Abstract
Symmetry and other derived stimulus relations are readily demonstrated in humans in a variety of experimental preparations. Comparable emergent relations are more difficult to obtain in other animal species and seem to require certain specialized conditions of training and testing. This article examines some of these conditions with an emphasis on what animal research may be able to tell us about the nature and origins of derived stimulus relations. We focus on two areas that seem most promising: 1) research generated by Urcuioli’s (2008) theory of the conditions necessary to produce symmetry in pigeons, and 2) research that explores the effects of multiple exemplar training on emergent relations. Urcuioli’s theory has successfully predicted emergent relations in pigeons by taking into account their apparent difficulty in abstracting the nominal training stimulus from other stimulus properties such as location and temporal position. Further, whereas multiple exemplar training in non-humans has not consistently yielded arbitrarily-applicable relational responding, there is a growing body of literature showing that it does result in abstracted same-different responding. Our review suggests that although emergent stimulus relations demonstrated in non-humans at present have not yet shown the flexibility or generativity apparent in humans, the research strategies reviewed here provide techniques that may permit the analysis of the origins of derived relational responding.
Keywords: Symmetry, Abstraction, Stimulus equivalence, Multiple exemplar training, Emergent stimulus relations
The remarkable range of complex human behavior has often been analyzed with the goal of assessing the fundamental differences between humans and other animals. Behavioral abilities thought to be uniquely and critically human have included tool-use, building fires, generative grammar, language in general, symbolic processes, mental time travel, theory of mind and dozens more (Deacon, 1998; Pinker, 1994; Suddendorf, 2013). Some of these may be central to human uniqueness, others perhaps epiphenomenal: “Philosophers have often looked for the defining feature of humans — language, rationality, culture, and so on. I’d stick with this: Man is the only animal that likes Tabasco sauce (Bloom, 2010 p. 52).”
Throughout its history, behavior analysis has emphasized the continuity of principles across species. Skinner’s (1956) famous presentation of three cumulative records showing identical patterns of fixed-interval responding from pigeon, rat and monkey set a tone that guided the field. The many examples of complex behavior that are unique to the repertoire of humans were noted by Skinner, but his strategy was always application of basic principles derived from the animal laboratory to account for more complex phenomena including verbal behavior (Skinner, 1957, 1976). However, in recent years, several behavior analysts have suggested a need to propose new, and perhaps uniquely human, processes to account for research on derived stimulus relations (e.g., Hayes, Barnes-Holmes, & Roche, 2001; Hayes & Sanford, 2014; Horne & Lowe, 1996). In this paper, we consider these issues in light of the growing literature on emergent relations in animals. Although there are relatively recent reviews of this literature (e.g., Lionello-DeNolf, 2009; Zentall, Wasserman, & Urcuioli, 2014), controversy remains (Dymond, 2014; Hughes & Barnes-Holmes, 2014; McIlvane, 2014), and the purpose of our paper is to briefly review and reconsider the current status. Our analysis leads us to a focus on two key emerging research areas: 1) studies based on Urcuioli’s (2008) theory and 2) analyses of multiple exemplar training in humans and animals. We believe that developments in these two areas may help to identify the place of animal research in the study of emergent relations.
Where do novel stimulus relations—indeed novel behaviors of any form—come from? This question has posed a major challenge from the earliest days of behavioral science. The way behavior analysts understand these issues changed fundamentally over 40 years ago with the pioneering work of Murray Sidman and the stimulus equivalence paradigm. Although Sidman demonstrated the basic features of stimulus equivalence in the early 1970s in children with intellectual disabilities (e.g., Sidman, 1971), it was the publication of back-to-back articles in the Journal of the Experimental Analysis of Behavior (Sidman & Tailby, 1982; Sidman et al., 1982) that really captured the attention of the larger scientific community. As is now well known, Sidman and Tailby demonstrated several emergent relations in children after conditional discrimination training with physically unrelated stimuli; these were termed reflexivity (in which a stimulus is matched to itself, given A select A), symmetry (after training given A select B, the reversed relation, given B select A emerges) and transitivity (after training given A select B and given B select C, the transitive relation, given A select C, emerges as well as a combined symmetry/transitive relation, given C, select A). The combined emergence of all three relations showed that the trained stimuli had become functionally interchangeable. In the companion piece, Sidman et al., (1982) tested for emergent symmetry in children, rhesus monkeys and baboons after training similar arbitrary conditional discriminations. Unlike most of the children, none of the non-human primates showed emergent symmetry. Taken together, these seminal studies suggested new directions for behavioral accounts of the origins of symbolic and complex verbal behavior in humans, as well as the intriguing possibility that emergent equivalence might be unique to humans.
An explosion of research on stimulus relations in laboratories around the world followed. Much of this research was with human subjects with stimulus equivalence relations of increasing complexity demonstrated in children and adults (McIlvane, 2013; Sidman, 1994). Numerous applications of these techniques were discovered across a wide variety of educational and therapeutic settings (Barnes & Rehfeldt, 2013; Critchfield & Fienup, 2010; O'Donnell & Saunders, 2003; Zinn, Newland, & Ritchie, 2015). The question of where equivalence relations come from led to important theoretical developments with implications for the behavioral analysis of language and cognition. For example, Sidman’s (2000) theory holds that equivalence relations are automatically generated by reinforcement contingencies. His view is that classes are formed relating all elements consistently associated with a given contingency (i.e., sample and comparison stimuli, response and reinforcer). Language and symbolic behavior are thought to be made possible by this process. Another theory contends that the acquisition of some features of language (naming relations) is prerequisite to the demonstration of equivalence relations (Horne & Lowe, 1996). Still another highly influential approach is Relational Frame Theory (RFT) developed by Hayes and colleagues (Hayes, 1991; Hayes et al., 2001). RFT views equivalence relations (termed coordination in RFT) as just one example of arbitrarily-applicable relational responding (AARR) which is seen as higher-order operant behavior shaped by reinforcement across different examples of the relation, i.e., multiple exemplar training. Many different types of AARR have now been explored by researchers working in this tradition, including opposition, hierarchical relations, comparison, distinction, deictics, and spatial/temporal relations (see Hughes & Barnes-Holmes, 2016 for a recent review).
The search for symmetry and other AARRs in non-humans continued as well, but was much less successful. Indeed, Lionello-DeNolf (2009) reviewed 24 published studies of various non-human species and found only two showing consistent evidence of symmetry. Given these difficulties, it is certainly possible that some aspects of derived stimulus relations are uniquely human; as Hayes and Sanford put it: “No nonhuman animal has yet shown the defining features of relational framing, and the centrality of relational framing to complex human behavior is very evident...” (Hayes & Sanford, 2014, p. 125). Hayes and Sanford argue that the development of cooperative social behavior in early humans created an environment in which the ability to derive simple forms of AARRs such as symmetry and equivalence (frames of coordination) was selected and this ability was refined over time to become a uniquely human behavioral process.
However, many researchers have not been willing to concede that the difficulties in demonstrating symmetry in non-humans reflect a fundamental difference in human-animal processes. There are a number of possible explanations for these negative results other than species differences (Sidman et al., 1982). There are many challenges in creating comparable conditions in the animal and human laboratories. For example, most human studies make heavy use of instructions to initiate behavior and sustain it during unreinforced probe trials making comparison with animal studies problematic. The issue most often raised is the difficulty of identifying the controlling stimuli which may not be those intended by the experimenter (Dube, McIlvane, Callahan, & Stoddard, 1993; McIlvane & Dube, 2003; McIlvane, Serna, Dube, & Stromer, 2000). Perhaps the problem is not with the limited abilities of our animal subjects, but with our lack of experimental sophistication in framing the question in such a way that animals can give us a meaningful answer. Indeed, over the years a number of different paradigms and procedures have been designed to assess such possibilities in animals.
Zentall et al., (2014) reviewed this literature and concluded that there is evidence for the emergence of arbitrary stimulus relations in nonhumans and that it is premature to conclude that different processes are required to account for derived relations in humans: “The research we have reviewed here argues against that human-animal distinction: animals can indeed acquire and adaptively deploy associative concepts” (p. 147). These conclusions generated considerable controversy (e.g., Dymond, 2014; Hughes & Barnes-Holmes, 2014; McIlvane, 2014), in part because the review considered a variety of procedures other than the traditional stimulus equivalence paradigm which is the focus here (e.g., Urcuioli, Zentall, Jackson-Smith, & Steirn, 1989; Vaughn, 1988). However, among the procedures Zentall et al., (2014) consider is a technique to demonstrate symmetry in pigeons that was both successful and replicable (Frank & Wasserman, 2005) and which has led to a novel theory of derived stimulus relations in pigeons (Urcuioli, 2008). We now consider the research that led to Urcuioli’s theory and additional studies generated by it that may provide a novel account of derived relations in animals.
