Abstract
Blood cell analysis is one of the standard clinical tests. Despite the widespread use of exogenous markers for blood cell quantification, label-free optical methods are still of high demand due to their possibility for in vivo application and signal specific to the biochemical state of the cell provided by native fluorophores. Here we report the results of blood cell characterization using label-free fluorescence imaging techniques and flow-cytometry. Autofluorescence parameters of different cell types – white blood cells, red blood cells, erythrophagocytic cells – are assessed and analyzed in terms of molecular heterogeneity and possibilities of differentiation between different cell types in vitro and in vivo.
1. Introduction
Quantification of white blood cells (WBC) content and subtypes in blood is a routine clinical test. It allows the fractionation and distributional analysis of immune cells within main subpopulations: granulocytes (polymorphonuclear leukocytes), divided into subsets of neutrophils, eosinophils, basophils, and agranulocytes (mononuclear leukocytes), divided into lymphocytes and monocytes [1].
Alterations of WBC count within subpopulations may indicate occurrence of pathological processes in the organism. In general, elevated level of leukocytes subpopulations points at allergic reactions, chronic inflammations, parasitic infections, etc. Alterations of the populational distributions of leukocytes may be observed during such severe disorders as ischemic necrosis (e.g. myocardial infarction), leukemia and several hereditary diseases, which are accompanied by increased number of neutrophils, decrease in lymphocytes and eosinophils, and the decrease in neutrophils count is associated with anemia and several cancer types [1]. Routine analysis of WBC subpopulations is commonly performed using flow cytometry, which allows for fast cell counting and differentiation using light scattering and fluorescence emission, mostly of exogenous stains.
In the era of high-throughput techniques for cells characterization and vast variety of available probes for specific staining of cells, label-free methods are still of interest due to their advantages in certain niches [2]. First, in vivo studies of process involving WBC are of interest, and non-invasive methods for WBC activity tracking and characterization in humans are of a high demand. During intravital imaging in humans, the possibilities of specific staining and use of exogenous labels are limited due to their adverse influence on the organism, which include such aspects as phototoxicity and are largely unknown. Hence, label-free methods, which relay on endogenous contrast, are superior for this purpose. Second, label-free methods may provide morphological, biochemical and molecular-specific information about the cells, which has an independent value or complements information extracted using exogenous probes. Finally, the lack of necessity of staining becomes a significant advantage when developing simple, compact and cheap devices for point-of-care diagnostics, e.g. based on microfluidic technologies, as in this case the absence of reagents and pre-treatment (staining procedure) simplifies the chip architecture [3].
Among label-free methods for studying processes at the subcellular level, optical techniques become indispensable due to their spatial and temporal resolution, as well as non-invasive functioning. In Table 1, we briefly summarize representative label-free optical methods for WBC detection and characterization in vivo and in vitro. Methods of label-free intravital imaging differ significantly in physical principles (type of contrast), information provided and simplicity of implementation. Generally, a micrometer resolution and ∼100 fps imaging rate are required to track WBC in the blood flow, while for imaging WBC out of the vessels such a high temporal resolution is not a necessary prerequisite. In vivo visualization of WBC can be performed using the confocal laser scanning microscopy (CLSM) in the reflection mode [4,5], multiphoton microscopy with third harmonics generation (THG) [6] or two-photon excited autofluorescence (TPEAF) as a contrast [7], spectrally encoded detection [8], photothermal flow cytometry [9] and wide field microscopy (capillaroscopy) [10]. These methods allow for detection and quantification [10] and determination of WBC subtypes in the human skin dermis and dermal capillaries.
Table 1. Optical methods for label-free in vivo and in vitro imaging of WBC.
| Method | Endogenous contrast | Application | Ref. |
|---|---|---|---|
| Third harmonic generation | Third order non-linear susceptibility | In vivo separation between lymphocytes, monocytes and neutrophils based on THG intensity and cell size in the human skin papillary dermis | [6] |
| Two-photon excited fluorescence | Tryptophan or NAD(P)H fluorescence | In vivo study of WBC movement in the mouse ear | [7] |
| Spectrally encoded detection | Refractive index at different wavelengths | In vivo counting of WBC and separation between granulocytes and monocytes | [8] |
| Video capillaroscopy | Contrast of light absorbance between RBC and WBC in capillaries | In vivo semi-quantitative counting of WBC, including the case of neutropenia performed on the human nailfold capillaries | [10] |
| Confocal laser scanning microscopy | Refractive index | In vivo observation of WBC interaction with the vessel wall [4] and macrophages infiltration during cutaneous wound healing [5] | [4,5] |
| Fluorescence lifetime imaging | Fluorescence lifetime | In vitro differentiation between WBC and leukemic cells after their separation on a microfluidic chip | [3] |
| Optical diffraction tomography | 3D refractive index maps | In vitro differentiation between WBC based on their morphology and extraction of morphological and biochemical information on WBC content | [11] |
| Hyperspectral imaging | Endogenous chromophores | WBC differentiation in vitro | [12] |
| Raman imaging | Molecule types, which differ in Raman spectra, e.g. DNA, proteins, lipids, etc. | Differentiation between two WBC subtypes | [13] |
In the case of in vitro label-free imaging, multiple spectroscopic and optical imaging methods can be used to characterize WBC, such as Raman microscopy [13], optical diffraction tomography [11], hyperspectral imaging [12], etc. Various types of endogenous contrast can be used, such as distributions of refractive index inside the cell or molecular-specific information, e.g. redox state of the cell as revealed by autofluorescence. Fluorescence lifetime imaging microscopy (FLIM) technique is an imaging modality, which uses excited state decay parameters as a contrast to assess different molecular species in tissues. FLIM is known to provide information about differentiation of cells [14,15], oxidative stress [16], metabolic state of cells [17,18], etc. The main advantage of FLIM is that it is extremely sensitive to the molecular environment of a fluorophore and depends on its conformation and chemical interaction [19]. This is especially important in the imaging of endogenous fluorophores since no much information can be obtained based only on their spectral data: in most cases the spectra are broad, poorly defined and do not depend on the interaction status of the fluorophore with the environment. Moreover, compared to conventional fluorescence imaging, FLIM generally does not depend on the concentration of the fluorophores, therefore, measurement artifacts related to the scattering and absorption of the light, as well as its intensity fluctuations, are not an issue. Importantly, FLIM can be used as an additional modality with other imaging techniques, e.g. multiphoton microscopy, providing for complementary biochemical information about the object under investigation, e.g. cells’ metabolism [18].
In this paper, we address the capabilities of fluorescence spectroscopy and FLIM in characterization of blood cells, first of all, WBC, in order to reveal specific optical parameters, which can be used to separate certain types of cells, assess their heterogeneity and, as a prospective, to analyze them in vivo. The obtained results allow to differentiate between different blood cells and demonstrate how the process of erythrophagocytosis can be studied using fluorescence methods.
2. Materials and methods
2.1 Sample preparation
Blood samples were collected into vacuum blood collection tubes with EDTA (PUTH Vacumine tubes, K2EDTA, 4 ml). Blood withdrawal was done after overnight fasting between 08:00 to 10:00 in a quiet environment at normal ambient temperature (21 ÷ 24°C) from antecubital vein according to required specifications for hematologic measurements [20]. All donors gave written informed consent in accordance with the Declaration of Helsinki. The study was approved by the local ethics committee of the Medical Research and Educational Center of the Lomonosov Moscow State University.
WBC-enriched samples (leukoconcentrates) were obtained according to the following protocol: (1) after blood sampling the containers were held vertically during 30 min at room temperature for sedimentation of RBC; (2) blood plasma was collected into the tubes and centrifuged for 10 min at 200 g; (3) after centrifuging, the supernatant was collected leaving 200 µl, which was used in experiments as leukoconcentrate.
