Abstract
Objectives
This feasibility study aimed to assess the acceptability of inspiratory muscle training (IMT) in people with chronic obstructive pulmonary disease (COPD) who declined pulmonary rehabilitation (PR) as a potential treatment option or precursor to PR. Objectives were to assess attitudes to IMT, PR and alternatives to PR; factors influencing adherence with IMT and acceptability of outcome measures, research tools and study protocol.
Design
A pragmatic, mixed methods, prepost feasibility study was conducted. Recruitment took place over a 4-month period. Participants were followed up for a period of 6 months.
Settings
IMT sessions and assessments were conducted in the domiciliary setting.
Participants
Inclusion criteria: people over the age of 35, stable COPD, Medical Research Council Dyspnoea scale of 3 or above, declined PR. Exclusion criteria: history of spontaneous pneumothorax, incomplete recovery from a traumatic pneumothorax, asthma, known recently perforated eardrum, unstable angina, ventricular dysrhythmias, cerebrovascular event or myocardial infarction within the last 2 months. Participants were selected from a purposive sample. Of the 22 potential participants screened, 11 were recruited and interviewed. Ten participants commenced IMT. Seven participants completed the follow-up assessment.
Intervention
Eight weeks of IMT twice a day, 5 days a week with visits once weekly by a physiotherapist. Unsupervised IMT twice a day three times a week until follow-up at 6 months.
Outcomes
Acceptability of IMT and the study process was explored via semi-structured interviews. Adherence with IMT was assessed by the Powerbreathe K3 device and participant diaries. Uptake of PR was identified.
Results
IMT was found to be acceptable. Adherence was explored. Four people went on to participate in PR.
Conclusions
Feasibility was established. A randomised controlled trial is warranted to establish efficacy and cost-effectiveness of IMT in those who decline PR and IMT as an intervention to promote uptake of PR.
Trial registration number
Keywords: chronic airways disease, pulmonary rehabilitation, inspiratory muscle training, adherence, rehabilitation medicine, uptake
Strengths and limitations of this study.
The prepost design was appropriate to establish feasibility.
Mixed methods allowed triangulation of data.
Objectives were met and inspiratory muscle training was found to be acceptable.
The feasibility study design generated sufficient data to warrant a larger pilot study.
Study design and sample size were appropriate to establish feasibility only, inference of results is not recommended.
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with high levels of morbidity and mortality and is a significant burden on health resources.1 Pulmonary rehabilitation (PR) is a highly evidence-based, cost-effective therapeutic intervention involving education and supervised exercise for people with COPD.2–4 PR should be offered to people with COPD with functionally limiting breathless and can be repeated annually.1 However, PR uptake is poor. The national PR audit5 identified that in the 2013/2014 financial year, of the estimated 446 000 people with COPD with Medical Research Council (MRC) Dyspnoea scale of 3 or above in England and Wales, there were approximately 68 000 referrals for PR and only 42% of those referred went on to complete the programme. Some factors associated with PR non-adherence have also been linked to higher levels of healthcare utilisation and poor compliance with other therapies.6–8 Interventions to enhance PR uptake9 10 must take into account factors associated with disengagement, such as anxiety and beliefs about capability.11
Inspiratory muscle training (IMT) may be an acceptable treatment choice for those who decline PR, particularly for those with severe COPD or those who fear exercise-induced breathlessness, as it can be performed while seated in the home.12 IMT involves increasing the workload of the inspiratory muscles by inspiring against an external targeted load.13 Inspiratory muscle weakness is common in COPD14 15 and IMT has been shown to strengthen the inspiratory muscles demonstrated by an increase in maximal inspiratory pressure (PiMax).16
There is some debate surrounding the theory of IMT17 and the British Thoracic Society guidelines18 do not currently advocate IMT as a precursor to PR or as an alternative for those who decline PR. However, meta-analyses of IMT for COPD has demonstrated clinical benefits.13 16 19–21 Dyspnoea has been seen to improve in people with COPD when IMT has been used alone,16 19 in conjunction with exercise13 and following PR.22 23 Significant improvements in health-related quality of life (HRQOL) of people with COPD have been demonstrated when IMT was compared with control16 19 and when IMT was used following PR.23 However HRQOL was not enhanced by adding IMT to exercise.16 Meta-analyses found exercise tolerance to improve significantly when IMT was compared with control.13 16 Evidence surrounding improvements in exercise tolerance when IMT is added to exercise therapy is conflicting and appears to be dependent on baseline inspiratory muscle weakness.13 16 20 A multicentre randomised controlled trial (RCT)24 added IMT to PR for patients with baseline weakness. Although no difference was found in the primary outcome measure (6 min walk test), they found a 50% increase in their secondary measure (constant workrate endurance cycling) and reduced dyspnoea at isotime on the cycle test in those receiving IMT in addition to PR. One meta-analysis has compared outcomes of IMT in those with a baseline inspiratory muscle pressure of above and below 60 cmH2O and found that initial PiMax did not seem to affect results.21 Evidence on the impact of IMT on healthcare utilisation in people with COPD is limited. One RCT25 found that IMT did not reduce admissions but reduced length of stay and primary care consultations. However, the study was small (n=42) and although participants were not receiving an additional exercise programme, it was not clear whether participants had declined PR.
If IMT is found to be acceptable as a precursor or alternative to PR for those who decline, there could be cost savings to the NHS and clinical benefits to users. A comprehensive analysis of the clinical-effectiveness and cost-effectiveness of IMT in people with COPD who decline PR is warranted, commencing with a feasibility study to establish adherence and acceptability in this potentially non-concordant and disengaged population.
Aim
This feasibility study aimed to assess the acceptability of IMT in people with COPD who declined PR.
Objectives
To assess attitudes to IMT, PR and other potential alternatives to PR.
To assess factors that influence adherence with IMT.
To assess acceptability of outcome measures, research tools and study protocol.
Methodology
The feasibility study was a pragmatic exploratory study incorporating a mixed methods approach using quantitative and qualitative research methods.26 27 The study design was a non-blinded pretest post-test single-group experimental design28 with qualitative data collected by conducting semi-structured interviews.29 The COM-B theoretical framework was used to aid coding and analysis.11 Triangulation was conducted using the protocol adopted by Farmer et al.30
Method
Sample
The feasibility study aimed to recruit 10 people declining PR who agreed to IMT. People who declined the feasibility study were asked if they would be interviewed to explore barriers to participation. A small sample size was deemed appropriate to establish acceptability. Purposive sampling31 was implemented, identifying service users declining PR. Inclusion criteria: people over the age of 35, with stable COPD (having had no exacerbation needing antibiotics or steroids in the preceding 4 weeks) with breathlessness on MRC Dyspnoea scale32 of 3 or above and who declined PR. Exclusion criteria were: history of spontaneous pneumothorax, incomplete recovery from a traumatic pneumothorax, asthma, known recently perforated eardrum, unstable angina, ventricular dysrhythmias, cerebrovascular event or myocardial infarction within the last 2 months. The research was performed within Community Services, Sheffield Teaching Hospitals (STH) NHS Foundation Trust. All assessments and IMT were conducted in the participant’s own home. Three of the interviews were conducted with spouses present.
