Abstract
Background
The integrin CD103 is proposed to be a potential therapeutical target in inflammatory bowel disease (IBD), as it can form a heterodimeric integrin with β7 (Etrolizumab, anti-β7 integrin) on epithelial T cells. Therefore, we aimed to study the frequencies of different intestinal CD103+T-cell subsets, both CD4+ and CD8+, in newly diagnosed, untreated IBD patients at baseline and during follow-up, compared with healthy controls.
Methods
Intestinal biopsies from inflamed segments during colonoscopy and peripheral blood samples were prospectively taken from IBD patients at diagnosis and during follow-up. Blood and single cell suspensions from biopsies were analyzed for CD103+ T-cell subpopulations by flow cytometry and expressed as median percentages of the total T-cell population.
Results
In total, 75 Crohn’s disease (CD) patients, 49 ulcerative colitis (UC) patients, and 16 healthy controls were included. At presentation, IBD patients displayed lower percentages of CD103+T-cell subsets in inflamed biopsies: 3% (1 to 5) CD103+CD4+ in IBD vs 5% (5 to 7) in healthy controls (P = 0.007) and 9% (4 to 15) CD103+CD8+ compared with 42% (23 to 57) in healthy controls (P = 0.001). The majority of intestinal T cells was composed of CD103-CD4+ T cells (65% [52 to 74]) in IBD compared with 30% (21 to 50) in healthy controls (P = 0.001). In patients with endoscopic remission during follow-up (n = 27), frequencies of CD103+ and CD103-T-cell subsets were comparable with healthy controls.
Conclusion
At diagnosis, active inflammation in IBD was associated with decreased percentages of both CD103+CD4+ and CD103+CD8+T-cell subsets in colon and ileum biopsies. In active disease during follow-up, these T-cell populations remained low but increased in remission to values comparable with healthy controls. A shift toward more CD103-T cells was observed during active inflammation.
Keywords: IBD, CD103, translational immunology, CD69
With the upcoming anti-β7 therapies, the frequencies of the targeted CD103+CD4+ and CD103+CD8+T-cell populations were studied in newly diagnosed, untreated IBD patients at diagnosis and during follow-up. In active disease, these T-cell populations remained low but increased in remission.
INTRODUCTION
Aberrant and inordinate immune responses to environmental triggers may cause chronic active mucosal inflammation in a genetically susceptible host, leading to chronic gut inflammation disorders such as Crohn’s disease (CD) and ulcerative colitis (UC). T lymphocytes are pivotal in the immune response associated with inflammatory bowel disease (IBD).1, 2
Regularly, the gut homing phenotype, characterized by expression of the α4β7 integrin, is induced on naïve T cells after encounter with their cognate antigen in the gut-associated lymphoid tissue (GALT), smaller lymph aggregates, and mesenteric lymph nodes.3 In IBD, increased recruitment of these primed effector T cells to the intestinal mucosa has been described by the binding of the α4β7 integrin to MAdCAM-1 on endothelial cells.3, 4 After entrance to the lamina propria, in the presence of TGF-beta, αEβ7+ may be brought to expression on specific T-cell subsets.5, 6
The αEβ7+ integrin is considered to be of importance in modulating homeostasis of memory T cells by mediating selective retention of these T lymphocytes in the intestinal epithelia and lamina propria through its binding to E-cadherin.7 Alfa E (αE) can only form a heterodimer with β7, whereas β7 can bind to both αE and α4. Gene expression of αEβ7+ differs from the expression of this integrin on the cell surface because intracellular regulatory events might inhibit translation of mRNA to αEβ7+ integrin present on the cell surface.8 The αEβ7+ integrin can be identified with monoclonal antibodies recognizing the CD103 subunit on the cell surface by flow cytometry; therefore, we refer to CD103+ when αE is expressed on T cells.9
Currently, treatment options preventing the gut-specific migration of T cells in IBD are being extended with antibodies against different integrins like α4β7 (anti-α4β7, Vedolizumab) and CD103+(αE)β7 (anti-β7, Etrolizumab) and adhesion molecules such as MAdCAM-1 (anti-MAdCAM-1, PF-00547659).3, 10 In animal models, blocking β7+ by a combination of anti-α4β7 and anti-αEβ7 antibodies suppressed accumulation of the CD103+CD8+ T cells but had no effect on the accumulation of the pro-inflammatory CD103+CD4+ T cells in the gut mucosa.11 Assessment of the relative numbers of different CD103+(αE)β7+ T-cell subsets in the inflamed gut mucosa could be useful to identify patients suitable for different treatment strategies.
The major T-cell subsets, including CD4+ and CD8+ T cells, may have different functions within the mucosal CD103+ T-cell population. A potential pro-inflammatory role was allocated exclusively for the CD103+CD4+ subset, whereas functional investigation could not confirm this role for the CD103+CD8+ T cells in the gut of UC patients.12 The latter seems to belong to the tissue-resident-memory T cells (TRMs) with an immunosurveillance and protective function in different human tissues, also presumable for the intestinal mucosa.13 In addition, CD69 is another marker for TRMs and distinguishes the resident population from circulating memory T cells together with CD103. Also, CD69 is well known as a marker of activated cells, most often lymphocytes and natural killer cells.14 However, the frequencies of the different CD103+(CD69+)CD4+ and CD103+(CD69+)CD8+ T-cell subsets have not been studied in newly diagnosed IBD patients so far.
In our previous study, we observed lower percentages of overall CD103+ T cells in IBD patients with active disease compared with healthy controls in biopsies of ileal and colonic mucosa analyzed together.15 The present study is a continuation of this work, expanding the patient population, analyzing ileal and colonic biopsies separately for the determination of the percentages of CD103+CD4+ and CD103+CD8+ subsets, performing additional phenotypical analysis, and studying both newly diagnosed, untreated IBD patients at presentation and during follow-up.
PATIENTS AND METHODS
Patients
Patients with IBD were prospectively recruited at the Department of Gastroenterology and Hepatology in Rijnstate Hospital in Arnhem, the Netherlands, a referral hospital for IBD patients. Patients suspected of IBD based on clinical symptoms (chronic diarrhea, rectal blood loss, abdominal pain, or weight loss) and/or calprotectin values underwent ileocolonoscopy as part of their initial diagnostic workup. The diagnosis of IBD was based on clinical, endoscopic, and histopathological features according to the ECCO guidelines.16, 17 Exclusion criteria consisted of concomitant or previous use of immunosuppressive medication and the presence of other autoimmune disorders or malignancies. We included healthy controls (HCs) that underwent ileocolonoscopy for polyp surveillance or iron deficiency. Enrolled healthy controls had no history of autoimmune diseases. During ileocolonoscopy, macroscopically normal colonic and ileal mucosa of HCs was identified and subsequently microscopically confirmed. At the time of inclusion, all IBD patients were naïve to immunosuppressive treatment (eg, steroids, thiopurines, aminosalicylates, and biologics). Follow-up ileocolonoscopy was performed as part of standard care in 42.7% of the included IBD patients.
After initial diagnosis of IBD, treatment was initiated according to the step-up approach. Crohn’s disease patients started with corticosteroids, followed by maintenance treatment with immunomodulators (primarily thiopurines) and when ineffective anti-TNFα treatment was prescribed. Preparations with aminosalicylates or corticosteroids were the first options of treatment in patients with UC. In case of nonresponse, thiopurines or anti-TNFα treatment was initiated. The follow-up period ranged from diagnosis until the last visit at the outpatient clinic. We reported the highest treatment step that was taken to reach remission in this follow-up period. This step was defined as the last treatment applied using the step-up approach.
