Abstract
Objective
To understand the importance of electronic nicotine delivery systems (ENDS) product attributes to adult consumers in the USA by age and gender.
Design
Cross-sectional survey with a discrete choice experiment (best–worst, case 2, scaling) of 19 choice tasks in which participants answered what would make them most want to use and least want to use an ENDS product.
Setting and participants
A national sample of adults (aged 18+ years) in the USA who had tried an ENDS product at least once.
Measures
We included 9 ENDS attributes with levels that varied across 19 choice tasks. We performed a multinomial logistic regression to obtain overall importance scores, attribute-level part-worth utilities and most important attribute.
Results
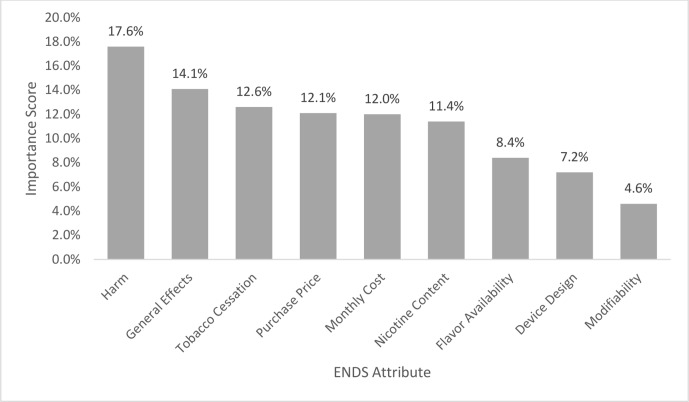
Of 660 participants, 81% were white, 51% women and 37% had at least a 4-year college degree with an average age of 42.0 years (SD ±19.4). The attributes had the following importance: harms of use 17.6%; general effects 14.1%; cessation aid 12.6%; purchase price 12.1%; monthly cost 12.0%; nicotine content 11.4%; flavour availability 8.4%; device design 7.2%; modifiability 4.6%. Harms of use was the most important attribute for all ages and genders (p<0.05); variation in other important attributes existed by age though not by gender.
Conclusion
This study identified the importance of nine ENDS attributes. Perceived harms of use of ENDS use appeared most important, and modifiability was least important. Variation by consumer group existed, which may allow for targeted interventions to modify ENDS use.
Keywords: electronic nicotine delivery system, vaping, e-cigarettes, attributes, preference
Strengths and limitations of this study:
Large US sample using a robust experimental design, a best–worst scaling, case 2 method, incorporating large numbers of relevant attributes of electronic nicotine delivery systems (ENDS).
Consumers found different attributes of ENDS products important to their use.
Perceived harms of ENDS use appeared most important to their choice of ENDS products, and modifiability was least important.
Variation by consumer group existed, which may allow for targeted interventions to modify ENDS use.
Limitations include the convenience sample of US ENDS users, and the use of an experimental design which may invite more socially acceptable responses as opposed to direct purchasing observations.
Introduction
Although its health effects remain unclear, the use of electronic nicotine delivery systems (ENDS) in the USA continues to grow even in the face of impending regulation.1 While the use of combustible tobacco cigarettes has declined, the US ENDS market now exceeds $8 billion.2 3 The rise in the use of ENDS has occurred despite mixed evidence about its harm reduction effects or use as a tobacco cessation aid.4 Given the uncertainty around ENDS, initial regulations were piecemeal and varied from state to state. However, in 2016, the Food and Drug Administration (FDA) issued a regulation deeming ENDS to fall under its authority.5 These regulations will impose a variety of restrictions on ENDS manufacturing, sales and marketing. Notably, however, the FDA has postponed implementation of some of the rules requirements, citing the possibility that ENDS have the ‘potential to make a notable public health difference’.6 As the potential benefits and harms of ENDS become clear, regulators and public health groups will need to understand how their proposed regulations will likely affect consumers’ use. Consumers use ENDS due to a variety of product attributes that may be amenable to regulation or public health campaigns.7 Regulators and public health groups need to know the incremental role of individual product attributes on the decision to use ENDS products so that they can make evidence-based policies depending on how they want to modify ENDS use.
A widely used method in behavioural economics to understand consumer use is discrete choice experiments (DCE).8 9 Once the relationship between consumers’ use and product attributes is understood, regulators can consider whether and how to address the most important attributes to most of the consumers to either increase or decrease product use. While DCEs have been conducted to examine consumers’ attitudes towards combustible tobacco regulations and smoking cessation,10–14 only one has examined potential ENDS use.15 This recently published study conducted in Canada examined only four ENDS attributes: flavour, nicotine content, health warnings and price15; finding that health risks and efficacy as a tobacco cessation aid were the two most important attributes to consumers. However, this study only examined four attributes and included non-smokers and non-ENDS users.
