Abstract
Recently, many interdisciplinary community researchers have focused their efforts on study of the low-level light irradiation effects (photobiomodulation, PBM) as a promising therapeutic technology. Among the priorities, a search of new wavelength ranges of laser radiation to enhance the laser prospects in treatment of autoimmune and cancer diseases commonly accompanied by disorders in the antioxidant system of the body. The laser wavelengths within 1265-1270 nm corresponds to the maximum oxygen absorption band. Therefore, PBM effects on a model organism within this spectrum range are of particular interest for preclinical research. Here, we report comprehensive biomolecular studies of the changes in the BALB/c nude mice skin after an exposure to the continuous laser radiation at the 1270 nm wavelength and energy densities of 0.12 and 1.2 J/cm2. Such regime induces both local and systemic PBM effects, presumably due to the short-term increase in ROS levels, which in turn activate the cell antioxidative system.
1. Introduction
For the last decades, laser systems have been enthusiastically studied and then used in different ways in industry, telecommunication, and biomedicine. One of fields where lasers have found very broad applications is biology and medicine utilizing them for microscopy, flowcytometry and in photodynamic therapy and diagnostics. Also the lasers are widely used in the wound healing, and recreational medicine, so-called photobiomodualtion (PBM), due to the absence of side effects, pain, and precise control of the irradiation dose. Some PBM effects such as photoinduced dissociation of oxyhemoglobin (HbO2), where molecular oxygen is released [1], light-oxygen and photodynamic effects related to the singlet oxygen formation [2], and biotissue heating associated with absorption of radiation and nonradiative energy loss in hemoglobin molecules [3] have been observed in cells after employing PBM at the wavelengths of 585-587 nm, 632 nm, 950-960 nm, and 1267-1270 nm. These processes are determined by the PBM wavelength and radiation energy density applied. The strongest resonance absorption band of oxygen is in the range of 1265-1270 nm, where no absorption of known chromophores occurs in the living cell. The light-oxygen effect (1265-1270 nm ranges) can activate or damage (depending on the energy density) the biosystems through a direct photoexcitation of molecular triplet oxygen into the singlet oxygen [4–9].
It has been demonstrated that in cells with no intrinsic photoreceptors laser photodynamic effects may activate through the oxidative stress gene expression and synthesis of enzyme cascade responsible for the cell protective responses [10–12]. These explain some biological and therapeutic effects induced by PBM in the visible spectrum range on cells and tissues with no exogenous dyes loaded in [13–15]. It has been found that laser irradiation at the wavelength within the infrared range (IR) at a dose up to 30 J/cm2 activates microcirculation, whereas at a dose higher than 70 J/cm2 may in contrary disorder microcirculation [16]. Irradiation in the energy density range of 0.005-0.05 J/cm2, 0.1-1.0 J/cm2, 0.1-3.0 J/cm2 stimulates cell proliferation, tissue metabolism and organ functioning, and analgesia, correspondently [17].
Near infrared (NIR) PBM effects on skin are deployed in cosmetology and wounds healing through antimicrobial, anti-inflammatory and regenerative effects [18]. Hypothesizing that the extensive number of the different skin receptors related to different organs can be the points through which the PBM has its systemic effects on organism. As such the PBM absorption depends on the tissue properties. At a wavelength of 0.6 – 1.4 µm and over, the skin, muscles and bones, parenchymal organs and highly vascularized tissue absorb 25-40%, 30-80%, and up to 100% of radiation, respectively [19]. IR laser radiation at the doses of 5 - 40 J/cm2 are often used for local laser hyperthermia of neoplasms inducing coagulative necrosis of the tumor with minimal damage to surrounding healthy tissues [20].
Laser irradiation effects at the wavelength within the resonance absorption band of molecular oxygen (1270 nm) on biochemical and physiological processes in Balb/c Nude mice are of particular interest of the medics and physiologists due to their unknown nature (mechanisms) related to the advantages of this wavelength irradiation in the tumor phototreatment and organism photobiomodulation [21–24]. This research is aimed to reveal the mechanisms of the non-thermal effects of the low intensity near-infrared irradiation on the skin cell/tissue for further development of the animal models which will be used in treatment of the melanoma.
It is well known, that proliferative activity of primary melanoma determined by the mitotic index is a strong and independent prognostic factor for survival [25]. However, there is an ambiguous effect of mitotic index extent on the frequency of regional metastasis [26]. Changes in the antioxidant defense system are among important indicators of tumor progress. An increase in the lipid peroxidation primary products and decrease in the activity of enzymatic and nonenzymatic antioxidant defense components have been observed in female mice with B16/F10 transplanted melanoma [27]. As the tumor thickness increases, levels of IL-1RA and TNF-α in the tumor tissue decrease with rising content of interleukins (IL-6, IL-8). Noteworthy, some of these changes affect peritumoral tissue [28].
Similar to miR-200c the miR-205 also acts as an onсo-suppressor. It has been revealed that only these two types of 735 miRNAs isolated from paraffin blocks have shown a statistically significant decrease in melanoma tissue comparing with nevi, also demonstrating significant differences between primary melanoma and its metastases [29]. These results show in agreement with the results earlier published by other authors [30,31]. The methylation of miR-31 also induces melanoma cell proliferation [32]. MiR-31 is involved in the migration and invasion of melanoma cells, which is similar to its role in breast cancer [33]. Melanocytes are normally enriched with miR-31 and during progression into melanoma, expression of miR-31 is suppressed either by loss of the gene or by epigenetic silencing. Targets for miR-31 include oncogenic kinases, such as SRC, MET, NIK (MAP3K14) and melanoma-specific oncogene RAB27a. MiR-130a is generally suppressed in patients with various types of cancer, including melanoma [34].
Therefore, we propose a comprehensive biomolecular analysis of the changes in the BALB/c Nude mice skin after an exposure to the continuous irradiation at the 1270 nm wavelength.
2. Materials and methods
Immunodeficient BALB/c Nude mice (“Pushchino” Ltd., Russia) were selected for control and experimental groups as an object of the study.The skin of these mice does not require hair removing, thus eliminating risk of additional skin irritation. Two experimental groups include animals (n = 8) irradiated by Raman laser operating at the wavelength of 1270 nm and power of 1.96 mW. Animals were exposed to a single irradiation session for 2 min at the intensity of 10 mW/cm2 and energy density (ED) of 1.2 J/cm2, and for 12 seconds at the intensity of 10 mW/cm2 and ED of 0.12 J/cm2. Control group animals (n = 7) were not exposed to laser irradiation.
