Abstract
Objective
Often unrecognized, obstructive sleep apnea (OSA) worsens over pregnancy and is associated with poorer perinatal outcomes. The association between OSA in late pregnancy and metabolic biomarkers remains poorly understood. We tested the hypothesis that OSA in pregnant women with obesity is positively correlated with 24-hour patterns of glycemia and IR despite controlling for diet.
Design
Pregnant women (32 to 34 weeks’ gestation; body mass index, 30 to 40 kg/m2) wore a continuous glucose monitor for 3 days. OSA was measured in-home by WatchPAT 200™ [apnea hypopnea index (AHI), oxygen desaturation index (ODI; number per hour)]. Fasting blood was collected followed by a 2-hour, 75-g, oral glucose tolerance test to measure IR. Association between AHI and 24-hour glucose area under the curve (AUC) was the powered outcome.
Results
Of 18 women (29.4 ± 1.4 years of age [mean ± SEM]), 12 (67%) had an AHI ≥5 (mild OSA). AHI and ODI were correlated with 24-hour glucose AUC (r = 0.50 to 0.54; P ≤ 0.03) and mean 24-hour glucose (r = 0.55 to 0.59; P ≤ 0.02). AHI and ODI were correlated with estimated hepatic IR (r = 0.59 to 0.74; P < 0.01), fasting free fatty acids (fFFAs; r = 0.53 to 0.56; P < 0.05), and waking cortisol (r = 0.49 to 0.64; P < 0.05).
Conclusions
Mild OSA is common in pregnant women with obesity and correlated with increased glycemic profiles, fFFAs, and estimates of hepatic IR. OSA is a potentially treatable target to optimize maternal glycemia and metabolism, fetal fuel supply, and pregnancy outcomes.
Obstructive sleep apnea, patterns of glycemia, and insulin action were measured in pregnant women with obesity. Obstructive sleep apnea was associated with 24-hour glycemia and insulin resistance.
Pregnancy requires substantial anatomical and physiologic adaptations that result in difficulty sleeping. Increasing weight and abdominal pressure make snoring, a symptom of sleep-disordered breathing, increasingly common as pregnancy progresses (1–3). Although these changes are largely seen as “normal,” epidemiologic data suggest that sleep disturbances during pregnancy are associated with negative ramifications for maternal and fetal health (4, 5). Obstructive sleep apnea (OSA), the most severe form of sleep-disordered breathing, worsens insulin resistance (IR), is associated with metabolic syndrome and hypertensive disorders, and is estimated to affect 9% to 50% of women (2, 6). This is concerning, as OSA is tightly linked to obesity (7) and type 2 diabetes (8) outside of pregnancy, and it is correlated with a higher risk of gestational diabetes mellitus (GDM) (9, 10) and poor pregnancy outcomes.
Given that >60% of women of childbearing age are overweight or obese (11) and that sleep disorders are underdiagnosed in pregnancy (6), it is likely that many pregnant women with obesity have preexisting undiagnosed OSA worsened by pregnancy. Many women with obesity enter pregnancy with a “background” of chronic preexisting IR, upon which the IR of normal pregnancy is additive (12, 13). In fact, we and others have published that pregnant women with obesity demonstrate ∼8% to 10% higher 24-hour glycemic profiles than do normal-weight mothers (14, 15), a strong risk factor for fetal overgrowth, yet they do not make criteria for GDM. Although women who qualify for a diagnosis of GDM by their glucose tolerance test will receive nutrition therapy, a glucometer, and intensified fetal surveillance, these pregnant women with obesity are considered “normal glucose tolerant” (NGT) and do not qualify for interventions. However, obesity alone accounts for the greatest number of cases of macrosomia (birth weight ≥4000 g) (16), which result in a high risk for fetal and newborn complications (17). These occult metabolic changes are rarely identified early and may result in an increased risk for metabolic disease and type 2 diabetes for the mother (18). Furthermore, being born either large or small for gestational age also increases the risk for metabolic disease later in life for the offspring (19, 20).
It is possible that worsening unrecognized OSA, particularly in women with obesity, may exacerbate IR after testing for GDM occurs. This is of particular concern during the third trimester when increasing IR, difficulties sleeping, changes in pulmonary vital capacity, and weight gain occur in parallel with accelerated fetal growth. Worsening OSA may lead to exacerbation of pregnancy IR, occult hyperglycemia, and hyperlipidemia, which are now understood to impart a potentially deleterious fetal–placental exposure to overnutrition and fetal overgrowth. Alternatively, or even synergistically, more severe OSA may also create fetal–placental exposures to heightened inflammation and brief but frequent episodes of maternal hypoxia that may impair placental function, affect oxygenation, and restrict nutrition supply to the fetus (4). Rodent models suggest that exposure to symptoms of OSA (intermittent hypoxia or sleep fragmentation) may increase the risk for infant and early childhood obesity in the offspring (21–24). Furthermore, OSA is a strong risk factor for metabolic disease in children and adults with obesity, particularly nonalcoholic fatty liver disease (25). Importantly, because OSA is potentially treatable, understanding the association between OSA and maternal metabolism creates an opportunity for targeted interventions with substantial potential to improve maternal–fetal outcomes.
A limitation of the existing evidence in humans is a lack of objective measures in controlled clinical studies to quantify the association between OSA and metabolism in pregnancy. Accordingly, the aim of the study was to determine the relationship between OSA and patterns of 24-hour glycemia and IR using controlled, objective measures of glucose and lipid metabolism in NGT women with obesity during late pregnancy while controlling for diet. We tested the hypothesis that increasing severity of OSA is positively correlated with 24-hour patterns of glycemia and is associated with heightened IR in NGT pregnant women with obesity during 32 to 34 weeks’ gestation, while controlling for diet. A secondary exploratory aim was to examine the association between OSA, fasting free fatty acids (fFFAs), nocturnal and early morning cortisol levels, and neonatal adiposity.
Methods
This prospective study was approved by the Colorado Multiple Institutional Review Board. Healthy pregnant women with current body mass index (BMI) in the obesity range (BMI at enrollment ≥30 to ≤40 kg/m2) receiving their obstetric care at the University of Colorado Hospital on the Anschutz Medical Campus (Aurora, CO) were recruited to participate during gestational weeks 32 to 34. Women were 20 to 39 years of age, had a singleton pregnancy, and had a normal glucose tolerance test at 24 to 28 weeks according to American College of Obstetricians and Gynecologists criteria for GDM diagnosis (26). Women were excluded when they had a diagnosis of diabetes (GDM, preexisting DM), were on medications for hypertension, or had diagnosed pulmonary or cardiovascular disease. Women with children aged ≤2 years old (due to disrupted sleep), or women with a diagnosed sleep disorder (e.g., OSA, insomnia, restless leg syndrome), who worked night or rotating shifts, or who reported use of sleep medications were also excluded.
