Abstract
Glycaemic control is the main focus of managing diabetes and its complications. Hyperglycaemia induces oxidative stress favouring cellular damage and subsequent diabetic complications. The present study was conducted to compare the plasma total antioxidant capacity (TAC) and individual antioxidant marker antioxidant status of type 2 diabetics (T2D) with good ((+) GC) and poor ((-) GC) glycaemic control with prediabetic (PDM) and normoglycaemic (NG) individuals. T2D (n = 147), PDM (n = 47), and NGC (n = 106) were recruited as subjects. T2D and PDM had lower plasma TAG than NG subjects. T2D and PDM had significantly higher GPx activity and plasma MDA concentrations than NG. PDM showed the highest SOD activity. T2D (-) GC showed significantly elevated GPx activity and higher MDA level and significantly lower SOD activity among all study groups. Lower plasma TAC and higher plasma MDA indicate the presence of oxidative stress in T2D and PDM. Elevated GPx activity in T2D, PDM, and particularly in T2D (-) GC suggests a compensatory response to counteract excess lipid peroxidation in the hyperglycaemic state. Decline in SOD activity advocates the presence of glycation and excess lipid peroxidation in T2D.
1. Introduction
Glycaemic control is the central focus of managing diabetes and its complications. Hyperglycaemia promotes oxidative stress through increased glucose autooxidation, protein glycation, and successive degradation of glycated proteins [1]. Excess ROS production and subsequent decline in cellular antioxidant defense facilitate the rise in systemic insulin resistance and lipid peroxidation leading to cellular damage and cytoprotective enzyme inactivation [2, 3].
Consequently, systemic oxidative stress and accompanied defects in antioxidant defense mechanisms promote the predisposition of diabetic complications, both micro and macro vascular [4]. Earlier tight glucose control has shown to improve cardiovascular morbidity in the United Kingdom Prospective Diabetes Study (UKPDS) [5]. Further, a recent follow-up to the same study was able to verify ability of long-term glycaemic control in type 2 diabetes in preventing the cardiovascular diseases [6]. However, the role of glycaemic control in improving the diabetes complications via oxidative stress pathways is not adequately inquired.
Previous findings on glycaemic control and oxidative stress in patients with type 2 diabetes (T2D) are ambiguous. There were depletions [7–15], improvements [16–20], and no change [21] in the total antioxidant status, and antioxidant enzyme activities in diabetics with poor glycaemic control have been disclosed. Therefore, diabetes patients with good and poor glycaemic control were used to address the unsolved interpretations of glycaemic control on plasma antioxidant capacity and antioxidant markers.
2. Materials and Methods
2.1. Subjects
One hundred and forty-seven (n = 147) type 2 diabetics (T2D), forty-seven (n = 47) prediabetics (PDM), and a hundred and six (n = 106) normoglycaemic (NG) apparently healthy individuals were recruited as subjects.
T2D patients were recruited from the diabetes clinical registries of selected Government Hospitals of North Western Province, Sri Lanka. Screening sessions were conducted in community centers and working places situated closer to the Wayamba University of Sri Lanka to identify PDM and NG individuals. Screened individuals were invited to participate in the study. Individuals who were already diagnosed by a clinician or identified by the researchers in screening sessions having impaired fasting glycaemia (6.1–6.9 mmol/L) [22] more than two consecutive occasions verified with glycated haemoglobin (HbA1c) measurement greater than 6% were considered as the PDM. Individuals with a fasting plasma glucose level below 6.1 mmol/L were considered as normoglycaemic [22].
T2D, PDM, and NG volunteers with known history of heart disease, liver disease, kidney diseases, or endocrine diseases other than diabetes and those who were ill for more than seven days during the last three months were excluded. Smokers, regular users of alcohol (more than 2 times per week), those who followed a weight loss regimen, users of antioxidant supplements (vitamins and minerals), and pregnant and lactating mothers were also excluded. None of the T2D patients selected were on insulin for glycaemic control during the study period.
2.1.1. Ethics
The study was approved by the ethics review committee of Sri Lanka Medical Association (ERC/07/007). The study protocol was in accordance with the principles described in the Declaration of Helsinki. Written informed consent was obtained from each participant after providing the detailed description of the study. Prior approval from the respective hospital authorities was taken for recruiting diabetics.
2.2. Data Collection
Details of general life style, disease, and medical history of the subjects were collected using a pretested questionnaire. Weight and height of the subjects were measured using standard methods in light indoor clothing. The waist circumference of the subjects was measured around the point of the umbilicus using a nonstretchable plastic tape. The hip circumference was measured at the widest point of the body.
2.2.1. Systolic and Diastolic Blood Pressures
Seated systolic (SBP) and diastolic (DBP) blood pressures were measured in the nondominant arm using a digital sphygmomanometer (Omron Ltd., Singapore) after resting for 10 minutes prior to the measurement. Average of the last two readings was considered. Subjects were refrained from speaking and moving during the measurement.
2.3. Blood Collection and Biochemical Analysis
2.3.1. Blood Collection
A single 10 mL of fasting venous blood sample was drawn by a phlebotomist from each participant following 12 hours of fasting. The blood sample was then transferred to blood collection tubes containing lithium heparin for glutathione peroxidase (GPx), superoxide dismutase (SOD), Trolox equivalent antioxidant capacity (TEAC) assay, oxygen radical absorption capacity (ORAC) assay, and malondialdehyde (MDA) analysis; EDTA for ferric reducing ability of plasma (FRAP) assay, HbA1c, fasting plasma glucose, and fasting lipids; and serum tubes for fasting serum insulin analysis.
