Abstract
Mesenchymal stromal cells (MSCs), formerly known as mesenchymal stem cells, are nonhematopoietic multipotent cells and are emerging worldwide as the most clinically used and promising source for allogeneic cell therapy. MSCs, initially obtained from bone marrow, can be derived from several other tissues, such as adipose tissue, placenta, and umbilical cord. Diversity in tissue sourcing and manufacturing procedures has significant effects on MSC products. However, in 2006, a minimal set of standard criteria has been issued by the International Society of Cellular Therapy for defining derived MSCs. These include adherence to plastic in conventional culture conditions, particular phenotype, and multilineage differentiation capacity in vitro. Moreover, MSCs have trophic capabilities, a high in vitro self-renewal ability, and immunomodulatory characteristics. Thus, immunosuppressive treatment with MSCs has been proposed as a potential therapeutic alternative for conditions in which the immune system cells influence outcomes, such as inflammatory and autoimmune diseases. The precise mechanism by which MSCs affect functions of most immune effector cells is not completely understood but involves direct contact with immune cells, soluble mediators, and local microenvironmental factors. Recently, it has been shown that their homeostatic resting state requires activation, which can be achieved in vitro with various cytokines, including interferon-γ. In the present review, we focus on the suppressive effect that MSCs exert on the immune system and highlight the significance of in vitro preconditioning and its use in preclinical studies. We discuss the clinical aspects of using MSCs as an immunomodulatory treatment. Finally, we comment on the risk of interfering with the immune system in regard to cancer formation and development.
1. Background
Mesenchymal stromal cells (MSCs) are nonhematopoietic cells which possess self-renewal, proliferative, and clonogenic potential and have the ability to commit to different cell types including adipocytes, chondrocytes, and osteocytes depending on the environmental conditions [1–3]. They can be easily isolated from human tissues and have exceptional biological properties for advanced therapies [4]. Traditionally derived from bone marrow (BM) [5], MSC populations may also be obtained from other various tissue sources, such as maternal decidua basalis of the placenta, adipose tissue (AT), foreskin, or neonatal birth-associated tissues (fetal part of the placenta and umbilical cord (UC)) [6, 7]. In 2006, the International Society for Cellular Therapy (ISCT) established the minimum criteria for designating MSCs derived from various origins: adherence to plastic in standard culture conditions; expression of different nonspecific surface molecules such as CD105/endoglin, CD90/Thy1, and CD73/5′-nucleotidase; lack of expression of CD34, CD45, CD14 or CD11b, CD79a or CD19, and HLA-DR (<2%); and trilineage differentiation potential due to the expression of several pluripotency genes. The weak expression of major histocompatibility complex (MHC) class I protects MSCs from natural killer (NK) cell-mediated killing; additionally, the lack of MHC class II expression confers to these cells the ability to evade immune recognition by CD4+ T cells. MSCs present minimal expression for HLA-DR (<2%) and do not express costimulatory proteins (CD80, CD86, and CD40), endothelial or hematopoietic surface molecule markers, such as CD31, CD45, CD34, CD14 or CD11b, and CD79a or CD19 [8]. New developments in characterization and marker profiling improve the methods of isolation, verification, and quality assessment of MSCs. In addition to hematopoietic support, tissue repair after injury, and use in regenerative medicine, the immunomodulatory properties of MSCs are attributes that represent the rationale for using MSCs as a novel therapy for many diseases, particularly disorders of the immune system [9–13]. Interestingly, the ISCT issued guidelines pertaining to MSC effector pathways such as immunomodulation, regeneration, and homing properties [14]. In 2002, for the first time, it was demonstrated that MSCs can modulate immunosuppression in vitro and in vivo [15]. For Caplan, the acronym MSC stands for “medicinal signaling cells,” indicating that the main attribute of MSC therapy is the secretion of bioactive molecules (extracellular vesicles (EVs), cytokines, growth factors, and chemokines) [16], and Caplan and Correa later proposed that the trophic and immunomodulatory properties of MSCs may function as site-regulated “drugstores” in vivo [17]. MSCs were also called the “guardians of inflammation” [18]. Those properties confer the clinical value of MSCs through the interaction with immune cells and the secretion of bioactive molecules leading to the suppression of lymphocyte proliferation, maturation of monocytes, and generation of regulatory T cells (Tregs) and M2 macrophages [19, 20]. In this review, we focus on the immunomodulatory effects of MSCs, the value of preconditioning, and its application in preclinical studies. We then comment on some clinical trials using MSCs and encountered hurdles. Finally, we discuss the risk of modulating the action of immune cells, which might theoretically favor the formation and development of cancer.
2. MSC-Mediated Immunomodulation of Immune Cells
MSCs were described as sensors of the inflammatory microenvironment in regard to their impact on the immune system [21]. Through cell-to-cell contact and regulatory molecule secretion which includes growth factors, chemokines, cytokines, and EVs, MSCs regulate both innate and adaptive immunity by affecting the activation, maturation, proliferation, differentiation, and effector functions of T and B lymphocytes (adaptive immune system), NK cells, neutrophils, and macrophages (innate immune system), as well as dendritic cells (DC), which link innate to adaptive immunity [22, 23].
2.1. T Lymphocytes
Activated T cells proliferate and release inflammatory cytokines and chemokines [24]. In the inflammatory environment, MSCs recruit local helper (Th) and effector T cells, via highly expressed chemokine (C-X-C motif) ligands CXCL9 and CXCL10, thus facilitating their immunomodulatory activity [25]. The intracellular enzymes indoleamine-2,3-dioxygenase (IDO) and inducible NO synthase (iNOS) produced by MSCs are some of the major mediators of T cell suppression, prompting their polarity shift from a proinflammatory Th1 state to an anti-inflammatory Th2 condition [26–28]. Galectin-1, abundantly expressed in and secreted by MSCs, also acts on T lymphocyte subpopulations and influences their cytokine production and release [29]. Interleukin- (IL-) 10, transforming growth factor- (TGF-) β, and the lipid mediator prostaglandin E2 (PGE2) secretion by MSCs inhibit Th17 cell differentiation and inhibit the production of IL-17, IL-22, interferon- (IFN-) γ, and tumor necrosis factor- (TNF-) α by mature Th17 cells [30–33]. In addition, TGF-β enhances T regulatory cell (Treg) function and differentiation, thus collectively modulating the Treg/Th17 balance [32]. Besides, the Notch 1 signaling pathway has been involved in MSC-mediated Treg differentiation [34], and the IL-10-dependent secretion of HLA-G5 further expands the Treg compartment [35].