Study protocol
Women were studied during gestational weeks 32 to 34 to avoid the confounding effect of increasing pregnancy IR seen when the gestational week is not controlled (27). After obtaining informed consent, a Dexcom G4™ real-time continuous glucose monitoring (CGM) sensor (DexCom, San Diego, CA) was inserted and worn for 72 hours. A 48-hour fixed eucaloric diet (50% carbohydrate, 35% fat, 15% protein; consumption began on day 2) was provided by the Colorado Clinical Translational Science Institute Bionutrition Department to control for the independent effect of diet on 24-hour glycemic patterns, fasting lipids, and IR. To monitor sleep, women wore the WatchPAT200™ (Itamar Medical, Caesarea, Israel) for three consecutive nights. Salivary cortisol was collected just prior to sleep and immediately on awakening on the second and third nights. Women were asked to record wake and sleep times as well as meal times in a journal provided. Women were instructed to not exercise vigorously while in the study. Following the third night, after a 10-hour overnight fast, women reported to the Colorado Clinical and Translational Science Institute outpatient research clinic. Fasting metabolic markers [glucose, insulin, fFFAs, glycerol, total triglycerides (TGs), cholesterol, high-density lipoprotein and low-density lipoprotein cholesterol, and HbA1c] were collected followed by a 75-g oral glucose tolerance test (OGTT). Glucose and insulin were collected following the protocol of Matsuda and DeFronzo (28) (t −15 minutes, t = 0, followed by blood collection every 30 minutes for 2 hours) to assess IR. Women were followed through delivery for collection of perinatal outcomes, and neonatal anthropometry and body composition by air displacement plethysmography (PEAPOD™; Cosmed, Rome, Italy) were completed at 2 weeks of life.
Sleep data
The WatchPAT 200™ is a wrist-worn device that includes pulse oximetry approved for ambulatory diagnosis of OSA (29) and has been validated against polysomnography in pregnant women (30). Sleep data collected during the three nights were averaged for each woman. Markers of severity of OSA measured included the apnea hypopnea index (AHI; number of apneas and hypopneas per hour of sleep) and oxygen desaturation index (ODI; number of times oxygen saturation dropped ≥4% from baseline per hour of sleep). If women had an AHI ≥5 [diagnostic of at least mild sleep apnea (31)] they were referred to their provider after participation in the study. Minimum overnight oxygen saturation (lowest oxygen saturation percentage reached overnight) as well as average oxygen saturation were also measured. Sleep measures also included: total sleep time (TST; total time spent asleep), sleep onset latency (amount of time it took to fall asleep), sleep efficiency (SE; percentage of night spent sleeping), and number of awakenings.
CGM
Real-time CGM (Dexcom G4™) was used to monitor interstitial glucose every 5 minutes during the 48 hours of provided diet. Women were blinded to CGM glucose concentrations. Glucometers were provided (OneTouch; LifeScan, Milpitas, CA) to calibrate the CGM sensor twice daily. Glucose data were analyzed during the 48-hour period of diet control. Pregnancy-specific CGM variables were extracted and analyzed as previously described (14, 32), including 24-hour glucose area under the curve (AUC), mean 24-hour glucose, and daytime and nocturnal glucose. The average from the 2 days of diet control for each glucose variable was used in the analyses. Intraclass coefficients between glucometer and CGM glucose concentrations ranged from 0.75 to 0.95.
Blood assays
Batched samples were run using well-established protocols in the Core Laboratory of the Colorado Clinical and Translational Science Institute. Plasma glucose was measured using hexokinase (Beckman Coulter, Brea, CA) and plasma insulin was measured by radioimmunoassay (Millipore, Billerica, MA). Fasting plasma TGs, direct low-density lipoprotein cholesterol (Beckman Coulter), and fFFAs (Wako Chemicals, Richmond, VA) were measured using an enzymatic method and lipids were measured by a spectrophotometric method (vanillin/sulfuric acid). HbA1c was measured using potassium ferricyanide (Siemens DCA Vantage, Malvern, PA). Whole-body IR was calculated using the Matsuda index (28), where a higher index indicates higher insulin sensitivity. Hepatic IR was estimated using the product of glucose and insulin AUC from the first 30 minutes of the OGTT (33), where a higher index indicates greater IR. The homeostatic model assessment of IR (HOMA-IR) was calculated as [fasting insulin (µU/mL)] × [fasting glucose (mg/dL) × 0.05551]/22.5 (34). To estimate adipose IR, the product of fFFAs and fasting insulin was calculated (35), where a higher index indicates greater IR.
Salivary cortisol
Salivary cortisol was immediately frozen at −80°C and samples were run in a batch at the end of the study. Cortisol was measured by enzyme assay (Salimetrics, Carlsbad, CA). The mean of the two morning and two evening values were used in the analysis.
Neonatal anthropometry and body composition
Birthweight and gestational age at birth were collected from self-report and when possible confirmed by medical record. At ∼2 weeks of life, women brought their infants in to measure body composition. Fat mass [newborn percent fat (NB%fat)] was assessed using air-displacement plethysmography (PEAPOD™) (36, 37). Weight was obtained with a calibrated scale at the same time. Length was obtained with a standardized length board.
Statistical analysis
Data are means ± SEM; all data approximated a normal distribution. The primary analysis for this prospective study was the correlation between AHI and 24-hour glucose AUC by CGM. Sample size was calculated a priori for the primary hypothesis that AHI would account for 50% of the variability in 24-hour glucose AUC (R2 = 0.50). N = 17 women would provide 80% power to detect a significant association between AHI and 24-hour glucose AUC (two-tailed significance, α = 0.05) (38). Pearson correlations and simple linear regression were first applied to determine the strength of the association between markers of OSA/sleep (e.g., AHI, ODI, TST, SE) with 24-hour glucose AUC (primary analysis) and mean 24-hour glucose from CGM. For exploratory analyses, Pearson correlations and simple linear regression were further applied between markers of OSA/sleep with whole-body IR, estimated hepatic IR, markers of metabolic function (e.g., fFFAs, glycerol, TGs), cortisol, and NB%fat. Sleep, glucose, and metabolic markers were also compared between women with an AHI ≥5 (OSA) and those with an AHI <5 (no OSA) using independent group t tests.