Symmetry in the Pigeon: Urcuioli’s Theory
Prior to Frank and Wasserman (2005) many studies had tested for symmetry in pigeons, but most were unsuccessful (e.g., Hogan & Zentall, 1977; Lipkens, Kop, & Matthijs, 1988; Rodewald, 1974), so their demonstration of symmetry was certainly surprising. Which of the several unusual features in the Frank and Wasserman study were critical to the successful outcome? Urcuioli (2008) isolated three aspects of their procedure that he hypothesized were necessary and which led him to a new theory of emergent relations. First, instead of the traditional simultaneous matching-to-sample (MTS) arrangement, Frank and Wasserman used a successive (go, no-go) discrimination training procedure in which both the sample and comparison stimuli were presented on the center key. The notion that presenting both sample and comparison stimuli in the same location might be critical came from several previous studies that showed that stimulus location can control responding in simultaneous MTS tasks in rats, monkeys and pigeons (Iversen, 1997; Iversen, Sidman, & Carrigan, 1986; Lionello & Urcuioli, 1998). These studies all demonstrated that successful matching broke down when the stimulus location was changed.
That location is part of the functional stimulus in MTS in animals may help explain the failure to obtain symmetry in most studies using simultaneous MTS procedures. If sample stimulus A is presented, say, on the center key and comparison stimulus B is presented on one of the side keys during training, note that during the symmetry test stimulus B is presented as a sample on the center key and comparison stimulus A is now presented on one of the side keys. From the pigeon’s perspective, these are simply not the same stimuli used in training: Acenter and Bcenter are not equivalent to Aside key or Bside key and so it is as if completely novel stimuli are presented on the symmetry test. No wonder that derived symmetry relations fail to emerge! Using a go, no-go procedure with a single stimulus location as Frank and Wasserman (2005) did does not remove the possibility of control by location, but it does mean that location is the same in training and testing with all stimuli and should not interfere with the emergence of symmetry.
A second feature of a successive discrimination procedure that Urcuioli (2008) considered critical to the demonstration of symmetry is that it ensures forced exposure to each trial, whether positive or negative, such that each negative trial is associated with extinction whereas each positive trial ends in reinforcement. In contrast, as more successful performances develop with simultaneous discrimination, there is less contact with incorrect comparisons and fewer unreinforced responses. This is highlighted by the fact that studies using simultaneous discriminations so often fail to result in symmetry even when training otherwise comparable to Frank and Wasserman’s (2005) was used (e.g., Lionello-DeNolf & Urcuioli, 2002).
The third important aspect of the Frank and Wasserman (2005) study was that they trained identity matching with the same stimuli used in the arbitrary conditional discrimination. Urcuioli (2008) hypothesized that identity training was critical to the demonstration of symmetry because the temporal position of the stimuli (e.g., samples always presented first, comparisons always presented second) might also come to control responding. If this is true, once again, symmetry would not be expected to occur even using a successive MTS procedure after arbitrary MTS training alone. For example, consider that the researcher trains the bird to select Bcomparison after Asample. On the symmetry test, Bsample is presented, but is a novel stimulus to the bird and is unrelated to Acomparison which is also a novel stimulus. Urcuioli hypothesized that identity training in which birds were trained to select Acomparison following Asample and Bcomparison following Bsample created two stimulus classes: one with A in both sample and comparison positions, and the other with B in both positions. The AB arbitrary training would create a third class including Asample and Bcomparison and class merger would then result in a four-member class including Asample, Acomparison, Bsample and Bcomparison. The formation of this class would predict a positive symmetry test because Bsample and Acomparison are now class members.
Urcuioli (2008) tested this hypothesis in two ways. First, he replicated the Frank and Wasserman (2005) study with different stimuli (Experiment 3). Using a go, no-go procedure, pigeons were trained on two arbitrary conditional discriminations (Redsample ➔Trianglecomparison and Greensample➔Horizontalcomparison) and all four identity relations (Redsample ➔Redcomparison, Greensample➔Greencomparison, etc.). This training was predicted to produce two, four-member classes (1: Greensample, Greencomparison, Horizontalsample, Horizontalcomparison; 2: Redsample, Redcomparison, Trianglesample, Trianglecomparison—see Fig. 1, left panel) and thus successful symmetry tests (Trianglesample ➔ Redcomparison and Horizontalsample ➔ Greencomparison) which were obtained in most birds. That is, Urcuioli found higher rates of responding on unreinforced symmetry probe trials than on non-symmetry probes.
Fig. 1.
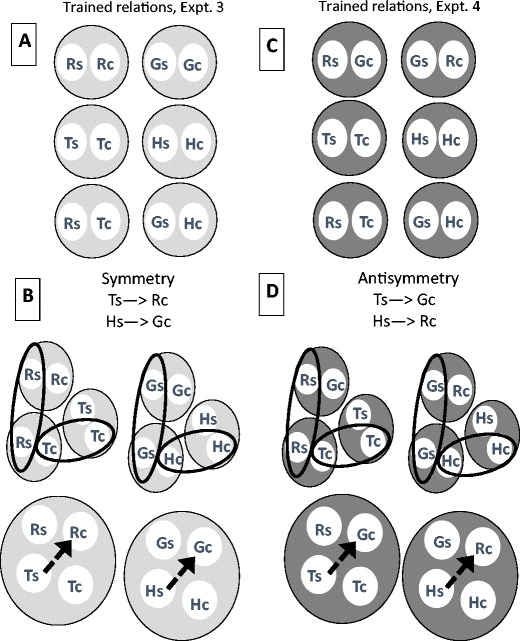
The left panels (Expt 3) provide an illustration of the trained and emergent relations in Urcuioli (2008, Experiment 3). The uppercase letters stand for characteristics of the training stimuli (R = red; G = green; T = triangle; H = horizontal line) and the lowercase letters “s” and “c” refer to the sample and comparison positions respectively. Trained relations and the two-member classes they form are depicted at the top (A). In the panels below (B), the common stimuli that result in class merger are circled, and the four-member classes that result are depicted. The dotted arrow shows that emergent symmetry is predicted. The right panels (Expt 4) provide a comparable illustration for Urcuioli (2008, Experiment 4) with trained relations at the top (C), and class merger and four-member classes below (D). The dotted line shows that anti-symmetry, not symmetry, is predicted
As a further test of the hypothesis, Urcuioli (2008) conducted a follow-up experiment (Experiment 4) which was a replication of the previous study except that for the colors an oddity relation was trained rather than an identity relation (Redsample➔Greencomparison; Greensample➔Redcomparison). Urcuioli’s theory now predicts that class merger will result in Trianglesample and Greencomparison in one class and Horizontalsample and Redcomparison in another (see Fig. 1, right panel). This leads to the remarkable prediction that pigeons should respond less to a reversal of the trained arbitrary relations (symmetry) and more to a reversal of the untrained relations (anti-symmetry), and indeed most of the pigeons tested did show evidence of anti-symmetry.
Thus, Urcuioli (2008) provided strong support for the hypothesis that in pigeons the functional stimulus in MTS training includes the nominal stimulus (what), the stimulus location (where), and the temporal position—sample or comparison (when). To Urcuioli, specialized training that takes these variables into consideration can produce symmetry, or indeed antisymmetry, through class merger. Since the formulation of this theory, Urcuioli and his colleagues have systematically tested the theory across a variety of conditions. These include replicating and extending the original findings of symmetry and anti-symmetry (Campos, Urcuioli, & Swisher, 2014; Urcuioli & Swisher, 2012a) and demonstrations of reflexivity (Sweeney & Urcuioli, 2010; Urcuioli, 2011; Urcuioli & Swisher, 2012b) and transitivity (Urcuioli & Swisher, 2015). Overall, these studies have provided consistent support for Urcuioli’s theory (see Urcuioli, 2015 for a more detailed review).
Urcuioli (2015) has been clear that his theory is an account of emergent relations in pigeons, but it certainly has potential to provide a more general account of why non-humans so often fail traditionally formulated symmetry tests. However, at present, all of the support for Urcuioli’s theory comes from research with pigeons and an important addition to the research agenda for students of stimulus relations is to extend these findings to other non-human species. A study in our laboratory attempted to replicate Urcuioli’s (2008) Experiment 3 in rats using odor stimuli (Prichard, Panoz-Brown, Bruce, & Galizio, 2015). Rats learned two arbitrary conditional discriminations along with identity relations between all stimuli, but none of the seven rats that completed training showed evidence of symmetry. One interpretation of these findings is that a different account of MTS may be required for rats, but an alternative view is that translating the procedures used in the pigeon laboratory to rats needs further refinement.
In any case, the importance of testing the hypothesis in other species is clear. If Urcuioli’s theory is applicable to other non-human animals, it suggests an answer to the question of what conditions are needed for non-humans to show emergent symmetry and other AARRs. This interpretation bears out the wisdom of the caveat noted by Sidman et al. (1982) in the original “Search for Symmetry…” paper that “Incorrect specification by the experimenter of the controlling stimuli in the conditional discrimination may be the most fundamental factor underlying the absence of symmetry (p. 43).” Urcuioli’s theory may thus be seen as consistent with Sidman’s (2000) hypothesis about the origin of equivalence relations adapted to the special case of stimulus control in pigeons.1 Why animals fail to show emergent symmetry relations has always been a puzzle with respect to Sidman’s account, and the Urcuioli theory potentially resolves it by showing that when the functional discriminative stimuli controlling the pigeon’s behavior are brought into line with those of the experimenter, symmetry and other derived relations indeed emerge (see Dube & McIlvane, 1996; McIlvane & Dube, 2003, and McIlvane et al., 2000, for more detailed discussion of these issues).