Knowledge from our formative qualitative work was used to create a DCE with a larger number of attributes focused on ENDS users. We examined the list of a dozen attributes that are important to consumers, which we developed from a recent structured content analysis.16 Among these 12 attributes, we found 9 attributes that appeared related to the ENDS device itself.16 We chose to look at the device-related attributes because these attributes may be more easily regulated than other psychosocial attributes such as the ability to vape in public or as a social outlet.17 18 We designed and fielded a DCE study using a best–worst scaling experiment among the national US sample of ENDS users.
Methods
Study design
We conducted a one-time survey including a best–worst scaling, case 2 design DCE of the importance of ENDS product attributes. The final version of the survey was fielded using the Research Now survey panel between 26 August and 31 August 2016. Participants were remunerated from Research Now in accordance with their usual rate.
Patient and public involvement
The attributes used in the DCE were developed through focus groups conducted as part of prior research.19 The study involved participants in a pretest phase using an academic mass email system. Twelve individuals pretested the survey between 9 May and 14 May 2016. Incentive for completing the survey included possibly receiving one of four gift cards valued at $50 each. Feedback from the pretest led to both condensing and simplifying the language of the survey. Clarity of the survey was again improved after another round of testing with 75 survey panellists recruited from Research Now, a research survey company, between 15 August and 16 August 2016. The second episode of testing also led to language alterations surrounding questions on the flavour attribute and its levels to ensure comprehension. Participants were not involved in the recruitment to or conduct of the study. Participants in the final version of the survey did not receive their study results.
Participants
We recruited 660 members of the Research Now survey panel aged 18 years and older who reported having used ENDS at least once in their lifetime to complete the ENDS survey. Research Now is one of the major online survey panel companies with over 140 million finished surveys annually. Participants had to live in the USA and be able to complete an electronic survey in English. We specifically oversampled for older adults and minimum quotas to ensure near equal balance of gender.
Survey design
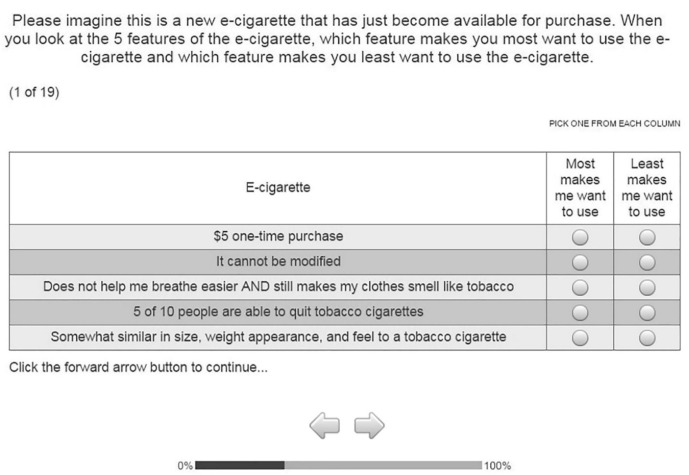
The survey was designed to include a best–worst scaling experiment related to ENDS use followed by a series of tobacco and ENDS-related questions. The survey included 19 best–worst scaling choice tasks, followed by questions on each participant’s demographics, personality, current and past use of ENDS and tobacco products and prior attempts to quit tobacco use. Sawtooth Software was used to design the survey. Prior to the best–worst tasks, the survey provided an explanation for each of the attributes, as well as an example task involving car attribute preference followed by an example of a best–worst scaling task (figure 1). The participants were told that some of the attributes were real but others were not and were asked, for the purposes of the study, to pretend they were all real and imagine each choice task as a new device. As opposed to other types of DCE methods where consumers choose between ENDS products, for each of the 19 choice tasks, participants selected one of five listed attribute levels that they felt most likely to encourage and least likely to encourage their use of a theoretical ENDS product. While other approaches such as a classical DCE would also yield importance scores, past studies demonstrated user fatigue and attribute dominance due to complex and overwhelming survey questions, whereas best–worst scaling, though not case 2, has been used in other areas of tobacco control.20–23 Additionally, best–worst scaling case 2 methodology was preferred because it leads to scores on a common scale, permitting direct comparison between all attribute levels within the study, but not just direct comparisons among levels within the same attribute (as is the case for other DCE methods).24 25 The 660 participants received 1 of 50 versions of the survey using a partial profile design. We sought efficiency by using a computer search algorithm to generate a design that showed each of a given attribute’s levels an equal number of times (one-way level balance) and each pairing of a given attribute level with the levels of other attributes an equal number of times (two-way level balance that reduces correlations among the attributes). These two criteria will maximise both level balance and orthogonality, the two constituents of design efficiency for experimental design for a set of single profiles. Overall, each attribute level was seen about three times per participant (2.97), and each valid cross-attribute pair was seen not quite half the time by each participant (0.42 times). Reliable best–worst utilities can be obtained as long as each participant sees each level about three times.26 The order of attributes varied across the survey blocks so that positional balance was maintained.
