Abstract
Background—
Patients experiencing major bleeding while taking vitamin K antagonists require rapid vitamin K antagonist reversal. We performed a prospective clinical trial to compare nonactivated 4-factor prothrombin complex concentrate (4F-PCC) with plasma for urgent vitamin K antagonist reversal.
Methods and Results—
In this phase IIIb, multicenter, open-label, noninferiority trial, nonsurgical patients were randomized to 4F-PCC (containing coagulation factors II, VII, IX, and X and proteins C and S) or plasma. Primary analyses examined whether 4F-PCC was noninferior to plasma for the coprimary end points of 24-hour hemostatic efficacy from start of infusion and international normalized ratio correction (≤1.3) at 0.5 hour after end of infusion. The intention-to-treat efficacy population comprised 202 patients (4F-PCC, n=98; plasma, n=104). Median (range) baseline international normalized ratio was 3.90 (1.8–20.0) for the 4F-PCC group and 3.60 (1.9–38.9) for the plasma group. Effective hemostasis was achieved in 72.4% of patients receiving 4F-PCC versus 65.4% receiving plasma, demonstrating noninferiority (difference, 7.1% [95% confidence interval, −5.8 to 19.9]). Rapid international normalized ratio reduction was achieved in 62.2% of patients receiving 4F-PCC versus 9.6% receiving plasma, demonstrating 4F-PCC superiority (difference, 52.6% [95% confidence interval, 39.4 to 65.9]). Assessed coagulation factors were higher in the 4F-PCC group than in the plasma group from 0.5 to 3 hours after infusion start (P<0.02). The safety profile (adverse events, serious adverse events, thromboembolic events, and deaths) was similar between groups; 66 of 103 (4F-PCC group) and 71 of 109 (plasma group) patients experienced ≥1 adverse event.
Conclusions—
4F-PCC is an effective alternative to plasma for urgent reversal of vitamin K antagonist therapy in major bleeding events, as demonstrated by clinical assessments of bleeding and laboratory measurements of international normalized ratio and factor levels.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: .
Keywords: anticoagulants, hemorrhage, plasma, prothrombin complex concentrates, vitamin K antagonist
Vitamin K antagonists (VKAs) are prescribed routinely for the treatment and prevention of thromboembolic events, with >22 million prescriptions issued for warfarin in the United States annually.1
In clinical practice in the United States, major hemorrhage in VKA-treated patients reportedly occurs at an annual rate of 1.7% to 3.4%.2 Furthermore, there are >60 000 annual emergency department visits for hemorrhagic complications in VKA-treated patients.3 Bleeding and supratherapeutic international normalized ratio (INR) are also common manifestations of adverse drug events in older adults, accounting for ≈42 000 hospitalizations annually in the United States.4 Patients presenting with acute hemorrhage require rapid VKA reversal via prompt restoration of vitamin K-dependent coagulation factors (VKDFs).5 The first step is the administration of vitamin K; however, reversal can take several hours, and therefore it is not recommended as monotherapy for acute bleeding.5,6
VKDF replacement can be achieved by administering plasma or prothrombin complex concentrate (PCC).7–9 Despite widespread use, the efficacy of plasma for urgent VKA reversal has not been established, and it has several drawbacks, including (1) time delays for ABO blood typing and thawing of frozen plasma; (2) large volumes and long infusion times to reach the factor levels necessary to correct coagulopathy; (3) risk of pathogen transmission; and (4) risk of transfusion-related acute lung injury and transfusion-associated circulatory overload, which are leading causes of transfusion-related deaths.7–10
An alternative to plasma is PCC. There are 2 types of PCCs: activated (licensed for treatment of hemophilia A or B with an inhibitor) and nonactivated. Nonactivated PCCs are lyophilized concentrates of VKDFs, referred to as 3-factor (contain significant quantities of factors II, IX, and X) or 4-factor (4F; also contain sufficient factor VII).7,11 PCCs can be administered promptly because of their relatively small infusion volume and because there is no need for thawing or blood-type matching.7 PCCs are effective for urgent VKA reversal12 and are considered preferable to plasma for rapid INR correction in many countries,7,13 which is highlighted in treatment guidelines from a number of organizations, including the American College of Chest Physicians, the British Committee for Standards in Haematology, and the Task Force for Advanced Bleeding Care in Trauma.5,6,14
This prospective, randomized, multinational clinical trial compared 4F-PCC with plasma for urgent VKA reversal in patients with acute major bleeding.
Methods
Study Design
This prospective, randomized, open-label, active-controlled, nonin-ferioiity phase IIIb trial was conducted at 36 sites across the United States and Europe. The study was sponsored by CSL Behring, registered at http://www.clinicaltrials.gov/ct2/show/NCT00708435, and performed in accordance with local ethics regulations; written informed consent was obtained from all patients.
Patients were randomly assigned (1:1) to receive either 4F-PCC (Beriplex P/N, CSL Behring, Marburg, Germany) or plasma. Patients were assigned by a centrally managed biased-coin minimization method,15 which controlled for balance in number of patients among treatment arms overall and per site as well as among bleeding type (comprising gastrointestinal, visible, intracranial hemorrhage, musculoskeletal, and other nonvisible bleeding). Study staff were not blinded to treatment allocation because of the inherent characteristics of the study drugs. Therefore, hemostatic efficacy was assessed by a blinded, independent Endpoint Adjudication Board (EAB). An independent Data Safety Monitoring Board reviewed unblinded data to assess patient safety. Serious adverse events (AEs) of interest to the Data Safety Monitoring Board (thromboembolic events, deaths, late bleeding events) were reviewed by a blinded, independent Safety Adjudication Board (SAB). Further details are in Table I in the online-only Data Supplement.
Efficacy End Points
Because of the complexity of assessing VKA reversal in a population experiencing diverse types of bleeding, the study was designed with 2 complementary coprimary end points. One coprimary study end point was hemostatic efficacy of the intervention (4F-PCC or plasma), assessed over a 24-hour period from the start of infusion. The other coprimary end point was rapid INR reduction (≤1.3) at 0.5 hour after the end of infusion. Secondary efficacy end points included plasma levels of VKDFs (factors II, VII, IX, and X) and natural anticoagulant proteins (proteins C and S) and time to INR correction (a complete list appears in Table II in the online-only Data Supplement).
Patients
Patients (≥18 years of age) receiving VKA therapy with an elevated INR (≥2.0 within 3 hours before study treatment) and experiencing an acute major bleeding event were eligible. Acute major bleeding was defined as 1 of the following: life-threatening or potentially life-threatening (according to the treating physician); acute bleeding associated with a fall in hemoglobin ≥2 g/dL; and bleeding requiring blood product transfusion. Exclusion criteria are listed in Table 1.
