Abstract
Background
PYRAMID was an international multicenter, noninterventional, postmarketing registry assessing long-term safety and effectiveness of adalimumab (Humira), as used in routine clinical practice.
Methods
Adult patients with moderately to severely active Crohn’s disease with or without prior adalimumab experience were enrolled in the registry and followed for up to 6 years. Effectiveness measurements included the Physician’s Global Assessment (PGA, a composite of Harvey Bradshaw Index [HBI] and rectal bleeding score), clinical remission (HBI < 5), Short Inflammatory Bowel Disease Questionnaire (SIBDQ), and Work Productivity and Activity Impairment (WPAI) questionnaire. Data were reported for adalimumab-naïve patients and analyzed by baseline immunomodulator use and disease duration.
Results
This study evaluated 2057 adalimumab-naïve patients. Mean PGA improved from 7.5 (baseline) to 3.9 (year 1) and 3.3 (year 6). The proportion of patients in HBI remission increased from 29% (573 of 1969; baseline) to 68% (900 of 1331; year 1) and 75% (625 of 831; year 6). Patients stratified by baseline immunomodulator use had similar HBI remission rates; patients with disease duration <2 years achieved numerically higher HBI remission rates than patients with longer disease duration. Patient-reported SIBDQ and WPAI scores improved at year 1; all WPAI subscore improvements were clinically meaningful (≥7% point change) at year 1 and maintained through year 6. Serious infections were reported in 11.1% of patients; incidence rates of malignancies, lymphoma, and demyelinating disorders were low.
Conclusion
Adalimumab therapy, as used in routine clinical practice, improved physician-reported and patient-reported disease outcomes and remission rates for up to 6 years. No new safety signals were observed.
Keywords: clinical practice, disease activity, long-term effectiveness, long-term safety, work productivity
Long-term effectiveness and safety of adalimumab were evaluated in routine clinical practice settings in patients with Crohn’s disease. Adalimumab improved disease activity, work productivity, and activity impairment in adalimumabnaïve patients enrolled in PYRAMID registry. No new safety signals were identified.
INTRODUCTION
Crohn’s disease is a chronic progressive disease that requires long-term treatment. The clinical symptoms can have a detrimental effect on patients’ emotional and social well-being and overall quality of life, including increased absenteeism rates, which affect work productivity.1, 2 The proposed treatment goal for Crohn’s disease is achievement of clinical and endoscopic remission (deep remission), an endpoint that usually leads to improved long-term outcomes.3 The efficacy and safety of adalimumab for inducing and maintaining remission in patients with moderately to severely active Crohn’s disease has been demonstrated in controlled clinical trials for up to 1 year;4–7 long-term adalimumab therapy has been shown to maintain remission and response in these patients for up to 4 years.8 Treatment with antitumor necrosis factor (TNF) agents, such as adalimumab, earlier in the course of the disease may lead to improved clinical outcomes.9 A randomized double-blind clinical trial showed that adalimumab therapy has also improved health-related quality of life and symptoms of depression and fatigue in patients with Crohn’s disease.10
Clinical practice can provide important supplemental data to support effectiveness and safety results in patients with Crohn’s disease from clinical trials.11 Registries provide real-world data outside of clinical trials and reflect the effectiveness observed in everyday practice. PYRAMID was an international, multicenter, noninterventional, postmarketing registry that evaluated the long-term safety and effectiveness of adalimumab, as used in routine clinical practice. The primary objective of the registry was to evaluate the long-term safety of adalimumab in adults with Crohn’s disease who were treated according to the local product label. The registry achieved its primary objective of ruling out a doubling of risk of lymphoma in patients receiving adalimumab compared with the estimated background rate. The final safety data from PYRAMID have been reported elsewhere.12 The secondary objective of the registry was to evaluate the long-term effectiveness of adalimumab. In this analysis, the long-term effectiveness of adalimumab is reported in adult patients with Crohn’s disease who did not receive adalimumab before enrolling in PYRAMID.
MATERIALS AND METHODS
Study Design and Patients
PYRAMID (NCT00524537) was a multicenter, uncontrolled, observational registry of adults with moderately to severely active Crohn’s disease who were started on adalimumab therapy, according to the local product label. Patients who had never been treated with adalimumab before entering the registry (adalimumab-naïve) or who were current or prior participants of adalimumab investigational Crohn’s disease trials or who were currently receiving adalimumab per local product label could enroll in the registry. Patients from the United States, Canada, Europe, South Africa, Australia, and New Zealand were enrolled. The first patient was enrolled on September 5, 2007, and the last patient was enrolled on December 14, 2009. All patients received adalimumab in a routine clinical practice setting and were followed during regular office visits for up to 6 years. Patients provided written informed consent at enrollment and signed a Patient Authorization for Use/Disclosure of Data form.
