Abstract
Objective
Inter-alpha-trypsin inhibitor heavy chain H3 (ITIH3) and inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4) are heavy chains of protein members belonging to the ITI family, which was associated with inflammation and carcinogenesis. However, the diagnostic value of ITIH3 and ITIH4 in human colorectal cancer (CRC) remains unknown.
Methods
In total, 101 CRC patients and 156 healthy controls were enrolled. The concentrations of ITIH3 and ITIH4 proteins in plasma samples of participants were assessed using enzyme-linked immunosorbent assay. ITIH3 and ITIH4 expressions in human CRC tissues were additionally assessed via immunohistochemical staining (IHC). Receiver operating characteristic (ROC) was applied to estimate the diagnostic power of the two proteins, and the net reclassification improvement (NRI) was adopted to evaluate the incremental predictive ability of ITIH3/ITIH4 when added to the tissue inhibitor of metalloproteinase-1 (TIMP-1).
Results
The plasma concentration of ITIH3 in CRC patients (median: 4.370 μg/mL; range: 2.152–8.170 μg/mL) was significantly lower than that in healthy subjects (median: 4.715 μg/mL; range: 2.665–10.257 μg/mL; p < 0.001), while the ITIH4 plasma level in subjects with CRC (median: 0.211 μg/mL; range: 0.099–0.592 μg/mL) was markedly increased relative to that in the control group (median: 0.134 μg/mL; range: 0.094–0.460 μg/mL, p < 0.001). Consistently, IHC score assessment showed a dramatic reduction in ITIH3 expression and, conversely, upregulation of ITIH4 in colorectal carcinoma specimens relative to adjacent normal colorectal tissues (p < 0.001 in both cases). The area under the curve (AUC) of the ROC for ITIH4 (AUC = 0.801, 95% CI: 0.745–0.857) was higher than that for ITIH3 (AUC = 0.638, 95% CI: 0.571–0.704, both p values < 0.001). The AUC of the ROC for combined ITIH3 and ITIH4 was even higher than that for carcinoembryonic antigen. NRI results showed that combining ITIH3 and ITIH4 with TIMP-1 significantly improved diagnostic accuracy (NRI = 17.12%, p = 0.002) for CRC patients compared to TIMP-1 alone.
Conclusions
Circulating ITIH3 and ITIH4 levels are associated with carcinogenesis in CRC, supporting their potential diagnostic utility as surrogate biomarkers for colorectal cancer detection.
1. Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer type in both men and women and the fourth leading cause of cancer-related mortality worldwide [1–3], causing more than 50,000 deaths in the USA each year [4]. The recent years have seen a continuing increase in the incidence and mortality of CRC in China [5, 6].
CRC often appears to develop and progress slowly over the years. In many cases, there is an initial noninvasive polyp stage in the setting of chronic inflammation, which presents a more convenient step for prevention screening relative to many other solid malignancies. Current screening strategies, such as the fecal occult blood test (FOBT), fecal immunochemical testing, and colonoscopy, have improved the effectiveness of CRC detection [7]. However, on average, only 65% of the elderly population have undergone CRC screening tests in the United States [8]. An effective alternative strategy may be to develop more specific biomarkers detectable in the peripheral blood for accurate and reliable detection of CRC.
The inter-alpha-trypsin inhibitor (ITI) family proteins which were originally isolated from human plasma are plasma serine protease inhibitor proteins [9]. ITIs are composed of one light chain (bikunin) and five homologous heavy chains [10]. The inter-alpha-trypsin inhibitor heavy chains (ITIHs) are involved in inflammation as well as tumorigenic and metastatic processes. The proteins are covalently linked to hyaluronic acid (HA), a major component of the extracellular matrix. Since HA linking and extracellular matrix stability are strongly dependent on ITIHs, dysregulation of ITIH family members could influence the vascularization process during tumor development [11]. In two proteomic studies, ITIH3/ITIH4, as one of the serum differential proteins, was detected from the patients of hepatocellular cancer or gastric cancer [12, 13], indicating a potential relation of ITIH3/ITIH4 to digestive system cancers. It has been reported that ITIH3 and ITIH4 serve as candidate plasma proteins indicative of early-stage intestinal cancer in a mouse model [14]. Additionally, ITIH4 was shown to be upregulated in plasma of mice with severe colitis [15] and had high sensitivity and specificity in the identification of early-stage colon adenoma [16]. Furthermore, the ITIH4 level was significantly elevated in THE serum of patients growing early colorectal adenomas, which was identified as a premalignant state [17]. Considering that there have been no studies aiming at ITIH3/ITIH4 in the human plasma of CRC, we therefore selected the two proteins as plasma biomarkers to determine their association with CRC risk.
In this case control study, we examined the levels of ITIH3 and ITIH4 proteins in human plasma and evaluated their diagnostic value as biomarkers for CRC. Expressions of ITIH3 and ITIH4 proteins in colorectal cancer and adjacent normal tissues were additionally examined via immunohistochemical (IHC) analysis.
2. Materials and Methods
2.1. Participants
In total, 101 patients diagnosed with CRC and treated between January 2017 and July 2018 at the Dalian Municipal Central Hospital Affiliated to Dalian Medical University (Dalian, China) participated in the study. CRC was diagnosed based on pathological findings from tissue specimens of patients. Staging information was determined histopathologically and combined with various imaging modalities, such as computed tomography, magnetic resonance imaging, and clinical information. According to disease stages based on the American Joint Committee on Cancer staging system [18], colorectal cancer patients were divided into two subgroups: nonmetastatic (stages I to III) and metastatic (stage IV).
The 156 healthy controls were randomly selected from participants subjected to health screening during the same period at the Medical Examination Center of Dalian Municipal Central Hospital. Our study was conducted with the human subjects' understanding and consent and was approved by the ethics committee of Dalian Municipal Central Hospital (no. YN2017-034-01). All work was carried out in accordance with the Helsinki declaration.