That being said, does Urcuioli’s theory permit us to reject the argument that humans are unique in deriving AARRs? That notion has become controversial. For example, Hughes and Barnes-Holmes (2014) have argued that the procedural restrictions required to produce symmetry in pigeons differ functionally from AARRs in humans. In humans, the minimal training to produce symmetry involves only two nominal stimuli (e.g., AsampleBcomparison➔BsampleAcomparison). But remember that from the perspective of Urcuioli’s theory, the above training given to pigeons involves not two, but four stimuli. If his theory is correct, it isn’t even possible to conduct a proper symmetry test in the pigeon. Indeed, the Urcuioli paradigm seems to fail to capture the essential feature of symmetry in humans: the reversibility of stimulus relations. To produce symmetry in pigeons, training requires not only AsampleBcomparison, but also Asample Acomparison and BsampleBcomparison, which leads through class merger to the formation of a four-member class including all four of the above terms (see Fig. 2). As depicted in Fig. 2, this class merger requires two-nodes (Asample and Bcomparison) to permit the symmetry relation to emerge, and what we see as symmetry between the nominal stimuli in fact requires combined equivalence relations. Making this same point, Urcuioli (2008) argued that although this may not be conventional Sidman symmetry, it still demonstrates stimulus relations that include both transitivity and symmetry in their derivation. That acknowledged, the symmetry demonstrated in the Urcuioli procedure still does not involve a straightforward reversibility of trained relations and in that sense, fails to meet the definition of symmetry as seen in humans. This issue is nicely illustrated in the case of anti-symmetry in which four-member classes are also posited to develop, but reversibility is clearly absent. Thus, when Urcuioli describes his research program as a successful search for symmetry (Urcuioli, 2015), he is speaking of the presence of symmetry in the combined equivalence relation that is required to produce the observed probe performances. When critics challenge the relevance of this account to AARR, they are focusing on the fact that bidirectional relations emerge in humans with no special training requirements. From our perspective, both points are valid and, importantly, suggest another critical question: why is it that humans show AARRs without these special conditions needed to bring them about in pigeons?
Fig. 2.
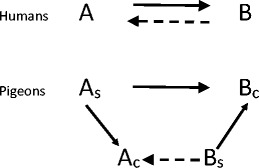
A comparison of the steps required to produce symmetry relations in a typical experiment with human participants (top) and in the Urcuioli procedures with pigeons (below). Uppercase letters A and B refer to the nominal stimuli and lowercase s and c refer to the sample and comparison positions respectively. Arrows point from sample to comparison stimuli. Solid arrows represent explicitly trained relations, whereas broken arrows represent emergent relations. Note that symmetry emerges in humans after training a single relation (top), but that three relations are trained in the Urcuioli procedure. The emergent Bs ➔ Ac relation is thus an example of a two-node (As and Bc) transitivity/equivalence relations in pigeons
Humans show emergent symmetry and other equivalence relations across a broad range of conditions including both simultaneous and successive conditional discrimination procedures, stimulus pairing, sorting, and a host of others (see Hughes & Barnes-Holmes, 2016; Pilgrim & Galizio, 1996, for reviews). Clearly humans do not need any special training to separate the nominal stimulus from its physical or temporal position in MTS training. With apologies to William Shakespeare and Gertrude Stein, a rose in any other place or time is still a rose. In humans, abstraction of an object from other stimulus properties, such as where or when it is presented, is evident very early in children’s development as shown by naming and object recognition. Developmental psychologists often attribute this early abstraction to innate dispositions and faculties (e.g., Baillargeon & Carey, 2012); however, behavior analysts would generally emphasize the child’s history of reinforcement for selection of stimuli independently of their spatial and temporal positions (among other properties).
From our perspective, this history is what non-human animals lack; importantly, Urcuioli’s training generates emergent relations through a different mechanism. The Urcuioli paradigm trains an explicit relation between a particular object and its temporal position rather than a more generalized form of abstraction. Hughes and Barnes-Holmes (2014) noted that the emergent relations demonstrated in pigeons lack flexibility and generativity relative to those in humans which are not tied to particular procedures (see also McIlvane, 2014). The requirement to train relations between nominal stimuli, locations and temporal positions before any test of derived relations would certainly limit the applicability of the Urcuioli paradigm to the study of the more complex relations observed in humans. To obtain the sort of flexibility seen in humans, training which results in a generalized abstraction of the nominal stimulus from other irrelevant features must be established. There is a growing literature on the conditions necessary to bring such abstraction about and it focuses on multiple exemplar training (MET) which is considered in the next section.
Multiple Exemplar Training
Catania (2013) defines abstraction as “…discrimination based on a single stimulus property, independent of other properties; thus generalization among all stimuli with that property” (p. 428). Behavior analytic accounts generally assume that multiple exemplar training is required to produce abstraction. Differential reinforcement of responding to a red ball and not to a blue truck would likely not be sufficient to bring behavior under the control of the color red. Rather reinforcement of responding to multiple examples of various red objects and non-reinforcement of responding to objects of other colors would eventually do the trick. That multiple exemplar training might also come to produce relational responding goes back at least to Skinner (1953) who noted that organisms can learn through differential reinforcement to respond on the basis of relations such as the size of an object. Learning to respond to relative size would be an example of non-arbitrarily-applicable relational responding (NAARR) because it is based strictly on the physical or formal relations between stimuli. Note that the sort of MET required to produce abstraction of a stimulus property such as color is somewhat different than that which produces NAARR. For an NAARR such as relative size MET would involve presentation of numerous different stimulus pairs rather than single objects. These stimuli might differ in many features in addition to size. Selection of the larger (or smaller) stimulus is consistently reinforced across pairs eventually giving rise to control by the size relation.
MET plays a special role in RFT which holds that it is necessary to create AARR. The way that MET is posited to create AARR is similar to the process of shaping NAARR, however in order to shape AARR, MET requires training of coordinated relations rather than a single relation. In the case of symmetry, bidirectional training is necessary: in a particular context, selection of B is reinforced given A as a sample, and selection of A is reinforced given B as a sample. For example, consider a young child looking at a picture book with a parent. The parent might point to a picture of a flower and ask the child “what is it?” and subsequently reinforce the child’s response “rose.” Alternatively, the parent might ask child “where is the rose?” and reinforce selection of the correct flower. This interrelated training differentially reinforces a specific symmetry relation between the word “rose” and the flower pictured, but if it is extended across many different words and pictures, then the AARR of generalized symmetry is thought to be eventually abstracted from the various exemplars.
RFT considers AARRs to be examples of higher order or overarching operant classes similar in nature to generalized imitation and operant variability (Neuringer, 2002; Baer & Sherman, 1964). The origins of these complex operant classes are controversial and poorly understood (see discussion by Galizio, 2003; Pilgrim & Galizio, 2000), but RFT researchers have been very active in recent years in efforts to clarify the origins of AARRs. There are now numerous studies that illustrate the development of a variety of different relational frames through MET (see Hughes & Barnes-Holmes, 2016, for a review). A limitation of many of these studies is that the relational operant is likely to have been initially shaped through the participant’s extra-experimental history. The laboratory training is providing new exemplars for an AARR (e.g., coordination, opposition) that was likely already established early in life. This issue is most clearly evident when adult participants are studied, but there is a growing literature on children and infants aimed to address this issue.
MET and Derived Stimulus Relations in Human Infants and Children
Because humans are typically exposed to verbal environments from birth, it is extraordinarily important to study the origins of AARR in infants who are more naïve with respect to them. There are several critical studies of equivalence relations in infants. The first was a study by Lipkens, Hayes and Hayes (1993) who began training with a 16-month old infant and showed the emergence of symmetry relations as early as 17 months. Pelaez, Gewirtz, Sanchez, and Mahabir (2000) conducted a more extensive equivalence study in nine infants aged 21–25 months. Interestingly, they found transitivity in eight of the nine infants, but, not unlike the animal research, symmetry was not consistently evident (only one of the eight infants tested averaged above 80% correct on all symmetry trials). Luciano, Becerra, and Valverde (2007) provided crucial evidence regarding MET in the youngest infant studied to date (15 months, 24 days). The infant initially twice failed a symmetry test—she was given the name of an object, but then failed to select it when asked to pick it up. Following MET with 10 new objects, she then showed emergent symmetry at the age of 16 months, 25 days. This study was among the first to provide direct evidence that MET can result in the emergence of a novel AARR of symmetry (mutual entailment in RFT terminology).
However, it may not be safe to assume that the MET provided by Luciano et al. (2007) was sufficient to produce the emergent relations. Infants at this age are certainly exposed to daily bidirectional word-object training from caregivers and have already developed a substantial receptive vocabulary. Indeed, like virtually all studies with infants and children, Luciano conducted training and testing with verbal prompts (e.g., look at this, give me the ____, what is it?). The use of verbal prompts, questions and instructions may be critical to obtaining emergent relations in infants and children. For example, developmental psychologists have found that the way in which questions are worded can play a significant role in concept and category learning in infants as young as 12 months (Waxman & Gelman, 2010). Further, Pilgrim, Jackson, and Galizio (2000) were unable to successfully train arbitrary conditional discriminations in three- to six-year old children until verbal prompts were added to the training procedure. The role of such verbal interventions in the acquisition of conditional discriminations and emergence of untrained relations is not well understood, but obviously of considerable importance. Since the Luciano et al. study, a number of additional experiments have been conducted with older children showing that, through MET, the development of AARRs that were not previously in the child’s repertoire, including opposition, comparison, and perspective-taking (deictic) relations, could be acquired (e.g., Barnes-Holmes, Barnes-Holmes, Smeets, Strand, & Friman, 2004; Berens & Hayes, 2007; Gorham, Barnes-Holmes, Barnes-Holmes, & Berens, 2009; Heagle & Rehfeldt, 2006; Weil, Hayes, & Capurro, 2011). However, it should be noted that the methodology of these studies relied extensively on verbal questions and prompts that required a relatively sophisticated repertoire of verbal behavior.