Definition of Disease Activity
The Mayo score (0 to 3 scale) was used to assess endoscopic severity of disease activity at primary diagnosis and during follow-up endoscopy in UC patients. Patients with inactive disease were in endoscopic remission, defined as a Mayo score of 0.18 The simple endoscopic score for CD patients (SES-CD, scale from <4 to >19) was used to assess endoscopic severity of disease activity at primary diagnosis and during follow-up in CD patients. Patients with inactive disease were in endoscopic remission, defined as a SES-CD <7 and <4 for ileum-only CD patients.19
Location and behavior of disease was reported for both entities according to the Montreal classification.18 The Harvey-Bradshaw index was reported for evaluation of clinical complaints in Crohn’s disease and Mayo severity score for UC patients.20
Methods
Tissue handling and flow cytometry
At the time of initial diagnosis and during follow-up ileocoloscopy, 4 to 6 biopsies were taken from the most inflamed ileal and/or colonic parts of the mucosa, respectively. When ulcerations were present, these biopsy specimens were taken from the inflamed mucosa on the edge of the ulcerations.
In case of remission during follow-up endoscopy, biopsied specimens were taken from the same segments as at initial investigation. If patients had active disease during follow-up endoscopy, biopsies were taken from the most inflamed mucosal areas. Biopsied specimens from healthy controls were taken from ileum and colon to perform flow cytometric analysis and regular histological evaluation. Parallel to the flow cytometric analysis, biopsies were histologically evaluated for the confirmation of chronic active inflammation. Specimens for flow cytometric analysis were collected in a phosphate-buffered saline solution at 2–8° and analyzed within 8 hours. To preserve all cell surface proteins for phenotyping, we avoided enzymatic digestion and used mechanical preparation of a single cell suspension. Specimens were pooled and blended in Hanks’ 1% bovine serum albumin using a 70-mm gaze and spatula followed by Ficoll density gradient centrifugation. The homogenate was resuspended after washing in 0.5 mL Hanks’ 1% bovine serum albumin. The concentration of mononuclear cells in the suspension was estimated by microscopic counting with a KOVA glasstic slide (Hycor Biomedical Ltd., Penicuik, United Kingdom), following the same protocol as in our previous study.15
Two hundred μL of the total cell suspension was used for flow cytometric analysis (FACSCanto, BD Biosciences). The total intestinal CD3+ T-cell population was reported as a percentage from the whole lymphocyte population. The different CD3+ T-cell subpopulations (ie, CD8+, CD4+, CD103+, CD103-CD69+, CD69-, FoxP3+, FoxP3- and Ki-67+ T cells) were reported as a percentage of the total CD3+ population. The CD103+(CD69+) and CD103-(CD69+) T cells were also expressed as a percentage of the CD3+CD4+ and the CD3+CD8+ subset, respectively. Antibodies and reagens used for flow cytometry were all obtained from Becton Dickinson Biosciences, USA (Supplementary Table S1).
Peripheral blood handling and flow cytometry
Peripheral blood was withdrawn after initial ileocolonoscopy for measurement of C-reactive protein (CRP) and for immunophenotyping. Immunophenotyping was performed on whole blood (100 μl per monoclonal combination), followed by erythrocyte lysing using FACS lysing solution (BD Biosciences) and permeabilization (in case of intracellular staining: FACS Fix/Perm solution BD Biosciences).
Statistical Analysis
The Shapiro-Wilk test was used to test whether the sample was distributed normally. Categorical characteristics of patients were presented as a number (n) with percentage and analyzed using the χ2 test in case of normal distribution; otherwise, the Fisher exact test was performed. Medians of continuous variables were reported with 25th and 75th percentile (interquartile range [IQR]) in case the distribution was not normal. In case of a normal distribution, different T-cell subsets were analyzed with the independent t test; otherwise, the Mann-Whitney U test was performed. Patients in the follow-up group were compared with their own baseline values using the Wilcoxon signed ranks test or the paired t test. The Spearman test was used to test the correlation between the different T-cell subsets and the SES-CD score in CD and the Mayo in UC patient; if both variables were continuous, we performed the Pearson rank test. Statistical significance was accepted if the probability of a type I error did not exceed 5%. Data were analyzed with SPSS statistics (version 22.0.0.0; IBM Corp, Armonk, NY, USA) and GraphPad Prism (GraphPad Software version 7.0, La Jolla, CA, USA).
Ethics
The study protocol (NL28761.091.09) was approved by the research ethics committee of the Radboud University Nijmegen Medical Centre (CMO Regio Arnhem-Nijmegen). Written informed consent was obtained from each participating patient before any study-related procedure was performed. The procedures were performed in accordance with the Declaration of Helsinki (version 9, 19 October 2013).
RESULTS
Study Population
The baseline characteristics of all patients and HsC are presented in Table 1. In total, 75 CD patients, 49 UC patients, and 16 HCs were included. Crohn’s disease and ulcerative colitis groups were comparable for age and gender (P = 0.37 and P = 0.15). Patients with CD had higher baseline CRP levels (P = 0.001), more extraintestinal manifestations (P = 0.004), and fewer family members with IBD (P = 0.005) compared with UC patients. More CD patients were smokers at initial presentation compared with UC patients (P = 0.004). Patients with CD also had a longer history of complaints before diagnosis during initial ileocolonoscopy (P = 0.017).
TABLE 1.