Effectiveness
Effectiveness was evaluated in all patients who received at least 1 dose of adalimumab in the registry and had at least 1 postenrollment assessment. For this report, only patients who were adalimumab-naïve at registry enrollment were analyzed. Patients in the registry were allowed to escalate and de-escalate adalimumab therapy per the investigator’s decision. The Physician’s Global Assessment (PGA) used in PYRAMID was a composite of the Harvey Bradshaw Index (HBI) and a rectal bleeding score.13 The PGA assessed general well-being (rated 0 to 4), abdominal pain (rated 0 to 3), diarrhea (the number of semisolid/liquid stools per day during the past 7 days), blood in stool (rated 0 to 2), abdominal mass (rated 0 to 3), and Crohn’s disease–related extraintestinal manifestation (rated 1 per complication). Patients with HBI <5 were considered to be in clinical remission. Analyses of change from baseline in HBI and of HBI remission were performed overall and also stratified by baseline (registry enrollment) immunomodulator use (methotrexate, azathioprine, 6-mercaptopurine, thioguanine) and disease duration (<2 years, 2 to <5 years, 5 to <10 years, and ≥10 years). Because patients could have already permanently discontinued adalimumab during the registry at the time of an effectiveness assessment, additional analyses of change from baseline in HBI and HBI remission were performed during the registry before permanent discontinuation of adalimumab (included in the analyses were HBI data from the first adalimumab dose through 14 days after the last adalimumab dose during the registry in patients receiving either continuous treatment or episodic dosing, excluding the time off for patients who discontinued from the registry and later re-enrolled); these analyses were also performed by baseline immunomodulator use and disease duration at enrollment.
The patient-reported Short Inflammatory Bowel Disease Questionnaire (SIBDQ) was used to assess the impact of inflammatory bowel disease on health-related quality of life; an increase in score indicated improvement.14 The SIBDQ includes bowel, social, systemic (fatigue, weight loss or gain), and emotional components.14 The SIBDQ scores range from 10 (worst health) to 70 (best health). A 9-point change in total SIBDQ score is correlated with a 100-point change in Clinical Disease Activity Index (CDAI).14 The patient-reported Work Productivity and Activity Impairment (WPAI) questionnaire was used to evaluate the effect of the patients’ disease on their ability to work and perform regular activities during the previous 7 days, measuring percentages of work time missed (absenteeism), impairment while working (presenteeism), overall work impairment, and daily activity impairment; higher values indicated greater impairment and less productivity.15 An absolute change in WPAI score of 7% was considered the minimum clinically important difference (MCID).16
Safety
Safety variables included adverse events (AEs) of special interest, serious AEs (SAEs), and AEs that led to permanent discontinuation of adalimumab. Safety data from PYRAMID were captured throughout the registry. Patients who discontinued adalimumab for any reason were encouraged to remain in the registry for the full 6 years. Patients who discontinued from the registry were offered participation in the direct to healthcare provider process, in which physicians completed a simplified questionnaire on an annual basis.
Registry treatment-emergent AEs (TEAEs) were defined as any event with an onset date on or after the first dose of adalimumab received in the registry until 70 days after the last adalimumab dose in the registry or up to first discontinuation from the registry, whichever came first (data were excluded after initial discontinuation for patients who were later re-enrolled or entered into the health care provider process). Exposure to adalimumab in the registry up to first discontinuation was defined as the time from first adalimumab dose through 14 days after the last dose date in the registry (up to first discontinuation from the registry), minus total days of any treatment interruptions. A treatment interruption was defined as a period of >70 consecutive days during which a patient did not receive any adalimumab injection. Pharmacokinetic data were not collected as part of this registry.
Statistical Analysis
Effectiveness measures were summarized descriptively with 95% confidence intervals (CIs). Change from baseline values were calculated for patients with available values at both baseline and study visit. Because data were collected from an observational registry designed to provide real-world clinical settings instead of a controlled clinical trial, data were reported as observed, and no imputation for missing data was performed. A multiple Cox regression analysis was used to determine predictors of time to first remission (defined as HBI <5) in patients without remission at baseline. Factors included in the Cox model were duration of Crohn’s disease at registry enrollment (years), prior infliximab use (yes/no), previous Crohn’s disease–related surgery (yes/no), and perianal disease (yes/no). Hazard ratios (HR) <1 were interpreted as being in favor of the “no” category for categorical variables coded “yes” versus “no” and in favor of shorter duration for the continuous variable duration of Crohn’s disease. P values were calculated using the Wald test of the null hypothesis that the predictor has no effect on hazard in the model adjusted for all other covariates. Median time to loss of first remission (defined as HBI ≥5) was evaluated using survival analyses in all adalimumab-naïve patients, in patients without treatment interruption, and in patients with ≥1 treatment interruption.