Autologous leukoconcentrates were mixed with blood samples obtained from another donor in not less than 10:1 proportion to initiate the immune response and erythrophagocytosis. After supplementation the leukoconcentrates were incubated for 30 minutes at 37°C. All measurements were performed no later than 3 hours after blood sampling. In total, 30 leukoconcentrate samples were measured.
2.2 Fluorescence microscopy and FLIM
Fluorescence imaging of WBC samples was performed using the Nikon A1 system (Nikon, Japan). Differential interference contrast (DIC), brightfield and epifluorescence images were taken using three spectral channels: DAPI (ex = 375 (28) nm / em = 460 (60) nm), FITC (ex = 480 (30) nm / em = 535(45) nm), TxRed (ex = 560 (40) / em = 630 (60) nm), hereinafter first number indicates center wavelength, number in parentheses – spectral full width at half-maximum of transmission for used bandpass filters. Fluorescent signal was detected using CMOS camera Nikon DS-Fi3 (Nikon, Japan) with acquisition time of 1 s, 2 s and 4 s for DAPI, FITC, TxRed channels respectively. During processing, differences in acquisition time were taken into account.
Confocal microscopy was performed at several excitation wavelengths (ex = 405 nm / em = 450 (50) nm; ex = 488 nm / 525 (50) nm; ex = 543 nm / em = 595 (50) nm; ex = 638 nm / em = 700 (80) nm). Imaging was performed with a 60× 1.4 NA objective (Nikon CFI Apo 60×H λS, maximum power 1 mW), image size was 512×512 pixels with collection time of 30 µs/pixel.
Fluorescence lifetime imaging was performed with the Microtime 200 setup (PicoQuant, Germany) with picosecond 402 nm excitation (40 MHz repetition rate, maximum power 50 µW, pulse duration = 40 ps). Imaging was performed with a 100× 1.4NA objective (Olympus UPlanSApo), image size was 400×400 pixels with collection time of 0.2 ms/pixel, i.e. collection time for the whole 80×80 µm image was 40 s. Detection was performed in two spectral channels, 440 (40) nm (channel 1) and >520 nm (channel 2).
Fluorescence decay curves were obtained using the time-correlated single photon counting technique and processed using the custom-made software written in Python programming language. The binning value was set to 3 (i.e. fluorescence signal was averaged over 49 pixels), and only the fluorescence decay profiles with ≥100 photon count in maximum were analyzed. Fluorescence decay curves were fitted with two exponential terms with respect to the instrument response function (IRF). Amplitudes (a1, a2) and decay lifetimes (τ1, τ2) were used to calculate mean lifetime as τmean = (a1τ1 + a2τ2)/(a1 + a2).
Phasor plot was calculated for each decay curve on binned FLIM image in a standard manner [21] with respect to the IRF in a time range of 10 ns for better representation of data on a phasor plane. Data clustering was made with the K-means algorithm with cluster number K = 5 using Phasor-C, Phasor-S and number of photon counts in fluorescence decay curves as features. Before clustering, each feature has been preprocessed to be in the (0, 1) range for equilibration of features contribution.
For dimensionality reduction, the t-distributed Stochastic Neighborhood Embedding (t-SNE) [22] algorithm was applied. Fluorescence features included mean, maximum, minimum and 25%, 50%, 75% percentiles of intensity in cells’ regions of interest (ROIs) for three spectral channels without additional preprocessing. Hyperparameter perplexity was set to 25. Standard implementations of the K-means and t-SNE algorithm were taken from the Scikit-learn python module [23].
2.3 Flow cytometry
Flow cytometry experiments were performed with the CytoFLEX system (Beckman Coulter, USA), equipped with three excitation sources (405, 488 and 635 nm). At least 105 events were detected in experiments with WBC. Spectral channels further encountered in the text correspond to the following excitation and emission wavelengths: PB450 (ex = 405 nm / em = 450 (45) nm), KO525 (ex = 405 nm / em = 525 (40) nm), FITC (ex = 488 nm/ em = 525 (40) nm), ECD (ex = 488 nm / em = 610 (20) nm), PC5.5 (ex = 488 nm / em = 690 (50) nm). FSC and SSC channels correspond to forward and side scattering at 488 nm, Violet SSC corresponds to side scattering signal at the 405 nm wavelength.
3. Results
3.1 Fluorescence microscopy of WBC: specific spectral features
When measuring fluorescence signal from the blood vessels, WBC signal is superimposed on the background signal of blood plasma, and exciting radiation and fluorescence emission are effectively absorbed by RBC. For instance, when performing imaging of blood vessels using bright field microscopy, WBC are detected as gaps between dark regions composed of RBC [10]. In the epifluorescence mode, the observed picture is similar: fluorescing spots can be detected from the area of blood capillaries, however, the origin of these spots, the possibility to attribute them to WBC and, moreover, to differentiate between WBC subtypes, is a challenging problem. To assess fluorescence properties of blood cells and verify possibilities of their classification, we performed examination of WBC-enriched samples (leukoconcentrates) using epifluorescence imaging in different spectral channels and confocal fluorescence imaging. Hereinafter, only the autofluorescence (AF) signal of cells was assessed, i.e. staining procedures were not performed.
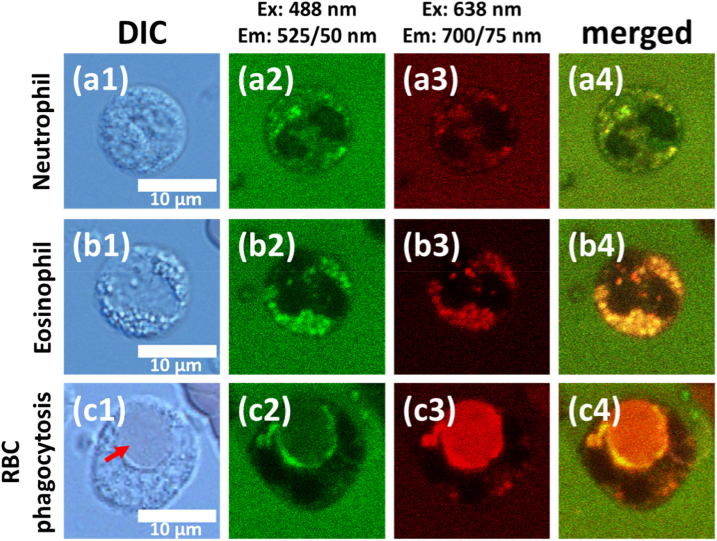
Figure 1 demonstrates DIC microscopy and confocal fluorescence images at 488 nm and 638 nm excitation of three cell types in the leukoconcentrate sample, which exhibited distinct spectral features: neutrophil (Fig. 1(a)), eosinophil (Fig. 2(b)) and erythrophagocytic cell (Fig. 1(c)). While at 488 nm excitation neutrophils exhibited a moderate (comparable to that of blood plasma, Fig. 1(a2)) fluorescence signal, which was localized in granules, almost no fluorescence was observed at 638 nm (Fig. 1(a3)). However, eosinophils and erythrophagocytic cell demonstrated emission at 638 nm excitation (Fig. 1(b3) and Fig. 1(c3) correspondingly). First, as described in literature [24–26], red fluorescence from granules was characteristic for eosinophils (Fig. 1(b)). Second, although rarely, a group of cells was present in all samples, for which the process of erythrophagocytosis, i.e. phagocytosis of RBC, was observed (Fig. 1(c)).
Fig. 1.
Autofluorescence confocal imaging of a) neutrophil, b) eosinophil, c) erythrophagocytic cell. (a1), (b1), (c1) correspond to DIC microscopy. (a2), (b2), (c2) represent fluorescence imaging at 488 nm excitation and 525(50) nm registration (“green” channel). (a3), (b3), (c3) represent fluorescence imaging at 638 nm excitation and 700(75) nm registration (“red” channel). (a4), (b4), (c4) represent the merged images of green and red channels, where the yellow color is due to overlay of the merged signals in different spectral channels.
Fig. 2.