Patient Demographics
| CD (n = 75) | UC (n = 49) | HC (n = 16) | P CD-UC | |
|---|---|---|---|---|
| Median age at diagnosis in years (IQR) | 27 (21–40) | 30 (26–40) | 43 (33–55) | 0.37 |
| Gender | ||||
| Female, n (%) | 49 (65.3%) | 30 (61.2%) | 14 (87.5%) | 0.15 |
| Male, n (%) | 26 (34.7%) | 19 (38.8%) | 2 (12.5%) | |
| Smoking at diagnosis | ||||
| Yes | 32 (42.7%) | 7 (14.3%) | 6 (37.5%) | 0.004* |
| No | 43 (57.3%) | 41 (83.7%) | 10 (62.5%) | |
| Extraintestinal manifestations | ||||
| Yes | 18 (24.0%) | 2 (4.1%) | 0 (0%) | 0.004* |
| IBD in family | ||||
| Yes | 7 (9.3%) | 15 (30.6%) | 1 (6.3%) | 0.005* |
| Duration of complaints before diagnosis | 0.017* | |||
| <3 months | 27 (36.0%) | 21 (42.9%) | — | |
| 3–6 months | 15 (20.0%) | 18 (36.7%) | ||
| > 6 months | 33 (44.0%) | 9 (18.4%) | ||
| CRP at initial diagnosis | 21 (9–61) | 3 (1–14) | 1 (1–6) | 0.001* |
| Median clinical follow-up period in months (IQR) | 32 (19–70) | 28 (9–41) | — | 0.601 |
| Baseline Mayo endoscopic score, n (%) | ||||
| Mayo 0 | 0 (0%) | — | — | |
| Mayo 1 | — | 9 (18.4%) | ||
| Mayo 2 | 30 (61.2%) | |||
| Mayo 3 | 10 (20.4%) | |||
| Baseline Montreal UC, n (%) | ||||
| Extent | ||||
| E1: Ulcerative proctitis | — | 11 (22.4%) | — | — |
| E2: Left-sided UC | 17 (34.7%) | |||
| E3: Extensive UC | 21 (42.9%) | |||
| Severity | ||||
| S0: Clinical remission | 0 (0%) | |||
| S1: Mild UC | 14 (28.6%) | |||
| S2: Moderate UC | 23 (46.9%) | |||
| S3: Severe UC | 12 (24.5%) | |||
| HBI score, n (%) | ||||
| <5, remission | 3 (4.0%) | — | — | — |
| 5–7 mild disease | 31 (41.3%) | |||
| 8–16 moderate disease | 33 (44.0%) | |||
| >16 severe disease | 8 (10.7%) | |||
| Baseline SES-CD, n (%) | ||||
| 0–3 inactive disease | 0 (0%) | — | — | — |
| 4–10 mild disease | 26 (34.7%) | |||
| 11–19 moderate disease | 33 (44.0%) | |||
| >19 severe disease | 16 (21.3%) | |||
| Baseline Montreal CD, n (%) | ||||
| Location | ||||
| L1: ileal | 26 (34.7%) | — | — | — |
| L2: colonic | 32 (42.7%) | |||
| L3: ilealcolonic | 17 (22.6%) | |||
| Behaviour | ||||
| B1: nonstricturing, nonpenetrating | 58 (77.3%) | |||
| B2: structuring | 13 (17.3%) | |||
| B3: penetrating | 4 (5.3%) | |||
| P: perianal disease | 11 (14.7%) | |||
| Highest step in treatment to reach remission | — | 0.001* | ||
| 5-ASA | 4 (5.3%) | 30 (61.2%) | ||
| Oral steroids | 6 (8%) | 4 (8.2%) | ||
| Immunomodulators | 41 (54.7%) | 12 (24.5%) | ||
| Anti-TNF | 20 (26.7%) | 3 (6.1%) | ||
| MTX | 3 (4%) | 0 (0%) | ||
| Anti-integrins | 0 (0%) | 0 (0%) | ||
| Resective surgery | 1 (1.3%) | 0 (0%) | ||
| Follow-up Mayo endoscopic score n = 20, n (%) [Baseline Mayo score of the follow-up group, n [%]] | — | |||
| Mayo 0 | 7 (35%) [0[0%]] | |||
| Mayo 1 | 5 (25%) [4[20%]] | |||
| Mayo 2 | 4 (20%) [10[50%]] | |||
| Mayo 3 | 4 (20%) [6[30%]] | |||
| Follow-up SES-CD n = 32, n (%) | ||||
| 0–3 inactive disease | 21(65.6%) | — | ||
| 4–10 mild disease | 7 (21.9%) | |||
| 11–19 moderate disease | 3 (9.4%) | |||
| >19 severe disease | 1 (3.1%) |
* Significant P value ≤0.05.
After diagnosis, the majority of CD patients needed immunomodulators (n = 41, 54.7%). The majority of UC patients reached remission on aminosalicylate preparations (n = 30, 61.2%). No patients were treated with Vedolizumab or Etrolizumab.
Frequencies of Intestinal CD103+T-Cell Subsets at Baseline
Ulcerative colitis
The baseline frequencies of the different intestinal T-cell subsets in UC can be found in Figure 1 and Supplementary Table S2.
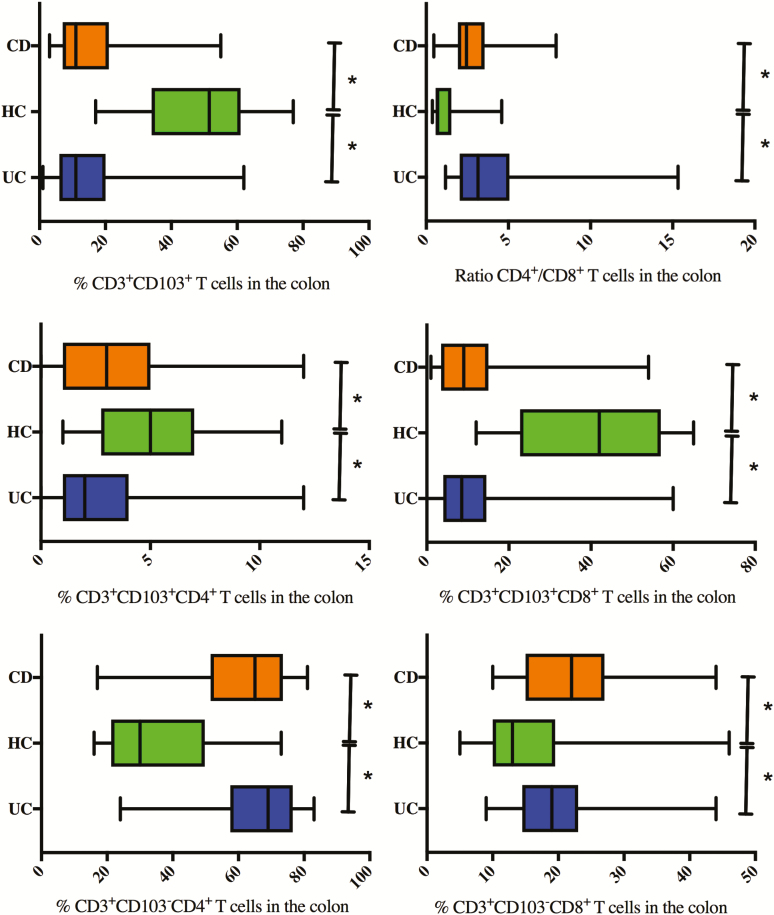
FIGURE 1.
Baseline percentages of CD103+, CD103+CD4+, CD103+CD8+, CD103-CD4+, and CD103-CD8+ within CD3+ T lymphocytes and the ratio CD4+/CD8+ T lymphocytes explored with FACS analysis on colonic biopsies of UC and CD patients with active colon disease compared with healthy controls. *Significant P value.
In colonic biopsies of UC patients, lower percentages of CD103+ T cells (11% [6 to 20]) were found compared with colonic biopsies of HC (52% [34 to 61], P = 0.001). Both CD103+CD4+ and CD103+CD8+ T-cell subsets were present in lower percentages (respectively 3% [1 to 4]) and 9% [5 to 14]) in UC compared with HCs (respectively 5% [5 to 7], P = 0.002 and 42% [23 to 57], P = 0.001). The CD103-CD4+ T-cell subpopulation predominates (69%) in colonic biopsies from UC patients at diagnosis compared with HCs, in concordance with a higher CD4+/CD8+ ratio of 3.0 (2.0 to 4.2) in patients vs 0.7 in HCs (0.6 to 1.5, P = 0.001).
There is no correlation between the severity of disease (mayo score) at diagnosis and the numbers of the different T-cell subsets (Supplementary Figure S1).
Crohn’s disease
The baseline frequencies of the different colon and ileum T-cell subsets in CD can be found in Figures 1 and 2 and Supplementary Table S2.