Figure 1.
Example of a best–worst scaling, case 2 task.
Best–worst scaling attributes and levels
The nine attributes were included with a definition in the survey. Their definitions can be found in online appendix table 1. For the rest of the paper, we will refer to these nine as harms of use, general effects, cessation aid, purchase price, monthly cost, nicotine content, flavour availability, device design and modifiiability.16 We chose to divide cost into a separate purchase price and monthly cost attributes because of the variation that can be seen in each; the focus group members mentioned the two attributes separately as well. Rates of tobacco cessation for ENDS products were drawn from the evidence that ENDS are often used as a tobacco cessation aid regardless of actual effectiveness. It is worth noting that medications prescribed for assisting in tobacco cessation (eg, bupropion, varenicline, nicotine containing products) have 2 out of 10 success rate at best.27 Nicotine content levels were drawn from the current spectrum of labelled nicotine concentrations.28 In addition to the actual concentration, we included a label of ‘none’, ‘low’, etc., to denote where a particular concentration falls in the range of concentrations.
bmjopen-2018-027247supp001.pdf (54.6KB, pdf)
Other measures
Participants responded to questions about sex, age, race and ethnicity, perceptions of general health,29 education level and yearly household income. The survey then collected details about each participants’ tobacco and ENDS use behaviours. Items included the heaviness of smoking index that asks participants ‘At present, how long after waking do you wait before having your first cigarette (in mins)?’ and ‘How many cigarettes do you smoke per day at present?’30 and other questions from the National Adult Tobacco Survey Questionnaire, 2012–2013, such as age of first cigarette, number of cigarettes smokes per day and smoking days per month.31
Analysis
In DCEs, respondents are given tasks that combine possible varieties of product attributes and asked to make a choice, in our case, which attribute of the product was important to their use. With enough choice tasks, estimated importance scores can be generated, indicating which attribute most influenced their choices. The statistical model underlying best–worst scaling assumes that the relative choice probability of a given pair of best–worst choices is proportional to the distance between the two attribute levels on the latent utility scale. The pair of attribute levels chosen maximises the difference in the part-worth utilities for a given choice task. These distances between attribute levels are modelled as a difference model, with variations on best–worst scaling sometimes called ‘maximum difference scaling’.32 Using a multinomial regression model, these differences can provide the part-worth utilities relative to a single attribute level rather than relative to the sample mean.24 Part-worth utilities are zero-centred numerical values that represent the relative desirability of the levels within each attribute. The higher the number, the more desirable the attribute’s level is to participants. Importance scores were then calculated based on the difference between minimum and maximum part-worth utilities within an attribute.25 33 The total importance of all attributes to a decision is 100%, with each attribute given a percentage of that total importance. Most important attribute was determined by comparing the importance scores for each individual, defining the attribute with the largest importance score as most important. In order to examine changes in attribute importance by age and gender, we performed a dependent z-test of proportions to compare the most important attribute with the next highest ranked attribute by age and gender.
Results
Of 900 individuals surveyed, 660 participants had used ENDS at least one time. Participants had a mean age of 42 years (SD ±19.4) with a range from 18 to 82 years and were evenly split women versus men (51% vs 49%) (table 1). Most participants were white (81%), making less than $60 000 annually (60%) and self-reported very good or excellent health (60%). Sixty-four percent had used ENDS in the last 30 days. Almost all participants (92%) reported a history of traditional tobacco products and most (85%) had tried to quit tobacco in the past 12 months. Pearson’s χ2 tests of the relation between age and tobacco use characteristics found statistically significant (p<0.05) differences between subgroups of young (18–24 years), middle-aged (25–49 years) and older (50+ years) adults. For example, young adult participants were more likely to have used ENDS in the last 30 days (p=0.036) and to have used flavoured ENDS (p=0.012). Older adults were less likely to have used ENDS in the past 30 days (p<0.001) and more likely to smoke one pack/day of traditional tobacco (p<0.001) (see table 2).