Results
Of 19 women with obesity who were enrolled in the study, valid sleep and glycemia data were available for 18 women. Table 1 provides demographic characteristics of the women as well as birth and neonatal outcomes (n = 16). Thirteen of the women reported their ethnicity to be white, four women reported to be black, and one woman reported to be “other.” Of the four women who had cesarean deliveries, only one was unplanned due to failure to progress. The single preterm birth was due to development of severe preeclampsia. Body composition testing was completed on 13 of 18 infants. Two mothers did not bring their infants back at 2 weeks postpartum, one infant was born preterm (<37 weeks) and infant body testing was not completed, and PEAPOD™ data for one infant were unrecoverable owing to a mechanical error. On average, infants were born full term and birthweights were appropriate for gestational age (no infants were born large or small for gestational age). Average infant fat mass at 2 weeks was 10.9% ± 1.3%, in the range of normal (8% to 12%) (39).
Table 1.
Demographic and Sleep Summary Data
| Maternal demographics (n = 18)a | |
| Age, y | 29.4 ± 1.2 |
| Gestational age, wk | 33 4/7 ± 1/7 |
| BMI, kg/m2 | 34.3 ± 0.57 |
| White | 13 (72) |
| Delivery outcomes (n = 16) | |
| Preeclampsia | 3 (19) |
| Cesarean-section delivery | 4 (25) |
| Preterm birth (<37 wk) | 1 (6) |
| Infant demographic information (n = 16) | |
| Female | 10 (63) |
| Birthweight, g | 3062 ± 76.9 |
| Gestational age at birth, wk | 38 4/7 ± 2/7 |
| Age at PEAPOD™,b d | 16.4 ± 0.72 |
| NB%fatc | 10.9 ± 1.3 |
| Length at 2 wk, cm | 50.9 ± 0.32 |
| Weight at 2 wk, g | 3383.1 ± 84.7 |
Data are means ± SEM or N (% of total).
n = 13 for PEAPOD™ data.
NB%fat was measured by PEAPOD™.
Sleep
Twelve of 18 women (67%) had an AHI ≥5 (range 0-35), indicative of at least mild OSA (31). Six of the 12 women had mild sleep apnea (AHI 5 to 14), 5 women had moderate sleep apnea (AHI 15 to 29), and 1 woman had severe sleep apnea (AHI ≥30). There were no differences in ethnicity, BMI, or age between women with OSA and women without OSA. Across the entire sample, women slept ∼6 hours per night (370.3 ±12.4 minutes), sleep efficiency was low (80.1% ± 1.5%), and average AHI and ODI were mildly elevated at 11.6 ± 2 and 5.2 ± 1.3. Table 2 provides the differences in sleep, glucose, and metabolic markers between women without OSA (AHI <5) and women with OSA (AHI ≥5). Although 12 of the mothers were told that they had OSA and were referred back to their primary care providers, none of the women received follow-up testing or treatment during pregnancy.
Table 2.
Comparison of Sleep, CGM, and OGTT Data Between Women With No OSA (AHI <5) and With OSA (AHI ≥5)
| AHI <5 (n = 6) | AHI ≥5 (n = 12) | |
|---|---|---|
| Sleep dataa | ||
| Total sleep time, min | 349 ± 20.1 | 380.9 ± 15.2 |
| Sleep efficiency, % | 80.0 ± 3.6 | 80.1 ± 1.4 |
| Sleep latency, min | 20.9 ± 3.0 | 25.4 ± 3.1 |
| Mean O2 saturation | 94.0 ± 0.4 | 94.2 ± 0.0.3 |
| Minimum saturation, % | 89.1 ± 1.3 | 87.9 ± 0.7 |
| Mean pulse, bpm | 77.8 ± 1.7 | 83.0 ± 2.9 |
| AHI (events per h) | 2.54 ± 0.58 | 16.1 ± 2.5b |
| ODI (events per h) | 0.7 ± 0.2 | 7.4 ± 1.7b |
| Glucose data from CGMa | ||
| Mean prebreakfast glucose, mg/dL (mmol/L) | 84.8 ± 5.9 (4.7 ± 0.32) | 86.5 ± 3.0 (4.8 ± 0.17) |
| Mean 24-h glucose, mg/dL (mmol/L) | 91.9 ± 3.8 (5.1 ± 0.21) | 100.9 ± 2.3 (5.6 ± 0.13)c |
| Mean daytime glucose, mg/dL (mmol/L) | 92.6 ± 3.7 (5.13 ± 0.21) | 101.4 ± 2.4 (5.6 ± 0.13)d |
| Mean nocturnal glucose, mg/dL (mmol/L) | 94.3 ± 4.2 (5.2 ± 0.23) | 98.7 ± 4.1 (5.5 ± 0.23) |
| 24-h Glucose AUC, mg⋅min/dL (mmol⋅min/L) | 131,693.7 ± 5402.6 (7309 ± 299.8) | 144.140.5 ± 3029.5 (7999.8 ± 168.1)c |
| Metabolic markersa | ||
| Fasting glucose, mg/dL (mmol/L) | 77.1 ± 2.9 (4.3 ± 0.16) | 76.0 ± 1.8 (4.2 ± 0.10) |
| Fasting insulin, μIU/L | 17.1 ± 2.0 | 16.6 ± 1.9 |
| 2-h Glucose, mg/dL (mmol/L) | 135.8 ± 15.1 (7.5 ± 0.84) | 125.1 ± 6.6 (6.94 ± 0.37) |
| 2-h Insulin, μIU/L | 146.5 ± 34.0 | 161.9 ± 17.9 |
| Glucose AUC, mg⋅min/dL (mmol⋅min/L) | 16,203.8 ± 1202.1 (899.3 ± 66.7) | 15,121.9 ± 475.3 (839.3 ± 26.4) |
| Insulin AUC, μIU⋅min/L | 13,638.8 ± 2185.6 | 18,129.6 ± 1810.5 |
| Fasting glycerol, μM/L | 101.5 ± 16.2 | 123.8 ± 12.7 |
| fFFAs, μEq/L | 500.4 ± 84.7 | 550.8 ± 44.2 |
| Fasting TGs, mg/dL (mmol/L) | 203.3 ± 21.4 (11.3 ± 1.2) | 221.7 ± 18.6 (12.3 ± 1.0) |
| HbA1c, % (mmol/mol) | 5.4 ± 0.08 (36 ± 0.9) | 5.5 ± 0.06 (37 ± 0.7) |
| Matsuda index | 2.5 ± 0.32 | 2.2 ± 0.17 |
| Hepatic IR [glucose0–30 (AUC) × insulin0–30 (AUC)] | 5.7 × 106 ± 6.0 × 105 | 8.2 × 106 ± 5.9 × 105c |
| Adipose IR (free fatty acids0 × insulin0) | 8611.9 ± 1708 | 9552.1 ± 139.6 |
| Waking cortisol, µg/dL (nmol/L) | 0.52 ± 0.06 (14.4 ± 1.7) | 0.56 ± 0.06 (15.5 ± 1.7) |
| Evening cortisol, µg/dL (nmol/L) | 0.19 ± 0.03 (5.2 ± 0.83) | 0.22 ± 0.02 (6.1 ± 0.55) |
Sleep data represent the average for each woman over the three nights. CGM data are the average of 2 d of CGM use on controlled diet. Prebreakfast glucose is the sum of six consecutive values starting at 6:00 am or after a 7-h fast (if food consumed after 11:00 pm). Mean daytime glucose is the mean glucose between 6:30 am and 11:30 pm; mean nocturnal glucose is the mean glucose between 11:30 pm and 6:30 am. For the Matsuda index, a higher index indicates greater insulin sensitivity. For hepatic and adipose IR, higher indices indicate greater IR.