2.2. B Lymphocytes
B cells are indispensable for humoral immunity and secrete antibodies when stimulated by antigens and inflammatory cytokines such as IL-10. Under quiescent conditions, MSCs trigger the differentiation into regulatory B cells (Bregs) [36]; while during inflammation, MSCs inhibit B cell proliferation, dampen the production of immunoglobulins (IgA, IgG, and IgM), and lose the capacity to induce Bregs [36–38]. While the potential of MSCs in B cell immunomodulation is not fully understood, it appears that inflammatory conditions are necessary for MSCs to exert their role through a combination of cell-cell contact (e.g., PD-L1 pathway) and soluble factors [39, 40].
2.3. NK Cells
Considered a subset of lymphocytes, NK cells are an important source of IFN-γ in addition to T cells [41]. MSCs are able to dampen the expansion of NK cells, effector functions, and cytotoxic production through the key mediators PGE2, IDO, and HLA-G5 [35, 42, 43].
2.4. Neutrophils
During inflammatory processes, neutrophils generate large concentrations of reactive oxygen intermediates and decrease the levels of antioxidants, which are regulators of the apoptotic cascade [44]. IL-6 produced by MSCs dampens respiratory bursts from neutrophils but does not affect phagocytic activity, matrix adhesion, and chemotaxis [45]. The suppression of their releasing destructive enzymes, such as peroxidases and proteases, rescues neutrophils from apoptosis [45, 46].
2.5. Macrophages
PGE2 secreted by MSCs influences the macrophage switch from an inflammatory M1 into an anti-inflammatory M2 state [47–49]. This M2 macrophage expresses high levels of CD206 and IL-10, reduces levels of TNF-α and IL-12, and shows higher phagocytic activity [50, 51]. In addition, the shift in macrophage polarization was observed in vitro and in vivo using EVs isolated from human AT-MSCs [52]. Morrison's group demonstrated this in an acute respiratory distress syndrome murine model using human-derived MSCs and postulated an EV-mediated mitochondrial transfer [53].
2.6. Dendritic Cells (DCs)
DCs, the most efficient antigen-presenting cells, prime naïve T cells to activate the adaptive immune cascade and interact with MSCs [54]. MSCs block the differentiation of monocytes towards DCs through a mechanism involving PGE2 [55] and prompt the differentiation of mature DCs into a regulatory subtype through cell-cell contact, involving Jagged-2 [56].
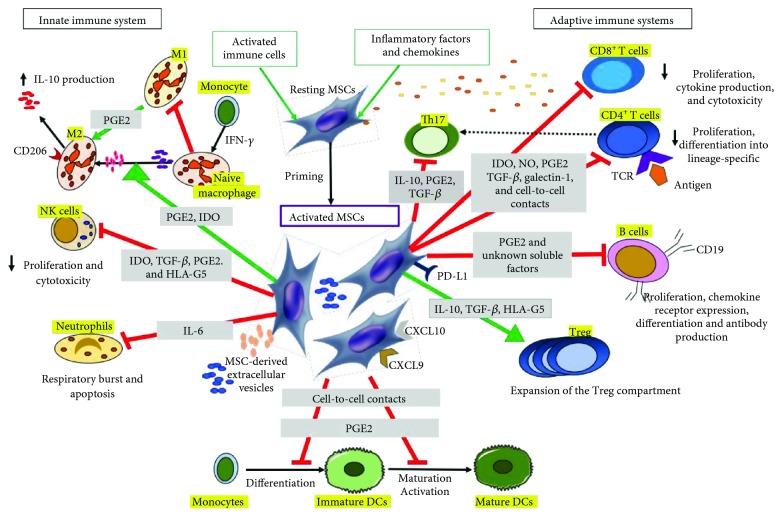
Figure 1 summarizes some of the mechanisms mediating immunomodulation.
Figure 1.
Mechanisms mediating immunomodulation. MSCs and their derived extracellular vesicles (EVs) exert their effect on innate (NK, neutrophils, monocytes, and macrophages) and adaptive (B and T cells) immune systems, as well as dendritic cells (DCs) through cell-to-cell interactions and several immunomodulatory factors. Activated T cells activate resting MSCs, which in turn facilitate the recruitment of helper and effector T cells via CXCL9 and CXCL10. Several immunomodulatory factors (TGF-β, PGE2, and HLA-G5) and membrane-bound molecules (PD-L1) suppress CD4+ and CD8+ T cell proliferation and induce the polarization of CD4+ T cells towards Th17 cells. NO and IDO released by MSCs act on the suppression of CD8+ T cell proliferation, cytokine production, and cytotoxicity. MSCs support the development of Treg populations via IL-10, TGF-β, and HLA-G5. In the context of B cells, MSCs inhibit activation, proliferation, chemokine receptor expression, and differentiation to antibody-secreting plasma cells. MSCs suppress naïve macrophage polarization to proinflammatory M1 macrophage and then favor anti-inflammatory M2 polarization. IL-6 secreted by MSCs suppresses neutrophil apoptosis and respiratory burst.
3. Value of Preconditioning MSCs
3.1. Preconditioning MSCs to Enhance Immunomodulation
MSCs do not inherently display immunosuppressive properties at baseline. To replicate the inflammatory environment of a patient suffering from immune dysfunction, they require activation to adopt an immunosuppressive phenotype [57, 58]. In addition to the inflammatory status of the recipient, the efficacy of MSC-based therapies is influenced by differences in tissue origin, donor-to-donor heterogeneity, and dearth of standardized manufacturing practices [19, 21]. Ongoing research efforts are focused on “licensing” or “priming” MSCs to display a more homogeneous immunosuppressive phenotype. This concept refers to an in vitro exposure of MSCs to proinflammatory cytokines such as IFN-γ, TNF-α, IL-1α, or IL-1β [14]. Other preconditioning cytokines and stimuli such as hypoxia and pharmacological agents can also be used during in vitro culture to modulate the MSC secretory profile [59] and thus impact their properties [60]. Preconditioning strategies also extend to methods of triggering the expression of cytoprotective genes that aim at prolonging the longevity of MSCs introduced to an adverse inflammatory milieu and therefore extend the duration of the immunomodulatory effect exerted [61]. These stimuli appear to potentially “correct” such variation and therefore allow the use of more uniform therapeutic products with enhanced immunosuppressive potential, which may lead to higher clinical benefits in patients. Although strategies for improving MSC function are advancing at the bench, there are other factors to be considered before their implementation in the clinic. Nowadays, the assessment of functionally relevant markers reflecting the immunoregulatory properties of MSCs should become the basis for their clinical use as therapeutic cell-based products. Scientists at the U.S. Food and Drug Administration (FDA) designed an assay that identifies morphological changes associated with the immunosuppressive capacity after priming. By integrating the analysis of cellular changes with high-dimensional flow cytometry data and quantification of IFN-γ-augmented immunosuppression from multiple experimental conditions into a singular experiment, they were able to obtain a predictive measurement of the immunosuppressive capabilities of the cells [62].