Table 1.
Participants’ characteristics, n (%)
| Characteristics | Total, n=660 | Age 18–24 years, n=169 | Age 25–49 years, n=242 | Age 50+ years, n=249 |
| Age, mean (SD) | 42.0 (19.4) | 22.2 (1.6) | 32.1 (7.7) | 65.0 (6.9) |
| Female | 334 (51%) | 90 (53%) | 120 (49%) | 124 (50%) |
| Race | ||||
| White | 532 (81%) | 113 (67%) | 191 (79%) | 228 (92%) |
| Black | 42 (6%) | 14 (8%) | 18 (7%) | 10 (4%) |
| Asian-American | 40 (6%) | 20 (12%) | 18 (7%) | 2 (1%) |
| Other | 46 (7%) | 22 (13%) | 15 (6%) | 9 (4%) |
| Hispanic or Latino/Latina | 94 (14%) | 42 (25%) | 45 (19%) | 7 (3%) |
| College degree or higher | 247 (37%) | 37 (22%) | 113 (47%) | 97 (38%) |
| Overall health, very good or excellent | 396 (60%) | 110 (65%) | 160 (66%) | 126 (51%) |
| Annual household income | ||||
| $0 to $29 999 | 149 (23%) | 52 (31%) | 51 (21%) | 46 (18%) |
| $30 000 to $59 999 | 247 (37%) | 75 (44%) | 85 (35%) | 87 (35%) |
| $60 000 to $89 999 | 156 (24%) | 25 (15%) | 73 (30%) | 58 (23%) |
| $90 000 or more | 106 (16%) | 17 (10%) | 33 (14%) | 58 (23%) |
| Knowledge that quitting smoking with help is more successful than without | 310 (47%) | 75 (44%) | 108 (45%) | 127 (51%) |
| Used ENDS in last 30 days† | 387 (64%) | 109 (71%) | 153 (69%)* | 125 (54%)* |
| Used flavoured ENDS in last 30 days, of current ENDS users‡ | 296 (70%) | 93 (79%)* | 134 (79%)* | 69 (52%)* |
| Anticipates ENDS use in next year | 478 (72%) | 121 (72%) | 193 (80%)* | 164 (66%)* |
| Ever used traditional tobacco products | 607 (92%) | 154 (91%) | 221 (91%) | 232 (93%) |
| Age at first cigarette, mean (SD)† | 17 (6) | 16 (3) | 18 (5) | 18 (8) |
| Smokes more than one pack/day§ | 54 (12%) | 3 (3%)* | 12 (8%)* | 39 (23%)* |
| First smoke within 30 min of wakingठ| 256 (59%) | 48 (44%)* | 89 (59%) | 119 (70%)* |
| Tobacco quit attempt in past 12 months§ | 369 (85%) | 87 (79%)* | 130 (85%) | 152 (89%) |
*P<0.05; test versus total.
†n=607, 154, 221 and 232, respectively.
‡n=420, 117, 170 and 133, respectively.
§n=432, 110, 152 and 170, respectively.
ENDS, electronic nicotine delivery systems.
Table 2.