The next day after irradiation, the animals from control and experimental groups were taken under ether anesthesia. The blood samples were taken and heparin-stabilized animal blood was used for further biochemical and immunological studies. Skin sample with a spot diameter of 0.5 cm exposed to laser irradiation on the lateral surface of the body was studied. The same skin area was explored in the control group animals. Unirradiated skin segments of control animals and irradiated skin regions of experimental animals were fixed in 10% neutral formalin for histological examination and in RNA-later solution for further studies on microRNA expression. All experiments were performed in accordance with World Medical Association Declaration of Helsinki (1964) amended in 1975, 1983 and 1989 and Ethical Approval Committee of Ulyanovsk State University (No. 9, September 15, 2017).
Morphological methods
The irradiated skin patch of 0.5 cm in diameter was taken for histological assessment. The biopsy material was fixed in 10% neutral formalin and series of skin sections were stained with hematoxylin-eosin. Morphometry of skin structures was carried out using a video-test system supplied with Motic microscope, JVC digital video camera (Japan), and density photometry software “Mekos-С1” (Russia) at 10 to 40 times magnification. The quantitative analysis was performed for a conventional unit of the sections. Morphometric data were recognized as statistically significant when P calculated by the two-sided Student t-test was <0.05.
Molecular genetics methods
The irradiated skin section 0.5 x 0.5 cm was fixed in the RNA-later solution (LLC “Eurogen”, Russia) and then was cut using sterile tweezers for an isolation of total RNA employing the “Extraction 100” kit (LLC “Vector Best”, Russia). The reverse transcription reaction was launched immediately after the RNA isolation (set MMLV Kit, LLC Eurogen, Russia) with miRprimer2 to design primers for the microRNAs examined. MicroRNAs miR-31, −130а, −191, −200с, −205 were used to investigate the expression pattern and its involvements in the skin physiological processes [35–43]. MicroRNA profile for the skin samples were evaluated with the CX96 nucleic acid PCR amplifier (BioRad, USA). To normalize the data, miRNA-191 was chosen as the skin-referred gene for skin cells. Calculation of the relative expression was performed according to the Qiagen protocol: ΔΔCt = ΔCt1 (experiment) - ΔCt2(normal).
Normalized target gene expression level = 2-ΔΔCt.
Biochemical methods
The level of lipid peroxidation products (diene conjugates (DC), ketodienes (KD), Schiff bases (SB), malondialdehyde (MDA)) and activity of the antioxidant system enzyme, catalase and reduced glutathione (GSH) level in the red blood cell hemolysate (1:10) were assessed. The DC, KD, conjugate trienes(CT) and SB levels were spectrophotometrically determined by Volchegorsky method [44] in heptane-isopropanol extracts. The concentrations of these products were calculated from the ratios between the optical densities of the heptane phase of E232/220 (DK), E278/220 (KD and CT), E400/E220 (SB) and were given in oxidation index units (O.I.U.). The MDA level (μmol/L) was determined in the test with thiobarbituric acid according to [45]. The catalase activity and GSH level were determined as referred [46].
The statistical significance of the results was estimated using the nonparametric Mann-Whitney U-test. Differences between groups were considered significant at p<0.05.
Immunological methods
To assay the cytokine levels in the skin homogenate and blood plasma from the control and experimental animals, immunoenzyme kits were used to quantify the murine tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) (eBioscience, USA).The statistical significance of the results was estimated using the nonparametric Mann-Whitney U-test. Differences between groups were considered significant at p<0.05.
3. Results and discussion
The laboratory BALB/c Nude mice used in the experiments are well known hairless and immunodeficient model which makes them suitable for research in dermatology, oncology, and immunobiology. The mice skin thickness measured by histology has been about 500 µm. Since the present research highlights the laser radiation effect on physiological processes in skin at the wavelength of 1270 nm, its results allow estimating laser potential for melanoma treating. So, the BALB/cNude mice used as a model are quite suitable for the experiment.
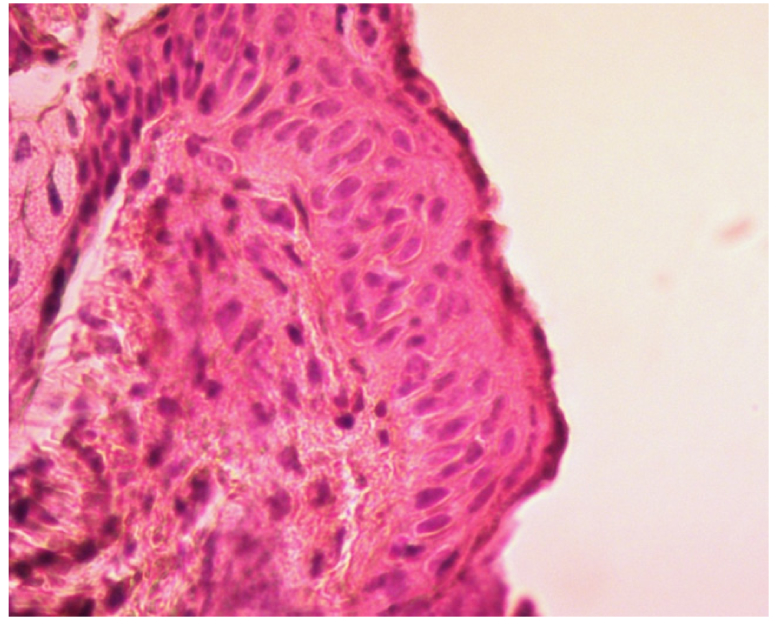
I. The histological analysis has shown that the back skin of the control group animals has a fine-folded relief shape and thin epidermis.
Epidermis usually comprises 2-3 rows of grown cell layers. In the first row (basal layer), the basal cells with dark purple round nuclei are clearly visible (Fig. 1). The second row (thorny layer) comprises 1-2 rows of keratinocytes. Next granular layer includes one row of strongly flattened cells with uneasily distinguished nuclei. However, the dark-violet keratohyalin granules are easily observed in the cytoplasm. The stratum corneum is thin and tightly adjacent to the granular layer peeling off in some places. A slight diffuse lymphoid-leukocyte infiltration is observed in dermis.
Fig. 1.
High-resolution histology image of Balb/cNude mouse skin from the control group. Light microscopy (LM) x400. The sample is stained with hematoxylin-eosin.
The dermis papillary layer is smoothed and papillae are sparse. Loose fibrous unformed connective tissue located sub-epidermally consists of thin collagen fibers and transfers into reticular dermis layer with no sharp boundaries.
The reticular dermis layer is composed of a dense fibrous unformed connective tissue consisting of multidirectional thick collagen fibrils. The connective tissue cells are mainly fibroblasts, macrophages, tissue basophils and some other differentiated cellular elements.