Recruitment
Eligible participants were identified by clinicians within Community Services and the Respiratory Medicine Directorate of Sheffield Teaching Hospitals. Participants expressing an interest were then approached by the research team via telephone and sent the participant information sheet and consent form. A mutually convenient date was set to gain informed consent in person. Recruitment took place over a 4-month period.
Intervention
Participants performed 8 weeks of IMT strength training using the Powerbreathe K3 device. Training was progressed within the participant’s capability up to 60% PiMax. Participants inhaled through the mouthpiece of the device at high velocity from residual volume to total lung capacity, 30 times, twice a day, 5 days per week.33 Inhalations through the device were initially paced every 30 seconds to assess symptoms and capability. Participants then aimed to progress to three sets of 10 consecutive inhalations through the device (reducing training time to under 5 min). Training was titrated and progressed in accordance with the weekly PiMax assessment by the physiotherapist (independent from the research team). This weekly visit for the first 8 weeks also allowed supervision of IMT to ensure optimal technique and that 30 inhalations could be achieved on the new settings. After 8 weeks training, the participants were reassessed and advised to continue training unsupervised, twice a day, three times per week for a further 18 weeks33 until 6-month follow-up. In the event that training was interrupted due to an exacerbation of COPD, participants had the option to recommence IMT and continue with the remainder of the study.
Patient and public involvement
The original research question was developed following anecdotal feedback from service users who had declined PR. The Sheffield ‘Breathe Easy Group’ and the STH Therapeutics and Palliative Care Patient Panel were consulted. Two people with COPD were also invited to be on the steering group. This PPI contributed to acceptability of study design, choice of outcome measures and development of participant information and consent forms. The results of the study were disseminated to the Breathe Easy Group. Study participants will each receive a copy of the publication.
Outcome measures
Recruitment
Recruitment and rate of attrition data were collected to establish feasibility.
Adherence
Adherence with IMT was measured using the electronic Powerbreathe K3 and participant diaries. The K3 records number of sessions, load, power, volume and T-Index (an indicator of effort). The diary was used to record the number of sessions completed and duration of training. Adherence data were collected at each visit during the 8-week training period by the physiotherapist and monthly in the follow-up period by a therapy assistant.
Acceptability
Semi-structured interviews lasting approximately an hour were conducted at baseline. The interview topic guide was piloted within the feasibility study and explored attitudes to IMT, treatment preferences and opinions regarding study design and outcome measures measured at baseline, 8 weeks and 6 months. Interviews were recorded and transcribed verbatim. A shorter follow-up interview was conducted at 6 months addressing attitudes to the IMT intervention and study design and engagement or future intention to engage with other services (eg, PR).
The acceptability of the following outcome measures (to be used in any subsequent RCT) were also assessed.
Validated questionnaires included the self-administered Chronic Respiratory Disease Questionnaire,34 the COPD Assessment Test,35 the Hospital Anxiety and Depression scale36 and the EQ5D.37 Participant diaries were used to measure healthcare utilisation (antibiotic/steroid use). Spirometry was performed according to professional38 and national39 guidelines using the MicroMedical MicroLab. Spirometry was used to establish severity and to monitor progression of COPD throughout the study. Inspiratory muscle strength (PiMax) was measured from residual volume using the MicroRPM (Micromedical). PiMax was also measured weekly during the first 8 weeks in order to establish progression of training and titrate training intensity. Limitations of measuring PiMax40 were addressed by measuring sniff nasal pressure which reduces the occurrence of falsely identifying inspiratory muscle weakness.41 Activity monitoring was measured using the validated Sensewear (Bodymedia) accelerometer.42 The device captured total energy expenditure in calories, number of steps, time spent sitting, time spent lying, time spent sleeping, average metabolic equivalent of task (METS), active energy expenditure (calories used above 3 METS) and duration of moderate and above moderate physical activity (time spent above 3 METS). The device was worn for 7 days with data analysed from five full days of use.
Outcome measures were measured at baseline, 8 weeks and 6 months, with the exception of the EQ5DL, which was measured at baseline and at 6 months as would reflect extrapolation of utility store in a larger study.
Data analysis
Qualitative data obtained from the semi-structured interviews was analysed using NVivo V.11 software (QSR International). Framework analysis43 using the COM-B theoretical framework11 was employed in the development of the coding tree. Adherence rate was calculated by dividing the number of sessions completed by the prescribed number of sessions. Descriptive statistics were used to interpret recruitment and attrition rate.
Reflexivity
The interviewer was Cath O’Connor, a female Clinical Specialist Respiratory Physiotherapist (MSc Clinical Research, MSc Respiratory Physiotherapy) with postgraduate training in qualitative research. The interviewer had clinical experience in both IMT and PR. The interviewer had no relationship with those involved prior to the study and the goals of the research were explained during the informed consent process.
Ethics
The study was conducted in accordance with standards set out in Good Clinical Practice (GCP)44 and Department of Health guidelines.45
Results
Recruitment and attrition
Eleven participants consented to IMT (figure 1) (recruitment rate 2.5 per month). An 11th participant was recruited due to the dropout of a participant before baseline assessment. Demographics are displayed in table 1. Attrition rate was 27 % at 8 weeks and 36% at 6 months (figure 1).
Figure 1.
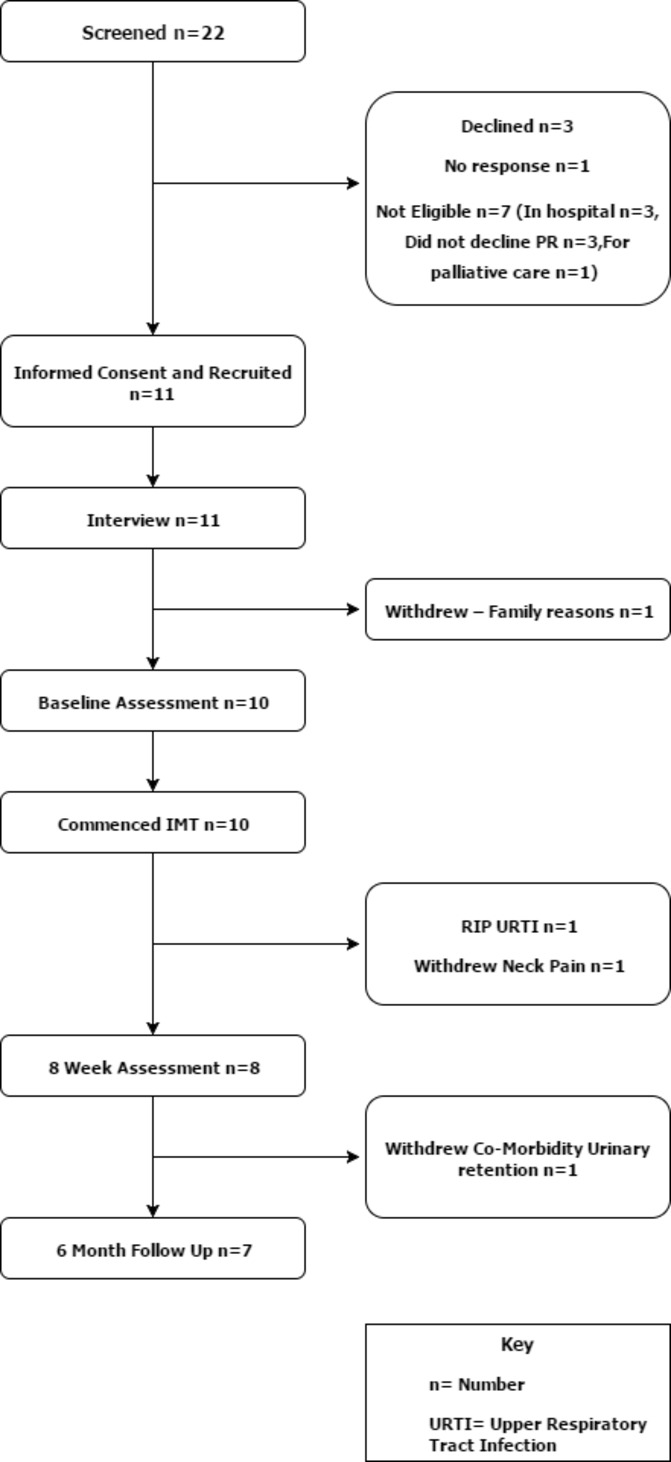
Consort diagram. IMT, inspiratory muscle training; n, number; URTI, upper respiratory tract infection.
