Abstract
Introduction
Sandwich-cultured human hepatocytes (SCHHs) are the most common in vitro hepatocyte model used for studying hepatic drug disposition and hepatotoxicity. Targeted quantification of key DME and transporter protein expression is useful for in vitro-in vivo extrapolation of drug and xenobiotic clearance and developing corresponding PBPK models. However, established methods for comprehensive quantification of drug metabolizing enzyme (DMEs) and transporter expression in SCHHs are lacking. In this study, a targeted quantitative proteomic isotope dilution nanoLC-MS/MS method developed in our laboratory was adapted to quantify a panel of phase I & II DMEs and transporter proteins in SCHHs under basal and induced conditions.
Methods
SCHHs were treated with known inducers of DMEs (Rifampin: PXR activator, CITCO: CAR activator) and transporters (CDCA: FXR activator) or with vehicle control (DMSO) for 72 h. Membrane protein was isolated from the SCHHs using a membrane extraction kit and 30 μg membrane protein was digested with trypsin. The resulting peptides were analyzed by isotope dilution nanoLC-MS/MS to quantify the DMEs and transporters.
Results
Using the method, we could quantify fourteen phase I and ten phase II DMEs, and twelve uptake/efflux transporters, under basal and induced conditions in the SCHHs. Analysis showed donor to donor variation in basal protein levels of CYP450s, UGTs and transporters, and that basal protein expression of CYP450s and UGTs was higher than that of transporters. In addition, induction of key proteins in response to rifampin, CITCO and CDCA was observed.
Discussion
We have successfully quantified protein abundance of multiple phase I and II DMEs and uptake and efflux transporters in SCHHs using a method previously developed in our laboratory. Our method is sufficiently sensitive to quantify inter-donor differences in protein concentrations at the basal level as well as changes in protein expression in response to endogenous and exogenous stimuli.
Keywords: Drug Metabolizing Enzymes, Hepatocytes, Proteomics, Quantification, SCHH Model, Targeted, Transporters
1. Introduction
Sandwich-cultured human hepatocytes (SCHHs) are considered a metabolically competent in vitro model to evaluate hepatic drug metabolism and transport and to study cytotoxicity and perturbation of biological processes by drugs and hepatically generated metabolites (Swift, Pfeifer, & Brouwer, 2010; Yang, et al., 2016). In addition to retaining expression of drug metabolizing enzymes (DMEs) and maintaining function for longer periods in culture, SCHHs retain polarity and proper localization of basolateral and canalicular transporters as well as formation of functional bile networks (Swift, et al., 2010; Yang, et al., 2016). Because of the extended life of the culture, SCHHs can be used to study the induction of DMEs and transporters by endogenous and exogenous molecules.
Quantification of DME and transporter mRNA levels does not directly reflect the concentration and functional activity of the proteins (Ohtsuki, et al., 2012). Thus, evaluating the expression of phase I and II DME and transporter (uptake and efflux) proteins is necessary to understand the underlying mechanism of drug metabolism and transporter mediated disposition that determine hepatic exposure and clearance of drugs and xenobiotics. Targeted quantitative proteomics by LC-MS/MS is a superior technique to immunoblotting that allows us to quantify multiple DMEs and transporters in a single analysis (Aebersold, Burlingame, & Bradshaw, 2013; Fallon, et al., 2008). Prior studies have shown that quantification of DMEs and transporters by targeted proteomics more accurately estimates the function of DMEs and transporters (Schaefer, et al., 2012) and provides better in vitro-in vivo predictions of liver clearance (A. Vildhede, Wisniewski, Noren, Karlgren, & Artursson, 2015). Protein abundance of DMEs and transporters in human liver tissue and isolated hepatocytes has been reported by some studies (Ohtsuki, Uchida, Kubo, & Terasaki, 2011; A. Vildhede, et al., 2015). Previous studies have examined the protein abundance of transporters (Bi, et al., 2012; Kimoto, et al., 2012; Kumar, et al., 2019; A. Vildhede, et al., 2016), some cytochrome P450 enzymes (CYP450s), UGTs 1A1 and 2B7 and transporters (Schaefer, et al., 2012) in SCHHs. However, simultaneous targeted proteomic quantification of multiple phase I and II DMEs, including UGTs, and transporters in SCHHs has not been investigated. Absolute quantification of DMEs and transporters in SCHHs will be useful for assessing mechanisms underlying the impact of endogenous (e.g. steroid hormones) and exogenous (e.g. xenobiotics) factors on changes in DME/transporter expression and hepatobiliary drug disposition. Furthermore, protein concentration of DMEs and transporters is a preferable parameter to, for example, mRNA determination for developing PBPK models for in vitro-in vivo extrapolation (IVIVE) (e.g. to per gram of liver) of drug and xenobiotic clearance and for accurately estimating the function of DMEs and transporters (Schaefer, et al., 2012; Xu, et al., 2018).
The aims of this study were to (1) quantify multiple phase I and II DMEs and transporters in the SCHH model employing our previously developed isotope dilution targeted quantitative proteomic nanoLC-MS/MS method (Fallon, Houvig, Booth-Genthe, & Smith, 2018; Fallon, Neubert, Goosen, & Smith, 2013; Fallon, Neubert, Hyland, Goosen, & Smith, 2013; Fallon, Smith, Xia, & Kim, 2016), (2) assess the variation in basal DME and transporter expression in SCHHs derived from three hepatocyte donors, and (3) quantify changes in expression of key DME and transport proteins in response to known inducers. Our method quantified fourteen phase I (mainly CYP450s) and ten phase II (UGTs) DMEs, and twelve uptake/efflux drug transporters in SCHHs under basal and induced conditions.
2. Materials and Methods
2.1. Materials
Cryopreserved human hepatocytes from three female donors were obtained from Sekisui XenoTech (Kansas City, KS) (Donors HC3–26 and HC5–40) and Fisher Scientific (Pittsburgh, PA) (Donor HU1880). Hepatocyte thawing media was purchased from Sekisui XenoTech. Seeding, culture and induction media were purchased from (BioIVT, Durham,NC). BioCoat™ (Beckton Dickinson) Collagen I Cellware 12-well plates and Corning® Matrigel® Matrix were obtained from Corning (Corning, NY). The (Calbiochem®) ProteoExtract® Native Membrane Protein Extraction Kit (Catalog # 444810) was obtained from MilliporeSigma (Burlington, MA). The Protein Assay Kit II (#5000002) was obtained from Bio-Rad (Hercules, CA). Rifampin, CITCO, CDCA, dimethyl sulfoxide (DMSO), ammonium bicarbonate, dithiothreitol, β-casein, sodium deoxycholate, iodoacetamide, formic, acetic and trifluoroacetic acids were purchased from Sigma-Aldrich (St Louis, MO). Acetonitrile (HPLC grade) and the 0.2 mL PCR tubes (part # 14230227) in which digestion reactions were performed were from Fisher Scientific. Trypsin Gold mass spectrometry grade was purchased from Promega (Madison, WI). Purified water was obtained from an in-house Picopure® 2 system (Hydro Service and Supplies, Inc. Durham, NC). All other chemicals were reagent grade. Stable isotope labeled (SIL) (13C and 15N) proteotypic tryptic peptide standards for most of the UGTs were obtained from Thermo Scientific Biopolymers (Rockford, IL) (AQUA Quant Pro) and the SIL standards for the remainder of the UGTs, the CYP450s, CESs and transporters were obtained from Theracode JPT Inc. (Acton, MA) (SpikeTides™_TQL; purified and calibrated). The JPT peptides each contained a tryptic linker at the C-terminus, used for quantification at the company after production, which needed to be removed during the digestion procedure. The Thermo peptides did not contain any such linker. Our laboratory HLM (human liver microsomes) QC (Gentest™; pool of 50) was obtained from BD Biosciences (now Corning® Gentest™, Corning, NY). The solid phase extraction (SPE) cartridges were Strata™-X 33u Polymeric Reversed Phase (10 mg/mL, part no. 8BS100AAK) obtained from Phenomenex (Torrance, CA).