Aliquots of whole blood were stored at -80°C for future analysis of erythrocyte GPx and SOD. For HbA1c determination, aliquot of whole blood was stored at -20°C. Heparinized plasma was separated for further analysis of TEAC, ORAC, and MDA using a centrifuge set at 4000 rpm for 10 minutes and stored at -80°C. Separated EDTA plasma was stored at -80°C for FRAP analysis. For the fasting plasma glucose, lipid profile, and insulin analyses, aliquots of plasma and serum were stored at -20°C.
2.3.2. Biochemical Analysis
(1) Plasma Total Antioxidant Capacity (TAC). Plasma FRAP assay. The plasma FRAP assay was performed according to the method described by Benzie and Strain [23]. Briefly, 3 mL of a working FRAP reagent was mixed with 100 μL of plasma followed by mixing and incubating for 6 minutes at 37°C. After the 6 minutes of incubation, absorption was read by a spectrophotometer (Labo Med Inc., UK) set at 593 nm. Plasma FRAP values were expressed as μmol of FeSO4 equivalents per litre.
Plasma TEAC assay. Plasma TEAC was measured using the method described by Re et al. [24]. ABTS radical cation was prepared by mixing equal volumes of 7 mM ABTS (2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid)) and 2.45 mM potassium persulfate solutions and keeping it for 16-24 hours in the dark. ABTS radical cation was diluted with PBS (pH 7.4) for an absorbance of 0.7 (±0.02) and equilibrated at 30°C. Then, 100 μL of diluted plasma was added to the ABTS cation solution and incubated for 6 minutes at 30°C. Absorbance readings were taken at one-minute intervals after initial mixing up to 6 min using a spectrophotometer set at 734 nm. Plasma TEAC values were expressed in Trolox equivalents per litre.
Plasma ORAC assay. Plasma ORAC was measured as the method described by Cao et al. [25]. Diluted heparinized plasma was mixed with 100 μL of fluorescein in black plates followed by preheating in the microplate reader set at 37°C for 10 minutes. After the addition of 50 μL of AAPH solution and mixing, reading was taken at one-minute intervals using a microplate reader set at excitation and emission wavelengths of 494 and 535 nm, respectively. The net area under the curve for each standard and sample was derived, and ORAC values were expressed as Trolox units per litre.
Erythrocyte GPx and SOD activities. Activities of erythrocyte glutathione peroxidase (GPx) (RANSEL RS 505, Randox Laboratories, UK) and superoxide dismutase (SOD) (RANSOD SD 125, Randox Laboratories, UK) were measured using the enzymatic reagent kits, following the manufacturer's instructions. The activities of both enzymes were normalized by the concentration of haemoglobin (Hb) and expressed as units per gram of Hb per min.
Plasma MDA level. Plasma lipid peroxidation marker, malondialdehyde (MDA), concentration was measured as a marker of lipid peroxidation using thiobarbituric acid reactive substances (TBARS) [26]. Briefly, 200 μL of heparinized plasma was first mixed with Butylated Hydroxytoluene (BHT), and plasma proteins were precipitated by adding trichloroacetic acid (TCA). Then, the thiobarbituric acid (TBA) was added to the mixture and incubated on a water bath set at 95°C for 45 minutes. The resulting chromogen was extracted using n-butanol and saturated NaCl solution. The organic mixture was separated by centrifugation. Intensity of the upper organic layer was measured spectrophotometrically (model: UV/Visible Auto PC Scanning spectrophotometer; Labo Med Inc., Germany) at 535 nm and 572 nm against the blank. The MDA level of the samples was determined using the linear standard curve developed by 0.5 to 32 μM of 1,1,3,3-tetraethoxypropane.
Glycaemic control. Fasting plasma glucose (FPG) and HbA1c levels of the subjects were measured using enzymatic reagent kits (Stanbio, USA). Fasting insulin was analyzed by an Enzyme-Linked Immunosorbent Assay (ELISA) kit (DRG, USA). Insulin resistance was derived using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) by multiplying fasting plasma glucose (mmol/L) and fasting insulin level (pmol/L) and dividing it by 135.
Fasting plasma lipids. Fasting total cholesterol and triacylglycerol levels were measured using the enzymatic reagent kits (Stanbio, USA). HDL cholesterol was determined using an enzymatic reagent kit after selective precipitation of VLDL and LDL [27]. The LDL cholesterol level was estimated using the Friedewald formula [28].
2.4. Data Analysis
T2D and PDM were further clustered into two groups based on the presence or absence of good glycaemic control (HbA1c < 7%; (+) GGC) and poor glycaemic control (HbA1c > 7%; (-) GGC).
BMI > 25 kg m−2 was used to define obesity (WHO Definition for BMI of Asians, 2004). The waist circumference (>85 cm for women and >90 for males) and waist to hip ratio (WHR > 0.85 for females and 0.9 for males) (WHO, 2008) were used to define central obesity. Metabolic syndrome was defined according to the International Diabetes Federation Definition for metabolic syndrome [29].
2.5. Statistical Analysis
Data were expressed in absolute numbers and percentages. Data were checked for normality using the Kolmogorov-Smirnov test. If the data of a variable were not normally distributed, they were transformed to natural logarithms and checked for normality, and a parametric test was used. One-way ANOVA was used in order to observe the differences in antioxidant markers and anthropometric and biochemical variables between groups. Effects of the study group and glycaemic control on total antioxidant status and individual antioxidant markers were assessed using factorial ANOVA with multiple comparisons. Post hoc tests (Tukey's b with Bonferroni correction) were performed to compare the subgroups with and without good glycaemic control for their total antioxidant status and individual antioxidant markers. Data were analyzed using the SPSS (Statistical Package for Social Sciences), version 16.