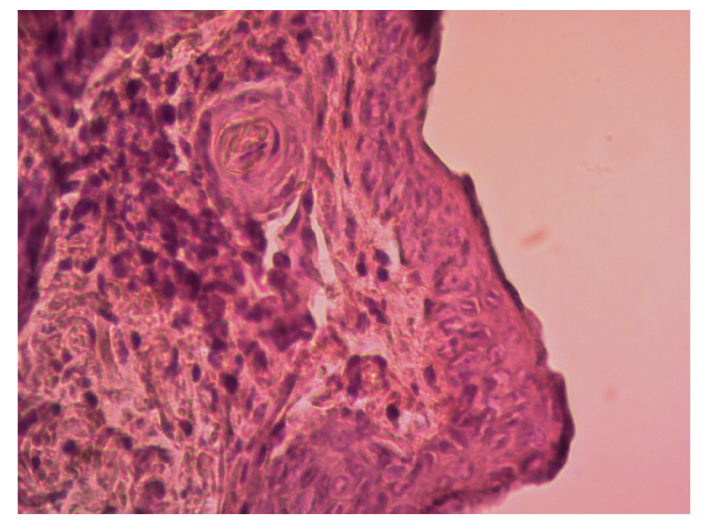
Noteworthy, histological analysis of skin samples in the second experimental group irradiated at the wavelength of 1270 nm and E = 0.12 J/cm2 demonstrated no differences from the control group animals (Fig. 2).
Fig. 2.
High-resolution histology image of Balb/c Nude mouse skin after a single exposure to 1270 nm laser irradiation at E = 0.12 J/cm2. LM x400. The sample is stained with hematoxylin-eosin.
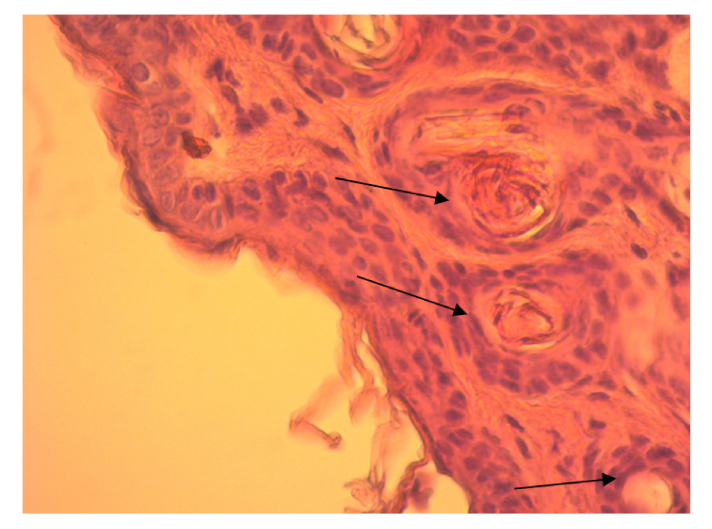
After laser exposure at the wavelength of 1270 nm and E = 1.2 J/cm2 the mice skin has also a fine-folded relief and the skin structure is not changed (Fig. 3).
Fig. 3.
High-resolution histology image of Balb/cNude mouse skin after a single exposure to 1270 nm laser irradiation at E = 1.2 J/cm2. LMx400. The sample is stained with hematoxylin-eosin (arrows point to the cells of epidermis growth layer).
The epidermis was not thickened over the normal size. The growth layer comprises 2-3 rows of keratinocytes with definably colored rounded nuclei which have clearly visible nucleoli. In some places, the growth layer cells are overlapping each other. In these regions, the nuclei are elongated. The granular layer and stratum corneum are kept unmodified. Like in the control group, the stratum corneum is thin with no signs of damage, peeling off, and vacuolization. The dermis papillary layer is not modified clearly indicating that the stratum corneum does not show any pathological signs. In the interlobular connective tissue of subcutaneous adipose tissue and in endomisis, perimisia (intermuscular connective tissue), moderate diffuse lymphoid-leukocyte infiltration was visualized.
In the first experimental group, the morphometric analysis reveals a significant proliferation of keratinocytes in the basal layer compared to the control group (Table 1). An increase in the number of basal epitheliocytes is probably due to a higher level of cell mitotic activity in this layer after exposure to 1270 nm laser irradiation. In the material, there are few mitotic figures, since the experimental material is taken 24 hours after irradiation. Mitotic division lasts some 6-8 hours. Thus, 24 hours after laser exposure more nuclei are observed per unit area.
Table 1. Number of basal epidermis layer cells per arbitrary unit area.
| Control group, (n = 7) | E = 0.12 J/cm2, (n = 8) | E = 1.2 J/cm2, (n = 8) | |
|---|---|---|---|
| Average number of cells | 7,22 ± 0,22 | 7,24 ± 0,20 | 7,92 ± 0,26 * |
- significant difference from the control value, р<0.05.
In the second experimental group, no significant differences from the control group were registered.
As it was reported, the PBM experiments on dog epidermal keratinocytes, at the energy densities of 0.1, 0.2, 1.2 and 10 J/cm2 (He-Ne laser, λ = 650 nm) stimulated the proliferative activity of skin cells. Therefore, these doses were chosen for experimental study on healing wounds in vivo models [19].
II. Experimental studies show the role of microRNAs in maintaining homeostasis of skin as an integral organ through their participation in regulation of proliferation, differentiation and apoptosis [16,35,36]. MicroRNAs are a relatively new class of endogenous, non-coding single-stranded small RNAs (18-22 nucleotides) participating in regulation of gene expression at the posttranscriptional level by inhibiting translation or destruction of specific transcripts of matrix RNA [37]. The pattern of microRNA expression has some tissue specificity. The expression pattern is studied for microRNAs, whose role in the physiological processes of skin has been demonstrated [39,47,48], namely, miR-31, −130а, −191, −200с, −205. For endogenous control, TaqMan Advanced miRNA Assays User Guide (ThermoFisher Scientific, USA, Cat. #A25576) recommends microRNA-191 to be used.
MicroRNA-31 is a key regulator of the proliferation and migration of keratinocytes (dividing cells of basal layer), differentiation and hair growth [38]. Increased expression of mir-31 appeared to be in some skin diseases characterized by excessive proliferation of keratinocytes, e.g., psoriasis, chronic inflammatory skin disease, long-term wound healing [39,40], and squamous cell carcinoma [41].