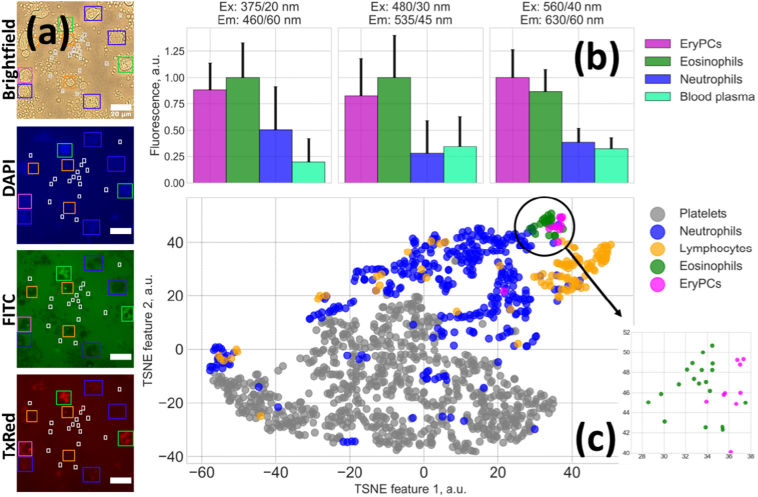
(a) Imaging of cells in transmission and in epifluorescence mode in DAPI, FITC, TxRed channels; cells’ ROIs were selected on the bright field image and applied to epifluorescence images. Scale bar is equal to 20 µm. (b) Average fluorescence intensity for different cell types. The number of cells used to calculate mean and STD error was 346, 96, 19 and 10 for neutrophils, lymphocytes, eosinophils and EryPC, respectively. (c) Visualization of fluorescent features of provided by the t-SNE algorithm. Clustering of different cell types is observed; the inset in the lower right corner shows enlarged image of eosinophils and EryPC separation.
For these erythrophagocytic cells (EryPC) fluorescence signal was originated from the area of phagocyted RBC, where fluorescing Hb degradation products, e.g. bilirubin, could be accumulated [27–29].
Typical fluorescence images obtained in a bright field transmission mode and in different spectral channels in epifluorescence mode are presented in Fig. 2(a). An average fluorescence intensity for different cell types obtained in different spectral channels is shown in Fig. 2(b).
Based on epifluorescence imaging data in different spectral channels, automatic clustering of blood cells could be performed using solely their fluorescence features (i.e. without taking into account cells’ morphology or size). For this, individual cells were manually selected on bright field images, and fluorescence parameters were calculated for the corresponding cells’ masks for epifluorescence images. The t-Distributed Stochastic Neighbor Embedding (t-SNE) algorithm was used to reduce the dimensionality of fluorescence features space. The diagram of cells distribution in the t-SNE space is presented in Fig. 2(c), which demonstrates the possibility to differentiate between blood cells types using epifluorescence microscopy.
3.2 Flow-cytometry of WBC: heterogeneity of fluorescence parameters and gating strategy to separate erythrophagocytosis and eosinophils
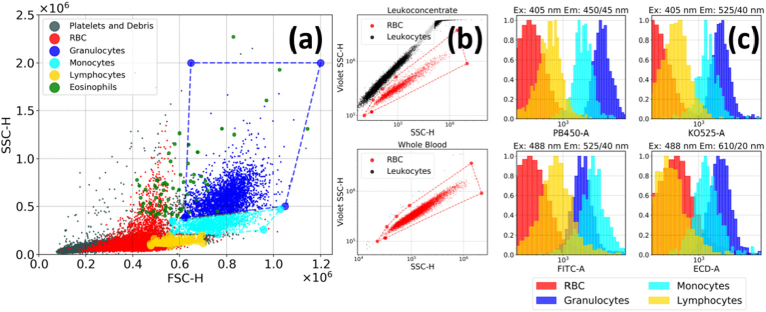
To assess AF properties of different WBC subtypes in a high-throughput manner, we performed measurements using flow cytometry, which, besides a much better statistics compared to microscopy experiments, allows for separation of cells based on their granularity and size by measuring side scattering (SSC) and forward scattering (FSC) from each cell, respectively. Figure 3(a) demonstrates a typical SSC/FSC diagram for a leukoconcentrate sample, where the areas corresponding to lymphocytes, monocytes and granulocytes, as well as for platelets and cell debris were marked according to the described procedure [30]. RBC can be separated from WBC and platelets using SSC-405/SSC-488 nm diagram [31] as shown in Fig. 3(b), where this diagram is plotted for leukoconcentrate and whole blood. By masking the corresponding regions, AF distribution in different spectral regions can be extracted for these cell types (Fig. 3(c)).
Fig. 3.
(a) The side (SSC)/forward (FSC) scattering diagram for a leukoconcentrate, where the regions corresponding to different blood cells are marked. (b) The SSC-405/SSC-488 scattering diagram for leukoconcentrate and whole blood used to select RBC in the SSC/FSC diagram. (c) Distribution of fluorescence intensity in different spectral channels for four blood cell types.
The obtained values of average intensities and full width at half maximum (FWHM) of AF distribution are presented in Table 2. It can be observed that while granulocytes AF is generally twofold higher than that of monocytes, the latter become slightly more intensively fluorescent in the ex = 488/ em = 525 (40) nm channel, presumably indicating differences in the fluorescing molecules content for these two WBC subtypes. We also note that RBC demonstrated low, although non-negligible fluorescence signal in all spectral channels (Fig. 3(c)).
Table 2. Fluorescence emission parameters for different blood cells and blood plasma.
| Cell type | Intensity (ex/em), a.u. |
Lifetime, ns | Notes | |||
|---|---|---|---|---|---|---|
| 405/450 | 405/525 | 488/610 | 488/690 | |||
| Neutrophils | 2.1 ± 0.5 | 1.4 ± 0.3 | 1.5 ± 0.4 | 2.9 ± 1.4 | 1.25 ± 0.15 | Biexponential decay at 402 nm excitation caused by enzyme-bound flavins |
| Monocytes | 1.4 ± 0.4 | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.5 ± 0.7 | – | |
| Lymphocytes | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.4 | – | |
| Eosinophils | 12 | 7.1 | 19.8 | 34 | 0.9 ± 0.1 | Highly fluorescent cells with red shifted emission |
| EryPC | 1.2 ± 0.4 | 0.8 ± 0.3 | 3.5 ± 2.6 | 21 | 0.7 ± 0.1 | High fluorescence in the red region of spectrum with a fast fluorescence decay caused by hemoglobin degradation products |
| RBC | 0.5 ± 0.2 | 0.4 ± 0.1 | 0.6 ± 0.2 | 1.1 ± 0.9 | ∼IRF | Weak ultrafast fluorescence emission caused by the formation of hemoglobin photoproduct |
| Blood plasma | - | - | - | - | 1.1 ± 0.2 | Background signal comparable to that of WBCs at 405 and 488 nm excitation |
To verify whether different blood cells can be separated using solely their autofluorescence signal, we’ve performed processing of the flow cytometry data without taking into account the scattering signals (FSC and SSC) using the t-SNE algorithm. As can be seen in Fig. 4, which demonstrates visualization of fluorescence features provided by t-SNE, a clear separation between different cell types is observed. This fact is intriguing, as using fluorescence microscopy and three spectral channels (Fig. 1 and Fig. 2) does not allow for separation between neutrophils subtypes as their fluorescence spectra are strongly overlapped, while it becomes possible when analyzing flow cytometry data, which contains information from a larger number of spectral channels. This example clearly demonstrates the possibility to separate between different blood cells using fluorescence spectroscopy.
Fig. 4.
Separation between different blood cells based solely on their autofluorescence signal obtained as a result of flow cytometry data processing using the t-SNE algorithm. We note that the scattering signals (FSC and SSC) were not considered when performing clustering of cells.