3. Results
General characteristics of the study subjects are shown in Table 1. Average clinical duration of diabetes among T2D was 5.0 ± 4.6 years. Nearly 99% of T2D were on oral hypoglycaemic agents (OHA) for glycaemic control. Only 1-2% reported that they were on dietary and physical activity modifications for glycaemic control. None of the T2D was on insulin for glycaemic control. Biguanide (metformin) was the main type (93%) of OHA used. In addition, 46% of diabetics used sulfonylureas (tolbutamide, glibenclamide, and Diaonil) along with biguanides. None of the PDM was on OHA for glycaemic control. However, only 61% of T2D and 66% of PDM were on proper glycaemic control (HbA1c < 7.0). Thirty-one percent (31%) of T2D were hypertensive and on antihypertensive drugs. The presence of dyslipidemia among T2D was 20%, and all were on lipid-lowering drugs (statins). Twenty-three percent (23%) of PDM and 12% of NG had dyslipidemia, and they were not on drug therapy.
Table 1.
Anthropometric, clinical, and biochemical characteristics of the study subjects (n = 300).
| Variable | T2D (n = 147) | PDM (n = 47) | NG (n = 106) | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 47.6 ± 8.3 | 45.7 ± 8.8 | 44.2 ± 8.2 | 0.125 1 |
| Sex, M/F (%) | 42/58 | 48/52 | 42/58 | 0.236 2 |
| BMI (kg m−2), mean ± SD | 24.7 ± 4.3 | 24.9 ± 4.1 | 25.2 ± 3.2 | 0.156 1 |
| WC (cm), mean ± SD | 87.7 ± 8.8 | 87.0 ± 9.1 | 85.0 ± 8.0 | 0.143 1 |
| WHR, mean ± SD | 0.96a ± 0.07 | 0.93b ± 0.06 | 0.92b ± 0.06 | 0.033 1 |
| Diabetes duration (years), mean ± SD | 5.0 ± 4.6 | — | — | |
| SBP (mmHg), mean ± SD | 127a ± 19 | 121b ± 17 | 121b ± 16 | 0.043 1 |
| DBP (mmHg), mean ± SD | 77 ± 13 | 76 ± 13 | 76 ± 11 | 0.237 1 |
| FPG (mmol/L), mean ± SD | 7.9a ± 2.80 | 5.9a ± 1.20 | 5.1a ± 0.56 | 0.031 1 |
| HbA1c (%), mean ± SD | 6.70a ± 1.50 | 6.70a ± 1.00 | 5.10b ± 0.70 | 0.037 1 |
| TC (mmol/L), mean ± SD | 4.6a ± 1.00 | 4.6a ± 0.70 | 4.50a ± 1.00 | 0.129 1 |
| HDL-C (mmol/L), mean ± SD | 0.94a ± 0.27 | 0.90a ± 0.21 | 0.92a ± 0.23 | 0.078 1 |
| LDL-C (mmol/L), mean ± SD | 3.30a ± 1.00 | 3.40a ± 0.70 | 3.30a ± 1.00 | 0.123 1 |
| TAG (mmol/L), mean ± SD | 1.60a ± 0.78 | 1.60a ± 0.85 | 1.30b ± 0.62 | 0.033 1 |
| HOMA-IR, mean ± SD | 3.60a ± 4.00 | 2.70b ± 2.80 | 2.0b ± 2.50 | 0.029 1 |
| Family history of T2DM (%) | 51a | 41a | 32b | 0.041 2 |
| Family history of HTN (%) | 39a | 37a | 21b | 0.043 2 |
| Family history of CVD (%) | 11 | 09 | 13 | 0.214 2 |
| Metabolic syndrome (%) | 78a | 52b | 47b | 0.037 2 |
1One-way ANOVA; 2Pearson's χ2 test. Values expressed in absolute numbers and percentages. Means with the different superscripts in a given row are significantly different at P < 0.05 (Tukey's post hoc test). BMI: body mass index; DBP: diastolic blood pressure; FPG: fasting plasma glucose; HbA1c: glycated haemoglobin concentration; HDL-C: high-density lipoprotein cholesterol; HOMA-IR: homeostatic model assessment for insulin resistance; LDL-C: low-density lipoprotein cholesterol; NG: normoglycaemic; PDM: prediabetic; SBP: systolic blood pressure; TC: total cholesterol; WC: waist circumference; WHR: waist to hip ratio; T2DM: type 2 diabetes mellitus.
T2D had a significantly (P < 0.05) higher waist to hip ratio (WHR), SBP, FPG, and HOMA-IR than the PDM and NG groups (Table 1). Both T2D and PDM had significantly higher (P < 0.05) HbA1c and plasma TAG compared with the NG group (Table 1).
3.1. Antioxidant Status
Table 2 shows the antioxidant status of T2D, PDM, and NG groups. T2D had significantly (P < 0.05) lower plasma FRAP, TEAC, and ORAC compared to NG. T2D and PDM had similar plasma TEAC and ORAC. However, the T2D group had significantly (P < 0.05) higher erythrocyte GPx activity. Significantly (P < 0.05) higher SOD activity was shown by the PDM than T2D and NG groups.
Table 2.
Plasma total antioxidant capacity and antioxidant markers of the study groups (n = 300).
