTA driver line is not inherently restricted to the entorhinal cortex. Brain Struct Funct (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jucker M & Walker LC Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501, 45–51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clavaguera F et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11, 909–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clavaguera F et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci U S A 110, 9535–40 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stancu IC et al. Templated misfolding of Tau by prion-like seeding along neuronal connections impairs neuronal network function and associated behavioral outcomes in Tau transgenic mice. Acta Neuropathol 129, 875–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridley RM, Baker HF, Windle CP & Cummings RM Very long term studies of the seeding of beta-amyloidosis in primates. J Neural Transm 113, 1243–51 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Luk KC et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–53 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Recasens A et al. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75, 351–62 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Terry RD Do neuronal inclusions kill the cell? J Neural Transm Suppl 59, 91–3 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Caughey B & Lansbury PT Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26, 267–98 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Santacruz K et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–81 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berger Z et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci 27, 3650–62 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wegmann S et al. Removing endogenous tau does not prevent tau propagation yet reduces its neurotoxicity. EMBO J (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benilova I, Karran E & De Strooper B The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci 15, 349–57 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Blennow K et al. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 26, 231–45 (1995). [DOI] [PubMed] [Google Scholar]
- 55.Motter R et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol 38, 643–8 (1995). [DOI] [PubMed] [Google Scholar]
- 56.Hawkes CH, Del Tredici K & Braak H Parkinson’s disease: the dual hit theory revisited. Ann N Y Acad Sci 1170, 615–22 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Barten DM et al. Tau transgenic mice as models for cerebrospinal fluid tau biomarkers. J Alzheimers Dis 24 Suppl 2, 127–41 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Chai X, Dage JL & Citron M Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol Dis 48, 356–66 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Mably AJ et al. Tau immunization: a cautionary tale? Neurobiol Aging 36, 1316–32 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Zetterberg H et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther 5, 9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borghi R et al. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson’s disease and normal subjects. Neurosci Lett 287, 65–7 (2000). [DOI] [PubMed] [Google Scholar]
- 62.El-Agnaf OM et al. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J 17, 1945–7 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Mollenhauer B et al. alpha-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol 10, 230–40 (2011). [DOI] [PubMed] [Google Scholar]
- 64.Masliah E et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One 6, e19338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Boutajangout A, Quartermain D & Sigurdsson EM Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci 30, 16559–66 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holmes BB et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci U S A 110, E3138–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caughey B & Baron GS Prions and their partners in crime. Nature 443, 803–10 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Borchelt DR, Rogers M, Stahl N, Telling G & Prusiner SB Release of the cellular prion protein from cultured cells after loss of its glycoinositol phospholipid anchor. Glycobiology 3, 319–29 (1993). [DOI] [PubMed] [Google Scholar]
- 69.Harris DA et al. Processing of a cellular prion protein: identification of N- and C-terminal cleavage sites. Biochemistry 32, 1009–16 (1993). [DOI] [PubMed] [Google Scholar]
- 70.Altmeppen HC et al. Proteolytic processing of the prion protein in health and disease. Am J Neurodegener Dis 1, 15–31 (2012). [PMC free article] [PubMed] [Google Scholar]
- 71.Halliday M, Radford H & Mallucci GR Prions: generation and spread versus neurotoxicity. J Biol Chem 289, 19862–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sandberg MK et al. Prion neuropathology follows the accumulation of alternate prion protein isoforms after infective titre has peaked. Nat Commun 5, 4347 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiesa R & Harris DA Prion diseases: what is the neurotoxic molecule? Neurobiol Dis 8, 743–63 (2001). [DOI] [PubMed] [Google Scholar]
- 74.Solomon IH, Schepker JA & Harris DA Prion neurotoxicity: insights from prion protein mutants. Curr Issues Mol Biol 12, 51–61 (2010). [PMC free article] [PubMed] [Google Scholar]
