Abstract
Purpose
To verify the accuracy and efficacy of three-dimensional printing individual template (3D-PIT) with computed tomography-magnetic resonance imaging (CT-MRI) fusion for radioactive iodine-125 (125I) seed implantation in high-grade brain gliomas.
Material and methods
Between June 2017 and June 2018, 16 patients with recurrent high-grade gliomas (rHGG) underwent radioactive seed implantation with 3D-PIT. The prescribed dose was 120-140 Gy. We compared the dose distribution of the postoperative plan with the preoperative plan. Dose parameters included D90, V100, V200, conformity index (CI), and external index of the target volume (EI). Local control and early complications were also analyzed.
Results
Sixteen treatment areas were reported in our study. Median gross tumor volume (preoperative) of patients was 64.2 cm3, median needle number was 8, and median number of implanted 125I seeds was 60. For postoperative plans, the median D90, V100, and V200 was 152.1 Gy, 96.8%, and 49.1%, respectively, and 151.7 Gy, 97.0%, and 48.9%, respectively, in preoperative plans. Comparing with the preplanned cases, the dose of the target volume was slightly higher; the high-dose area of the target volume was larger in postoperative cases, but the difference was not statistically significant (p > 0.05). Actual dose conformity of the target volume was greater than preplanned, and the difference was not statistically significant (p > 0.05). Local control was 81.25% and 75% at 3 and 6 months after implantation, respectively. No serious early toxicities were observed.
Conclusions
3D-PIT based on the CT-MRI fusion images can result in good accuracy for positioning and dose distribution in radioactive seed implantation for treatment of rHGG.
Keywords: 3D printing template, brachytherapy, dosimetry, glioma, computed tomography, magnetic resonance
Purpose
Permanent interstitial brachytherapy has been cumulatively used and resulted in good outcomes for primary or adjuvant treatment of prostate cancer, gynecologic malignancies, certain head and neck tumors, and nervous system tumors [1,2,3,4,5,6]. The therapeutic effect of radioactive iodine-125 (125I) seeds (RIS) interstitial implantation brachytherapy depends primarily on dose distribution. The dose distribution is mainly achieved by preoperative planning and intraoperative optimization of brachytherapy treatment planning systems (BTPSs) based on images [7]. Although computer and imaging technologies have been improved in recent years, computed tomography (CT) or magnetic resonance imaging (MRI) still has insurmountable shortcomings, which may affect treatment effects of RIS implantation.
With the rapidly advancing technique of imaging technology, three-dimensional printing individual template (3D-PIT) has been applied to surgery and brachytherapy. Some brachytherapy scholars tried to implant 125I seeds guided by 3D-PIT in treating tumors of head and neck, thorax, abdomen, and pelvic cavity, and found that 3D-PIT-guided seed implantation could ensure the consistency of the needles pathway and dose distribution with preplan precisely, resulting in a significantly reduced error [7,8,9]. In 2017, based on the current situation of Chinese brachytherapy, an expert consensus based on 3D printing was developed [10,11].
Since recurrent high-grade glioma (rHGG) is a relatively complicated brain tumor, the outcome of brachytherapy treatment may be affected by CT’s unclear boundary. Our previous research has introduced CT-MRI images into BTPSs for the treatment of rHGG, proving good results [12]. In previous brachytherapy studies, 3D-PIT was usually based on 3D CT image reconstruction, but no data was available for 3D-PIT based on CT-MRI fusion.
Our study compared the results of preoperative dosimetry and the results of postoperative dosimetry of RIS implantation for rHGG, assisted by 3D-PIT and guided by CT-MRI fusion images. Also, we verified the application accuracy of this technology at the planning level. To our knowledge, this is the first report on 3D-PIT based on CT-MRI fusion images.
Material and methods
Ethical approval of the study protocol
Our study protocol was approved by the ethics committee, and an informed consent was obtained from every participant of this study.
General clinical data
Sixteen patients with a single rHGG, undergoing 125I seeds implantation with 3D-PIT, guided by CT-MRI fusion images in our center from June 2017 to June 2018 were involved in the study. The basic information of patients and tumors are presented in Table 1. According to the previous study, 110 to 160 Gy is the dose range that results in a good therapeutic effect for head and neck tumor [13]. The prescribed dose range in our study was 120 to 140 Gy according to above study and our clinical experience.
Table 1.
