Abstract
Background:
The New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial vs. ASA to Prevent Embolism in Embolic Stroke of Undetermined Source (NAVIGATE-ESUS) trial is a randomized phase-III trial comparing rivaroxaban versus aspirin in patients with recent ESUS.
Aims:
We aimed to describe the baseline characteristics of this large ESUS cohort to explore relationships among key subgroups.
Methods:
We enrolled 7213 patients at 459 sites in 31 countries. Prespecified subgroups for primary safety and efficacy analyses included age, sex, race, global region, stroke or transient ischemic attack prior to qualifying event, time to randomization, hypertension, and diabetes mellitus.
Results:
Mean age was 66.9 ± 9.8 years; 24% were under 60 years. Older patients had more hypertension, coronary disease, and cancer. Strokes in older subjects were more frequently cortical and accompanied by radiographic evidence of prior infarction. Women comprised 38% of participants and were older than men. Patients from East Asia were oldest whereas those from Latin America were youngest. Patients in the Americas more frequently were on aspirin prior to the qualifying stroke. Acute cortical infarction was more common in the United States, Canada, and Western Europe, whereas prior radiographic infarctions were most common in East Asia. Approximately forty-five percent of subjects were enrolled within 30 days of the qualifying stroke, with earliest enrollments in Asia and Eastern Europe.
Conclusions:
NAVIGATE-ESUS is the largest randomized trial comparing antithrombotic strategies for secondary stroke prevention in patients with ESUS. The study population encompasses a broad array of patients across multiple continents and these subgroups provide ample opportunities for future research.
Keywords: Stroke, cryptogenic stroke, cerebral embolism, Embolic Stroke of Undetermined Source (ESUS), stroke prevention, rivaroxaban, aspirin, randomized trial
Introduction
Embolic stroke of undetermined source (ESUS) is a subset of cryptogenic stroke, and a diagnostic label proposed for an ischemic stroke that occurs without an identifiable and specifically treatable underlying stroke etiology, including greater than 50% stenosis in a large proximal artery in the territory of ischemia, atrial fibrillation or other major-risk cardioembolic source, lacunar (small vessel occlusive) disease, or identified uncommon cause.1 ESUS accounts for 15% to 30% of all ischemic strokes.2 A wide range of potential cardiac, arterial, paradoxical, and hematological sources have been proposed that might be amenable to treatment with an anticoagulant.1,3,4 The New Approach Rivaroxaban Inhibition of Factor Xa in a Global trial vs. ASA to Prevent Embolism in Embolic Stroke of Undetermined Source (NAVIGATE-ESUS) trial is an international randomized phase-III trial comparing rivaroxaban with aspirin in patients with recent ESUS. The design of the trial has previously been reported,5 enrollment of 7213 subjects has recently been completed, and participant features are reported here.
Although the NAVIGATE-ESUS participants share a common diagnosis of ESUS, they likely vary with respect to the underlying potential embolic sources,6 and therefore subgroup analyses may be especially important.7,8 Subgroup analysis in clinical trials is often performed for 2 key purposes. One major goal is to explore the consistency of a treatment effect among different subpopulations that are defined at baseline. The other is to investigate whether there are specific groups that are more or less likely to receive benefit or harm from the treatment. Together, these assessments of both homogeneity and heterogeneity can yield valuable information for clinicians and future research, but these analyses must be interpreted cautiously, mitigated by reduced statistical power and the play of chance.9 Subgroup analysis can also help identify populations at greatest risk of a recurrent event. Clinical characteristics of selected subgroups pre-specified in the NAVIGATE ESUS trial statistical analysis plan are provided.
Methods
NAVIGATE ESUS Study Design
The design of NAVIGATE ESUS (clinicaltrials.gov.NCT02313909) has previously been published.5 In brief, it is an international, double-blinded, randomized phase-III superiority trial comparing rivaroxaban 15 mg once daily (immediate-release, film-coated tablets) with aspirin (enteric-coated) 100 mg once daily, both to be taken with food, in patients with recent ESUS. Target enrollment was approximately 7000, and the study was designed to continue until at least 450 primary events have occurred. Key eligibility criteria for NAVIGATE ESUS are summarized in the Appendix (Supplementary Table S1). The primary efficacy outcome is time to recurrent stroke, comprising ischemic, hemorrhagic, and undefined stroke, including transient ischemic attacks (TIAs) with positive neuroimaging10 or systemic embolism. The primary safety outcome is major bleeding as defined by the criteria of the International Society of Thrombosis and Haemostasis.11 The main efficacy and safety results will be available in 2018.