3.2. Preclinical Studies Using Primed MSCs
Recent preclinical reports in the literature have demonstrated the significance of MSC priming with inflammatory cytokines for future clinical use. In addition to the aforementioned agents, others such as hyaluronan, polyinosinic acid, and polycytidylic acid have been used to prime MSCs for several forms of connective tissue repair in mice [63, 64]. These primed MSCs exhibit enhanced therapeutic properties with minimal or no significant adverse effects when compared to unprimed (naïve) counterparts [65, 66]. MSCs from multiple sources such as AT, BM, and Wharton's Jelly (WJ) primed with IFN-γ displayed gene expression profiles consistent with an immunosuppressive potential [67]. The immunomodulatory properties of MSCs derived from UC, AT, and periodontal ligaments presented comparable immunosuppressive capacities in vitro; however, UC-MSCs had shorter expansion time, predominantly utilized HLA-G as an immunosuppressive mechanism, and upon activation with IFN-γ did not express further HLA-DR, which would lower the risk of triggering an allogeneic immune response [68]. When IFN-γ-primed BM-MSCs isolated and cultured under good manufacturing practice (GMP) conditions were infused into murine models, no adverse effects related to primed BM-MSCs administration were found. Furthermore, the comparison of phenotypic profiles between primed and unprimed MSCs from the same donor demonstrated that the changes were due to IFN-γ priming rather than genetic variability [66]. In the context of graft versus host disease (GvHD), GvHD-mice injected with IFN-γ-primed MSCs had improved survival rates when compared to the group injected with naïve cells, and this was attributed to the activation of the IFN-γ-Janus kinase- (JAK-) signal transducer and activator of transcription 1 (STAT 1) pathway, which suppressed T cell proliferation [65].
4. Clinical Applications of MSCs in Diseases Mediated by Immune Cells
Culture-expanded MSCs are classified by both the FDA and European Medicines Agency (EMA) as more than minimally manipulated cellular and gene therapy (CGT) products [69]. The earliest therapeutic attempts at using autologous MSC infusion after ex vivo culture expansion showed an acceleration of the hematopoietic reconstitution after hematopoietic stem cell transplantation [70] and high-dose chemotherapy in breast cancer [71]. In both studies, no treatment-associated adverse effects were reported, thus these results laid the foundation for ex vivo cell expansion and administration. While the majority of MSC applications so far have relied on BM being the gold standard source, other adult and fetal tissues such as AT, UC, and WJ have gained popularity because of their comparable or even superior immunomodulatory profiles and their accessibility as medical waste products [72, 73]. For early phase human clinical trials, several factors including identity, viability, and sterility are established as release criteria [8]. However, for advanced-phase clinical trials, regulatory authorities additionally required the development of potency assays as part of the release criteria [74]. Additionally, the EMA has provided multiple guidelines to ensure quality, safety, and efficacy, including the “Guideline on Human Cell-Based Medicinal Products,” “Guideline on Strategies to Identify and Mitigate Risk for First-in-Human Clinical Trials with Investigational Medicinal Products,” and “Reflection Paper on Stem Cell-Based Medicinal Products,” among others [75].
4.1. Broad Range of Applications
Most of the clinical trials performed to date have showed the feasibility and safety of the approach with however conflicting results in terms of efficacy, partially explicable with methodological biases (i.e., small cohorts, lack of control groups, variability of source, and preparation and routes of administration). Also, the use of autologous vs. allogeneic MSC is still controversial with no univocal data on the immunological properties of MSCs derived from patients suffering from autoimmune disorders compared to healthy donors [76, 77]. We provide a brief overview of clinical trials performed or ongoing in the setting of immune-related disorders. However, a more comprehensive picture is beyond the scope of the current review.
Results of clinical trials in inflammatory bowel disease have been recently reviewed by Algeri et al. [76]. MSCs have been administered intravenously to control luminal inflammatory disease or locally in perianal fistulizing Crohn's disease (CD), in cases of refractory disease or acute flares not responsive to conventional methods of treatment such as steroids and immunosuppressive drugs. The two largest studies conducted on systemic administration of allogeneic MSCs have reached conflicting conclusions: Lazebnik et al. showed clinical response in all treated patients (39 Ulcerative Colitis and 11 CD, [78]), while Pfizer did not succeed to demonstrate any clinical benefit in 48 treated Ulcerative Colitis patients compared to 40 placebo [79].
More homogenous positive results have been obtained for the treatment of fistulizing CD where MSCs promote the healing of rectal mucosa, without any observable adverse events [80–82]. A phase III randomized, double blind, controlled trial with allogeneic, adipose-derived MSCs (Cx601) demonstrated a higher remission rate in 107 patients treated vs. 105 placebo [81]. Alofisel or Cx601 is going to be the first off-the-shelf MSC therapy to be approved by EMA for complex perianal fistulas in adult CD [83].
Since 2004, allogeneic MSCs have been used in the treatment of GvHD in several patients enrolled in a multitude of trials worldwide [10, 84]. Osiris sponsored a phase III trial of allogeneic BM-MSCs from random donors for the treatment of steroid-refractory GvHD (NCT00366145). Unfortunately, it was considered a failure due to a lack of positive outcomes [85]. This was due to inconsistencies in sourcing, isolation and manufacturing methods, passage numbers used, and fresh vs. thawed cells [86, 87]. Despite this, the Osiris-backed BM-MSC product has been approved in Canada, New Zealand, and Japan (on an insurance-dependent basis) for restricted use in children with GvHD [88]. Alternative sources have also been tested, and placenta-derived decidua stromal cells seem to hold promise of better response rates compared to BM-MSCs for severe acute GvHD [89].