As it has been demonstrated in the fluorescence microscopy experiments, two types of cells in blood exhibit very distinct optical features: eosinophils and EryPC. To assess distributions of their optical properties, leukoconcentrate samples were measured using flow cytometry. Normally erythrophagocytosis is a relatively rare observation: the proportion of RBC that is daily cleared is approx. 0.8% per day [32]. In order to initiate the immune response and stimulate erythrophagocytosis in the experimental autologous leukoconcentrate, RBC from another donor were added.
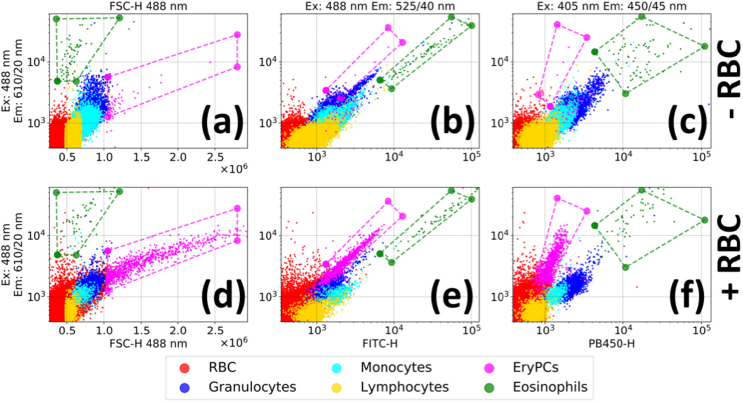
Figure 5 shows the ECD (ex = 488 nm/ em = 610 (20) nm)/FSC diagram, where it can be seen that addition of RBC resulted in the appearance of a clearly visible cluster of cells (shown in magenta) characterized by higher values of forward scattering typical for phagocytosis [33]. As expected from fluorescent imaging experiments (Fig. 1(c), Figs. 2(b)-(c)), EryPC were characterized by red-shifted intense fluorescence signal. According to these optical properties, a mask for EryPC was proposed and overlaid on the other diagrams (Fig. 5(e)-(f)), demonstrating how EryPC can be gated in flow cytometry experiments using solely their AF.
Fig. 5.
A gating strategy to detect erythrophagocytes and eosinophils by forward scattering and red autofluorescence ((a) and (d)) and using AF solely AF ((b),(e);(c),(f)). (a),(b),(c) represent 2D diagrams for autologous leukoconcentrates; (d),(e),(f) – leukoconcentrates with addition of RBC from another donor.
Eosinophils can be distinguished from other cells by their high fluorescent signals in the FITC and ECD channels or by high fluorescent signal and relatively low forward scattering (green points in Fig. 5) [34]. Generally, EryPC can be distinguished from the eosinophils by their lower fluorescence in blue (ex = 405 / em = 450 (45) nm) and green (ex = 488 / em = 525 (40) nm) spectral regions (almost a magnitude lower) (Fig. 5). Data points corresponding to eosinophils were also visualized in Fig. 3(a). Based on the described gating strategy, distribution of fluorescence parameters for EryPC and eosinophils was assessed and presented in Table 2.
3.3 Fluorescence lifetime imaging of WBC. Clustering of different cell types and assessment of molecular heterogeneity
FLIM was performed to assess fluorescence decay parameters of different WBC, which could be expected due to differences in their molecular content. Excitation was performed at 402 nm, and fluorescence signal was detected in two spectral channels: 440 (40) nm (channel 1) and >520 nm (channel 2). At this excitation wavelength, both NAD(P)H (nicotinamide adenine dinucleotide (phosphate) reduced) and bound flavins (mononucleotide FMN and adenine dinucleotide FAD) were reported to be responsible for fluorescence signal with their emission centered at 450 and 520 nm [35]. However, fluorescence intensity in channel 1 was not sufficient to obtain reliable FLIM fits, hence, only the signal obtained in the channel 2 will be discussed below.
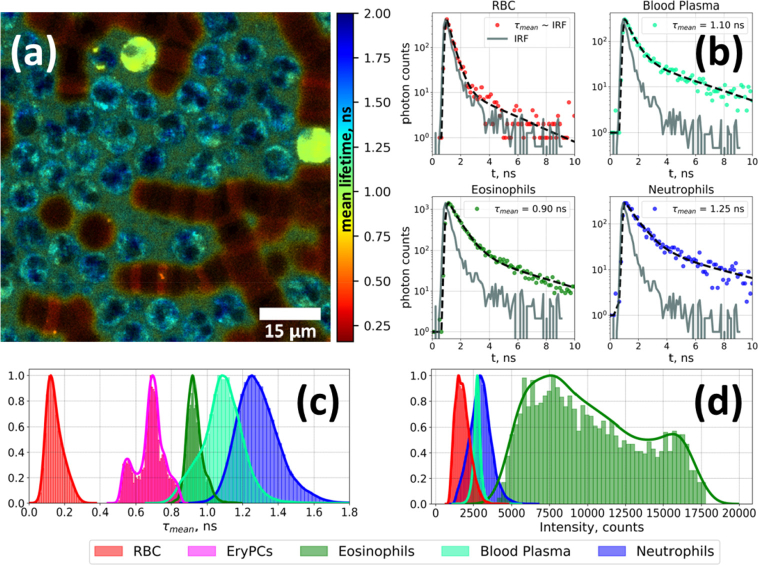
Typical FLIM image of WBC-enriched sample is presented in Fig. 6(a), representative fluorescence decay curves and their biexponential fits are shown in Fig. 6(b). Several objects can be identified based on their fluorescence lifetimes distributions. That is, RBC are characterized by faster decay comparable to IRF, in agreement with the literature data [36]. Neutrophils exhibited the slowest decay (τmean = 1.25 ± 0.15 ns), while for eosinophils the mean fluorescence lifetime was shorter (τmean = 0.9 ± 0.1 ns). EryPC were characterized by τmean = 0.7 ± 0.1 ns.
Fig. 6.
(a) FLIM of a leukoconcentrate sample, color-coded according to the mean fluorescence lifetime (τmean) value. (b) Representative fluorescence decay curves for RBC (red), blood plasma (cyan), eosinophils (green) and neutrophils (blue) and their fits to the biexponential decay law. Distribution of (c) τmean and (d) integral intensity for RBC, blood plasma, eosinophils, erythrophagocytic cells and neutrophils.
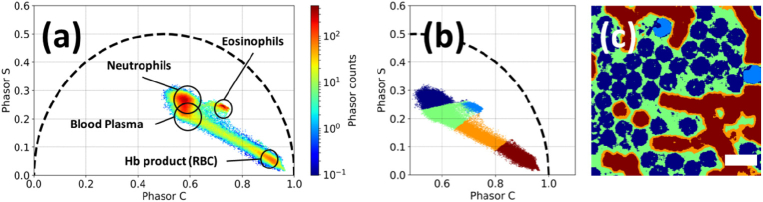
To further analyze the FLIM data, we depicted them in the form of phasor plots, which enable fit-free representation on the phasor plane, where different coordinates correspond to different fluorescence decay parameters (ai and τi). Phasor plot of a typical FLIM of a leukoconcentrate sample is presented in Fig. 7(a), where the areas corresponding to different cell types are marked based on their spatial localization. We also tested whether the FLIM data can be used to automatically segment the image into areas corresponding to different objects by applying the K-means clustering algorithms to the dataset, where each element was characterized by two coordinates in the phasor plot and intensity. The results are presented in Figs. 7(b)-(c), where the clustering of points on the phasor plot (Fig. 7(b)) and on the initial image (Fig. 7(c)) is shown. It can be seen that this approach allows for reliable separation of RBC, blood plasma, eosinophils and neutrophils.
Fig. 7.
(a) FLIM of WBC subtypes, RBC and blood plasma represented in the form of phasor plot. (b) Clustering of objects using the K-means (K = 5) algorithm on the phasor plot. (c) The initial image (Fig. 6(a)) colored corresponding to the cluster colors. Scale bar is equal to 15 µm.