Registry treatment-emergent AEs were summarized by number and percentage of patients with at least 1 AE and as incidence rates of events (E) per 100 patient years (PY) of exposure to adalimumab in the registry up to first discontinuation.
To determine standardized mortality ratio, expected deaths were calculated using the most recent country-specific World Health Organization mortality rates through 2006. When country-specific rates were not available, rates from a country within the same geographical region with similar life-expectancy were used (rates from Hungary were used for Romania and Russia; rates from China were used for Korea; rates from Ireland were used for Iceland; rates from Greece were used for Turkey; rates from the United States were used when country was unknown). The 95% CIs for standardized mortality ratios were calculated using the Byar approximation.
RESULTS
There were a total of 5025 patients evaluated in the registry. Of these patients, 2057 (41%) were adalimumab-naïve at baseline and were the population analyzed in this report. Patient disposition is summarized in Fig. 1. Among the adalimumab-naïve patients, 58% were female, and 95% were white (Table 1). Less than 20% of patients had Crohn’s disease for <2 years. Approximately half of the patients (53%) had received at least 1 prior biologic therapy, with infliximab as the most common (51%) treatment. Additionally, 25% of patients received concomitant immunomodulators at baseline (methotrexate, azathioprine, 6-mercaptopurine, thioguanine), 24% received concomitant corticosteroids at baseline, and 17% of patients received both immunomodulators and corticosteroids at baseline. Because PYRAMID was an uncontrolled, observational registry, physicians were free to determine the appropriate therapy for each patient in accordance with the locally approved label. As such, 75% of patients (1531 of 2056) received 160/80 mg induction dosing at registry enrollment; 23% of patients (477 of 2056) received 80/40 mg induction dosing; 2% of patients (48 of 2056) received other induction dosing; and 1 patient had missing data. Mean duration ± SD of exposure to adalimumab in the registry was 1119 ± 842 days. There were 593 patients in the registry who escalated from maintenance (40 mg every other week) to weekly dosing. The median time to first escalation from 40 mg every other week to weekly dosing was 335 days (n = 593; range, 17 to 2217 days), and the median time to first de-escalation was 694 days (n = 217; range, 59 to 2255 days).
FIGURE 1.
Patient disposition for adalimumab-naïve patients enrolled in the PYRAMID registry.
TABLE 1.
Patient Demographics and Baseline Characteristics
| Characteristic | Adalimumab-naïve Patients N = 2057 |
|---|---|
| Sociodemographic parameters | |
| Female, n (%) | 1199 (58.3) |
| White, n (%) | 1964 (95.5) |
| Age, mean ± SD (median), y | 37.1 ± 12.7 (35.0) |
| Duration of Crohn’s disease, n (%)a | N = 1980 |
| <2 years | 373 (18.8) |
| 2 to <5 years | 334 (16.9) |
| 5 to <10 years | 512 (25.9) |
| ≥10 years | 761 (38.4) |
| Prior biologic use, n (%)b | 1082 (54.6) |
| Infliximab | 1049 (51.0) |
| Certolizumab pegol | 103 (5.0) |
| Concomitant IMM and/or CS, n (%) | |
| IMM only | 512 (24.9) |
| CS only | 490 (23.8) |
| IMM and CS | 341 (16.6) |
| Crohn’s disease location, n (%)c | |
| Anal | 365 (17.7) |
| Colon | 1331 (64.7) |
| Gastroduodenum | 88 (4.3) |
| Ileum | 1509 (73.4) |
| Jejunum | 127 (6.2) |
| Rectum | 323 (15.7) |
CS = corticosteroids; IMM = immunomodulators (methotrexate, azathioprine, 6-mercaptopurine, thioguanine).
aPercentages calculated on nonmissing values.
bPatients could have received ≥1 biologic. Shown here are biologics used in >1% patients.
cPatients could have more than 1 location of Crohn’s disease.