Table 1.
Exclusion Criteria
| • Expected survival of <3 d or expected surgery* in <1 d |
| • Acute trauma for which reversal of vitamin K antagonists alone would not be expected to control or resolve the acute bleeding event |
| • Use of unfractionated or low-molecular-weight heparin <24 h before enrollment or expected need <24 h after start of infusion† |
| • History of thrombotic event, myocardial infarction, disseminated intravascular coagulation, cerebral vascular accident, transient ischemic attack, unstable angina pectoris, severe peripheral vascular disease at ≤ 3 mo of enrollment |
| • Known history of antiphospholipid antibody syndrome |
| • Suspected/confirmed sepsis at enrollment |
| • Administration of plasma, plasma fractions, or platelets ≤2 wk before study (administration of packed red blood cells permitted) |
| • Large blood vessel rupture (eg, aortic dissection or ruptured aortic aneurysm) |
| • Preexisting progressive fatal disease with a life expectancy of <2 mo |
| • Known inhibitors to factors II, VII, IX, or X; or hereditary protein C or S deficiency; or heparin-induced, type II thrombocytopenia |
| • Treatment with any other investigational medicinal product ≤30 d before study |
| • Presence or history of hypersensitivity to components of the study medication |
| • Patients with intracranial hemorrhage: |
| ⚬ Glasgow Coma Scale score <7‡ |
| ⚬ Intracerebral hematoma volume >30 cm3 (assessed by ABC/2 formula) |
| ⚬ For subdural hematomas: maximum thickness ≥10 mm, midline shift ≥5 mm |
| ⚬ For subarachnoid hemorrhage: any evidence of hydrocephalus |
| ⚬ Infratentorial intracranial hemorrhage location |
| ⚬ Epidural hematomas |
| ⚬ Intraventricular extension of hemorrhage |
| ⚬ Modified Rankin Scale score >3 before intracranial hemorrhage |
Patients with acute major bleeding requiring minimally invasive procedures (eg, endoscopy, bronchoscopy, central lines) that were indicated for diagnostic or therapeutic reasons were not excluded per protocol, as long as plasma was intended to be given for treatment of major bleeding.
Exclusion added at time of amendment.
Modified from Glasgow Coma Scale score <9 to Glasgow Coma Scale score <7 on request of Food and Drug Administration.
Treatment
On day 1, each patient received his or her assigned study treatment according to baseline INR and body weight (Table 2).16 4F-PCC was administered as a single intravenous dose, with a maximum infusion rate of 3 IU/kg per minute. The quantity (IU) of active ingredients per vial of 4F-PCC was approximately as follows: factor II, 380 to 800; factor VII, 200 to 500; factor IX, 400 to 620; factor X, 500 to 1020; protein C, 420 to 820; and protein S, 240 to 680. Plasma was infused intravenously with a study protocol-recommended rate of 1 U per 30-minute interval.
Table 2.
Dose of Study Treatment per Baseline INR
| Baseline INR | 4F-PCC Dose, IU of Factor IX per kg Body Weight* |
Plasma Dose, mL per kg Body Weight* |
|---|---|---|
| 2 to <4 | 25 | 10 |
| 4–6 | 35 | 12 |
| >6 | 50 | 15 |
4F-PCC indicates 4-factor prothrombin complex concentrate; and INR, international normalized ratio.
Dose calculation based on 100 kg body weight for patients weighing >100 kg. Maximum dose ≤5000 IU of factor IX (4F-PCC) or ≤1500 mL (plasma).
All patients were to receive vitamin K by slow intravenous infusion dosed according to 2008 American College of Chest Physicians guidelines (5–10 mg)17 or local clinical practice if different.
Assessments
Traditional trials for evaluating coagulation factor products (eg, hemophilia treatments) are single-arm designs with subjective hemostasis end points.18–20 To reduce potential investigator bias and to increase end point objectivity, a hemostatic efficacy scale was developed in discussion with the Food and Drug Administration for adjudication by a blinded EAB. Hemostatic efficacy was rated by the EAB as excellent, good, or poor/none. Effective hemostasis was defined as a rating of excellent or good over a 24-hour period from the start of infusion; noneffective was defined as a rating of poor/ none. Data provided to the EAB for assessment included hemoglobin, hematocrit, any additional hemostatic treatments, AE data, and clinical outcome over the 24-hour assessment period. Objective pre-defined hemostasis criteria specific to each category of bleeding were used (Table III in the online-only Data Supplement). Patients were assigned a poor/none hemostatic efficacy rating if their management required administration of any hemostatic products other than study product or packed red blood cells within 24 hours after the start of study product infusion.
At the request of the Food and Drug Administration, the time points used for assessment of the primary rating of hemostatic efficacy for patients with musculoskeletal or visible bleeding were modified (from 3 and 6 hours after the start of infusion to 1 and 4 hours after the end of infusion) during the study. Consent was reobtained from the majority of affected patients before the assessment of hemostatic efficacy. Two patients were assessed with the use of the preamendment time points and therefore had missing data for the hemostatic efficacy end point. These patients were excluded from the intention-to-treat (ITT) efficacy (ITT-E) and per protocol analyses.
The total volume and total infusion time of each study treatment were recorded. Blood samples were drawn for determination of INR and levels of VKDFs, protein C, and protein S before study product infusion and 0.5, 1, 3, 6, 12, and 24 hours after start of infusion, as well as for determination of INR at 0.5 hour after end of infusion. Additional hematology parameters (hemoglobin and platelet count) were measured before infusion and 3 and 6 hours after start of infusion.
Baseline INR was assessed ≤3 hours before start of infusion. AEs and serious AEs were recorded by study investigators. AEs and concomitant medications were recorded at every time point up to day 10 (visit window days 7–11); serious AEs were recorded up to day 45 (visit window days 43–51).
Statistical Analysis
Data were analyzed with the use of SAS (SAS Institute Inc) version 9.1.3 and version 9.2. Sample size was based on assumptions of effective hemostasis for 85% in patients in the plasma group and 90% in the 4F-PCC group. It was originally estimated that (including a 10% dropout rate) 92 patients per group (total=184) would yield 84% power to show noninferiority of 4F-PCC versus plasma. The significance level used for the power calculation of hemostatic efficacy was 1-sided 0.025. After the protocol amendment to the hemostatic efficacy end point, target enrollment was increased to 212 to preserve study power. No power calculation was made for the INR end point measured at 0.5 hour after end of infusion.