General status of the 16 patients in our study
| Characteristics | General |
|---|---|
| Sex | |
| Male | 11 |
| Female | 5 |
| Age (years) | 28-69 |
| Karnofsky performance status | Median score, 80 (70-90) |
| Primary treatment | |
| Surgery | 1 |
| Radiotherapy | 5 |
| Combination therapy | 10 |
| Initial staging | |
| III | 6 |
| VI | 10 |
| Prescribed dose (Gy) | 120-140 |
| Seed air-kerma strength (μGy.m2.h-1) | Median, 0.76 (0.60-0.94) |
Material and equipment
Iodine-125 seeds (model 6711, 4.5 mm long, 0.8 mm in diameter, half value of 0.025 mm in lead, Beijing Atom and High Technique Industries Inc., Beijing, China), with a half-life of 59.4 days and dose-rate constant of 0.965 cGy/(h.U) were implanted [14]. Mick radio-nuclear instrument (Eckert & Ziegler BEBIG, Berlin, Germany) was used for all treatments. The fusion software, which we developed, was used to combine the images of CT and MRI [12]. Brachytherapy treatment planning system – 3D BTPS (KLSIRPS-3D, Beijing University of Aeronautics and Astronautics, Beijing, China) was employed and the dose calculation algorithm of the TG-43 report was used [15]. 3D imaging and reverse engineering software (Magics 19.01; Materialise, Leuven, Belgium) was used. 3D printer (UnionTech RS6000; Shanghai Union Technology, Shanghai, China), with an accuracy of 0.1 mm was applied, and light-cured resin was used as the printing material.
Design of preoperative plan
All patients underwent both CT (GE Lightspeed 16-slice CT scanner, Milwaukee, WI, USA) and MRI (GE Signa HDxt, Milwaukee, WI, USA) 2 days before brachytherapy. The slice thickness was 5 mm, and a vacuum pad was used to fix the body position. The positioning point and the alignment reference point of the template were marked on the surface of the patient. MRI and CT data were fused by the method reported in our previous study [12]. The fused data was transmitted to BTPS to design the preoperative plan by forward planning approach, which included contouring of gross tumor volume (GTV), setting the prescribed dose and activity of 125I seeds, determination of the implantation needle path (direction, depth, and distribution), simulation of the spatial distribution of 125I seeds, and calculation of dose distribution of the target volume and organs at risk (brain stem). The dose received by 90% of GTV (GTV D90) achieved the prescribed dose as much as possible. Also, doses received by brain stem were as low as possible, using optimization.
Design and fabrication of 3D-PIT
The fused data in the BTPS was imported to 3D imaging and reverse engineering software (Magics 19.01; Materialise, Leuven, Belgium) to construct digital modeling for a 3D-PIT. Two types of 3D-PIT for one patient were created, comprising of the drilling 3D-PIT and the planning 3D-PIT. They were scanned by CT, followed by a 3D reconstruction. 3D reconstruction images were transferred to BTPS to compare whether they were completely consistent with the preoperative plan. Information about the needle-path direction was added to the model. Usually, it takes about 5-6 hours for a 3D-PIT to be printed by the 3D light-cured rapid forming printer. The biologic surface characteristics of the therapy area, a registered mark, and information on the simulated needle path were included in the 3D-PIT.
Radioactive 125I seeds implantation
The RIS implantation was performed under local anesthesia. The drilling 3D-PIT was aligned to the surface of therapy region, and it was placed into position in three steps: first, it was placed on the patient’s craniofacial area according to the skin contour; second, the position of 3D-PIT was finely adjusted under the assistance of positioning point marked on the patient and 3D-PIT alignment reference point, confirming that the actual position of 3D-PIT was in line with the preoperative plan; and third, the operator implanted target needles with shallow depths in the margin area of the individual template to accurately determine the relative position of the 3D-PIT and tumor. If an error occurred, the error between the actual image and positioning image was measured and adjusted in real time. The total tunnel was perforated using a medical bone drill through the drilling 3D-PIT guidance. The planning 3D-PIT was aligned to the surface of therapy region by above positioning method. The implantation needle was used to puncture into the predetermined depth percutaneously through a guide hole in 3D-PIT, when the latter was exactly aligned. CT scan was performed during the puncture to verify the position of the implantation needle and seeds. The intraoperative CT was combined with the preoperative MRI, and additional seeds were implanted if dose loss areas were seen in the target volume. CT was performed again upon completion of RIS implantation to observe the actual distribution of 125I seeds, until the actual distribution was consistent with that of the preoperative plan.