Final ENDS attributes, levels and mean utilities, n=660
| ENDS attributes | Levels of ENDS attributes | Part-worth utilities, mean (95% CI) |
| Harms of use | Less harmful on my body as compared with tobacco cigarettes | 88.96 (84.79 to 93.13) |
| Unknown harm on my body as compared with tobacco cigarettes | −19.24 (−21.32 to 17.17) | |
| Same amount of harm on my body as compared with tobacco cigarettes | −22.45 (−24.90 to 19.99) | |
| More harm on my body as compared with tobacco cigarettes | −47.27 (−49.69 to 44.86) | |
| General effects of use | Helps me breathe easier and my clothes do not smell like tobacco | 58.37 (55.07 to 61.68) |
| Helps me breathe easier but my clothes smell like tobacco | −3.50 (−5.78 to 1.22) | |
| Does not help me breathe easier but my clothes do not smell like tobacco | −12.35 (−14.78 to 9.93) | |
| Does not help me breathe easier but still makes my clothes smell like tobacco | −42.52 (−45.15 to 39.89) | |
| Tobacco cessation | 7 of 10 people are able to quit tobacco cigarettes | 41.92 (39.36 to 44.47) |
| 5 of 10 people are able to quit tobacco cigarettes | 19.98 (18.20 to 21.76) | |
| 2 of 10 people are able to quit tobacco cigarettes | −12.79 (−14.84 to 10.73) | |
| People are not able to quit smoking tobacco cigarettes | −49.11 (−52.46 to 45.75) | |
| Purchase price of product | $5 one-time purchase | 52.41 (49.12 to 55.70) |
| $55 one-time purchase | 12.40 (11.02 to 13.77) | |
| $115 one-time purchase | −26.04 (−27.92 to 24.17) | |
| $175 one-time purchase | −38.76 (−40.95 to 36.57) | |
| Monthly cost of use | $5 per month to use | 47.50 (44.72 to 50.29) |
| $25 per month to use | 16.68 (15.00 to 18.37) | |
| $65 per month to use | −22.16 (−23.91 to 20.42) | |
| $100 per month to use | −42.03 (−44.37 to 39.68) | |
| Nicotine content* | None (0 mg/mL) | 10.89 (6.83 to 14.96) |
| Low (6 mg/mL) | 18.29 (16.16 to 20.41) | |
| Medium (12 mg/mL) | 2.11 (−0.01 to 4.23) | |
| High (24 mg/mL) | −31.30 (−35.12 to 27.47) | |
| Flavour availability | Available in fruit, candy, coffee, wine and other flavours | 15.88 (13.32 to 18.44) |
| Available in tobacco and menthol flavours | 13.50 (11.94 to 15.05) | |
| Available without any flavours | −29.38 (-32.27 to 26.48) | |
| Device design | Very similar in size, weight, appearance, and feel to a tobacco cigarette | 12.85 (11.16 to 14.54) |
| Somewhat similar in size, weight, appearance, and feel to a tobacco cigarette | 14.47 (12.87 to 16.07) | |
| Not similar at all in size, weight, appearance and feel to a tobacco cigarette | −27.32 (-29.98 to 24.66) | |
| Modifiability | Various parts can be modified | 12.46 (10.68 to 14.23) |
| It cannot be modified | −12.46 (-14.23 to 10.68) |
*Nicotine levels corresponded to what the current literature designated as low, medium and high levels of nicotine.
ENDS, electronic nicotine delivery systems.
Importance of ENDS attributes overall and by subgroup
The overall importance scores for the nine attributes are found in figure 2. The attributes had the following importance distribution: harms of use 17.6%; general effects 14.1%; cessation aid 12.6%; purchase price 12.1%; monthly cost 12.0%; nicotine content 11.4%; flavour availability 8.4%; device design 7.2% and modifiability 4.6%. Independent t-tests of the importance scores by gender found that when compared with men, women participants were more likely to give importance to harms of uses (∆+9.7%; p<0.001) and general effects (∆+8.1%; p=0.002) and less likely to give importance to purchase price (∆−8.3%; p=0.011) and monthly cost (∆−6.8%; p=0.008).
Figure 2.
Importance of ENDS characteristics, n=660. ENDS, electronic nicotine delivery systems.
Numerous statistically significant differences were found by age subgroups. Younger adults compared with middle-aged and older adults together were more likely to give importance to modifiability (∆+22%; p=0.004) and less likely to give importance to purchase price (∆−11%; p=0.006). Middle-aged adults compared with young and older adults together were more likely to give importance to modifiability (∆+24%; p=0.001) and less likely to give importance to harms of use (∆−5%; p=0.027). Older adults compared with middle-aged and young adults together were more likely to give importance to purchase price (∆+11%; p=0.004) and less likely to give importance to modifiability (∆−34%; p<0.001).
Part-worth utilities of ENDS attribute levels
The part-worth utility scores for the levels of the nine attributes are shown in table 2. Harms of use had the level with the highest part-worth utility of 88.96 (90% CI: 84.79, 93.13), followed by general effects with a level at 58.37 (90% CI: 55.07, 61.68) and purchase price at 52.41 (90% CI: 49.12, 55.70). Cessation aid had the level with the lowest part-worth utility of −49.11 (90% CI: −52.46,–45.75), followed by harms of use at −47.27 (90% CI: −49.69,–44.86).