Noninferiority analyses were conducted on the ITT-E population via calculation of the 2-sided 95% confidence interval (CI) for the difference in the proportions of patients with effective hemostasis and separately for the coprimary end point of INR reduction in the 2 treatment groups (4F-PCC minus plasma) according to the method of Farrington-Manning.21 Noninferiority of 4F-PCC was demonstrated if the lower limit of the 95% CI for the between-group difference was greater than −10%. 4F-PCC would be successfully claimed noninferior to plasma if noninferiority was shown for both the hemostatic efficacy and INR reduction end points. If noninferiority was shown, there would be an additional test for superiority of the effect of 4F-PCC compared with that of plasma for each of the coprimary end points. Superiority for an end point would be declared if the lower bound of the 95% CI exceeded zero. No adjustment to the type I error was required for this closed test procedure after the establishment of noninferiority.
The ITT population comprised all patients randomized to a treatment group. The ITT safety (ITT-S) population comprised all patients from the ITT population who had received any portion of study product. The ITT-E population comprised all patients from the ITT population who had received any portion of study product, who presented with acute major bleeding, and who had an INR >1.3 before infusion with study product. The per protocol population comprised all patients from the ITT-E population, excluding those with major protocol deviations. Patients with missing data for the hemostatic efficacy assessment were scored as noneffective for the ITT and ITT-E analyses and excluded from the per protocol analysis; if the INR measurement at 0.5 hour after infusion was missing, the patient was counted as having no rapid INR reduction for the ITT-E analysis.
Analyses of secondary end points were considered supportive, and no corrections for multiple comparisons were performed. A secondary analysis of the hemostatic efficacy end point was performed with the use of the original rating categories. Each category was compared between treatments with a Cochran-Mantel-Haenszel test. VKDF/protein levels were analyzed at each time point with a 2-tailed Wilcoxon rank sum test for between-group comparisons and a signed rank test for within-group changes from baseline. Time to INR correction data was summarized per treatment group in life tables and respective survival time graphs. Missing event time data or patients without events were censored at the longest nonmissing time. Between-group comparisons of INR per time point were analyzed by the 2-tailed Wilcoxon rank sum test. Safety data were analyzed descriptively.
Results
Demographics and Treatment
Two hundred sixteen patients were randomized; 103 patients received 4F-PCC, and 109 received plasma (Figure 1). The proportions of patients meeting the acute major bleeding criteria are detailed in Table IV in the online-only Data Supplement. The ITT-E population comprised 202 patients (4F-PCC, n=98; plasma, n=104); reasons for loss and exclusion are in Figure 1. Baseline data and characteristics were similar between groups (Table 3).
Figure 1.
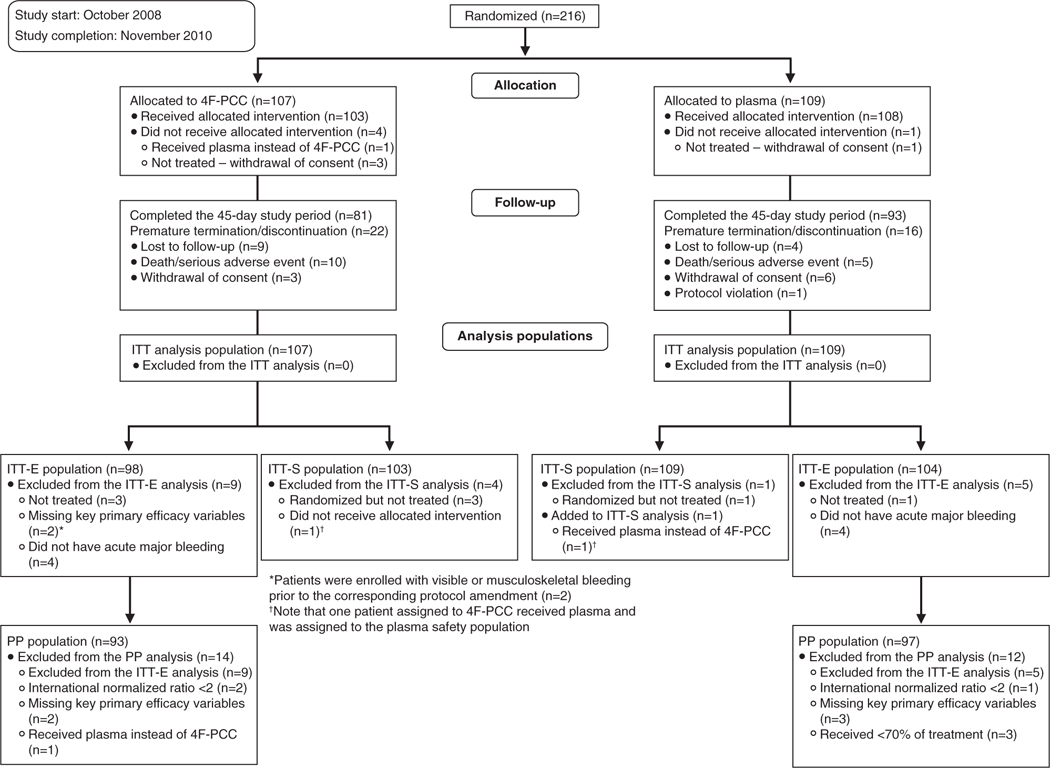
Patient flow. 4F-PCC indicates 4-factor prothrombin complex concentrate; ITT-E, intention-to-treat efficacy; ITT-S, intention-to-treat safety; and PP, per protocol.
Table 3.