Table 1.
Demographics and baseline observations
| Participant | Age (years) |
Gender | BMI | Modified Borg dyspnoea score at rest | SaO2 on air (%) | FEV1 (L) | FEV1 % predicted | VC (L) | PiMax (cmH2O) | Sniff pressure (cmH2O) |
| 1 | 59 | M | 23.2 | 2 | 95 | 0.85 | 31 | 3.64 | 58 | Declined |
| 2 | 72 | F | x | 4.5 | 90 | 0.58 | 35 | 1.56 | 39 | Declined |
| 3 | 65 | M | 22 | 3 | 94 | 0.84 | 29 | 2.86 | 57 | 11 |
| 4 | 62 | M | 22.6 | 3 | 93.5 | 1.47 | 52 | 2.91 | 80 | 59 |
| 5 | 75 | F | 38.7 | 0 | 92 | 0.84 | 40 | 1.85 | 39 | 15 |
| 6 | 68 | M | 32.7 | 1 | 88.5 | 0.61 | 23 | 1.35 | 31 | Declined |
| 7 | 58 | F | 32 | 2 | 94 | 1.35 | 59 | 3.34 | 63 | Unrepeatable |
| 8* | 56 | F | x | x | x | x | x | x | x | X |
| 9 | 71 | M | 24.7 | 3 | 90 | 0.66 | 25 | 1.53 | 72 | Declined |
| 10 | 67 | F | 29 | 0 | 95 | 1.1 | 62 | 2.33 | 60 | 52 |
| 11 | 71 | M | 23.7 | 0.5 | 88.5 | 0.75 | 30 | 3.21 | 36 | Unrepeatable |
*Participant dropped out after initial interview prior to baseline measures.
BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PiMax, maximum inspiratory pressure; SaO2, oxygen saturation; VC, vital capacity.
Adherence
Eight participants completed the 6 months IMT training programme, however, only seven were available for follow-up assessment. Adherence data are reported in table 2. Results were skewed due to overtraining by some participants. Participants training ranged between 48% and 126% of their expected sessions in the first 8 weeks with 481 sessions out of an expected 579 completed. Overall adherence with prescribed sessions for the 6-month period was 76% (not including overtraining). In the supervised period, three participants (33%) performing IMT had completed all of their prescribed sessions and seven (77%) completed >70% of sessions. In the unsupervised period (weeks 9–26), 905 sessions out of an expected 987 were completed. Three participants (37.5%) had completed all of the prescribed sessions in the follow-up period and four (50%) had completed >70%. Delays surrounding device retrieval accounted for an increase in expected sessions for some participants; this was attributed to staff availability.
Table 2.
Adherence
| Participant | Weeks 1–8 | Weeks 9–26 | ||||||
| Days | Sessions | Expected sessions | Adherence (%) | Days | Sessions | Expected sessions | Adherence (%) | |
| 1 | 30 | 53 | 42 | 126 | x | x | x | x |
| 2 | 58 | 63 | 81 | 77.8 | 185* | 70 | 159.1 | 44 |
| 3 | 58 | 52 | 81.2 | 64 | 120 | 67 | 103.2 | 65 |
| 4 | 45 | 57 | 63 | 90 | 120 | 58 | 103.2 | 56 |
| 6 | 52 | 77 | 72.8 | 106 | 144 | 201 | 123.84 | 162 |
| 7 | 50 | 64 | 70 | 91.4 | 154 | 95 | 132.44 | 72 |
| 9 | 47 | 59 | 65.8 | 90 | 142 | 188 | 122.12 | 154 |
| 10 | 50 | 73 | 70 | 104 | 152 | 201 | 130.72 | 154 |
| 11 | 54 | 36 | 75.6 | 48 | 131 | 25 | 112.66 | 22 |
*Delay in equipment collection.
Intensity and effects of IMT
Intensity of IMT was recorded weekly for the first 8 weeks and then after approximately every 30 sessions (K3 data storage 36 sessions). The load for IMT was progressed weekly for the first 8 weeks. Progression of training (figure 2) was limited by exacerbations of COPD and other comorbidities. P9 was ill in week 1 and therefore commenced training in week 2. P11 was ill in week 3 and did not perform any sessions, therefore the load is recorded as 0. P3 developed comorbidities that impacted on training and worsened as the study progressed. Data regarding intensity of IMT are displayed in figures 3 and 4. Figures 5-7 display training data (based on the load set at the 8-week assessment) in relation to PR uptake and improvement in PiMax. P7 was ill at the 8-week assessment and therefore training load for weeks 9–26 was titrated according to the previous training session’s recorded PiMax. One participant particularly struggled with training progression due to anxiety and recurrent exacerbation (P11).
Figure 2.
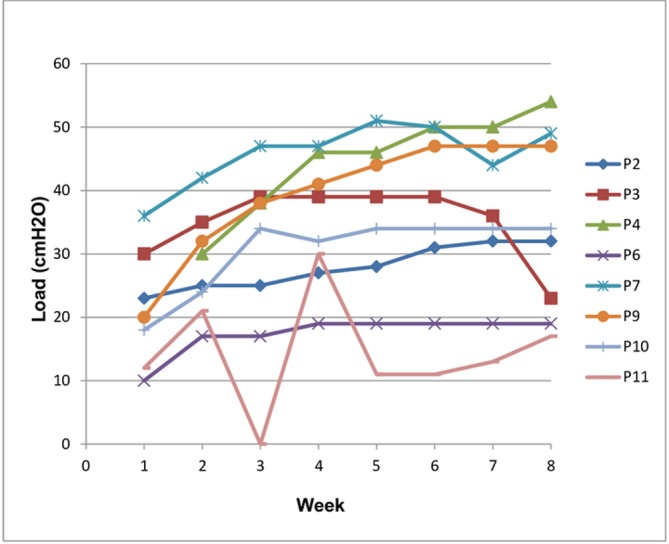
Training progression: load (weeks 1–8). P, participant.