2.2. SCHH and induction treatment
Human hepatocytes (1 × 106 per well) were seeded on the Biocoat™ Collagen I Cellware 12-well plates on day 1. Hepatocytes were overlaid with Corning® Matrigel® Matrix on day 2. On day 3 the SCHHs were treated with 10 μM rifampin, a known PXR activator (LeCluyse, 2001), 1.0 μM CITCO, a known CAR activator (Maglich, et al., 2003), 100 μM CDCA, a known FXR activator (Liu, et al., 2014; Makishima, et al., 1999; Parks, et al., 1999; Zhang, et al., 2017), or vehicle control (0.1% DMSO). Media was replaced at 8, 24, 32, 48 and 56 h with fresh treatment-containing media. All sample treatment conditions were duplicated (i.e. two independent wells were used for each treatment group). The procedure was repeated with hepatocytes from three female donors (HC3–26, HC4–15 and HU1880).
2.3. Membrane protein isolation
SCHHs were harvested on day 6, and the ProteoExtract® Native Membrane Protein Extraction Kit was used to isolate membrane protein. The kit employs differential surfactant extraction and the manufacturer’s protocol was followed with minor modifications. Briefly, after the 72 h induction, hepatocytes were washed twice with cold wash buffer and lysed in 750 uL of Extraction Buffer I. Cell lysate was incubated in ice for 10 min with gentle vortexing at every 5 min, then centrifuged at 16,000 × g for 15 min at 4°C. The resulting cell pellets were suspended in 100 uL of Extraction Buffer II and frozen at −80°C for 30 min. The suspension was thawed and incubated in ice for 15 min with gentle vortexing every 5 min. This was followed by centrifugation at 16,000 × g for 15 min at 4°C. The supernatant was collected as a membrane fraction, which was quantified using the Bio-Rad Protein Assay Kit II. Membrane protein recovery per one million cells ranged from 84 – 141 μg for Donor HC3–26, 38 – 83 μg for Donor HC4–15 and 101 – 141 μg per for Donor HU1880.
2.4. Sample batching for trypsin digestion and analysis
Thirty micrograms of membrane protein from each (duplicate) well was digested with trypsin. Samples were analyzed in three batches corresponding to the three SCHH donor preparations. Our laboratory HLM QC (Corning® Gentest™) was analyzed in duplicate with each batch with 20 μg of total QC microsomal protein being used in each digestion. The QC had been analyzed on many occasions in the laboratory and provided an interday measure of assay performance. The protein solution for each digestion was evaporated to dryness at the beginning so that the volumes of reagents used in each digestion reaction were consistent. The 0.2 mL Fisher PCR tubes were used for conducting the digestions. All samples were solubilized in 1% sodium deoxycholate, a detergent used to aid solubility and denaturation.
2.5. Sample denaturation, reduction, blocking and digestion
After evaporation of sample protein solution to dryness in a ThermoSavant SpeedVac 100 μL of 50 mM ammonium bicarbonate, 10 μL of 40 mM dithiothreitol, 10 μL of β-casein (0.5 μg/10 μL) (an indicator of digestion and a chromatography retention time marker) and 13.3 μL of 10% sodium deoxycholate were added to each tube. Samples were denatured and reduced for 40 min at 60 °C in an Isotemp Thermal Mixer (Fisher Scientific) shaking at 500 rpm. After cooling to room temperature, 10 μL of 135 mM iodoacetamide was added and tubes were incubated for 30–40 min in the dark at room temperature. One pmol of each JPT Theracode SIL peptide was then added from mixes that were routinely prepared and used in the lab (concentrations of peptides in the mixes was 1 pmol/10 μL). Trypsin was then added to each sample to give a trypsin:protein ratio of 1:20 (w/w) (concentration 0.1 μg/μL in 50 mM acetic acid; 15 μL to study samples, 10 μL to QC duplicates). Samples were then vortexed and digested at 37 °C overnight (20 h) using the Isotemp Thermal Mixer shaking at 300 rpm. The reaction was stopped by the addition of 10% trifluoroacetic acid, such that the volume added was 10% of the total reaction volume, which was 21 μL for each study sample and 20 μL for the QC duplicates. A sodium deoxycholate precipitate formed. One pmol of each Thermo Scientific UGT SIL peptide was then added in a 10 μL volume (1.0 pmol of each peptide in 10 μL of mix). Samples were vortex mixed and centrifuged at 13.3K × g for 5 min to pellet the precipitate. The supernatant was transferred to fresh tubes (Eppendorf Protein LoBind, 0.5 mL) and the samples were evaporated for 10 min in the ThermoSavant SpeedVac to remove excess acetonitrile that may have been present from the heavy labeled peptide mixes.
2.6. Post-Digestion (SPE; Solid Phase Extraction)
Samples were then treated with SPE. The SPE cartridges (10 mg/mL; polymeric reversed phase) were conditioned with methanol and purified water. Sample was added and the cartridges were washed with water. Elution was with 60% acetonitrile/40% formic acid (0.1% solution) into Eppendorf 0.5 mL LoBind tubes. The eluate was evaporated to dryness and reconstituted in 50 μL modified mobile phase A (water/acetonitrile/formic acid 98/2/0.1, i.e. 2% acetonitrile). Following centrifugation at 13.3K × g for 5 min, the supernatant was transferred to deactivated vial inserts for analysis by nanoLCMS/MS.