Adalimumab Effectiveness in Adalimumab-naïve Patients
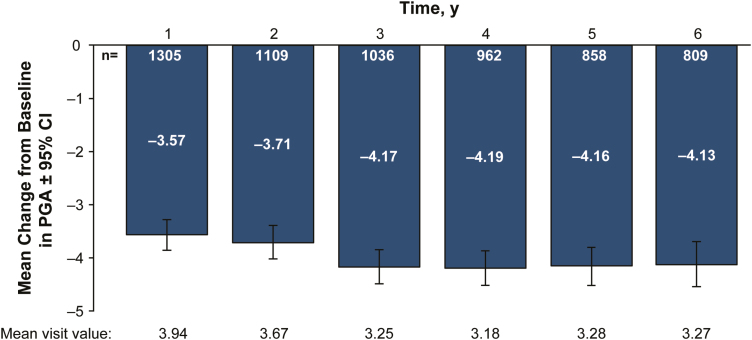
In patients with available baseline and year 1 data, mean PGA improved from 7.51 (95% CI, 7.23–7.79) at enrollment to 3.94 (95% CI, 3.71–4.18) at 1 year after entering the registry (n = 1305; Fig. 2) and to 3.27 (95% CI, 2.96–3.58) at year 6 (n = 809). Mean HBI improved from 7.24 (95% CI, 6.97–7.51) at enrollment to 3.82 (95% CI, 3.59–4.04) at 1 year, for an overall mean change from baseline in HBI of −3.42 (95% CI, −3.71 to −3.14; n = 1305; Fig. 3A). At year 6, mean HBI improved to 3.18 (95% CI, 2.88–3.49), for an overall mean change from baseline in HBI of −3.94 (95% CI, −4.35 to −3.53; n = 809). Similar improvement in mean HBI was observed during the registry before permanent discontinuation of adalimumab; mean HBI improved from 7.19 (95% CI, 6.91–7.47) at enrollment to 3.69 (95% CI, 3.45–3.92) at 1 year, for an overall mean change from baseline in HBI of −3.51 (95% CI, −3.80 to −3.21; n = 1200; Supplementary Fig. S1). At year 6, mean HBI in these patients improved to 2.72 (95% CI, 2.36–3.08), for an overall mean change from baseline in HBI of −4.21 (95% CI, −4.74 to −3.68; n = 412).
FIGURE 2.
Mean change from baseline in PGA scores (with 95% CI) of patients in the registry. PGA was calculated at all registry visits starting at study enrollment. Assessment of current disease activity included general well-being, abdominal pain, diarrhea, blood in stool, abdominal mass, and Crohn’s disease–related complications. PGA = Physician Global Assessment.
FIGURE 3.
Mean change from baseline in HBI (with 95% CI). (A) All patients, (B) patients stratified by immunomodulator use at enrollment, (C) patients stratified by disease duration at enrollment. HBI, Harvey-Bradshaw Index.
In subgroup analyses, mean changes in HBI values over time were similar in patients with and without baseline immunomodulator use (Fig. 3B). Mean changes from baseline in HBI ranged from −3.83 (95% CI, −4.27 to −3.38) at year 1 to −4.23 (95% CI, −4.87 to −3.59) at year 6 in patients with baseline immunomodulator use and −3.11 (95% CI, −3.48 to −2.75) at year 1 and −3.70 (95% CI, −4.23 to −3.17) at year 6 in patients without baseline immunomodulator use. When patients were stratified by disease duration at enrollment, no differences in mean changes from baseline in HBI were observed across the disease groups during years 1 through 6 (Fig. 3C). In all subgroups, improvements in HBI were sustained from year 1 through year 6. Similar improvements in HBI were observed during the registry before permanent discontinuation of adalimumab, stratified by baseline immunomodulator use and disease duration at enrollment (Supplementary Fig. S1B and S1C).
At year 1, the proportion of patients in HBI remission increased to 68% (900 of 1331), and remission rates were sustained through year 6 (Fig. 4A). Similar rates of HBI remission were observed for patients with and without baseline immunomodulator use at registry enrollment. Remission rates were sustained from year 1 through year 6 (year 1, 71% [417 of 588] and 65% [483 of 743]; year 6, 78% [296 of 378] and 73% [329 of 453]) in patients with and without baseline immunomodulator use, respectively; Fig. 4B).
FIGURE 4.
HBI remission. (A) All patients, (B) patients stratified by immunomodulator use at enrollment, (C) patients stratified by disease duration at enrollment. Remission was defined as HBI <5. HBI, Harvey-Bradshaw Index.
Patients with the shortest disease duration (<2 years) achieved the highest rates of HBI remission from year 1 through year 6 relative to patients with longer disease duration (Fig. 4C). The percentage of patients in remission with <2 years disease duration at registry enrollment increased to 81% at year 1 (209 of 258) and remained consistent through year 6 (84%, 129 of 153). Remission rates in patients with the longest disease duration (≥10 years) increased to 58% (279 of 479) at year 1 and to 68% (215 of 314) at year 6 (Fig. 4C).
Patients receiving adalimumab had similar improvements in remission rates during the registry before permanent discontinuation of adalimumab, stratified by immunomodulator use and disease duration at enrollment (Supplementary Fig. S2A–C).