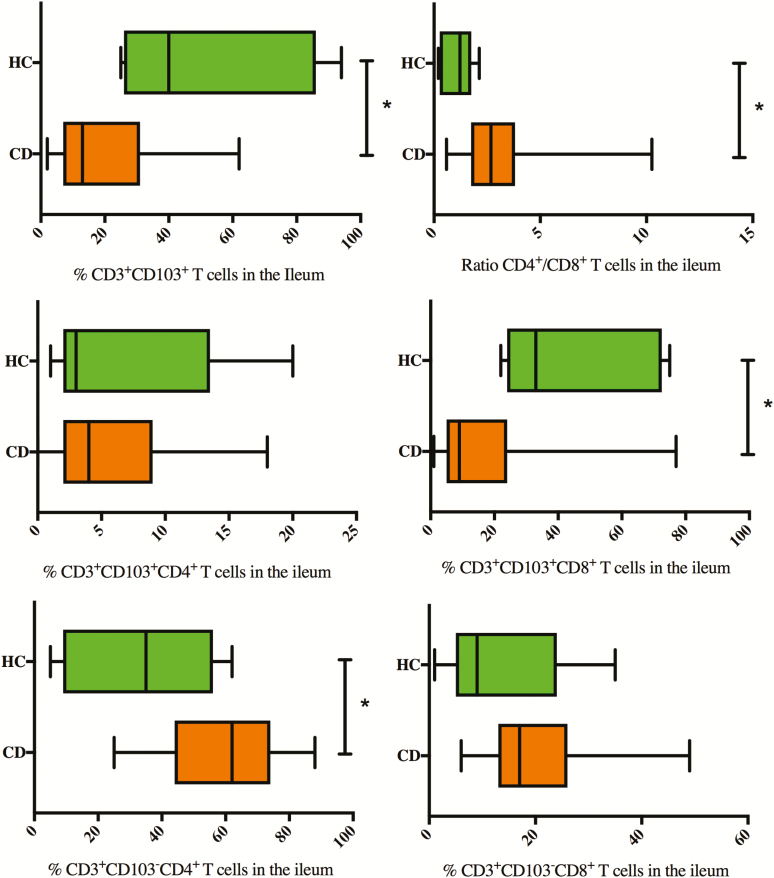
FIGURE 2.
Baseline percentages of CD103+, CD103+CD4+, CD103+CD8+, CD103-CD4+, and CD103-CD8+ within CD3+ T-lymphocytes and the ratio CD4+/CD8+ T-lymphocytes explored with FACS analysis on ileal biopsies of CD patients with active ileal disease compared with healthy controls. *Significant P value.
Colonic biopsies of active CD patients showed lower percentages of CD3+CD103+ T cells (11% [7 to 21]) compared with colonic biopsies of HCs (52% [34 to 61], P = 0.001). Numbers of both CD103+CD4+ and CD103+CD8+ T cells were decreased compared with HC (42% [23 to 57]), with a more pronounced decrease in the CD103+CD8+ subset (9% [4 to 15], P = 0.001). In line with these results, the majority of the colonic CD8+ T cells (86% [66 to 100]) and CD4+ T cells (92% [85 to 97]) were CD103-. The CD4+/CD8+ ratio within the total T-cell population in the colon of CD patients was 2.4 (1.9 to 3.5) vs 0.7 in colon biopsies taken from healthy controls (0.6 to 1.5, P = 0.001).
There were no statistical differences for any of the analyzed subsets between ileal and colonic location in CD patients, nor between colonic CD and UC.
Concomitant perianal disease at initial diagnosis of CD was associated with lower percentages of CD103+ T cells, both CD103+CD4+ and CD103+CD8+ T-cell subsets, in ileal biopsies (respectively, 5.5% [2.75 to 8.25], 1.5% [1 to 2] and 4.25% [2.25 to 6.5]) compared with active ileal CD without perianal activity (respectively 15.0% [7 to 35] P = 0.018, 4.0% [2–10], P = 0.039 and 11.0% [6 to 24] P = 0.027). However, there was no significant correlation between the SES-CD score and the numbers of the different T-cell subsets (Supplementary Fig. S1).
Smoking in CD was associated with higher CD103-CD8+ T cells (25.0% [17 to 31]) in the colon biopsies and higher CD103+CD4+ T cells (6.0% [3 to 13]) in ileal biopsies compared with nonsmoking CD patients (respectively 19.5% [14 to 24] P = 0.011 and 3.0% [1.0 to 5.75] P = 0.03).
Additional Phenotypical Analysis of the CD103+ T-Cell Subsets at Baseline
CD69 as a marker of cell activation
In a smaller cohort of newly diagnosed IBD patients (UC, n = 10; CD, n = 1; and HCs, n = 4), CD69 staining was performed. In the colonic biopsies of UC and CD patients, lower percentages of CD103+CD8+CD69+ T cells (respectively 7% [5 to 12] and 6% [4 to 10]) were demonstrated compared with HCs (14% [8 to 21], UC vs HC, P = 0.14; CD vs HC, P = 0.05), similar to lower percentages of CD103+CD4+CD69+ T cells (UC 3% [2 to 6], CD 2% [1 to 4], HC 5% [3 to 9]; UC vs HC, P = 0.37; CD vs HC, P = 0.11) (Table 2).
TABLE 2.
Baseline Percentages of CD103+CD4+CD69+, CD103+CD8+CD69+, CD103-CD4+CD69+, and CD103-CD8+CD69+ Within CD3+ T Lymphocytes Explored With FACS Analysis in Crohn’s Disease, Ulcerative Colitis and Healthy Controls. Median percentages (IQR). *Significant P value ≤0.05. N = amount of analyzed biopsies.
| Location of biopsies inflamed mucosa at first presentation | CD | UC | HC | P | ||
|---|---|---|---|---|---|---|
| Colon (n = 10) | Ileum (n = 10) | Colon (n = 10) | Colon (n = 4) | Ileum (n = 4) | ||
| CD3 + CD103 + CD8 + CD69 + % d | 6.42 (4.3–10.4) | 18.7 (1.5–27.8) | 7.5 (4.6–11.8) | 14.1 (8.3–21.2) | 39.7 (29.1–60.6) | a0.14 |
| b0.05* | ||||||
| c0.05* | ||||||
| CD3 + CD103 - CD8 + CD69 + % d | 4.4 (3.3–7.2) | 2.4 (1.7–5.7) | 5.7 (4.2–6.4) | 3.1 (1.7–3.7) | 1.6 (1.4–1.9) | a0.01* |
| b0.05* | ||||||
| c0.08 | ||||||
| CD3 + CD103 + CD8 + CD69 - % d | 0.6 (0.4–0.8) | 1.1 (0.4–1.6) | 0.8 (0.4–1.8) | 0.8 (0.6–0.9) | 0.5 (0.1–0.9) | a1.01 |
| b0.24 | ||||||
| c0.11 | ||||||
| CD3 + CD103 - CD8 + CD69 - % d | 11.9 (7.9–12.7) | 6.2 (2.1–9.4) | 6.0 (3.5–9.0) | 4.6 (3.9–6.9) | 1.2 (0.6–5.6) | a0.73 |
| b0.01* | ||||||
| c0.11 | ||||||
| CD3 + CD103 + CD4 + CD69 + % d | 2.4 (1.3–4.0) | 5.3 (1.8–7.7) | 2.7 (1.8–6.1) | 5.1 (2.6–9.0) | 13.9 (8.8–20.9) | a0.37 |
| b0.11 | ||||||
| c0.01* | ||||||
| CD3 + CD103 - CD4 + CD69 + % d | 32.0 (27.0–38.5) | 32.7 (19.2–37.9) | 50.4 (34.3–58.1) | 45.7 (33.3–47.8) | 18.4 (12.3–32.2) | a0.37 |
| b0.14 | ||||||
| c0.19 | ||||||
| CD3 + CD103 + CD4 + CD69 - % d | 0.0 (0.0–0.7) | 0.4 (0.0–0.7) | 0.7 (0.0–0.8) | 0.0 (0.0–0.5) | 0.4 (0.1–0.6) | a0.24 |
| b0.84 | ||||||
| c1.01 | ||||||
| CD3 + CD103 - CD4 + CD69 - % d | 39.9 (32.5–45.2) | 33.3 (14.1–50.0) | 23.2 (18.2–25.4) | 32.1 (20.9–33.3) | 17.5 (7.7–23.7) | a0.24 |
| b0.08 | ||||||
| c0.11 |
a P value comparison active UC colon with HC colon.