The final postoperative CT images were fused with preoperative MRI images. The combined data was transmitted to the BTPS. The actual dose distribution in the target volume was evaluated by means of a dose-volume histogram. The verification result after brachytherapy treatment was compared with the parameters corresponding to preoperative plan: D90 (dose received by 90% of GTV), V100 (volume percentage of GTV receiving 100% of prescribed dose), and V200 (volume percentage of GTV receiving 200% of prescribed dose). The conformity of the dose distribution was evaluated using conformity index (CI) and external index of the target volume (EI). CI and EI were calculated referring to published literature [16,17]. CI = (VT, ref/VT) × (VT, ref/Vref), wherein VT, ref and Vref were the volume of GTV with the prescribed dose and the total volume covered by prescribed dose (cc, cm3). EI = (Vref – VT, ref)/VT × 100%. The ideal CI is 1 and EI is 0, showing the prescribed dose just covering GTV and the dose outside GTV less than the prescribed dose.
Follow-up
Magnetic resonance imaging of the brain was done at 3 and 6 months after implantation of 125I seeds to ascertain changes in tumor diameter. Local control was determined at 3 and 6 months after implantation. Local control of rHGG was calculated according to the modified response evaluation criteria in solid tumors [18]. Treatment toxicity was evaluated using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 [19], and the radiotherapy-related morbidity was measured using the Radiation Therapy Oncology Group (RTOG) scale [20].
Statistical analysis
Non-parametric Wilcoxon test was used to compare the differences between each fraction of parameters because not all samples were in compliance with normal distribution. Differences with a p value < 0.05 were considered statistically significant. All calculations were performed with SPSS Statistics for Windows, version 21.0 (SPSS Inc, Chicago, Ill, USA).
Results
Image advantages of 3D-PIT based on CT-MRI fusion images
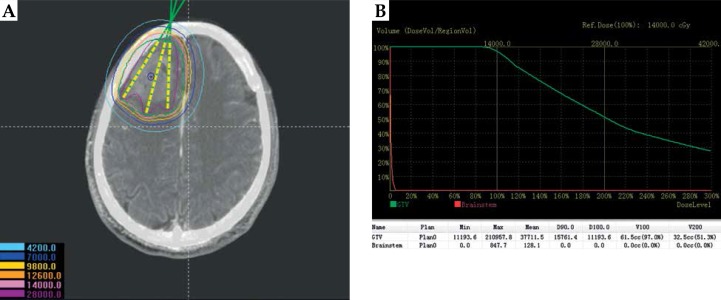
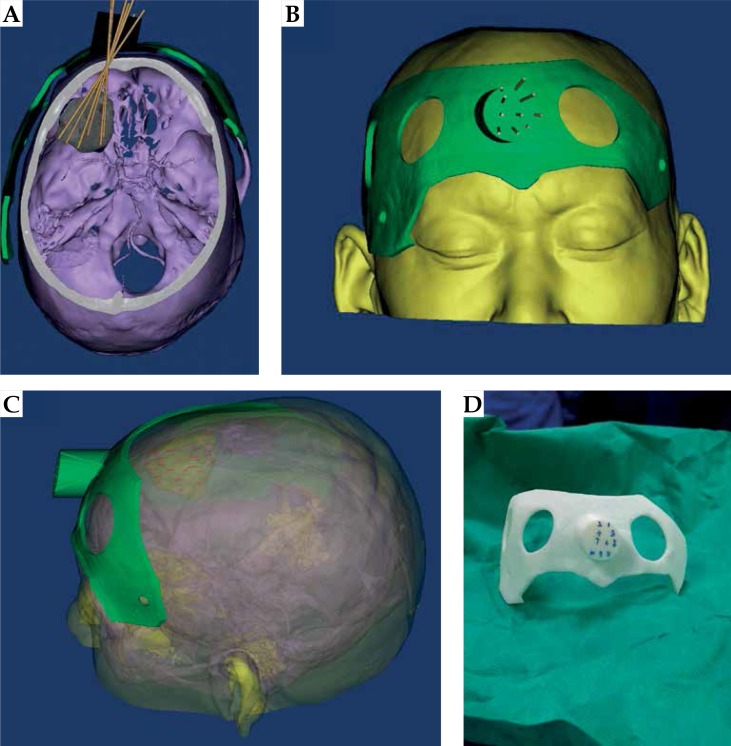