It has been demonstrated that miR-130a level increases in chronic wounds and delayed reepithelization [35]. Both MicroRNAs-200c and −205 participate in regulation of the cell cycle [42], as well as in the epithelial-mesenchymal transition and cell differentiation to the embryonic phenotype through direct (miRNA-200c) and intermediary (miRNA-205) regulation of ZEB1 and ZEB2 gene expression which suppresses E-cadherin gene transcription [43]. Thus, these miRNAs were chosen to study the genetic mechanisms involved in regulating the response to PBM 1270 nm.
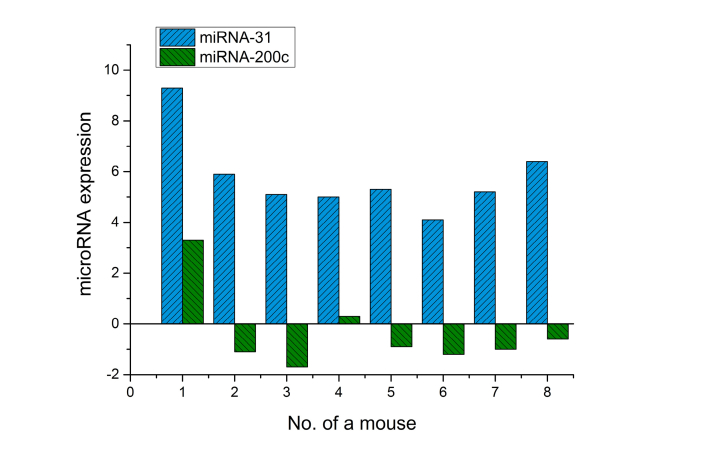
Real-time PCR is used to assay miRNA expression in irradiated skin areas and intact area from the opposite side after laser exposure at E = 1.2 J/cm2. Figure 4 shows the expression of miR-31, −200c in skin after laser exposure.
Fig. 4.
Expression of miR-31 and miR-200c in mouse skin after irradiation at 1270 nm (Log2 value 2-ΔΔCt).
In all skin samples irradiated with 1270 nm laser (E = 1.2 J/cm2) a significant increase in miR-31 expression has been observed (Fig. 4), whereas microRNA-200c do not demonstrate such an overall change. Expression of these genes has only been observed in two animals (No.1, 4) in the experimental group. However, in most cases (75%), the mir-200c expression went down. The data are calculated for endogenous control of miR-191 and are given in relative units to compare expression dynamics in the irradiated and non-irradiated skin region of the same mouse.
No significant changes in the expression profile of miR-205 and miR-130a were observed in any sample.
Thus, only two miRNAs (miR-31 and −200c) change in the first experimental group. These post-transcriptional activity gene regulators control keratinocyte division and differentiation of other cell types. The changes registered in the skin of BALB/c Nude mice after 1270 nm laser applied with ED of 1.2 J/cm2 indicates a biostimulating effect of PBM associated with an increase in proliferative activity of the basal layer cells in epidermis and posttranscriptional factor of microRNA-31 regulating this process in the skin.
In the second experimental group, there are no changes in miRNA expression.
III. Biochemical studies on blood hemolysate of BALB/c Nude mice show that PBM of 1270 nm and E = 1.2 J/cm2 leads to a significant increase of the reduced glutathione level (GSH) in erythrocytes of irradiated mice (Table 2), whereas the level of primary lipid oxidation products (diene conjugates) increases slightly and the levels of ketodienes and Schiff bases in contrast demonstrate significant decrease (Table 3). Among the products of protein oxidative modifications, the level of primary ketone groups (530 nm) decrease significantly in mouse erythrocytes (Table 4). The obtained data assume the increase in the antioxidant status of mice red blood cells under PBM of 1.2 J/cm2.
Table 2. Activity of catalase and GSH in the erythrocytes of BALB/c Nude mice after PBM at 0.12 and 1.2 J/cm2.
| Control, (n = 7) | E = 0.12 J/cm2, (n = 8) | E = 1.2 J/cm2, (n = 8) | |
|---|---|---|---|
| Catalase, mmol/min/l | 6245.0 ± 97.7 | 6246.1 ± 164.2 | 6241.4 ± 235.6 |
| GSH, μmol/l | 0.92 ± 0.066 | 0.99 ± 0.025 | 1.35 ± 0.074* |
- the data are significantly different from the control group results.
Table 3. Level of lipid peroxidation products in erythrocytes of BALB/c Nudemouse after PBM at 0.12 and 1.2 J/cm2.
| Control, (n = 7) | E = 0.12 J/cm2, (n = 8) | E = 1.2 J/cm2, (n = 8) | |
|---|---|---|---|
| MDA, μmol / l | 570.9 ± 24,4 | 572.4 ± 31.8 | 583.0 ± 39.9 |
|
DC, (heptane phase),
units of oxidation index 232/220 |
1.053 ± 0.011 | 1.048 ± 0.015 | 1.119 ± 0.008* |
|
KD, (heptane phase),
units of oxidation index 278/220 |
0.173 ± 0.003 | 0.170 ± 0.006 | 0.105 ± 0.007* |
|
SB, (heptane phase),
units of oxidation index 400/220 |
0.050 ± 0.004 | 0.049 ± 0.002 | 0.021 ± 0.003* |
- the data are significantly different from the control group results.
Table 4. Protein oxidative modification in erythrocytes after PBM at 0.12 and 1.2 J/cm2.
| Control, (n = 7) | E = 0.12 J/cm2, (n = 8) | E = 1.2 J/cm2, (n = 8) | |
|---|---|---|---|
| Neutral aldehyde groups | 2.908 ± 0.193 | 2.884 ± 0.107 | 2.375 ± 0.056* |
| Neutral ketone groups | 3.412 ± 0.222 | 3.452 ± 0.149 | 2.780 ± 0.054* |
| Primary aldehyde groups | 2.382 ± 0.199 | 2.307 ± 0.126 | 1.850 ± 0.069* |
| Primary ketone groups | 0.752 ± 0.079 | 0.721 ± 0.093 | 0.558 ± 0.025* |
- the data are significantly different from the control group results.
This irradiation regime (1.2 J/cm2) leads to protection of the membrane proteins and lipids, decrease in the pro-oxidant effect due to activation of antioxidant mechanisms, in particular, the endogenous glutathione system. The observed pronounced increase in GSH, as the main red blood cell antioxidant, could indicate an increase in its synthesis by glutathione-dependent enzymes and through its participation in utilization of pro-oxidants. The most pronounced changes are observed in the level of oxidative modification of membrane proteins (Table 4). It can indicate a greater sensitivity of protein molecules to PBMat the wavelength of 1270 nm. Twenty-four hours after laser exposure, detoxification of reactive oxygen species intensifies that could indirectly indicate an increase of the singlet oxygen generation in response to laser exposure.