2.2. Colorectal Cancer Plasma and Tissue Samples
All 257 blood samples used in the study were collected into EDTA-containing tubes, which were centrifuged at 3000 × g for 15 min to separate blood cells. Plasma was collected into another tube and stored at −80°C until experimental use.
For immunohistochemical analysis, matched malignant and adjacent normal colorectal tissues were obtained from patients who accepted surgery. Tissue specimens were fixed in 10% buffered formalin solution, dehydrated, and embedded in paraffin.
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) for ITIH3, ITIH4, and Tissue Inhibitor of Metalloproteinase-1 (TIMP-1) Plasma Levels
The plasma levels of ITIH3, ITIH4, and TIMP-1 were detected independently by two researchers. Commercial ELISA kits (Lifespan Biosciences Inc., Seattle, WA, USA) were employed for identifying the plasma concentrations of ITIH3 (catalog no. LS-F7346), ITIH4 (catalog no. LS-F6535), and TIMP-1 (catalog no. LS-F24684). All steps were conducted according to the manufacturer's instructions: (1) Standard, blank, and experimental samples (pretreated with sample diluents) were added to individual wells of 96-well microplates precoated with the target-specific capture antibodies (anti-ITIH3 or anti-ITIH4) and incubated for 1 h at 37°C. (2) After aspirating the liquid of each well, a biotin-conjugated detection secondary antibody was added to the wells and incubated for 1 h at 37°C. (3) The liquid was aspirated again and washed three times per well with Wash Buffer solution. Next, the fluid in each well was removed, and after washing three times with Wash Buffer, each well was filled with HRP conjugate for 30 min at 37°C. (4) Similarly, after washing at least five times, wells were incubated with tetramethyl benzidine (TMB) substrate for 10–20 min at 37°C. (5) Finally, 50 μL stop solution was used to terminate the color reaction, and the optical density value of each well was determined immediately using a microplate reader at a wavelength of 450 nm. The concentrations of ITIH3 and ITIH4 (μg/mL) in each well were calculated using the standard curve.
2.4. Determination of Carcinoembryonic Antigen (CEA) Plasma Concentrations
CEA is the most widely used biomarker for CRC in the clinical setting. The CEA plasma level was analyzed using the specific electrochemiluminescence immunoassay and measured by the Roche Cobas e601 system (Roche Diagnostics Inc., Indianapolis, IN, USA).
2.5. IHC Staining
Paraffin block-embedded human tissues were cut into 5 μm sections using a microtome. IHC was conducted with primary ITIH3 (Proteintech Group Inc., Chicago, IL, USA, catalog no. 21247-1-AP) and ITIH4 (Proteintech Group Inc., Chicago, IL, USA, catalog no. 24069-1-AP) antibodies at a 1 : 500 dilution. The antigen-antibody complex was visualized using diaminobenzidine chromogen. The immunoreactivity intensity of ITIH3 or ITIH4 in cancer and adjacent normal colorectal tissues was evaluated via light microscopy. Immunohistochemical staining intensity of ITIH3 or ITIH4 was scored as negative (0), weak (1), moderate (2), and strong (3), and the percentage of positive cells as 5% (0), 5–30% (1), 31–50% (2), and >50% (3); the IHC score of each slide was calculated by multiplying these two values (ranging from 0 to 9). This is the method of Zhao et al., and the method description partly reproduces their wording [19]. All individuals who donated tissues for this study provided written informed consent. A total of 20 colorectal carcinoma and adjacent normal colorectal tissue specimens were analyzed.
2.6. Statistical Analysis
Statistical analysis was performed with SPSS software (SPSS Inc., SPSS Standard version 22.0, Chicago, IL, USA) and GraphPad Prism (GraphPad Software Inc., GraphPad Prism version 5.01, La Jolla, CA, USA). Data which did not follow normal distribution based on the Kolmogorov-Smirnov test are presented as median values with ranges. Nonparametric statistical analyses (the Mann–Whitney U or the Kruskal-Wallis test) were used to compare the differences between two independent groups. The ages of individuals in the two groups, which followed a normal distribution, were presented as the mean and standard deviation (SD) and compared using Student's t-test. Pearson's chi-squared test was adopted to evaluate the differences in characteristics of patients compared with healthy controls. Receiver operating characteristic (ROC) curves were generated to estimate the sensitivity and specificity of the biomarkers in diagnosing CRC. A binomial logistic regression model was fitted to combine the diagnostic performance of different biomarkers. The net reclassification improvement (NRI) was applied to estimate the incremental predictive ability of ITIH3/ITIH4 based when added to TIMP-1. The value of NRI is the overall reclassification sum of differences, in proportions of individuals reclassified upward minus the proportion reclassified downward for people who developed events and the proportion of individuals moving downward minus the proportion moving upward for those who did not develop events, and the statistical significance of the overall improvement is assessed with an asymptotic test, as described by Pencina et al. [20]. The survival rates were calculated by the Kaplan-Meier method, and differences between survival curves were analyzed by the log-rank tests. IHC scores in different groups were compared using a paired t-test. A two-sided probability value of less than 0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics of Patients and Controls
The clinicopathologic characteristics of patients and control subjects are described in Table 1. A total of 101 patients (57 male and 44 female) with CRC were diagnosed between January 2017 and July 2018 at the Dalian Municipal Central Hospital Affiliated to Dalian Medical University. The mean patient age was 61.089 ± 8.505 years. One hundred and fifty-six noncancer subjects of similar ages with the same ethnicity were selected from the checkup population of the hospital as the normal control group. The mean age of the control group was 59.359 ± 7.792 years. No significant differences between the patient and control groups were identified in terms of sex (p = 0.363), age (p = 0.095), smoking (p = 0.172), or drinking (p = 0.345) status.
Table 1.