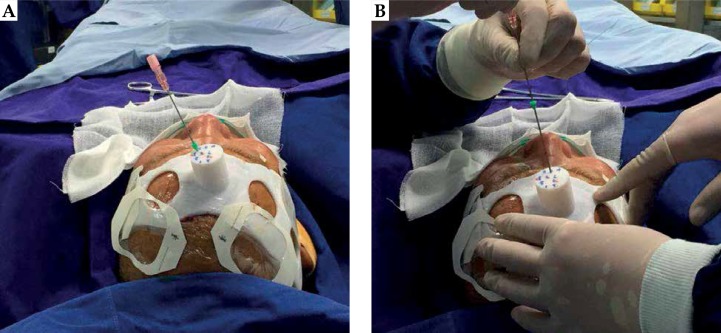
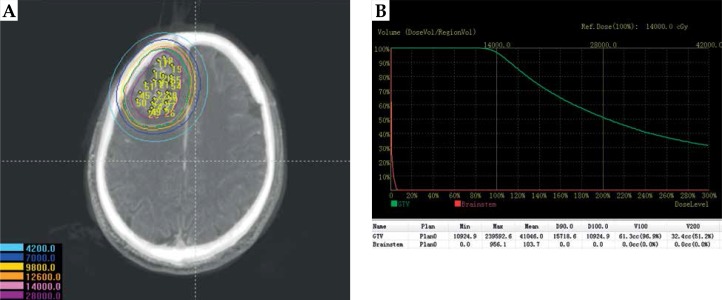
During the preoperative plan, the tumor target area was accurately delineated in the preoperative CT-MRI fusion images (Figure 1). Figure 2 shows the 3D reconstruction of the fused image and the design of 3D-PIT template to avoid blood vessels visibility on the images. The 3D-PIT contained the tumor location information, the needle point, the needle track, and the possible particle distribution. The needle was punctured through the needle tract of the 3D-PIT and seeds were implanted by surgeons (Figure 3). After the operation, postoperative CT and preoperative MRI fusion images were imported into BTPS for postoperative verification (Figure 4).
Fig. 1.
Preoperative planning design. A) Determining delineation of the target area, needle tract, calculating the dose distribution of target volume based on CT-MRI fusion images by BTPS; B) Dose volume histogram of preoperative plan
Fig. 2.
3D-PIT design with CT-MRI guidance. A) Needle tract avoidance of large blood vessels; B) 3D-PIT appearance structure; C) Possible distribution of seeds in the tumor; D) Actual printed planning 3D-PIT
Fig. 3.
Seed implantation with the planning 3D-PIT guidance. A) inserting a needle according to the needle tract of 3D-PIT; B) Seed implantation into the tumor
Fig. 4.
Postoperative dosimetry verification. A) Actual seed distribution of postoperation; B) Dose volume histogram of postoperative plan
Characteristics of implantation guided by 3D-PIT
3D-PITs were designed and created for 16 therapy areas. Each 3D-PIT had, on median, 8.0 implantation needle paths (range, 5-13). All patients received RIS under CT and MRI fusion guidance, with the assistance of a 3D-PIT. The preoperative median volume of GTV of patients was 64.2 cm3 (23.0-150 cm3). The median activity of single 125I seed used was 0.76 μGy.m2.h-1 (0.60-0.94 μGy.m2.h-1). The median number of seeds implanted was 60.0 (25-126). The median D90 of the postoperative GTV was 152.1 Gy, and 100% of postoperative D90 was higher than the prescribed dose (Table 2).
Table 2.