Figure 3.
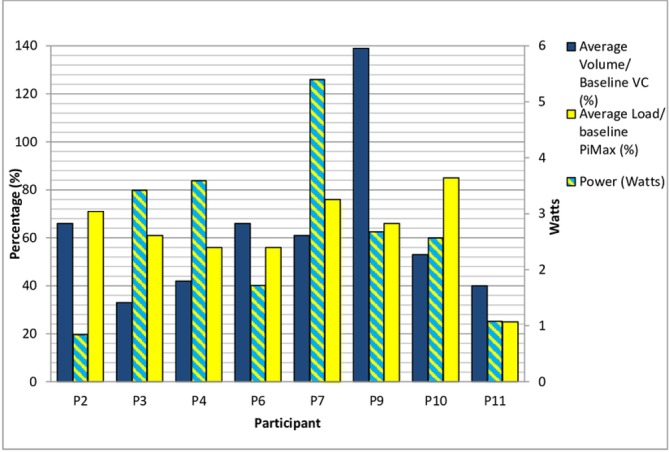
Training intensity: average load, volume and power (weeks 1–8). PiMax, maximal inspiratory pressure; VC, vital capacity.
Figure 4.
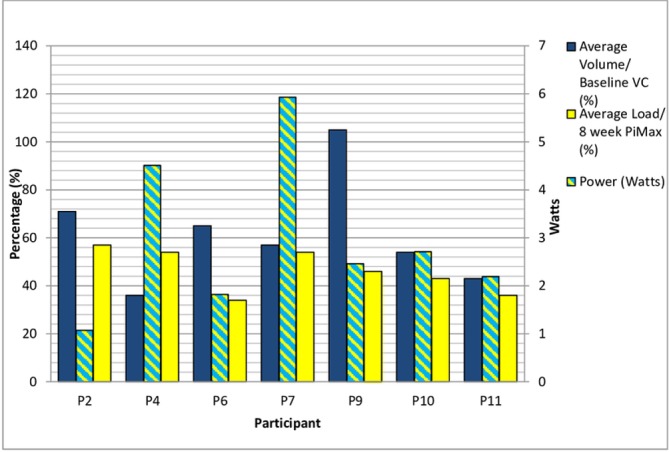
Training intensity: average load, volume and power (weeks 9–26). PiMax, maximal inspiratory pressure; VC, vital capacity.
Figure 5.
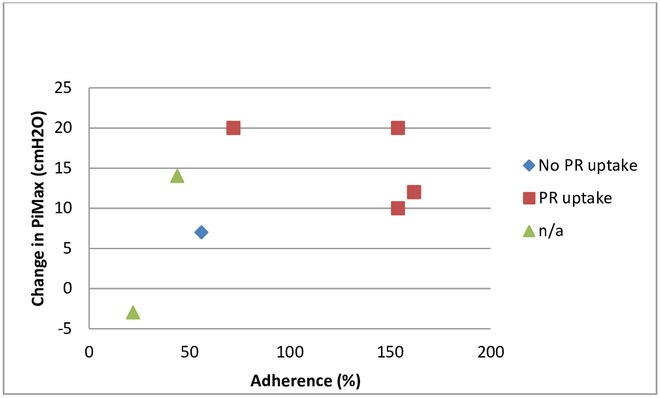
PR uptake, adherence (weeks 9–26) and change in PiMax at 6 months. n/a, not appropriate; PiMax, maximal inspiratory pressure; PR, pulmonary rehabilitation.
Figure 6.
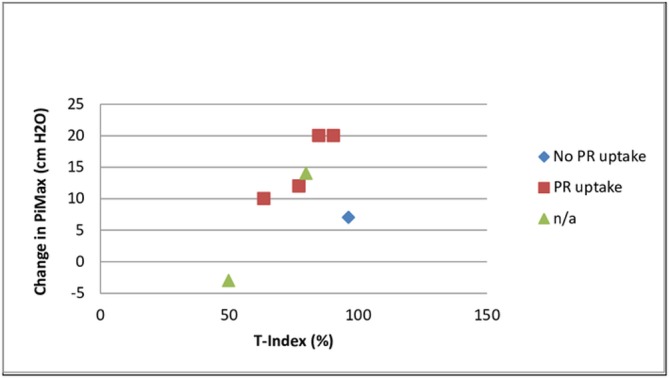
PR uptake, T-Index (weeks 9–26) and change in PiMax at 6 months. n/a, not appropriate; PiMax, maximal inspiratory pressure; PR, pulmonary rehabilitation; T-Index, amount of work achieved during a session expressed as a percentage of the maximal potentially achievable work.
Figure 7.
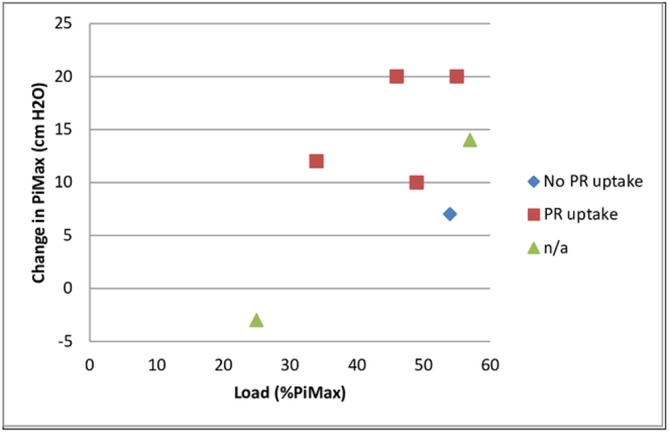
PR uptake, training load (weeks 9–26) and change in PiMax at 6 months. n/a, not appropriate; PiMax, maximal inspiratory pressure; PR, pulmonary rehabilitation.
Interview data
Interview data surrounding acceptability was analysed using the COM-B theoretical framework,11 which defines data in terms of Capability (psychological and physical), Opportunity (social and physical) and Motivation (reflective and automatic) and the impact that these have on Behaviour. The COM-B framework was used to establish why PR had been declined and whether IMT was acceptable. Table 3 summarises the key barriers to PR and IMT arising from the qualitative analysis.
Table 3.
Barriers to IMT and PR
| COM-B domain | |||
| Capability | Opportunity | Motivation | |
| PR | Poor retention of PR information Breathlessness Physical limitation |
Travel/Transport Age stigma Lack of support Time |
PR not seen to be necessary Concerns about breathlessness on exercise |
| IMT | Physical limitation Recurrent infections |
Technical issues | No barriers identified |
IMT, inspiratory muscle training; PR, pulmonary rehabilitation.