The baseline characteristics of patients with colorectal cancer and healthy controls.
| Characteristics | CRC patients | Healthy controls | p value∗ |
|---|---|---|---|
| (n = 101) | (n = 156) | ||
| Gender | 0.363 | ||
| Female | 44 | 77 | |
| Male | 57 | 79 | |
| Age | 0.095 | ||
| Mean ± SD | 61.089 ± 8.505Δ | 59.359 ± 7.792Δ | |
| Smoking status | 0.172 | ||
| Yes | 58 | 76 | |
| No | 43 | 80 | |
| Drinking status | 0.345 | ||
| Yes | 47 | 82 | |
| No | 54 | 74 | |
| Tumor stage (AJCC) | |||
| Stage I | 10 | ||
| Stage II | 15 | ||
| Stage III | 19 | ||
| Stage IV | 57 |
∗ p < 0.05: statistically significant. ΔYears are presented as mean ± SD. CRC: colorectal cancer.
3.2. Relationship between Plasma ITIH3 and ITIH4 Expression Patterns of CRC Patients and Clinicopathological Features of Tumors
The concentrations of ITIH3 and ITIH4 were estimated in all preoperative plasma samples with the aid of ELISA. Moreover, to determine the effects of clinicopathological features on the concentration of ITIH3 or ITIH4 in the case group, the associations between ITIH3 and ITIH4 concentrations and clinicopathological features were analyzed in CRC patients. No statistical significances were detected in the mean plasma levels of ITIH3 or ITIH4 between various subgroups stratified by clinical characteristics, and all corresponding p values were greater than 0.05 (Table 2). Our results suggest that clinical features have no obvious influence on the plasma ITIH3 or ITIH4 concentrations in CRC patients.
Table 2.
Serum levels of biomarkers tested in CRC patients in relation to clinicopathological features of tumor.
| Variable analyzed | No. | ITIH3 (μg/mL) | p value∗ | ITIH4 (μg/mL) | p value∗ |
|---|---|---|---|---|---|
| Median (range) | Median (range) | ||||
| Age | 0.830 | 0.586 | |||
| ≤60 | 48 | 4.429 (2.152–8.170) | 0.205 (0.110–0.592) | ||
| >60 | 53 | 4.370 (2.852–8.070) | 0.213 (0.099–0.415) | ||
| Gender | 0.133 | 0.574 | |||
| Male | 57 | 4.170 (2.152–8.170) | 0.216 (0.106–0.592) | ||
| Female | 44 | 4.577 (2.862–7.098) | 0.203 (0.099–0.422) | ||
| Smoking | 0.183 | 0.452 | |||
| Yes | 58 | 4.204 (2.162–8.170) | 0.204 (0.103–0.592) | ||
| No | 43 | 4.569 (2.152–8.070) | 0.221 (0.099–0.409) | ||
| Alcohol | 0.734 | 0.240 | |||
| Yes | 47 | 4.260 (2.152–8.170) | 0.222 (0.103–0.592) | ||
| No | 54 | 4.411 (2.352–7.098) | 0.207 (0.099–0.421) | ||
| Tumor localization | 0.771 | 0.446 | |||
| Rectum | 34 | 4.266 (2.152–8.170) | 0.206 (0.106–0.429) | ||
| Colon | 67 | 4.385 (2.162–8.070) | 0.211 (0.099–0.592) | ||
| Tumor size | 0.307 | 0.872 | |||
| ≤3 cm | 45 | 4.576 (2.352–8.170) | 0.210 (0.103–0.592) | ||
| >3 cm | 56 | 4.204 (2.152–8.070) | 0.212 (0.099–0.587) | ||
| Tumor stage (AJCC) | 0.404 | 0.278 | |||
| Stage I | 10 | 4.473 (3.820–4.820) | 0.185 (0.110–0.263) | ||
| Stage II | 15 | 4.585 (3.788–8.170) | 0.210 (0.127–0.315) | ||
| Stage III | 19 | 4.207 (2.162–6.898) | 0.213 (0.162–0.587) | ||
| Stage IV | 57 | 4.219 (2.152–8.070) | 0.219 (0.099–0.592) | ||
| Distant metastases | 0.261 | 0.617 | |||
| Nonmetastatic group | 44 | 4.512 (2.162–8.170) | 0.206 (0.110–0.587) | ||
| Metastatic group | 57 | 4.219 (2.152–8.070) | 0.219 (0.099–0.592) | ||
| MSI status | 0.680 | 0.692 | |||
| MSS | 55 | 4.556 (2.162–8.070) | 0.211 (0.099–0.592) | ||
| MSI-H | 8 | 4.401 (2.852–5.265) | 0.232 (0.127–0.409) | ||
| Unknown | 38 | 4.177 (2.152–8.170) | 0.207 (0.103–0.432) |
∗ p < 0.05: statistically significant. CRC: colorectal cancer; ITIH3: inter-alpha-trypsin inhibitor heavy chain H3; ITIH4: inter-alpha-trypsin inhibitor heavy chain H4; MSI: microsatellite instability status; MSS: microsatellite stable; MSI-H: microsatellite instability status high.
3.3. Significant Alterations in Plasma ITIH3 and ITIH4 Expressions in CRC Patients
Next, we evaluated the expression levels of ITIH3 and ITIH4 in the plasma of both CRC patients and normal control subjects to establish their utility as potential biomarkers for CRC detection. The statistical results for comparison of the ITIH3 or ITIH4 plasma concentrations between CRC patients and controls are shown in Table 3. The plasma ITIH3 level in CRC patients (median: 4.370 μg/mL; range: 2.152–8.170 μg/mL) was significantly lower than that in the control group (median: 4.715 μg/mL; range: 2.665–10.257 μg/mL; p < 0.001; Figure 1(a)). The median plasma levels of ITIH4 in colorectal cancer patients and healthy controls were 0.211 μg/mL (range: 0.099–0.592 μg/mL) and 0.134 μg/mL (range: 0.094–0.460 μg/mL), respectively. A box plot (Figure 1(b)) further revealed that ITIH4 expression in the plasma of CRC patients is significantly upregulated, compared with that in normal subjects (p < 0.001). Our data collectively suggest that plasma ITIH3 and ITIH4 may be useful as biomarkers for differentiating CRC patients from healthy subjects.