Rheumatic disorders are also considered another potential area for MSC application. Since 2010, more than 300 patients with relapsing systemic lupus erythematosus (SLE) have been reported in the same center in Nanjing, China. However, the presence of multiple biases in the study design (i.e., lack of endpoint definition and of randomization) and in data analyses renders the study inconclusive in proving efficacy. Regardless, the use of pooled allogeneic MSCs derived from healthy donors was also shown to regulate and normalize lymphocyte counts and differentials in SLE patients [90].
Similarly, phase I/II uncontrolled clinical trials have been conducted in other inflammatory rheumatic diseases, such as systemic sclerosis, Sjögren syndrome, dermatomyositis/polymyositis, and rheumatoid arthritis with promising results, although bigger randomized prospective controlled studies are mostly warranted [91, 92]. Several ongoing clinical trials are exploring the efficacy and toxic effects of MSCs in patients with multiple sclerosis [93]; however, phase I/II studies have not brought significant positive results and further investigations are warranted [94, 95]. In a large nonrandomized comparative trial in 173 patients with active rheumatoid arthritis, the intravenous treatment with UC-MSCs succeeded in inducing a substantial remission of the disease as per the American College of Rheumatology improving standards [96]. Based on the fact that several studies in animal models of Type 1 Diabetes (T1D) have shown MSCs to ameliorate or reverse overt diabetes, also demonstrating their successful engraftment in the pancreatic islets [97, 98], Carlsson et al. performed a phase I clinical trial showing for the first time the opportunity to interfere with the progression of T1D by systemic infusion of MSCs. Autologous BM-MSCs were administered to adult patients recently diagnosed with T1D. Strikingly, during the first year postdiagnosis, no adverse events were disclosed and a conserved or improved C-peptide response to a mixed-meal tolerance test in the patient cohort was demonstrated [99].
Table 1 summarizes other clinical trials of MSCs on diseases mediated by the immune system not previously discussed (http://www.clinicaltrials.gov, [100–106]).
Table 1.
Clinical trials of MSCs on diseases mediated by the immune system.
| Trial no. | Phase | Commencement year | Targeted disease | Status | Patient enrollment (n) | Country |
|---|---|---|---|---|---|---|
| NCT00447460 | I/II | 2007 | Graft vs. host disease (GvHD) | Completed [100] | 15 | Spain |
| NCT01522716 | I | 2011 | Unknown (NRP) | 11 | Sweden | |
| NCT01764100 | I | 2013 | Completed [101] | 40 | Italy | |
| NCT02032446 | I/II | Recruiting | 47 (estimated) | |||
| NCT02291770 | III | 2014 | Unknown (NRP) | 130 (estimated) | China | |
| NCT02055625 | I/II | Suspended (NRP) | 11 | Sweden | ||
| NCT02359929 | I | 2015 | Recruiting | 24 (estimated) | USA | |
| NCT01741857 | I/II | Systemic lupus erythematosus (SLE) | Completed [102] | 40 | China | |
| NCT03171194 | I | Active, not recruiting | 6 (estimated) | USA | ||
| NCT03673748 | II | 2019 | SLE/lupus nephritis | Not yet recruiting | 36 (estimated) | Spain |
| NCT00781872 | I/II | 2006 | Multiple sclerosis (MS) | Completed [103] | 20 | Israel |
| NCT00395200 | I/II | 2008 | Completed [104, 105] | 10 | UK | |
| NCT01730547 | I/II | 2013 | Unknown | 15 (estimated) | Sweden | |
| NCT02495766 | I/II | 2015 | Unknown | 8 (estimated) | Spain | |
| NCT03799718 | II | 2019 | Not yet recruiting | 20 (estimated) | USA | |
| NCT02893306 | II | 2012 | Type 1 diabetes mellitus (T1DM) | Unknown (NRP) | 10 | Chile |
| NCT02940418 | I | 2017 | Recruiting | 20 (estimated) | Jordan | |
| NCT03406585 | I/II | Recruiting | 24 (estimated) | Sweden | ||
| NCT02249676 | II | 2013 | Devic syndrome/neuromyelitis optica | Completed | 15 | China |
| NCT01659762 | I | 2012 | Crohn's disease | Completed [106] | 16 | USA |
NRP: no results posted.
4.2. Current Challenges in Clinical Use
4.2.1. Fate of the Infused MSCs
A factor that influences the future of MSC application in the clinic is that the exact fate of the cells postinfusion is yet to be completely elucidated. There are multiple reports in both human and animal models that point to sequestration of the cells in the lungs following systemic administration and their complete disappearance within 7 days of treatment [9, 107, 108]. Another study showed that allogeneic donor MSC DNA was found engrafted into the recipient's digestive tract via chromosomal fluorescence studies [10]. This is in support of the theory that MSCs are capable of escaping sequestration and migrating to sites of inflammation, homing to released cytokines and other inflammatory molecules. If this is the case, this will facilitate the administration of MSCs to patients with multisystemic or disseminated involvement, e.g., SLE and rheumatoid arthritis, with gross effects including treating inflammation, regulating lymphocyte function, and stimulating tissue repair, including regeneration of cartilage [109]. Other theories suggest that MSCs prior to apoptosis release EVs that are capable of migrating to inflamed tissues and exerting the same anti-inflammatory effects of viable MSCs. This alternative approach highlights the potential of cell-free MSC-based therapy [52, 107].