Reasons for declining PR
Capability
The interviews highlighted a lack of information or poor retention of information surrounding PR. Reasons for declining PR included reporting no recollection of being offered the service, physical limitations and misinformation surrounding PR location. One participant dropped out after the assessment session of PR, as they had not perceived that the PR group exercise sessions would be different to the assessment walk test:
I thought I could do that at home…but they didn’t explain anything else after… I just come home and I just though well I could walk round cones in here can’t I…they didn’t explain anything to me. P10 baseline
Some participants did not feel that they had the physical capability to do PR. Three participants were housebound. One participant had an above knee amputation, one had severe arthritis and another suffered from angina and had restricted mobility. Breathlessness was perceived to be a significant physical barrier to exercise, although one participant felt that this could have been addressed if exercise was performed at the right level. Two participants had been advised against doing exercise in relation to physical capability, one by a physiotherapist and another by a doctor.
Opportunity
Participants did not feel that they were socially supported to attend PR. Those with supportive families were reluctant to ask for help. Others reported less support and understanding from family members.
Some of the younger participants felt that age influenced their decision to decline group PR:
I just sometimes think when I go to places like that, it’s telling me I’m past my sell by date (laughs). P10 baseline
One participant overcame their concerns about the age group by making a ‘buddy’. Recommendations were made by one of the younger participants for a PR group orientated towards those under 60 years. However, two of those stating that they felt too young for the PR group were over 60 years. It was perceived that a group for younger participants, or those with less severe COPD, might have allayed fears that participants expressed about the prognosis of COPD and prospective use of oxygen.
Eight participants interviewed at baseline mentioned that physical opportunity in terms of transport was a barrier to group PR. Concerns were raised about having to get more than one bus to their nearest venue, distances between drop-off and venue, especially if a hill was involved, and the additional weight of carrying oxygen on the bus:
If I was to go to Springs (PR venue) now, because it’s right at the top of the hill I would have to get a bus into town, then get the tram to Manor Top. P01 baseline
One participant stated that the combined travel time and class time was a waste and that the exercise could be performed at home. Not all participants were aware of community transport. Of those who knew about community transport, one participant felt that it would be too expensive, and two others felt that there was an age stigma associated with the service. The two participants who owned their own car did not perceive transport to be a barrier to PR.
Motivation
Participants declining PR felt that they were doing activities and did not all feel that PR was necessary:
Well I go out every day for a walk round anyway so… I can do it I’ll do it myself. P04 baseline
Breathlessness on exercise was a concern to the majority of participants, although it was unclear from the interviews whether these emotional concerns were reflective or automatic. Supervision by staff experienced with COPD management and tailored programmes were felt to be a necessity with regard to safety of exercise. However, concerns associated with travelling to a PR venue superseded this need for supervision.
Acceptability of IMT
Capability
Knowledge of IMT was extremely limited, most having never heard of the device prior to the study yet despite this, lack of knowledge was not perceived to be a barrier to IMT:
I only saw it in paper…and I thought oh that’s alright…so I think it was £135.99 or something like that but I’d have paid it…If I’d thought it were going to do the trick…you’d pay it wouldn’t you. P10 baseline
Participants were not aware that IMT devices were available on prescription and concern was raised about the lack of information about the options available given to people with COPD. Participants stated that they would like to be made aware of all management options open to them.
Prior to the study, participants felt that if a prompt to use IMT was required it would be from a spouse. Participants mentioned that the development of routine would reinforce their behaviour and this was reiterated at follow-up.
The majority of those interviewed felt that they had the physical capability and skills to use IMT. Problems encountered included initial difficulty with the IMT technique (quickly resolved) and thirst on performing the training (alleviated by having a drink at hand). Neck pain after using the device was reported by a participant with severe arthritis and one participant cited recurrent infections as a barrier to adherence. No other physical limitations were reported.
Opportunity
IMT participants felt supported by staff, yet some felt they would be reluctant to ring staff if there was a problem.
Factors that influenced acceptability and adherence of IMT included problems with the IMT device included the inspiratory valve sticking and problems with the filters, particularly when training load exceeded 50% PiMax. These issues were addressed by changing the filters, having the devices checked and, where appropriate, lowering the level of resistance. One participant reported that the battery ran out quickly. Others reported no problems.
Interviewees felt that they would have the time to do IMT, although commitments varied significantly with some doing voluntary work or attending appointments and others having no commitments and welcoming a new activity. The environment of therapy appeared to have a high impact on acceptability of IMT. Interviewees liked the fact that they could do the treatment in their own home:
(It’s) a lot better really because I haven’t go the get up and go to go (out) and it frustrates me, everything does so therefore with the way I am, it’s been better. P02 follow-up
One participant would not have got involved in the study if it had not been in their home due to issues with the weather and time of year. If a treatment was to be in a venue, most participants felt that this should be at their local GP surgery.
Motivation
A number of participants reported symptoms of depression, including low self-esteem. However, confidence to undertake IMT was high. Goals of IMT included being in control of the breathlessness, associated with activities of daily living, hobbies and social and leisure activities:
I just would love to be that little bit better…so I can do more…makes me feel good, makes my family proud of me… and spend more time with my grandson. P07 baseline
Participants wanted to improve their health and goals linked closely with beliefs about the consequences of IMT.11 46 Participants viewed IMT as a novel therapy and were willing to give IMT ‘a chance’. In contrast to peripheral exercises, it was recognised that IMT could directly impact on breathing. Despite several of the participants suffering from anxiety, no specific concerns about IMT were raised. Participants reported that subjective and objective improvements in health status would reinforce IMT behaviour and felt that IMT was important if it could help their symptoms.
At 6 months follow-up, three participants described benefits at the end of the study in terms of breathing efficiency, increased exercise tolerance, improved inhaler technique and reduced inhaler usage:
I weren’t going out at all…something’s given me the confidence and I think it was this (IMT) you know… Oh I’m more or less going out once a week now. P02 follow-up
One participant found benefits with IMT initially in terms of increased activity, but felt that these did not last. Three others perceived no benefit with IMT. Adherence with IMT was affected for one particularly anxious participant who had not used the device due to fear that it would cause infection; however, at 6 months follow-up this fear was reported to have been unfounded. Two participants had used the device but attributed lack of benefit to other comorbidities or infections during the study.
Behaviour
The target behaviour was adherence with the IMT intervention. Adherence was variable and reduced once supervision ceased (table 2). However, some participants overtrained during the unsupervised maintenance period due to perceived benefits. Five participants accepted the offer for an IMT device to be prescribed for long-term usage when offered and four participants went on to participate in PR. All four participants who went on to attend PR had an IMT adherence rate of >70% (three were 100% adherent) at the end of the 6-month study period and two had the highest gains in PiMax (20 cmH2O) (figure 5).
Triangulation
Convergence between findings regarding acceptability of the IMT intervention and study protocol is presented in table 4. Agreement was found on five components with partial agreement on four components.30 The IMT intervention was found to be acceptable by the majority of participants who commenced treatment, and this was supported by adherence with the device. The study protocol was generally acceptable with the exception of the paper diary and sniff testing (table 4).
Table 4.