Thus, PBM causes an activation of the endogenous antioxidant defense systems and suppresses the action of reactive oxygen species generated in BALB/c Nude mice red blood cells immediately after laser exposure. This effect is consistent with the reported data highlighting the PBM-induced membrane-stabilizing effect and enhanced antioxidant blood properties reported earlier [49–51]. Thus, PBM stimulates the protective effect in red blood cells even at short exposure time and low irradiation dose (1.2 J/cm2).
IV. The cytokines usually act through interaction with specific receptors at the plasma membrane rafts, which are expressed in small amounts on the cells [52]. They regulate the activation, differentiation and proliferation of immunocompetent and somatic cells. These regulatory proteins are involved in modulation both inflammatory and immune responses and are critical elements in the skin response to damage or viral-infection. IL-6 belongs to potent pro-inflammatory cytokines that stimulates B-cell proliferation and differentiation. It is involved in the production of multipotent colony-forming factors and megakaryocytes. IL-6 is found in normal-skin fibroblasts and sweat ducts [53]. It is shown that IL-6 is secreted by almost all cells, including keratinocytes, melanocytes and Langerhans cells after their stimulation with various factors, including cytokines, tumor necrosis factor (TNF), platelet-derived growth factor (PDGF), bacterial and virus infection, and microbial lipopolysaccharides (LPS).
TNF-α is produced by monocytes, macrophages, T cells (Th1), keratinocytes, neutrophils, natural killers, endothelial, mast and myeloid cells, and lymphokine-activated killer (LAK) cells playing an important physiological role in immunoregulation [54]. TNF-α stimulates lymphocytes, neutrophils, eosinophils, endothelial cells, fibroblasts, inducing the production of IL-6 in many cell types [55]. However, it has been shown that TNF-α does not affect proliferation and differentiation of normal keratinocytes in a skin model [56]. TNF-α participates in inflammation and its level increases with the inflammation progression [57]. The experimental data report that PBM decreases production of IL-6 and TNF-α in the supernatant of activated mononuclear cells [58,59].
Twenty-four hours after exposure to a diode laser at 780 and 660 nm, in brain and blood tissues, a decrease in the level of IL-6 and TNF-α has been observed [60]. When analyzing the immunomodulatory PBM effect on the skin contact hypersensitivity reaction, no significant changes have been registered in TNF-α concentration [61]. Despite the progress achieved in studying the mechanisms of immunogenesis regulation, the immunomodulatory effect of laser radiation in vivo on the pro-inflammatory cytokine synthesis is still unclear.
The analysis of the results shows that the level of IL-6 in skin irradiated with λ = 1270 nm is significantly lower than its level in the non-irradiated area of the BALB/c Nude mouse skin, whereas TNF-α level increases slightly in response to laser exposure (Table 5). No significant differences are observed in the animal group irradiated at E = 0.12 J/cm2.
Table 5. Level of cytokines in homogenate of irradiated BALB/c Nude mouse skin.
| Control, (n = 7) | E = 0.12 J/cm2, (n = 8) | E = 1.2 J/cm2), (n = 8) | |
|---|---|---|---|
|
IL-6, pg/ml/mg
protein |
3499.198 (1610.85 – 6916.44) |
3346.673 (1390.62 – 5816.57) |
2446.90* (1674.28 – 3145.37) |
|
TNF-α, pg/ml/mg
protein |
2652.553 (961.54 – 4000) |
2599.464 (905.68 – 4000) |
2839.86 (1363.64 – 4000) |
The data are significantly different from the control group results(p<0.05).
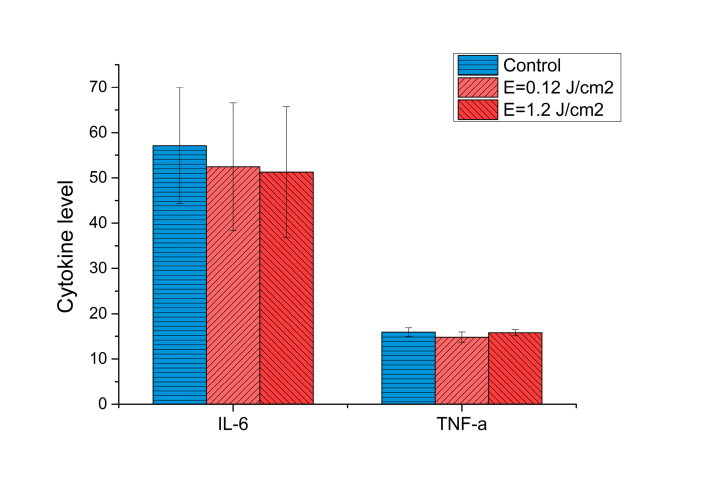
In the first experimental group the level of IL-6 decreases in blood plasma (51.26 ± 14.45 pg/ml versus 57.14 ± 12.82 pg/ml in the control group). TNF-α level in the blood plasma was the same in both experimental and control groups (Fig. 5). This is a positive dynamic, since laser radiation being 1.2 J/cm2 does not induce the inflammatory reaction in the skin and the blood. Moreover, laser radiation reduces to some extent production of pro-inflammatory cytokines, in particularly, interleukin-6.
Fig. 5.
Cytokine level in the blood plasma of BALB/c Nude mouse after laser exposure.
4. Conclusion
In the BALB/c Nude mouse model, PBM has been shown to induce a photo-stimulating effect of the 1270 nm radiation at the dose of 1.2 J/cm2 in both an irradiated skin spot and peripheral blood cells. This effect is associated with biochemical reactions changing cell redox state, synthesis of signal molecules (cytokines and microRNAs) and also responsible for consequent physiological effects. In this case, the laser triggers cell protective effects through direct excitation of oxygen molecules. Nonetheless, low intensity and short exposure time of laser radiation cause pronounced effects on the membrane-protective action and enhanced antioxidant system. Also this irradiation decreases the synthesis of pro-inflammatory cytokines in blood plasma, mainly IL-6. Locally, in the region of laser exposure, the proliferative processes intensify with no signs of inflammation. Thus, in the BALB/c Nude mouse model the laser at the wavelength of 1270 nm and dose of 1.2 J/cm2 produces local and systemic stimulation effect highly likely due to a short-term increase in the ROS levels which turns active the cell antioxidative system.
The further studies should be done with higher radiation doses for revealing whether 1270 nm radiation can treat the melanoma planted on BALB/c Nude mouse model.
Acknowledgments
The work of Igor Zolotovskii, Dmitrii Stoliarov, Andrei Fotiadi, and Anna Khohklova was supported by the Ministry of Education and Science of the Russian Federation under Government Assignment No. 3.3889.2017.