4.2.2. Practical Decisions Impacting MSC-Based Therapy Outcome
Other dilemmas impacting the widespread clinical use of MSCs that researchers have yet to reach a consensus for are which tissue source yields the most effective product, combined with the significant impact of donor variability and continued passaging on cell growth, protein production, and EV release [85, 110]. Furthermore, there is a lack of standardized disease-specific procedures and clinical trial regulations regarding the magnitude (average of 1-2 million cells/kg body weight) and frequency of dose administration, the use of allogeneic vs. autologous MSCs, systemic vs. local administration, and primed vs. naïve cells, and the use of freshly cultured vs. frozen and thawed cells [76]. Functional differences were observed between in vivo and in vitro contexts and between species (murine vs. human) in terms of susceptibility to undergoing oncogenic transformation during expansion, and effector molecules used in T cell suppression mechanisms have to be taken into account [21]. This is highlighted by the reported discrepancies between what is described in in vitro and animal models vs. what is reported in the literature of later-phase clinical trials and by the publishing bias (few or no reports on negative outcomes and/or failed trials) [92]. Interestingly, the lack of consistent benefit seen in late phase human clinical trials may also be explained by the fact that the injected cell products were “naïve or resting” MSCs; therefore, the immunosuppressive potential of the cells is entirely depending on an individual patient's microenvironment and immune status [19, 21, 111]. These variables collectively hinder the production of reliable “off-the-shelf” cell therapy products that produce sustainable and consistent results among patients.
5. Risk of Modulating the Action of Immune Cells and the Dilemma of Cancer Formation and Development
One of the main concerns in MSC-based therapy is that tumorigenicity could result from MSC malignant transformation during in vitro culture expansion or following infusion, or the immunosuppressive effects exerted by MSCs could allow tumor formation and development of already existing malignant cells in the host/recipient [112]. Similarly to murine MSCs readily undergoing spontaneous transformation in vitro [113], Rosland et al. demonstrated spontaneous malignant transformation of BM-derived human MSCs after in vitro cultures leading to an aggressively metastatic disease in immunodeficient mice [114]. However, the impaired immunological status of the recipient was likely more prone to initiate or develop cancer [115]. In humans, MSCs are minimally susceptible to oncogenic transformation in vivo, and long-term culture either does not affect MSC morphology or cause chromosomal alterations [116]. Furthermore, continued passaging leads to loss of already existing aneuploidy, or any resulting aneuploidy leads to senescence, negating the risk of cancer formation [117]. The Committee for Advanced Therapies and the Cell Product Working Party organized a meeting to discuss the risk of tumor formation following MSC-based therapies, with a focus on regulatory and scientific aspects. When discussing the influence of the manufacturing process on inducing cytogenetic abnormalities, it was highlighted that culture duration and conditions present critical risk factors for producing chromosomal aberrations. The committee also suggested that long-term expansion could mostly cause chromosomal aberrations rather than donor-derived factors [112]. However, in a study by Tarte et al., aneuploidy without risk of transformation occurring in a long-term culture of clinical grade MSCs was most likely donor dependent (3 out of 5 aberrations were derived from the same donor) [118]. Thus, donor screening and monitoring of the long-term expansion and integrity of the cells are a requirement [119].
MSCs exhibit a tropism for the tumor microenvironment niche [120], and selective homing into inflammatory tumor sites has been established in various types of cancer [121]. Even if MSCs have intrinsic antitumor properties, they can potentially alter their phenotype towards a protumorigenic role including proangiogenic and immunosuppressive capabilities. Thus, the presence of MSCs within the cancerous stroma has been a matter of contradictory reports [122]. There is no official statement on the potential of tumorigenicity in MSC-based therapies, and no observation of tumor formation of MSC origin in patients given cellular therapy. Despite these facts, one cannot rule out the possibility of MSC-derived tumors developing in vivo. Interestingly, there are reports of spontaneous MSC transformation resulting from MSC culture cross-contamination with malignant cells emphasizing the importance of maintaining good manufactory practice conditions in the production of cell therapy products [123, 124]. While MSC therapy has been qualified as safe by both FDA and EMA, the potential long-term risks still have to be considered.
6. Conclusion
In the last 10 years, MSCs have been a promising treatment for a plethora of immune-related conditions, through the regulation of inflammation and the support of tissue homeostasis. Despite having been unanimously deemed safe, clinical trials report conflicting data in terms of efficacy in several clinical settings. Inconsistencies can be ascribed to limitations in the design of clinical trials and translation of successful preclinical models, discrepancies in the source, preparation and handling of the MSC product, route of administration, and type of donor (autologous vs. allogeneic).
Moreover, the lack of in vitro biomarkers correlating with the in vivo activity of MSCs has so far hindered the progress towards uniformly potent cell products. MSC priming or licensing, before administration, might offer the possibility to enhance their effectiveness in vivo, limiting the variability inherent to the inflammatory status of the patients.
Acknowledgments
The authors wish to thank the Qatar National Library for funding the publication of this article.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Wei X., Yang X., Han Z. P., Qu F. F., Shao L., Shi Y. F. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacologica Sinica. 2013;34(6):747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciuffreda M. C., Malpasso G., Musarò P., Turco V., Gnecchi M. Protocols for in vitro differentiation of human mesenchymal stem cells into osteogenic, chondrogenic and adipogenic lineages. In: Gnecchi M., editor. Mesenchymal Stem Cells. Vol. 1416. New York, NY, USA: Humana Press; 2016. pp. 149–158. (Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 3.Horwitz E. M., le Blanc K., Dominici M., et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 4.Deola S., Guerrouahen B. S., Sidahmed H., et al. Tailoring cells for clinical needs: meeting report from the advanced therapy in healthcare symposium (October 28–29 2017, Doha, Qatar) Journal of Translational Medicine. 2018;16(1, article 276) doi: 10.1186/s12967-018-1652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittenger M. F., Mackay A. M., Beck S. C., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Marquez-Curtis L. A., Janowska-Wieczorek A., McGann L. E., Elliott J. A. W. Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology. 2015;71(2):181–197. doi: 10.1016/j.cryobiol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling. 2011;9(1, article 12) doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominici M., le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 9.Ankrum J. A., Ong J. F., Karp J. M. Mesenchymal stem cells: immune evasive, not immune privileged. Nature Biotechnology. 2014;32(3):252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Blanc K., Rasmusson I., Sundberg B., et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. The Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 11.Le Blanc K., Frassoni F., Ball L., et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. The Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 12.Jitschin R., Mougiakakos D., Von Bahr L., et al. Alterations in the cellular immune compartment of patients treated with third-party mesenchymal stromal cells following allogeneic hematopoietic stem cell transplantation. Stem Cells. 2013;31(8):1715–1725. doi: 10.1002/stem.1386. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Gan Y., Li W., et al. The interaction between mesenchymal stem cells and steroids during inflammation. Cell Death & Disease. 2014;5(1, article e1009) doi: 10.1038/cddis.2013.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galipeau J., Krampera M., Barrett J., et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartholomew A., Sturgeon C., Siatskas M., et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology. 2002;30(1):42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 16.Caplan A. I. What's in a name? Tissue Engineering. Part A. 2010;16(8):2415–2417. doi: 10.1089/ten.tea.2010.0216. [DOI] [PubMed] [Google Scholar]
- 17.Caplan A. I., Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prockop D. J., Youn Oh J. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Molecular Therapy. 2012;20(1):14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Wang M., Yuan Q., Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells International. 2018;2018:12. doi: 10.1155/2018/3057624.3057624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernardo M. E., Fibbe W. E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nature Immunology. 2014;15(11):1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 23.Lin L., Du L. The role of secreted factors in stem cells-mediated immune regulation. Cellular Immunology. 2018;326:24–32. doi: 10.1016/j.cellimm.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Dimeloe S., Burgener A. V., Grählert J., Hess C. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology. 2017;150(1):35–44. doi: 10.1111/imm.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuang J., Shan Z., Ma T., et al. CXCL9 and CXCL10 accelerate acute transplant rejection mediated by alloreactive memory T cells in a mouse retransplantation model. Experimental and Therapeutic Medicine. 2014;8(1):237–242. doi: 10.3892/etm.2014.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keating A. How do mesenchymal stromal cells suppress T cells? Cell Stem Cell. 2008;2(2):106–108. doi: 10.1016/j.stem.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Su J., Chen X., Huang Y., et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death and Differentiation. 2014;21(3):388–396. doi: 10.1038/cdd.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato K., Ozaki K., Oh I., et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 29.Gieseke F., Bohringer J., Bussolari R., Dominici M., Handgretinger R., Muller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116(19):3770–3779. doi: 10.1182/blood-2010-02-270777. [DOI] [PubMed] [Google Scholar]
- 30.Qu X., Liu X., Cheng K., Yang R., Zhao R. C. H. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Experimental Hematology. 2012;40(9):761–770. doi: 10.1016/j.exphem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Ghannam S., Pène J., Torcy-Moquet G., Jorgensen C., Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. Journal of Immunology. 2010;185(1):302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 32.Wang D., Huang S., Yuan X., et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cellular & Molecular Immunology. 2017;14(5):423–431. doi: 10.1038/cmi.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baratelli F., Lin Y., Zhu L., et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. The Journal of Immunology. 2005;175(3):1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 34.Del Papa B., Sportoletti P., Cecchini D., et al. Notch1 modulates mesenchymal stem cells mediated regulatory T‐cell induction. European Journal of Immunology. 2013;43(1):182–187. doi: 10.1002/eji.201242643. [DOI] [PubMed] [Google Scholar]
- 35.Selmani Z., Naji A., Zidi I., et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 36.Luk F., Carreras-Planella L., Korevaar S. S., et al. Inflammatory conditions dictate the effect of mesenchymal stem or stromal cells on B cell function. Frontiers in Immunology. 2017;8:p. 1042. doi: 10.3389/fimmu.2017.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corcione A., Benvenuto F., Ferretti E., et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 38.Franquesa M., Mensah F. K., Huizinga R., et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells. 2015;33(3):880–891. doi: 10.1002/stem.1881. [DOI] [PubMed] [Google Scholar]
- 39.Wang H., Qi F., Dai X., et al. Requirement of B7-H1 in mesenchymal stem cells for immune tolerance to cardiac allografts in combination therapy with rapamycin. Transplant Immunology. 2014;31(2):65–74. doi: 10.1016/j.trim.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Fan L., Hu C., Chen J., Cen P., Wang J., Li L. Interaction between mesenchymal stem cells and B-cells. International Journal of Molecular Sciences. 2016;17(5):p. 650. doi: 10.3390/ijms17050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nature Immunology. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 42.Spaggiari G. M., Capobianco A., Becchetti S., Mingari M. C., Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107(4):1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 43.Spaggiari G. M., Capobianco A., Abdelrazik H., Becchetti F., Mingari M. C., Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 44.Watson R. W. G. Redox regulation of neutrophil apoptosis. Antioxidants & Redox Signaling. 2002;4(1):97–104. doi: 10.1089/152308602753625898. [DOI] [PubMed] [Google Scholar]
- 45.Raffaghello L., Bianchi G., Bertolotto M., et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26(1):151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 46.Jiang D., Muschhammer J., Qi Y., et al. Suppression of neutrophil-mediated tissue damage—a novel skill of mesenchymal stem cells. Stem Cells. 2016;34(9):2393–2406. doi: 10.1002/stem.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasandan A. B., Jahnavi S., Shashank C., Prasad P., Kumar A., Prasanna S. J. Human mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Scientific Reports. 2016;6(1, article 38308) doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiossone L., Conte R., Spaggiari G. M., et al. Mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. Stem Cells. 2016;34(7):1909–1921. doi: 10.1002/stem.2369. [DOI] [PubMed] [Google Scholar]