| Variable | T2D (n = 147) | PDM (n = 47) | NG (n = 106) | P 1 |
|---|---|---|---|---|
| Plasma TAC | ||||
| FRAP (μmol/L) | 787a ± 180 | 857b ± 211 | 847b ± 215 | 0.033 |
| TEAC (μmol/L) | 1216a ± 484 | 1180a ± 388 | 1436b ± 592 | 0.027 |
| ORAC (μmol/L) | 4496a ± 1523 | 4555a ± 2042 | 5072b ± 1904 | 0.019 |
| GPx activity (U/g Hb/min) | 326a ± 249 | 279b ± 203 | 244b ± 159 | 0.023 |
| SOD activity (U/g Hb/min) | 797a ± 560 | 4418b ± 3888 | 3611c ± 2918 | 0.011 |
| Albumin (g/dL) | 5.8 ± 1.0 | 5.5 ± 1.2 | 5.7 ± 1.9 | 0.072 |
| Bilirubin (mg/dL) | 0.9 ± 0.5 | 0.9 ± 0.2 | 0.9 ± 0.8 | 0.134 |
| Total protein (g/dL) | 7.1a ± 1.5 | 7.9a ± 1.8 | 6.5b ± 1.2 | 0.047 |
| Uric acid (μmol/L) | 283a ± 72 | 398b ± 179 | 387b ± 146 | 0.031 |
| MDA (μmol/L) | 6.4a ± 5.1 | 6.0a ± 5.7 | 4.7b ± 2.9 | 0.036 |
All values are mean ± SD. 1One-way ANOVA. Means with the different superscripts in a given row are significantly different at P < 0.05 (Tukey's post hoc test). NS: not significant; FRAP: ferric reducing antioxidant power assay; GPx: glutathione peroxidase; MDA: malondialdehyde; NG: normoglycaemic subjects; ORAC: oxygen radical absorption capacity; PDM: prediabetics; TEAC: Trolox equivalent antioxidant capacity assay; TAC: total antioxidant capacity; T2DM: type 2 diabetes mellitus.
Table 3 shows the results of correlation analysis between antioxidant markers and glycaemic control parameters of T2D and PDM groups. Plasma ORAC was negatively correlated with FPG, HbA1c of T2D, and HOMA-IR of PDM. GPx activity had a positive correlation with HbA1c of T2D. SOD activity showed negative relationship with FPG, HbA1c in T2D, and FPG in PDM (Table 3).
Table 3.
Correlation coefficients (r) between glycaemic control parameters, plasma TAC, and other antioxidant markers of T2D and PDM groups (n = 194).
(a).
| Parameter | T2D group (n = 147) | |||||
|---|---|---|---|---|---|---|
| Ln FRAP | TEAC | ORAC | GPx | SOD | MDA | |
| FPG | -0.08 | 0.08 | -0.20∗ | 0.02 | -0.20∗ | 0.20∗ |
| Ln HbA1c | 0.10 | 0.02 | -0.20∗ | 0.20∗ | -0.20∗ | 0.02 |
| HOMA-IR | 0.15 | -0.14 | 0.13 | -0.08 | -0.06 | -0.13 |
(b).
| Parameter | PDM group (n = 47) | |||||
|---|---|---|---|---|---|---|
| Ln FRAP | Ln TEAC | Ln ORAC | GPx | SOD | MDA | |
| FPG | -0.07 | -0.28∗ | 0.11 | 0.14 | 0.15∗ | 0.14 |
| Ln HbA1c | -0.30∗ | 0.30∗ | -0.10 | 0.06 | 0.12 | 0.03 |
| HOMA-IR | 0.20∗ | -0.18 | -0.30∗ | 0.11 | 0.04 | 0.24∗ |
∗Correlations significant at P < 0.05. FPG: fasting plasma glucose; HbA1c: glycated haemoglobin level; HOMA-IR: insulin resistance; PDM: prediabetics; T2D: type 2 diabetics.
Effects of glycaemic control on plasma TAC and other antioxidant markers are given in Table 4. PDM subgroup with good glycaemic control had significantly (P < 0.05) lower TEAC compared to all the other subgroups. PDM subgroup without good glycaemic control and NG group had significantly (P < 0.05) higher plasma TEAC than T2D and PDM subgroups with good glycaemic control. A significantly (P < 0.05) higher ORAC was observed in NG compared to T2D subgroups with and without good glycaemic control (Table 4).
Table 4.
Plasma total antioxidant capacity and antioxidant markers of subgroups with and without good glycaemic control (n = 300).
| Variable | T2D | PDM | NG (n = 106) | P 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (+) GC (n = 97) | (-) GC (n = 50) | (+) GC (n = 31) | (-) GC (n = 16) | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Plasma TAC | |||||||||||
| FRAP (μmol/L) | 773 | 181 | 812 | 177 | 883 | 205 | 813 | 220 | 847 | 215 | 0.317 |
| TEAC (μmol/L) | 1200a | 440 | 1240ab | 650 | 1120c | 450 | 1320b | 446 | 1400b | 490 | 0.007 |
| ORAC (μmol/L) | 4509a | 15182 | 4146a | 1299 | 4594ab | 2051 | 4476ab | 2093 | 5156b | 1844 | 0.004 |
| Antioxidant markers | |||||||||||
| Uric acid (μmol/L) | 288a | 75 | 278a | 70 | 384b | 135 | 429b | 240 | 387b | 146 | 0.005 |
| Albumin (g/L) | 5.8 | 1.0 | 5.8 | 1.0 | 5.5 | 1.2 | 5.4 | 1.0 | 5.7 | 1.9 | 0.254 |
| Bilirubin (g/L) | 0.90 | 0.24 | 0.80 | 0.22 | 0.81 | 0.20 | 0.90 | 0.80 | 0.90 | 0.80 | 0.323 |
| Total protein (g/L) | 7.1a | 1.4 | 7.2a | 1.6 | 8.0b | 1.7 | 7.6b | 1.8 | 6.5c | 1.2 | 0.007 |
1One-way ANOVA. Means with the different superscripts in a given row are significantly different (Tukey's post hoc t-test). (+) GC: with good glycaemic control; (-) GC: without good glycaemic control (poor glycaemic control); FRAP: ferric reducing ability of plasma; NG: normoglycaemic; ORAC: oxygen radical absorptive capacity; PDM: prediabetics; TEAC: Trolox equivalent antioxidant capacity; T2D: type 2 diabetics.