The work of Sergei Sokolovski and Edik Rafailov was supported by EU H2020 FET research and innovation program MESO-BRAIN under Grant No. 713140.
The work of Edik Rafailov was supported by the Russian Science Foundation (RSF) under Grant No. 18-15-00172.
The work of Andrei Fotiadi was supported by the Leverhulme Trust under Grant No. VP2-2016-042 (Visiting Professorship, ASTON University, Birmingham, U.K.).
Funding
Ministry of Education and Science of the Russian Federation (Government Assignment # 3.3889.2017); Russian Science Foundation (RSF) (# 18-15-00172); EU H2020 FET research and innovation program MESO-BRAIN (#713140); Leverhulme Trust (#VP2-2016-042).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Asimov M. M., “Biomedical effects of in vivo laser induced oxyhemoglobin photodissociation,” Opt. Spectrosc. 115(1), 867–872 (2013). 10.1134/S0030400X13110027 [DOI] [Google Scholar]
- 2.Zakharov S. D., Ivanov A. V., “Light-oxygen effect in cells and its potential applications in tumor therapy (review),” Quantum Electron. 29(12), 1031–1053 (1999). 10.1070/QE1999v029n12ABEH001629 [DOI] [Google Scholar]
- 3.Krasnovsky A. A., Jr., “Luminescence and photochemical studies of singlet oxygen photonics,” J. Photochem. Photobiol. Chem. 196(2-3), 210–218 (2008). 10.1016/j.jphotochem.2007.12.015 [DOI] [Google Scholar]
- 4.Sokolovski S. G., Zolotovskaya S. A., Goltsov A., Pourreyron C., South A. P., Rafailov E. U., “Infrared laser pulse triggers increased singlet oxygen production in tumour cells,” Sci. Rep. 3(1), 3484 (2013). 10.1038/srep03484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anquez F., Courtade E., Sivéry A., Suret P., Randoux S., “A high-power tunable Raman fiber ring laser for the investigation of singlet oxygen production from direct laser excitation around 1270 nm,” Opt. Express 18(22), 22928–22936 (2010). 10.1364/OE.18.022928 [DOI] [PubMed] [Google Scholar]
- 6.Gening T. P., Voronova O. S., Dolgova D. R., Abakumova T. V., Zolotovskii I. O., Sholokhov E. M., Kurkov A. S., Gening S. O., “Analysis of the efficiency of using 1265-nm CW laser radiation for initiating oxidative stress in the tissue of a solid malignant tumor,” Quantum Electron. 42(9), 805–807 (2012). 10.1070/QE2012v042n09ABEH014961 [DOI] [Google Scholar]
- 7.Saenko Y. V., Glushchenko E. S., Zolotovskii I. O., Sholokhov E., Kurkov A., “Mitochondrial dependent oxidative stress in cell culture induced by laser radiation at 1265 nm,” Lasers Med. Sci. 31(3), 405–413 (2016). 10.1007/s10103-015-1861-z [DOI] [PubMed] [Google Scholar]
- 8.Khokhlova A., Zolotovskii I., Stoliarov D., Vorsina S., Liamina D., Pogodina E., Fotiadi A., Sokolovski S., Saenko Y., Rafailov E., “The photobiomodulation of vital parameters of the cancer cell culture by low dose of Near-IR laser irradiation,” IEEE J. Sel. Top. Quantum Electron. 25(1), 7201510 (2019). 10.1109/JSTQE.2018.2854539 [DOI] [Google Scholar]
- 9.Khokhlova A., Zolotovskii I., Pogodina E., Saenko Y., Stoliarov D., Vorsina S., Fotiadi A., Liamina D., Sokolovski S., Rafailov E., “Effects of high and low level 1265 nm laser irradiation on HCT116 cancer cells,” Proc. SPIE 10861, 108610L (2019). 10.1117/12.2509529 [DOI] [Google Scholar]
- 10.Briviba K., Klotz L. O., Sies H., “Toxic and signaling effects of photochemically or chemically generated singlet oxygen in biological systems,” Biol. Chem. 378(11), 1259–1265 (1997). [PubMed] [Google Scholar]
- 11.Klotz L. O., “Oxidant-induced signaling: effects of peroxynitrite and singlet oxygen,” Biol. Chem. 383(3-4), 443–456 (2002). 10.1515/BC.2002.047 [DOI] [PubMed] [Google Scholar]
- 12.Klotz L. O., Kröncke K. D., Sies H., “Singlet oxygen-induced signaling effects in mammalian cells,” Photochem. Photobiol. Sci. 2(2), 88–94 (2003). 10.1039/B210750C [DOI] [PubMed] [Google Scholar]
- 13.Karu T. I., “Cellular mechanisms of low-level laser therapy,” Biol. Bull. Rev. 121, 110–120 (2001). [Google Scholar]
- 14.Klebanov G. I., Poltanov E. A., “Primary free radical and secondary cell molecular mechanisms of laser therapy,” Laser Phys. 13(1), 1–14 (2003). [Google Scholar]
- 15.Vladimirov IuA., Klebanov G. I., Borisenko G. G., Osipov A. N., “Molecular and cellular mechanisms of the low intensity laser radiation effect,” Biofizika 49(2), 339–350 (2004). [PubMed] [Google Scholar]
- 16.Banerjee J., Chan Y. C., Sen C. K., “MicroRNAs in skin and wound healing,” Physiol. Genomics 43(10), 543–556 (2011). 10.1152/physiolgenomics.00157.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuzmina I. Yu., Krauze T. M., “Up-to-date aspects of laser therapy,” Int. Med. J. 2, 106–110 (2006). [Google Scholar]
- 18.Illarionov V., “Laser therapy (clinical lecture),” Vrach 3, 11–15 (1993). [Google Scholar]
- 19.Gagnon D., Gibson T. W. G., Singh A., zur Linden A. R., Kazienko J. E., LaMarre J., “An in vitro method to test the safety and efficacy of low-level laser therapy (LLLT) in the healing of a canine skin model,” BMC Vet. Res. 12(1), 73 (2016). 10.1186/s12917-016-0689-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschberg H., Madsen S. J., “Cell Mediated Photothermal Therapy of Brain Tumors,” J. Neuroimmune Pharmacol. 12(1), 99–106 (2017). 10.1007/s11481-016-9690-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Liu L., You Q., Song Y., Sun Q., Wang Y., Cheng Y., Tan F., Li N., “All-in-One Theranostic Nanoplatform Based on Hollow MoSx for Photothermally-maneuvered Oxygen Self-enriched Photodynamic Therapy,” Theranostics 8(4), 955–971 (2018). 10.7150/thno.22325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou C. N., Milanesi C., Jori G., “An ultrastructural comparative evaluation of tumors photosensitized by porphyrins administered in aqueous solution, bound to liposomes or to lipoproteins,” Photochem. Photobiol. 48(4), 487–492 (1988). 10.1111/j.1751-1097.1988.tb02850.x [DOI] [PubMed] [Google Scholar]