Table 3.
The serum concentrations of ITIH3 and ITIH4 between CRC patients and healthy subjects.
| Diagnosis | No. of cases | ITIH3 (μg/mL) | ITIH4 (μg/mL) | ||
|---|---|---|---|---|---|
| Median (range) | p value∗ | Median (range) | p value∗ | ||
| CRC patients | 101 | 4.370 (2.152–8.170) | p < 0.001∗ | 0.211 (0.099–0.592) | p < 0.001∗ |
| Healthy controls | 156 | 4.715 (2.665–10.257) | 0.134 (0.094–0.460) | ||
∗ p < 0.05: statistically significant. CRC: colorectal cancer; ITIH3: inter-alpha-trypsin inhibitor heavy chain H3; ITIH4: inter-alpha-trypsin inhibitor heavy chain H4.
Figure 1.
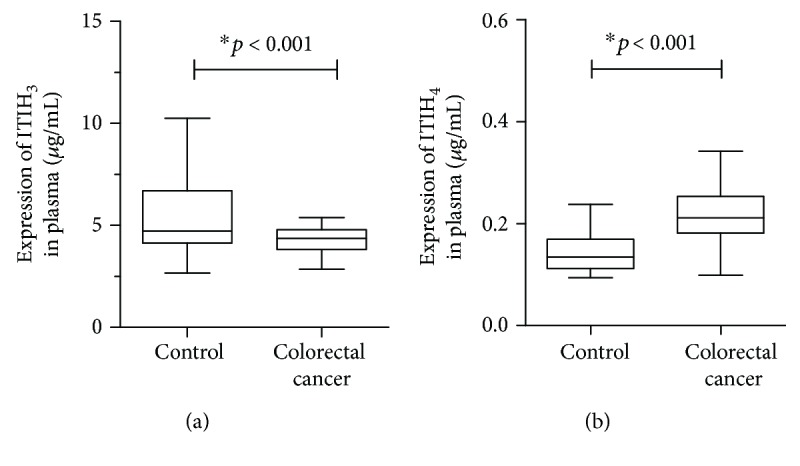
The plasma expression of inter-alpha-trypsin inhibitor heavy chain H3/H4 (ITIH3/ITIH4) in colorectal cancer (CRC) patients (n = 101) was compared with that of healthy subjects (n = 156). (a) The box plot showed the distributions of the plasma ITIH3 level in CRC patients and the normal controls. (b) The other box plot described the ITIH4 expression in the plasma of CRC patients relative to the normal subjects.
3.4. Diagnostic Efficiency of ITIH3/ITIH4 for CRC Patients
We conducted ROC curve analysis to determine the sensitivity and specificity of ITIH3 and ITIH4 in the detection of CRC. The ROC curve of CEA was additionally obtained to compare the efficacy of these two plasma proteins with that of the classical clinical biomarker in CRC diagnosis. Recently, emerging evidence has shown that TIMP-1 is a promising biomarker in the early diagnosis of CRC and more superior to CEA [21]. Therefore, we also conducted the ROC curve for TIMP-1 and consider it as an important comparison.
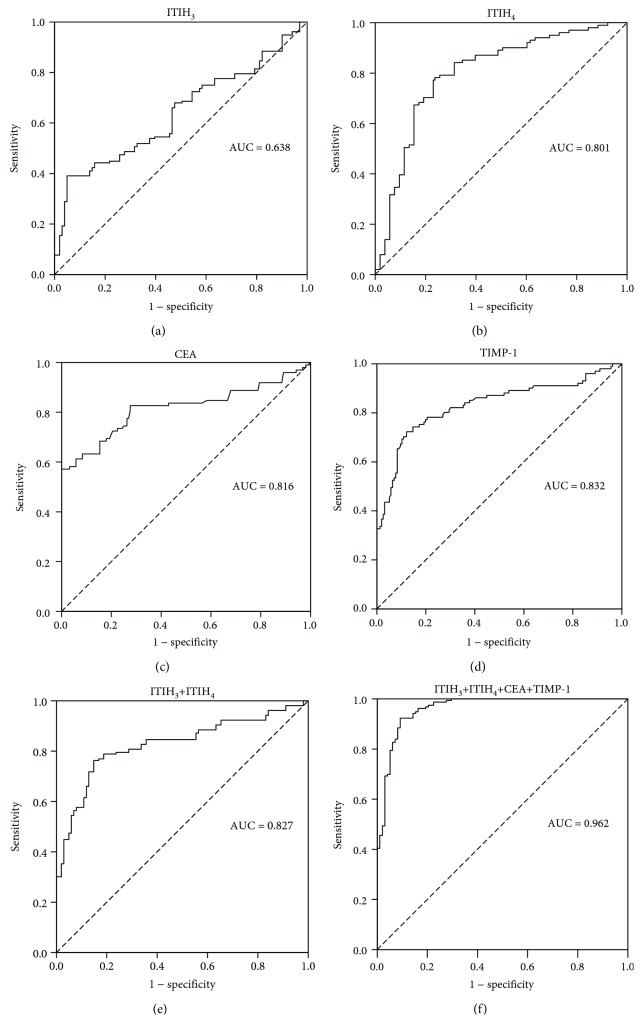
The area under the curve (AUC) for ITIH4 (AUC = 0.801, 95% confidence interval (CI): 0.745–0.857, p < 0.001) (Figure 2(b)) was higher than that for ITIH3 (AUC = 0.638, 95% CI: 0.571–0.704, p < 0.001) (Figure 2(a)) while those for CEA and TIMP-1 were 0.816 (95% CI: 0.754–0.878, p < 0.001) (Figure 2(c)) and 0.832 (95% CI: 0.776–0.888, p < 0.001) (Figure 2(d)).