Baseline Characteristics and Subgroup Analyses
Baseline characteristics collected in the trial include demographic features, medical history, qualifying stroke information, and baseline functional and cognitive status. Prespecified participant subgroup analyses for which the treatment effects will be presented in the main results publication were chosen for presentation here, in accordance with the statistical analysis plan. These included the following, based on the data collected at the time of randomization: age, sex, race, global region, stroke or TIA prior to qualifying event, time from qualifying stroke to randomization, hypertension, and diabetes mellitus.
Statistical Analysis
We describe the features of all subjects and compare the baseline characteristics for selected prespecified subgroups. Descriptive statistics use mean ± standard deviation, median (interquartile range [IQR]), or proportion as appropriate. Univariate comparisons were made using t-tests for continuous variables and chi-square tests for categorical variables, and we present nominal 2-sided P values. For comparisons within subgroups, we consider only P values less than .01 to be significant to account for the multiple comparisons.
Results
A total of 7213 subjects were randomized in the NAVIGATE ESUS trial between December 24, 2014 and September 20, 2017. The major baseline characteristics for the entire study population are summarized in Table 1. The mean age was 66.9 ± 9.8 years, and 62% were men. Median baseline National Institutes of Health Stroke Scale score was 1 (IQR 0, 2) and was less than or equal to 5 in 96% of patients. All subjects had extracranial vascular imaging, echocardiography, and initial cardiac rhythm monitoring as required by protocol, and 78% had intra-cranial vascular imaging. Forty-three percent of patients were enrolled from Western Europe (Figure 1). Characteristics of prespecified selected subgroups are summarized in Tables 2–4 and the Supplementary Tables. Key differences among subgroups are described below. Of note, only 7% of participants had a history of coronary artery disease due to protocol stipulation excluding patients who require single or dual antiplatelet therapy.
Table 1.
Baseline characteristics of the complete NAVIGATE-ESUS study population
| Characteristic | N with Data | Summary (N = 7214) |
|---|---|---|
| Age, years (mean ± s.d.) | 7213 | 66.9 ± 9.8 |
| Age<60 years | 7213 | 1716 (24) |
| Male sex | 7213 | 4437 (62) |
| Race: | ||
| White only | 7213 | 5219 (72) |
| Black only | 7213 | 111 (2) |
| East Asian only | 7213 | 1414 (20) |
| Others (includes not reported/multiracial) | 7213 | 470 (7) |
| BMI, kg/m2 (mean ± s.d.) | 7182 | 27.2 ± 5.0 |
| <25 kg/m2 | 7182 | 2505 (35) |
| ≥25–<30 kg/m2 | 7182 | 2970 (41) |
| ≥30 kg/m2 | 7182 | 1708 (24) |
| <30 kg/m2 | 7182 | 5475 (76) |
| ≥30 kg/m2 | 7182 | 1708 (24) |
| Weight, kg (mean ± s.d.) | 7189 | 76.2 ± 16.5 |
| <70 kg | 7189 | 2535 (35) |
| 70–90 kg | 7189 | 3479 (48) |
| >90 kg | 7189 | 1176 (16) |
| <50 kg | 7189 | 199 (3) |
| 50–100 kg | 7189 | 6467 (90) |
| >100 kg | 7189 | 524 (7) |
| Estimated glomerular filtration rate (eGFR), mL/min per 1.73 m2 | 7209 | 78.6 ± 20.6 |
| <50 mL/min | 7209 | 419 (6) |
| 50–80 mL/min | 7209 | 3531 (49) |
| >80 mL/min | 7209 | 3260 (45) |
| Medical history: | ||
| Hypertension | 7213 | 5586 (77) |