- 49.Manferdini C., Paolella F., Gabusi E., et al. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: in vitro evaluation. Osteoarthritis and Cartilage. 2017;25(7):1161–1171. doi: 10.1016/j.joca.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Kim J., Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Experimental Hematology. 2009;37(12):1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magatti M., Vertua E., De Munari S., et al. Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. Journal of Tissue Engineering and Regenerative Medicine. 2017;11(10):2895–2911. doi: 10.1002/term.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lo Sicco C., Reverberi D., Balbi C., et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Translational Medicine. 2017;6(3):1018–1028. doi: 10.1002/sctm.16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison T. J., Jackson M. V., Cunningham E. K., et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. American Journal of Respiratory and Critical Care Medicine. 2017;196(10):1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spaggiari G. M., Moretta L. Interactions between mesenchymal stem cells and dendritic cells. In: Weyand B., Dominici M., Hass R., Jacobs R., Kasper C., editors. Mesenchymal Stem Cells - Basics and Clinical Application II. Vol. 130. Berlin, Heidelberg: Springer; 2012. pp. 199–208. (Advances in Biochemical Engineering/Biotechnology). [DOI] [PubMed] [Google Scholar]
- 55.Spaggiari G. M., Abdelrazik H., Becchetti F., Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113(26):6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 56.Zhang B., Liu R., Shi D., et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113(1):46–57. doi: 10.1182/blood-2008-04-154138. [DOI] [PubMed] [Google Scholar]
- 57.Krampera M., Galipeau J., Shi Y., Tarte K., Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells--the International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15(9):1054–1061. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Krampera M. Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia. 2011;25(9):1408–1414. doi: 10.1038/leu.2011.108. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira J. R., Teixeira G. Q., Santos S. G., Barbosa M. A., Almeida-Porada G., Gonçalves R. M. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Frontiers in Immunology. 2018;9:p. 2837. doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu C., Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. Journal of Cellular and Molecular Medicine. 2018;22(3):1428–1442. doi: 10.1111/jcmm.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva L. H. A., Antunes M. A., Dos Santos C. C., Weiss D. J., Cruz F. F., Rocco P. R. M. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Research & Therapy. 2018;9(1, article 45) doi: 10.1186/s13287-018-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klinker M. W., Marklein R. A., Lo Surdo J. L., Wei C. H., Bauer S. R. Morphological features of IFN-γ–stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(13):E2598–E2607. doi: 10.1073/pnas.1617933114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Succar P., Medynskyj M., Breen E. J., Batterham T., Molloy M. P., Herbert B. R. Priming adipose-derived mesenchymal stem cells with hyaluronan alters growth kinetics and increases attachment to articular cartilage. Stem Cells International. 2016;2016:13. doi: 10.1155/2016/9364213.9364213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saether E. E., Chamberlain C. S., Aktas E., Leiferman E. M., Brickson S. L., Vanderby R. Primed mesenchymal stem cells alter and improve rat medial collateral ligament healing. Stem Cell Reviews. 2016;12(1):42–53. doi: 10.1007/s12015-015-9633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim D. S., Jang I. K., Lee M. W., et al. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-γ. EBioMedicine. 2018;28:261–273. doi: 10.1016/j.ebiom.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guess A. J., Daneault B., Wang R., et al. Safety profile of good manufacturing practice manufactured interferon γ-primed mesenchymal stem/stromal cells for clinical trials. Stem Cells Translational Medicine. 2017;6(10):1868–1879. doi: 10.1002/sctm.16-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Q., Yang Q., Wang Z., et al. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton’s jelly as sources of cell immunomodulatory therapy. Human Vaccines & Immunotherapeutics. 2016;12(1):85–96. doi: 10.1080/21645515.2015.1030549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J. H., Jo C. H., Kim H. R., Hwang Y. I. Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical cord, and adipose tissue. Stem Cells International. 2018;2018:12. doi: 10.1155/2018/8429042.8429042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mendicino M., Bailey A. M., Wonnacott K., Puri R. K., Bauer S. R. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14(2):141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 70.Lazarus H. M., Haynesworth S. E., Gerson S. L., Rosenthal N. S., Caplan A. I. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplantation. 1995;16(4):557–564. [PubMed] [Google Scholar]
- 71.Koç O. N., Gerson S. L., Cooper B. W., et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. Journal of Clinical Oncology. 2000;18(2):307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 72.Yoo K. H., Jang I. K., Lee M. W., et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cellular Immunology. 2009;259(2):150–156. doi: 10.1016/j.cellimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Melief S. M., Zwaginga J. J., Fibbe W. E., Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Translational Medicine. 2013;2(6):455–463. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Wolf C., van de Bovenkamp M., Hoefnagel M. Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy. 2017;19(7):784–797. doi: 10.1016/j.jcyt.2017.03.076. [DOI] [PubMed] [Google Scholar]
- 75.Ancans J. Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Frontiers in Immunology. 2012;3:p. 253. doi: 10.3389/fimmu.2012.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Algeri M., Conforti A., Pitisci A., et al. Mesenchymal stromal cells and chronic inflammatory bowel disease. Immunology Letters. 2015;168(2):191–200. doi: 10.1016/j.imlet.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 77.Serena C., Keiran N., Madeira A., et al. Crohn’s disease disturbs the immune properties of human adipose-derived stem cells related to inflammasome activation. Stem Cell Reports. 2017;9(4):1109–1123. doi: 10.1016/j.stemcr.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lazebnik L. B., Konopliannikov A. G., Kniazev O. V., et al. Use of allogeneic mesenchymal stem cells in the treatment of intestinal inflammatory diseases. Terapevticheskiĭ Arkhiv. 2010;82(2):38–43. [PubMed] [Google Scholar]
- 79.Pfizer A. I. A study to investigate the safety and possible clinical benefit of Multistem(r) in patients with moderate to severe ulcerative colitis. 2014. https://clinicaltrials.gov/ct2/show/NCT01240915.