Demographics and Baseline Characteristics (Intention-to-Treat Efficacy Population)
| 4F-PCC (n=98) | Plasma (n=104) | |
|---|---|---|
| Female sex, n (%) | 48 (49.0) | 53 (51.0) |
| Age, mean (SD; range), y | 69.8 (13.93; 29–96) | 69.8 (12.78; 26–92) |
| Age group, n (%), y | ||
| <65 | 33 (33.7) | 31 (29.8) |
| ≥65 to <75 | 24 (24.5) | 29 (27.9) |
| ≥75 | 41 (41.8) | 44 (42.3) |
| Race, n (%) | ||
| White | 93 (94.9) | 88 (84.6) |
| Nonwhite | 5 (5.1) | 16 (15.4) |
| Region, n (%) | ||
| United States | 68 (69.4) | 72 (69.2) |
| Europe | 30 (30.6) | 32 (30.8) |
| Body mass index, mean (SD), kg/m2 | 27.66 (8.54) | 27.64 (6.47) |
| Baseline INR, median (range) | 3.90 (1.8–20.0) | 3.60 (1.9–38.9) |
| Type of bleeding, n (%) | ||
| Gastrointestinal/other nonvisible | 63 (64.3) | 64 (61.5) |
| Visible | 16 (16.3) | 21 (20.2) |
| Intracranial hemorrhage | 12 (12.2) | 12 (11.5) |
| Musculoskeletal | 7 (7.1) | 7 (6.7) |
| Reason for oral VKA therapy, n (%) | ||
| Arrhythmia | 56 (57.1) | 53 (51.0) |
| Thromboembolic event | 18 (18.4) | 21 (20.2) |
| Artificial heart valve/joint | 13 (13.3) | 13 (12.5) |
| Vascular disease | 10 (10.2) | 13 (12.5) |
| Other | 1 (1.0) | 4 (3.8) |
| Time from first VKA dose to start of study | 720 | 757 |
| product infusion, median (range), d | (3–8476) | (3–10 734) |
| Previous antiplatelet therapy (<2 wk before study entry), n (%) | ||
| Clopidogrel | 3 (3.1) | 5 (4.8) |
| Prasugrel | 1 (1.0) | 0 |
| Cilostazol | 0 | 1 (1.0) |
| Medical history (most frequently listed terms), n (%)* | ||
| Hypertension | 87 (84.5) | 88 (80.7) |
| Atrial fibrillation | 69 (67.0) | 63 (57.8) |
| Anemia | 42 (40.8) | 35 (32.1) |
| Coronary artery disease | 38 (36.9) | 33 (30.3) |
| Cardiac failure congestive | 34 (33.0) | 33 (30.3) |
| Hyperlipidemia | 26 (25.2) | 33 (30.3) |
| Myocardial infarction | 25 (24.3) | 20 (18.3) |
| Chronic obstructive pulmonary disease | 23 (22.3) | 26 (23.9) |
| Appendectomy | 14 (13.6) | 27 (24.8) |
| Type 2 diabetes mellitus | 18 (17.5) | 24 (22.0) |
| Baseline hemoglobin, mean (SD), g/dL* | 9.33 (2.526) | 9.86 (2.817) |
| Baseline platelet count, mean (SD), ×109/L* | 228.0 (95.37) | 218.3 (81.19) |
4F-PCC indicates 4-factor prothrombin complex concentrate; INR, international normalized ratio; and VKA, vitamin K antagonist.
Intention-to-treat safety population.
Infusion durations, volumes, and rates reflected the different study substances involved (Table 4).
Table 4.
Study Product Infusion Details and Details of Concomitant Packed Red Blood Cell Use (Intention-to-Treat Efficacy Population)
| Parameter | 4F-PCC (n=98) | Plasma (n=104) |
|---|---|---|
| Study product* | ||
| Duration, median (range), min | 17.0 (7–288) | 148.0 (26–928) |
| Total volume, median (range), mL | 99.4 (50–230) | 813.5 (400–1525) |
| Infusion rate, median (range), IU/min for 4F-PCC, mL/min for plasma | 154.1 (7.3–307.1) | 6.6 (1.1–38.8) |
| Packed red blood cells | ||
| Patients receiving ≥1 transfusion, n (%) | 48 (49.0) | 47 (45.2) |
| Units transfused, mean (SD) | 1.4 (1.77) | 1.2 (1.57) |
4F-PCC indicates 4-factor prothrombin complex concentrate.
The patient randomized to 4F-PCC who received plasma was excluded
Four patients in the 4F-PCC group and 2 in the plasma group did not receive vitamin K during the study. Eight patients in the 4F-PCC group and 3 in the plasma group received vitamin K by a nonintravenous route. The timing of vitamin K therapy in relation to study product infusion was comparable between groups (data not shown).
Most hematology variables were within the same ranges at baseline in both groups (data not shown). There was no difference between groups in the mean number of packed red blood cell units transfused in the 24 hours after the start of infusion (Table 4). Lengths of hospital stays were similar between groups (Table V in the online-only Data Supplement).
Efficacy
Hemostatic Efficacy
Effective hemostasis (efficacy rating excellent or good) was achieved in 71 patients (72.4%) in the 4F-PCC group versus 68 (65.4%) in the plasma group (Table 5). Analysis of the group difference confirmed the noninferiority of 4F-PCC to plasma according to the predefined criterion (difference, 7.1% [−5.8 to 19.9]). The treatment difference did not demonstrate superiority.
Table 5.
Hemostatic Efficacy (Intention-to-Treat Efficacy Population)
| Primary Rating | No. (%) of Patients [95% CI] |
Difference 4F-PCC Minus Plasma, % (95% CI) |
|
|---|---|---|---|
| 4F-PCC (n=98) |
Plasma (n=104) |
||
| Hemostatic efficacy rating by category* | |||
| Excellent | 44† (44.9) | 45† (43.3) | |
| Good | 27 (27.6) | 23 (22.1) | |
| Poor/none | 27 (27.6) | 36 (34.6) | |
| Noneffective | 25 (25.5) | 33 (31.7) | |
| Missing primary rating | 2 (2.0) | 3 (2.9) | |
| rating | |||
| Effective hemostasis | 71 (72.4) | 68 (65.4) | 7.1‡ (−5.8 to 19.9) |
| [63.6 to 81.3] | [56.2 to 74.5] | ||
Effective hemostasis indicates hemostatic efficacy rated as excellent or good. 4F-PCC indicates 4-factor prothrombin complex concentrate; and CI, confidence interval.
Hemostatic efficacy assessed by a blinded independent board
P=0.50 by Cochran–Mantel–Haenszel test.
4F-PCC noninferior to plasma: lower limit of 95% CI more than −10% Farrington–Manning P value for noninferiority P=0.0045 rejecting null hypothesis of inferiority of 4F-PCC.
There was no difference between groups in the number of patients with an excellent hemostatic efficacy rating at 24 hours (P=0.50). A subanalysis stratified by type of bleed did not reveal statistically significant differences between interventions for any category of bleed (Table VI in the online-only Data Supplement). However, visible and musculoskeletal bleeding could be assessed earlier than other bleeding types (at 4 hours compared with 24 hours). In a post hoc analysis, significantly more patients had effective hemostasis in the 4F-PCC group than the plasma group when visible and musculoskeletal bleeding (rated at 4 hours) were analyzed (P=0.0200; Table 6).
Table 6.
Hemostatic Efficacy by Time of Rating (Post Hoc Analysis; Intention-to-Treat Efficacy Population)
| Treatment Group |
Difference 4F-PCC Minus Plasma, % (95% CI)* |
||
|---|---|---|---|
| 4F-PCC (n=98) |
Plasma (n=104) |
||
| No. of bleeds assessed for hemostatic efficacy at 4 h (visible, musculoskeletal) | 23 | 28 | |
| No. (%) of patients with effective hemostasis | 19 (82.6) | 14 (50.0) | 32.6 (4.5 to 60.7; P=0.0200) |
| No. of bleeds assessed for hemostatic efficacy at 24 h (gastrointestinal, intracranial, other nonvisible) | 75 | 76 | |
| No. (%) of patients with effective hemostasis | 52 (69.3) | 54 (71.1) | −1.7 (−17.6 to 14.2; P=−0.95) |
4F-PCC indicates 4-factor prothrombin complex concentrate; and CI, confidence interval.