In sum, although evidence is accumulating that links MET with the emergence of a variety of different types of relations in infants and children, it may yet be premature to conclude that MET is sufficient to produce AARRs de novo. While neither the difficulties nor the value of these studies on stimulus relations in infants and young children can be over-emphasized, still, as noted above, there are limits on the control that can be exerted in such research. Control over the subject’s behavioral history is one of the central virtues of animal research, and it is here that studies of MET in non-humans can make an important contribution. Hughes and Barnes-Holmes (2016) make this point well: “Animal preparations and populations offer an opportunity to ask questions about AARR that cannot be answered with humans for ethical and practical reasons. This work could help us disentangle the history of learning involved in establishing and manipulating relational responding as generalized operant behavior” (p. 160). However, only a few studies of MET of AARRs in non-human subjects have been conducted, and these are considered next.
MET and Derived Stimulus Relations in Animals
Perhaps the first study to provide a convincing demonstration of stimulus equivalence in non-humans was Schusterman and Kastak’s (1993) classic study of the sea lion Rio. Schusterman and Kastak initially trained 30 AB relations between black and white visual stimuli. Rio failed tests of BA symmetry with five out of the first six stimulus pairs. Only after MET with these pairs did Rio show successful emergent symmetry on subsequent tests. Subsequently 30 BC relations were trained and Rio passed tests for emergent CB symmetry as well as transitivity (AC) and equivalence (CA). Each test was followed by additional training with derived relations and accuracy on new relations continued to improve with increased MET. This outcome is often used to make the point that MET is critical to demonstrating derived relations in animals. However, it is worth noting that Rio had an extensive history with similar procedures prior to the experiment including demonstration of generalized identity matching (Schusterman & Kastak, 1993). In a subsequent study, Kastak, Schusterman, and Kastak (2001) were able to demonstrate symmetry in a different sea lion (Rocky) and replicate it in Rio after initial training on a functional equivalence task. Rocky also had a complex pre-experimental history including a failure to show symmetry in the Schusterman and Kastak (1993) study.
Unfortunately, there are only a few studies that have attempted to replicate the effects of MET in other species. Some groups have examined MET in pigeons. Lionello-DeNolf and Urcuioli (2002) trained 12 birds on a conditional discrimination (e.g., AB) and were tested for symmetry under conditions in which reinforcement was provided for symmetry responses in one group (consistent), but responses inconsistent with symmetry were reinforced for the other group (inconsistent). More rapid acquisition in the consistent group compared to the inconsistent group would have suggested the emergence of symmetry, but this failed to occur. Lionello-DeNolf and Urcuioli then continued to train the symmetry relation (i.e., AB and BA relations were both reinforced) in the consistent group and trained the opposite relations in the inconsistent group. A new set of conditional discriminations was then established (e.g., BC) followed by a CB symmetry test conducted in the same way. The consistent group differed from the inconsistent group on the first two test sessions but overall evidence for symmetry was weak. An additional round of symmetry training (both BC and CB relations reinforced in the consistent group, the opposite in the inconsistent group) was followed by training another new conditional discrimination (e.g., AD), but despite the MET, now with two stimulus sets, there was no evidence of DA symmetry.
Velasco, Huziwara, Machado, and Tomanari (2010) noted that in the Lionello-DeNolf and Urcuioli (2002) study the C and D stimuli used in the second and third symmetry tests were presented as sample stimuli for the first time in those tests. They reasoned that this might have limited the emergence of symmetry because this required the birds to make successive discriminations between the C and D stimuli for the first time. Velasco, et al. developed a procedure that provided experience with both simultaneous and successive discriminations among all stimuli. Four pigeons were first tested after training two conditional discriminations and none showed evidence of symmetry. After training these symmetry relations to criterion, two new conditional discriminations were trained and symmetry was assessed again. Of the four birds, one bird showed fairly strong evidence of symmetry, and another two showed at least some trend toward symmetry following MET training with only two exemplars, which was interpreted as supporting the claim that MET increased the likelihood of observing symmetry.
However, a more recent study with pigeons (Gomez, Garcia, & Perez, 2014) did not find evidence of improvement in emergent symmetry after MET. This study was quite extensive in that pigeons were trained on 4 to 24 different conditional discriminations over a period of four years, yet none of the pigeons showed evidence of symmetry throughout the study. Although the design used by Gomez et al. did not permit training of both successive and simultaneous discriminations with the stimuli prior to the symmetry tests as did Velasco et al., the failure to observe symmetry here is still striking. None of the other pigeon studies provided as much MET over such an extensive time period as was accomplished in Gomez et al. Indeed, the difficulty of studying MET in some species due to the length of time required to learn multiple conditional discriminations is well-illustrated by this study.
Several laboratories have studied MET in non-human primates with somewhat mixed results. Yamamoto and Asano (1995) trained arbitrary colorsample-lexigramcomparison conditional discriminations in a chimpanzee and found no evidence of symmetry until they had trained symmetry relations (lexigramsample-colorcomparison) for six pairs. Subsequently, training of three new colorsample-lexigramcomparison pairs led to above chance accuracy on untrained symmetry tests. These findings provided some support for the hypothesis that MET had led to generalized symmetry, but follow-up testing suggested that this was highly limited. A new set of lexigramsample-Chinese charactercomparison was trained, but symmetry failed to emerge. Thus, evidence of generalized symmetry in Yamamoto and Asano’s study was, at best, constrained to color-lexigram stimulus pairs.
Dugdale and Lowe (2000) tested for symmetry in two chimpanzees with extensive histories of lexigram training as part of a well-known language training project (Savage-Rumbaugh, 1986). These animals were trained to select arbitrary lexigrams in the presence of particular objects (generally food items or other reinforcers). They were also trained on the reverse of these tests with selection of the object in the presence of the lexigrams reinforced, i.e., symmetry was trained. As these chimpanzees received training on more than 90 different lexigram-object relations over a period of 10 years, this study is likely to be the most extensive extant example of MET. Dugdale and Lowe trained arbitrary conditional discriminations with characters (Y or zig-zag) as samples and colors (red or green) as comparisons. After meeting accuracy criteria on the baseline conditional discriminations (curiously only after much difficulty), symmetry tests were given and both animals performed at chance levels. This study is frequently considered among the more spectacular failures to demonstrate symmetry in animals because of the species studied and the extensive MET and related language-training experiences in the chimpanzees’ history.
Finally, a more recent experiment has examined symmetry after MET in a capuchin monkey with an extensive history of MTS training yielding generalized identity matching, but also with previous failures to show symmetry (Brino et al., 2014a; Brino, Campos, Galvão, & McIlvane, 2014b). Three arbitrary (AB) relations were trained in a simultaneous MTS procedure along with two reversed (BA relations). In this way, symmetry relations were reinforced through MET. Subsequently, symmetry tests were given for the remaining untrained BA relation. Responding consistent with symmetry was observed until special test trials were introduced. On these test trials the negative comparison stimulus was blank (a white square). On tests that presented only the blank stimulus along with the A stimulus, selection of the A stimulus would indicate symmetry responding, but the capuchin reliably chose the blank stimulus. This finding indicates that the apparent demonstration of symmetry on other test trial types was a “false positive.” The A stimulus was selected when the other comparison stimuli were negative comparisons, but not when there were no negative stimuli to reject. Thus, responding was not controlled by selection of the A stimulus (symmetry), but rather by the presence of negative comparison stimuli—a “reject” relation. Thus, the Brino et al. study must be considered another failure of MET to produce emergent symmetry in non-humans, as well as a cautionary tale of the complex forms of stimulus control that may emerge in experiments such as these and the need for appropriate controls to detect them.
In summary, most studies with non-humans have failed to find that MET of bidirectional stimulus relations can produce a generalized form of symmetry. However, there are significant procedural limitations in these studies that may have hindered an effective demonstration of emergent symmetry. For example, only six or fewer exemplars were trained in most of the studies (Brino et al., 2014a, 2014b; Lionello-DeNolf & Urcuioli, 2002; Velasco et al., 2010; Yamamoto & Asano, 1995). Of course there is no real way to know how many exemplars of reinforced symmetry might be needed to produce AARR. Schusterman and Kastak’s (1993) successful demonstration occurred after MET training with only six stimulus pairs. Yet even after bidirectional training with more than 90 stimulus pairs, Dugdale and Lowe’s chimpanzees failed subsequent symmetry tests. It should be noted, however, that their MET was with lexigrams and objects, but the apparatus and types of stimuli used during the symmetry tests (colors and arbitrary shapes) were quite different. A similar issue might be involved in the Yamamoto and Asano study as their one successful demonstration of symmetry followed MET with the same general form of stimuli used on the symmetry test (colors/lexigrams) and the failed symmetry test involved a different type of stimuli (Chinese characters). As relational responding in humans is generally understood to be under contextual control, it seems possible that contextual changes of these sorts might have disrupted stimulus control in the chimpanzees and prevented the observation of emergent symmetry. There is still work to do before the question of whether MET can produce AARR in non-humans can be put to rest.