Figure 2.
The receiver operating characteristic (ROC) curves were plotted for the biomarkers. (a) The ROC curve for ITIH3 (area under the curve (AUC) = 0.638). (b) The ROC curve for ITIH4 (AUC = 0.801). (c) The ROC curve for CEA (AUC = 0.816). (d) The ROC curve for TIMP-1 (AUC = 0.832). (e) The diagnostic accuracy of ITIH3 and ITIH4 combinations was assessed by a logistic regression model. The ROC curve for combined ITIH3 and ITIH4 (AUC = 0.827). (f) The combinations of CEA, TIMP-1, ITIH3, and ITIH4 yielded the highest diagnostic accuracy (AUC = 0.962).
Using a logistic regression model, the diagnostic capabilities of ITIH3 and ITIH4 were combined, generating a ROC curve, with an AUC of 0.827 (95% CI: 0.776–0.877, p < 0.001) (Figure 2(e)), which was even higher than that of CEA, indicating that the combined two biomarkers could be representative of greater effectiveness in disease diagnosis. In particular, the combined ROC analysis of TIMP-1, CEA, ITIH3, and ITIH4 revealed the highest diagnostic accuracy (AUC = 0.962, 95% CI: 0.940–0.985, p < 0.001) (Figure 2(f)) was at the cutoff value of 0.705, with the corresponding sensitivity of 0.917 and specificity of 0.908.
ROC analyses indicated that plasma ITIH3 and ITIH4 levels may be successfully employed to discriminate patients with CRC from control subjects and ITIH3/ITIH4 has the potential to serve as a diagnostic marker in colorectal cancer. All the results of ROC analysis are shown in Table 4.
Table 4.
The ROC curves for differentiating CRC patients from healthy subjects.
| Biomarker | No. of cases (CRC patients/controls) | AUC (95% CI) | Cutoff value | Sensitivity | Specificity | p value∗ |
|---|---|---|---|---|---|---|
| ITIH3 | 101/156 | 0.638 (0.571-0.704) | 4.441 (μg/mL) | 0.679 | 0.525 | p < 0.001∗ |
| ITIH4 | 101/156 | 0.801 (0.745-0.857) | 0.170 (μg/mL) | 0.782 | 0.763 | p < 0.001∗ |
| CEA | 101/156 | 0.816 (0.754-0.878) | 3.515 (ng/mL) | 0.633 | 0.897 | p < 0.001∗ |
| TIMP-1 | 101/156 | 0.832 (0.776-0.888) | 205.680 (μg/mL) | 0.723 | 0.878 | p < 0.001∗ |
| ITIH3+ITIH4 | 101/156 | 0.827 (0.776-0.877) | 0.674 | 0.763 | 0.851 | p < 0.001∗ |
| ITIH3+ITIH4+CEA+TIMP-1 | 101/156 | 0.962 (0.940-0.985) | 0.705 | 0.917 | 0.908 | p < 0.001∗ |
∗ p < 0.05: statistically significant. CRC: colorectal cancer; ITIH3: inter-alpha-trypsin inhibitor heavy chain H3; ITIH4: inter-alpha-trypsin inhibitor heavy chain H4; CEA: carcinoembryonic antigen; TIMP-1: tissue inhibitor of metalloproteinase-1.
3.5. NRI Analysis for Combining ITIH3/ITIH4 and TIMP-1
Since the ROC curve of TIMP-1 showed the highest diagnostic accuracy among the overall biomarkers we detected, we chose TIMP-1 as a reliable biomarker for further analysis. The NRI analysis was conducted to estimate the incremental predictive ability combining ITIH3/ITIH4 with TIMP-1 compared to TIMP-1 alone.
The NRI for reclassification showed significant improvements for CRC detection when ITIH3 was added to TIMP-1 (NRI = 13.6%, p = 0.006). Additionally, there was a relatively small improvement in the predictive value of ITIH4 combined with TIMP-1 compared to TIMP-1 alone (NRI = 6.2%, p = 0.241). Finally, we combined both ITIH3 and ITIH4 into TIMP-1 and yielded the highest NRI of 17.1% (p = 0.002).
Taken together, these results suggested that in CRC patients, ITIH3/ITIH4 could significantly add the diagnostic accuracy beyond that provided by TIMP-1 alone.
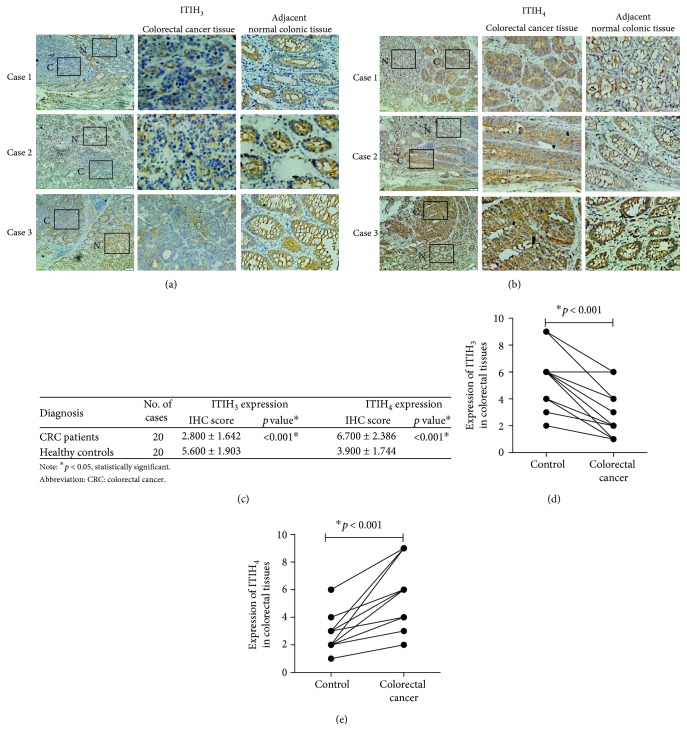
3.6. Altered Expression of ITIH3/ITIH4 in Human CRC Tissues
Expression of ITIH3 or ITIH4 in colorectal cancer and adjacent normal colorectal tissues was analyzed via IHC staining. According to the IHC score assessment, ITIH3 expression was dramatically reduced in colorectal cancer, compared with that in normal tissues (p < 0.001) (Figures 3(a) and 3(d)). Conversely, ITIH4 was upregulated in colorectal carcinoma specimens relative to adjacent normal colorectal tissues (p < 0.001) (Figures 3(b) and 3(e)). Analysis of the scores (listed in Figure 3(c)) confirmed that the altered trends in ITIH3 and ITIH4 expressions between the case and control groups in colorectal tissue are consistent with those in plasma.