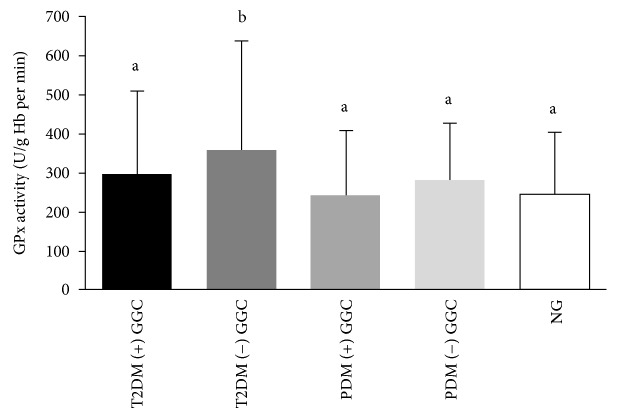
Effects of glycaemic control on erythrocyte GPx and SOD activities and plasma MDA concentration are illustrated in Figures 1–3, respectively. T2D subgroup without good glycaemic control had significantly higher GPx activity compared with the PDM subgroups with and without good glycaemic control and the NG group (Figure 1).
Figure 1.
Erythrocyte GPx activity among subgroups with good glycaemic control ((+) GC) and without good glycaemic control ((-) GC) of T2D, PDM, and NG study groups. Means with the different superscripts are significantly different at P < 0.05 (Tukey's post hoc test).
Figure 2.
Erythrocyte SOD activity among subgroups with good glycaemic control ((+) GC) and without good glycaemic control ((-) GC) of T2D, PDM, and NG study groups. Means with the different superscripts are significantly different at P < 0.05 (Tukey's post hoc test).
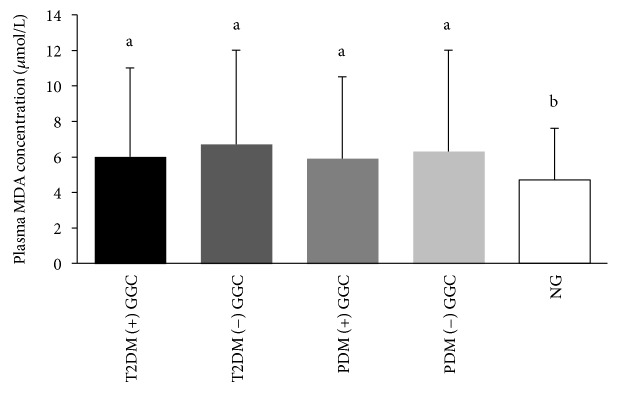
Figure 3.
Plasma MDA concentration among subgroups with good glycaemic control ((+) GC) and without good glycaemic control ((-) GC) of T2DM, PDM, and NG study groups. Means with the different superscripts are significantly different at P < 0.05 (Tukey's post hoc test).
PDM subgroups had the highest SOD activity, and they were significantly higher than T2D and NG. The lowest SOD activity was observed in T2D subgroups, and it was significantly lower than other subgroups of PDM and NG (Figure 2).
There was no significant effect of glycaemic control on plasma MDA concentration. However, the NG group had the significantly lowest level of plasma MDA compared to T2D and PDM subgroups with and without good glycaemic control (Figure 3).
4. Discussion
Both T2D and PDM groups had lower plasma TAC compared with the NG subjects. Hyperglycaemia induces the excess ROS generation thus depleting the plasma TAC in T2D and PDM [30–33]. T2D and PDM subgroups with poor glycaemic control had depleted plasma TAC compared to the NG similar to previous studies [9, 31].
T2D and PDM subjects exhibited higher levels of the plasma lipid peroxidation marker, MDA. The rise in plasma MDA among T2D has been previously reported similar to our findings [34, 35]. Hyperglycaemia promotes ROS-mediated lipid peroxidation via nonenzymatic and autoglycation pathways [1, 7]. Hyperlipidemia experienced by the T2D and PDM could be one of the reasons for increased production of lipid peroxides [7]. Although we could not obtain significantly different MDA levels in T2D with good and poor glycaemic control, previous authors have demonstrated raised MDA levels among T2D with poor glycaemic control [35, 36]. It also highlights the presence of lipid peroxidation and systemic inflammation that contributes to the metabolic imbalance even in well-controlled T2D as suggested by previous authors [37, 38].
Hence, depleted plasma TAC and increased MDA represent the presence of oxidative stress in T2D and PDM of the present study. MDA levels of T2D and PDM were positively correlated with fasting plasma glucose and HOMA-IR and therefore show the association of hyperglycaemia with oxidative stress. It further verifies that persistent hyperglycaemia promotes ROS production, leading to lipid peroxidation and depletion of endogenous antioxidants demonstrating the increased oxidative stress in T2D [39]. Differences in sample sizes of T2D and PDM and use of oral hypoglycaemic agents only by the T2D might have contributed to the identical HbA1c levels in T2D and PDM of the present study.
Erythrocyte GPx activity has elevated in T2D and PDM and particularly the T2D and PDM with poor glycaemic control. GPx responses in T2D were controversial [15, 40–42]. Our findings on GPx activity are in agreement with previous authors [40, 41]. GPx is thought to represent the initial protective response required for adjusting hydrogen peroxide concentration in normal physiological conditions and after oxidative insult [43]. In the present study, the rise of erythrocyte GPx activity in T2D and PDM was parallel to that of MDA. Therefore, we can hypothesize that the excess hydrogen peroxide generation resulted from lipid peroxidation in T2D and PDM, which may have stimulated the GPx activity.
Further, T2D and PDM with poor glycaemic control showed the higher GPx activity parallel to the MDA levels. T2D with poor glycaemic control had the highest GPx activity in the present study. As the primary function of GPx is to achieve the hydrogen peroxide balance, once the hydrogen peroxide production is exaggerated via the lipid peroxidation pathway, GPx activity would have been raised. It suggests an adaptive response against the excess lipid peroxidation and accumulation of hydrogen peroxide in a dysglycaemic state [44]. Therefore, the T2D with poor glycaemic control tend to exhibit the higher GPx activity among all groups. Positive correlations existed between HbA1c and GPx activity; FPG and MDA of T2D further verify the above findings.