Data are means ± SEM.
P ≤ 0.01.
P < 0.05.
P = 0.057.
Twenty-four–hour patterns of glycemia, metabolic markers, and cortisol
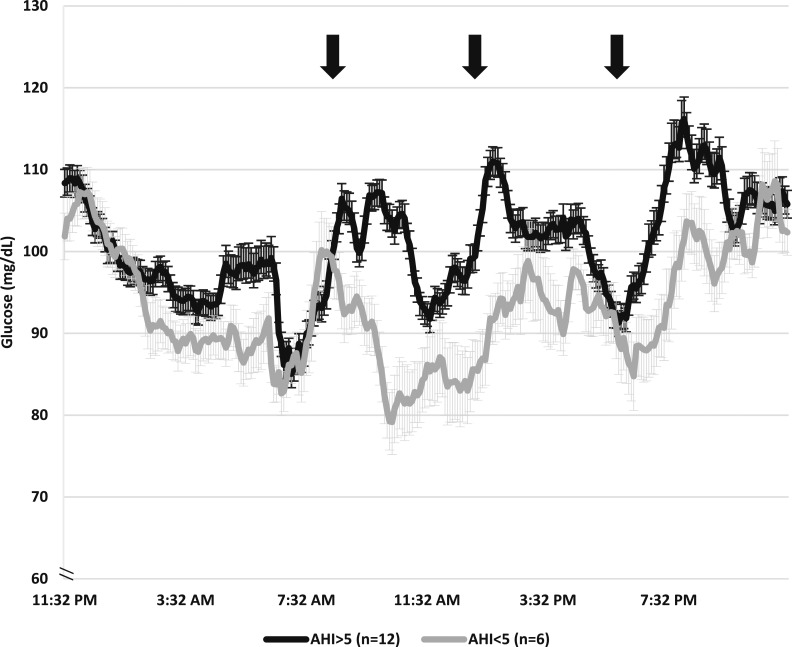
Across women and during the 2 days of controlled diet, the prebreakfast (6:00 to 6:30 am) glucose by CGM was 85.9 ± 2.7 mg/dL (4.8 ± 0.15 mmol/L), and the mean 24-hour glucose was 97.9 ± 2.2 mg/dL (5.44 ± 0.12 mmol/L). At the OGTT, fasting plasma insulin was elevated at 16.8 ± 1.4 μIU/L whereas fasting plasma glucose was normal at 76.2 ± 1.5 mg/dL (4.2 ± 0.08 mmol/L) for the entire group. Waking and evening cortisol levels were in the normal range. Compared with women without OSA, mean 24-hour glucose AUC [144,140.5 ± 3029.5 (8007.8 ± 168.3 mmol⋅min/L) vs 131,697.1 ± 5402.6 mg⋅min/dL (7316.5 ± 300.14 mmol⋅min/dL)] and 24-hour mean glucose (100.9 ± 2.3 (5.6 ± 0.13 mmol/L) vs 91.9 ± 3.8 mg/dL (5.11 ± 0.21 mmol/L)] were substantially higher in women with OSA (P < 0.05 for both). Figure 1 demonstrates the significant difference in 24-hour glucose patterns between groups. As highlighted in Table 2, fasting metabolic markers were similar between women with OSA and women without OSA. Women with OSA had higher estimated hepatic IR (P = 0.02); however, glucose and insulin were not different between groups throughout the OGTT.
Figure 1.
Twenty-four–hour patterns of glycemia higher in women with OSA compared with women without OSA. Black line represents women with at least mild OSA (AHI ≥5), and gray line represents women with no OSA (AHI <5) (mean ± SEM). Mean 24-h glucose AUC was significantly higher in women with OSA (144,140.5 ± 3029.5 mg⋅min/dL) compared with women without OSA (131,697.1 ± 5402.6 mg⋅min/dL, P < 0.05). Black arrows indicate approximate meal start times.
Correlation/regression analyses between sleep and 24-hour patterns of glycemia
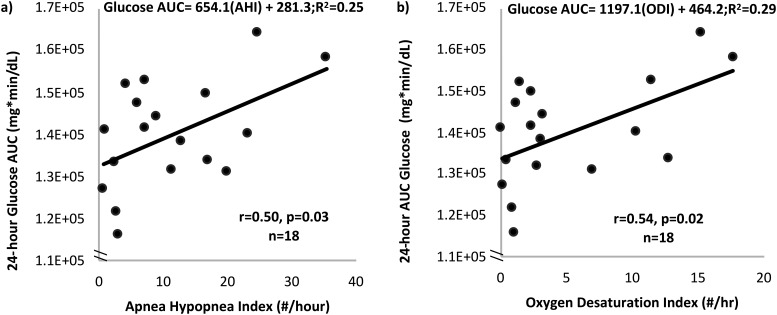
The primary analysis showed that AHI was associated with 24-hour glucose AUC (r = 0.50, P = 0.03; β = 654.1, R2 = 0.25, P = 0.03; Fig. 2a) and mean 24-hour glucose (r = 0.55, P = 0.02; β = 0.52, R2 = 0.33, P = 0.02). ODI was also associated with both 24-hour glucose AUC (r = 0.54, P = 0.02; β = 0.93, R2 = 0.30, P = 0.02; Fig. 2b) and mean 24-hour glucose (r = 0.58, P = 0.01; β = 1191.1, R2 = 0.29, P = 0.02), but no significant correlations were found between AHI and ODI with nocturnal glucose from CGM. Figure 2 demonstrates the correlations between AHI and ODI with the 24-hour glucose AUC. Conversely, total sleep time was negatively correlated with 24-hour glucose AUC (r = −0.46, P = 0.05) and mean 24-hour glucose (r = −0.50, P = 0.04), indicating that less total sleep was related to higher patterns of glucose, as expected.