2.7. Nano LC-MS/MS Analysis
Analysis was performed on a nanoAcquity (Waters, Milford, MA) coupled to a SCIEX QTRAP 5500 hybrid mass spectrometer (Framingham, MA) equipped with a NanoSpray III source. Control was with Analyst 1.5 software (SCIEX) and nanoAcquity UPLC Console. Mobile phase A consisted of 1% acetonitrile and 0.1% formic acid. Mobile phase B was 100% acetonitrile. For the study samples, an injection volume of 0.1 or 0.2 μL (0.06 or 0.12 μg or 0.3 or 0.6% of the sample) was loaded onto a Symmetry® C18 trap column, 2G-VM, 180 μm × 20 mm, 5 μm particle size (Waters) (part # 186006527) at a trapping flow of 15 μL/min of mobile phase A for 1 min. For the (20 μg) QC duplicates the volume injected was 0.15 or 0.2 μL. Peptides were eluted from the trap column and separated at a flow rate of 1.3 μL/min on a BEH130 C18 column, 150 μm × 100 mm, 1.7 μm particle size (Waters) (part # 186003550). Separation conditions were 100% A at start, 58% A at 24 min, 5% A at 24.5 min for 3 min and 100% A at 28 min for 7 min (total run time 35 min). The analytical column temperature was set to 35°C.
MS/MS analysis was conducted in the positive mode with ion spray voltage at 4000. An uncoated PicoTip emitter (20 μm inner diameter, 10 μm tip; New Objective, Woburn, MA) was used to produce the nanospray. The heavy labeled (SIL) peptides used to report DME and transporter concentrations quantified, and the MRMs acquired for each peptide, are shown in Tables 1a (DMEs) and 1b (transporters and membrane marker). Other, confirmatory, heavy labeled peptides were added and the MRMs acquired for each are shown in Table S1. Peptide selection was based on in silico assessment, crude peptide evaluation and available literature (Fallon, et al., 2018; Fallon, Neubert, Goosen, et al., 2013; Ohtsuki, et al., 2012; Shawahna, et al., 2011). Individual peptide MRM collision energies for all peptides had been optimized during method development using crude (unlabeled) peptides (occasionally the heavy labeled peptides had been used) and employing Skyline software (v2.6, University of Washington) for initial prediction of MRM lists. Equality of MRM response between the heavy labeled (SIL) and unlabeled peptides was assumed. The product ion for most of the heavy labeled peptide MRMs contains the heavy label (Tables 1a, 1b & S1). Data for the samples was acquired in three nanoLC-MS/MS injections with a method containing approximately 200 MRMs being used for each injection (i.e. total of ~600 MRMs).
Table 1a.
Human proteotypic tryptic peptides used to report DME concentrations that were above the LLOQ of ∼0.1 pmol/mg protein in the SCHHs, and MRMs acquired.
| DME | Peptide Sequence | MRM1 (product ion) (mass spec specific) | MRM2 (product ion) (mass spec specific) |
|---|---|---|---|
| CYP1A2 | Y244LPNPALQR252 | 541.31/403.23 (y7) | 541.31/594.36 (y5) |
| CYP2A6 | G162TGGANIDPTFFLSR176 | 781.90/877.48 (y7) | 781.90/992.51 (y8) |
| CYP2B6 | G379YIIPK384 | 349.73/478.36 (y4) | 349.73/365.27 (y3) |
| CYP2C8 | N466LNTTAVTK474 | 485.28/742.43 (y7) | 485.28/628.39 (y6) |
| CYP2C9 | S460LVDPK465 | 333.70/252.18 (y2) | 333.70/466.28 (y4) |
| CYP2C19 | G98HFPLAER105 | 312.84/385.21 (y3) | 312.84/439.21 (b4) |
| CYP2D6 | S116QGVFLAR123 | 444.25/672.41 (y6) | 444.25/516.32 (y4) |
| CYP2E1 | F360ITLVPSNLPHEATR374 | 568.98/566.29 (y10) | 568.98/720.37 (y6) |
| CYP2J2 | D276FIDAYLK283 | 496.76/730.42 (y6) | 496.76/617.34 (y5) |
| CYP3A4 | L477SLGGLLQPEKPVVLK492 | 567.03/693.43 (y13) | 567.03/664.92 (y12) |
| CYP3A5 | D244TINFLSK251 | 473.30/217.10 (b2) | 473.26/729.44 (y6) |
| CYP4F2 | S109VINASAAIAPK120 | 575.35/850.50 (y9) | 575.35/665.42 (y7) |
| CES1 | E394LIPEATEK402 | 519.29/682.36 (y6) | 519.29/341.68 (y6) |
| CES2 | T35THTGQVLGSLVHVK49 | 396.98/430.78 (y8) | 528.97/747.47 (y7) |
| UGT1A1 | D70GAFYTLK77 | 462.75/681.39 (y5) | 462.75/524.31 (y4) |
| UGT1A3 | Y164LSIPTVFFLR174 | 683.39/1089.63 (y9) | 683.39/889.52 (y7) |
| UGT1A4 | Y164LSIPAVFFWR174 | 704.89/1132.61 (y9) | 704.89/932.51 (y7) |
| UGT1A6 | D44IVEVLSDR52 | 528.28/728.38 (y6) | 528.28/599.34 (y5) |
| UGT1A9 | G171ILCHYLEEGAQCPAPLSYVPR192 | 847.41/1009.56 (y9) | 847.41/841.48 (y7) |
| UGT2A3 | V41ILEELIVR49 | 547.35/881.53 (y7) | 547.35/768.45 (y6) |
| UGT2B4 | F174SPGYAIEK182 | 510.27/785.42 (y7) | 510.27/688.41 (y6) |
| UGT2B7 | A253DVWLIR259 | 441.76/597.39 (y4) | 441.76/696.43 (y5) |
| UGT2B10 | G49HEVTVLASSASILFDPNDSSTLK72 | 832.77/869.48 (y8) | 832.77/1131.54 (y10) |
| UGT2B15 | F175SVGYTFEK183 | 543.28/752.38 (y6) | 543.28/851.44 (y7) |
MRMs are roughly in order of highest intensity.
Amino acids in bold are 13C and 15N heavy labeled.
Mass differences between labeled (shown) and unlabeled (not shown) R, K and F are 10, 8 and 10 respectively (m/z differences also depend on charge state).
KP is not cleavable with trypsin. P prevents trypsin cleavage at the adjacent preceding K.
Table 1b.