b P value comparison active CD colon with HC colon.
c P value comparison active CD ileum with HC ileum.
dPercentages within CD3+ T cells
Overall percentages of CD103+CD69+ T-cell subsets were higher in the ileum of CD patients and HCs compared with percentages in their colonic mucosa. Ileal CD was associated with statistic significant lower percentages of CD103+CD69+, both CD8+ (19% [2 to 28]) and CD4+ (5% [2 to 8]) T cells compared with the ileum of HC (respectively 40% [29 to 61], P = 0.05, and 14% [9 to 21], P = 0.01) (Table 2).
FoxP3 as a marker for regulatory CD4+ T cells
We found low percentages of CD103+FoxP3+CD25+CD4+ T cells both in colon and ileum, ranging from 0%–2% of the total lymphocyte population (with no differences between HCs and IBD patients, P = 0.98). The majority of FoxP3+CD25+CD4+ regulatory T cells (Tregs) turned out to be CD103-.
Ki-67 as a marker for cell proliferation
CD103+ T-cell subsets had a 2-fold higher fluorescence intensity of Ki-67 compared with CD103- T-cell subsets (Table 3). This suggests a higher proliferation rate of the CD103+ T cells compared with the CD103- T cells.
TABLE 3.
Ki-67 Expression on CD8+CD103+, CD8+CD103-, CD4+CD103+, and CD4+CD103- T Cells. Geometric Mean Fluorescence Intensity (IQR).
| Location of biopsies inflamed mucosa at first presentation | UC colon (n = 11) | CD colon (n = 4) | CD ileum (n = 4) |
|---|---|---|---|
| CD3+CD8+CD103+ki67 | 848 (709–1263) | 804 (573–1229) | 624 (530–727) |
| CD3+CD4+CD103+ki67 | 475 (334–894) | 510 (384–847) | 472 (357–704) |
| CD3+CD8+CD103-ki67 | 539 (454–666) | 453 (443–480) | 594 (445–699) |
| CD3+CD4+CD103-ki67 | 226 (221–309) | 275 (264–388) | 317 (240–434) |
CD103+ T-Cell Subsets in Peripheral Blood at Baseline
Percentages of the CD103+ T-cell subsets in peripheral blood mononuclear cells (PBMCs) were very low (<1% within the CD3+ population), with no differences between IBD patients and HCs. (Supplementary Table S3)
Frequencies of Intestinal CD103+T-Cell Subsets During Follow-up
Ulcerative colitis
The frequencies of different intestinal T-cell subsets in UC during follow-up can be found in Supplementary Table S4.
Percentages of different intestinal T-cell subsets from UC patients in remission during follow-up were compared with their own baseline and with HCs. In these patients, the percentages shifted to levels comparable to HC (Figs. 3–5 and Supplementary Table S4). There was a statistically significant increase in percentage of CD103+ T cells (both CD4+ and CD8+) in patients in remission during follow-up when compared with their own baseline values. The CD4+/CD8+ ratio in remission (0.7 [0.57 to 1.53]) was comparable to the ratio in healthy controls (0.7 [0.6 to 1.5]). In UC patients with active disease during follow-up, T-cell subpopulations were comparable to their baseline levels.
FIGURE 3.
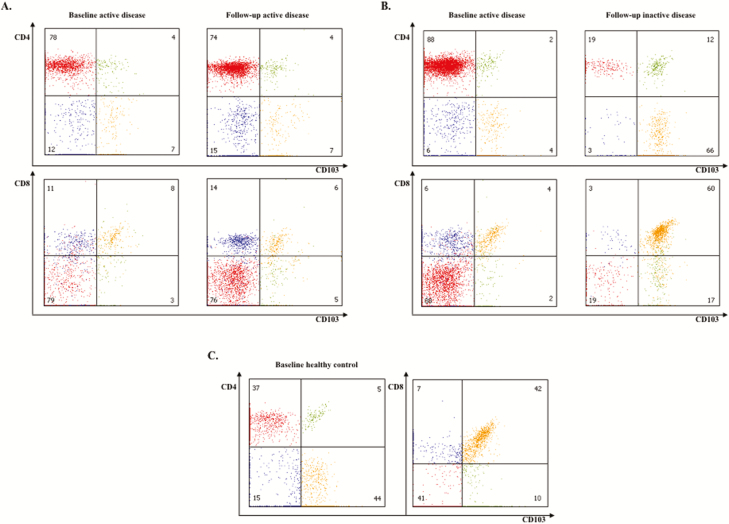
Representative flow cytometric analyses of CD103+CD4+, CD103+CD8+, CD103-CD4+, and CD103-CD8+ within CD3+ T-lymphocytes on (A) colonic biopsies of a CD patient with moderate to severe colonic disease during baseline and follow-up endoscopy, (B) colonic biopsies of a CD patient with moderate to severe colonic disease during baseline endoscopy and inactive disease during follow-up endoscopy, and (C) colonic biopsies of a healthy control.
FIGURE 4.
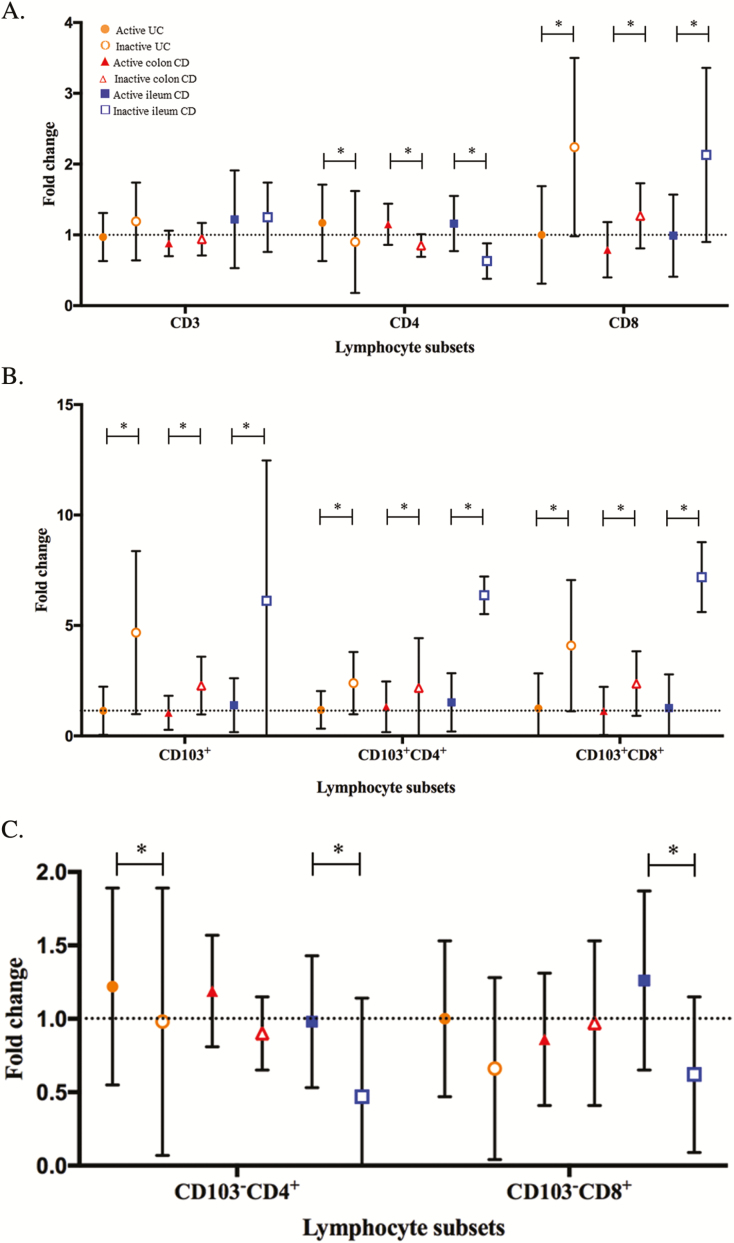
Intra-individual fold change, expressing the ratio between follow-up and baseline percentages of the different intestinal lymphocyte subsets with either active or inactive endoscopic disease (A, CD3, CD4, CD8; B, CD103+, CD103+CD4+, CD103+CD8+; C, CD103-CD4+, CD103-CD8+). ● Active UC (n = 13). ○ Inactive UC (n = 7). ▲Active colon CD (n = 9). ∆ Inactive colon CD (n = 10). ■ Active ileum CD (n = 6). □ Inactive ileum CD (n = 17). *Significant P value ≤0.05.