Figure 3.
The expressions of ITIH3 or ITIH4 in human CRC tissues and their adjacent normal colorectal tissues were analyzed by immunohistochemical (IHC) staining. (a) The expressions of ITIH3 in CRC tissues and the normal colorectal tissues. The boxed areas lined with black color in the left images of (a, b) were magnified in the middle and right ones. N: adjacent normal tissue (shown in the right column); C: CRC tissue (shown in the middle column). Original magnification, ×100-fold. (b) The expressions of ITIH4 in CRC tissues compared with the normal colorectal tissues. (c) The IHC scores of the expressions for ITIH3 and ITIH4; the p values were acquired by paired t-test. (d, e) The individual line plot diagrams described the IHC scores of ITIH3 and ITIH4 expressions.
4. Discussion
While the increasing use of colonoscopy has led to a reduction in mortality of CRC patients [22], more precise and noninvasive methods, such as the identification of reliable blood biomarkers that can stably detect CRC, are essential for improving diagnosis.
CEA is one of the most extensively studied serological tumor markers and has been widely used in the clinical setting, despite the low sensitivity of serum CEA for early-stage CRC [23]. Recently, dozens of more protein biomarkers in serum have been detected for distinguishing the CRC patients from healthy individuals. Among these, TIMP-1, soluble CD26 (sCD26), and M2-pyruvate kinase (M2-PK) have shown relatively promising results [24, 25]. Particularly, TIMP-1 is the only one which has been regarded as an available marker in many clinical researches and could be detected at early stages of CRC. Functionally, TIMP-1 is a multifunctional glycoprotein which can inhibit most matrix metalloproteinases (MMPs) and stimulate tumor growth as well as malignant transformation [26]. Emerging evidence has identified that TIMP-1 is a reliable biomarker with relatively stable and high sensitivity of 65% and specificity of 95%, which exhibits a more superior detecting ability, compared to CEA [26]. As “preclinical development” serum protein biomarkers, the M2-PK and sCD26 still need more evidence for validation [24].
In the present study, we showed for the first time that the plasma concentrations of ITIH3 are significantly decreased in CRC patients relative to normal controls (p < 0.001), consistent with ITIH3 mRNA expression patterns in tissues of multiple solid cancer types, such as breast, uterus, colon, ovary, lung, and rectum cancers [11]. Earlier, Paris and coworkers revealed the roles of ITIH1 and ITIH3 in reducing the metastasis of lung cancer in mice while increasing cell attachment in vitro [27]. Inhibition of tumor growth and metastasis mediated by ITIH3 is related to its stabilizing effects on the extracellular matrix as well as covalent linkage of HA [28]. Therefore, downregulation of ITIH3 in plasma appears to be a reasonable step for CRC progression. Data from our experiments support the utility of plasma ITIH3 as a potential biomarker for detection of CRC.
The plasma concentration of ITIH4 in CRC patients showed a tendency of upregulation (p < 0.001). ITIH4 protein is closely related to carcinogenesis, development, and metastasis of many solid tumor types. The plasma level of ITIH4 is reported to be significantly higher in prediagnostic breast cancer samples and identified as a potential diagnostic marker for breast cancer, consistent with our current findings [29].
In a rat model, ITIH4 was upregulated in early intestinal tumors, indicative of a role in extracellular matrix remodeling in colon tumor tissue [16]. In addition, the elevated level of serum ITIH4 was associated with early colonic adenomagenesis, which served as the most important premalignant state for CRC [17]. These results indicate that upregulation of plasma ITIH4 is closely related to the carcinogenesis of CRC, supporting its utility as an indicator of tumorigenesis in clinical practice.
In our study, no significant differences in ITIH3 and ITIH4 were observed between the invasive and noninvasive subgroups in CRC patients (p values were 0.261 and 0.617, respectively), further supporting the theory that the ITIH3/ITIH4 biomarker set is related to carcinogenesis rather than prediction of prognosis or metastasis in CRC.
ROC curve analysis was further performed for determining the sensitivity and specificity of plasma ITIH3 and ITIH4 in distinguishing between CRC patients and healthy subjects. The AUC values of ITIH3 and ITIH4 were significantly greater than 0.5 (0.638 and 0.801, respectively), supporting their effectiveness in CRC detection. The AUC value of plasma ITIH4 was similar to that of the classical biomarker CEA (AUC = 0.816), suggesting that this protein ITIH4 can be reliably applied to distinguish CRC. The combination of ITIH3 and ITIH4 (AUC = 0.827) could be representative of greater effectiveness in disease diagnosis than each protein alone, and the fitting AUC of the two proteins was even higher than that of CEA. In particular, the combination of CEA, TIMP-1, ITIH3, and ITIH4 would significantly enhance the diagnostic performance (AUC = 0.962), which might provide a more reliable strategy for disease screening.
The NRI analysis showed the range from 6.2% to 17.1% for CRC detective improvement when adding ITIH3 and/or ITIH4 to TIMP-1, compared with TIMP-1 alone, indicating the highly significant effects of ITIH3 and/or ITIH4 on diagnosis accuracy improvement.