Additionally, a multiple Cox regression analysis was performed to identify predictors associated with the time to first HBI remission. In patients without remission at enrollment, shorter disease duration in years (HR, 0.99; 95% CI, 0.98–1.00; P = 0.005), no prior infliximab use (HR, 0.72; 95% CI, 0.63–0.82; P < 0.001), and no prior Crohn’s disease-related surgery (HR, 0.76; 95% CI, 0.66–0.89; P < 0.001) were independent predictors for remission, adjusted for the other covariates of the model. In patients with ≥1 treatment interruption, only no prior infliximab use was a predictor for remission (HR, 0.59; 95% CI, 0.39–0.89; P = 0.011), adjusted for the other covariates in the model.
During the time on adalimumab in the registry, the median time to loss of remission after first remission for all adalimumab-naïve patients was 1085 days (95% CI, 937–1316 days; patients with loss of remission, n = 817 of 1591). For patients without treatment interruption, the median time to loss of remission after first remission was 1237 days (95% CI, 1014–1606 days; patients with loss of remission, n = 701 of 1433). For patients with ≥1 treatment interruption, median time to loss of remission after first remission was 522 days (95% CI, 379–736 days; patients with loss of remission, n = 116 of 158).
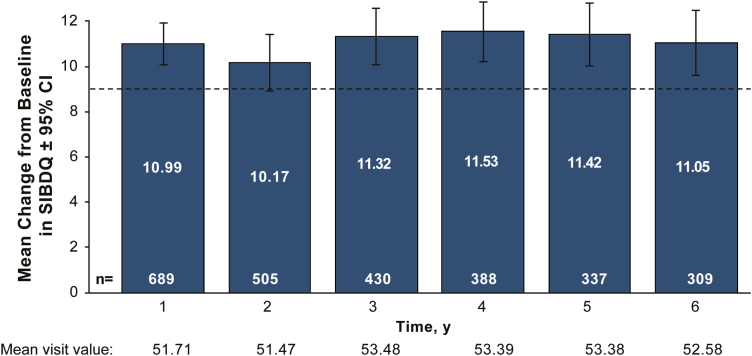
Clinically meaningful improvement in SIBDQ scores was observed from years 1 through 6 (Fig. 5). Mean total SIBDQ scores improved from 40.72 (95% CI, 39.77–41.67) at baseline to 51.71 (95% CI, 10.06–11.92) at 1 year in patients who had baseline and year 1 data; these improvements were sustained through year 6.
FIGURE 5.
Mean change from baseline in patient-reported SIBDQ scores (with 95% CI). Total SIBDQ score ranged from 10 (poor health-related quality of life) to 70 (optimum health-related quality of life). Dashed line indicates a 9-point change in total SIBDQ score (Irvine et al, 1996). SIBDQ, Short Inflammatory Bowel Disease Questionnaire.
All 4 mean WPAI subscores showed improvement from baseline to year 1 in patients who had both baseline and year 1 data available (Fig. 6). The mean absenteeism subscore improved from 20.18 (95% CI, 16.63–23.73) at baseline to 8.01 (95% CI, 5.49–10.53) at year 1. The mean presenteeism subscore improved from 39.53 (95% CI, 36.35–42.70) at baseline to 20.68 (95% CI, 18.04–23.33) at year 1. The mean overall work impairment improved from 47.68 (95% CI, 43.97–51.39) at baseline to 24.30 (95% CI, 21.02–27.58) at year 1. And the mean activity impairment improved from 50.45 (95% CI, 48.16–52.75) at baseline to 28.52 (95% CI, 26.37–30.67) at year 1. For all WPAI subscores, clinically meaningful improvements represented by a ≥7% point change in WPAI scores were achieved at year 1 and were maintained through year 6.
FIGURE 6.
Mean change from baseline in patient-reported WPAI scores (with 95% CI). Effects of patients’ Crohn’s disease on their ability to work and perform regular activities during the previous 7 days were evaluated. Dashed line indicates a 7% point change in WPAI score, representing the minimum clinically important difference. WPAI, work productivity and activity impairment.