In contrast to the GPx activity, SOD activity was intensely reduced in T2D irrespective of their glycaemic control. PDM had the highest erythrocyte SOD activity in the present study. Blunting of the SOD activity due to the poor glycaemic control has already been studied and proven by previous studies [15, 45, 46]. Our findings are also in agreement with previous findings [15, 45, 46] as shown by negative relationship between the HbA1c and SOD activity in T2D. Low activity of the SOD can be partly explained by the enzymatic glycation of SOD due to the higher circulating levels of glucose [15]. Nearly 50% of SOD in diabetics are glycated and made the SOD inactive [47]. In addition, excess hydrogen peroxide produced in lipid peroxidation and autooxidation of glucose has contributed to the lower SOD activity in T2D [48].
However, the upsurge of SOD among PDM suggests an endogenous adaptive response on metabolic alterations underneath the prediabetic stage [49]. Less exposure to the hyperglycaemic spikes by the PDM than T2D might have not suppressed the SOD activity. Increased production of superoxide radical and an accompanied rise of hydrogen peroxide may be the reason for exaggerated SOD activity in PDM. A proposed mechanism is further supported by the increased GPx activity in PDM.
Hence, the upsurge of erythrocyte GPx activity in parallel to the lipid peroxidation in T2D and PDM and particularly in T2D with poor glycaemic control drives the adaptive response for counteracting the excess hydrogen peroxide and other free radicals generated. Glycation and excess accumulation of hydrogen peroxide have made the part of SOD inactive in T2D. The rise in SOD among PDM suggests the potential metabolic adaptation. Increased GPx activity in T2D and PDM and SOD activity in PDM suggest the concept of reductive stress as a temporary defense against the oxidative stress in hyperglycaemia.
Reductive stress followed by oxidative stress may serve as one of the major mechanisms of glucotoxicity in a persistent hyperglycaemic state [50]. Hence, the oxidative stress is believed to be originated from NADH-imposed reductive stress thus would remain temporary [51]. Attenuated hyperglycaemia-induced reductive stress may provide therapeutic approaches for preventing diabetes and diabetic complications [50].
Almost all of the T2D of the present study were on oral hypoglycaemic agents for glycaemic control. Metformin and gliazide drugs of the sulphonylurea group were shown to improve the antioxidant status of T2D [52, 53]. Further, the use of antihypertensive drugs [54, 55] and statins can [56, 57] enhance the antioxidant status of T2D. Therefore, the use of oral hypoglycaemic agents, particularly the metformin, antihypertensive drugs, and statins, might have contributed to the pronounced antioxidant activities shown by T2D. However, the life style pattern (dietary and physical activity patterns) of subjects may have exerted beneficial effects on antioxidant status. Therefore, it would be further useful to assess the contribution of the life style pattern on antioxidant status.
5. Conclusions
T2D and PDM had depleted plasma TAC compared to the NG. It suggests that the hyperglycaemia induced ROS generation and overuse of endogenous antioxidants available in plasma. Poor glycaemic control has further shown to deteriorate the plasma TAC of T2D and PDM. Lower plasma TAC and higher plasma MDA levels further confirm the presence of oxidative stress in T2D and PDM. Increased erythrocyte GPx activity found in T2D and PDM and further rise of GPx in T2D and PDM with poor glycaemic control suggest a compensatory adaptive response for poor glycaemic control and accompanied excess lipid peroxidation. Hence, the parallel rise of the lipid peroxidation marker and GPx activity is seen. Decline in SOD activity advocates the excess hydrogen peroxide generation due to glucose autoxidation, lipid peroxidation, and glycation present in hyperglycaemic status.
Acknowledgments
All the subjects are kindly acknowledged for their voluntary participation. The authors would like to acknowledge the National Research Council of Sri Lanka (Grant No.: 09-48) and Wayamba University of Sri Lanka Research Grant Scheme (SRHDC/RP/04/15-09) for financial assistance.
Data Availability
The biochemical and anthropometric data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
All authors declare that there is no conflict of interest.
References
- 1.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 2.Bruce C. R., Carey A. L., Hawley J. A., Febbraio M. A. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52(9):2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A., Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(5):816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 4.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circulation Research. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) The Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 6.Holman R. R., Paul S. K., Bethel M. A., Neil H. A. W., Matthews D. R. Long-term follow-up after tight control of blood pressure in type 2 diabetes. The New England Journal of Medicine. 2008;359(15):1565–1576. doi: 10.1056/NEJMoa0806359. [DOI] [PubMed] [Google Scholar]
- 7.Kesavulu M. M., Giri R., Kameswara Rao B., Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes & Metabolism. 2000;26(5):387–392. [PubMed] [Google Scholar]
- 8.Song F., Jia W., Yao Y., et al. Oxidative stress, antioxidant status and DNA damage in patients with impaired glucose regulation and newly diagnosed type 2 diabetes. Clinical Science. 2007;112(12):599–606. doi: 10.1042/CS20060323. [DOI] [PubMed] [Google Scholar]