- 23.Lu C., Zhou F., Wu S., Liu L., Xing D., “Phototherapy-induced antitumor immunity: long-term tumor suppression effects via photoinactivation of respiratory chain oxidase-triggered superoxide anion burst,” Antioxid. Redox Signal. 24(5), 249–262 (2016). 10.1089/ars.2015.6334 [DOI] [PubMed] [Google Scholar]
- 24.Novoselova E. G., Glushkova O. V., Cherenkov D. A., Chudnovsky V. M., Fesenko E. E., “Effects of low-power laser radiation on mice immunity,” Photodermatol. Photoimmunol. Photomed. 22(1), 33–38 (2006). 10.1111/j.1600-0781.2006.00191.x [DOI] [PubMed] [Google Scholar]
- 25.Mandalà M., Galli F., Cattaneo L., Merelli B., Rulli E., Ribero S., Quaglino P., De Giorgi V., Pigozzo J., Sileni V. C., Chirco A., Ferrucci P. F., Occelli M., Imberti G., Piazzalunga D., Massi D., Tondini C., Queirolo P., “Mitotic rate correlates with sentinel lymph node status and outcome in cutaneous melanoma greater than 1 millimeter in thickness: a multi-institutional study of 1524 cases,” J. Am. Acad. Dermatol. 76(2), 264–273 (2017). 10.1016/j.jaad.2016.08.066 [DOI] [PubMed] [Google Scholar]
- 26.Pak M. B., Mudunov A. M., Demidov L. V., Azizyan R. I., Brzhezovskiy V. Z., Stelmakh D. K., Bozhchenko Y. A., Ignatova A. V., “Effect of morphological prognostic factors on long-term treatment results in patients with head and neck skin melanoma,” Head and Neck Tumors 7(1), 61–68 (2017). 10.17650/2222-1468-2017-7-1-61-68 [DOI] [Google Scholar]
- 27.Zarkovic N., Tillian M. H., Schaur J., Waeg G., Jurin M., Esterbauer H., “Inhibition of Melanoma B16-F10 Growth by Lipid Peroxidation Product 4-Hydroxynonenal,” Cancer Biother. 10(2), 153–156 (1995). 10.1089/cbr.1995.10.153 [DOI] [PubMed] [Google Scholar]
- 28.Donia M., Kjeldsen J. W., Svane I. M., “The controversial role of TNF in melanoma,” OncoImmunology 5(4), e1107699 (2016). 10.1080/2162402X.2015.1107699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y., Brenn T., Brown E. R., Doherty V., Melton D. W., “Differential expression of microRNAs during melanoma progression: miR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumour suppressors,” Br. J. Cancer 106(3), 553–561 (2012). 10.1038/bjc.2011.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J., Feilotter H. E., Paré G. C., Zhang X., Pemberton J. G., Garady C., Lai D., Yang X., Tron V. A., “MicroRNA-193b represses cell proliferation and regulates cyclin D1 in melanoma,” Am. J. Pathol. 176(5), 2520–2529 (2010). 10.2353/ajpath.2010.091061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philippidou D., Schmitt M., Moser D., Margue C., Nazarov P. V., Muller A., Vallar L., Nashan D., Behrmann I., Kreis S., “Signatures of microRNAs and selected microRNA target genes in human melanoma,” Cancer Res. 70(10), 4163–4173 (2010). 10.1158/0008-5472.CAN-09-4512 [DOI] [PubMed] [Google Scholar]
- 32.Asangani I. A., Harms P. W., Dodson L., Pandhi M., Kunju L. P., Maher C. A., Fullen D. R., Johnson T. M., Giordano T. J., Palanisamy N., Chinnaiyan A. M., “Genetic and epigenetic loss of microRNA-31 leads to feed-forward expression of EZH2 in melanoma,” Oncotarget 3(9), 1011–1025 (2012). 10.18632/oncotarget.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valastyan S., Reinhardt F., Benaich N., Calogrias D., Szász A. M., Wang Z. C., Brock J. E., Richardson A. L., Weinberg R. A., “A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis,” Cell 137(6), 1032–1046 (2009). 10.1016/j.cell.2009.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Pan Y., Wang R., Zhang F., Chen Y., Lv Q., Long G., Yang K., “MicroRNA-130a inhibits cell proliferation, invasion and migration in human breast cancer by targeting the RAB5A,” Int. J. Clin. Exp. Pathol. 8(1), 384–393 (2015). [PMC free article] [PubMed] [Google Scholar]
- 35.Pastar I., Khan A. A., Stojadinovic O., Lebrun E. A., Medina M. C., Brem H., Kirsner R. S., Jimenez J. J., Leslie C., Tomic-Canic M., “Induction of specific microRNAs inhibits cutaneous wound healing,” J. Biol. Chem. 287(35), 29324–29335 (2012). 10.1074/jbc.M112.382135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aftab M. N., Dinger M. E., Perera R. J., “The role of microRNAs and long non-coding RNAs in the pathology, diagnosis, and management of melanoma,” Arch. Biochem. Biophys. 563, 60–70 (2014). 10.1016/j.abb.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inui M., Martello G., Piccolo S., “MicroRNA control of signal transduction,” Nat. Rev. Mol. Cell Biol. 11(4), 252–263 (2010). 10.1038/nrm2868 [DOI] [PubMed] [Google Scholar]
- 38.Mardaryev A. N., Ahmed M. I., Vlahov N. V., Fessing M. Y., Gill J. H., Sharov A. A., Botchkareva N. V., “Micro-RNA-31 controls hair cycle-associated changes in gene expression programs of the skin and hair follicle,” FASEB J. 24(10), 3869–3881 (2010). 10.1096/fj.10-160663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y., Zhou B., Wu D., Yin Z., Luo D., “Baicalin modulates microRNA expression in UVB irradiated mouse skin,” J. Biomed. Res. 26(2), 125–134 (2012). 10.1016/S1674-8301(12)60022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morhenn V. B., Nahm W. J., Mansbridge J. N., “Psoriatic keratinocytes are resistant to tumor necrosis factor alpha’s induction of mRNA for the NMDA-R2C subunit,” Exp. Dermatol. 22(11), 750–751 (2013). 10.1111/exd.12242 [DOI] [PubMed] [Google Scholar]