95% CI and P value based on a Wald test with continuity correction.
INR Correction
Rapid INR reduction (INR ≤1.3 at 0.5 hour after the end of infusion) was achieved in 61 patients (62.2% [95% CI, 52.6 to 71.8]) in the 4F-PCC group versus only 10 (9.6% [95% CI, 3.9 to 15.3]) in the plasma group (Table 7). Analysis of the group difference demonstrated superiority of 4F-PCC over plasma (difference, 52.6% [39.4 to 65.9]).
Table 7.
Rapid INR Reduction (Intention-to-Treat Efficacy Population)
| No. (%) of Patients [95% CI] |
|||
|---|---|---|---|
| 4F-PCC (n=98) |
Plasma (n=104) |
Difference 4F-PCC Minus Plasma, % (95% CI) |
|
| Rapid INR reduction* | 61 (62.2) | 10 (9.6) | 52.6† |
| [52.6 to 71.8] | [3.9 to 15.3] | (39.4 to 65.9) | |
4F-PCC indicates 4-factor prothrombin complex concentrate; CI, confidence interval; and INR, international normalized ratio.
INR <1.3 at 0.5 h after end of infusion.
4F-PCC noninferior to plasma: lower limit of 95% CI more than −10% Farrington-Manning P value for noninferiority P<0.0001 rejecting null hypothesis of inferiority of 4F-PCC; 4F-PCC superior to plasma: lower limit of 95% CI >0.
Patients in the 4F-PCC group achieved INR correction more rapidly than those in the plasma group; 1 hour after the start of infusion, 68 patients (69%) in the 4F-PCC group had an INR ≤1.3 compared with none in the plasma group. This trend continued at the subsequent time points and was still evident at 24 hours after start of infusion (88% versus 58%, respectively; Figure 2A). Furthermore, median INR was significantly lower in the 4F-PCC group compared with the plasma group until 12 hours after the start of infusion (Figure 2B).
Figure 2.
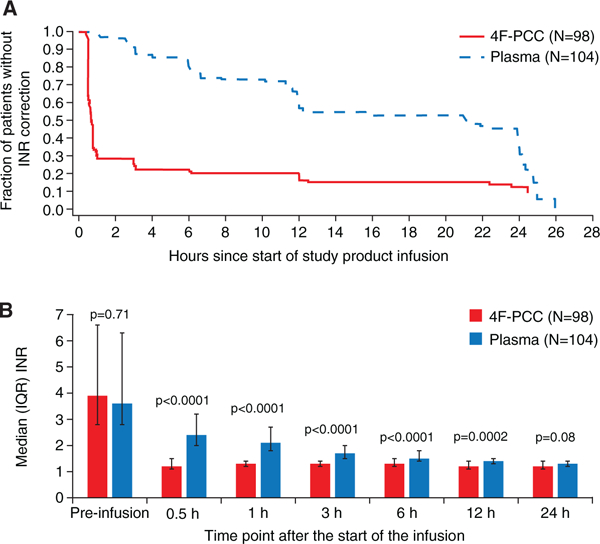
A, Time to international normalized ratio (INR) correction (intention-to-treat efficacy population). B, Median INR by time point (intentionto-treat efficacy population). 4F-PCC indicates 4-factor prothrombin complex concentrate; and IQR, interquartile range.
In a post hoc analysis, the 97.5% Farrington-Manning risk difference CIs for hemostatic efficacy and rapid INR reduction were also calculated with the assumption that the noninferiority boundary was −10%. These 97.5% CIs are equivalent to testing each of the 2 end points at individual 1-sided levels of α=0.0125. In this way, the multiplicity of testing for superiority in 2 coprimary end points was addressed with preservation of the type I error of a 0.025 significance level. For hemostatic efficacy, the 97.5% CI was −7.6% to 21.7%. For rapid INR reduction, the 97.5% CI was 37.5% to 67.7%. The lower bound of this CI is greater than zero, and therefore superiority can be declared for 4F-PCC for the rapid INR reduction end point.
Coagulation Factor/Protein Levels
Mean preinfusion levels of VKDFs, protein C, and protein S were similar between groups (P>0.05). Figure 3 shows changes in factor levels over time. Mean factor levels were significantly higher in the 4F-PCC group than the plasma group at 0.5, 1, 3, and 6 hours (P<0.05) apart from factor VII at 6 hours (not significantly different between groups; P=0.19).
Figure 3.
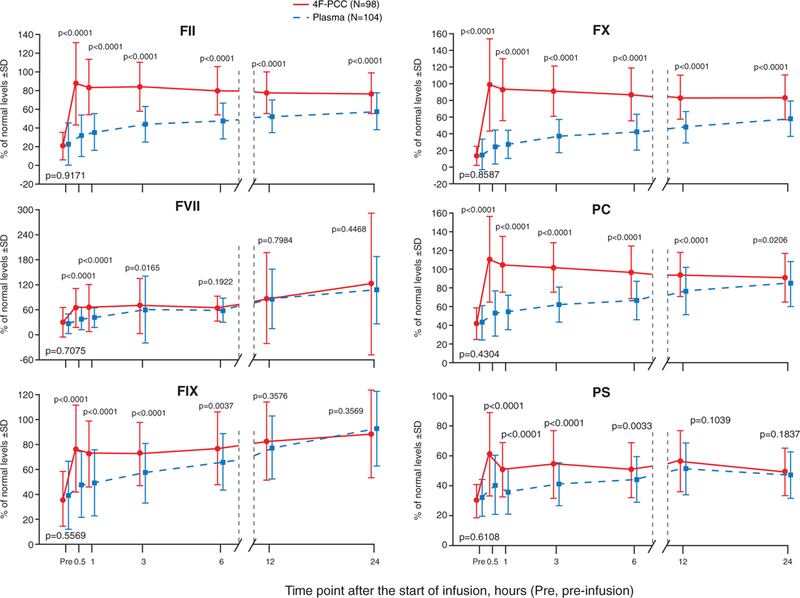
Mean coagulation protein levels before and after infusion (intention-to-treat efficacy population). 4F-PCC indicates 4-factor prothrombin complex concentrate; F, factor; PC, protein C; and PS, protein S.