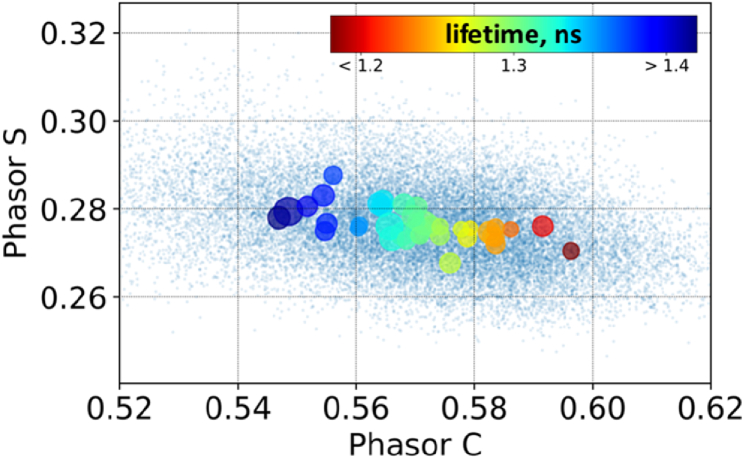
A broad distribution of FLIM parameters for WBC (Fig. 6) suggests the existence of heterogeneity of fluorescing molecular content for individual cells. To assess this heterogeneity quantitatively, we calculated the positions of weighted averages of phasor-C and phasor-S components belonging to a single cell with weights proportional to number of photon counts in corresponding fluorescent decay curves. The circles in Fig. 8 correspond to single cells, while their color and size correspond to their phasor time and FWHM of its distribution (Fig. 8).
Fig. 8.
Assessment of the molecular heterogeneity of neutrophils based on the analysis of phasor plot. Point colors correspond to different fluorescence lifetime values (from less than 1.2 (red) to 1.4 ns (blue), as shown on the scale bar), point sizes correspond to fluorescence lifetimes heterogeneity for single cells (scale bar). The small blue dots correspond to calculated phasor values for the different pixels of the FLIM image, while large dots correspond to the mean phasor values for the pixels that belong to single cells.
Usually, distribution of fluorescence decay rate across the phasor plot for different cells is analyzed in terms of their metabolic heterogeneity, e.g. differences of metabolic status determined as the ratio of free and enzyme-bound NAD(P)H impacts to the overall AF signal. However, at the 402 nm excitation (single photon) NAD(P)H excitation cross-section is low (155 M−1cm−1, which is only ∼2.5% of the maximum of molar extinction of NAD(P)H at 340 nm [37]), thus, its fluorescence is negligible and the observed heterogeneity is attributed to other fluorophores and related effects.
4. Discussion
4.1 WBC: heterogeneity of fluorescence parameters
AF of blood plasma [38] and cells, including WBC, is a well-studied phenomenon [39], which is the basis of several diagnostic approaches. At >350 nm (single photon) excitation, fluorescence emission of cells is attributed to free and enzyme-bound NAD(P)H and flavins (FMN, FAD), riboflavin and, more rarely, lipofuscin [40]. Using the NAD(P)H and FAD signals, both steady-state and time-resolved, several ratiometric indicators of the redox or metabolic state of the cell were suggested, being the basis of the wide-spread optical metabolic imaging [41,42]. For instance, the ratio of free and bound NAD(P)H extracted from the amplitudes of fast and slow fluorescence components excited (single photon) at 360 ± 20 nm (or 740 ± 40 nm at two-photon excitation) reveal the major metabolic pathway in cell, i.e. glycolytic or oxidative phosphorylation [17]. Moreover, analysis of NAD(P)H lifetimes distribution allows for assessment of cells differentiation and phenotype, e.g. for macrophages [43] or stem cells [14].
The 402 nm wavelength fits the tail of NAD(P)H absorption spectrum with an absorption cross-section almost two orders of magnitude lower than that in the absorption maximum at 340 nm (155 and 6220 M−1cm−1 respectively) [37]. Nevertheless, some papers still relay on NAD(P)H fluorescence when analyzing emission at >400 nm excitation, e.g. by decomposing the spectrum into several gaussian-shaped bands and attributing the most short wave component to NAD(P)H [35]. The validity of such interpretation is verified by controlled changes of the abovementioned signal to application of external chemical agents such as respiratory chain inhibitors [35]. However, in our experiments no significant signal was detected in the NAD(P)H fluorescence band at 402 nm excitation, even when the laser power was increased 50-fold (from 20 µW to 1 mW). In other words, in our case low absorption cross-section of NAD(P)H was not compensated by its presumably high (∼100 µM in mitochondria) concentration. As a result, we assume that the detected WBC fluorescence was predominantly originated from flavins.
It is common that fluorescence decay curves of the cellular flavins are best fit by a bi-exponential model, where the short lifetime component corresponds to the quenched state of FAD (“closed” or stacked form) and the long lifetime component contains contributions from unquenched FAD (“open” or unstacked form), FMN and riboflavin [41,42,44]. Given that the discrimination of different flavins within cells is problematic, biochemical interpretation of the fluorescence lifetime measurements remains a challenge.
The observed absence of NAD(P)H fluorescence in FLIM experiments is also interesting in the context of interpreting flow cytometry data. Namely, at 405 nm excitation AF signal is observed both in 450 (45) nm (PB450) and 525 (40) nm (KO525) channels, hence, by attributing these signals to NAD(P)H and FAD, respectively, the redox ratio can be calculated and assessed for different WBC subtypes. Based on this ratio, metabolic status of immune cells can be characterized in a high-throughput manner. However, taking into account the results of FLIM measurements, this interpretation should be treated with a certain care and direct evidence that the ex = 405 / em = 450 (45) nm signal is originated from NAD(P)H should be provided. Of note, label-free assessment of cells metabolic status using flow cytometry has been performed in [45] using NAD(P)H excitation with a 378 nm laser and time-resolved detection, where it has been demonstrated that this approach allows for assessment of apoptotic processes in cells. Overall, we consider flow cytometry-based assessment of WBC metabolic status to be a prospective task for biomedical diagnostics [46].
Flow cytometry also allowed for assessment of relative fluorescence intensities of different WBC subtypes and its heterogeneity across the whole ensemble (Table 2). Although fluorescence intensity of a cell is a function of both its molecular content, size and morphology, which has been shown to vary dramatically even for a certain WBC subtype [11], further studies are required to verify whether distributions of WBC AF can serve as a marker of pathological processes.
Finalizing, we would like to mention several cases when label-free characterization of cells based on AF in flow cytometry could complement or even outperform the standard approach, which is based on specific staining with fluorescent dyes. In [47], using AF-based flow cytometry sorting of heterogeneous mixture of breast cancer cells with different phenotype was performed. In [18] it was observed that high AF signal in macrophages populations is associated with high granularity caused by the significant presence of cellular organelles in the cytoplasm. Finally, in [48] it was observed that cancer stem cells can be detected and separated from other tumor cells using a specific AF, which was shown to be due to enhanced riboflavin accumulation – the authors argue that this method is superior over the protocols involving staining of specific cancer stem cells receptors. Collectively, these results indicate that there is still a place for label-free AF characterization and development of new gating strategies in analysis of cellular and subcellular processes, e.g. various metabolic pathways, phagocytosis in cancer-related inflammations. On the other hand, this demand requires detailed investigation of biochemical processes and origin of fluorophores responsible for selective characterization of processes in cells.
4.2 Autofluorescence of red blood cells
As can be seen from fluorescence imaging of blood samples (Fig. 6), RBC exhibit weak emission signal with a fast decay (comparable to IRF). Although it is known that hemoglobin (Hb) molecules are essentially non-fluorescent due to the electron transfer from porphyrin to the Fe ion on fs-timescale [49], it was reported that RBC emission at two-photon excitation in the 700-800 nm range is originated from hemoglobin [36,50]. It has also been shown that the two-photon excited fluorescence of RBC can be used for in vivo imaging of blood vessels due to its very distinct FLIM signature, i.e. ultrafast decay [51]. Further studies revealed that single (in the 400-500 nm range) and two-photon excited fluorescence of RBC is due to the formation of Hb photoproduct [29]. As a consequence, fluorescence intensity of RBC suspension and Hb aqueous solution increases linearly with the irradiation time [29].