The statistical differences of circulating ITIH3 expression have been detected in the tumors of gastric [30] and pancreatic [31] tumors, compared to healthy controls. Also, the serum expression of ITIH4 significantly changed in cancers of hepatocellular carcinoma [32], breast [29], and ovarian [33], in comparison to normal individuals. Therefore, the specificity of ITIH3/ITIH4 for CRC detection could not reach the absolute value of 100%, due to the changed expression of the two markers across multiple other solid cancers. It should be noted that the quantitative measurements of posttranslational modifications for ITIH4/ITIH3 might improve the classification of multiple cancers [34], which might improve the specificity of ITIH3 and ITIH4 in detecting cancers including CRC.
We additionally conducted IHC assessments for the two biomarkers. The IHC score indicated a similar decreasing trend of ITIH3 expression along with a dramatic increase in ITIH4 expression in CRC tissues relative to that in adjacent normal colorectal tissue. These trends were consistent with serological results, further verifying the reliability of plasma assessments for ITIH3 and ITIH4.
Kaplan-Meier curves were generated to analyze the prognostic value of ITIH3 and ITIH4 in CRC metastasis and prognosis (date not shown in results). Notably, the p values obtained from the log-rank test for ITIH3 and ITIH4 (0.570 and 0.511) were not statistically significant, confirming a role of these biomarkers in tumorigenesis rather than prediction of neoplasm metastasis.
The current study has several limitations worth noting. Firstly, a relatively small sample size was examined and further studies with larger sample sizes are thus required for confirmation of our findings. Secondly, only two members of the ITIH family were investigated. Detailed molecular studies should be conducted to clarify the roles and specific mechanisms of ITIH3 and ITIH4 proteins in tumorigenesis and development of CRC.
In summary, ITIH3 is downregulated while ITIH4 is upregulated in the plasma of CRC patients, similar to the expression trends observed in CRC tissues. Our findings collectively support the utility of plasma ITIH3 and ITIH4 proteins as novel tumor biomarkers for diagnosis of CRC.
Acknowledgments
We thank Dr. Tiangang Xie (Dalian Municipal Central Hospital, Dalian, China) for his assistance in plasma preparation. We also thank Dr. Yue Xin (Dalian Municipal Central Hospital, Dalian, China) for carrying out the immunohistochemical analysis. This work was supported by the National Natural Science Foundation of China (grant numbers 81872263 and 81672792 to H.W.) and LiaoNing Revitalization Talents Program for H.W.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors have no conflict of interest to declare.
Authors' Contributions
All authors made substantial contributions to the conception, design, acquisition, analysis, and interpretation of data and took part in drafting the article or revising it critically for important intellectual content. All of the authors have given their approval for this version of the manuscript to be published and have agreed to be accountable for all aspects of the work.
References
- 1.Arnold M., Sierra M. S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Zhang A., Sun H., Yan G., Wang P., Han Y., Wang X. Metabolomics in diagnosis and biomarker discovery of colorectal cancer. Cancer Letters. 2014;345(1):17–20. doi: 10.1016/j.canlet.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 3.He Y., Sun L. Y., Wang J., et al. Hypermethylation of Apc2 is a predictive epigenetic biomarker for Chinese colorectal cancer. Disease Markers. 2018;2018:7. doi: 10.1155/2018/8619462.8619462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overholt B. F., Wheeler D. J., Jordan T., Fritsche H. A. Ca11-19: a tumor marker for the detection of colorectal cancer. Gastrointestinal Endoscopy. 2016;83(3):545–551. doi: 10.1016/j.gie.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 5.Jia J., Zhang P., Gou M., Yang F., Qian N., Dai G. The role of serum Cea and Ca19-9 in efficacy evaluations and progression-free survival predictions for patients treated with cetuximab combined with Folfox4 or Folfiri as a first-line treatment for advanced colorectal cancer. Disease Markers. 2019;2019:8. doi: 10.1155/2019/6812045.6812045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W. Cancer statistics: updated cancer burden in China. Chinese Journal of Cancer Research. 2015;27(1):p. 1. doi: 10.3978/j.issn.1000-9604.2015.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai L., Pan G., Liu X., et al. High expression of Aldoa and Ddx5 are associated with poor prognosis in human colorectal cancer. Cancer Management and Research. 2018;10:1799–1806. doi: 10.2147/CMAR.S157925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winawer S. J., Fischer S. E., Levin B. Evidence-based, reality-driven colorectal cancer screening guidelines: the critical relationship of adherence to effectiveness. JAMA. 2016;315(19):2065–2066. doi: 10.1001/jama.2016.3377. [DOI] [PubMed] [Google Scholar]
- 9.Bost F., Diarra-Mehrpour M., Martin J. P. Inter-α-trypsin inhibitor proteoglycan family: a group of proteins binding and stabilizing the extracellular matrix. European Journal of Biochemistry. 1998;252(3):339–346. doi: 10.1046/j.1432-1327.1998.2520339.x. [DOI] [PubMed] [Google Scholar]