| Diabetes mellitus | 7213 | 1805 (25) |
| Current tobacco use | 7212 | 1484 (21) |
| Coronary artery disease | 7213 | 473 (7) |
| Heart failure | 7213 | 238 (3) |
| Cancer | 7213 | 620 (9) |
| Bioprosthetic heart valve | 7213 | 21 (0) |
| Prior stroke or TIA | 7213 | 1263 (18) |
| Global region: | ||
| U.S.A. and Canada | 7213 | 918 (13) |
| Latin America | 7213 | 746 (10) |
| Western Europe | 7213 | 3081 (43) |
| Eastern Europe | 7213 | 1119 (16) |
| East Asia | 7213 | 1350 (19) |
| Qualifying stroke: | ||
| Clinical TIA with imaging-confirmed infarction as qualifying event: | 7213 | 521 (7) |
| Arterial territory of qualifying stroke: | ||
| Anterior circulation | 7213 | 5187 (72) |
| Posterior circulation | 7213 | 2269 (31) |
| Location of qualifying stroke: | ||
| Single Location: | ||
| Cerebral hemisphere with cortical involvement | 7213 | 4036 (56) |
| Cerebral hemisphere, subcortical only | 7213 | 1518 (21) |
| Brainstem only | 7213 | 331 (5) |
| Cerebellum only | 7213 | 562 (8) |
| Multiple Locations: | 7213 | 762 (11) |
| Chronic infarct on imaging (in addition to index stroke) | 7212 | 2350 (33) |
| Aspirin use prior to qualifying stroke | 7213 | 1247 (17) |
| Statin use prior to randomization | 7213 | 4425 (61) |
| Treated with intravenous tPA for qualifying stroke | 7213 | 1255 (17) |
| Treated with endovascular intervention for qualifying stroke | 7213 | 300 (4) |
| NIHSS score at randomization (median, IQR) | 7209 | 1.0 (0.0, 2.0) |
| NIHSS score ≤5 | 7209 | 6927 (96) |
| Modified Rankin Scale (mRS) at randomization: | ||
| mRS 0 or 1 | 7212 | 4670 (65) |
| mRS 2 | 7212 | 1673 (23) |
| mRS ≥3 | 7212 | 870 (12) |
| MoCA score at randomization (median, IQR) | 6531 | 25.0 (21.0, 27.0) |
| Time from qualifying stroke to randomization, days (median, IQR) | 7213 | 37.0 (14.0, 88.0) |
| Extracranial vascular imaging completed: | ||
| CTA | 7211 | 2743 (38) |
| MRA | 7212 | 2380 (33) |
| Carotid ultrasound | 7212 | 4553 (63) |
| Conventional angiography | 5583 | 121 (2) |
| Intracranial vascular imaging completed: | ||
| CTA but not MRA or Transcranial Doppler | 7213 | 2586 (36) |
| MRA but not Transcranial Doppler | 7213 | 2201 (31) |
| Transcranial Doppler | 7213 | 857 (12) |
| None | 7213 | 1570 (22) |
| Transthoracic echocardiography: | 7213 | 6885 (95) |
| Left atrial diameter, cm (mean ± s.d.) | 4009 | 3.8 ± 1.4 |
| Left ventricular ejection fraction, % (mean ± s.d.) | 5761 | 62.3 ± 8.1 |
| Transesophageal echocardiography | 7211 | 1382 (19) |
| Patent foramen ovale present | 1382 | 372 (27) |
| Duration of cardiac rhythm monitoring ≥48 hours | 7207 | 2438 (34) |
Abbreviations: BMI, body mass index; CTA, computed tomographic angiography; IQR, interquartile range; MoCA, Montreal Cognitive Assessment; MRA, magenetic resonance angiography; NAVIGATE-ESUS, New Approach Rivaroxaban Inhibition of Factor Xa in a Global trial vs. ASA to Prevent Embolism in Embolic Stroke of Undetermined Source; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation; TIA, transient ischemic attack; tPA, tissue plasminogen activator.
Figure 1.
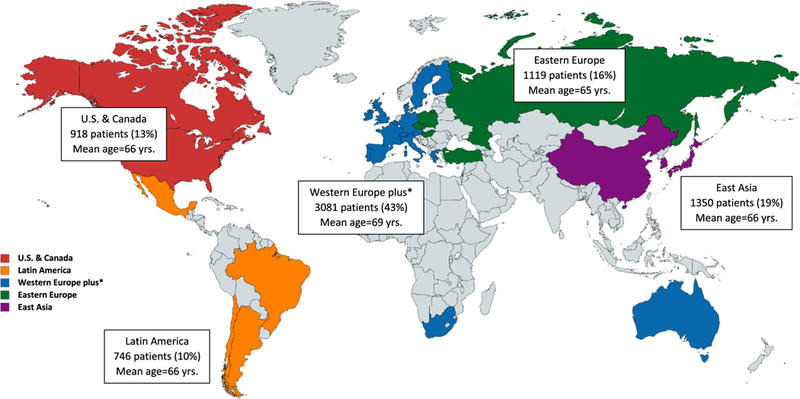
Enrollment by global region in the NAVIGATE-ESUS trial. Abbreviation: NAVIGATE-ESUS, New Approach Rivaroxaban Inhibition of Factor Xa in a Global trial vs. ASA to Prevent Embolism in Embolic Stroke of Undetermined Source.
Table 2.