FIGURE 5.
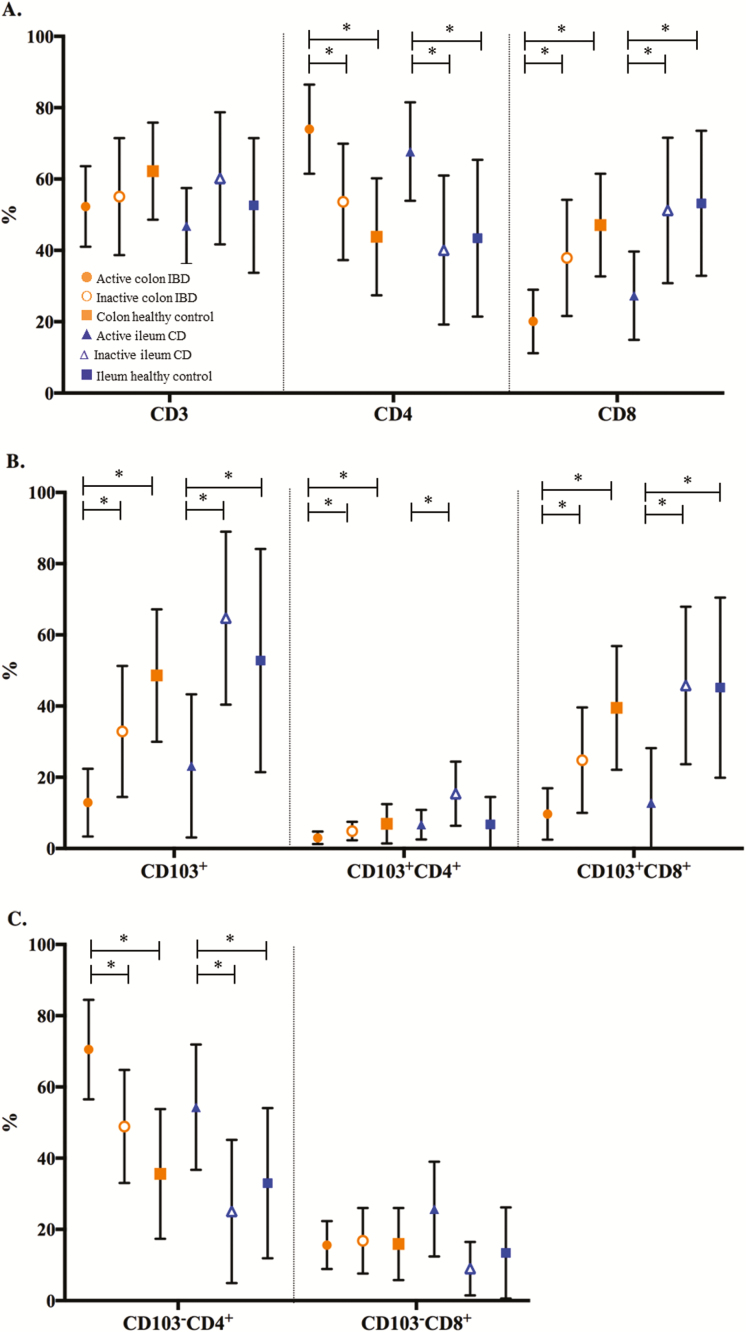
Percentages of the different intestinal lymphocyte subsets at follow-up endoscopy (A, CD3, CD4, CD8; B, CD103+, CD103+CD4+, CD103+CD8+; C, CD103-CD4+, CD103-CD8+). ● Active colon IBD (n = 22). ○ Inactive colon IBD (n = 17). ■ Colon healthy control (n = 16). ▲Active ileum CD (n = 6). ∆ Inactive ileum CD (n = 17). ■ Ileum healthy control (n = 5). *Significant P value ≤0.05.
Patients with UC with active endoscopic disease during follow-up had statistically significant lower percentages of CD103+, both CD4+ (3.0% [1 to 4]) and CD8+ (6% [2 to 13]) compared with patients with inactive endoscopic disease (5.0% [3 to 8] and 38% [18 to 45], P = 0.002). In active disease during follow-up, the CD103- CD4+ T-cell subpopulation was predominant (76.0% [69 to 86]).
Crohn’s disease
The frequencies of different intestinal T-cell subsets in CD during follow-up can be found in Supplementary Table S5.
Percentages of different intestinal T-cell subsets in colonic and ileal tissue from CD patients in remission were compared with their own baseline levels and HCs. In the ileal tissue of these CD patients, the percentages of the different intestinal subsets reached levels comparable to HCs (Figs. 3–5 and Supplementary Table S5). This was a statistically significant increase of CD103+ T cells, both CD4+ and CD8+, in the ileum of CD patients with inactive disease during follow-up (P = 0.001, P = 0.003, and P = 0.002). In line with these findings, the percentages of CD103- T-cell subsets were decreased. The CD4+/CD8+ ratio in the ileum of patients with inactive disease was 0.73 (0.46 to 1.15), comparable to the ratio in healthy controls (0.7 [0.6–1.5]).
In comparison with the ileum, the percentages of CD103+CD4+ T-cell subsets in the colonic tissue of CD patients with inactive disease only showed a trend toward levels seen in HCs. The percentage of CD103+CD8+ T cells in the colon increased statistically significant from baseline (P = 0.025). The CD4+/CD8+ ratio in the colon of patients with inactive disease was 2.08 (1.3 to 2.33), statistically not different from healthy controls (Figs. 3–5 and Supplementary Table S5). There were also no differences between T-cell subpopulations in colon of CD patients with active disease during follow-up compared with their baseline levels.