Preoperative and postoperative dosimetry parameters for the 16 patients
| Pt | D90 (Gy) | V100 (%) | V200 (%) | CI | EI (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | 157.6 | 157.2 | 97.0 | 96.9 | 51.3 | 51.2 | 0.83 | 0.81 | 13.2 | 15.8 |
| 2 | 148.0 | 148.2 | 95.0 | 94.1 | 42.3 | 41.9 | 0.87 | 0.90 | 9.6 | 12.3 |
| 3 | 144.0 | 142.6 | 97.6 | 98.0 | 51.6 | 51.4 | 0.75 | 0.69 | 15.2 | 18.6 |
| 4 | 136.7 | 136.1 | 96.5 | 96.4 | 49.8 | 52.1 | 0.86 | 0.81 | 10.8 | 9.5 |
| 5 | 168.1 | 162.4 | 97.6 | 97.8 | 54.4 | 54.0 | 0.79 | 0.72 | 18.3 | 15.2 |
| 6 | 162.4 | 161.8 | 97.8 | 98.3 | 46.9 | 49.5 | 0.80 | 0.78 | 13.5 | 11.8 |
| 7 | 161.6 | 161.9 | 97.5 | 97.6 | 50.2 | 52.5 | 0.83 | 0.85 | 12.1 | 11.9 |
| 8 | 139.2 | 139.8 | 94.7 | 95.1 | 37.0 | 40.1 | 0.65 | 0.75 | 17.4 | 15.7 |
| 9 | 148.5 | 150.2 | 97.5 | 97.9 | 48.4 | 48.7 | 0.79 | 0.85 | 13.2 | 16.3 |
| 10 | 157.8 | 159.3 | 96.8 | 96.7 | 44.2 | 43.5 | 0.81 | 0.85 | 20.8 | 16.7 |
| 11 | 146.8 | 146.5 | 95.6 | 96.3 | 43.9 | 43.2 | 0.76 | 0.70 | 12.7 | 11.5 |
| 12 | 153.7 | 153.9 | 97.0 | 97.1 | 46.2 | 45.6 | 0.75 | 0.80 | 11.4 | 12.3 |
| 13 | 158.9 | 158.7 | 96.9 | 96.5 | 50.5 | 50.4 | 0.83 | 0.80 | 18.7 | 15.9 |
| 14 | 149.6 | 150.0 | 95.2 | 95.5 | 46.3 | 46.5 | 0.78 | 0.82 | 13.9 | 12.6 |
| 15 | 159.9 | 159.6 | 97.2 | 97.3 | 49.4 | 48.2 | 0.70 | 0.90 | 12.9 | 12.0 |
| 16 | 148.2 | 148.5 | 96.8 | 96.2 | 50.3 | 50.6 | 0.85 | 0.83 | 8.5 | 9.8 |
Dosimetry verification and comparison results
D90, V100, and V200 as well as EI and CI of the 16 lesions before and after brachytherapy were compared using non-parametric Wilcoxon test with p < 0.05 considered significant (Table 3). The mean values of V100 after brachytherapy were smaller than those before brachytherapy, and the mean values of D90 and V200 after brachytherapy were greater than those before brachytherapy. All parameters in the 2 groups were not significantly different (p > 0.05). The mean values of CI after brachytherapy were greater compared with that before brachytherapy (0.79 and 0.80, respectively), and the difference was not significant (p > 0.05). The mean values of EI after brachytherapy were greater than that before brachytherapy (13.9% and 13.6%, respectively), but the difference was not significant (p > 0.05). In addition, we analyzed the mean dose of the maximum dose received by brain stem and found that the doses received by brain stem after brachytherapy decreased slightly compared with those before brachytherapy. This minor decrease was not statistically significant (p > 0.05).
Table 3.
Comparison of preoperative and postoperative dosimetry parameters (16 cases)
| Parameter | Preoperative plan | Postoperative plan | P | ||||
|---|---|---|---|---|---|---|---|
| Range | Median | M (SD) | Range | Median | M (SD) | ||
| D90 (Gy) | 136.7-168.1 | 151.65 | 152.56 (8.83) | 136.1-162.4 | 152.05 | 152.29 (8.33) | 0.815 |
| V100 (%) | 94.7-97.8 | 96.95 | 96.69 (1.00) | 94.1-98.3 | 96.80 | 96.73 (1.14) | 0.376 |
| V200 (%) | 37.0-54.4 | 48.90 | 47.67 (4.30) | 40.1-54.0 | 49.10 | 48.09 (4.17) | 0.816 |
| CI | 0.65-0.87 | 0.80 | 0.79 (0.06) | 0.69-0.90 | 0.81 | 0.80 (0.06) | 0.697 |
| EI (%) | 8.5-20.8 | 13.20 | 13.89 (3.41) | 9.5-18.6 | 12.45 | 13.62 (2.68) | 0.605 |
V100%, V200% – volume of the GTV receiving 100%, 200% of the prescribed dose, D90 – dose covering 90% of the GTV, CI – conformity index, EI – external index of the target volume, M – mean, SD – standard deviation
Local control
All patients were followed up with the use of MRI. There was 1 case of complete response (CR), 12 cases of partial response (PR), and 3 cases of stable disease (SD) at 3 months after implantation. There was 1 case of CR, 11 cases of PR, 3 cases of SD, and 1 case of PD at 6 months after implantation. Local control was 81.25% and 75% at 3 and 6 months after implantation, respectively.