Human proteotypic tryptic peptides used to report transporter and membrane marker concentrations that were above the LLOQ of ∼0.1 pmol/mg protein in the SCHHs, and MRMs acquired.
| Transporter (Gene) | Peptide Sequence | MRMs 1 and 2 (product ion)a [mass spectrometer specific] |
|---|---|---|
| OATP1B1 (SLCO1B1) | N321VTGFFQSFK330 | 591.81/969.50 (y8), 591.81/868.46 (y7) |
| OATP1B3 (SLCO1B3) | I615YNSVFFGR623 | 556.79/836.43 (y7), 556.79/722.34 (y6) |
| OATP2B1 (SLCO2B1) | Y641YNNDLLR648 | 540.77/754.41 (y6), 540.77/917.47 (y7) |
| OCT3 (SLC22A3) | G522IALPETVDDVEK534 | 697.37/1039.51 (y9), 697.37/355.23 (b4) |
| NTCP (SLC10A1) | G144IYDGDLK151 | 444.73/718.35 (y6), 444.73/555.29 (y5) |
| ENT1 (SLC29A1) | W360LPSLVLAR368 | 532.83/765.49 (y7), 532.83/383.25 (y7) |
| MRP2 (ABCC2) | L1377TIIPQDPILFSGSLR1392 | 890.52/441.31 (b4), 890.52/999.59 (y9) |
| MRP3 (ABCC3) | G654ALVAVVGPVGCGK667 | 646.37/682.35 (y7), 646.37/781.42 (y8) |
| MRP6 (ABCC6) | T1142QAPFVAQNNAR1153 | 663.84/301.15 (b3), 663.84/1026.54 (y9) |
| P-gp (MDR1, ABCB1) | I368IDNKPSIDSYSK380 | 496.60/631.32 (y11), 496.60/904.46 (y8) |
| BCRP (ABCG2) | S87SLLDVLAAR96 | 527.81/654.38 (y6), 527.81/767.47 (y7) |
| BSEP (ABCB11) | S462TALQLIQR470 | 520.31/539.35 (y4), 520.31/667.41 (y5) |
| Na+/K+-ATPase (ATP1A1) | V213DNSSLTGESEPQTR227 | 543.92/511.29 (y4), 815.38/511.29 (y4) |
MRMs are roughly in order of highest intensity.
C-terminus amino acids in bold are 13C and 15N heavy labeled.
Mass differences between labeled (shown) and unlabeled (not shown) R and K are 10 and 8 respectively (m/z differences also depend on charge state).
KP is not cleavable with trypsin. P prevents trypsin cleavage at the adjacent preceding K.
2.8. Treatment of data and statistical analysis
MRM data were processed by MultiQuant 2.0.2 (SCIEX). One peptide was used for reporting of concentration of the respective protein, with the second, and sometimes third, proteotypic peptide, when available, providing confirmatory information. Concentrations were calculated for each individual peptide using area ratios of unlabeled (endogenous) to labeled MRM responses (2 unlabeled and 2 labeled MRMs summed per peptide). Rationale for selection of peptides for reporting protein concentration has been previously published (Fallon et al., 2013) and includes stability to digestion relative to other peptides, limit of detection (LOD) and statistical analysis of the variance distribution for each peptide using selected sample sets (e.g. using the quality control sample). Generally, the peptide selected is that which gives the highest concentration.
For analysis purposes, protein concentrations between the lower limit of quantification (LLOQ) of 0.1 pmol/mg protein (signal to noise ratio > 3) and 0.02 pmol/mg protein were presented as is, and concentrations that below 0.02 pmol/mg protein were imputed as 0.02 pmol/mg protein. Detection and LLOD can vary across tissue matrix examined, fractionation method and species, but are typlically in the range of 0.1 – 0.2 pmole/mg total protein. Student’s t-tests, after log10 transformation of the data, were used to test for statistical differences in protein concentrations between basal and induced conditions (p<0.05 was considered significant). Graphs were generated using Prism 7.0 (GraphPad software, La Jolla. CA), and statistical analysis was performed using SAS-JMP Pro 12.0 (SAS Institute, Cary, NC).
3. Results
3.1. Isotope dilution targeted quantitative proteomic method
We have successfully applied an isotope dilution targeted quantitative proteomic method developed in our laboratory (Fallon, et al., 2018; Fallon, Neubert, Goosen, et al., 2013; Fallon, Neubert, Hyland, et al., 2013; Fallon, et al., 2016) to quantify several DMEs, including hUGTs and CYP450s, and transporters in SCHHs. The method involves extracting membrane protein from the SCHHs using a ProteoExtract® Native Membrane Protein Extraction Kit, adding a known amount of SIL peptide standard to the membrane protein, digesting with trypsin and analyzing by nanoLC-MS/MS, with the mass spectrometer being operated in the MRM mode. We employed three separate MRM methods, each containing approximately 200 MRMs, to acquire data and thus each sample was injected three times onto the system. A total ion chromatogram showing data from one of the three MRM methods, mainly focusing on transporters, is shown in Fig. 1. Response from the SIL peptides dominates the chromatogram and thus chromatograms for the other two MRM methods (i.e. for UGTs/CYP450s and CESs/various other proteins) look similar and are not shown. Extracted ion chromatograms for one peptide, in basal and induced samples, demonstrating the sensitivity of our method are shown in Figure S1. Our laboratory HLM QC performed as expected, the sample having been analyzed on many previous occasions in the laboratory. Inter- and intraday variation for the method has previously been published (Fallon, et al., 2018; Fallon, Neubert, Hyland, et al., 2013).
Fig. 1. Representative total ion chromatogram for transporter peptides in trypsin digested membrane protein isolated from SCHHs (0.1 % DMSO treated sample).
Time is on the x-axis and response (cps) on the y-axis. 30 μg of total membrane protein was digested for 20 h and 0.1 μL (0.06 μg, 0.2% of the total) of the digest was injected onto the nanoAcquity-QTRAP 5500 system. Most of the visible peaks in the chromatogram represent heavy labeled proteotypic tryptic peptide standards and so the chromatograms for the UGT/CYP450, and CESs and Various, MRM methods look similar. Two MRMs were acquired per peptide. Heavy labeled and unlabeled peptides co elute. This is a zoom of the total ion chromatogram (8 to 23 min) for the transporters MRM method. Examples of extracted ion chromatograms for one peptide are shown in Figure S1.
3.2. Targeted proteomic quantification of phase I DMEs in SCHHs
We successfully quantified (concentrations >0.1 pmol/mg membrane protein) twelve CYP450 enzymes and two CESs in the SCHHs from all three donors (Fig. 2). CYP2E1 showed the highest mean protein expression (22 pmol/mg protein) followed by CYPs 2A6 and 3A4 (18 pmol/mg protein for both). CYP2B6 exhibited the lowest protein concentration, ranging from 0.3 to 1.5 pmol/mg protein across donors, which was comparable with the reported data (Schaefer, et al., 2012). For carboxylesterases, the mean protein abundance of CES1 was at least 20 times higher than that of CES2 in all donors (Fig.2B). Concentrations of CYPs 2B6, 2C8, 2C9, 2D6 and 3A4 were lower in the SCHHs from each donor than in the pooled HLM QC. Protein concentrations of the CYPs 2C19 and 2J2, CES1 and CES2, and membrane protein marker Na+/K+-ATPase in SCHHs were similar to those measured in the HLM QC (Fig. 2).
Fig. 2. Absolute protein quantification of key phase I DMEs at basal level in SCHHs.
Protein expression of 12 CYP isoforms and the membrane protein marker Na+/K+-ATPase (A), and carboxylesterase isoforms (B) under basal conditions are presented. Protein concentration was quantified in membrane protein fractionated from SCHHs from three donors, and in HLM QC samples analyzed in duplicate with each donor. Each SCHH data point represents DME protein concentration for an individual donor (n=2 biological replicates). Data are plotted on a log scale, and the dotted line indicates the lower limit of quantification.