Safety in Adalimumab-naïve Patients
Registry treatment-emergent AEs are summarized in Table 2. Adverse events were reported in 47.8% of the patients (incidence, 40.4 E/100 PY). Serious AEs were reported in 36.6% of patients (26.7 E/100 PY), and SAEs that were at least possibly related to adalimumab were reported in 9.4% of patients (4.7 E/100 PY). Serious infections were reported in 11.2% of patients (5.0 E/100 PY). Intestinal stricture occurred in 199 patients (258 events; 4.1 E/100 PY). There were 7 events of tuberculosis (0.1 E/100 PY), including 4 active (<0.1 E/100 PY) and 3 latent tuberculosis events (<0.1 E/100 PY). A total of 47 malignancies were reported in 39 patients (1.9%), including 14 nonmelanoma skin cancers, 7 melanomas, 1 lymphoma, 1 leukemia, and 24 other malignancies. The rates of malignancies, lymphoma, and demyelinating disorders were low (0.7, <0.1, and <0.1 E/100 PY, respectively). All other AEs occurred at the rate of <1 E/100 PY. Nineteen AEs led to the deaths of 15 patients (0.3 E/100 PY). The number of observed deaths in PYRAMID was not different from what was expected in the general population; the standardized mortality ratio was 0.93 (95% CI, 0.52–1.54). The safety profile of adalimumab in the registry was consistent with published data in patients with Crohn’s disease.4 Additionally, the safety profile was no different in adalimumab-naïve patients than in patients with a prior history of adalimumab therapy.12
TABLE 2.
Registry Treatment-emergent Adverse Events in Adalimumab-naïve Patients
| Adalimumab | ||
|---|---|---|
| N = 2057 | PYs = 6299.2 | |
| n (%) | Events (E/100 PY) | |
| Any AE | 983 (47.8) | 2545 (40.4) |
| Serious AE | 752 (36.6) | 1682 (26.7) |
| Serious AE at least possibly related to adalimumaba | 193 (9.4) | 293 (4.7) |
| AE leading to adalimumab discontinuation | 268 (13.0) | 351 (5.6) |
| AE leading to death | 15 (0.7) | 19 (0.3) |
| Severe AEs | 442 (21.5) | 829 (13.2) |
| Injection site reactions | 6 (0.3) | 14 (0.2) |
| Infection | 355 (17.3) | 524 (8.3) |
| Serious infection | 230 (11.2) | 312 (5.0) |
| Opportunistic infection (excluding oral candidiasis and TB) | 7 (0.3) | 7 (0.1) |
| Any TB | 7 (0.3) | 7 (0.1) |
| Active TB | 4 (0.2) | 4 (<0.1) |
| Latent TB | 3 (0.1) | 3 (<0.1) |
| Any malignancy | 39 (1.9) | 47 (0.7) |
| Nonmelanoma skin cancer | 10 (0.5) | 14 (0.2) |
| Melanoma | 7 (0.3) | 7 (0.1) |
| Leukemia | 1 (<0.1) | 1 (<0.1) |
| Lymphoma | 1 (<0.1) | 1 (<0.1) |
| Other malignancy | 24 (1.2) | 24 (0.4) |
| Intestinal stricture | 199 (9.7) | 258 (4.1) |
| Worsening or new onset of psoriasis | 40 (1.9) | 42 (0.7) |
| Demyelinating disorder | 3 (0.1) | 3 (<0.1) |
E/100 PY = event per 100 patient-years; TB = tuberculosis
aAs assessed by investigator
DISCUSSION
This analysis of adalimumab-naïve patients with moderately to severely active Crohn’s disease enrolled in the PYRAMID registry demonstrated that improvements in disease activity, work productivity, and activity impairment in patients who remained in the study regardless of receiving adalimumab were achieved after 1 year in the registry and that these improvements were maintained through 6 years of the registry. Furthermore, no new safety signals were identified with long-term, real-world adalimumab use for up to 6 years.
Data collected from the 2057 patients newly receiving adalimumab therapy for up to 6 years provided an extended look at efficacy and safety of adalimumab in real-world settings. In these patients, disease activity improved as measured by HBI, independent of immunomodulator use at baseline or disease duration. Patients with disease duration <2 years achieved numerically higher remission rates compared with patients with longer disease duration. These results are consistent with findings in the placebo-controlled CHARM study, which showed that CDAI-based remission rates were higher in patients with disease duration <2 years compared with longer disease duration.17 Early introduction of anti-TNF therapy in patients with Crohn’s disease may be beneficial for improvement of long-term outcomes.