That being said, it must be conceded that a replicable procedure in which MET yields AARR in non-humans has yet to be demonstrated convincingly. This failure may well be seen as consistent with the possibility that AARR is a uniquely human characteristic, at least when developed through MET (Hayes & Sanford, 2014). However, does this imply that the traditional behavior analytic strategy of identifying and analyzing basic principles and processes in non-humans is invalid in the search to understand derived stimulus relations? Does it follow, as Hayes (2016) noted, that in the wake of these developments “Animal laboratories were immediately much less important…” (p. 14)?
We certainly believe that an increased emphasis on research with adult humans, children and infants in basic research laboratories and applied settings is a most welcome and necessary development for the field of behavior analysis (cf. Baron, Perone, & Galizio, 1991a, 1991b). However, the logistic and ethical difficulties in studying the acquisition of AARRs in naïve infants (those without a pre-existing verbal repertoire) highlight the potential value of continuing the search in non-humans. Further, even if it turns out that AARRs of the sort described in RFT represent a uniquely human trait, the animal laboratory may yet play a crucial role. As discussed above, there is value in the development of strategies and procedures like those of Urcuioli (2008) that provide examples of AARR-like behavior in animals, even though these behaviors may develop differently from those in humans (see Zentall et al., 2014, for several additional examples). Another potential role for additional research with non-humans may be in the analysis of more basic forms of relational responding as a model preparation.
NAARR in Animals
The most likely candidate for a rudimentary or prerequisite process on which selection might have operated in early humans is relational responding based on physical or non-arbitrary stimulus dimensions, that is, NAARR. RFT theorists have noted that NAARR is found in a wide range of species and represent examples of overarching higher-order operant behavior that is shaped by MET which, except for the level of abstraction, is similar to AARR. As Hayes et al. (2001) put it:
Organisms learn to discriminate the relevant stimulus relation, as well as the formal dimension along which the relation is relevant, through multiple training trials in which the relata vary. If selecting only the larger of two stimulus objects is reinforced over a series of trials with varying objects, there is no reason to be surprised if an organism begins to respond to the relation between the stimuli rather than their absolute characteristics. The consequences have shaped just such a response class. (p. 25)
This certainly seems plausible, but ironically, it might just make the study of NAARR in non-humans immediately more important! This is because the literature available on the role of MET in developing NAARRs is, at best, rather scant. There is a relatively untapped potential to learn more about the determinants of relational operant behavior through research on NAARRs in the animal laboratory. Here we will briefly discuss research on two topics that are probably the most widely studied NAARRs: transposition and identity matching.
MET and Transposition
The question of whether relational responding is possible in non-humans was famously addressed in the analysis of the phenomenon termed transposition which became the experimental battleground for Gestalt and S-R psychology. Wolfgang Kohler (1918/1938) trained simultaneous discriminations between two shades of gray in chickens and chimpanzees. After training, organisms were tested with different stimulus pairs and Kohler found that they responded relationally—selecting the lighter or darker shades depending on the direction of training. Borrowing from the musical term, he labeled this effect transposition. However, Spence’s (1937) elegant mathematical model showed that transposition could be predicted by the interaction of gradients of excitation and inhibition without recourse to relational responding. Decades of research on transposition and the related phenomenon of peak shift (Hanson, 1959) followed and, although the Spence model successfully accounted for many of the experimental outcomes, at least some seem to require a relational account (see reviews by Lazareva, 2012, and Reese, 1968).
Some of the classic studies in the transposition literature employed multiple relational examples in training (e.g., Lawrence & DeRivera, 1954), but most studies used only a single positive and negative stimulus—that is, they did not provide MET. So, to the extent that relational responding did occur in these experiments, it seems to have developed without MET. More recently, three studies assessed the effects of MET on transposition in pigeons trained to respond to circles varying in size (Lazareva, Miner, Wasserman, & Young, 2008; Lazareva, Young & Wasserman, 2014; Lazareva, Wasserman, & Young, 2005). In these studies, selection of the larger circle (or smaller in different groups) was reinforced with one, two or three training stimulus pairs. In all three studies, percent of transposition responses increased as a function of the number of training exemplars, suggesting that MET increased relational responding. A follow-up experiment by Lazareva et al. (2014) extended the analysis to a novel faster-slower discrimination with the speed of object motion in a video frame, but results here were less supportive; percent transposition responses was fairly high after training with just one stimulus pair and did not show much increase in birds trained with two pairs. The circle size studies are consistent with the hypothesis that transposition can be viewed as an NAARR that is at least enhanced by MET, but the object motion study suggests that there is much we have yet to learn about the determinants of relational responding in the transposition paradigm. Overall, these findings suggest the possibility that research on transposition and peak shift might become useful to address a new set of theoretical issues—they point the way to a methodology that can be used to study MET and higher-order operants in animals (see Lazareva, 2012).
Is MET Necessary for Generalized Identity Matching?
The same-different relation is perhaps the most frequently studied example of abstract concept learning in non-humans. It is also the area in which the most systematic research on MET has been conducted. Before we discuss the MET research, some background is needed. Two general procedures (MTS and same-different) have become widely used to study same-different relations. Identity (MTS) and oddity (non-matching-to sample; NMTS) are often studied using either simultaneous or successive discrimination procedures (McIlvane, 2013). In these procedures, responding to the stimulus that is identical to (or different from) the sample is reinforced. However, in the same-different procedure, two separate responses are available to the animal, and on trials when the sample and comparison stimuli are identical, responding on the “same” lever or response key is reinforced, but when sample and comparison differ, responding on the “different” lever or response key produces reinforcement (Daniel et al., 2016). With either procedure, development of accurate responding with the training stimuli is not sufficient to permit the inference that behavior is under the control of the same-different relation. Control by stimulus configuration or by specific stimulus-stimulus relations commonly develops; although there may be the appearance of relational responding, these forms of stimulus control can be unmasked by testing with novel stimuli (Carter & Werner, 1978; Cumming & Berryman, 1965; McIlvane, 2013). Indeed, because most early studies failed to demonstrate generalized identity MTS with novel stimuli, it was generally believed that non-verbal organisms were not capable of same-different concept learning (e.g., Premack, 1978). However, advances in stimulus control research using MTS, NMTS and same-different procedures have since demonstrated accurate responding with novel sample and comparison stimuli in a wide variety of non-human species. Same-different relational responding/concept learning is often inferred from these outcomes (for a review see Daniel et al., 2016), although such conclusions remain controversial (Mackintosh, 2000; Penn, Holyoak, & Povinelli, 2008).
In most cases, training with a single pair of stimuli is not sufficient to bring about same-different learning, but rather, training with multiple exemplars is required. Katz, Wright and their colleagues (Katz, Wright, & Bachevalier, 2002; Katz & Wright, 2006; Katz, Wright, & Bodily, 2007; Wright, Rivera, Katz, & Bachevalier, 2003) have developed a paradigm to study MET in same-different concept learning. In these studies, capuchin monkeys, rhesus monkeys, and pigeons were trained on the same-different procedure with a small set of complex visual stimuli (e.g., travel slides). When accurate responding was acquired, the stimulus set was expanded, i.e., new stimuli were added to the mix. This set expansion procedure has two important features: first, it provides a test for same-different responding to novel stimuli; second, as responses to the new stimuli are reinforced, it provides MET with an increased number of examples. Set expansion can be continued as accuracy criteria are met with a progressively increasing set size. Generally a control group is included for which the initial small stimulus set is held constant, but is matched with the expansion group for number of training sessions.
Using such procedures, monkeys required exposure to at least 32 exemplars before showing much evidence of above chance same-different responding to novel stimuli (about 70% correct) and exposure to 128 different exemplars was required before accuracy to novel stimuli matched baseline levels of 80% correct or higher (Katz et al., 2002; Wright et al., 2003). Pigeons required 64 or more exemplars to reach above chance (70% correct) performance on novel stimuli, and 256 exemplars or more before performance on novel stimuli matched baseline levels of accuracy (Katz & Wright, 2006). In control conditions matched for number of training sessions, training with the initial small set of exemplars did not result in transfer to novel stimuli. This shows that MET, not just extended training, was necessary to produce the NAARR. Thus, the set expansion procedure might well be viewed as a model for MET research in that it permits analysis of the emergence of relational responding in individual subjects as the number of trained exemplars is progressively increased.
The set expansion procedure has also been applied to MTS and NMTS procedures in pigeons (Daniel, Wright & Katz, 2015; Bodily, Katz, & Wright, 2008; see also Brino et al., 2014a, b) with similar outcomes. Above chance accuracy was seen with fewer exemplars using matching relative to same-different procedures, but the function relating accuracy with novel stimuli to number of exemplars was similar. More recently, Wright and his colleagues (Wright, Magnotti, Katz, Leonard, & Kelly, 2016; Wright et al., 2017) used the set expansion procedure with corvids (Clark’s nutcracker and Black-billed magpies), a family of birds known for tool-use, highly developed spatial memory, and other intelligent behaviors. In both studies, corvids showed transfer that was somewhat above chance after training with only eight exemplars and functions similar to those of monkeys were obtained as the stimulus set was expanded.