DISCUSSION
In the present study, we found substantial differences in the intestinal CD103+ T lymphocyte subsets of patients with active CD and UC compared with healthy controls and patients in endoscopic remission. Percentages of CD103- T-cell subsets were higher in inflamed ileum and colon of newly diagnosed IBD patients compared with HCs and IBD patients in remission, with the majority consisting of CD103-CD4+ T cells. Baseline numbers in active disease of both CD103+CD4+ and CD103+CD8+ T cells were decreased compared with HCs, with a more pronounced decrease in the CD103+CD8+ subset. The same differences were found in the CD103+CD69+ T-cell subsets. Percentages of CD103+CD4+ and CD103+CD8+ T cells were low in patients with active disease during follow-up endoscopy, comparable to active disease at baseline. In patients with endoscopic remission during follow-up, the proportion of CD103+CD4+ and CD103+CD8+ T cells increased to levels comparable to HCs.
The intestine of healthy humans comprises almost equal proportions of CD4+ and CD8+ T cells, with marginally higher numbers of CD8+ T cells.21 The majority of intestinal CD103+ T cells belongs to the CD8+ subset (76%).21 The present results are in line with these findings, as we demonstrated a CD4/CD8 ratio of 0.7 to 1.2 and a predominance (75%–80%) of CD8+ T cells within the CD103+ T-cell subset. The CD103 expression is confined to a small subset (<2%) of circulating T lymphocytes, which was confirmed in our cohort.15, 22 After migration to the mucosal tissue, CD103 expression on T cells is induced and maintained by TGF-β, produced by epithelial and dendritic cells.23 The CD103 is expressed at high levels on T cells in the skin, eyes, and in the mucosa of the gut and lungs of healthy patients,21 but also on dendritic cells, innate lymphoid cells and natural killer cells.23 Its function is exerted through binding to E-cadherin on intestinal epithelial cells (IECs), mediating cell adhesion and retention of T cells within the mucosa. On the other hand, the IECs themselves are able to interact and activate different T-cell subsets and were demonstrated to induce a CD103+CD8+ subset with regulatory function.24
In sarcoidosis, reduced frequencies of CD103+CD4+ and CD103+CD8+ T cells were found in bronchoalveolar lavage fluid compared with patients with other interstitial lung disease (eg, hypersensitivity pneumonitis, idiopathic pulmonary fibrosis, nonspecific interstitial pneumonia, and cryptogenic organizing pneumonia).25 These reduced CD103+ T-cell percentages, which are accompanied by a peripheral CD4+ T-cell lymphopenia, suggest a recruitment of CD4+ T cells towards lung tissue.
Other studies on CD103 expression in IBD showed contradictory results. Flow cytometric analysis by Elewaut et al showed decreased percentages of CD103+ T cells within intraepithelial lymphocytes (IELs) in ileal and colonic tissue of CD patients compared with HCs.26 No differences in numbers of CD103+ T cells were seen in the lamina propria (LP) of CD patients compared with HCs.26 Analysis with immunohistochemistry in another study showed no differences between IBD and HC in frequencies of epithelial CD103+T cells but demonstrated higher numbers of lamina propria CD103+ T cells in IBD.27 Using immunohistochemistry and quantitative reverse transcription polymerase chain reaction, Ichikawa et al showed a higher proportion of αE+ T cells in the ileum compared with the colon of patients with long-standing CD and UC, as well as in HCs. In CD patients with active colon disease, reduced numbers of the total amount of αE+ T cells were seen compared with HCs and UC patients. Overall, αE levels were not affected by inflammation and did not differ from HC.28
Regarding different CD103+ T-cell subsets in IBD, higher percentages of intestinal CD103+CD4+ subsets were recently described by performing immunohistochemistry in a small number of UC and CD patients vs HCs. No difference was found for the percentages of CD103+CD8+ T cells in neither epithelium nor lamina propria of IBD patients compared with HCs.11 However, these patients were under treatment with anti-α4β7 antibodies (Vedolizumab), which could have influenced the frequencies of β7+ T-cell subsets in gut mucosa and peripheral blood. Therefore, these findings are not comparable with our results in untreated patients. Further experiments in dextran sulfate sodium mice models by the same authors demonstrated an inhibitory effect of treatment with anti-β7 antibody (Etrolizumab) on the accumulation of CD103+CD8+ but not on the CD103+CD4+ T cells in the mucosa.11 Nevertheless, the percentage of CD103+CD4+ of all intestinal CD103+ T cells has been reported to be very low; the majority of the CD103+ T cells is CD8+.29 In previously mentioned studies,12, 26–28 inflamed and uninflamed biopsies of long-standing IBD patients under treatment were statistically analyzed together, which could have influenced the numbers of αE+ T cells. The discrepancies between studies can also be explained by the method used (immunohistochemistry vs FACS) and the number of included patients.
The protein CD69 is a marker for early lymphocyte activation and tissue retention.14 We observed, however, that T cells with a TRM phenotype (CD103+ and CD69+) were present in lower percentages in untreated newly diagnosed IBD patients compared with patients with inactive disease and HCs. It is mainly the CD103-(CD4+) T-cell population that is clearly increased in active IBD. Expression of CD69, which also may suggest activation, was not different on this CD103-CD4+ T-cell population in active IBD compared with HCs.
In a TNF-driven mice model of chronic ileitis, CD103+CD8+ T cells exerted regulatory functions by producing TGF-β, inhibiting proliferation of CD4+ T cells and attenuating transferred ileitis in vivo.30 Another study displayed an essential role in mucosal immune regulation for CD103+ T cells, explained by regulatory T cell–mediated suppression of colitis, which was absent in CD103-deficient mice.31 Recently, a pro-inflammatory role has been suggested for the CD103+CD4+ T cells in IBD, explained by expression of higher levels of IFNy and TNFα; although this could not be demonstrated for CD103+CD8+T cells.12 The functional features of CD103+CD8+ are in need of further investigation in the future, as this subset has been demonstrated to possess substantial regulatory capacities in mice models.
In the present study, frequencies of CD103+CD4+ and CD103+CD8+ T-cell subsets are described for the first time in a large cohort of newly diagnosed patients, in inflamed and noninflamed ileum and colon samples analyzed separately, at baseline and during follow-up. Our results are in line with previous studies, showing that CD103+ T cells in colon of HCs and IBD patients in remission mainly consist of CD8+ cells.21 The largest intestinal T-cell subset involved in active IBD is the CD103-CD4+ subset. These higher numbers of CD103- T cells next to their low proliferation level (Ki-67 fluorescence intensity) confirm a redistribution from peripheral origin of this subset instead of a local proliferation. Furthermore in our patients, frequencies of both CD103+CD4+ and CD103+CD8+ subsets did not increase in the inflamed gut mucosa during follow-up endoscopy. Therefore, these findings do not underline an upregulation of CD103 on intestinal T cells in the inflamed gut mucosa in time.
The proportion of CD103+ T cells, CD4+, and CD8+ subsets increased in our patients reaching endoscopic remission. We can also confirm that CD patients in remission and HCs express higher percentages of CD103+ T cells in the ileum mucosa compared with the colon. This is in line with previous studies describing a linear decrease in the number of T cells expressing CD103 from the ileum and ascending colon to rectum.28, 32
A limitation of the present study might be the use of intestinal biopsies that do not reach the deepest layers of the intestinal wall such as the muscularis layer. This might lead to an underestimation of the T-cell infiltration of the deepest layers in CD patients with transmural inflammation. The mechanical method used to preprocess the biopsy specimens before flow cytometry might have had an influence on the absolute cell numbers. However, in a recent comparison of different methods (mechanical, enzymatic and organ culture protocols), they all proved to have their limitations.33 In line with this method, we were not able to distinguish the lymphocyte infiltrate in lamina propria from the epithelium.