Figure 2.
Increasing severity of OSA is related to higher patterns of 24 glucose AUC. (a) Increasing AHI (number of apneas and hypopneas per hour of sleep) was significantly correlated with increasing 24-h glucose AUC measured using continuous glucose monitors. (b) Increasing ODI (number of times oxygen saturation drops ≥4% from baseline per hour of sleep) substantially was significantly correlated with increasing 24-h glucose AUC measured using continuous glucose monitors.
Correlations/regression analyses between OSA, metabolic markers, and cortisol
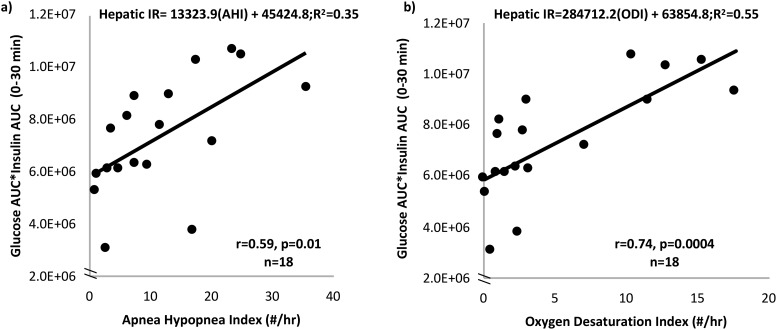
Table 3 provides the Pearson correlation r values between OSA and metabolic markers. As demonstrated in Fig. 3, AHI and ODI were strongly associated with estimated hepatic IR (AHI, β = 133,323.9, R2 = 0.35, P = 0.01, Fig. 3a; ODI, β = 284,712.2, R2 = 0.55, P < 0.001, Fig. 3b). No significant correlations were found between markers of OSA, whole-body IR (Matsuda index), or HOMA-IR. However, AHI and ODI were positively correlated with fasting markers of lipolysis, including fFFAs (r = 0.53 and r = 0.56, respectively; P < 0.05) and glycerol (r = 0.60 and r = 0.66, respectively; P < 0.01). ODI was positively correlated with estimated adipose IR (r = 0.49, P < 0.05). AHI was positively correlated with waking cortisol (r = 0.64, P < 0.01), and there was a trend association between ODI and waking cortisol (r = 0.49, P = 0.05).
Table 3.
Correlations Between Sleep and Fasting Metabolic Markers (r Values)
| AHI | ODI | |
|---|---|---|
| Matsuda index | −0.07 | −0.18 |
| Hepatic IR | 0.59a | 0.74a |
| HOMA-IR | 0.09 | 0.15 |
| Adipose IR | 0.39 | 0.49b |
| Insulin, μIU/L | 0.12 | 0.22 |
| Glucose, mg/dL | −0.09 | −0.33 |
| Free fatty acids, μEq/L | 0.53b | 0.56b |
| Glycerol, μM/L | 0.70a | 0.75a |
| TGs, mg/dL | 0.45 | 0.16 |
| Waking cortisol, µg/dL | 0.64a | 0.49b |
| Evening cortisol, µg/dL | 0.22 | 0.29 |
For the Matsuda index, a higher index indicates greater insulin sensitivity. For hepatic and adipose IR, higher indices indicate greater IR. Adipose IR refers to an estimate of adipose IR.
P ≤ 0.001.
P ≤ 0.05.
Figure 3.
Increasing severity of OSA is related to higher hepatic IR. (a) Increasing AHI (number of apneas and hypopneas per hour of sleep) was significantly correlated with increasing hepatic IR measured using a 75-g OGTT. (b) Increasing ODI (number of times oxygen saturation drops ≥4% from baseline per hour of sleep) was significantly correlated with increasing hepatic IR measured using a 75-g OGTT.
Exploratory correlations between markers of sleep quality with metabolic markers
Although not powered to detect these associations, we found that better sleep quality was related to improved metabolic measures. SE was positively correlated with the Matsuda index (higher value indicates higher insulin sensitivity) (r = 0.48, P < 0.05) whereas the correlation between number of awakenings and the Matsuda index was negative (r = −0.54, P < 0.05; Table 3), suggesting that more continuous sleep is correlated with better insulin sensitivity. TST was negatively correlated with fasting insulin (r = −0.50, P = 0.03) as expected, and the numbers of awakenings were positively correlated with fasting TGs (r = 0.58, P = 0.01) and adipose IR (r = 0.56, P = 0.02). Better sleep efficiency was associated with less adipose IR (r = −0.53, P < 0.05).
Exploratory correlations between OSA and infant adiposity
Thirteen of 18 infants had body composition assessed. Severity of OSA as measured by AHI and ODI was positively, but not significantly, correlated with NB%fat (r = 0.34 and r = 0.22, respectively; P > 0.05 for both). However, overnight minimum oxygen saturation was negatively correlated with NB%fat, suggesting that lower overnight oxygen was related to higher newborn fat (r = −0.63, P = 0.02; β = −1.5, R2 = 0.39, P = 0.02). No other markers of sleep were associated with NB%fat (P > 0.05).
Discussion
The goal of this prospective study was to test the hypothesis that increasing severity of OSA, as measured by AHI, is associated with higher glycemic patterns in glucose-tolerant women with obesity during late pregnancy. Using objective measures during a tight gestational window and controlling for diet in the ambulatory setting, we have demonstrated that increasing severity of OSA (within a group of women with mild-to-moderate OSA) was related to higher levels of 24-hour glucose by CGM (Fig. 2). Strikingly, 12 of 18 of these healthy women with obesity had occult OSA, and as a group, their TST (∼6 hours per night) and sleep efficiency (∼80%) were low. The American Academy of Sleep Medicine recommends that adults obtain 7 to 9 hours of sleep to achieve maximum health benefits (40). Moreover, strong positive associations were observed between AHI and ODI with markers of lipolysis and estimated hepatic IR, suggesting a strong connection between sleep disorders and IR in pregnancy. Although exploratory, the association between severity of oxygen desaturation and NB%fat further supports a link between OSA and fetal growth. Although two-thirds of these mothers had some level of OSA, these women with obesity were relatively healthy, as demonstrated by their low fasting plasma glucose. Fasting TGs were elevated, but consistent with our previous cohorts of women with obesity in late pregnancy (13, 41). Taken together, these data suggest that occult OSA is common and may contribute to higher patterns of glycemia and IR. The findings underscore the importance of recognizing patterns of OSA in pregnancy as a treatable condition that, if targeted and attenuated, may mitigate fetal overnutrition secondary to elevated glucose, fFFAs, and heightened maternal IR, potentially improving perinatal outcomes.