One important finding in all of the set expansion studies was that there appeared to be an intermediate pattern of responding between absence of transfer to novel stimuli and levels of transfer that were equal to baseline levels. Katz and colleagues refer to this final level as “full concept learning” and the intermediate pattern as “partial concept learning” (Daniel et al., 2016; Katz & Wright, 2006). From a behavior analytic perspective, these patterns might be hypothesized to reflect a change in stimulus control topography (Dube & McIlvane, 1996; McIlvane & Dube, 2003). The hypothesis would be that after training with only a few exemplars, responding to novel stimuli is primarily controlled by generalization from specific features of previous encountered stimuli, thus transfer to novel stimuli is poor. With exposure to more exemplars, relational responding begins to develop and novel stimuli generate a mixture of both item-specific and relational responding. However, as multiple exemplar training continues, relational responding is now fully applicable to both novel and familiar stimuli and accuracy reaches baseline levels. Viewed from this perspective, partial concept learning is better described as a partial application of relational responding to novel stimuli, and the function relating number of exemplars to transition from partial to full concept learning as the learning curve for a relational operant.
Although training with a large number of exemplars is generally required to observe generalized MTS/NMTS and same-different responding, there are some curious exceptions. For example, Oden, Thompson, and Premack (1988) trained MTS with two objects to young chimpanzees and found very high levels of accuracy to a variety of novel objects. Oden et al. suggest that ability to “spontaneously” match after few exemplars may be a capacity limited to apes and humans, but the possibility that some features of the procedure or the animals’ histories were important still needs to be ruled out. Other studies have observed the emergence of generalized same-different or MTS responding after training with few exemplars. For example, Cook, Kelly, and Katz (2003) found transfer of same-different responding to novel stimuli in pigeons after training with only two exemplars, and above chance generalized identity matching was also observed by Urcuioli and Swisher (2012b) in pigeons after training with only two stimuli. Prichard et al. (2015) obtained responding to novel stimuli that matched baseline accuracy in rats after training with only four exemplars. All three of these studies used successive (go, no-go) discrimination procedures, so it may be that some features of this procedure accelerate the development of relational responding. Alternatively, it is possible that non-relational cues rather than the identity relation may have come to control behavior in these studies (but note that Cook et al. ruled out several possible sources of non-relational control). There is still much to learn about the role of MET in the development of same-different relational responding in animals, but methodological tools such as the set-size expansion procedure provide experimental paradigms that permit quantitative and cross-species comparisons of the effects of MET.
Where do We go from Here?
The search for symmetry and other AARRs in non-humans has generated a fairly extensive literature since Sidman et al. (1982). At that time it may have seemed a straightforward matter to assess emergent relations or their absence following carefully contrived conditional discrimination training in the standard species of the operant animal laboratories to quickly resolve the issues. The results of 35 years of research have turned out to be a bit more complex. On the one hand, we count a number of apparently successful demonstrations of symmetry and other emergent stimulus relations in this review. These procedures may provide us with animal models that might be used to increase our understanding of the neurobiological underpinnings of relational responding and symbolic behavior as well as to generate new applications to teaching such skills to children who fail to develop them naturally. On the other hand, however, we note that the conditions and procedures under which these relations emerge are highly restrictive and inflexible. The ability to abstract the features of experimental stimuli from the location in which they are displayed and from their temporal position as sample or comparison appears to be critical in explaining successful and more flexible human performances. Further, these observations suggest analysis of the roots of this form of abstraction as a target for future research with animals.
The limitations noted above have led some to argue that AARR that is functionally similar to that seen in humans has yet to be demonstrated in animals (Dymond, 2014; Hayes & Sanford, 2014; Hughes & Barnes-Holmes, 2014; Hayes, 2016). Flexible and rapidly-developed AARR may indeed be uniquely human, but is it the essence of what makes humans unique? As we have discussed, the difficulties in demonstrating emergent responding in animals are not limited to AARR; demonstrations of NAARRs have been controversial as well (e.g., Mackintosh, 2000). Indeed, many theorists have argued that same-different concept learning is uniquely human (e.g., Penn et al., 2008). However, a growing literature demonstrating generalized same-different responding in an increasing variety of non-human species continues to become more convincing. This progress has been facilitated by techniques such as the set-size expansion methodology which permit experimental analysis of the role of MET in the development of NAARR (cf. Daniel et al., 2016). Extending such research to additional non-human species, types of relations, and to the study of variables affecting the development of relational responding seems an important addition to our research agenda. Application of such techniques to pre-verbal infants could increase understanding of the role of MET in the development of human AARR as well. Research with non-verbal humans and animals offers the possibility of moving us toward an answer to the question of whether the ability to derive stimulus relations is fundamental to the various forms of human behavioral uniqueness. Many basic questions remain to be answered about the development and properties of NAARR and AARR and some of these may best be answered in the animal laboratory.
Acknowledgements
The authors thank Katherine Dyer, Madeleine Mason, Simone Nguyen, and Tiffany Phasukkan for their helpful comments on an earlier version of this manuscript.
Conflict of Interest
Both Mark Galizio and Katherine Bruce declare that they have no conflicts of interest related to the material in this manuscript.
Funding
The first author was supported by grant DA 29252 during the preparation of this manuscript.
Footnotes
Contributor Information
Mark Galizio, Phone: 910-962-3813, Email: galizio@uncw.edu.
Katherine E. Bruce, Phone: 910-962-3813, Email: bruce@uncw.edu
References
- Baer DM, Sherman JA. Reinforcement control of generalized imitation in young children. Journal of Experimental Child Psychology. 1964;1(1):37–49. doi: 10.1016/0022-0965(64)90005-0. [DOI] [Google Scholar]
- Baillargeon, R., & Carey, S. (2012). Core cognition and beyond: the acquisition of physical and numerical knowledge. In S. M. Pauen & S. M. Pauen (Eds.), Early childhood development and later outcome (pp. 33–65). New York, NY: Cambridge University Press.
- Barnes CS, Rehfeldt RA. Advances in language interventions based on relational frame theory for individuals with developmental disorders. In: Dymond S, Roche B, editors. Advances in relational frame theory: research and application. Oakland, CA: New Harbinger; 2013. pp. 151–177. [Google Scholar]
- Barnes-Holmes Y, Barnes-Holmes D, Smeets PM, Strand P, Friman P. Testing and training relational responding in accordance with the relational frame of opposite in young children. International Journal of Psychology and Psychological Therapy. 2004;4:559–586. [Google Scholar]
- Baron A, Perone M, Galizio M. Analyzing the reinforcement process at the human level:can application and behavioristic interpretation replace laboratory research? The Behavior Analyst. 1991;14:95–105. doi: 10.1007/BF03392557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A, Perone M, Galizio M. The experimental analysis of human behavior: indispensible, ancillary, or irrelevant. The Behavior Analyst. 1991;14:145–155. doi: 10.1007/BF03392565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens NM, Hayes SC. Arbitrarily applicable comparative relations: experimental evidence for a relational operant. Journal of Applied Behavior Analysis. 2007;40:45–71. doi: 10.1901/jaba.2007.7-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom P. How pleasure works: the new science of why we like what we like. New York, NY: Random House; 2010. [Google Scholar]
- Bodily KD, Katz JS, Wright AA. Matching-to-sample abstract-concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34(1):178–184. doi: 10.1037/0097-7403.34.1.178. [DOI] [PubMed] [Google Scholar]
- Brino AL, Campos RS, Galvão OF, McIlvane WJ. Blank-comparison matching-to-sample reveals a false positive symmetry test in a capuchin monkey. Psychology & Neuroscience. 2014;7:193–198. doi: 10.3922/j.psns.2014.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brino AL, Galvão OF, Picanco CRF, Barros RS, Souza CBA, Goulart BRK, McIlvane WJ. Generalized identity matching after multiple exemplar-training in capuchin monkeys. Psychological Record. 2014;64:693–704. doi: 10.1007/s40732-014-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos HC, Urcuioli PJ, Swisher M. Concurrent identity training is not necessary for associative symmetry in successive matching. Journal of the Experimental Analysis of Behavior. 2014;101:10–25. doi: 10.1002/jeab.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DE, Werner TJ. Complex learning and information processing by pigeons: a critical analysis. Journal of the Experimental Analysis of Behavior. 1978;29:565–601. doi: 10.1901/jeab.1978.29-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania, A. C. (2013). Learning, 5th Ed. Cornwall on Hudson, NY: Sloan Publishing.