Results were similar for the coprimary end points when analyzed by country/region (Tables VII and VIII in the online-only Data Supplement). Similar results were also seen for the ITT (Tables IX and X in the online-only Data Supplement) and per protocol populations (data not shown).
Safety
Safety outcomes were assessed with the use of the ITT-S population (Figure 1). There were 66 of 103 patients in the 4F-PCC group and 71 of 109 patients in the plasma group with ≥1 AE (Table 8). AEs considered by investigators to be treatment related were reported for 10 patients in the 4F-PCC group and 23 in the plasma group. Serious AEs were reported for 32 patients in the 4F-PCC group and 26 in the plasma group, of which 2 (ischemic stroke, deep vein thrombosis in the 4F-PCC group) and 4 (myocardial ischemia [n=2], respiratory failure, fluid overload in the plasma group) were considered treatment related by investigators (Table XI in the online-only Data Supplement).
Table 8.
Summary of AEs (Intention-to-Treat Safety Population)
| No. (%) of Patients |
||
|---|---|---|
| AE | 4F-PCC (n=103) |
Plasma (n=109) |
| Any nonserious AE* | 66 (64.1) | 71 (65.1) |
| Related AE† | 10 (9.7) | 23 (21.1) |
| AE leading to treatment discontinuation | 0 | 3 (2.8) |
| Serious AE* | 32 (31.1) | 26 (23.9) |
| Related serious AE† | 2 (1.9) | 4 (3.7) |
| AEs of interest | ||
| Deaths to day 30 | 6 (5.8) | 5 (4.6) |
| Deaths to day 45 | 10 (9.7) | 5 (4.6) |
| Related deaths (to day 45)‡ | 1 (1.0) | 0 |
| Thromboembolic AE | 8 (7.8) | 7 (6.4) |
| Related thromboembolic AE† | 4 (3.9) | 3 (2.8) |
| Fluid overload or similar cardiac event | 5 (4.9) | 14 (12.8) |
| Related fluid overload or similar cardiac event† | 0 | 7 (6.4) |
4F-PCC indicates 4-factor prothrombin complex concentrate; and AE, adverse event.
Defined in Table XIV in the online-only Data Supplement.
Defined as events for which there was a relationship to study treatment in the opinion of the investigator. AEs with missing relationship were considered treatment related.
As assessed by the Safety Adjudication Board; no deaths in either group were classified as related by an investigator.
Thromboembolic AEs were reported during the study for 8 patients in the 4F-PCC group and 7 in the plasma group (Table 8). Four patients in the 4F-PCC group and 3 patients in the plasma group had events considered treatment related by investigators. There were 5 patients in the 4F-PCC group and 3 patients in the plasma group with serious thromboembolic events, which were reviewed by the SAB. The SAB adjudicated that 2 patients in the 4F-PCC group and 2 in the plasma group had serious throm-boembolic events that were treatment related. The SAB did not confirm 1 investigator-listed serious AE of myocardial infarction (4F-PCC group) as a thromboembolic event.
Five patients (4.9%) in the 4F-PCC group had fluid overload or similar cardiac events (Table 8 and Table XII in the online-only Data Supplement; preferred terms: fluid overload, pulmonary edema, cardiac failure congestive, cardiac failure chronic, and cardiac failure), and none were considered treatment related by investigators. Fourteen patients (12.8%) had fluid overload or similar cardiac events after administration of plasma, and 7 were considered treatment related by investigators.
By day 30 after infusion, there were 6 deaths in the 4F-PCC group and 5 in the plasma group, of which 1 (sudden death after hospital discharge, 7 days after receiving 4F-PCC, cause unknown) was considered by the SAB (but not by the investigator) to be treatment related. Four additional deaths occurred in the 4F-PCC group between days 30 and 45; no additional deaths were recorded for the plasma group. Deaths are detailed in Table XIII in the online-only Data Supplement. Notably, 8 of 10 patients in the 4F-PCC group and 4 of 5 patients in the plasma group died after being placed on comfort care.
Discussion
This was the first randomized clinical trial to compare 4F-PCC and plasma for urgent VKA reversal in patients with major bleeding. The study met the coprimary end points: 4F-PCC was noninferior to plasma for hemostatic efficacy and for rapid INR reduction. Consistent with the rapid reduction in INR, mean plasma levels of VKDFs markedly increased to >60% at 0.5 hour after the start of 4F-PCC infusion, whereas increases in factor levels after administration of plasma were significantly slower. Between 3 and 24 hours (when vitamin K begins to take effect), factor levels in the plasma group trended toward those seen in the 4F-PCC group.
The rapid INR reduction and factor level increments are consistent with other studies demonstrating that PCCs are a clinically useful means to rapidly replace VKDFs and effectively lower INR.16,22–25 A number of hypotheses might explain why this 4F-PCC achieved superiority to plasma for rapid INR reduction and achieved noninferiority to plasma for hemostatic efficacy. Although it is possible that patients with less severe coagulopathies (INR 2–4) benefited from supportive measures aimed at stopping bleeding, there was no evidence of a difference in hemostatic efficacy between 4F-PCC and plasma for each baseline INR group. The hemostatic efficacy rating might have been higher for the 4F-PCC group if only patients with severe bleeding were included. However, it would not have been practical or feasible to enroll enough patients if the stringency of the entry criteria was increased. The clinical hemostasis assessments used data collected throughout the 24-hour period after study product administration; this timing might have limited our ability to detect differences. Because of the longer plasma infusion time, concomitant administration of vitamin K was a potential confounding factor because of its greater contribution in the plasma group; by 24 hours, factor levels in both groups had reached hemostatic levels. Interestingly, for bleeding types for which an early assessment was possible (visible and musculoskeletal bleeding), there was a more pronounced clinical effect of 4F-PCC compared with plasma (Table 6).
Historically, clinical trials of hemostasis have had limitations, some of which were present in this trial. Commonly, trials with bleeding end points lack defined efficacy criteria or defined time points for analysis, and most hemostatic efficacy scales have some subjective elements. In this trial, end points consistent with standard clinical assessments and time points were explicitly defined for the blinded EAB. This was the first clinical trial to use an EAB for this indication, which may explain why the frequency of hemostatic efficacy in this trial was different than anticipated on the basis of other studies in which nonstandardized assessments were used. Another challenge in clinical bleeding trials is the inability to perform direct assessments of hemostasis (continuously and in real time) for many types of bleeding, such as gastrointestinal hemorrhage. The timed nature of the assessments in this trial meant that actual hemostasis may have been achieved earlier than recorded.