- 75.Collinge J & Clarke AR A general model of prion strains and their pathogenicity. Science 318, 930–6 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Holmqvist S et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128, 805–20 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Wakabayashi K, Takahashi H, Ohama E & Ikuta F Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol 79, 581–3 (1990). [DOI] [PubMed] [Google Scholar]
- 78.Hawkes CH, Shephard BC & Daniel SE Is Parkinson’s disease a primary olfactory disorder? QJM 92, 473–80 (1999). [DOI] [PubMed] [Google Scholar]
- 79.Wang N, Gibbons CH, Lafo J & Freeman R alpha-Synuclein in cutaneous autonomic nerves. Neurology 81, 1604–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lemere CA et al. Sequence of deposition of heterogeneous amyloid β-peptides and Apo E in Down syndrome: Implications for initial events in amyloid plaque formation. Neurobiol. Dis 3, 16–32 (1996). [DOI] [PubMed] [Google Scholar]
- 81.Frost B, Ollesch J, Wille H & Diamond MI Conformational diversity of wild-type Tau fibrils specified by templated conformation change. J Biol Chem 284, 3546–51 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Desplats P et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A 106, 13010–5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watts JC et al. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci U S A 110, 19555–60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo JL & Lee VM Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med 20, 130–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mirbaha H, Holmes BB, Sanders DW, Bieschke J & Diamond MI Tau Trimers Are the Minimal Propagation Unit Spontaneously Internalized to Seed Intracellular Aggregation. J Biol Chem 290, 14893–903 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Usenovic M et al. Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells. J Neurosci 35, 14234–50 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown P et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology 55, 1075–81 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Wroe SJ et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet 368, 2061–7 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Collinge J Variant Creutzfeldt-Jakob disease. Lancet 354, 317–23 (1999). [DOI] [PubMed] [Google Scholar]
- 90.Will RG et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347, 921–5 (1996). [DOI] [PubMed] [Google Scholar]
- 91.Beekes M, Thomzig A, Schulz-Schaeffer WJ & Burger R Is there a risk of prion-like disease transmission by Alzheimer- or Parkinson-associated protein particles? Acta Neuropathol 128, 463–76 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Irwin DJ et al. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol 70, 462–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goudsmit J et al. Evidence for and against the transmissibility of Alzheimer disease. Neurology 30, 945–50 (1980). [DOI] [PubMed] [Google Scholar]
- 94.Godec MS et al. Evidence against the transmissibility of Alzheimer’s disease. Neurology 41, 1320 (1991). [DOI] [PubMed] [Google Scholar]
- 95.Brown P et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol 35, 513–29 (1994). [DOI] [PubMed] [Google Scholar]
- 96.Baker HF, Ridley RM, Duchen LW, Crow TJ & Bruton CJ Induction of beta (A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Mol Neurobiol 8, 25–39 (1994). [DOI] [PubMed] [Google Scholar]
- 97.Baker HF, Ridley RM, Duchen LW, Crow TJ & Bruton CJ Evidence for the experimental transmission of cerebral beta-amyloidosis to primates. Int J Exp Pathol 74, 441–54 (1993). [PMC free article] [PubMed] [Google Scholar]
- 98.Rewcastle N, Gibbs CJ & Gajdusek D Transmission of familial Alzheimer’s disease to primates. Journal of Neuropathology & Experimental Neurology 37, 679 (1978). [Google Scholar]
- 99.Viola KL et al. Towards non-invasive diagnostic imaging of early-stage Alzheimer’s disease. Nat Nanotechnol 10, 91–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moreno JA et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med 5, 206ra138 (2013). [DOI] [PubMed] [Google Scholar]
- 101.Greig JR Scrapie: Observations on the Transmission of the Disease by Mediate Contact. Veterinary Journal 96, 203–206 (1940). [Google Scholar]
- 102.Cuille J & Chelle PL Investigations of Scrapie in Sheep. Veterinary Medicine 34, 417 (1939). [Google Scholar]
- 103.Chandler RL Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet 1, 1378–9 (1961). [DOI] [PubMed] [Google Scholar]
- 104.Gajdusek DC & Zigas V Degenerative disease of the central nervous system in New Guinea; the endemic occurrence of kuru in the native population. N Engl J Med 257, 974–8 (1957). [DOI] [PubMed] [Google Scholar]
- 105.Gajdusek DC, Gibbs CJ & Alpers M Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature 209, 794–6 (1966). [DOI] [PubMed] [Google Scholar]
- 106.Klatzo I, Gajdusek DC & Zigas V Pathology of Kuru. Lab Invest 8, 799–847 (1959). [PubMed] [Google Scholar]
- 107.Gibbs CJ Jr. et al. Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science 161, 388–9 (1968). [DOI] [PubMed] [Google Scholar]
- 108.Griffith JS Self-replication and scrapie. Nature 215, 1043–4 (1967). [DOI] [PubMed] [Google Scholar]
- 109.Bolton DC, McKinley MP & Prusiner SB Identification of a protein that purifies with the scrapie prion. Science 218, 1309–11 (1982). [DOI] [PubMed] [Google Scholar]
- 110.Oesch B et al. A cellular gene encodes scrapie PrP 27–30 protein. Cell 40, 735–46 (1985). [DOI] [PubMed] [Google Scholar]
- 111.Chesebro B et al. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature 315, 331–3 (1985). [DOI] [PubMed] [Google Scholar]