Most important ENDS attribute overall and by subgroup
After examining the importance scores for each individual, harms of uses was the most important attribute for 49% of participants (table 3). Nicotine content was next most frequent (13%) and purchase price was third (12%). A goodness-of-fit χ2 test determined that the nine attributes differed from expected and thus were not equally distributed among participants (p<0.001). Also, dependent z-tests of the proportions of the second through ninth ranked attributes against harms of use found that all were significantly lower ranked and were less likely to be chosen as most important (range: ∆−83%–99%; p<0.001). Aside from nicotine content compared with harms of use, stepped tests of each attribute against its nearest found that only device design (p=0.005) and modifiability (p=0.020) were statistically significantly less important.
Table 3.
Most important attribute, n (%)
| ENDS characteristics | Total n=660 |
Age 18–24 years n=169 | Age 25–49 years n=242 | Age 50+ years n=249 |
Male n=326 |
Female n=334 |
| Harms of use | 326 (49%) | 74 (44%) | 114 (47%) | 138 (55%)† | 149 (46%) | 177 (53%) |
| Nicotine content | 85 (13%)* | 26 (15%) | 32 (13%) | 27 (11%) | 42 (13%) | 43 (13%) |
| Purchase price of product | 77 (12%) | 14 (8%) | 30 (12%) | 33 (13%) | 42 (13%) | 35 (10%) |
| General effects of use | 56 (9%) | 26 (15%)† | 13 (5%)† | 17 (7%) | 24 (7%) | 32 (10%) |
| Tobacco cessation aid | 45 (7%) | 11 (6%) | 16 (7%) | 18 (7%) | 28 (9%) | 17 (5%) |
| Flavour availability | 32 (5%) | 10 (6%) | 18 (7%)† | 4 (2%)† | 18 (5%) | 14 (4%) |
| Monthly cost of use | 27 (4%) | 7 (4%) | 11 (5%) | 9 (4%) | 17 (5%) | 10 (3%) |
| Device design | 10 (2%)* | 1 (1%) | 6 (2%) | 3 (1%) | 6 (2%) | 4 (1%) |
| Modifiability | 2 (0%)* | 0 (0%) | 2 (1%) | 0 (0%) | 0 (0%) | 2 (1%) |
* P<0.05 versus next higher ranked attribute for total sample.
†P<0.05 versus total sample for age groups.
The nine attributes also statistically significantly differed from the expected distribution by the subgroups for age and gender (p<0.001). Pearson’s Χ2 tests of the relation between the age subgroups and most important attribute found that general effects was the most important attribute for younger adults as compared with other ages (p<0.001). Compared with younger and older adults, general effects was less likely to be the most important attribute for middle-aged adults (p=0.029) and flavour availability was more likely to be most important (p=0.018). Compared with both younger and middle-aged adults, harms of use was more likely to be important (p=0.016) and flavour availability was less likely to be important (p=0.003). There were no statistically significant differences in the distribution of most important attributes by gender although harms of use (p=0.061) and cessation aid (p=0.075) approached significance.
Discussion
In our study of US adult ENDS consumers, we found that harms of use had the highest importance to consumers’ choice of an ENDS product. Other than cost (ie, purchase price and monthly cost), the attributes with the highest importance scores hinged on consumers’ perceptions of efficacy as a harm reduction strategy (harms of use), health benefit (general effects) and tobacco cessation (cessation aid), respectively. Consumers of different ages varied in importance they placed on different ENDS product characteristics. As more evidence about these products’ ability to benefit or harm consumers is more fully understood, public health initiatives could target these perceptions. Variation by consumer group existed, which may allow for targeted interventions to reduce or enhance ENDS use in any given consumer group.
ENDS products are marketed as healthier than combustible tobacco products,34 but the evidence regarding the impact of ENDS products on human health is slowly emerging. National Academies of Science, Engineering, and Medicine recently released a report on the public health consequences of ENDS products.35 While there is substantial evidence that, except for nicotine, exposure to potentially toxic substances from ENDS is significantly lower compared with combustible tobacco products, there is also substantial evidence that exposure to ENDS aerosols can induce lung dysfunction and oxidative stress in human tissue.36 The long-term effects on cardiovascular outcomes, cancer or other health conditions are unclear. Our work should add a sense of urgency to the push for ongoing research into the evidence for and against ENDS products as a harm reduction strategy. The idea that an ENDS product was less harmful than tobacco cigarettes was extremely important to participants’ choice. The level that the product was ‘less harmful on my body as compared with tobacco cigarettes’ had the highest part-worth utility of any attribute (88.96). Moreover, the level that the product ‘had the same amount of harm on my body as compared with tobacco cigarettes’ caused people to avoid choosing that product and had a negative utility (−22.45). Both the health effects and the use as a cessation aid had levels that are likely healthier and of more help in cessation than the evidence suggests. Yet in general, perceptions, including misperceptions, affect smokers’ behaviour.37 The look and feel of cigarette packaging appears to influence consumers’ use and may affect their perceptions’ of the healthiness and harm of the cigarettes within.38 A study of combustible tobacco labelling revealed that ‘additive-free’ or ‘natural’ labels on current cigarette brands were misperceived to be possibly less harmful than other brands of cigarettes and may reduce the efficacy of public health initiatives.39 Even efforts by the Federal Trade Commission to prohibit language that might create misperceptions of reduced harm in tobacco cigarettes has been unsuccessful.37 This (mis)perception of harm and health appears to strongly influence the choice of ENDS products. Further efforts, including those studying clear labelling and health warnings, are needed to explore how to align ENDS users’ perceptions of ENDS products with the evidence.