Comparisons by Age
| Characteristic | <60 yrs (N = 1716) |
60–75 yrs (N = 4012) |
>75 yrs (N = 1485) |
P trend^ |
|---|---|---|---|---|
| Age, years (mean ± s.d.) | 54.1 ± 4.5 | 67.4 ± 4.4 | 80.5 ± 3.7 | |
| Male sex | 1211 (71) | 2525 (63) | 701 (47) | <.001 |
| Race: | ||||
| White only | 1143 (67) | 2933 (73) | 1143 (77) | |
| Black only | 43(3) | 58(1) | 10(1) | |
| East Asian only | 410 (24) | 764(19) | 240 (16) | |
| Others (includes not reported/multiracial) | 120(7) | 258 (6) | 92 (6) | <.001 |
| BMI, kg/m2 (mean ± s.d.) | 27.9 ± 5.4 | 27.3 ± 5.0 | 26.3 ± 4.5 | <.001 |
| Weight, kg (mean ± s.d.) | 80.5 ± 17.3 | 76.6 ± 16.2 | 69.9 ± 14.5 | <.001 |
| Estimated glomerular filtration rate (eGFR), mL/min per 1.73 m2 | 88.1 ± 21.0 | 78.7 ± 19.3 | 67.5 ± 17.4 | <.001 |
| Medical history: | ||||
| Hypertension | 1344 (78) | 3005 (75) | 1237 (83) | 0.002 |
| Diabetes mellitus | 456 (27) | 1014 (25) | 335 (23) | 0.01 |
| Current tobacco use | 648 (38) | 760 (19) | 76 (5) | <.001 |
| Coronary artery disease | 79 (5) | 254 (6) | 140 (9) | <.001 |
| Heart failure | 65 (4) | 113 (3) | 60 (4) | 0.80 |
| Cancer | 43 (3) | 358 (9) | 219 (15) | <.001 |
| Bioprosthetic heart valve | 3 (0) | 12 (0) | 6 (0) | 0.23 |
| Prior stroke or TIA | 331 (19) | 625 (16) | 307 (21) | 0.44 |
| Global region: | ||||
| U.S.A. and Canada | 251 (15) | 502 (13) | 165 (11) | |
| Latin America | 177 (10) | 429 (11) | 140 (9) | |
| Western Europe | 595 (35) | 1672 (42) | 814 (55) | |
| Eastern Europe | 306 (18) | 681 (17) | 132 (9) | |
| East Asia | 387 (23) | 729 (18) | 234 (16) | <.001 |
| Qualifying stroke: | ||||
| Clinical TIA with imaging-confirmed infarction as qualifying event: | 96 (6) | 311 (8) | 114 (8) | 0.02 |
| Arterial territory of qualifying stroke: | ||||
| Anterior circulation | 1224 (71) | 2868 (71) | 1095 (74) | 0.14 |
| Posterior circulation | 538 (31) | 1280 (32) | 451 (30) | 0.58 |
| Location of qualifying stroke: | ||||
| Single Location: | ||||
| Cerebral hemisphere with cortical involvement | 828 (48) | 2276 (57) | 932 (63) | <.001 |
| Cerebral hemisphere, subcortical only | 461 (27) | 839 (21) | 218 (15) | <.001 |
| Brainstem only | 95 (6) | 191 (5) | 45 (3) | <.001 |
| Cerebellum only | 148 (9) | 302 (8) | 112 (8) | 0.23 |
| Multiple Locations: | 184 (11) | 403 (10) | 175 (12) | 0.38 |
| Chronic infarct on imaging (in addition to index stroke) | 528 (31) | 1278 (32) | 544 (37) | <.001 |
| Aspirin use prior to qualifying stroke | 216 (13) | 673 (17) | 358 (24) | <.001 |
| Treated with intravenous tPA for qualifying stroke | 271 (16) | 736 (18) | 248 (17) | 0.42 |
| Treated with endovascular intervention for qualifying stroke | 69 (4) | 174 (4) | 57 (4) | 0.83 |
| NIHSS score at randomization (median, IQR) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | <.001 |
| Modified Rankin Scale (mRS) at randomization: | ||||
| mRS 0 or 1 | 1091 (64) | 2704 (67) | 875 (59) | |
| mRS 2 | 436 (25) | 877 (22) | 360 (24) | |
| mRS ≥3 | 189 (11) | 431 (11) | 250 (17) | <.001 |
| MoCA score at randomization (median, IQR) | 26.0 (22.0, 28.0) | 25.0 (21.0, 27.0) | 22.0 (18.0, 26.0) | <.001 |
| Time from qualifying stroke to randomization, days (median, IQR) | 35.0 (14.0, 87.5) | 37.0 (15.0, 90.0) | 36.0 (14.0, 86.0) | 0.42 |
Abbreviations: BMI, body mass index; IQR, interquartile range; MoCA, Montreal Cognitive Assessment; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation; TIA, transient ischemic attack; tPA, tissue plasminogen activator.