- 112.Hsiao KK et al. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science 250, 1587–90 (1990). [DOI] [PubMed] [Google Scholar]
- 113.Lloyd SE, Mead S & Collinge J Genetics of prion diseases. Curr Opin Genet Dev 23, 345–51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bueler H et al. Mice devoid of PrP are resistant to scrapie. Cell 73, 1339–47 (1993). [DOI] [PubMed] [Google Scholar]
- 115.Come JH, Fraser PE & Lansbury PT Jr. A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci U S A 90, 5959–63 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eigen M Prionics or the kinetic basis of prion diseases. Biophys Chem 63, A1–18 (1996). [DOI] [PubMed] [Google Scholar]
- 117.Cohen SI et al. Proliferation of amyloid-beta42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci U S A 110, 9758–63 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Silveira JR et al. The most infectious prion protein particles. Nature 437, 257–61 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Safar J et al. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4, 1157–65 (1998). [DOI] [PubMed] [Google Scholar]
- 120.Cronier S et al. Detection and characterization of proteinase K-sensitive disease-related prion protein with thermolysin. Biochem J 416, 297–305 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sandberg MK, Al-Doujaily H, Sharps B, Clarke AR & Collinge J Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature 470, 540–2 (2011). [DOI] [PubMed] [Google Scholar]
- 122.Kane MD et al. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci 20, 3606–11 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meyer-Luehmann M et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science 313, 1781–4 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Rosen RF et al. Exogenous seeding of cerebral beta-amyloid deposition in betaAPP-transgenic rats. J Neurochem 120, 660–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stohr J et al. Purified and synthetic Alzheimer’s amyloid beta (Abeta) prions. Proc Natl Acad Sci U S A 109, 11025–30 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harper JD & Lansbury PT Jr. Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem 66, 385–407 (1997). [DOI] [PubMed] [Google Scholar]
- 127.Arosio P, Knowles TP & Linse S On the lag phase in amyloid fibril formation. Phys Chem Chem Phys 17, 7606–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Esparza TJ et al. Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol 73, 104–19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shankar GM et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14, 837–42 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cummings CJ et al. Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice. Neuron 24, 879–92 (1999). [DOI] [PubMed] [Google Scholar]
- 131.Arrasate M, Mitra S, Schweitzer ES, Segal MR & Finkbeiner S Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431, 805–10 (2004). [DOI] [PubMed] [Google Scholar]
- 132.Serrano-Pozo A et al. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain 133, 1312–27 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koffie RM et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A 106, 4012–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Langer F et al. Soluble Abeta seeds are potent inducers of cerebral beta-amyloid deposition. J Neurosci 31, 14488–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eisele YS et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 330, 980–2 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jaunmuktane Z et al. Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature 525, 247–50 (2015). [DOI] [PubMed] [Google Scholar]
- 137.Jaunmuktane Z et al. Erratum: Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature 526, 595 (2015). [DOI] [PubMed] [Google Scholar]
- 138.Thery C et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 166, 7309–18 (2001). [DOI] [PubMed] [Google Scholar]
- 139.Rustom A, Saffrich R, Markovic I, Walther P & Gerdes HH Nanotubular highways for intercellular organelle transport. Science 303, 1007–10 (2004). [DOI] [PubMed] [Google Scholar]
- 140.Caughey B, Baron GS, Chesebro B & Jeffrey M Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu Rev Biochem 78, 177–204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hashimoto M & Masliah E Alpha-synuclein in Lewy body disease and Alzheimer’s disease. Brain Pathol 9, 707–20 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ittner LM et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 142, 387–97 (2010). [DOI] [PubMed] [Google Scholar]
- 143.Zempel H et al. Amyloid-beta oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J 32, 2920–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kanmert D et al. C-Terminally Truncated Forms of Tau, But Not Full-Length Tau or Its C-Terminal Fragments, Are Released from Neurons Independently of Cell Death. J Neurosci 35, 10851–65 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cho Y et al. Personalized medicine approach for optimizing the dose of tafamidis to potentially ameliorate wild-type transthyretin amyloidosis (cardiomyopathy). Amyloid 22, 175–80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dettmer U et al. Parkinson-causing alpha-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun 6, 7314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bartels T, Choi JG & Selkoe DJ alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477, 107–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]