Donor to donor variation in the protein expression of CYPs 1A2, 2A6, 2B6, 3A4 and 3A5 was high; whereas, less donor to donor variation was observed with CYPs 2C8, 2C9, 2C19, 2J2 and 4F2 (Fig.2A). CYP3A4 concentrations were lower in Donor HC3–26 (5.2 pmol/mg protein) compared to in Donor HU1880 (18 pmol/mg protein) and Donor HC4–15 (32 pmol/mg protein). Basal protein levels of CYP3A5 was markedly higher in Donor HU1880 (21 pmol/mg protein) compared to Donor HC4–15 (1.8 pmol/mg protein) and Donor HC3–26 (1.6 pmol/mg protein).
3.3. Targeted proteomic quantification of UGTs in SCHHs
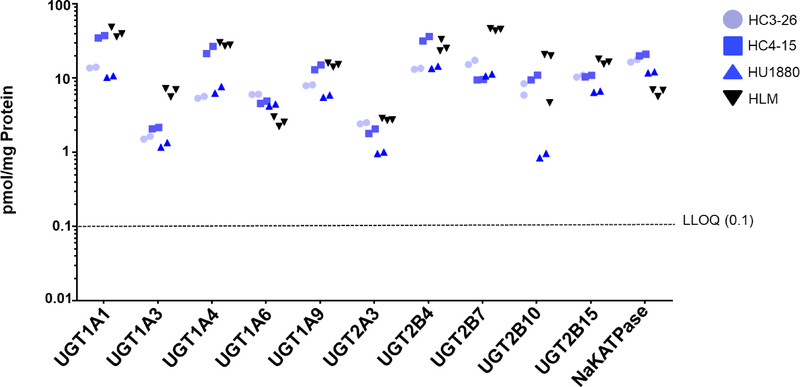
We attempted to determine the protein concentration of seventeen UGTs in the SCHHs, and successfully quantified (concentrations >0.1 pmol/mg membrane protein) ten isoforms in all three donors (Fig. 3). The mean protein expression levels of UGTs 1A1 and 2B4 were highest (>20 pmol/mg protein), whereas UGTs 1A3 and 2A3 demonstrated the lowest mean protein expression (<2.0 pmol/mg protein). Unlike that of CYP450s, the protein abundance of UGTs in the SCHHs was generally comparable to abundance in the HLM QC.
Fig. 3. Absolute protein quantification of UGTs at basal level in SCHHs.
Protein expression of 10 UGT isoforms and the membrane protein marker Na+/K+-ATPase under basal conditions are presented. Protein concentration was quantified in membrane protein fractionated from SCHHs from three donors, and in HLM QC samples analyzed in duplicate with each donor. Each SCHH data point represents DME protein concentration for an individual donor (n=2 biological replicates) and is plotted on a log scale. Dotted line indicates the lower limit of quantification.
Among the three donors, the protein expression of UGTs 1A1 (HC4–15: 36 pmol/mg protein, HC3–26: 14 pmol/mg protein, HU1880: 11 pmol/mg protein) and 1A4 (HC4–15: 24 pmol/mg protein HC3–26: 5.5 pmol/mg protein, HU1880: 7.0 pmol/mg protein) varied most compared to the other UGTs. Protein expression of UGT2B10 in HU1880 (0.9 pmol/mg protein) and in the HLM QC analyzed at the same time was lower than expected (Fig. 3), possibly due to performance of the peptide, the chromatography peaks having previously been noted to be broad. Long-term storage of the 24-mer peptide, possibly causing degradation, could also be a contributory factor.
3.4. Targeted proteomic quantification of drug transporters in SCHHs
Of twenty-nine transporters screened for, in the MRM method, we quantified (concentrations >0.1 pmol/mg protein) six uptake and six efflux transporters, and Na+/K+ ATPase (which is an uptake and efflux transporter, and a membrane marker), in the SCHHs from all three donors (Fig. 4). Uptake transporter OATP1B1 showed the highest mean protein expression level of 3.1 pmol/mg protein. The mean protein concentration of the transporter P-gp (2.2 pmol/mg protein) was highest for the efflux transporters quantified. Although protein abundance of BCRP, NTCP and OCT3 were the lowest among the transporters, their mean protein concentrations for each donor were above the LLOQ of 0.1 pmol/mg protein. ENT1 was not detected in Donor HC3–26. Among the three donors, high variation in the protein expression of OATP1B3 and BCRP was observed, with the concentrations of all transporters being much lower than those of the CYP450s and UGTs.
Fig. 4. Protein expression of uptake and efflux transporters at basal level in SCHHs.
Protein expression of 6 uptake and 6 efflux transporters and the membrane protein marker Na+/K+-ATPase under basal conditions are presented. Protein concentration was quantified in membrane protein fractionated from SCHHs from three donors, and in HLM QC samples analyzed in duplicate with each donor. Each SCHH data point represents transporter protein concentration for an individual donor (n=2 biological replicates) and is plotted on a log scale. Dotted line indicates the lower limit of quantification. Protein concentrations below 0.02 pmol/mg protein are identified by the dashed line.
3.5. Induced expression of DMEs and transporters in SCHHs
To test the dynamic sensitivity of our targeted proteomic method, we examined changes in expression of all of the proteins quantified in response to known inducers in the SCHHs. The key changes noted are shown in Fig. 5. The prototypical PXR activator rifampin significantly increased CYP3A4, UGTs 1A1 and 1A4, and P-gp protein concentrations compared to DMSO treatment (Fig. 5A). Similarly to rifampin, the prototypical CAR activator CITCO significantly increased CYP2B6 protein concentrations (Fig. 5B), while the prototypical FXR activator CDCA significantly increased BSEP protein expression (Fig. 5C). Inter-donor variation in the degree of induction is presented in Fig. S2. These data illustrate that induction in donor HC4–15 was lower in magnitude or absent when compared to the other two donors, which was possibly due to higher basal expression of each protein in this donor.
Fig. 5. Induction of key DME and transport proteins in SCHHs.
SCHHs from three donors were treated with vehicle control (0.1% DMSO) or known inducers for 72 h, and membrane protein was isolated. (A) Protein concentrations of CYP3A4, UGTs 1A1 and 1A4, and P-gp following treatment with rifampin (10 μM). (B). Protein concentration of CYP2B6 following treatment with CITCO (1.0 μM). (C) Protein concentration of BSEP following treatment with CDCA (100 μM). Data are expressed as the mean ± SEM fold change relative to vehicle control (n=6 per group; n=2 per donor). Student’s t-test, *p<0.05 versus DMSO. Inter-donor differences in induction are shown in Figure S2.