Table 4.
Comparisons by global region
| Characteristic | U.S.A. & Canada (N = 918) |
Latin America (N = 746) |
Western Europe (N = 3081) |
Eastern Europe (N = 1118) |
East Asia (N = 1350) |
P value^ |
|---|---|---|---|---|---|---|
| Age, years (mean ± s.d.) | 66.2 ± 9.8 | 66.4 ± 10.0 | 68.7 ± 9.8 | 64.7 ± 8.5 | 65.6 ± 10.1 | <.001 |
| Age<60 years | 251 (27) | 177 (24) | 595 (19) | 306 (27) | 387 (29) | <.001 |
| Male sex | 533 (58) | 417 (56) | 1869 (61) | 687 (61) | 931 (69) | <.001 |
| Race: | ||||||
| White only | 783 (85) | 605 (81) | 2714 (88) | 1117 (100) | 0 (0) | |
| Black only | 59 (6) | 29 (4) | 22 (1) | 1 (0) | 0 (0) | |
| East Asian only | 42 (5) | 2 (0) | 23 (1) | 0 (0) | 1347 (100) | |
| Others (includes not reported/multiracial) | 34 (4) | 110 (15) | 322 (10) | 1 (0) | 3 (0) | <.001 |
| BMI, kg/m2 (mean ± s.d.) | 29.3 ± 6.4 | 28.1 ± 4.6 | 27.5 ± 4.8 | 28.3 ± 4.5 | 23.8 ± 3.2 | <.001 |
| Weight, kg (mean ± s.d.) | 83.4 ± 19.2 | 74.5 ± 14.5 | 78.1 ± 15.7 | 81.1 ± 14.6 | 63.7 ± 11.2 | <.001 |
| Estimated glomerular filtration rate (eGFR), mL/min per 1.73 m2 | 73.3 ± 17.8 | 80.9 ± 22.0 | 77.5 ± 19.2 | 78.6 ± 20.1 | 83.5 ± 23.4 | <.001 |
| Medical history: | ||||||
| Hypertension | 672 (73) | 621 (83) | 2334 (76) | 995 (89) | 964 (71) | <.001 |
| Diabetes mellitus | 220 (24) | 230 (31) | 690 (22) | 305 (27) | 360 (27) | <.001 |
| Current tobacco use | 154 (17) | 96 (13) | 567 (18) | 275 (25) | 392 (29) | <.001 |
| Coronary artery disease | 114 (12) | 27 (4) | 158 (5) | 114 (10) | 60 (4) | <.001 |
| Heart failure | 30 (3) | 16 (2) | 81 (3) | 102 (9) | 9 (1) | <.001 |
| Cancer | 120 (13) | 41 (5) | 308 (10) | 49 (4) | 102 (8) | <.001 |
| Bioprosthetic heart valve | 6 (1) | 0 (0) | 11 (0) | 2 (0) | 2 (0) | 0.10 |
| Prior stroke or TIA | 215 (23) | 136 (18) | 508 (16) | 153 (14) | 251 (19) | <.001 |
| Qualifying stroke: | ||||||
| Clinical TIA with imaging-confirmed infarction as qualifying event: | 71 (8) | 41 (5) | 289 (9) | 30 (3) | 90 (7) | <.001 |
| Arterial territory of qualifying stroke: | ||||||
| Anterior circulation | 657 (72) | 525 (70) | 2229 (72) | 789 (71) | 987 (73) | 0.51 |
| Posterior circulation | 303 (33) | 239 (32) | 915 (30) | 339 (30) | 473 (35) | 0.006 |
| Location of qualifying stroke: | ||||||
| Single Location: | ||||||
| Cerebral hemisphere with cortical involvement | 595 (65) | 417 (56) | 1973 (64) | 518 (46) | 533 (39) | <.001 |
| Cerebral hemisphere, subcortical only | 124 (14) | 183 (25) | 449 (15) | 364 (33) | 398 (29) | <.001 |
| Brainstem only | 19 (2) | 56 (8) | 99 (3) | 76 (7) | 81 (6) | <.001 |
| Cerebellum only | 70 (8) | 51 (7) | 247 (8) | 92 (8) | 102 (8) | 0.81 |
| Multiple Locations: | 109 (12) | 39 (5) | 309 (10) | 69 (6) | 236 (17) | <.001 |
| Chronic infarct on imaging (in addition to index stroke) | 261 (28) | 253 (34) | 836 (27) | 414 (37) | 586 (43) | <.001 |
| Aspirin use prior to qualifying stroke | 196 (21) | 168 (23) | 551 (18) | 185 (17) | 147 (11) | <.001 |
| Treated with intravenous tPA for qualifying stroke | 191 (21) | 57 (8) | 684 (22) | 205 (18) | 118 (9) | <.001 |
| Treated with endovascular intervention for qualifying stroke | 69 (8) | 7 (1) | 159 (5) | 20 (2) | 45 (3) | <.001 |
| NIHSS score at randomization (median, IQR) | 0.0 (0.0, 1.0) | 2.0 (1.0, 4.0) | 0.0 (0.0, 2.0) | 2.0 (0.0, 3.0) | 1.0 (0.0, 2.0) | <.001 |
| Modified Rankin Scale (mRS) at randomization: | ||||||
| mRS 0 or 1 | 636 (69) | 411 (55) | 2051 (67) | 633 (57) | 939 (70) | |
| mRS 2 | 207 (23) | 218 (29) | 682 (22) | 308 (28) | 258 (19) | |
| mRS ≥3 | 75 (8) | 117 (16) | 347 (11) | 178 (16) | 153 (11) | <.001 |
| MoCA score at randomization (median, IQR) | 26.0 (23.0, 28.0) | 22.0 (17.0, 25.5) | 25.0 (21.0, 27.0) | 25.0 (22.0, 27.0) | 23.0 (19.0, 26.0) | <.001 |