Although it is known that OSA increases the odds of being diagnosed with GDM (9), our women with a current BMI in the obesity range had passed their diagnostic test for GDM and were considered to be NGT. Despite normal glucose tolerance, we still found that increasing severity of OSA using AHI and ODI was positively correlated with 24-hour patterns of glycemia, and women with at least mild OSA (AHI ≥5) had substantially higher patterns of glucose throughout the 24-hour period as compared with women with no OSA (Figs. 1 and 2). Although the CGM glucose ranges for the study sample were consistent with our other cohorts of mothers with obesity (13, 14, 41), the average CGM concentrations were higher than those in normal-weight women (14, 27). The severity of OSA found in these women was also mild (average AHI of 11.6); however, it is plausible to speculate that if we had captured women with more severe OSA, the glucose patterns may have been higher. Women wore the CGM device while consuming a controlled healthy diet, which may not be reflective of ad libitum dietary intake. However, consistent with our previous studies (41–43), diet was controlled to characterize the metabolic phenotype independent of the significant confounding effects of nutrition. If normal diet quality is poor, glucose levels may also be higher. We have previously published that women with obesity have ∼8% to 10% higher 24-hour glucose profiles than do normal-weight women (14, 15). Our current findings suggest that occult OSA may be one potential reason for these higher glucose levels. In our study, daytime glucose was higher in women with OSA compared with women with no OSA (101.4 ± 2.4 vs 92.6 ± 3.7 mg/dL) whereas nocturnal glucose was similar between groups (98.7 ± 4.1 vs 94.3 ± 4.2 mg/dL). Higher maternal glucose in combination with heightened IR either due to maternal obesity, OSA, or both may have negative implications for maternal and fetal metabolic health. Considering that most macrosomic babies appear to be born to mothers who are obese and do not meet criteria for GDM (16), these findings support that undiagnosed OSA may be a contributing factor. During the OGTT, there were no statistically significant differences in the fasting and 2-hour glucose levels; however, the insulin levels of the women with OSA were higher. This finding suggests that the women with OSA were more insulin resistant but their glucose levels were maintained by the high levels of circulating insulin.
As previously stated, OSA is also related to an increased risk for GDM (2, 9). In the current study, although increasing severity of OSA was related to higher glycemic patterns, we did not find a significant correlation between severity of OSA and whole-body insulin sensitivity (Matsuda index). However, there was a significant correlation between severity of OSA and estimated hepatic IR (Fig. 3). Outside of pregnancy, experimental sleep fragmentation as well as intermittent hypoxia (the two main defects in OSA) are associated with increased IR in healthy adults (44, 45). The mechanisms behind the relationship between sleep disruption and increasing IR have not been fully elucidated; however, sleep fragmentation and intermittent hypoxia activate the sympathetic nervous system (46) and hypothalamic–pituitary–adrenal axis (47, 48), both of which can exacerbate IR. Indeed, in our current study, waking salivary cortisol levels, a marker hypothalamic–pituitary–adrenal axis activation, were associated with severity of OSA. Cortisol increases gluconeogenesis in the liver and decreases insulin sensitivity (49). The lack of a correlation between OSA and whole-body IR in the current study may be due to the small sample size or the fact that all of the women were highly insulin resistant as a function of pregnancy. Future studies with larger numbers or more precise measures of IR, such as hyperglycemic-euglycemic clamps, may help to further elucidate the mechanisms behind the correlations observed herein.
Outside of pregnancy, it has been suggested that increasing severity of OSA is associated with lack of suppression of lipolysis and high nocturnal free fatty acids (47), which contributes to lipotoxicity (50). There is also support that OSA is related to nonalcoholic fatty liver disease (25, 51). A significant correlation between severity of OSA with increased fFFA and glycerol, markers of lipolysis, and adipose IR is reported in pregnant women. These findings suggest that worsening severity of OSA may contribute to increased lipolysis in pregnancy. This is concerning, as increased free fatty acids can further worsen maternal IR, facilitating fetal overnutrition. Importantly, free fatty acids can be used as a source of fuel for fetal growth, increasing the risk of macrosomia or excess fetal fat accretion (41, 52).
Interestingly, although two-thirds of this relatively small sample of women demonstrated mild sleep apnea and many of the women reported that they snored, none thought they had sleep apnea or had been screened for sleep apnea. Not surprisingly, the women in our study had poor sleep quality. Sleep quality and symptoms of OSA (i.e., snoring) are known to worsen during the course of pregnancy (3, 53).
Although not powered to detect an association, and being exploratory in nature, minimum overnight oxygen saturation, a marker of severity of hypoxia, in the mother was correlated with NB%fat, the strongest modifiable predictor of childhood obesity (54). As previously stated, our sample of women had mild to moderate OSA, and the lowest oxygen saturation was only 84.5; thus, hypoxia was not severe in these women. A positive trend association was seen for AHI and ODI with NB%fat. In humans, OSA has been linked to low birth weight and small-for-gestational-age infants and to babies born large for gestational age (55). It is possible that the risk of small for gestational age or large for gestational age in the infant is based on the timing and severity of OSA development. Severe OSA, especially early in pregnancy, with high levels of hypoxia likely results in altered placental function, which leads to a baby born small for gestational age; however, this study was not intended to identify women with more severe OSA early in pregnancy. Mild OSA, as seen in our women, resulted in more frequent mild hypoxic events and sleep fragmentation, worsening IR of pregnancy, and it could result in nutrient overexposure and fetal overgrowth or altered body composition. No studies in humans, to our knowledge, have investigated the relationship between OSA and infant adiposity, which has been shown to be a strong predictor of childhood obesity (54). Future studies with larger samples of women and powered on infant outcomes are needed to fully elucidate the mechanisms behind these relationships.