- Cook RG, Kelly DM, Katz JS. Successive two-item same-different discrimination and concept learning by pigeons. Behavioural Processes. 2003;62:125–144. doi: 10.1016/S0376-6357(03)00022-6. [DOI] [PubMed] [Google Scholar]
- Critchfield TS, Fienup DM. Using stimulus equivalence technology to teach statistical inference in a group setting. Journal of Applied Behavior Analysis. 2010;43:763–768. doi: 10.1901/jaba.2010.43-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming W, Berryman R. The complex discriminated operant: studies of matching-to-sample and related problems. In: Mostovsky DI, editor. Stimulus generalization. Stanford, CA: Stanford University Press; 1965. pp. 284–330. [Google Scholar]
- Daniel TA, Goodman AM, Thompkins AM, Forloines MR, Lazarowski L, Katz JS. Generalization cannot predict abstract-concept learning. In: Olmstead MC, editor. Animal cognition: principles, evolution and development. New York: Nova Science Publishers; 2016. pp. 131–145. [Google Scholar]
- Daniel, T. A., Wright, A. A., & Katz, J. S. (2015). Abstract-concept learning of difference in pigeons. Animal cognition, 18, 831–837. [DOI] [PubMed]
- Deacon TW. The symbolic species: the co-evolution of language and the brain. New York, NY: WW Norton & Company; 1998. [Google Scholar]
- Dube WV, McIlvane WJ. Implications of stimulus control topography analysis for emergent behavior and stimulus classes. In: Zentall TR, Smeets PM, editors. Advances in psychology: stimulus class formation in humans and animals. Amsterdam: Elsevier; 1996. pp. 197–220. [Google Scholar]
- Dube, W. V., McIlvane, W. J., Callahan, T. D., & Stoddard, L. T. (1993). The search for stimulus equivalence in nonverbal organisms. The Psychological Record, 43, 761–778.
- Dugdale N, Lowe CF. Testing for symmetry in the conditional discriminations of language-trained chimpanzees. Journal of the Experimental Analysis of Behavior. 2000;73:5–22. doi: 10.1901/jeab.2000.73-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S. Meaning is more than associations: relational operants and the search for derived relations in nonhumans. Journal of the Experimental Analysis of Behavior. 2014;101:152–155. doi: 10.1002/jeab.57. [DOI] [PubMed] [Google Scholar]
- Frank AJ, Wasserman EA. Associative symmetry in the pigeon after successive matching-to-sample training. Journal of the Experimental Analysis of Behavior. 2005;84(2):147–165. doi: 10.1901/jeab.2005.115-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio, M. (2003). The abstracted operant: a review of Relational frame theory: a post-Skinnerian account of human language and cognition. The Behavior Analyst, 26, 159–169. [DOI] [PMC free article] [PubMed]
- Gomez J, Garcia A, Perez V. Failure to find symmetry in pigeons after multiple exemplar training. Psicothema. 2014;26:435–441. doi: 10.7334/psicothema2013.352. [DOI] [PubMed] [Google Scholar]
- Gorham M, Barnes-Holmes Y, Barnes-Holmes D, Berens N. Derived comparative and transitive relations in young children with and without autism. The Psychological Record. 2009;59:221–246. doi: 10.1007/BF03395660. [DOI] [Google Scholar]
- Hanson HM. Effects of discrimination training on stimulus generalization. Journal of Experimental Psychology. 1959;58:321–334. doi: 10.1037/h0042606. [DOI] [PubMed] [Google Scholar]
- Hayes SC. A relational control theory of stimulus equivalence. In: Hayes LJ, Chase PN, editors. Dialogues on verbal behavior: the first international institute on verbal relations. Reno, NV: Context Press; 1991. pp. 19–40. [Google Scholar]
- Hayes SC. Why contextual behavior science exists: an introduction to part 1. In: Zettle RD, Hayes SC, Barnes-Holmes D, Biglan A, editors. The Wiley handbook of contextual behavioral science. Hoboken, NJ: Wiley; 2016. pp. 9–16. [Google Scholar]
- Hayes SC, Barnes-Holmes D, Roche B. Relational frame theory: a post-Skinnerian account of human language and cognition. New York: Plenum Press; 2001. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Sanford BT. Cooperation came first: evolution and human cognition. Journal of the Experimental Analysis of Behavior. 2014;101:112–129. doi: 10.1002/jeab.64. [DOI] [PubMed] [Google Scholar]
- Heagle AI, Rehfeldt RA. Teaching perspective-taking skills to typically developing children through derived relational responding. Journal of Early and Intensive Behavior Intervention. 2006;3:1–34. doi: 10.1037/h0100321. [DOI] [Google Scholar]
- Hogan, D. E., & Zentall, T. R. (1977). Backward associations in the pigeon. The American Journal of Psychology, 3–15.
- Horne PJ, Lowe CF. On the origins of naming and other symbolic behavior. Journal of the Experimental Analysis of Behavior. 1996;65(1):185–241. doi: 10.1901/jeab.1996.65-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Barnes-Holmes D. Associative concept learning, stimulus equivalence, and relational frame theory: working out the similarities and differences between human and nonhuman behavior. Journal of the Experimental Analysis of Behavior. 2014;101:156–160. doi: 10.1002/jeab.60. [DOI] [PubMed] [Google Scholar]
- Hughes S, Barnes-Holmes D. Relational frame theory: the basic account. In: Zettle RD, Hayes SC, Barnes-Holmes D, Biglan A, editors. The Wiley handbook of contextual behavioral science. Hoboken, NJ: Wiley; 2016. pp. 129–178. [Google Scholar]
- Iversen IH. Matching-to-sample performance in rats: a case of mistaken identity? Journal of the Experimental Analysis of Behavior. 1997;68(1):27–45. doi: 10.1901/jeab.1997.68-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen IH, Sidman M, Carrigan P. Stimulus definition in conditional discriminations. Journal of the Experimental Analysis of Behavior. 1986;45:297–304. doi: 10.1901/jeab.1986.45-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastak CR, Schusterman RJ, Kastak D. Equivalence classification by California sea lions using class-specific reinforcers. Journal of the Experimental Analysis of Behavior. 2001;76:131–158. doi: 10.1901/jeab.2001.76-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz, J. S., & Wright, A. A. (2006). Same/different abstract-concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes, 32, 80–86. [DOI] [PubMed]
- Katz JS, Wright AA, Bachevalier J. Mechanisms of same/different abstract-concept learning by rhesus monkeys (Macaca mulatta) Journal of Experimental Psychology: animal Behavior Processes. 2002;28:358–368. [PubMed] [Google Scholar]
- Katz JS, Wright AA, Bodily KD. Issues in the comparative cognition of abstract-concept learning. Comparative Cognition & Behavior Reviews. 2007;2:79–92. doi: 10.3819/ccbr.2008.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler W. Simple structural functions in the chimpanzee and in the chicken. In: Ellis WD, editor. A source book of gestalt psychology. London: Routledge & Kegan Paul; 1918. pp. 217–227. [Google Scholar]
- Lawrence DH, DeRivera J. Evidence for relational transposition. Journal of Comparative and Physiological Psychology. 1954;47:465–471. doi: 10.1037/h0063235. [DOI] [PubMed] [Google Scholar]
- Lazareva OF. Relational learning in a context of transposition: a review. Journal of the Experimental Analysis of Behavior. 2012;97:231–248. doi: 10.1901/jeab.2012.97-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazareva OF, Miner M, Wasserman EA, Young ME. Multiple-pair training enhances transposition in pigeons. Learning & Behavior. 2008;36:174–187. doi: 10.3758/LB.36.3.174. [DOI] [PubMed] [Google Scholar]
- Lazareva OF, Wasserman EA, Young ME. Transposition in pigeons: reassessing Spence (1937) with multiple discrimination training. Animal Learning & Behavior. 2005;33:22–46. doi: 10.3758/BF03196048. [DOI] [PubMed] [Google Scholar]
- Lazareva, O. F., Young, M. E., & Wasserman, E. A. (2014). A three-component model of relational responding in the transposition paradigm. Journal of Experimental Psychology: Animal Learning and Cognition, 40, 63–80. [DOI] [PubMed]
- Lionello, K. M., & Urcuioli, P. J. (1998). Control by sample location in pigeons matching to sample. Journal of the Experimental Analysis of Behavior, 70, 235–251. [DOI] [PMC free article] [PubMed]
- Lionello-DeNolf KM. The search for symmetry: 25 years in review. Learning & Behavior. 2009;37:188–203. doi: 10.3758/LB.37.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf KM, Urcuioli PJ. Stimulus control topographies and tests of symmetry in pigeons. Journal of the Experimental Analysis of Behavior. 2002;78:467–495. doi: 10.1901/jeab.2002.78-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkens, R., Hayes, S. C., & Hayes, L. J. (1993). Longitudinal study of the development of derived relations in an infant. Journal of Experimental Child Psychology, 56, 201–239. [DOI] [PubMed]
- Lipkens R, Kop PF, Matthijs W. A test of symmetry and transitivity in the conditional discrimination performances of pigeons. Journal of the Experimental Analysis of Behavior. 1988;49:395–409. doi: 10.1901/jeab.1988.49-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano C, Becerra IG, Valverde MR. The role of multiple-exemplar training and naming in establishing derived equivalence in an infant. Journal of the Experimental Analysis of Behavior. 2007;87:349–365. doi: 10.1901/jeab.2007.08-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. Abstraction and discrimination. In: Heyes C, Huber L, editors. The evolution of cognition. Cambridge, MA: MIT Press; 2000. pp. 123–141. [Google Scholar]
- McIlvane WJ. Simple and complex discrimination learning. In: Madden GJ, Dube WV, Hackenberg TD, Hanley GP, Lattal KA, editors. APA handbook of behavior analysis. Washington, DC: American Psychological Association; 2013. pp. 129–163. [Google Scholar]
- McIlvane, W. J. (2014). “Associative concept learning in animals” by Zentall, Wasserman, and Urcuioli: a commentary. Journal of the Experimental Analysis of Behavior, 101, 161–164. [DOI] [PubMed]
- McIlvane, W. J., & Dube, W. V. (2003). Stimulus control topography coherence theory: foundations and extensions. The Behavior Analyst, 26, 195–213. [DOI] [PMC free article] [PubMed]
- McIlvane WJ, Serna RW, Dube WV, Stromer R. Stimulus control topography coherence and stimulus equivalence: reconciling test outcomes with theory. In: Leslie JC, Blackman D, editors. Experimental and applied analysis of human behavior. Reno, NV: Context Press; 2000. pp. 85–110. [Google Scholar]
- Neuringer A. Operant variability: evidence, functions, and theory. Psychonomic Bulletin & Review. 2002;9:672–705. doi: 10.3758/BF03196324. [DOI] [PubMed] [Google Scholar]
- Oden DL, Thompson RK, Premack D. Spontaneous transfer of matching by infant chimpanzees (Pan troglodytes) Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:140–145. [PubMed] [Google Scholar]
- O'Donnell J, Saunders KJ. Equivalence relations in individuals with language limitations and mental retardation. Journal of the Experimental Analysis of Behavior. 2003;80(1):131–157. doi: 10.1901/jeab.2003.80-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaez M, Gewirtz JL, Sanchez A, Mahabir NM. Exploring stimulus equivalence formation in infants. Behavioral Development Bulletin. 2000;9:20–25. doi: 10.1037/h0100534. [DOI] [Google Scholar]
- Penn DC, Holyoak KJ, Povinelli DJ. Darwin's mistake: explaining the discontinuity between human and nonhuman minds. Behavioral and Brain Sciences. 2008;31:109–130. doi: 10.1017/S0140525X08003543. [DOI] [PubMed] [Google Scholar]
- Pilgrim, C., & Galizio, M. (1996). Stimulus equivalence: a class of correlations, or a correlation of classes? In T. R. Zentall & P. M. Smeets (Eds.), Advances in psychology: stimulus class formation in humans and animals (Vol. 117, pp. 173–195). Amsterdam: Elsevier.