Triangulation—acceptability of treatment and protocol
| Quantitative | Qualitative | Convergence | |
| IMT acceptability/adherence | High adherence rates. Few devices replaced due to malfunction. One withdrawal due to adverse effect. Five requested an IMT device. Four participants went on to participate in PR. |
Participants motivated at baseline to use the device with positive beliefs about consequences, goals and reinforcement. Few technical problems reported. Feedback surrounding IMT was positive, particularly that treatment was within the home setting. |
Partial agreement |
| Interviews | All IMT participants agreed to interview at baseline. | Well received. | Agreement |
| Diary | Few completed. | Diaries acceptable at baseline. At follow-up, participants forgot or did not wish to fill in the diary. Mixed views given regarding its value. | Partial agreement |
| Questionnaires | Completed—few missing values. | No problems reported. | Agreement |
| Accelerometer | Majority had no problem and were adherence with the device. One participant had eczema and could not wear the armband. | Some concerns raised at baseline. One participant thought it may be mistaken for an ‘ASBO’ device. No problems voiced by those who wore device. Accelerometry was preferable to a walk test. | Partial agreement |
| PiMax testing | Completed with no adverse effects. | No problems were reported in performing the inspiratory muscle strength manoeuvres. | Agreement |
| Sniff test | Varied completion and poor reliability. | Most participants were accepting of the sniff test. | Partial agreement |
| Spirometry | Completed with no adverse effects. | Participants reported that they had previously had difficulties with spirometry within their general care. No problems experienced during study spirometry. | Agreement |
| Visits | Visits cancelled mainly due to participant illness. | Satisfied with physiotherapists. The duration of the IMT session was acceptable. The number and duration of visits were acceptable. Feedback about staff was positive. | Agreement |
IMT, inspiratory muscle training.
Discussion
Barriers to PR echoed previous findings6 47 with transport difficulties in particular a significant limitation to group PR attendance, but not an issue for receiving IMT. A key area that limited acceptance of PR was lack of knowledge of both its benefits and what the programme entails. Knowledge and comprehension of therapies was poor, whereas the desire to know about potential treatment options was high. This may potentially mean that information was not given or as others have found that it was not retained.48 This highlights the need for effective methods of education and information delivery.
IMT was found to be acceptable. Fewer barriers to IMT were identified in comparison to PR in terms of Capability, Opportunity and Motivation. Participants were capable of learning the skills of IMT and, with the exception of one participant who dropped out with severe arthritis, physically capable of using the device. Participants had the physical and social opportunity to use IMT in terms of space and time. In keeping with barriers to PR,6 depression and anxiety were cited as factors associated with dropout and non-adherence with IMT in this study. These barriers must be addressed in order to promote engagement with therapies. Despite depression and frustration associated with their condition, participants were enthused and felt capable to try a device that did not involve a physical exercise assessment or general physical activity. Self-efficacy varied depending on the prospective target behaviour,49 participants reported low self-efficacy with PR and higher self-efficacy with IMT. All participants were motivated to use IMT at baseline, however, motivation varied at follow-up and was associated with reinforcement in terms of perceived benefit. There are few qualitative studies surrounding IMT to compare these findings. Hoffman et al50 studied qualitative outcomes rather than acceptability.
In keeping with the findings of Hoffman et al50 (who conducted interviews with participants after 8 weeks of IMT), some participants reported improvements with IMT in terms of breathlessness and activity. In addition, Hoffman et al50 found improvements in communication, whereas we found self-reported improvement in improved inhaler technique and reduced inhaler usage. Communication was not explored in this study, but may be explored in prospective research.
IMT was felt to hold greater value than PR and lack of perceived benefit of PR reflected previous findings.6 People felt that they were already as active as they could be, whereas IMT was novel. In addition, the value of IMT in terms of targeting the inspiratory muscles to relieve breathlessness was more readily perceived than exercising peripheral muscles, such as the quadriceps.
To the authors’ knowledge, PR uptake following IMT has not been measured previously. Four of the participants in this feasibility study went on to participate in PR after commencing IMT. This may have been due to increased knowledge and sense of importance of PR following input from the physiotherapist, enhancement in self-efficacy and confidence by achieving goals in terms of IMT performance or because health gains due to IMT enhanced physical capability to attend. Although not conclusive, there does appear to be a coherent ‘trend’ for those who were more adherent with IMT to attend PR (figure 5). Further research is warranted to identify which specific components of complex interventions meet the needs of the individual in order to enhance participation.
Concerns prior to the feasibility study that by declining PR participants may not be adherent with IMT were unfounded. Adherence with IMT varied and some participants overtrained (weeks 9–26). Several studies have evaluated adherence.24 25 51–57 However, comparison to these studies is limited as they measured supervised sessions only, reported adherence by diary alone and conducted studies of shorter duration. Of those measuring supervised and unsupervised sessions electronically, our adherence at 8 weeks (85%) was lower than the findings of Langer et al58 (95%), but similar at follow-up (76%) with the findings of Charususin et al24 (79.9%). Charususin et al24 did not follow participants beyond 3 months and therefore adherence cannot be directly compared. However, the findings are promising as populations of both Langer et al58 and Charususin et al’s studies24 may have been more motivated as they included people attending PR.
A limitation of the Powerbreathe K3 device was that although it captured the number of sessions performed, it did not report on whether the prescribed 30 inhalations were achieved within each session. The T-Index (figure 3) did to some extent indicate training quality as it represents the amount of work achieved during a session expressed as a percentage of the maximal potentially achievable work. Charususin et al24 identified a significant relationship between improvement in PiMax and training quality during IMT (ie, total inspiratory work performed/session, average peak power/session) as well as progression of training intensity. No formal statistics were performed as our study’s aim was to establish feasibility rather than efficacy of IMT, as reflected by sample size and single group design. However, there do appear to be positive trends between change in PiMax and parameters associated with training intensity and quality (figures 6 and 7). Median change in PiMax was 17 cmH2O (31%) at 8 weeks and 12 cmH2O at 6 months (28%). This compares well with improvements in PiMax of 25% and 29% on systematic reviews of IMT,16 21 but is lower than improvements of IMT being added to PR in the study by Charususin et al (42%).24
Feedback regarding the study process was positive and the IMT recruitment target was met. The main study processes requiring review are two of the outcome measures. The sniff test was declined by some participants. Others received it well, but some scores were unrepeatable. It was felt that this measure was not feasible to be used in any subsequent pilot study. Participant diaries were described as acceptable at the baseline interview, however, at follow-up completion rate was poor. This was in keeping previous findings8 and possibly because the value of the diaries was not clearly perceived by participants. Healthcare utilisation will be measured using healthcare records in any subsequent study.