4. Discussion
Protein abundance of both drug metabolizing enzymes and transporters are factors for understanding the mechanisms that underlie changes, or inter-patient variability, in drug disposition (International Transporter, et al., 2010; Schaefer, et al., 2012; Shitara, Horie, & Sugiyama, 2006). We attempted to quantify/screen for fourteen phase I DMEs, seventeen phase II DMEs (UGTs) and twenty-nine drug transporters (including Na+/K+ ATPase) by nano-LC/MS/MS targeted proteomics in the SCHH model. We were able to quantify, above concentrations of 0.1 pmol/mg protein, all of the phase I DMEs, ten of the UGTs and twelve drug transporters. Our method is not only suitable for quantifying protein concentrations of these key DMEs and transporters at basal levels in SCHHs, but also for detecting inter-donor differences in expression as well as changes in expression in response to known stimuli. To the best of our knowledge, this is the first study that reports the protein concentration of a comprehensive panel that includes multiple UGTs, CYPs 2J2 and 4F2, and CESs 1 and 2 in the SCHH model, and the effect of known inducers on the protein concentrations of the selected DMEs and transporters quantified (Table S2 and Fig. 5A–C). Though our method employed one million hepatocytes per sample extraction we recovered sufficient amounts of protein (~100 μg) to analyze many replicates and thus could envision applying the method to a lower number of cells (e.g. 250,000 or less).
Immunoblotting is a commonly used technique for identifying and quantifying individual protein concentrations (Mahmood & Yang, 2012). However, the generation of specific antibodies for the DME isoforms is difficult due to the high sequence similarity among the isoforms (Ohtsuki, et al., 2011). In contrast to immunoblotting, isotope dilution targeted quantitative proteomics by nanoLC-MS/MS is a superior technique that quantifies protein concentrations of multiple DMEs and transporters in a single analysis over a wide dynamic range (Aebersold, Burlingame, & Bradshaw, 2013; Fallon, et al., 2008). Although targeted quantitative proteomic methods have demonstrated promise in liver tissue studies (Ohtsuki, et al., 2012; A. Vildhede, et al., 2015), few have been reported for quantifying DMEs and transporters in SCHHs from cryopreserved hepatocytes (A. Vildhede, et al., 2016). Knowledge of protein concentrations of DMEs and transporters in SCHHs allows us to understand the underlying mechanisms of endogenous and exogenous factor-induced changes in expression and function, including hepatobiliary drug disposition. In addition, protein concentrations of DMEs and transporters are useful for developing PBPK models for in vitro-in vivo extrapolation of drug and xenobiotic clearance and for accurately estimating the function of DMEs and transporters (Schaefer, et al., 2012; Xu, et al., 2018).
The SCHH model is a dynamic in vitro hepatocyte model that offers distinct advantages over traditional hepatocyte cultures to study metabolism and transport mechanisms and predict hepatobiliary drug disposition in vivo (Brouwer, et al., 2013; Swift, et al., 2010; Yang, et al., 2016). The SCHHs regain in vivo like properties in culture, including the formation of intact canalicular networks with properly localized transporters (Swift, et al., 2010). In addition to the previously reported protein concentrations of CYPs and transporters, this study reports, for the first time, the targeted proteomic quantification of CYPs 2J2 and 4F2, CESs 1 and 2, and UGTs 1A3, 1A4, 1A6, 1A9, 2A3, 2B4, 2B10 and 2B15, in the SCHH model. We observed higher protein concentrations of DMEs compared to transporters (Fig. 2–4), and CYP450s were more inducible with known inducers than the UGTs and transporters (Fig. 5A–C). The magnitude of induction appeared to vary between donors, illustrating that our method has the sensitivity and precision to discern donor to donor differences in concentration under basal and induced conditions.
To assess the quality of the membrane protein, we included membrane protein markers Na+/K+-ATPase and gamma-GTP in our method. Higher protein abundance of Na+/K+-ATPase in the SCHHs than in our HLM QC indicated enrichment of membrane protein in our samples (Fig. 2–4). Unlike HLM preparations, normally prepared by differential centrifugation, our membrane protein fraction was prepared using a ProteoExtract® Native Membrane Protein Extraction Kit, which employs a differential surfactant fractionation method. The kit extracted samples have greater membrane protein content than microsomal preparations (Xu et al., 2018). Comparison of the mean concentration of Na+/K+-ATPase in our study with Schaefer et al. (2012) revealed that plasma membrane enrichment for transporters in our study was less than half of that observed in the Schaefer et al. study (Table S2). The two step enrichment method (additional ultracentrifugation followed by sucrose gradient enrichment) of Schaefer et al. (2012) therefore enriches cell membrane protein to a greater extent than the method used in the current study. In addition, Schaefer et al. (2012) use 10 million cells per sample, while 1 million cells per sample were used in this study. Given the expense of human hepatocytes, the use of lower numbers of cells is certainly a factor when designing experiments and we have found that the sequential detergent extraction methods provide more efficient membrane fraction recovery, thus allowing for smaller numbers of cells or amounts of tissue. Their enrichment method for CYP450s and UGTs, on the other hand, was just differential centrifugation and thus the Na+/K+-ATPase concentrations that they determined in those extracts were more similar to ours (Table S2).
The basal level of CYP3A4 was highest in the HC4–15 SCHHs. As a result, CYP3A4 was more inducible in response to the known inducer rifampin in the HU1880 and HC3–26 SCHHs than in HC4–15. High protein concentration of CYP3A5 in HU1880 SCHHs suggested that Donor HU1880 was a CYP3A5 expresser (Fig. 2). CYP3A5 protein expression was less inducible by rifampin than CYP3A4 in all three donors (data not shown). As summarized in Table S2, we compared the protein levels of DMEs and transporters in our study with literature data generated in the SCHH model. Expression levels of some of the CYP450s and transporters were comparable to the data previously reported (Badee, Achour, Rostami-Hodjegan, & Galetin, 2015; Bi, et al., 2012; Kimoto, et al., 2012; A. Vildhede, et al., 2016). Comparison of protein concentration of UGTs was only possible for UGTs 1A1 and 2B7 (Schaefer, et al., 2012). While the mean protein concertation of UGT1A1 in our study was similar, the mean protein concentration of UGT2B7 was much lower than that of a previous study (Schaefer, et al., 2012). This could have been because a different peptide was used in their study to report UGT2B7 concentration, illustrating the importance of peptide selection for the targeted proteomic technique (Chiva & Sabido, 2017; Fallon, Neubert, Hyland, et al., 2013). The mean protein concentrations of CYPs 1A2, 2C8, 2C9 and 2D6 were lower than in the previous study, but the concentrations of CYPs 2A6, 2C19, 2E1, 3A4 and 3A5 were higher in our study than in the reported study (Table S2).