The importance of the timing of assessments can also be seen for the rapid INR reversal (INR ¿1.3) end point. This end point, measured from the end of the infusion, did not capture key differences between the 2 treatments: preparation and administration time. The median infusion time was >8 times longer in the plasma group than the 4F-PCC group. Therefore, there was a substantial difference between initiation of treatment and the assessment time point. In a clinical context, within 1 hour of the start of infusion, more than two thirds of patients had INR ≤1.3 in the 4F-PCC group compared with none in the plasma group.
This study was not powered to demonstrate significant differences between groups for safety outcomes. Overall, the AEs observed in the 4F-PCC and plasma groups were consistent with their respective safety profiles and this population with multiple comorbidities. The SAB reviewed all study deaths and adjudicated that 1 death (patient died at home with unknown cause 7 days after administration of 4F-PCC) was related to study product. There was no unifying pattern to the deaths except for the frequent finding of a high comorbid burden, advanced age, and death after being placed on comfort care (12 of 15 patients).
Historically, the association of PCCs with thromboembolic events has been a concern. Two recent comprehensive reviews, based on single-arm studies of PCCs, concluded that there is a low risk of thromboembolic events in patients treated with PCCs for VKA reversal and that underlying disease and dosing may be important factors in increasing risk.13,26 This study found no evidence of an increased thromboembolic risk associated with this 4F-PCC compared with plasma; thromboembolism might occur in this patient population because of underlying risks irrespective of the means used to reverse VKA.27,28
Fluid overload and similar cardiac events occurred relatively frequently after plasma transfusion for VKA reversal, as described previously.7,29 PCCs have several safety advantages compared with plasma with respect to rare but important AEs, many of which are not measurable in a trial such as this. Plasma transfusions are associated with allergic reactions, risk of transfusion-associated circulatory overload, transfusion-related acute lung injury, and pathogen transmission30–34; however, several strategies have reduced many of these risks.33 This study was not powered to assess the incidence of transfusion-related acute lung injury, and therefore specific data are not available. PCCs undergo a series of pathogen-reduction and -inactivation steps and are associated with a minimal risk of pathogen transmission.13,26 The low infusion volume of PCCs avoids transfusion-associated circulatory overload; the median volume of plasma administered in this study was >8 times greater than the volume of 4F-PCC (813.5 versus 99.4 mL).
Overall, these results add to the existing evidence that 4F-PCC is an acceptable alternative to plasma for VKA reversal in time-and volume-critical situations. PCCs normalize the INR more rapidly than plasma,35–38 which has led to their widespread use for VKA reversal throughout Europe for >20 years.12,39 This preference for PCCs over plasma is reflected in many guidelines for VKA reversal.5,6,14
Conclusions
This was the first randomized, controlled study comparing PCC with plasma in patients receiving VKAs who presented with acute major bleeding. The efficacy of 4F-PCC was demonstrated by clinical assessments of bleeding and supported by laboratory measurements of INR and factor levels. The study showed that 4F-PCC is an effective alternative to plasma for the urgent reversal of VKA therapy in major bleeding events and that this 4F-PCC has an acceptable safety profile compared with plasma.
Supplementary Material
CLINICAL PERSPECTIVE.
The administration of vitamin K antagonists (VKAs) for the management of thromboembolic events is associated with hemorrhagic risk. Patients presenting with acute bleeding require rapid VKA reversal, which can be achieved by administration of either plasma or prothrombin complex concentrate (containing vitamin K-dependent factors). Despite widespread use in the United States, the efficacy of plasma has not been established and is associated with safety concerns such as fluid overload. We report the findings of the first prospective, controlled, randomized, multicenter clinical trial comparing a 4-factor prothrombin complex concentrate with plasma for the urgent reversal of VKAs in patients with acute major bleeding. The study demonstrated that compared with plasma, 4-factor prothrombin complex concentrate was noninferior for the primary end point of hemostatic efficacy at 24 hours, was superior for the coprimary end point of rapid international normalized ratio reduction, and had a similar safety profile. Thus, 4-factor prothrombin complex concentrate can be considered an efficacious alternative to plasma for VKA reversal in patients presenting with major bleeding in time-and volume-critical situations. We believe that this report will be of significant interest to clinicians involved in the management of VKA-related bleeding complications because VKA remains an important anticoagulant therapy for numerous clinical conditions.
Acknowledgments
Editorial assistance was provided by Rianne Stacey, PhD (Fishawack Communications).
Sources of Funding
This research was sponsored by CSL Behring. A steering committee of investigators, academic medical experts, and representatives of the sponsor oversaw the trial design and conduct with the assistance of the independent Data Safety Monitoring Board, SAB, and EAB. The sponsor participated in the selection of the board members. The sponsor was responsible for data processing, management, analysis of the data according to a predefined statistical analysis plan, and reporting of the results. Editorial assistance was funded by the sponsor. All authors had access to the data and jointly decided to submit the manuscript for publication. All authors contributed to interpretation of data and drafting of the manuscript. The authors vouch for the accuracy and completeness of the reported data and analyses, as well as the fidelity of this report to the protocol. All authors read and approved the final manuscript for publication.
Footnotes
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA. 113.002283/-/DC1.
Disclosures
Dr Sarode received consulting fees and honoraria from CSL Behring GmbH. Dr Mangione and B. Durn are employees of CSL Behring LLC. Dr Schneider is an employee of CSL Behring GmbH. Dr Goldstein received consulting fees, honoraria, and a research grant from CSL Behring GmbH. Dr Milling received consulting fees from CSL Behring. Dr Refaai reports no conflicts..
References
- 1.IMS data US National Prescription Audit; MAT (moving annual total) August 2011-July 2012.