- Pilgrim C, Galizio M. Stimulus equivalence and units of analysis. In: Leslie JC, Blackman D, editors. Experimental and applied analysis of human behavior. Reno, NV: Context Press; 2000. pp. 111–126. [Google Scholar]
- Pilgrim C, Jackson J, Galizio M. Acquisition of arbitrary conditional discriminations by young normally developing children. Journal of the Experimental Analysis of Behavior. 2000;73:177–193. doi: 10.1901/jeab.2000.73-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinker S. The language instinct: the new science of language and mind. New York, NY: HarperCollins; 1994. [Google Scholar]
- Premack D. On the abstractness of human concepts: why it would be difficult to talk to a pigeon. In: Hulse SH, Fowler HE, Honig WK, editors. Cognitive processes in animal behavior. Hillsdale, NJ: Lawrence Erlbaum; 1978. pp. 423–451. [Google Scholar]
- Prichard A, Panoz-Brown D, Bruce K, Galizio M. Emergent identity but not symmetry following successive olfactory discrimination training in rats. Journal of the Experimental Analysis of Behavior. 2015;104(2):133–145. doi: 10.1002/jeab.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese HW. The perception of stimulus relations: discrimination learning and transposition. New York: Academic; 1968. [Google Scholar]
- Rodewald HK. Symbolic matching-to-sample by pigeons. Psychological Reports. 1974;34:987–990. doi: 10.2466/pr0.1974.34.3.987. [DOI] [Google Scholar]
- Savage-Rumbaugh ES. Ape language: from conditioned response to symbol. New York: Columbia University Press; 1986. [Google Scholar]
- Schusterman, R. J., & Kastak, D. (1993). A California sea lion (Zalophus californianus) is capable of forming equivalence relations. The Psychological Record, 43, 823.
- Sidman M. Reading and auditory-visual equivalences. Journal of Speech, Language, and Hearing Research. 1971;14:5–13. doi: 10.1044/jshr.1401.05. [DOI] [PubMed] [Google Scholar]
- Sidman M. Equivalence relations and behavior: a research story. Boston: Authors Cooperative; 1994. [Google Scholar]
- Sidman M. Equivalence relations and the reinforcement contingency. Journal of the Experimental Analysis of Behavior. 2000;74:127–146. doi: 10.1901/jeab.2000.74-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Rauzin R, Lazar R, Cunningham S, Tailby W, Carrigan P. A search for symmetry in the conditional discriminations of rhesus monkeys, baboons, and children. Journal of the Experimental Analysis of Behavior. 1982;37:23–44. doi: 10.1901/jeab.1982.37-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Tailby W. Conditional discrimination vs. matching to sample: an expansion of the testing paradigm. Journal of the Experimental Analysis of Behavior. 1982;37:5–22. doi: 10.1901/jeab.1982.37-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF. Science and human behavior. New York: MacMillan; 1953. [Google Scholar]
- Skinner, B. F. (1956). A case history in scientific method. American Psychologist, 11, 221–233.
- Skinner BF. Verbal behavior. New York: Vintage; 1957. [Google Scholar]
- Skinner BF. About behaviorism. New York: Appleton- Century-Crofts; 1976. [Google Scholar]
- Spence KW. The differential response in animals to stimuli varying within a single dimension. Psychological Review. 1937;44:430–444. doi: 10.1037/h0062885. [DOI] [Google Scholar]
- Suddendorf T. The gap: the science of what separates us from other animals. Philadelphia, PA: Basic Books; 2013. [Google Scholar]
- Sweeney, M. M., & Urcuioli, P. J. (2010). Reflexivity in pigeons. Journal of the Experimental Analysis of Behavior, 94, 267–282. [DOI] [PMC free article] [PubMed]
- Urcuioli PJ. Associative symmetry, antisymmetry, and a theory of pigeon’s equivalence class formation. Journal of the Experimental Analysis of Behavior. 2008;90:257–282. doi: 10.1901/jeab.2008.90-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ. Emergent identity matching after successive matching training, I: reflexivity or generalized identity? Journal of the Experimental Analysis of Behavior. 2011;96:329–341. doi: 10.1901/jeab.2011.96-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ. A successful search for symmetry. Conductual. 2015;3:4–25. [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Swisher M. A replication and extension of the antisymmetry effect in pigeons. Journal of the Experimental Analysis of Behavior. 2012;98:283–293. doi: 10.1901/jeab.2012.98-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Swisher M. Emergent identity matching after successive matching training. II: reflexivity or transitivity? Journal of the Experimental Analysis of Behavior. 2012;97:5–27. doi: 10.1901/jeab.2012.97-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Swisher MJ. Transitive and anti-transitive emergent relations in pigeons: support for a theory of stimulus-class formation. Behavioural Processes. 2015;112:49–60. doi: 10.1016/j.beproc.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli, P. J., Zentall, T. R., Jackson-Smith, P., & Steirn, J. N. (1989). Evidence for common coding in many-to-one matching: retention, intertrial interference, and transfer. Journal of Experimental Psychology: Animal Behavior Processes, 15(3), 264–273.
- Vaughn W. Formation of equivalence sets in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:36–42. [Google Scholar]
- Velasco SM, Huziwara EM, Machado A, Tomanari GY. Associative symmetry by pigeons after few-exemplar training. Journal of the Experimental Analysis of Behavior. 2010;94:283–295. doi: 10.1901/jeab.2010.94-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SR, Gelman SA. Different kinds of concepts and different kinds of words: what words to for human cognition. In: Mareschal D, Quinn PC, Lea SEG, editors. The making of human concepts. NY: Oxford University Press; 2010. pp. 99–130. [Google Scholar]
- Weil TM, Hayes SC, Capurro P. Establishing a deictic relational repertoire in young children. The Psychological Record. 2011;61:371–390. doi: 10.1007/BF03395767. [DOI] [Google Scholar]
- Wright AA, Magnotti JF, Katz JS, Leonard K, Kelly DM. Concept learning set-size functions for Clark’s nutcrackers. Journal of the Experimental Analysis of Behavior. 2016;105(1):76–84. doi: 10.1002/jeab.174. [DOI] [PubMed] [Google Scholar]
- Wright, A. A., Magnotti, J. F., Katz, J. S., Leonard, K., Vernouillet, A., & Kelly, D. M. (2017). Corvids outperform pigeons and primates in learning a basic concept. Psychological Science, 28, 437–444. [DOI] [PubMed]
- Wright AA, Rivera JJ, Katz JS, Bachevalier J. Abstract-concept learning and list-memory processing by capuchin and rhesus monkeys. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29(3):184–198. doi: 10.1037/0097-7403.29.3.184. [DOI] [PubMed] [Google Scholar]
- Yamamoto JI, Asano T. Stimulus equivalence in a chimpanzee (Pan troglodytes) The Psychological Record. 1995;45:3–21. [Google Scholar]
- Zentall TR, Wasserman EA, Urcuioli PJ. Associative concept learning in animals. Journal of the Experimental Analysis of Behavior. 2014;101(1):130–151. doi: 10.1002/jeab.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn TE, Newland MC, Ritchie KE. The efficiency and efficacy of equivalence-based learning: a randomized controlled trial. Journal of Applied Behavior Analysis. 2015;48:865–882. doi: 10.1002/jaba.258. [DOI] [PubMed] [Google Scholar]