Early toxicity
Despite close vicinity of many important structures in brain, no mechanical injury of these organs was observed in course of implantation procedure. None of the patients suffered from paralysis in result of mechanical damage of nerves. Due to abundant vascularity of brain tumors, there was a small degree of bleeding at the time of seed implantation, which resolved spontaneously. No hemorrhagic foci were found on CT scans taken after the implantation. No clinical features of internal hemorrhage were observed in these patients. There were no serious complications such as meningitis, cerebrospinal fluid leakage, and cognitive impairment in all cases. In 4 patients (patient 1, 5, 11, 14), a few days after completion of brachytherapy, brain edema around the target area requiring mannitol dehydration, CTCAE scale grade 1 or 2 occurred. No radiotherapy-related morbidity occurred. We focused only on acute reactions because of short follow-up.
Discussion
The manufacture of 3D-PIT included several key procedures. Firstly, by digital processing, tumor information (image information) was transmitted to BTPS. Secondly, the treating physician and a medical physicist defined related parameters such as target volume, organs at risk, prescribed dose, and design of the needle channel. Thirdly, a 3D printer was used to print a 3D-PIT [21,22,23]. A 3D-PIT possesses information on the needle channel, coordinate system for laser positioning, and a laser-identification system.
The 3D-PIT technology can reduce an error of brachytherapy caused by the doctor’s technical reasons. A 3D-PIT includes information of the path of the implantation needle, characteristics of the surface of the therapy area of the patient, and positioning and orientation effects. In this way, the 3D-PIT fully reflects the individual characteristics and realizes accurate alignment between the 3D-PIT and therapy area as well as accurate control of the implantation needle. It was first reported in 2012 for treatment of head and neck tumors guided by 3D-PIT [24]. However, only needle path was available and detailed information of the dose distribution calculated by BTPS was not presented at that time. It was concluded that the postoperative dose could achieve the dose requirement (D90 of the target volume was mostly higher than the prescribed dose), but the results were not compared with the preoperative plan.
Subsequent studies included preoperative and postoperative comparisons due to the emergence of BTPS. The preoperative parameters and postoperative parameters of implantation of 125I seeds under the guidance of 3D-PIT for malignant tumors were studied and compared in Zhang et al.’s study [25]. There were no significant differences for D90, V90, V100, and V150 between preoperation and postoperation, which indicated that the preoperative plan could be properly completed. However, the sample size was only 9 cases, which involved four treatment sites. Ji et al. compared dose distributions of postoperative plans with preoperative plans for radioactive seed implantation of paravertebral/retroperitoneal tumors assisted by 3D-PIT and they found that the dose of the target volume was slightly lower and the high-dose area of the target volume was larger in postoperative cases compared with the preplanned cases; however, the difference was not statistically significant, which suggested that the CT-guided RIS using 3D-PIT can enable exact positioning and orientation for paravertebral/retroperitoneal lesions [26].
Previous studies of 3D-PIT were always CT-guided, but CT-MRI-guided 3D-PIT was never reported. However, brachytherapy based purely on CT image guidance suffers from several defects for brain tumors. Firstly, unclear borders may cause residual treatment and lead to tumor recurrence. Secondly, accurate control of the implantation needle is difficult. Thirdly, the angle and depth of the needle must be adjusted under CT guidance during the procedure, which increases the possibility of complications. Also, the limited display of vessels may increase the risk of cerebral bleeding. There is no doubt that there are disadvantages to rely solely on MRI images. Firstly, seed and catheter localization in MRI images is challenging. Secondly, MRI image is not recommended routinely for guiding implantation in real time. The previous research of our center realized the fusion of CT and MRI images, making their advantages and disadvantages complementary [12].
CT-MRI fusion images can reduce the error of brachytherapy caused by images. An earlier study in our center reported the application of the CT-MRI fusion images to monitor the operation. The CI in the CT-MRI fusion group (0.85) was higher compared to CI in CT alone group (0.75), with statistical significance. The higher CI rate verified postoperatively suggested that the tumor volume received a dose that was closer to the ideal treatment dose in the CT-MRI fusion group than that of the CT alone group. CT-MRI image fusion during the operation was useful to determine the border of glioma and can support to adjust the position of the catheters and seeds in real time. The image fusion protocol is an essential component of our intraoperative treatment and postoperative dose verification effort [12].