- 41.Bruegger C., Kempf W., Spoerri I., Arnold A. W., Itin P. H., Burger B., “MicroRNA expression differs in cutaneous squamous cell carcinomas and healthy skin of immunocompetent individuals,” Exp. Dermatol. 22(6), 426–428 (2013). 10.1111/exd.12153 [DOI] [PubMed] [Google Scholar]
- 42.Saydam O., Shen Y., Würdinger T., Senol O., Boke E., James M. F., Tannous B. A., Stemmer-Rachamimov A. O., Yi M., Stephens R. M., Fraefel C., Gusella J. F., Krichevsky A. M., Breakefield X. O., “Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway,” Mol. Cell. Biol. 29(21), 5923–5940 (2009). 10.1128/MCB.00332-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng X., Wang Z., Fillmore R., Xi Y., “MiR-200, a new star miRNA in human cancer,” Cancer Lett. 344(2), 166–173 (2014). 10.1016/j.canlet.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volchegorsky A., Nalimov A. G., Yarovinsky B. G., Lifshits R. I., “Comparison of various approaches to definition of products of peroxidation of lipids in geptan-isopropanol extracts of blood,” Quest. of Medical chemistry 35(1), 127–131 (1989). [PubMed] [Google Scholar]
- 45.Andreeva L. I., Kozhemiakin L. A., Kishkun A. A., “Modification of the method of determining lipid peroxidation in a test using thiobarbituric acid,” Lab. Delo 11(11), 41–43 (1988). [PubMed] [Google Scholar]
- 46.Kashuro V. A., Karpishchenko A. I., Glushkov S. I., Novikova T. M., Minaeva L. V., Glushkova T. I., Aksenov V. V., “State of the system of glutathione and lipid peroxidation in tissues of the liver and kidneys of the rats with acute cyclophosphamide poisoning,” Nephrology (Carlton) 1(2), 81–85 (2006).16669965 [Google Scholar]
- 47.Xu L. F., Wu Z. P., Chen Y., Zhu Q. S., Hamidi S., Navab R., “MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China,” PLoS One 9(8), e103698 (2014). 10.1371/journal.pone.0103698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruksha T. G., Sergeeva E. Yu., Palkina N. V., Aksenenko M. B., Komina A. V., Klimina G. M., Belonogov R. N., “MicroRNAs as ultraviolet irradiation effects regulators in skin cells,” Tsitologiia 58(10), 733–743 (2016). [PubMed] [Google Scholar]
- 49.Armichev A. V., Ivanov A. V., Panasenko N. A., Perov S. N., Zakharov S. D., “Spectral dependence of erythrocyte response to low-intensity irradiation at 570–590 nm,” J. Russ. Laser Res. 16(2), 186–187 (1995). 10.1007/BF02580877 [DOI] [Google Scholar]
- 50.Anquez F., Sivéry A., El Yazidi-Belkoura I., Zemmouri J., Suret P., Randoux S., Courtade E., “Chapter 4:Production of singlet oxygen by direct photoactivation of molecular oxygen”, inSinglet Oxygen: Applications in Biosciences and Nanosciences, Volume 1, Nonell S., Flors C., eds. (The Royal Society of Chemistry, 2016), pp. 75–91. [Google Scholar]
- 51.Gening T., Sysolyatin A., Arslanova D., Voronova O., Zolotovsky I., Ostatochnikov V., Yavtushenko M., “Effects of femtosecond laser radiation on blood cell suspensions,” Proc. SPIE 9701, 79010K (2011). [Google Scholar]
- 52.Belova O. V., Arion V. Y., Sergienko V. I., “Role of cytokines in immunological function of the skin,” Immunopathology, allergology, infectology 1, 41–55 (2008). [Google Scholar]
- 53.Kishimoto T., “Interleukin-6: from basic science to medicine-40 years in immunology,” Annu. Rev. Immunol. 23(1), 1–21 (2005). 10.1146/annurev.immunol.23.021704.115806 [DOI] [PubMed] [Google Scholar]
- 54.Zimina I. V., Lopukhin Yu. M., Arion V. Ya., “The skin as an immune organ: cellular elements and cytokines,” Immunologiya 1, 8–13 (1994). [Google Scholar]
- 55.Singh S., Singh N., Handa R., “Tumor necrosis factor-alpha in patients with malaria,” Indian J. Malariol. 37(1-2), 27–33 (2000). [PubMed] [Google Scholar]
- 56.Fransson J., “Tumour necrosis factor-alpha does not influence proliferation and differentiation of healthy and psoriatic keratinocytes in a skin-equivalent model,” Acta Derm. Venereol. 80(6), 416–420 (2000). 10.1080/000155500300012792 [DOI] [PubMed] [Google Scholar]
- 57.Kwon T. R., Oh C. T., Choi E. J., Kim S. R., Jang Y. J., Ko E. J., Suh D., Yoo K. H., Kim B. J., “Ultraviolet light-emitting-diode irradiation inhibits TNF-α and IFN-γ-induced expression of ICAM-1 and STAT1 phosphorylation in human keratinocytes,” Lasers Surg. Med. 47(10), 824–832 (2015). 10.1002/lsm.22425 [DOI] [PubMed] [Google Scholar]
- 58.Gavish L., Perez L. S., Reissman P., Gertz S. D., “Irradiation with 780 nm diode laser attenuates inflammatory cytokines but upregulates nitric oxide in lipopolysaccharide-stimulated macrophages: implications for the prevention of aneurysm progression,” Lasers Surg. Med. 40(5), 371–378 (2008). 10.1002/lsm.20635 [DOI] [PubMed] [Google Scholar]
- 59.Sousa L. R., Cavalcanti B. N., Marques M. M., “Effect of laser phototherapy on the release of TNF-alpha and MMP-1 by endodontic sealer-stimulated macrophages,” Photomed. Laser Surg. 27(1), 37–42 (2009). 10.1089/pho.2007.2220 [DOI] [PubMed] [Google Scholar]
- 60.Moreira M. S., Velasco I. T., Ferreira L. S., Ariga S. K., Barbeiro D. F., Meneguzzo D. T., Abatepaulo F., Marques M. M., “Effect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in rat,” J. Photochem. Photobiol. B 97(3), 145–151 (2009). 10.1016/j.jphotobiol.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 61.Kandolf-Sekulovic L., Kataranovski M., Pavlovic M. D., “Immunomodulatory effects of low-intensity near-infrared laser irradiation on contact hypersensitivity reaction,” Photodermatol. Photoimmunol. Photomed. 19(4), 203–212 (2003). 10.1034/j.1600-0781.2003.00040.x [DOI] [PubMed] [Google Scholar]