Adalimumab therapy also improved patient-reported SIBDQ and WPAI scores at year 1 compared with baseline; both SIBDQ and WPAI remained stable through year 6. This is consistent with previously published effects of adalimumab therapy on SIBDQ and WPAI in patients with Crohn’s disease. In the 20-week, multicenter CARE (Crohn’s Treatment With Adalimumab, Patient Response to a Safety and Efficacy Study) trial, adalimumab therapy improved total SIBDQ scores at weeks 4 and 20 in patients with and without prior infliximab exposure.18 Additionally, adalimumab was shown to improve WPAI presenteeism and total work productivity impairment in CARE.18 The open-label multicenter CHOICE (Crohn’s Disease WHO Failed Prior Infliximab to Collect Safety Data and Efficacy via Patient-Reported Outcome measures) trial that addressed effects of adalimumab on quality of life and work productivity in patients with Crohn’s disease who failed prior infliximab therapy showed a significant improvement in mean SIBDQ total score at year 1 compared with baseline (mean change ± SD, 9.5 ± 11.7),1 which was comparable to the improvement in SIBDQ total scores at year 1 vs baseline in the current study (10.99 ± 12.43). The CHOICE trial also demonstrated an adalimumab-dependent improvement in all components of WPAI.1 However, the CHOICE trial was limited in its duration, with <50% of patients receiving adalimumab therapy for 2 years.
The safety profile of adalimumab in the PYRAMID registry was consistent with the safety profile of up to 4 years of adalimumab therapy from Crohn’s disease and ulcerative colitis trials and with the overall safety profile of adalimumab across multiple indications. An analysis of global clinical trials that assessed adalimumab safety in 3160 patients found that the rate of SAEs was 34.4 E/100 PY, similar to the 26.7 E/100 PY reported for adalimumab-naïve patients in the current study.4 Consistent with the rates of AEs reported in this study, previous clinical trials of adalimumab found that infections were the most common AEs, whereas malignancies and demyelinating disorders occurred at low rates.4, 5 Additional safety data from the PYRAMID registry have been reported elsewhere.12
PYRAMID is one of the largest registries of patients with Crohn’s disease receiving a biologic; it includes both physician- and patient-reported outcomes to present a more complete picture of the long-term, real-world impact of adalimumab on symptoms associated with Crohn’s disease. To eliminate the confounding factor of previous adalimumab treatment, this analysis only included patients who were adalimumab-naïve. A limitation of this study was a possible underestimation of the number of nonserious AEs of special interest because they were not initially included within the event categories at the start of the registry. Additionally, because outcome data from this registry were evaluated using as observed analysis, there may have been a selection bias if patients who dropped out of the registry did so because of inadequate improvement. Additional limitations, owing to the nature of a registry, included the open-label study design, lack of control groups, lack of patient randomization to groups with and without immunomodulators, and confounding variables in subgroups. Pharmacokinetic endpoints such as serum levels were not captured.
In conclusion, data from the PYRAMID registry support the known efficacy and safety profile of adalimumab in Crohn’s disease. Clinically meaningful improvements in disease activity, work productivity, and activity impairment were achieved in adalimumab-naïve patients with moderately to severely active Crohn’s disease and were maintained for up to 6 years. No new safety signals were observed.
Supplementary Material
ACKNOWLEDGMENTS
AbbVie Inc. funded the registry and the current analyses. Medical writing support was provided by Natalia Zhukovskaya, PhD, of Complete Publication Solutions, LLC (North Wales, PA, USA), an ICON plc Company; this support was funded by AbbVie Inc. AbbVie reviewed and approved the publication. The authors would like to thank Martha Skup, PhD, of AbbVie Inc. (North Chicago) for her contributions to the study.
AbbVie is committed to responsible data sharing regarding the clinical trials they sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets) and other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. The clinical trial data can be requested by any qualified researcher who engages in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Presented at the American College of Gastroenterology annual meeting, October 13–18, 2017, Orlando, Florida, USA, and at the United European Gastroenterology Week, October 28 to November 1, 2017, Barcelona, Spain.
Conflicts of interest: EVL, Jr. has received consulting fees from AbbVie, Allergan, Amgen, Celgene, Celltrion Healthcare, eli Lilly, Janssen, Napo Pharmaceuticals, Pfizer, Takeda, and UCB; and research support from AbbVie, Amgen, Celgene, Genentech, Gilead, Janssen, MedImmune, Robarts Clinical Trials, Seres Therapeutics, Takeda, and UCB.