In this study, we combined CT-MRI fusion images with 3D-PIT for the first time. D90 and V200 in the postoperative verification were higher than those of preoperation. These findings suggested that the prescribed dose and high-dose range received by the target volume after brachytherapy were more than that in the preoperative plan, and the parameters of the two groups were not significantly different (p > 0.05), which suggested that the preoperative plan can be well implemented under the guidance of CT-MRI-based 3D-PIT. The median CI was 0.6 and EI was 0.4 in postoperation plan in the previous study [26]. The CI was higher and EI was lower in our study than those. We may conclude that 3D-PIT with the guidance of CT-MRI is better than 3D-PIT guided by CT alone for brain tumors.
The CT-MRI-guided RIS using a 3D-PIT resulted in good local control with few toxicities for rHGG. Regardless of the preoperative and postoperative planning, the doses received by brain stem were much lower than brain stem tolerance dose. The doses received by brain stem after brachytherapy were decreased slightly compared with those before brachytherapy, and this slight decrease was not statistically significant. Serious complications related to brachytherapy were not observed. Hence, we believe that toxicity of brachytherapy and damage to brain stem can be controlled under CT-MRI guidance using 3D-PIT. However, stereotactic body radiation therapy (SBRT), volumetric modulated arc therapy (VMAT), which has achieved good results as the main external radiotherapy for rHGG, may result in some complications, especially brain necrosis [27]. In addition, 125I seed brachytherapy can be repeated at short intervals as it causes milder tissue damage compared with SBRT at the time of tumor recurrence [28].
Our research still had shortcomings. Firstly, the sample size was small. Secondly, the overall survival rate could not be analyzed and only local control was assessed because of the limited follow-up period. Thirdly, no comparation with SBRT was done in our study. It is necessary to expand the sample size, follow-up time, and survival of all patients in our further research.
The CT-MRI-guided RIS using a 3D-PIT reduces both image and technical errors to enable exact positioning and orientation for rHGG. The parameters of the postoperative plan are mostly consistent with the preoperative plan. The conformity between the preoperative plan and postoperative plan was satisfactory.
Disclosure
Authors report no conflict of interest.
References
- 1.Skowronek J. Current status of brachytherapy in cancer treatment-short overview. J Contemp Brachytherapy. 2017;9:581–589. doi: 10.5114/jcb.2017.72607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao M, Spencer S, Guerrieri M, et al. A single institution analysis of low-dose-rate brachytherapy: 5-year reported survival and late toxicity outcomes. J Contemp Brachytherapy. 2018;10:155–161. doi: 10.5114/jcb.2018.75600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skowronek J. Brachytherapy in the treatment of lung cancer – a valuable solution. J Contemp Brachytherapy. 2015;7:297–311. doi: 10.5114/jcb.2015.54038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Wang J, Jiang Y, et al. The investigation of 125I seed implantation as a salvage modality for unresectable pancreatic carcinoma. J Exp Clin Cancer Res. 2013;32:106. doi: 10.1186/1756-9966-32-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodin J, Bar-Ad V, Cognetti D, et al. A systematic review of treating recurrent head and neck cancer: a reintroduction of brachytherapy with or without surgery. J Contemp Brachytherapy. 2018;10:454–462. doi: 10.5114/jcb.2018.79399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nachbichler SB, Kreth FW. Brachytherapy of intracranial gliomas. Prog Neurol Surg. 2018;31:72–86. doi: 10.1159/000467114. [DOI] [PubMed] [Google Scholar]
- 7.Hongtao Z, Xuemin D, Huimin Y, et al. Dosimetry study of three-dimensional print template-guided precision 125I seed implantation. J Cancer Res Ther. 2016;12:C159–C165. doi: 10.4103/0973-1482.200607. [DOI] [PubMed] [Google Scholar]