- 9.Lodovici M., Giovannelli L., Pitozzi V., Bigagli E., Bardini G., Rotella C. M. Oxidative DNA damage and plasma antioxidant capacity in type 2 diabetic patients with good and poor glycaemic control. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2008;638(1-2):98–102. doi: 10.1016/j.mrfmmm.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Surapaneni K. M., Venkataramana G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with osteoarthritis. Indian Journal of Medical Sciences. 2007;61(1):9–14. doi: 10.4103/0019-5359.29592. [DOI] [PubMed] [Google Scholar]
- 11.Arif M., Islam M. R., Waise T. M. Z., Hassan F., Mondal S. I., Kabir Y. DNA damage and plasma antioxidant indices in Bangladeshi type 2 diabetic patients. Diabetes & Metabolism. 2010;36(1):51–57. doi: 10.1016/j.diabet.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Fujita H., Sakamoto T., Komatsu K., et al. Reduction of circulating superoxide dismutase activity in type 2 diabetic patients with microalbuminuria and its modulation by telmisartan therapy. Hypertension Research. 2011;34(12):1302–1308. doi: 10.1038/hr.2011.127. [DOI] [PubMed] [Google Scholar]
- 13.Rani A. J., Mythili S. V. Study on total antioxidant status in relation to oxidative stress in type 2 diabetes mellitus. Journal of Clinical and Diagnostic Research. 2014;8(3):108–110. doi: 10.7860/JCDR/2014/7603.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisalkar P., Patne A., Karnik A., Fawade M., Mumbare S. Ferric reducing ability of plasma with lipid peroxidation in type 2 diabetes. International Journal of Pharmacy and Biological Sciences. 2012;2(2):53–56. [Google Scholar]
- 15.Kumawat M., Sharma T. K., Singh I., et al. Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. North American Journal of Medical Sciences. 2013;5(3):213–219. doi: 10.4103/1947-2714.109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaji H., Kurasaki M., Ito K., et al. Increased lipoperoxide value and glutathione peroxidase activity in blood plasma of Type 2 (non-insulin-dependent) diabetic women. Klinische Wochenschrift. 1985;63(16):765–768. doi: 10.1007/BF01733829. [DOI] [PubMed] [Google Scholar]
- 17.Kimura F., Hasegawa G., Obayashi H., et al. Serum extracellular superoxide dismutase in patients with type 2 diabetes: Relationship to the development of micro- and macrovascular complications. Diabetes Care. 2003;26(4):1246–1250. doi: 10.2337/diacare.26.4.1246. [DOI] [PubMed] [Google Scholar]
- 18.Jandric-Balen M., Bozikov B., Jadranka B., Metelko Z., Jandric I., Romic Z. Impact of glycaemic control on antioxidant enzyme activity in patients with type 2 diabetes mellitus. Diabetologia Croatica. 2004;33(4):131–135. [Google Scholar]
- 19.Savu O., Ionescu-Tirgoviste C., Atanasiu V., Gaman L., Papacocea R., Stoian I. Increase in total antioxidant capacity of plasma despite high levels of oxidative stress in uncomplicated type 2 diabetes mellitus. Journal of International Medical Research. 2012;40(2):709–716. doi: 10.1177/147323001204000235. [DOI] [PubMed] [Google Scholar]
- 20.Kharroubi A. T., Darwish H. M., Akkawi M. A., et al. Total antioxidant status in type 2 diabetic patients in Palestine. Journal of Diabetes Research. 2015;2015:7. doi: 10.1155/2015/461271.461271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarah N., Anaja H., Akuyam S., Bakari G. Serum total antioxidant status in type 2 diabetic Nigerians. IOSR Journal of Nursing & Health Sciences. 2014;3(1):61–65. [Google Scholar]
- 22.WHO Expert Consultation. Definitions and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. 2006.
- 23.Benzie I. F. F., Strain J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 24.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26(9-10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 25.Cao G., Verdon C. P., Wu A. H., Wang H., Prior R. L. Automated assay of oxygen radical absorbance capacity with the COBAS FARA II. Clinical Chemistry. 1995;41(12) Part 1:1738–1744. [PubMed] [Google Scholar]
- 26.Jentzsch A. M., Bachmann H., Fürst P., Biesalski H. K. Improved analysis of malondialdehyde in human body fluids. Free Radical Biology and Medicine. 1996;20(2):251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 27.Seigler L., Wu W. T. Separation of serum high-density lipoprotein for cholesterol determination: ultracentrifugation vs precipitation with sodium phosphotungstate and magnesium chloride. Clinical Chemistry. 1981;27(6):838–841. [PubMed] [Google Scholar]
- 28.Friedewald W., Levy R., Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 29.International Diabetes Federation. International Diabetes Federation consensus worldwide definition of the metabolic syndrome. 2003.
- 30.Opara E. C., Abdel-Rahman E., Soliman S., et al. Depletion of total antioxidant capacity in type 2 diabetes. Metabolism. 1999;48(11):1414–1417. doi: 10.1016/S0026-0495(99)90152-X. [DOI] [PubMed] [Google Scholar]
- 31.Bigagli E., Raimondi L., Mannucci E., et al. Lipid and protein oxidation products, antioxidant status and vascular complications in poorly controlled type 2 diabetes. The British Journal of Diabetes & Vascular Disease. 2012;12(1):33–39. doi: 10.1177/1474651411435588. [DOI] [Google Scholar]
- 32.Mohieldein A. H., Hasan M., al-Harbi K. K., Alodailah S. S., Azahrani R. M., al-Mushawwah S. A. Dyslipidemia and reduced total antioxidant status in young adult Saudis with prediabetes. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2015;9(4):287–291. doi: 10.1016/j.dsx.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Pieme C. A., Tatangmo J. A., Simo G., et al. Relationship between hyperglycemia, antioxidant capacity and some enzymatic and non-enzymatic antioxidants in African patients with type 2 diabetes. BMC Research Notes. 2017;10(1):p. 141. doi: 10.1186/s13104-017-2463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhutia Y., Ghosh A., Sherpa M. L., Pal R., Mohanta P. K. Serum malondialdehyde level: surrogate stress marker in the Sikkimese diabetics. Journal of Natural Science, Biology and Medicine. 2011;2(1):107–112. doi: 10.4103/0976-9668.82309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikekpeazu E. J., Neboh E. E., Ejezie F. E., Ibegbu M. D., Ike I. E. Oxidative stress and glycaemic control in type 2 diabetic patients in Enugu, South-East Nigeria. Annals of Medical and Health Sciences research. 2011;1(1):123–128. [PMC free article] [PubMed] [Google Scholar]