Transporters OATPs 1B1,1B3 and 2B1, NTCP, BCRP, BSEP, P-gp, MRP2 and MRP6 mean protein concentrations in our study were similar to those reported by Vildhede et al. (2016), but lower than those reported by Schaefer et al. (2012) (Table S2). Higher CYP3A4 activity (Scandlyn, Stuart, & Rosengren, 2008; Tanaka, 1999) and lower protein expression of P-gp (Schuetz, Furuya, & Schuetz, 1995) in females than in males has been previously reported. Vildhede et al. (2016) used hepatocytes from 2 male and 2 female donors, whereas Schaefer et al. (2012) used hepatocytes from 1 female and 2 male donors. In our study, we used hepatocytes from only female donors. Gender associated differences in protein expression of DMEs and transporters might be one of the reasons why the concentrations of some proteins, such as CYPs 2A6, 2C19, 2E1, 3A4 and 3A5 were higher, but others, such as the transporters, were generally lower in our study than in Schaefer et al. (2012), their two-step sucrose enrichment method for transporters mentioned above notwithstanding. Genetic polymorphisms are another possible factor that determine the protein expression of individual genes. For example, protein expression of CYP3A5 is highly variable among ethnic populations. 80–85% of the Caucasian population are homozygous for the variant CYP3A5*3 allele, resulting in lower protein expression (Birdwell, et al., 2015; Moilanen, et al., 2007). In our study, of the three Caucasian female donors studied, HU1880 seemed to be a CYP3A5 expresser (Fig. 2). However, genotypes of the hepatocytes used in the study were not available.
Not all transporters could be quantified in our study. For instance, MRP4 was not quantifiable in the SCHHs, which is consistent with previous study carried out by Schaefer et al. (2012). They reported a low concentration of MRP4 (0.6 pmol/mg protein) in SCHHs from one of three donors after two-step sucrose enrichment. Additionally, emerging transporters such as OAT2 and OSTα/β are not yet included on our panel. However, we have developed the method to quantify cytosolic drug metabolizing enzymes such as AOX1 and SULTs, but this study did not include the cytosolic fraction for the targeted proteomics.
5. Conclusion
We have successfully quantified the protein concentrations of multiple phase I and II DMEs and transporters in SCHHs from three female Caucasian donors using a targeted quantitative proteomic nanoLC-MS/MS isotope dilution method developed in our laboratory. Since our method can quantify the proteins at basal level and quantify both inter-donor differences in basal expression and changes in expression in response to stimuli, it can serve as a valuable tool in SCHH experiments for studying DME and transporter mediated drug disposition and hepatotoxicity mechanisms. Targeted proteomic quantification of key DMEs and transporters in SCHHs is useful for in vitro-in vivo extrapolation of drug and xenobiotic clearance and developing corresponding PBPK models.
Supplementary Material
Acknowledgments
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the EII and AHA.
Funding
This work was supported by a pilot grant from the Eshelman Institute of Innovation (EII), Chapel Hill, North Carolina to C.R.L., and by American Heart Association (AHA) grants 16GRNT29300003 to C.R.L. and 18POST33960403 to R.K.
Abbreviations
- BCRP
breast cancer resistance protein
- BSEP
bile salt export pump
- CAR
constitutive androstane receptor
- CDCA
chenodeoxycholic acid
- CES
carboxylesterase
- CITCO
6-(4-Chlorophenyl)imidazo[2,1-b] [1,3] thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl) oxime
- Cps
counts per second
- CYP
cytochrome P450
- DME
drug metabolizing enzyme
- DMSO
dimethyl sulfoxide
- FXR
farnesoid x receptor
- HLM
human liver microsomes
- IVIVE
in vitro-in vivo extrapolation
- MRM
multiple reaction monitoring
- MRP
multidrug resistance-associated protein
- Na+/K+-ATPase
sodium potassium ATPase
- NTCP
sodium taurocholate cotransporting polypeptide
- OATP
organic anion transporting polypeptides
- P-gp
permeability glycoprotein
- PXR
pregnane x receptor
- QC
quality control
- SCHH
sandwich-cultured human hepatocytes
- SIL
stable isotope label(ed)
- SPE
solid phase extraction
- UGT
uridine diphosphate-glucuronyltransferase.
Footnotes
Conflict of interest
The authors declared no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aebersold R, Burlingame AL, & Bradshaw RA (2013). Western blots versus selected reaction monitoring assays: time to turn the tables? Mol Cell Proteomics, 12, 2381–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badee J, Achour B, Rostami-Hodjegan A, & Galetin A (2015). Meta-Analysis of Expression of Hepatic Organic Anion-Transporting Polypeptide (OATP) Transporters in Cellular Systems Relative to Human Liver Tissue. Drug Metabolism and Disposition, 43, 424–432. [DOI] [PubMed] [Google Scholar]
- Bi YA, Kimoto E, Sevidal S, Jones HM, Barton HA, Kempshall S, Whalen KM, Zhang H, Ji CJ, Fenner KS, El-Kattan AF, & Lai YR (2012). In Vitro Evaluation of Hepatic Transporter-Mediated Clinical Drug-Drug Interactions: Hepatocyte Model Optimization and Retrospective Investigation. Drug Metabolism and Disposition, 40, 1085–1092. [DOI] [PubMed] [Google Scholar]
- Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ, Leeder JS, van Schaik R, Thummel KE, Klein TE, Caudle KE, & MacPhee IA (2015). Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther, 98, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer KLR, Keppler D, Hoffmaster KA, Bow DAJ, Cheng Y, Lai Y, Palm JE, Stieger B, Evers R, & Consortium IT (2013). In Vitro Methods to Support Transporter Evaluation in Drug Discovery and Development. Clinical Pharmacology & Therapeutics, 94, 95–112. [DOI] [PubMed] [Google Scholar]
- Chiva C, & Sabido E (2017). Peptide Selection for Targeted Protein Quantitation. Journal of Proteome Research, 16, 1376–1380. [DOI] [PubMed] [Google Scholar]
- Fallon JK, Harbourt DE, Maleki SH, Kessler FK, Ritter JK, & Smith PC (2008). Absolute quantification of human uridine-diphosphate glucuronosyl transferase (UGT) enzyme isoforms 1A1 and 1A6 by tandem LC-MS. Drug Metab Lett, 2, 210–222. [DOI] [PubMed] [Google Scholar]
- Fallon JK, Houvig N, Booth-Genthe CL, & Smith PC (2018). Quantification of membrane transporter proteins in human lung and immortalized cell lines using targeted quantitative proteomic analysis by isotope dilution nanoLC-MS/MS. J Pharm Biomed Anal, 154, 150–157. [DOI] [PubMed] [Google Scholar]