The observations reported herein support that screening for OSA, and potentially treatment of OSA, during pregnancy may result in improved glycemic control in mothers. Outside of pregnancy, OSA is related to decreased glucose tolerance; treatment of OSA using continuous positive airway pressure has been shown to improve glycemic control using CGM during sleep (56). Future studies investigating the effects of continuous positive airway pressure in pregnant women with OSA and its effect on glycemic control in pregnancy are needed.
Although the findings are provocative, there are limitations. The sample size was small, although it was in accordance with our a priori power analysis, and there was not adequate representation of the spectrum of OSA (most women with OSA in our sample had mild OSA). We did not collect prepregnancy BMI data for all of the women, so it is possible that some of the women in our study were overweight and not obese because obesity is usually defined as a prepregnancy BMI ≥30 kg/m2. However, this would suggest that even women who are overweight before pregnancy may be at risk for worsening OSA, which could lead to worsened glycemic control. Furthermore, this study was completed in late gestation, and it is unknown when OSA developed in these women. Some of our observations were exploratory, but they provide patterns for hypothesis generation. Future prospective studies are needed to identify whether development of OSA in earlier pregnancy or prior to pregnancy has similar or potentially worse effects on maternal metabolic outcomes. Most importantly, adequately powered intervention trials to determine whether treatment of OSA improves maternal metabolic parameters and pregnancy outcomes are warranted.
In conclusion, despite having a limited sample size, this study demonstrates that poor sleep and undiagnosed mild OSA are common in late pregnancy complicated by obesity and may contribute to higher patterns of glycemia, increased fFFA availability, and IR, even when diet is controlled. OSA is underdiagnosed, particularly in women with obesity who are pregnant. These findings suggest that screening for OSA in pregnancies complicated by obesity may be beneficial for maternal metabolic health and be modifiable, potentially resulting in improved maternal and infant outcomes.
Acknowledgments
We thank the Obstetrics Research Team under the direction of Jocelyn Phipers (University of Colorado Anschutz Medical Campus) for helping to recruit participants for the study. We also thank Laurie Moss at the University of Colorado Anschutz Medical Campus for help with data management and storage.
Financial Support: This work was supported by the University of Colorado Center for Women’s Health Research, National Institutes of Health/National Centers for Advancing Translational Sciences Grant 5KL2TR001080, a University of Colorado College of Nursing Area of Excellence Grant, National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK101659, and by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant T32DK007446.
Clinical Trial Information: ClinicalTrials.gov no. NCT03248219 (registered 14 August 2017).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AHI
apnea hypopnea index
- AUC
area under the curve
- BMI
body mass index
- fFFA
fasting free fatty acid
- CGM
continuous glucose monitoring
- GDM
gestational diabetes mellitus
- HOMA-IR
homeostatic model assessment of IR
- IR
insulin resistance
- NB%fat
newborn percent fat
- NGT
normal glucose tolerant
- ODI
oxygen desaturation index
- OGTT
oral glucose tolerance test
- OSA
obstructive sleep apnea
- SE
sleep efficiency
- TG
triglyceride
- TST
total sleep time
References
- 1. Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203(2):142.e1–5. [DOI] [PMC free article] [PubMed]
- 2. Facco FL, Ouyang DW, Zee PC, Strohl AE, Gonzalez AB, Lim C, Grobman WA. Implications of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2014;210(6):559.e1–6. [DOI] [PMC free article] [PubMed]
- 3. Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28(10):1299–1305. [DOI] [PubMed] [Google Scholar]
- 4. Ding XX, Wu YL, Xu SJ, Zhang SF, Jia XM, Zhu RP, Hao JH, Tao FB. A systematic review and quantitative assessment of sleep-disordered breathing during pregnancy and perinatal outcomes. Sleep Breath. 2014;18(4):703–713. [DOI] [PubMed] [Google Scholar]
- 5. Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;210(1):52.e1–14. [DOI] [PubMed]
- 6. Wimms A, Woehrle H, Ketheeswaran S, Ramanan D, Armitstead J. Obstructive sleep apnea in women: specific issues and interventions. BioMed Res Int. 2016;2016:1764837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med. 2013;1(4):329–338. [DOI] [PubMed] [Google Scholar]
- 9. Luque-Fernandez MA, Bain PA, Gelaye B, Redline S, Williams MA. Sleep-disordered breathing and gestational diabetes mellitus: a meta-analysis of 9,795 participants enrolled in epidemiological observational studies. Diabetes Care. 2013;36(10):3353–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Facco FL, Parker CB, Reddy UM, Silver RM, Koch MA, Louis JM, Basner RC, Chung JH, Nhan-Chang CL, Pien GW, Redline S, Grobman WA, Wing DA, Simhan HN, Haas DM, Mercer BM, Parry S, Mobley D, Hunter S, Saade GR, Schubert FP, Zee PC. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hernandez TL, Friedman JE, Barbour LA. Insulin resistance in pregnancy: implications for mother and offspring. In: Zeitler P, Nadeau KJ, eds. Insulin Resistance: Childhood Precursors to Adult Disease. 2nd ed. New York, NY: Springer, 2019. [Google Scholar]
- 14. Harmon KA, Gerard L, Jensen DR, Kealey EH, Hernandez TL, Reece MS, Barbour LA, Bessesen DH. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care. 2011;34(10):2198–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hernandez TL, Farabi SS, Van Pelt RE, Haugen E, Hirsch N, Reece MS, Chartier-Logan C, Friedman JE, Barbour LA. Early and late glycemic patterns in normal weight vs obese pregnancies: influence on neonatal adiposity. Diabetes. 2017;66(Suppl 1):A397. [Google Scholar]
- 16. Ryan EA. Diagnosing gestational diabetes. Diabetologia. 2011;54(3):480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barbour LA. Changing perspectives in pre-existing diabetes and obesity in pregnancy: maternal and infant short- and long-term outcomes. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):257–263. [DOI] [PubMed] [Google Scholar]
- 18. Coustan DR, Lowe LP, Metzger BE, Dyer AR; International Association of Diabetes and Pregnancy Study Groups. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol. 2010;202(6):654.e1–6. [DOI] [PMC free article] [PubMed]
- 19. Barker DJ. The developmental origins of insulin resistance. Horm Res. 2005;64(Suppl 3):2–7. [DOI] [PubMed] [Google Scholar]