In this work, we detected RBC fluorescence in a wide range of conditions. Namely, RBC were observed during confocal fluorescence imaging (Fig. 6) and were characterized by low intensity (∼20% of that for blood plasma) and the fastest fluorescence decay (∼IRF). A significant heterogeneity in fluorescence intensity was observed for single RBC (Fig. 5): while some cells were bright, others exhibited almost no emission signal. This fact suggests that the observed emission could be caused not only by Hb photoproduct.
RBC fluorescence was also detected when using flow cytometry, where low, although clearly detectable signal in all channels was observed (see Fig. 3 and Table 2). As the irradiation dose during flow cytometry measurement of a single cell is not high, one may assume that the detected signal in this case may partly originate from an internal fluorophore different to Hb photoproduct. This is in line with the fact that certain Hb degradation products may exhibit fluorescence signal [27].
4.3 Erythrophagocytosis: specific fluorescence features and prospects for in vivo diagnostics
It was shown in [27] that AF in the NIR range can be used to characterize atherosclerotic plaques according to the risk of rupture from the blood vessel wall. The authors demonstrated that the NIR AF (ex = 785 nm / em = 850 nm) originated from intraplaque hemorrhages and suggested that Hb degradation product, namely, bilirubin, was the possible source of the detected signal.
Generally, accumulation of heme degradation products occurs during the erythrophagocytosis process. In the organism, senescent and defective RBCs should be eliminated in order to prevent hemolysis with a consequent toxic Hb release [52]. An important stage in RBC removal is expressing certain signal molecules to initiate RBC clearance by macrophages (monocytes) or microphages (eosinophils and neutrophils). Effete RBC, recognized and engulfed by phagocytes, are degraded in phagolysosomes where they are exposed to various enzymes. The hemés porphyrin ring is oxidized by microsomal enzyme heme oxygenase, releasing the ferrous iron (Fe2+), CO and heme derivative (biliverdin) [53]. Biliverdin is subsequently converted by biliverdin reductase to the antioxidant and anti-inflammatory bilirubin, which is further released in blood plasma [54]. Free iron can be either stored in the cell (bound by ferritin), used for biosynthesis of iron-containing cytochromes, or released in plasma via iron transporters (ferroportins) where it can be bound by transferrin [53].
In this work, we observed the formation of Hb-derived fluorophore during erythrophagocytosis and studied its optical properties. Although rarely, the erythrophagocytosis events were observed in all leukoconcentrate samples, and to enhance the number of erythropagocytes (EryPC) immune response was stimulated by addition of RBCs from another sample (see e.g. Fig. 5).
EryPC were characterized by a set of very specific optical features, making them distinct from other blood components (Table 2). Namely, EryPC demonstrated the most red shifted fluorescence spectra (their emission could be excited at 638 nm, Fig. 3), high fluorescence intensity at 638 nm excitation and fast fluorescence decay at 402 nm excitation (Table 2). A set of model experiments was performed (addition of RBC from other donor) to develop a gating strategy for label-free detection of EryPC in blood. It was revealed that EryPC can be separated by a stronger forward scattering and intense AF in the 500-600 nm spectral region at 488 nm excitation (Fig. 4).
We note that several observations make the direct assignment of the observed EryPC fluorescence solely to bilirubin ambiguous, as while bilirubin can be efficiently excited at 405 nm, no strong EryPC fluorescence was observed at this wavelength (Fig. 5). Although the origin of EryPC fluorescence requires further investigation, it can be argued that they can be readily distinguished from other cells by the abovementioned fluorescence features. This could be of value for in vivo imaging of a number of processes accompanied by EryPC, e.g. wound healing, including the case of bruises, and inflammatory processes.
4.4 Eosinophils
Like EryPC, eosinophils are also characterized by distinct fluorescence properties, making it easy to separate them from other WBC: almost a tenfold higher intensity (Fig. 1 and Fig. 6(d)), red-shifted emission spectrum, shorter fluorescence lifetime (τm = 0.9 ± 0.1 ps compared to τm = 1.25 ± 0.15 ns for neutrophils). Properties of eosinophils AF is well documented in the literature [24]. In [39] excitation and emission spectra of eosinophils were recorded: at excitation from 330 to 400 nm emission was centered at 440 nm, while longer wavelength nm excitation led to the appearance of additional long wave fluorescence contributions in 500-520 and 540-560 ranges. These spectral components were significantly different for granulocyte and agranulocyte families. The authors explained this with a favoring condition for the emission of flavins and flavin coenzymes autofluorescence signal. At 445 nm excitation and 527 nm emission it was demonstrated using additional chromatographic experiments that the eosinophil granule-associated fluorophore is FAD [25]. However, the settings for the FAD autofluorescence may result in recording not solely FAD autofluorescence signal due to the presence of mitochondrial monoamine oxidase’s flavoproteins and the contribution of lipids peroxidation products. To clarify the source of fluorescence, mitochondria respiratory chain uncoupling agents (e.g. CCCP, FCCP, NaCN) should be used. Overall, specific fluorescence properties of eosinophils make them a prominent candidate for in vivo tracking and detection using intravital microscopy techniques, that could be of value e.g. for studying allergic reactions, which are usually accompanied by an increased number of eosinophils count. For instance, it was observed that in that human angioimmunoblastic T-cell lymphoma tissues highly autofluorescent cell are present, which were identified as eosinophils [26].
5. Conclusion
Several fluorescence-based techniques were applied for blood cells characterization in this work. We made use of epifluorescence and confocal imaging to extract specific fluorescence features of cells to verify whether differentiation between cell types is possible based solely on their autofluorescence parameters. After that, heterogeneity of fluorescence parameters was assessed using flow cytometry, which allowed for label-free characterization of six cell types. Finally, fluorescence lifetime imaging (FLIM) was performed to analyze molecular heterogeneity of different cell types based on distributions of fluorescence decay parameters within single cells. We observed that while fluorescence intensity of single WBC is usually comparable to that of blood plasma, eosinophils and erythrophagocytic cells can be readily distinguished by their enhanced emission in the red spectral region, that can be used for in vivo localization of these cells during allergic reactions, wound healing and inflammatory processes. Flow cytometry allowed assessment of spectral differences and fluorescence intensity distribution between major WBC subtypes: lymphocytes, monocytes and granulocytes. It was also shown that red blood cells exhibit low, although detectable fluorescence emission, that originates mainly from hemoglobin (photo)products. Finally, it was shown that automatic differentiation between blood cell types is possible by analysis of fluorescence decay data on the phasor plot, and that significant molecular heterogeneity between single neutrophil cells exists, that, at the excitation and detection parameters used, could be attributed to flavin molecules binding to different enzymes at a different extent. We consider that the obtained results are of use for development of novel methods for in vivo and in vitro characterization of WBC that will be of interest to a broad audience involved into the biomedical optics area.
Acknowledgments
The work was supported by the Russian Science Foundation (grant No. 17-75-10215). The experiments on erythrophagocytosis were supported by the Russian Foundation for Basic Research (grant No. 19-02-00947). The work on flow cytometry measurements and sample preparation was supported by the Russian Science Foundation (grant No. 18-15-00422)
Funding
Russian Science Foundation (RSF)10.13039/501100006769 (17-75-10215, 18-15-00422); Russian Foundation for Basic Research (RFBR)10.13039/501100002261 (19-02-00947-a).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Sakata T., Kuroda T., “Method for classifying leukocytes by flow cytometry,” U.S. patent 5,296,378 (Sep. 18, 1994).