- Fallon JK, Neubert H, Goosen TC, & Smith PC (2013). Targeted precise quantification of 12 human recombinant uridine-diphosphate glucuronosyl transferase 1A and 2B isoforms using nano-ultra-high-performance liquid chromatography/tandem mass spectrometry with selected reaction monitoring. Drug Metab Dispos, 41, 2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JK, Neubert H, Hyland R, Goosen TC, & Smith PC (2013). Targeted quantitative proteomics for the analysis of 14 UGT1As and −2Bs in human liver using NanoUPLC-MS/MS with selected reaction monitoring. J Proteome Res, 12, 4402–4413. [DOI] [PubMed] [Google Scholar]
- Fallon JK, Smith PC, Xia CQ, & Kim MS (2016). Quantification of Four Efflux Drug Transporters in Liver and Kidney Across Species Using Targeted Quantitative Proteomics by Isotope Dilution NanoLC-MS/MS. Pharm Res, 33, 2280–2288. [DOI] [PubMed] [Google Scholar]
- International Transporter C, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, & Zhang L (2010). Membrane transporters in drug development. Nat Rev Drug Discov, 9, 215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto E, Yoshida K, Balogh LM, Bi YA, Maeda K, El-Kattan A, Sugiyama Y, & Lai YR (2012). Characterization of Organic Anion Transporting Polypeptide (OATP) Expression and Its Functional Contribution to the Uptake of Substrates in Human Hepatocytes. Molecular Pharmaceutics, 9, 3535–3542. [DOI] [PubMed] [Google Scholar]
- Kumar V, Salphati L, Hop C, Xiao G, Lai Y, Mathias A, Chu X, Humphreys WG, Liao M, Heyward S, & Unadkat JD (2019). A Comparison of Total and Plasma Membrane Abundance of Transporters in Suspended, Plated, Sandwich-Cultured Human Hepatocytes vs. Human Liver Tissue Using Quantitative Targeted Proteomics and Cell-Surface Biotinylation. Drug Metab Dispos. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL (2001). Pregnane × receptor: molecular basis for species differences in CYP3A induction by xenobiotics. Chemico-Biological Interactions, 134, 283–289. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu H, Lu YF, Lei X, Cui JY, Ellis E, Strom SC, & Klaassen CD (2014). Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol Sci, 141, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, Stoltz CA, Kliewer SA, Lambert MH, Willson TM, & Moore JT (2003). Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. Journal of Biological Chemistry, 278, 17277–17283. [DOI] [PubMed] [Google Scholar]
- Mahmood T, & Yang PC (2012). Western blot: technique, theory, and trouble shooting. N Am J Med Sci, 4, 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, & Shan B (1999). Identification of a nuclear receptor for bile acids. Science, 284, 1362–1365. [DOI] [PubMed] [Google Scholar]
- Moilanen AM, Hakkola J, Vaarala MH, Kauppila S, Hirvikoski P, Vuoristo JT, Edwards RJ, & Paavonen TK (2007). Characterization of androgen-regulated expression of CYP3A5 in human prostate. Carcinogenesis, 28, 916–921. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Schaefer O, Kawakami H, Inoue T, Liehner S, Saito A, Ishiguro N, Kishimoto W, Ludwig-Schwellinger E, Ebner T, & Terasaki T (2012). Simultaneous absolute protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases as a novel approach for the characterization of individual human liver: comparison with mRNA levels and activities. Drug Metab Dispos, 40, 83–92. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Uchida Y, Kubo Y, & Terasaki T (2011). Quantitative targeted absolute proteomics-based ADME research as a new path to drug discovery and development: methodology, advantages, strategy, and prospects. J Pharm Sci, 100, 3547–3559. [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, & Lehmann JM (1999). Bile acids: natural ligands for an orphan nuclear receptor. Science, 284, 1365–1368. [DOI] [PubMed] [Google Scholar]
- Scandlyn MJ, Stuart EC, & Rosengren RJ (2008). Sex-specific differences in CYP450 isoforms in humans. Expert Opinion on Drug Metabolism & Toxicology, 4, 413–424. [DOI] [PubMed] [Google Scholar]
- Schaefer O, Ohtsuki S, Kawakami H, Inoue T, Liehner S, Saito A, Sakamoto A, Ishiguro N, Matsumaru T, Terasaki T, & Ebner T (2012). Absolute quantification and differential expression of drug transporters, cytochrome P450 enzymes, and UDP-glucuronosyltransferases in cultured primary human hepatocytes. Drug Metab Dispos, 40, 93–103. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Furuya KN, & Schuetz JD (1995). Interindividual Variation in Expression of P-Glycoprotein in Normal Human Liver and Secondary Hepatic Neoplasms. Journal of Pharmacology and Experimental Therapeutics, 275, 1011–1018. [PubMed] [Google Scholar]
- Shawahna R, Uchida Y, Decleves X, Ohtsuki S, Yousif S, Dauchy S, Jacob A, Chassoux F, Daumas-Duport C, Couraud PO, Terasaki T, & Scherrmann JM (2011). Transcriptomic and Quantitative Proteomic Analysis of Transporters and Drug Metabolizing Enzymes in Freshly Isolated Human Brain Microvessels. Molecular Pharmaceutics, 8, 1332–1341. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Horie T, & Sugiyama Y (2006). Transporters as a determinant of drug clearance and tissue distribution. European Journal of Pharmaceutical Sciences, 27, 425–446. [DOI] [PubMed] [Google Scholar]
- Swift B, Pfeifer ND, & Brouwer KLR (2010). Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metabolism Reviews, 42, 446–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E (1999). Gender-related differences in pharmacokinetics and their clinical significance. Journal of Clinical Pharmacy and Therapeutics, 24, 339–346. [DOI] [PubMed] [Google Scholar]
- Vildhede A, Mateus A, Khan EK, Lai YR, Karlgren M, Artursson P, & Kjellsson MC (2016). Mechanistic Modeling of Pitavastatin Disposition in Sandwich-Cultured Human Hepatocytes: A Proteomics-Informed Bottom-Up Approach. Drug Metabolism and Disposition, 44, 505–516. [DOI] [PubMed] [Google Scholar]
- Vildhede A, Wisniewski JR, Noren A, Karlgren M, & Artursson P (2015). Comparative Proteomic Analysis of Human Liver Tissue and Isolated Hepatocytes with a Focus on Proteins Determining Drug Exposure. Journal of Proteome Research, 14, 3305–3314. [DOI] [PubMed] [Google Scholar]
- Xu MJ, Saxena N, Vrana M, Zhang HY, Kumar V, Billington S, Khojasteh C, Heyward S, Unadkat JD, & Prasad B (2018). Targeted LC-MS/MS Proteomics-Based Strategy To Characterize in Vitro Models Used in Drug Metabolism and Transport Studies. Analytical Chemistry, 90, 11873–11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Guo C, Woodhead JL, St Claire RL, Watkins PB, Siler SQ, Howell BA, & Brouwer KLR (2016). Sandwich-Cultured Hepatocytes as a Tool to Study Drug Disposition and Drug-Induced Liver Injury. Journal of Pharmaceutical Sciences, 105, 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, LaCerte C, Kansra S, Jackson JP, Brouwer KR, & Edwards JE (2017). Comparative potency of obeticholic acid and natural bile acids on FXR in hepatic and intestinal in vitro cell models. Pharmacol Res Perspect, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.