- 20. Friedman JE. Obesity and gestational diabetes mellitus pathways for programming in mouse, monkey, and man—where do we go next? The 2014 Norbert Freinkel Award Lecture. Diabetes Care. 2015;38(8):1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cortese R, Khalyfa A, Bao R, Andrade J, Gozal D. Epigenomic profiling in visceral white adipose tissue of offspring of mice exposed to late gestational sleep fragmentation [published correction appears in I nt J Obes (Lond). 2015;39(9):1432]. Int J Obes. 2015;39(7):1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iqbal W, Ciriello J. Effect of maternal chronic intermittent hypoxia during gestation on offspring growth in the rat. Am J Obstet Gynecol. 2013;209(6):564.e1–9. [DOI] [PubMed]
- 23. Khalyfa A, Mutskov V, Carreras A, Khalyfa AA, Hakim F, Gozal D. Sleep fragmentation during late gestation induces metabolic perturbations and epigenetic changes in adiponectin gene expression in male adult offspring mice. Diabetes. 2014;63(10):3230–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mutskov V, Khalyfa A, Wang Y, Carreras A, Nobrega MA, Gozal D. Early-life physical activity reverses metabolic and Foxo1 epigenetic misregulation induced by gestational sleep disturbance. Am J Physiol Regul Integr Comp Physiol. 2015;308(5):R419–R430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2018;22(3):841–851. [DOI] [PubMed] [Google Scholar]
- 26. Lewis RM, Wadsack C, Desoye G. Placental fatty acid transfer. Curr Opin Clin Nutr Metab Care. 2018;21(2):78–82. [DOI] [PubMed] [Google Scholar]
- 27. Hernandez TL, Friedman JE, Van Pelt RE, Barbour LA. Patterns of glycemia in normal pregnancy: should the current therapeutic targets be challenged? Diabetes Care. 2011;34(7):1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 29. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343–1350. [DOI] [PubMed] [Google Scholar]
- 30. O’Brien LM, Bullough AS, Shelgikar AV, Chames MC, Armitage R, Chervin RD. Validation of Watch-PAT-200 against polysomnography during pregnancy. J Clin Sleep Med. 2012;8(3):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong T, Loube DL, Owens J, Pancer JP, Wise M. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. [DOI] [PubMed] [Google Scholar]
- 32. Hernandez TL, Barbour LA. A standard approach to continuous glucose monitor data in pregnancy for the study of fetal growth and infant outcomes. Diabetes Technol Ther. 2013;15(2):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30(1):89–94. [DOI] [PubMed] [Google Scholar]
- 34. Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19(7):527–534. [DOI] [PubMed] [Google Scholar]
- 35. Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84(1):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85(1):90–95. [DOI] [PubMed] [Google Scholar]
- 37. Barbour LA, Hernandez TL, Reynolds RM, Reece MS, Chartier-Logan C, Anderson MK, Kelly T, Friedman JE, Van Pelt RE. Striking differences in estimates of infant adiposity by new and old DXA software, PEAPOD and skin-folds at 2 weeks and 1 year of life. Pediatr Obes. 2016;11(4):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed New York, NY: Routledge; 2013. [Google Scholar]
- 39. Hawkes CP, Hourihane JO, Kenny LC, Irvine AD, Kiely M, Murray DM. Gender- and gestational age-specific body fat percentage at birth. Pediatrics. 2011;128(3):e645–e651. [DOI] [PubMed] [Google Scholar]
- 40. Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E, Twery M, Croft JB, Maher E, Barrett JA, Thomas SM, Heald JL; Consensus Conference Panel; Non-Participating Observers; American Academy of Sleep Medicine Staff. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med. 2015;11(6):591–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barbour LA, Farabi SS, Friedman JE, Hirsch NM, Reece MS, Van Pelt RE, Hernandez TL. Postprandial triglycerides predict newborn fat more strongly than glucose in women with obesity in early pregnancy. Obesity (Silver Spring). 2018;26(8):1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hernandez TL, Van Pelt RE, Anderson MA, Daniels LJ, West NA, Donahoo WT, Friedman JE, Barbour LA. A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: a randomized crossover study. Diabetes Care. 2014;37(5):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hernandez TL, Van Pelt RE, Anderson MA, Reece MS, Reynolds RM, de la Houssaye BA, Heerwagen M, Donahoo WT, Daniels LJ, Chartier-Logan C, Janssen RC, Friedman JE, Barbour LA. Women with gestational diabetes mellitus randomized to a higher-complex carbohydrate/low-fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: a pilot study. Diabetes Care. 2016;39(1):39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol (1985). 2009;106(5):1538–1544. [DOI] [PMC free article] [PubMed]
- 45. Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chouchou F, Pichot V, Pepin JL, Tamisier R, Celle S, Maudoux D, Garcin A, Levy P, Barthelemy JC, Roche F, Group PS. Sympathetic overactivity due to sleep fragmentation is associated with elevated diurnal systolic blood pressure in healthy elderly subjects: the PROOF-SYNAPSE study. Eur Heart J. 2013;34(28):2122–2131. [DOI] [PubMed]
- 47. Chopra S, Rathore A, Younas H, Pham LV, Gu C, Beselman A, Kim IY, Wolfe RR, Perin J, Polotsky VY, Jun JC. Obstructive sleep apnea dynamically increases nocturnal plasma free fatty acids, glucose, and cortisol during sleep. J Clin Endocrinol Metab. 2017;102(9):3172–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chou KT, Shiao GM. Increased morning cortisol level: effect of sleep fragmentation or stress response to the last annoying stimulus? Chest. 2010;138(2):460. [DOI] [PubMed] [Google Scholar]
- 49. Khani S, Tayek JA. Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin Sci (Lond). 2001;101(6):739–747. [DOI] [PubMed] [Google Scholar]
- 50. Gu C, Younas H, Jun JC. Sleep apnea: an overlooked cause of lipotoxicity? Med Hypotheses. 2017;108:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mirrakhimov AE, Polotsky VY. Obstructive sleep apnea and non-alcoholic fatty liver disease: is the liver another target? Front Neurol. 2012;3:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barbour LA, Hernandez TL. Maternal lipids and fetal overgrowth: making fat from fat. Clin Ther. 2018;40(10):1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95(1):14–18. [DOI] [PubMed] [Google Scholar]
- 54. Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Warland J, Dorrian J, Morrison JL, O’Brien LM. Maternal sleep during pregnancy and poor fetal outcomes: a scoping review of the literature with meta-analysis. Sleep Med Rev. 2018;41:197–219. [DOI] [PubMed] [Google Scholar]
- 56. Dawson A, Abel SL, Loving RT, Dailey G, Shadan FF, Cronin JW, Kripke DF, Kline LE. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4(6):538–542. [PMC free article] [PubMed] [Google Scholar]