While harms of use had the highest importance score, the combined importance of purchase price and monthly cost was greater than harms of use, so the importance of financial burden on ENDS use should not be underestimated. If we combine the two cost-related attributes, purchase price and monthly cost, overall cost’s importance score would be 24.1% as compared with 17.6% for health effects. A recent study of the cross-price elasticity of ENDS and tobacco cigarettes found that ENDS are partially substitutable for cigarettes.40 However, the availability of ENDS also reduced the number of individuals who reported they would quit smoking if cigarette costs increased by 20% (50.2% to 30.0%), revealing that ENDS may discourage smokers from quitting completely.41 Additionally, increase in the cost of ENDS products may shift consumers back towards combustible tobacco, though recent simulations found no relationship between cigarette prices and ENDS use.42 Taxation may reduce ENDS use but further work is needed to model the consequences of cost increase on ENDS use.
Potentially meaningful differences were found in the importance of ENDS product attributes and the most important attribute by different age groups. Younger and middle-aged adults found modifiability to be more important than older adults. While we did not see gender differences for attribute preferences, a Canadian DCE study of young women found that pack structure was the most important attribute driving ENDS use.14 The shape and structure of the device and packaging may be more important to a younger population. Younger adults were more likely to have general effects as their most important attribute while flavour availability was more likely to be most important to middle-aged adults and significantly less likely to be most important to older adults. A systematic review of studies of consumers’ preference for flavour found it to be likely important to young people.7 However, as with our own qualitative study, which was included in the review, many of the included studies did not have experimental designs.16 Interestingly, another Canadian DCE study of ENDS use found that younger smokers perceived cherry flavour as less harmful while older adults found tobacco flavour less harmful.15 While we found flavour availability was more important to middle-aged adults, we did not find older adults favoured tobacco flavour. However, both of these studies found that attributes related to the users’ health (harms of use in our case and health warnings in the Canadian study) were more important than flavour, and thus efforts to regulate flavours may not reduce ENDS use as much as other regulations on other attributes, such as harms of use.
Our study has several important limitations. First, our study examines choice behaviour and not actual purchase behaviour. While we drew from a national online survey panel, our respondent population is limited to a convenience sample. Additionally, best–worst scaling can be subject to attribute non-attendance where participants either fail to pay attention to an attribute or attributes, or attribute dominance where participants only pay attention to a single attribute. We found that only about 2% of participants did not attend to the majority of attributes, though about 16% failed to attend to at least one attribute. No participant showed dominance behaviour. While there was some attribute non-attendance, best–worst scaling inclines participants to make judgements about more attributes and does not invite as much attribute non-attendance or dominance as can be seen in other standard DCEs. However, it seems more likely that socially desirable responses could bias respondents’ choices within best–worst scaling than other DCEs.24 Lastly, while we did extensive pretesting, it is possible that different participants interpreted different attributes and levels differently. For example, it is possible that participants viewed the monthly cost of use in relation to their own use and thus the responses to monthly cost may need to be viewed with caution.
Conclusion
A variety of ENDS product attributes are important to consumers. Harms of use had the highest importance to consumers’ choice of an ENDS product. Other than cost, the attributes with the highest importance scores hinged on consumers’ perceptions of efficacy as a harm reduction strategy (harms of use), general benefit (general effects) and tobacco cessation (cessation aid), respectively. Consumers differed by age group in some of the more important attributes. Though the overall importance of ENDS product attributes was similar, efforts to increase or decrease ENDS use could be tailored to these group differences.
Supplementary Material
Acknowledgments
We would also like to thank CHAI Core’s Maihan Vu (MV) and Randall Teal (RT) for qualitative coding expertise. We would like to thank Trisha Crutchfield for her project management.