- 2.Tuchin V. V., Tárnok A. A., Zharov V. P., “In vivo flow cytometry: a horizon of opportunities,” Cytometry 79A(10), 737–745 (2011). 10.1002/cyto.a.21143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee D. H., Li X., Ma N., Digman M. A., Lee A. P., “Rapid and label-free identification of single leukemia cells from blood in a high-density microfluidic trapping array by fluorescence lifetime imaging microscopy,” Lab Chip 18(9), 1349–1358 (2018). 10.1039/C7LC01301A [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez S., Sackstein R., Anderson R. R., Rajadhyaksha M., “Real-time evidence of in vivo leukocyte trafficking in human skin by reflectance confocal microscopy,” J. Invest. Dermatol. 117(2), 384–386 (2001). 10.1046/j.0022-202x.2001.01420.x [DOI] [PubMed] [Google Scholar]
- 5.Lange-Asschenfeldt S., Bob A., Terhorst D., Ulrich M., Fluhr J. W., Mendez G., Roewert-Huber H.-J., Stockfleth E., Lange-Asschenfeldt B., “Applicability of confocal laser scanning microscopy for evaluation and monitoring of cutaneous wound healing,” J. Biomed. Opt. 17(7), 0760161 (2012). 10.1117/1.JBO.17.7.076016 [DOI] [PubMed] [Google Scholar]
- 6.Wu C. H., Wang T. D., Hsieh C. H., Huang S. H., Lin J. W., Hsu S. C., Wu H. T., Wu Y. M., Liu T. M., “Imaging cytometry of human leukocytes with third harmonic generation microscopy,” Sci. Rep. 6(1), 37210 (2016). 10.1038/srep37210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C., Pastila R. K., Pitsillides C., Runnels J. M., Puoris’haag M., Côté D., Lin C. P., “Imaging leukocyte trafficking in vivo with two-photon-excited endogenous tryptophan fluorescence,” Opt. Express 18(2), 988–999 (2010). 10.1364/OE.18.000988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winer M. M., Zeidan A., Yeheskely-Hayon D., Golan L., Minai L., Dann E. J., Yelin D., “In vivo noninvasive microscopy of human leucocytes,” Sci. Rep. 7(1), 13031 (2017). 10.1038/s41598-017-13555-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zharov V. P., Galanzha E. I., Tuchin V. V., “In vivo photothermal flow cytometry: Imaging and detection of individual cells in blood and lymph flow,” J. Cell. Biochem. 97(5), 916–932 (2006). 10.1002/jcb.20766 [DOI] [PubMed] [Google Scholar]
- 10.Bourquard A., Pablo-Trinidad A., Butterworth I., Sánchez-Ferro Á., Cerrato C., Humala K., Urdiola M. F., Del Rio C., Valles B., Tucker-Schwartz J. M., Lee E. S., Vakoc B. J., Padera T. P., Ledesma-Carbayo M. J., Chen Y., Hochberg E. P., Gray M. L., Castro-González C., “Non-invasive detection of severe neutropenia in chemotherapy patients by optical imaging of nailfold microcirculation,” Sci. Rep. 8(1), 5301 (2018). 10.1038/s41598-018-23591-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon J., Kim K., Park H., Choi C., Jang S., Park Y., “Label-free characterization of white blood cells by measuring 3D refractive index maps,” Biomed. Opt. Express 6(10), 3865–3875 (2015). 10.1364/BOE.6.003865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verebes G. S., Melchiorre M., Garcia-Leis A., Ferreri C., Marzetti C., Torreggianim A., “Hyperspectral enhanced dark field microscopy for imaging blood cells,” J. Biophotonics 6(11-12), 960–967 (2013). 10.1002/jbio.201300067 [DOI] [PubMed] [Google Scholar]
- 13.Ramoji A., Neugebauer U., Bocklitz T., Foerster M., Kiehntopf M., Bauer M., Popp J., “Toward a spectroscopic hemogram: Raman spectroscopic differentiation of the two most abundant leukocytes from peripheral blood,” Anal. Chem. 84(12), 5335–5342 (2012). 10.1021/ac3007363 [DOI] [PubMed] [Google Scholar]
- 14.Stringari C., Nourse J. L., Flanagan L. A., Gratton E., “Phasor fluorescence lifetime microscopy of free and protein-bound NADH reveals neural stem cell differentiation potential,” PLoS One 7(11), e48014 (2012). 10.1371/journal.pone.0048014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meleshina A. V., Dudenkova V. V., Shirmanova M. V., Shcheslavskiy V. I., Becker W., Bystrova A. S., Zagaynova E. V., “Probing metabolic states of differentiating stem cells using two-photon FLIM,” Sci. Rep. 6(1), 21853 (2016). 10.1038/srep21853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datta R., Alfonso-García A., Cinco R., Gratton E., “Fluorescence lifetime imaging of endogenous biomarker of oxidative stress,” Sci. Rep. 5(1), 9848 (2015). 10.1038/srep09848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blacker T. S., Duchen M. R., “Investigating mitochondrial redox state using NADH and NADPH autofluorescence,” Free Radical Biol. Med. 100, 53–65 (2016). 10.1016/j.freeradbiomed.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szulczewski J. M., Inman D. R., Entenberg D., Ponik S. M., Aguirre-Ghiso J., Castracane J., Condeelis J., Eliceiri K. W., Keely P. J., “In vivo visualization of stromal macrophages via label-free FLIM-based metabolite imaging,” Sci. Rep. 6(1), 25086 (2016). 10.1038/srep25086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker W., “Fluorescence lifetime imaging–techniques and applications,” J. Microsc. 247(2), 119–136 (2012). 10.1111/j.1365-2818.2012.03618.x [DOI] [PubMed] [Google Scholar]
- 20.Baskurt O. K., Boynard M., Cokelet G. C., Connes P., Cooke B. M., Forconi S., Liao F., Hardeman M. R., Jung F., Meiselman H. J., Nash G., Nemeth N., Neu B., Sandhagen B., Shin S., Thurston G., Wautier J. L., “New guidelines for hemorheological laboratory techniques,” (2009). [DOI] [PubMed]
- 21.Digman M. A., Caiolfa V. R., Zamai M., Gratton E., “The phasor approach to fluorescence lifetime imaging analysis,” Biophys. J. 94(2), L14–L16 (2008). 10.1529/biophysj.107.120154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Maaten L., Hinton G., “Visualizing data using t-SNE,” J. Mach. Learn. Res. 9(Nov), 2579–2605 (2008). [Google Scholar]
- 23.Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., Blondel M., Prettenhofer P., Weiss R., Dubourg V., Vanderplas J., Passos A., Cournapeau D., Brucher M., Perrot M., Duchesnay E., “Scikit-learn: Machine learning in Python,” J. Mach. Learn. Res. 12(Oct), 2825–2830 (2011). [Google Scholar]
- 24.Monici M., Agati G., Mazzinghi P., Fusi F., Bernabei P. A., Landini S., Pratesi R., “Image analysis of cell natural fluorescence: diagnostic applications in haematology,” Proc. SPIE 2928, 180–187 (1996). 10.1117/12.259969 [DOI] [Google Scholar]
- 25.Mayeno A. N., Hamann K. J., Gleich G. J., “Granule-associated flavin adenine dinucleotide (FAD) is responsible for eosinophil autofluorescence,” J. Leukocyte Biol. 51(2), 172–175 (1992). 10.1002/jlb.51.2.172 [DOI] [PubMed] [Google Scholar]
- 26.Buchwalow I., Atiakshin D., Samoilova V., Boecker W., Tiemann M., “Identification of autofluorescent cells in human angioimmunoblastic T-cell lymphoma,” Histochem. Cell Biol. 149(2), 169–177 (2018). 10.1007/s00418-017-1624-y [DOI] [PubMed] [Google Scholar]