- 2.Schulman S, Beyth RJ, Kearon C, Levine MN; American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 suppl):257S–298S. [DOI] [PubMed] [Google Scholar]
- 3.Shehab N, Sperling LS, Kegler SR, Budnitz DS. National estimates of emergency department visits for hemorrhage-related adverse events from clopidogrel plus aspirin and from warfarin. Arch Intern Med. 2010;170:1926–1933. [DOI] [PubMed] [Google Scholar]
- 4.Budnitz D, Lovegrove M, Shehab N, Richards C. Emergency hospitalizations for adverse events in older Americans. N Engl J Med. 2011; 365:2002–2012. [DOI] [PubMed] [Google Scholar]
- 5.Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e152S–e184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, Kitchen S, Makris M. Guidelines on oral coagulation with warfarin: fourth edition. Br J Haematol. 2011;154:311–324. [DOI] [PubMed] [Google Scholar]
- 7.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e44S–e88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bechtel BF, Nunez TC, Lyon JA, Cotton BA, Barrett TW. Treatments for reversing warfarin anticoagulation in patients with acute intracranial hemorrhage: a structured literature review. Int J Emerg Med. 2011;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vang ML, Hvas AM, Ravn HB. Urgent reversal of vitamin K antagonist therapy. Acta Anaesthesiol Scand. 2011;55:507–516. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Stanworth S, Hopewell S, Doree C, Murphy M. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion. 2012;52:1673–1686; quiz 1673. [DOI] [PubMed] [Google Scholar]
- 11.Holland L, Warkentin TE, Refaai M, Crowther MA, Johnston MA, Sarode R. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion. 2009;49:1171–1177. [DOI] [PubMed] [Google Scholar]
- 12.Vigué B Bench-to-bedside review: optimising emergency reversal of vitamin K antagonists in severe haemorrhage—from theory to practice. Crit Care. 2009;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sørensen B, Spahn DR, Innerhofer P, Spannagl M, Rossaint R. Clinical review: prothrombin complex concentrates—evaluation of safety and thrombogenicity. Crit Care. 2011;15:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Rossaint R. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2013;17:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 16.Pabinger I, Brenner B, Kalina U, Knaub S, Nagy A, Ostermann H; Beriplex P/N Anticoagulation Reversal Study Group. Prothrombin complex concentrate (Beriplex P/N) for emergency anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost. 2008;6:622–631. [DOI] [PubMed] [Google Scholar]
- 17.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 suppl):160S–198S. [DOI] [PubMed] [Google Scholar]
- 18.Bray GL, Gomperts ED, Courter S, Gruppo R, Gordon EM, Manco-Johnson M, Shapiro A, Scheibel E, White G III, Lee M; The Recombinate Study Group. A multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. Blood. 1994;83:2428–2435. [PubMed] [Google Scholar]
- 19.Gringeri A, Tagliaferri A, Tagariello G, Morfini M, Santagostino E, Mannucci P; ReFacto-AICE Study Group. Efficacy and inhibitor development in previously treated patients with haemophilia A switched to a B domain-deleted recombinant factor VIII. Br J Haematol. 2004;126:398–404. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro AD, Ragni MV, Lusher JM, Culbert S, Koerper MA, Bergman GE, Hannan MM. Safety and efficacy of monoclonal antibody purified factor IX concentrate in previously untreated patients with hemophilia B. Thromb Haemost. 1996;75:30–35. [PubMed] [Google Scholar]
- 21.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med. 1990;9:1447–1454. [DOI] [PubMed] [Google Scholar]
- 22.Kalina M, Tinkoff G, Gbadebo A, Veneri P, Fulda G. A protocol for the rapid normalization of INR in trauma patients with intracranial hemorrhage on prescribed warfarin therapy. Am Surg. 2008;74:858–861. [PubMed] [Google Scholar]
- 23.Khorsand N, Veeger NJ, van Hest RM, Ypma PF, Heidt J, Meijer K. An observational, prospective, two-cohort comparison of a fixed versus variable dosing strategy of prothrombin complex concentrate to counteract vitamin K antagonists in 240 bleeding emergencies. Haematologica. 2012;97:1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce D, Nokes TJ. Prothrombin complex concentrate (Beriplex P/N) in severe bleeding: experience in a large tertiary hospital. Crit Care. 2008;12:R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott LJ. Prothrombin complex concentrate (Beriplex P/N). Drugs. 2009;69:1977–1984. [DOI] [PubMed] [Google Scholar]
- 26.Dentali F, Marchesi C, Pierfranceschi MG, Crowther M, Garcia D, Hylek E, Witt DM, Clark NP, Squizzato A, Imberti D, Ageno W. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists: a meta-analysis. Thromb Haemost. 2011;106:429–438. [DOI] [PubMed] [Google Scholar]
- 27.Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, Iotti M, Tormene D, Simioni P, Pagnan A. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism: a prospective cohort study in 1,626 patients. Haematologica. 2007;92:199–205. [DOI] [PubMed] [Google Scholar]
- 28.Witt DM, Delate T, Garcia DA, Clark NP, Hylek EM, Ageno W, Dentali F, Crowther MA. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012:1–8. [DOI] [PubMed] [Google Scholar]
- 29.Narick C, Triulzi DJ, Yazer MH. Transfusion-associated circulatory overload after plasma transfusion. Transfusion. 2012;52:160–165. [DOI] [PubMed] [Google Scholar]
- 30.Boulis NM, Bobek MP, Schmaier A, Hoff JT. Use of factor IX complex in warfarin-related intracranial hemorrhage. Neurosurgery. 1999; 45: 1113–1118; discussion 1118. [DOI] [PubMed] [Google Scholar]
- 31.Khan H, Belsher J, Yilmaz M, Afessa B, Winters JL, Moore SB, Hubmayr RD, Gajic O. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131:1308–1314. [DOI] [PubMed] [Google Scholar]
- 32.Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, Montori VM, Roback JD. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion. 2010;50:1370–1383. [DOI] [PubMed] [Google Scholar]
- 33.O’Shaughnessy DF, Atterbury C, Bolton Maggs P, Murphy M, Thomas D, Yates S, Williamson LM; British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28. [DOI] [PubMed] [Google Scholar]
- 34.Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, Chapman CE, Davison K, Gerrard R, Gray A, Knowles S, Love EM, Milkins C, McClelland DB, Norfolk DR, Soldan K, Taylor C, Revill J, Williamson LM, Cohen H; SHOT Steering Group. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–282. [DOI] [PubMed] [Google Scholar]
- 35.Cartmill M, Dolan G, Byrne JL, Byrne PO. Prothrombin complex concentrate for oral anticoagulant reversal in neurosurgical emergencies. Br J Neurosurg. 2000;14:458–461. [DOI] [PubMed] [Google Scholar]
- 36.Huttner HB, Schellinger PD, Hartmann M, Kohrmann M, Juettler E, Wikner J, Mueller S, Meyding-Lamade U, Strobl R, Mansmann U, Schwab S, Steiner T. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral frozen plasma, and prothrombin complex concentrates. Stroke. 2006;37:1465–1470. [DOI] [PubMed] [Google Scholar]
- 37.Makris M, Greaves M, Phillips WS, Kitchen S, Rosendaal FR, Preston EF, Emergency oral anticoagulant reversal: the relative efficacy of infusions of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thromb Haemost. 1997;77:477–480. [PubMed] [Google Scholar]
- 38.Yasaka M, Sakata T, Naritomi H, Minematsu K. Optimal dose of prothrombin complex concentrate for acute reversal of oral anticoagulation. Thromb Res. 2005;115:455–459. [DOI] [PubMed] [Google Scholar]
- 39.Leissinger CA, Blatt PM, Hoots WK, Ewenstein B. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol. 2008;83:137–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