In conclusion, CD103+CD4+ and CD103+CD8+ subsets represent only a minority of the T-cell infiltrate in the inflamed gut, and maintenance of these low numbers was seen in active disease during follow-up. The majority of the mucosal infiltrating T cells in active IBD consists of the CD103-CD4+ subset both at diagnosis and follow-up. Therefore, we found no evidence for an upregulation of CD103+ on intestinal CD4+ and CD8+ T cells during chronic inflammation in time.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. A. A. van Bodegraven for his helpful suggestions during the writing of the manuscript.
Glossary
Abbreviations
- α4β7
alfa4beta 7
- CD103
αE, alfa E
- CD
Crohn’s disease
- CRP
C-reactive protein
- DSS
Dextran Sulfate Sodium
- GALT
gutaAssociated lymphoid tissue
- HC
healthy control
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cells
- IEL
intraepithelial lymphocytes
- IHC
immunohistochemistry
- SES-CD
simple endoscopic score for Crohn’s disease
- PBMC
peripheral blood mononuclear cells
- RT-PCR
Reverse Transcription Polymerase Chain Reaction
- Tem
effector memory T cells
- Treg
regulatory T cells
- TRM
tissue-resident-memory T cells
- UC
ulcerative colitis
Author Contribution: All authors participated in the conception and design of the study. BR, MG, CH, CS, and PW participated in patient recruitment and material collection. BR, CH, EvK, and EvL were responsible for flow cytometric immunophenotyping of the mucosal lymphocytes. BR, MG, CH, EvK, EvL, CS, and PW were responsible for collecting data. BR was responsible for statistical analysis. All authors were members of the writing group and participated in the drafting and revision of the manuscript. All authors approved the final version of the manuscript.
REFERENCES
- 1. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. . Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15:39–49. [DOI] [PubMed] [Google Scholar]
- 2. Park JH, Peyrin-Biroulet L, Eisenhut M, et al. . IBD immunopathogenesis: a comprehensive review of inflammatory molecules. Autoimmun Rev. 2017;16:416–426. [DOI] [PubMed] [Google Scholar]
- 3. Zundler S, Neurath MF. Novel insights into the mechanisms of gut homing and antiadhesion therapies in inflammatory bowel diseases. Inflamm Bowel Dis. 2017;23:617–627. [DOI] [PubMed] [Google Scholar]
- 4. Perez-Jeldres T, Tyler CJ, Boyer JD, et al. . Cell traficking interference in inflammatory bowel disease: therapeutic interventions base don basic pathogenesis concepts. Inflamm Bowel Dis 2019;25:270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kilshaw PJ, Murant SJ. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol. 1990;20:2201–2207. [DOI] [PubMed] [Google Scholar]
- 6. Austrup F, Rebstock S, Kilshaw PJ, et al. . Transforming growth factor-beta 1-induced expression of the mucosa-related integrin alpha E on lymphocytes is not associated with mucosa-specific homing. Eur J Immunol. 1995;25:1487–1491. [DOI] [PubMed] [Google Scholar]
- 7. Schön MP, Arya A, Murphy EA, et al. . Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 8. Smids C, Horjus Talabur Horje CS, van Wijk F, et al. . The complexity of alpha E beta 7 blockade in inflammatory bowel diseases. J Crohns Colitis. 2017;11:500–508. [DOI] [PubMed] [Google Scholar]
- 9. Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vermeire S, Sandborn WJ, Danese S, et al. . Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:135–144. [DOI] [PubMed] [Google Scholar]
- 11. Zundler S, Schillinger D, Fischer A, et al. . Blockade of αEβ7 intergin suppresses accumulation of CD8+ and Th9 lymphocytes from patients with IBD in the inflamed gut in vivo. Gut. 2017; 66(11):1936–1948. [DOI] [PubMed] [Google Scholar]
- 12. Lamb CA, Mansfield JC, Tew GW, et al. . Αeβ7 integrin identifies subsets of pro-inflammatory colonic CD4+ T lymphocytes in ulcerative colitis. J Crohns Colitis. 2017;11:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. [DOI] [PubMed] [Google Scholar]
- 14. Cibrián D, Sánchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol. 2017;47:946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smids C, Horjus Talabur Horje CS, Drylewicz J, et al. . Intestinal T cell profiling in inflammatory bowel disease: linking T cell subsets to disease activity and disease course. J Crohns Colitis. 2018;12:465–475. [DOI] [PubMed] [Google Scholar]
- 16. Magro F, Gionchetti P, Eliakim R, et al. ; European Crohn’s and Colitis Organisation [ECCO] . Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–670. [DOI] [PubMed] [Google Scholar]
- 17. Gomollón F, Dignass A, Annese V, et al. ; ECCO . 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 18. Silverberg MS, Satsangi J, Ahmad T, et al. . Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 19. Daperno M, D’Haens G, Van Assche G, et al. . Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. [DOI] [PubMed] [Google Scholar]
- 20. Harvey RF, Bradshaw JM. A simple index of crohn’s-disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 21. Sathaliyawala T, Kubota M, Yudanin N, et al. . Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cepek KL, Parker CM, Madara JL, et al. . Integrin alpha E beta 7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150:3459–3470. [PubMed] [Google Scholar]
- 23. Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412–419. [DOI] [PubMed] [Google Scholar]
- 24. Allez M, Brimnes J, Dotan I, et al. . Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123:1516–1526. [DOI] [PubMed] [Google Scholar]
- 25. Mota PC, Morais A, Palmares C, et al. . Diagnostic value of CD103 expression in bronchoalveolar lymphocytes in sarcoidosis. Respir Med. 2012;106:1014–1020. [DOI] [PubMed] [Google Scholar]
- 26. Elewaut D, Van Damme N, De Keyser F, et al. . Altered expression of alpha E beta 7 integrin on intra-epithelial and lamina propria lymphocytes in patients with Crohn’s disease. Acta Gastroenterol Belg. 1998;61:288–294. [PubMed] [Google Scholar]
- 27. Oshitani N, Watanabe K, Maeda K, et al. . Differential expression of homing receptor CD103 on lamina propria lymphocytes and association of CD103 with epithelial adhesion molecules in inflammatory bowel disease. Int J Mol Med. 2003;12:715–719. [PubMed] [Google Scholar]
- 28. Ichikawa R, Lamb CA, Eastham-Anderson J, et al. . AlphaE integrin expression is increased in the ileum relative to the colon and unaffected by inflammation. J Crohns Colitis. 2018;1–9. doi: 10.1093/ecco-jcc/jjy084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lamb CA, Kirby JA, Keir ME, et al. . T lymphocytes expressing alphae beta7 integrin in ulcerative colitis: associations with cellular lineage and phenotype. J Crohns Colitis. 2017;11:1504–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ho J, Kurtz CC, Naganuma M, et al. . A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol 2008;180:2573–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Annacker O, Coombes JL, Malmstrom V, et al. . Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirby JA, Bone M, Robertson H, et al. . The number of intraepithelial T cells decreases from ascending colon to rectum. J Clin Pathol. 2003;56:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carrasco A, Mañe J, Santaolalla R, et al. . Comparison of lymphocyte isolation methods for endoscopic biopsy specimens from the colonic mucosa. J Immunol Methods. 2013;389:29–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.