| Time from qualifying stroke to randomization, days (median, IQR) | 70.0 (41.0, 123.0) | 54.0 (31.0, 102.0) | 42.0 (14.0, 100.0) | 22.0 (13.0, 57.0) | 18.0 (11.0, 37.0) | <.001 |
Abbreviations: BMI, body mass index; IQR, interquartile range; MoCA, Montreal Cognitive Assessment; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation; TIA, transient ischemic attack; tPA, tissue plasminogen activator.
Age
As summarized in Table 2, 24% of patients were under 60 and 21% were older than 75 years. Older subjects were more likely to be women, of white race, and had lower weight and BMI values as well as lower estimated glomerular filtration rates. Older subjects had a greater burden of hypertension, coronary artery disease, cancer, but there were fewer smokers and less diabetes. Cognitive function as assessed by the Montreal Cognitive Assessment (MoCA) was more impaired with increasing age. Aspirin use prior to the qualifying stroke was more common with increasing age. The qualifying strokes in older subjects more frequently involved the cerebral cortex and were more often accompanied by evidence of prior or chronic infarcts observed on neuroimaging.
Sex
As shown in Table 3, compared with men, the 39% of subjects who were women were older, more likely to be white and less likely to be Asian. Women had lower weight but slightly greater BMI than men. Women had modestly more hypertension but were less likely to be current smokers. Women were more commonly treated with acute thrombolysis or endovascular therapy.
Table 3.
Comparisons by Sex
| Characteristic | Male (N = 4436) |
Female (N = 2777) |
P value^ |
|---|---|---|---|
| Age, years (mean ± s.d.) | 65.6 ± 9.4 | 69.1 ± 10.0 | <.001 |
| Age<60 years | 1211(27) | 505 (18) | <.001 |
| Race: | |||
| White only | 3107 (70) | 2112(76) | |
| Black only | 64(1) | 47 (2) | |
| East Asian only | 980 (22) | 434 (16) | |
| Others (includes not reported/multiracial) | 286 (6) | 184 (7) | <.001 |
| BMI, kg/m2 (mean ± s.d.) | 27.1 ± 4.6 | 27.5 ± 5.6 | <.001 |
| Weight, kg (mean ± s.d.) | 80.2 ± 15.7 | 69.7 ± 15.6 | <.001 |
| Estimated glomerular filtration rate (eGFR), mL/min per 1.73 m2 | 80.7 ± 20.7 | 75.4 ± 19.9 | <.001 |
| Medical history: | |||
| Hypertension | 3346 (75) | 2240 (81) | <.001 |
| Diabetes mellitus | 1156 (26) | 649 (23) | 0.01 |
| Current tobacco use | 1159 (26) | 325 (12) | <.001 |
| Coronary artery disease | 320 (7) | 153 (6) | 0.004 |
| Heart failure | 155 (3) | 83 (3) | 0.24 |
| Cancer | 366 (8) | 254 (9) | 0.19 |
| Bioprosthetic heart valve | 15(0) | 6(0) | 0.35 |
| Prior stroke or TIA | 735 (17) | 528 (19) | 0.008 |
| Global region: | |||
| U.S.A. and Canada | 533 (12) | 385 (14) | |
| Latin America | 417 (9) | 329 (12) | |
| Western Europe | 1869 (42) | 1212 (44) | |
| Eastern Europe | 687 (15) | 432 (16) | |
| East Asia | 931 (21) | 419 (15) | <.001 |
| Qualifying stroke: | |||
| Clinical TIA with imaging-confirmed infarction as qualifying event: | 331 (7) | 190 (7) | 0.32 |
| Arterial territory of qualifying stroke: | |||
| Anterior circulation | 3135 (71) | 2052 (74) | 0.003 |
| Posterior circulation | 1461 (33) | 808 (29) | <.001 |
| Location of qualifying stroke: | |||
| Single Location: | |||
| Cerebral hemisphere with cortical involvement | 2449 (55) | 1587 (57) | 0.10 |
| Cerebral hemisphere, subcortical only | 925 (21) | 593 (21) | 0.61 |
| Brainstem only | 213 (5) | 118 (4) | 0.28 |
| Cerebellum only | 354 (8) | 208 (7) | 0.45 |
| Multiple Locations: | 494 (11) | 268 (10) | 0.05 |
| Chronic infarct on imaging (in addition to index stroke) | 1481 (33) | 869 (31) | 0.06 |
| Aspirin use prior to qualifying stroke | 745 (17) | 502 (18) | 0.16 |
| Treated with intravenous tPA for qualifying stroke | 730 (16) | 525 (19) | 0.007 |
| Treated with endovascular intervention for qualifying stroke | 148 (3) | 152 (5) | <.001 |