- 80.Ciccocioppo R., Bernardo M. E., Sgarella A., et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60(6):788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 81.Panés J., García-Olmo D., van Assche G., et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. The Lancet. 2016;388(10051):1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 82.de la Portilla F., Alba F., García-Olmo D., Herrerías J. M., González F. X., Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. International Journal of Colorectal Disease. 2013;28(3):313–323. doi: 10.1007/s00384-012-1581-9. [DOI] [PubMed] [Google Scholar]
- 83.Sheridan C. First off-the-shelf mesenchymal stem cell therapy nears European approval. Nature Biotechnology. 2018;36(3):212–214. doi: 10.1038/nbt0318-212a. [DOI] [PubMed] [Google Scholar]
- 84.Dunavin N., Dias A., Li M., McGuirk J. Mesenchymal stromal cells: what is the mechanism in acute graft-versus-host disease? Biomedicines. 2017;5(4):p. 39. doi: 10.3390/biomedicines5030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galipeau J. The mesenchymal stromal cells dilemma--does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15(1):2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Galipeau J. Concerns arising from MSC retrieval from cryostorage and effect on immune suppressive function and pharmaceutical usage in clinical trials. ISBT Science Series. 2013;8(1):100–101. doi: 10.1111/voxs.12022. [DOI] [Google Scholar]
- 87.Moll G., Alm J. J., Davies L. C., et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32(9):2430–2442. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galipeau J., Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ringden O., Baygan A., Remberger M., et al. Placenta-derived decidua stromal cells for treatment of severe acute graft-versus-host disease. Stem Cells Translational Medicine. 2018;7(4):325–331. doi: 10.1002/sctm.17-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cras A., Farge D., Carmoi T., Lataillade J. J., Wang D. D., Sun L. Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis Research & Therapy. 2015;17(1, article 301) doi: 10.1186/s13075-015-0819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tyndall A. Mesenchymal stem cell treatments in rheumatology: a glass half full? Nature Reviews Rheumatology. 2014;10(2):117–124. doi: 10.1038/nrrheum.2013.166. [DOI] [PubMed] [Google Scholar]
- 92.Tyndall A. Mesenchymal stromal cells and rheumatic disorders. Immunology Letters. 2015;168(2):201–207. doi: 10.1016/j.imlet.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 93.Joyce N., Annett G., Wirthlin L., Olson S., Bauer G., Nolta J. A. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regenerative Medicine. 2010;5(6):933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ng T. K., Fortino V. R., Pelaez D., Cheung H. S. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World Journal of Stem Cells. 2014;6(2):111–119. doi: 10.4252/wjsc.v6.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gharibi T., Ahmadi M., Seyfizadeh N., Jadidi-Niaragh F., Yousefi M. Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of multiple sclerosis. Cellular Immunology. 2015;293(2):113–121. doi: 10.1016/j.cellimm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 96.Wang L., Wang L., Cong X., et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy. Stem Cells and Development. 2013;22(24):3192–3202. doi: 10.1089/scd.2013.0023. [DOI] [PubMed] [Google Scholar]
- 97.Lee R. H., Seo M. J., Reger R. L., et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jurewicz M., Yang S., Augello A., et al. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes. 2010;59(12):3139–3147. doi: 10.2337/db10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carlsson P. O., Schwarcz E., Korsgren O., le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587–592. doi: 10.2337/db14-0656. [DOI] [PubMed] [Google Scholar]
- 100.Perez-Simon J. A., Lopez-Villar O., Andreu E. J., et al. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial. Haematologica. 2011;96(7):1072–1076. doi: 10.3324/haematol.2010.038356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Introna M., Lucchini G., Dander E., et al. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biology of Blood and Marrow Transplantation. 2014;20(3):375–381. doi: 10.1016/j.bbmt.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 102.Wang D., Li J., Zhang Y., et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Research & Therapy. 2014;16(2):p. R79. doi: 10.1186/ar4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karussis D., Karageorgiou C., Vaknin-Dembinsky A., et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Archives of Neurology. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Connick P., Kolappan M., Patani R., et al. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials. 2011;12(1, article 62) doi: 10.1186/1745-6215-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Connick P., Kolappan M., Crawley C., et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. The Lancet Neurology. 2012;11(2):150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dhere T., Copland I., Garcia M., et al. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn's disease - a phase 1 trial with three doses. Alimentary Pharmacology & Therapeutics. 2016;44(5):471–481. doi: 10.1111/apt.13717. [DOI] [PubMed] [Google Scholar]
- 107.Galleu A., Riffo-Vasquez Y., Trento C., et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Science Translational Medicine. 2017;9(416, article eaam7828) doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 108.Lee R. H., Pulin A. A., Seo M. J., et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kurtz A. Mesenchymal stem cell delivery routes and fate. International Journal of Stem Cells. 2008;1(1):1–7. doi: 10.15283/ijsc.2008.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maziarz R. T. Mesenchymal stromal cells: potential roles in graft-versus-host disease prophylaxis and treatment. Transfusion. 2016;56(4):9S–14S. doi: 10.1111/trf.13563. [DOI] [PubMed] [Google Scholar]
- 111.Shi Y., Kirwan P., Livesey F. J. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nature Protocols. 2012;7(10):1836–1846. doi: 10.1038/nprot.2012.116. [DOI] [PubMed] [Google Scholar]
- 112.Barkholt L., Flory E., Jekerle V., et al. Risk of tumorigenicity in mesenchymal stromal cell–based therapies—bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15(7):753–759. doi: 10.1016/j.jcyt.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 113.He L., Zhao F., Zheng Y., Wan Y., Song J. Loss of interactions between p53 and survivin gene in mesenchymal stem cells after spontaneous transformation in vitro. The International Journal of Biochemistry & Cell Biology. 2016;75:74–84. doi: 10.1016/j.biocel.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 114.Røsland G. V., Svendsen A., Torsvik A., et al. Long-term cultures of bone marrow–derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Research. 2009;69(13):5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 115.Lee H. Y., Hong I. S. Double-edged sword of mesenchymal stem cells: cancer-promoting versus therapeutic potential. Cancer Science. 2017;108(10):1939–1946. doi: 10.1111/cas.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mareschi K., Ferrero I., Rustichelli D., et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. Journal of Cellular Biochemistry. 2006;97(4):744–754. doi: 10.1002/jcb.20681. [DOI] [PubMed] [Google Scholar]
- 117.Stultz B. G., McGinnis K., Thompson E. E., Lo Surdo J. L., Bauer S. R., Hursh D. A. Chromosomal stability of mesenchymal stromal cells during in vitro culture. Cytotherapy. 2016;18(3):336–343. doi: 10.1016/j.jcyt.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tarte K., Gaillard J., Lataillade J. J., et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115(8):1549–1553. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- 119.Cornélio D. A., de Medeiros S. R. B. Genetic evaluation of mesenchymal stem cells. Revista Brasileira de Hematologia e Hemoterapia. 2014;36(4):238–240. doi: 10.1016/j.bjhh.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kidd S., Spaeth E., Dembinski J. L., et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27(10):2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beckermann B. M., Kallifatidis G., Groth A., et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. British Journal of Cancer. 2008;99(4):622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Waterman R. S., Henkle S. L., Betancourt A. M. Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One. 2012;7(9, article e45590) doi: 10.1371/journal.pone.0045590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de la Fuente R., Bernad A., Garcia-Castro J., Martin M. C., Cigudosa J. C. Retraction: spontaneous human adult stem cell transformation. Cancer Research. 2010;70(16, article 6682) doi: 10.1158/0008-5472.CAN-10-2451. [DOI] [PubMed] [Google Scholar]
- 124.Torsvik A., Rosland G. V., Svendsen A., et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track - letter. Cancer Research. 2010;70(15):6393–6396. doi: 10.1158/0008-5472.CAN-10-1305. [DOI] [PubMed] [Google Scholar]