Strengths and limitations
Strengths of the study were that the aims of the study were met and recruitment to the IMT intervention was successful with limited promotion of the study. In addition, rich data were generated surrounding the acceptability of IMT, the study itself and how IMT may overcome some of the perceived barriers to PR. Although not formally assessed, saturation of data appeared to be met despite the small sample.59 Limitations included sample size and potential bias. Attrition rate was difficult to compare with other studies due to the variations in population and study duration. Our attrition rate was 36% at 6 months compared with 19% at 3 months in a recent multicentre study adding IMT to PR.24 Attrition with IMT was considerably lower than attrition data surrounding PR. High levels of PR attrition in the clinical setting have reflected findings from research.60 Given that attrition from PR was identified as 58% on the National Audit5 and our population were anticipated to have high risk of comorbidity, exacerbations of COPD and social barriers contributing to their declining PR, a high level of attrition might be expected. However, in such a small sample our findings are not transferable. A pilot study is warranted prior to any larger RCT to clarify attrition rate and provide data for sample size calculation. Retention and adherence in a larger RCT may be enhanced by incorporating a behaviour change component to the intervention. Bias may have been introduced in the following ways: respondent validation61 was not employed, coding was conducted by the interviewer alone, reasons for declining the study were not explored and purposive sampling is subject to selection bias.31 Electronic monitoring was used to assess adherence and is the ‘gold standard’ of measuring adherence in inhalation therapy8; however, the K3 device only recorded the number of sessions performed and not the date or time of training limiting interpretation of results.
The pragmatic method and sample size was deemed appropriate to establish feasibility. However, a multiple case study approach31 may have aided linkage of the COM-B domains to adherence with IMT facilitating the identification of barriers to IMT and triangulation. Further research may incorporate realist evaluation in order to illuminate the mechanisms of this complex intervention and the context of implementation.62 63
Conclusions
This feasibility study of IMT in those who declined PR met its aims and was successful in showing that IMT could be easily implemented and IMT proved acceptable to participants. Adherence levels varied and the use of behaviour change techniques may need to be incorporated with IMT in future research. Descriptions of clinical improvement were sufficient to justify a larger randomised trial in order to formally assess clinical and cost-effectiveness in those who decline PR. Barriers to PR were highlighted and consistent with previous findings. Of interest, four participants significantly altered healthcare behaviour and went on to participate in full PR programmes despite their initial refusal, warranting further investigation.
Supplementary Material
Acknowledgments
The authors would like to thank Sarah Lee, Helen Lawrence, Ian Gambling, Ursula Freeman, Lisa Farndon, Irene Mabbott, Paul Norman, Caroline Driscoll, Joan Egley, Julie Rees, Sheffield Breathe Easy Group.
Footnotes
Contributors: CO’C: substantial contributions to the conception and design of the work; the acquisition, analysis, the interpretation of data for the work. Drafting the work and revising it critically for important intellectual content. Final approval of the version to be published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RL andJW: substantial contributions to the design of the work and the interpretation of data for the work. Revising the work critically for important intellectual content. Final approval of the version to be published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. GHM: substantial contributions to the design of the work and revising it critically for important intellectual content. Final approval of the version to be published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: Funding was secured from a Sheffield Teaching Hospitals Medicine Directorate Charitable Fund.
Competing interests: None declared.
Ethics approval: The feasibility study was approved by Sheffield NHS Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon reasonable request.
Patient consent for publication: Not required.
References
- 1. NICE. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care: National Clinical Guideline Centre, 2010. [Google Scholar]
- 2. Puhan M, Scharplatz M, Troosters T, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2009:CD005305 10.1002/14651858.CD005305.pub2 [DOI] [PubMed] [Google Scholar]
- 3. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006:CD003793 10.1002/14651858.CD003793.pub2 [DOI] [PubMed] [Google Scholar]
- 4. Griffiths TL, Phillips CJ, Davies S, et al. Cost effectiveness of an outpatient multidisciplinary pulmonary rehabilitation programme. Thorax 2001;56:779–84. 10.1136/thorax.56.10.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steiner M. Pulmonary Rehabilitation: Time to breathe better. National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: Resources and organisation of Pulmonary Rehabilitation services in England and Wales 2015, 2015. [Google Scholar]
- 6. Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis 2011;8:89–99. 10.1177/1479972310393756 [DOI] [PubMed] [Google Scholar]
- 7. Hunter LC, Lee RJ, Butcher I, et al. Patient characteristics associated with risk of first hospital admission and readmission for acute exacerbation of chronic obstructive pulmonary disease (COPD) following primary care COPD diagnosis: a cohort study using linked electronic patient records. BMJ Open 2016;6:e009121 10.1136/bmjopen-2015-009121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. George J, Kong D, Stewart K. Adherence to Disease Management Programs in Patients with COPD. Int. J. COPD 2007;2:253–62. [PMC free article] [PubMed] [Google Scholar]
- 9. Jones AW, Taylor A, Gowler H, et al. Systematic review of interventions to improve patient uptake and completion of pulmonary rehabilitation in COPD. ERJ Open Res 2017;3:00089-2016 10.1183/23120541.00089-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Young J, Jordan RE, Adab P, et al. Interventions to promote referral, uptake and adherence to pulmonary rehabilitation for people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2017:1–14. [Google Scholar]
- 11. Michie S, Atkins L, West R. The behaviour change wheel a guide to designing interventions: Silverback, 2014. [Google Scholar]
- 12. Thomas MJ, Simpson J, Riley R, et al. The impact of home-based physiotherapy interventions on breathlessness during activities of daily living in severe COPD: a systematic review. Physiotherapy 2010;96:108–19. 10.1016/j.physio.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 13. Lötters F, van Tol B, Kwakkel G, et al. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J 2002;20:570–7. 10.1183/09031936.02.00237402 [DOI] [PubMed] [Google Scholar]
- 14. Bégin P, Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. Am Rev Respir Dis 1991;143:905–12. 10.1164/ajrccm/143.5_Pt_1.905 [DOI] [PubMed] [Google Scholar]
- 15. Polkey MI, Kyroussis D, Hamnegard CH, et al. Diaphragm strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;154:1310–7. 10.1164/ajrccm.154.5.8912741 [DOI] [PubMed] [Google Scholar]
- 16. Gosselink R, De Vos J, van den Heuvel SP, et al. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J 2011;37:416–25. 10.1183/09031936.00031810 [DOI] [PubMed] [Google Scholar]
- 17. Polkey MI, Moxham J, Green M. The case against inspiratory muscle training in COPD. Against. Eur Respir J 2011;37:236–7. 10.1183/09031936.00095510 [DOI] [PubMed] [Google Scholar]
- 18. Bolton CE, Bevan-Smith EF, Blakey JD, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013;68(Suppl 2):ii1–ii30. 10.1136/thoraxjnl-2013-203808 [DOI] [PubMed] [Google Scholar]