| NIHSS score at randomization (median, IQR) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 0.83 |
| Modified Rankin Scale (mRS) at randomization: | |||
| mRS 0 or 1 | 2951 (67) | 1719 (62) | |
| mRS 2 | 994 (22) | 679 (24) | |
| mRS ≥3 | 492 (11) | 378 (14) | <.001 |
| MoCA score at randomization (median, IQR) | 25.0 (21.0, 27.0) | 24.0 (20.0, 27.0) | <.001 |
| Time from qualifying stroke to randomization, days (median, IQR) | 36.0 (14.0, 90.0) | 37.0 (15.0, 85.0) | 0.76 |
Abbreviations: BMI, body mass index; IQR, interquartile range; MoCA, Montreal Cognitive Assessment; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation; TIA, transient ischemic attack; tPA, tissue plasminogen activator.
Region
As summarized in Table 4, patients enrolled in Western Europe tended to be the oldest. Smoking was more common in Eastern Europe and East Asia. Patients in the Americas were more likely to be on aspirin prior to the qualifying stroke than those from Europe and East Asia. Patients were less likely to be treated with thrombolysis for the qualifying stroke in Latin America and East Asia. Acute cortical infarction was more common in North America and Western Europe, whereas chronic infarctions in addition to the index stroke were most commonly observed in East Asia.
Race
The majority of subjects were white (72%) or Asian (20%). Variability in baseline characteristics by race is shown in Supplementary Table S2, with notably lower BMI values among Asians and more current tobacco use. Asians also appeared more likely to have subcortical infarctions, more chronic infarctions, and suffered less disability from their strokes (mRS 0–1). Blacks were enrolled mainly in the Americas and had the highest prevalence of hypertension.
Hypertension
A history of hypertension was reported in 77% of subjects, and was associated with older age and the presence of diabetes and coronary artery disease, as shown in Supplementary Table S3. Patients with hyper-tension were more likely to be taking aspirin prior to the qualifying stroke, had more evidence of chronic radiographic infarction, and mildly lower MoCA scores.
Diabetes mellitus
Diabetes mellitus was present in 25% of subjects at enrollment and characteristics are summarized in Supplementary Table S4. Patients with diabetes were younger, had greater BMI values, and were more likely to have hypertension, coronary disease, prior stroke as well as chronic radiographic infarction, and mildly lower MoCA scores. Aspirin use prior to the index stroke was more common and thrombolysis use less common in patients with diabetes.
Stroke or TIA prior to index event
As shown in Supplementary Table S5, 17% of subjects had a prior clinical stroke or TIA. These patients were similar with respect to demographic features, but had a greater burden of coronary artery disease. Aspirin use prior to the index stroke and chronic infarcts on imaging were more than twice as common in this group, and they had slightly lower MoCA scores.
Time from qualifying stroke to randomization
As indicated in Supplementary Table S6, 45% of subjects were enrolled within 30 days of the qualifying stroke, 31% between 30 and 90 days, and 24% between 3 and 6 months. Patients in East Asia and Eastern Europe were enrolled earlier than in other regions.
Discussion
NAVIGATE ESUS is the largest randomized trial comparing antithrombotic therapeutic strategies for secondary stroke prevention in patients with ESUS. The study population encompasses a wide spectrum of patients across multiple continents. Moreover, the population is similar to published smaller cohorts of patients with cryptogenic stroke or ESUS,2,12–15 supporting the external validity and generalizability of the ESUS concept and its implementation in this trial.