- 10.Salier J. P., Rouet P., Raguenez G., Daveau M. The inter-α-inhibitor family: from structure to regulation. The Biochemical Journal. 1996;315(1):1–9. doi: 10.1042/bj3150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamm A., Veeck J., Bektas N., et al. Frequent expression loss of inter-alpha-trypsin inhibitor heavy chain (Itih) genes in multiple human solid tumors: a systematic expression analysis. BMC Cancer. 2008;8(1):p. 25. doi: 10.1186/1471-2407-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W., Yang Q., Liu B., Zhu Z. Serum proteomics for gastric cancer. Clinica Chimica Acta. 2014;431:179–184. doi: 10.1016/j.cca.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Lee E. J., Yang S. H., Kim K. J., et al. Inter-alpha inhibitor H4 as a potential biomarker predicting the treatment outcomes in patients with hepatocellular carcinoma. Cancer Research and Treatment. 2018;50(3):646–657. doi: 10.4143/crt.2016.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivancic M. M., Huttlin E. L., Chen X., et al. Candidate serum biomarkers for early intestinal cancer using 15N metabolic labeling and quantitative proteomics in the Apcmin/+ mouse. Journal of Proteome Research. 2013;12(9):4152–4166. doi: 10.1021/pr400467c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viennois E., Baker M. T., Xiao B., Wang L., Laroui H., Merlin D. Longitudinal study of circulating protein biomarkers in inflammatory bowel disease. Journal of Proteomics. 2015;112:166–179. doi: 10.1016/j.jprot.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivancic M. M., Irving A. A., Jonakin K. G., Dove W. F., Sussman M. R. The concentrations of Egfr, Lrg1, Itih4, and F5 in serum correlate with the number of colonic adenomas in Apcpirc/+ rats. Cancer Prevention Research. 2014;7(11):1160–1169. doi: 10.1158/1940-6207.CAPR-14-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivancic M. M., Anson L. W., Pickhardt P. J., et al. Conserved serum protein biomarkers associated with growing early colorectal adenomas. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(17):8471–8480. doi: 10.1073/pnas.1813212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edge S. B., Compton C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of Tnm. Annals of Surgical Oncology. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y. R., Liu H., Xiao L. M., Jin C. G., Zhang Z. P., Yang C. G. The clinical significance of CCBE1 expression in human colorectal cancer. Cancer Management and Research. 2018;10:6581–6590. doi: 10.2147/CMAR.S181770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pencina M. J., D'Agostino R. B., Sr., D'Agostino R. B., Jr., Vasan R. S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in Medicine. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 21.Mroczko B., Groblewska M., Okulczyk B., Kedra B., Szmitkowski M. The diagnostic value of matrix metalloproteinase 9 (Mmp-9) and tissue inhibitor of matrix metalloproteinases 1 (Timp-1) determination in the sera of colorectal adenoma and cancer patients. International Journal of Colorectal Disease. 2010;25(10):1177–1184. doi: 10.1007/s00384-010-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alonso-Abreu I., Alarcon-Fernandez O., Gimeno-Garcia A. Z., et al. Early colonoscopy improves the outcome of patients with symptomatic colorectal cancer. Diseases of the Colon and Rectum. 2017;60(8):837–844. doi: 10.1097/DCR.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 23.Moertel C. G., O'Fallon J. R., Go V. L. W., O'Connell M. J., Thynne G. S. The preoperative carcinoembryonic antigen test in the diagnosis, staging, and prognosis of colorectal cancer. Cancer. 1986;58(3):603–610. doi: 10.1002/1097-0142(19860801)58:3<603::AID-CNCR2820580302>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.Hundt S., Haug U., Brenner H. Blood markers for early detection of colorectal cancer: a systematic review. Cancer Epidemiology, Biomarkers & Prevention. 2007;16(10):1935–1953. doi: 10.1158/1055-9965.EPI-06-0994. [DOI] [PubMed] [Google Scholar]
- 25.Nikolaou S., Qiu S., Fiorentino F., Rasheed S., Tekkis P., Kontovounisios C. Systematic review of blood diagnostic markers in colorectal cancer. Techniques in Coloproctology. 2018;22(7):481–498. doi: 10.1007/s10151-018-1820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holten-Andersen M. N., Christensen I. J., Nielsen H. J., et al. Total levels of tissue inhibitor of metalloproteinases 1 in plasma yield high diagnostic sensitivity and specificity in patients with colon cancer. Clinical Cancer Research. 2002;8(1):156–164. [PubMed] [Google Scholar]
- 27.Paris S., Sesboue R., Delpech B., et al. Inhibition of tumor growth and metastatic spreading by overexpression of inter-alpha-trypsin inhibitor family chains. International Journal of Cancer. 2002;97(5):615–620. doi: 10.1002/ijc.10120. [DOI] [PubMed] [Google Scholar]
- 28.Chen L., Mao S. J., McLean L. R., Powers R. W., Larsen W. J. Proteins of the inter-alpha-trypsin inhibitor family stabilize the cumulus extracellular matrix through their direct binding with hyaluronic acid. Journal of Biological Chemistry. 1994;269(45):28282–28287. [PubMed] [Google Scholar]
- 29.Opstal-van Winden A. W. J., Krop E. J. M., Kåredal M. H., et al. Searching for early breast cancer biomarkers by serum protein profiling of pre-diagnostic serum; a nested case-control study. BMC Cancer. 2011;11(1):p. 381. doi: 10.1186/1471-2407-11-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong P. K., Lee H., Zhou J., et al. Itih3 is a potential biomarker for early detection of gastric cancer. Journal of Proteome Research. 2010;9(7):3671–3679. doi: 10.1021/pr100192h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X., Zheng W., Wang W., et al. A new panel of pancreatic cancer biomarkers discovered using a mass spectrometry-based pipeline. British Journal of Cancer. 2017;117(12):1846–1854. doi: 10.1038/bjc.2017.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Li B., Li B., et al. Itih4: effective serum marker, early warning and diagnosis, hepatocellular carcinoma. Pathology Oncology Research. 2018;24(3):663–670. doi: 10.1007/s12253-017-0285-4. [DOI] [PubMed] [Google Scholar]
- 33.Clarke C. H., Yip C., Badgwell D., et al. Proteomic biomarkers apolipoprotein A1, truncated transthyretin and connective tissue activating protein Iii enhance the sensitivity of Ca125 for detecting early stage epithelial ovarian cancer. Gynecologic Oncology. 2011;122(3):548–553. doi: 10.1016/j.ygyno.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fung E. T., Yip T. T., Lomas L., et al. Classification of cancer types by measuring variants of host response proteins using SELDI serum assays. International Journal of Cancer. 2005;115(5):783–789. doi: 10.1002/ijc.20928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.