WR has served as a speaker for Abbott Laboratories, AbbVie, Aesca, Aptalis, Astellas, Centocor, Celltrion, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor, Yakult; he has served as a consultant for Abbott Laboratories, AbbVie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Roland Berger GmBH, Bioclinica, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, Elan, Ernest & Young, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, Mallinckrodt, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Robarts Clinical Trial, Schering-Plough, Second Genome, Setpointmedical, Sigmoid, Takeda, Therakos, Tigenix, UCB, Vifor, Zyngenia, and 4SC; he has served as an advisory board member for Abbott Laboratories, AbbVie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zyngenia, and 4SC; and he has received research funding from Abbott Laboratories, AbbVie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnsotik, MSD. RP has received consultant and/or lecture fees from AbbVie, Amgen, AstraZeneca, Axcan Pharma (now Aptalis), Biogen Idec, Bristol-Myers Squibb, Centocor, ChemoCentryx, Eisai Medical Research Inc, Elan Pharmaceuticals, Ferring, Genentech, GlaxoSmithKline, Janssen, Merck Sharp & Dohme Corp, Millennium Pharmaceuticals Inc (now Takeda), Ocera Therapeutics Inc, Otsuka America Pharmaceutical, Pfizer, Shire Pharmaceuticals, Prometheus Laboratories, Schering-Plough Corporation, Synta Pharmaceuticals Corp, Teva, UCB Pharma, and Warner Chilcott. SB, GA, MB, JK, JP, RT, and AMR are AbbVie employees and may own AbbVie stock and/or options. GDH has received consulting and/or lectures fees from AbbVie, ActoGeniX, AIM, Boehringer Ingelheim GmbH, Centocor, Chemo Centryx, Cosmo Technologies, Elan Pharmaceuticals, enGene, Dr Falk Pharma, Ferring, Galapagos, Giuliani SpA, Given Imaging, GlaxoSmithKline, Janssen Biologics, MSD, Neovacs, Novo Nordisk, Otsuka, PDL BioPharma, Pfizer, Receptos, Salix, SetPoint, Shire Pharmaceuticals, Schering-Plough, Takeda, Tillotts Pharma, UCB Pharma, Versant, and Vifor Pharma; he has received research grants from AbbVie, Janssen, Given Imaging, MSD, Dr Falk Pharma, and PhotoPill and speaking honoraria from AbbVie, Tillotts, Tramedico, Ferring, MSD, UCB Pharma, Norgine, and Shire.
REFERENCES
- 1. Lichtiger S, Binion DG, Wolf DC, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn’s disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32:1228–1239. [DOI] [PubMed] [Google Scholar]
- 2. Longobardi T, Jacobs P, Bernstein CN. Work losses related to inflammatory bowel disease in the United States: results from the National Health Interview Survey. Am J Gastroenterol. 2003;98:1064–1072. [DOI] [PubMed] [Google Scholar]
- 3. Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:414–422.e415. [DOI] [PubMed] [Google Scholar]
- 4. Colombel JF, Sandborn WJ, Panaccione R, et al. Adalimumab safety in global clinical trials of patients with Crohn’s disease. Inflamm Bowel Dis. 2009;15:1308–1319. [DOI] [PubMed] [Google Scholar]
- 5. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 6. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333; quiz 591. [DOI] [PubMed] [Google Scholar]
- 7. Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Panaccione R, Colombel JF, Sandborn WJ, et al. Adalimumab maintains remission of Crohn’s disease after up to 4 years of treatment: data from CHARM and ADHERE. Aliment Pharmacol Ther. 2013;38:1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D’Haens G, Baert F, van Assche G, et al. ; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–667. [DOI] [PubMed] [Google Scholar]
- 10. Loftus EV, Feagan BG, Colombel JF, et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: patient-reported outcomes of the CHARM trial. Am J Gastroenterol. 2008;103:3132–3141. [DOI] [PubMed] [Google Scholar]
- 11. Trotter JP. Patient registries: a new gold standard for “real world” research. Ochsner J. 2002;4:211–214. [PMC free article] [PubMed] [Google Scholar]
- 12. D’Haens G, Reinisch W, Panaccione R, et al. Lymphoma risk and overall safety profile of adalimumab in patients with Crohn’s disease with up to 6 years of follow-up in the pyramid registry. Am J Gastroenterol. 2018;113:872–882. [DOI] [PubMed] [Google Scholar]
- 13. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 14. Irvine EJ, Zhou Q, Thompson AK. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT investigators. Canadian Crohn’s relapse prevention trial. Am J Gastroenterol. 1996;91:1571–1578. [PubMed] [Google Scholar]
- 15. Reilly MC, Gerlier L, Brabant Y, et al. Validity, reliability, and responsiveness of the work productivity and activity impairment questionnaire in Crohn’s disease. Clin Ther. 2008;30:393–404. [DOI] [PubMed] [Google Scholar]
- 16. Reilly MC, Brown MC, Brahant Y, et al. Defining the minimally important difference for WPAI: CD Scores: what is a relevant impact on work productivity in active Crohn’s disease. Gut. 2007;56 Suppl 13:A159. Accessed Suppl 3A, 56.
- 17. Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J Crohns Colitis. 2013;7:213–221. [DOI] [PubMed] [Google Scholar]
- 18. Louis E, Löfberg R, Reinisch W, et al. Adalimumab improves patient-reported outcomes and reduces indirect costs in patients with moderate to severe Crohn’s disease: results from the CARE trial. J Crohns Colitis. 2013;7:34–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.