- 36.Manohar S. M., Vaikasuvu S. R., Deepthi K., Sachan A., Narasimha S. R. An association of hyperglycemia with plasma malondialdehyde and atherogenic lipid risk factors in newly diagnosed type 2 diabetic patients. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences. 2013;18(2):89–93. [PMC free article] [PubMed] [Google Scholar]
- 37.Cakatay U. Protein oxidation parameters in type 2 diabetic patients with good and poor glycaemic control. Diabetes & Metabolism. 2005;31(6):551–557. doi: 10.1016/S1262-3636(07)70230-6. [DOI] [PubMed] [Google Scholar]
- 38.de Souza Bastos A., Graves D. T., de Melo Loureiro A. P., et al. Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients. Journal of Diabetes and its Complications. 2016;30(8):1593–1599. doi: 10.1016/j.jdiacomp.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whiting P. H., Kalansooriya A., Holbrook I., Haddad F., Jennings P. E. The relationship between chronic glycaemic control and oxidative stress in type 2 diabetes mellitus. British Journal of Biomedical Science. 2008;65(2):71–74. doi: 10.1080/09674845.2008.11732800. [DOI] [PubMed] [Google Scholar]
- 40.Matkovics B., Varga S., Szabo L., Witas H. The effect of diabetes on the activities of the peroxide metabolism enzymes. Hormone and Metabolic Research. 1982;14(2):77–79. doi: 10.1055/s-2007-1018928. [DOI] [PubMed] [Google Scholar]
- 41.Rema M., Mohan V., Bhaskar A., Shanmugasundaram K. R. Does oxidant stress play a role in diabetic retinopathy? Indian Journal of Ophthalmology. 1995;43(1):17–21. [PubMed] [Google Scholar]
- 42.Gawlik K., Naskalski J. W., Fedak D., et al. Markers of antioxidant defense in patients with type 2 diabetes. Oxidative Medicine and Cellular Longevity. 2016;2016:6. doi: 10.1155/2016/2352361.2352361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y. S., Kim A. Y., Choi J. W., et al. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Molecular Endocrinology. 2008;22(9):2176–2189. doi: 10.1210/me.2008-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Likidlilid A., Patchanans N., Peerapatdit T., Sriratanasathavorn C. Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. Journal of the Medical Association of Thailand. 2010;93(6):682–693. [PubMed] [Google Scholar]
- 45.Jain S. K. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. The Journal of Biological Chemistry. 1989;264(35):21340–21345. [PubMed] [Google Scholar]
- 46.Michiels C., Raes M., Toussaint O., Remacle J. Importance of SE-glutathione peroxidase, catalase, and CU/ZN-SOD for cell survival against oxidative stress. Free Radical Biology and Medicine. 1994;17(3):235–248. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 47.Arai K., Iizuka S., Tada Y., Oikawa K., Taniguchi N. Increase in the glucosylated form of erythrocyte Cu-Zn-superoxide dismutase in diabetes and close association of the nonenzymatic glucosylation with the enzyme activity. Biochimica et Biophysica Acta (BBA) - General Subjects. 1987;924(2):292–296. doi: 10.1016/0304-4165(87)90025-0. [DOI] [PubMed] [Google Scholar]
- 48.Fajans S. Endocrinology Degroat L, 3rd Ed. USA: Saunders Co; 1995. Diabetes mellitus, definition, classification, tests; pp. 1411–1422. [Google Scholar]
- 49.Bandeira S. D. M., Guedes G. d. S., Fonseca L. J. S. d., et al. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and SOD activity. Oxidative Medicine and Cellular Longevity. 2012;2012:13. doi: 10.1155/2012/819310.819310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan L. J. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. Journal of Diabetes Research. 2014;2014:11. doi: 10.1155/2014/137919.137919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teodoro J. S., Rolo A. P., Palmeira C. M. The NAD ratio redox paradox: why does too much reductive power cause oxidative stress? Toxicology Mechanisms and Methods. 2013;23(5):297–302. doi: 10.3109/15376516.2012.759305. [DOI] [PubMed] [Google Scholar]
- 52.Signorini A. M., Fondelli C., Renzoni E., Puccetti C., Gragnoli G., Giorgi G. Antioxidant effects of gliclazide, glibenclamide, and metformin in patients with type 2 diabetes mellitus. Current Therapeutic Research. 2002;63(7):411–420. doi: 10.1016/S0011-393X(02)80047-9. [DOI] [Google Scholar]
- 53.Esteghamati A., Eskandari D., Mirmiranpour H., et al. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: a randomized clinical trial. Clinical Nutrition. 2013;32(2):179–185. doi: 10.1016/j.clnu.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Chandran G., Sirajudeen K. N. S., Nik Yusoff N. S., Swamy M., Samarendra M. S. Effect of the antihypertensive drug enalapril on oxidative stress markers and antioxidant enzymes in kidney of spontaneously hypertensive rat. Oxidative Medicine and Cellular Longevity. 2014;2014:10. doi: 10.1155/2014/608512.608512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suliburska J., Krejpcio Z., Staniek H., et al. The effects of antihypertensive drugs on chromium status, glucose metabolism, and antioxidant and inflammatory indices in spontaneously hypertensive rats. Biological Trace Element Research. 2014;157(1):60–66. doi: 10.1007/s12011-013-9864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davignon J., Jacob R. F., Mason R. P. The antioxidant effects of statins. Coronary Artery Disease. 2004;15(5):251–258. doi: 10.1097/01.mca.0000131573.31966.34. [DOI] [PubMed] [Google Scholar]
- 57.Stoll L. L., McCormick M. L., Denning G. M., Weintraub N. L. Antioxidant effects of statins. Drugs of Today. 2004;40(12):975–990. doi: 10.1358/dot.2004.40.12.872573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The biochemical and anthropometric data used to support the findings of this study are available from the corresponding author upon request.