- 8.Huang MW, Zhang JG, Zheng L, et al. Accuracy evaluation of a 3D-printed individual template for needle guidance in head and neck brachytherapy. J Radiat Res. 2016;57:662–667. doi: 10.1093/jrr/rrw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji Z, Jiang Y, Guo F, et al. Dosimetry verification of radioactive seed implantation for malignant tumors assisted by 3D printing individual templates and CT guidance. Appl Radiat Isot. 2017;124:68–74. doi: 10.1016/j.apradiso.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Saito S, Ye X. Expert consensus workshop report: Guideline for three-dimensional-printing template-assisted computed tomography-guided 125I seeds interstitial implantation brachytherapy. J Can Res Ther. 2017;13:605–606. doi: 10.4103/jcrt.JCRT_540_17. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Zhang F, Guo J, et al. Expert consensus workshop report: Guideline for three-dimensional printing template-assisted computed tomography-guided 125I seeds interstitial implantation brachytherapy. J Can Res Ther. 2017;13:607–612. doi: 10.4103/jcrt.JCRT_412_17. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Han Y, Nan G, et al. Value of CT-MRI fusion in iodine-125 brachytherapy for high-grade glioma. Oncotarget. 2017;8:112883–112892. doi: 10.18632/oncotarget.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu L, Jiang Y, Wang J, et al. An investigation of 125I seed permanent implantation for recurrent carcinoma in the head and neck after surgery and external beam radiotherapy. World J Surg Oncol. 2013;11:60. doi: 10.1186/1477-7819-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka K, Tateoka K, Asanuma O, et al. A dosimetry study of the Oncoseed 6711 using glass rod dosimeters and EGS5 Monte Carlo code in a geometry lacking radiation equilibrium scatter conditions. Med Phys. 2011;38:3069–3076. doi: 10.1118/1.3590370. [DOI] [PubMed] [Google Scholar]
- 15.Nath R, Anderson LL, Luxton G, et al. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys. 1995;22:209–234. doi: 10.1118/1.597458. [DOI] [PubMed] [Google Scholar]
- 16.Van’t Riet A, Mak AC, Moerland MA, et al. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys. 1997;37:731–736. doi: 10.1016/s0360-3016(96)00601-3. [DOI] [PubMed] [Google Scholar]
- 17.Saw CB, Suntharalingam N. Quantitative assessment of interstitial implants. Int J Radiat Oncol Biol Phys. 1991;20:135–139. doi: 10.1016/0360-3016(91)90149-x. [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 19. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 20.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 21.Zhe J, Yu LJ, Fu XG. Dosimetry verification of radioactive seed implantation for malignant tumor assisted by 3D printing individual guide template. Zhonghua Fang She Yi Xue Yu Fang Hu Za Zhi. 2016;36:662–666. [Google Scholar]
- 22.Lee WR, deGuzman AF, Tomlinson SK, et al. Radioactive sources embedded in suture are associated with improved postimplant dosimetry in men treated with prostate brachytherapy. Radiother Oncol. 2002;65:123–127. doi: 10.1016/s0167-8140(02)00305-5. [DOI] [PubMed] [Google Scholar]
- 23.Jun JW. 3D Printing Technology and Precise Radioactive Seeds Implantation Therapy. Beijing: Peking University Medical Press; 2016. [Google Scholar]
- 24.Huang MW, Liu SM, Zheng L, et al. A digital model individual template and CT-guided 125I seed implants for malignant tumors of the head and neck. J Radiat Res. 2012;53:973–977. doi: 10.1093/jrr/rrs046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang HT, Di XM, Yu HM, et al. Dose comparison between pre and post operation of 125I seeds implantation guided by 3D print template. Zhonghua Yi Xue Za Zhi. 2016;96:712–715. doi: 10.3760/cma.j.issn.0376-2491.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Ji Z, Jiang Y, Su L, et al. Dosimetry verification of 125I seeds implantation with three-dimensional printing noncoplanar templates and CT guidance for paravertebral/retroperitoneal malignant tumors. Technol Cancer Res Treat. 2017;16:1044–1050. doi: 10.1177/1533034617723221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanabe S, Takahashi H, Saito H, et al. Selection criteria for 3D conformal radiotherapy versus volumetric-modulated arc therapy in high-grade glioma based on normal tissue complication probability of brain. J Radiat Res. 2019;60:249–256. doi: 10.1093/jrr/rry106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Zhang LJ, Xie QG, et al. Comparison of clinical efficacy and complications of 125I seed brachytherapy and stereotactic body radiation therapy for recurrent pulmonary metastases from colorectal carcinoma. J Contemp Brachytherapy. 2018;10:360–367. doi: 10.5114/jcb.2018.77956. [DOI] [PMC free article] [PubMed] [Google Scholar]