The hypothesis of the NAVIGATE-ESUS trial is that rivaroxaban would be associated with a substantially lower risk of recurrent embolic events without a clinically unacceptable increase of major hemorrhages relative to aspirin, with relatively consistent treatment effects across the subgroups described here. However, higher event rates would be anticipated for older patients, women, and those with stroke or TIA prior to qualifying event, hypertension, and diabetes. Observed baseline differences across subgroups may be important in the assessment of treatment effects within these groups. Some of these are potentially relevant confounders, such as the relationships between older age and prior antiplatelet agent use, or between global region and time from qualifying event to randomization, because those relationships within subgroups could also be associated with outcome events. Others are statistically significant relationships that are unlikely to confound a treatment effect, such as the apparent relationship between smoking and time from the qualifying event, but nevertheless provide important descriptions of the cohort. The relevance of these factors will need to be weighed in the context of the overall and subgroup analyses of treatment effects in NAVIGATE ESUS, which are anticipated in the near future. Further, these data will provide a robust opportunity to determine if different ESUS subgroups have varying risks of recurrent stroke and other major vascular events. ESUS is a broad definition and description of the full cohort across multiple baseline characteristics helps to understand the inherent heterogeneity and perhaps guide future trials about optimal patient selection.
This study has potential limitations. Despite the large size of NAVIGATE cohort, it represents patients who are willing and able to participate in a clinical trial and therefore may be subject to limitations on generalizability, both overall and within these subgroups. Differences in the acute treatment of stroke and variations in risk factors based on racial and genetic predispositions may introduce heterogeneity among these subgroups. Further, diagnostic testing may affect outcome analysis as patients in higher income countries may have undergone more extensive pre-enrollment investigation than those in middle or lower income countries.
NAVIGATE ESUS is the largest randomized trial in ESUS and the first to address new paradigms for stroke diagnosis and prevention. The results, particularly in key subgroups, are expected to shed new light on the treatment and prognosis of ESUS.
Supplementary Material
Acknowledgments
Grant support: Sponsored by Bayer AG and Janssen Research and Development; partial funding for the Biomarker, Genetics, Gene Expression Substudy from the Canadian Stroke Prevention Intervention Network.
Footnotes
Appendix: Supplementary Material
Supplementary data to this article can be found online at doi:10.1016/j.jstrokecerebrovasdis.2018.01.027.
References
- 1.Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–438. [DOI] [PubMed] [Google Scholar]
- 2.Perera KS, Vanassche T, Bosch J, et al. Embolic strokes of undetermined source: prevalence and patient features in the ESUS Global Registry. Int J Stroke 2016;11:526–533. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Yiin GS, Geraghty OC, et al. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol 2015;14:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS stroke data bank. Ann Neurol 1989;25:382–390. [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for secondary stroke prevention in patients with embolic strokes of undetermined source: design of the NAVIGATE ESUS randomized trial. Eur Stroke J 2016;1:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Age Charidimou A. and the fuzzy edges of embolic stroke of undetermined source: implications for trials. Neurology 2017;89:526–527. [DOI] [PubMed] [Google Scholar]
- 7.Ntaios G, Lip GYH, Vemmos K, et al. Age- and sex-specific analysis of patients with embolic stroke of undetermined source. Neurology 2017;89:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet 2005;365:176–186. [DOI] [PubMed] [Google Scholar]
- 9.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med 2007;357:2189–2194. [DOI] [PubMed] [Google Scholar]
- 10.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulman S, Kearon C. Subcommittee on control of anticoagulation of the S, Standardization Committee of the International Society on T and haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb haemost 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 12.Arauz A, Morelos E, Colin J, et al. Comparison of functional outcome and stroke recurrence in patients with Embolic Stroke of Undetermined Source (ESUS) vs. Cardioembolic Stroke Patients. PLoS ONE 2016;11: e0166091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diener HC, Bernstein R, Hart R. Secondary stroke prevention in Cryptogenic Stroke and Embolic Stroke of Undetermined Source (ESUS). Curr Neurol Neurosci Rep 2017;17:64. [DOI] [PubMed] [Google Scholar]
- 14.Hart RG, Catanese L, Perera KS, et al. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke 2017;48:867–872. [DOI] [PubMed] [Google Scholar]
- 15.Montero MV, Pastor AG, Cano BC, et al. The A-S-C-O classification identifies cardioembolic phenotypes in a high proportion of Embolic Stroke of Undetermined Source (ESUS). J Neurol Sci 2016;367:32–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