- 27.Htun N. M., Chen Y. C., Lim B., Schiller T., Maghzal G. J., Huang A. L., Stocker R., “Near-infrared autofluorescence induced by intraplaque hemorrhage and heme degradation as marker for high-risk atherosclerotic plaques,” Nat. Commun. 8(1), 1–16 (2017). 10.1038/s41467-016-0009-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dybas J., Grosicki M., Baranska M., Marzec K. M., “Raman imaging of heme metabolism in situ in macrophages and Kupffer cells,” Analyst 143(14), 3489–3498 (2018). 10.1039/C8AN00282G [DOI] [PubMed] [Google Scholar]
- 29.Shirshin E. A., Yakimov B. P., Rodionov S. A., Omelyanenko N. P., Priezzhev A. V., Fadeev V. V., Darvin M. E., “Formation of hemoglobin photoproduct is responsible for two-photon and single photon-excited fluorescence of red blood cells,” Laser Phys. Lett. 15(7), 075604 (2018). 10.1088/1612-202X/aac003 [DOI] [Google Scholar]
- 30.Fujimoto H., Sakata T., Hamaguchi Y., Shiga S., Tohyama K., Ichiyama S., Houwen B., “Flow cytometric method for enumeration and classification of reactive immature granulocyte populations,” Cytometry 42(6), 371–378 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Ost V., Neukammer J., Rinneberg H., “Flow cytometric differentiation of erythrocytes and leukocytes in dilute whole blood by light scattering,” Cytometry 32(3), 191–197 (1998). [DOI] [PubMed] [Google Scholar]
- 32.de Back D. Z., Kostova E. B., van Kraaij M., van den Berg T. K., van Bruggen R., “Of macrophages and red blood cells; a complex love story,” Front. Physiol. 5, 9 (2014). 10.3389/fphys.2014.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bratosin D., Mazurier J., Slomianny C., Aminoff D., Montreuil J., “Molecular mechanisms of erythrophagocytosis: flow cytometric quantitation of in vitro erythrocyte phagocytosis by macrophages,” Cytometry 30(5), 269–274 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Weil G. J., Chused T. M., “Eosinophil autofluorescence and its use in isolation and analysis of human eosinophils using flow microfluorometry,” Blood 57(6), 1099–1104 (1981). [PubMed] [Google Scholar]
- 35.Wu Y., Qu J. Y., “Autofluorescence spectroscopy of epithelial tissues,” J. Biomed. Opt. 11(5), 054023 (2006). 10.1117/1.2362741 [DOI] [PubMed] [Google Scholar]
- 36.Zheng W., Li D., Zeng Y., Luo Y., Qu J. Y., “Two-photon excited hemoglobin fluorescence,” Biomed. Opt. Express 2(1), 71–79 (2011). 10.1364/BOE.2.000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakowicz J. R., Principles of Fluorescence Spectroscopy (Springer Science and Business Media, 2013). [Google Scholar]
- 38.Shirshin E. A., Cherkasova O. P., Tikhonova T., Berlovskaya E., Priezzhev A. V., Fadeev V., “Native fluorescence spectroscopy of blood plasma of rats with experimental diabetes: identifying fingerprints of glucose-related metabolic pathways,” J. Biomed. Opt. 20(5), 051033 (2015). 10.1117/1.JBO.20.5.051033 [DOI] [PubMed] [Google Scholar]
- 39.Monici M., Pratesi R., Bernabei P. A., Caporale R., Ferrini P. R., Croce A. C., Bottiroli G., “Natural fluorescence of white blood cells: spectroscopic and imaging study,” J. Photochem. Photobiol., B 30(1), 29–37 (1995). 10.1016/1011-1344(95)07149-V [DOI] [PubMed] [Google Scholar]
- 40.Croce A., Bottiroli G., “Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis,” Eur. J. Histochem. 58(4), 2461 (2014). 10.4081/ejh.2014.2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang S., Heikal A. A., Webb W. W., “Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein,” Biophys. J. 82(5), 2811–2825 (2002). 10.1016/S0006-3495(02)75621-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skala M. C., Riching K. M., Gendron-Fitzpatrick A., Eickhoff J., Eliceiri K. W., White J. G., Ramanujam N., “In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia,” Proc. Natl. Acad. Sci. 104(49), 19494–19499 (2007). 10.1073/pnas.0708425104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alfonso-García A., Smith T. D., Datta R., Luu T. U., Gratton E., Potma E. O., Liu W. F., “Label-free identification of macrophage phenotype by fluorescence lifetime imaging microscopy,” J. Biomed. Opt. 21(4), 046005 (2016). 10.1117/1.JBO.21.4.046005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galbán J., Sanz-Vicente I., Navarro J., De Marcos S., “The intrinsic fluorescence of FAD and its application in analytical chemistry: a review,” Methods Appl. Fluoresc. 4(4), 042005 (2016). 10.1088/2050-6120/4/4/042005 [DOI] [PubMed] [Google Scholar]
- 45.Alturkistany F., Nichani K., Houston K. D., Houston J. P., “Fluorescence lifetime shifts of NAD(P)H during apoptosis measured by time-resolved flow cytometry,” Cytometry 95(1), 70–79 (2019). 10.1002/cyto.a.23606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaefer P. M., Kalinina S., Rueck A., von Arnim C. A., von Einem B., “NADH Autofluorescence — A Marker on its Way to Boost Bioenergetic Research,” Cytometry 95(1), 34–46 (2019). 10.1002/cyto.a.23597 [DOI] [PubMed] [Google Scholar]
- 47.Shah A. T., Cannon T. M., Higginbotham J. N., Coffey R. J., Skala M. C., “Autofluorescence flow sorting of breast cancer cell metabolism,” J. Biophotonics 10(8), 1026–1033 (2017). 10.1002/jbio.201600128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda-Lorenzo I., Dorado J., Lonardo E., Alcala S., Serrano A. G., Clausell-Tormos J., Cioffi M., Megias D., Zagorac S., Balic A., Hidalgo M., Erkan M., Kleeff J., Scarpa A., Sainz B., Jr, Heeschen C., “Intracellular autofluorescence: a biomarker for epithelial cancer stem cells,” Nat. Methods 11(11), 1161–1169 (2014). 10.1038/nmeth.3112 [DOI] [PubMed] [Google Scholar]
- 49.Tripathy U., Steer R. P., “The photophysics of metalloporphyrins excited in their Soret and higher energy UV absorption bands,” J. Porphyrins Phthalocyanines 11(04), 228–243 (2007). 10.1142/S1088424607000291 [DOI] [Google Scholar]
- 50.Li D., Zheng W., Zeng Y., Luo Y., Qu J. Y., “Two-photon excited hemoglobin fluorescence provides contrast mechanism for label-free imaging of microvasculature in vivo,” Opt. Lett. 36(6), 834–836 (2011). 10.1364/OL.36.000834 [DOI] [PubMed] [Google Scholar]
- 51.Shirshin E. A., Gurfinkel Y. I., Priezzhev A. V., Fadeev V. V., Lademann J., Darvin M. E., “Two-photon autofluorescence lifetime imaging of human skin papillary dermis in vivo: assessment of blood capillaries and structural proteins localization,” Sci. Rep. 7(1), 1171 (2017). 10.1038/s41598-017-01238-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Briglia M., Rossi M. A., Faggio C., “Eryptosis: ally or enemy,” Curr. Med. Chem. 24(9), 937–942 (2017). 10.2174/0929867324666161118142425 [DOI] [PubMed] [Google Scholar]
- 53.Delaby C., Rondeau C., Pouzet C., Willemetz A., Pilard N., Desjardins M., Canonne-Hergaux F., “Subcellular localization of iron and heme metabolism related proteins at early stages of erythrophagocytosis,” PLoS One 7(7), e42199 (2012). 10.1371/journal.pone.0042199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gozzelino R., Jeney V., Soares M. P., “Mechanisms of cell protection by heme oxygenase-1,” Annu. Rev. Pharmacol. Toxicol. 50(1), 323–354 (2010). 10.1146/annurev.pharmtox.010909.105600 [DOI] [PubMed] [Google Scholar]