- 19. Geddes EL, O’Brien K, Reid WD, et al. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respir Med 2008;102:1715–29. 10.1016/j.rmed.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 20. O’Brien K, Geddes EL, Reid WD, et al. Inspiratory muscle training compared with other rehabilitation interventions in chronic obstructive pulmonary disease: a systematic review update. J Cardiopulm Rehabil Prev 2008;28:128–41. 10.1097/01.HCR.0000314208.40170.00 [DOI] [PubMed] [Google Scholar]
- 21. Beaumont M, Forget P, Couturaud F, et al. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin Respir J 2018;12:2178–88. 10.1111/crj.12905 [DOI] [PubMed] [Google Scholar]
- 22. Weiner P, Magadle R, Berar-Yanay N, et al. The cumulative effect of long-acting bronchodilators, exercise, and inspiratory muscle training on the perception of dyspnea in patients with advanced COPD. Chest 2000;118:672–8. 10.1378/chest.118.3.672 [DOI] [PubMed] [Google Scholar]
- 23. Magadle R, McConnell AK, Beckerman M, et al. Inspiratory muscle training in pulmonary rehabilitation program in COPD patients. Respir Med 2007;101:1500–5. 10.1016/j.rmed.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 24. Charususin N, Gosselink R, Decramer M, et al. Randomised controlled trial of adjunctive inspiratory muscle training for patients with COPD. Thorax 2018;73:942–50. 10.1136/thoraxjnl-2017-211417 [DOI] [PubMed] [Google Scholar]
- 25. Beckerman M, Magadle R, Weiner M, et al. The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest 2005;128:3177–82. 10.1378/chest.128.5.3177 [DOI] [PubMed] [Google Scholar]
- 26. Medical Research Council. A Framework for development and evaluation of RCTs for complex interventions to improve health, 2000. [Google Scholar]
- 27. Campbell NC, Murray E, Darbyshire J, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455–9. 10.1136/bmj.39108.379965.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robson C. Real world research: A resource for social scientists and practitioners-researchers. Oxford (Blackwell: Blackwell Publishers Ltd, 1993. [Google Scholar]
- 29. Pope C, Ziebland S, Mays N. Analysing qualitative data; Qualitative research in health care. BMJ 2000;320:114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farmer T, Robinson K, Elliott SJ, et al. Developing and implementing a triangulation protocol for qualitative health research. Qual Health Res 2006;16:377–94. 10.1177/1049732305285708 [DOI] [PubMed] [Google Scholar]
- 31. Bryman A. Social research methods: University press, 2012. [Google Scholar]
- 32. Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581–6. 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McConnell A, Romer L, Weiner P. Inspiratory muscle training in obstructive lung disease : how to implement and what to expect. Breathe 2005;2:38–49. [Google Scholar]
- 34. Williams JE, Singh SJ, Sewell L, et al. Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR). Thorax 2001;56:954–9. 10.1136/thorax.56.12.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648–54. 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 36. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 37. Wailloo A, Davis S, Tosh J. The incorporation of health benefits in cost utility analysis using the EQ-5D, 2010. [Google Scholar]
- 38. Police Clearance Services. A Guide to Performing Quality Assured Diagnostic Spirometry.
- 39. Improving the quality of diagnostic spirometry in adults : the National Register of certified professionals and operators. 2016.
- 40. Gibson GJ. American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002;166:518–624. 10.1164/rccm.166.4.518 [DOI] [PubMed] [Google Scholar]
- 41. Steier J, Kaul S, Seymour J, et al. The value of multiple tests of respiratory muscle strength. Thorax 2007;62:975–80. 10.1136/thx.2006.072884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Langer D, Gosselink R, Sena R, et al. Validation of two activity monitors in patients with COPD. Thorax 2009;64:641–2. 10.1136/thx.2008.112102 [DOI] [PubMed] [Google Scholar]
- 43. Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13:117 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. ICH. ICH Harmonised Tripartite Guideline for Good Clinical Practice, 1996. [Google Scholar]
- 45. Department of Health. Research governance framework for health and social care. 2nd edn, 2005. [Google Scholar]
- 46. Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7:37 10.1186/1748-5908-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cox NS, Oliveira CC, Lahham A, et al. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: a systematic review using the Theoretical Domains Framework. J Physiother 2017;63:84–93. 10.1016/j.jphys.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 48. Fleming SL, Jones SE, Green S, et al. P43 Patients’ experiences of early post-hospitalisation pulmonary rehabilitation: A quality improvement initiative. Thorax 2013;68:P43–A94. 10.1136/thoraxjnl-2013-204457.193 [DOI] [Google Scholar]
- 49. Bandura A. Self-efficacy - the exercise of control. WH Freeman; 1997. [Google Scholar]
- 50. Assis MG, Augusto VM, Silveira BMF, et al. The effects of inspiratory muscle training based on the perceptions of patients with advanced lung disease: a qualitative study. Brazilian J. Phys. Ther 2017;22:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Larson JL, Kim MJ, Sharp JT, et al. Inspiratory muscle training with a pressure threshold breathing device in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1988;138:689–96. 10.1164/ajrccm/138.3.689 [DOI] [PubMed] [Google Scholar]
- 52. Harver A, Mahler DA, Daubenspeck JA. Targeted inspiratory muscle training improves respiratory muscle function and reduces dyspnea in patients with chronic obstructive pulmonary disease. Ann Intern Med 1989;111:117–24. 10.7326/0003-4819-111-2-117 [DOI] [PubMed] [Google Scholar]
- 53. Covey MK, Larson JL, Wirtz SE, et al. High-intensity inspiratory muscle training in patients with chronic obstructive pulmonary disease and severely reduced function. J Cardiopulm Rehabil 2001;21:231–40. 10.1097/00008483-200107000-00008 [DOI] [PubMed] [Google Scholar]
- 54. Weiner P, Magadle R, Beckerman M, et al. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest 2003;124:1357–64. 10.1378/chest.124.4.1357 [DOI] [PubMed] [Google Scholar]
- 55. Ramirez-Sarmiento A, Orozco-Levi M, Guell R, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med 2002;166:1491–7. 10.1164/rccm.200202-075OC [DOI] [PubMed] [Google Scholar]
- 56. Hsiao SF, Wu YT, Wu HD, et al. Comparison of effectiveness of pressure threshold and targeted resistance devices for inspiratory muscle training in patients with chronic obstructive pulmonary disease. J Formos Med Assoc 2003;102:240–5. [PubMed] [Google Scholar]
- 57. Hill K, Jenkins SC, Philippe DL, et al. High-intensity inspiratory muscle training in COPD. Eur Respir J 2006;27:1119–28. 10.1183/09031936.06.00105205 [DOI] [PubMed] [Google Scholar]
- 58. Langer D, Charususin N, Jácome C, et al. Efficacy of a novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Phys Ther 2015;95:1264–73. 10.2522/ptj.20140245 [DOI] [PubMed] [Google Scholar]
- 59. Morse JM. Determining sample size. Qual Health Res 2000;10:3–5. 10.1177/104973200129118183 [DOI] [Google Scholar]
- 60. Goyder EC, Strong M, Green A, et al. Is a large scale community programme as effective as a community rehabilitation programme delivered in the setting of a clinical trial? BMC Med Res Methodol 2013;13:1–9. 10.1186/1471-2288-13-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lewis J, Ritchie J, Ormston R, et al. Generalising from qualitative reseaarch : Ritchie J, Lewis J, McNaughton Nicholls C, Ormston R, Qualitative research practice: Sage, 2014:347–66. [Google Scholar]
- 62. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Feather JL. Developing programme theories as part of a realist evaluation of a healthcare quality improvement programme. Int J Care Coord 2018;21:68–72. 10.1177/2053434518779753 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