The work reported in this paper was conducted by CHAI Core (Communication for Health Applications and Interventions), who is supported in part by a grant from NIH (DK056350) to the University of North Carolina Nutrition Obesity Research Center and from NCI (P30-CA16086) to the UNC Lineberger Comprehensive Cancer Center.
Footnotes
Contributors: CEK, LMR, ELS, KC, MB, GAZ and AOG all contributed to the conceptualisation and design of the survey. CK and KC assisted with data collection. CK, LMR, ELS, KC, CJW, CE, CM, MB, GAZ and AOG participated in the analysis of the data. All authors contributed to the content and reviewed the manuscript prior to submission.
Funding: The University of North Carolina at Chapel Hill University Cancer Research Fund at the Lineberger Comprehensive Cancer Center supported this work. The attributes and factors in the experiment were developed with the assistance of the CHAI Core (Communication for Health Applications and Interventions), who is supported by the National Institutes of Health (DK056350) and the National Institutes for Health at the National Cancer Institute (P30 CA16086).
Competing interests: None declared.
Ethics approval: The institutional review board at the University of North Carolina at Chapel Hill approved this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data can be requested from Christine_Kistler@med.unc.edu.
Patient consent for publication: Not required.
References
- 1. Pauly J, Li Q, Barry MB. Tobacco-free electronic cigarettes and cigars deliver nicotine and generate concern. Tob Control 2007;16:357–57. 10.1136/tc.2006.019687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67 53–9. 10.15585/mmwr.mm6702a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacGuill S. What is the new tobacco data telling us: Euromonitor International. 2017. updated 20 Jun 2016 https://blog.euromonitor.com/2016/06/what-is-the-new-tobacco-data-telling-us.html (Accessed 21 Jan 2018).
- 4. Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control 2014;23 Suppl 2:ii36–ii40. 10.1136/tobaccocontrol-2013-051470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Backinger CL, Meissner HI, Ashley DL. The FDA “Deeming Rule” and tobacco regulatory research. Tob Regul Sci 2016;2 290–3. 10.18001/TRS.2.3.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Administration USFaD. FDA’s Plan for Tobacco and Nicotine Regulation.
- 7. Zare S, Nemati M, Zheng Y. A systematic review of consumer preference for e-cigarette attributes: flavor, nicotine strength, and type. PLoS One 2018;13:e0194145 10.1371/journal.pone.0194145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Bekker-Grob EW, Hol L, Donkers B, et al. Labeled versus unlabeled discrete choice experiments in health economics: an application to colorectal cancer screening. Value Health 2010;13:315–23. 10.1111/j.1524-4733.2009.00670.x [DOI] [PubMed] [Google Scholar]
- 9. Bech-Larsen T, Nielsen NA. A comparison of five elicitation techniques for elicitation of attributes of low involvement products. Journal of Economic Psychology 1999;20:315–41. [Google Scholar]
- 10. Marti J. Assessing preferences for improved smoking cessation medications: a discrete choice experiment. Eur J Health Econ 2012;13:533–48. 10.1007/s10198-011-0333-z [DOI] [PubMed] [Google Scholar]
- 11. Marti J. A best–worst scaling survey of adolescents' level of concern for health and non-health consequences of smoking. Soc Sci Med 2012;75:87–97. 10.1016/j.socscimed.2012.02.024 [DOI] [PubMed] [Google Scholar]
- 12. Goto R, Takahashi Y, Ida T. Changes in smokers' attitudes toward intended cessation attempts in Japan. Value Health 2011;14:785–91. 10.1016/j.jval.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 13. Goto R, Nishimura S, Ida T. Discrete choice experiment of smoking cessation behaviour in Japan. Tob Control 2007;16:336–43. 10.1136/tc.2006.019281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kotnowski K, Fong GT, Gallopel-Morvan K, et al. The impact of cigarette packaging design among young females in Canada: findings from a discrete choice experiment. Nicotine Tob Res 2016;18 10.1093/ntr/ntv114 [DOI] [PubMed] [Google Scholar]
- 15. Czoli CD, Goniewicz M, Islam T, et al. Consumer preferences for electronic cigarettes: results from a discrete choice experiment. Tob Control 2016;25 10.1136/tobaccocontrol-2015-052422 [DOI] [PubMed] [Google Scholar]
- 16. Kistler C, Crutchfield T, Sutfin E, et al. Consumers’ preferences for electronic nicotine delivery system product features: a structured content analysis. International Journal of Environmental Research and Public Health 2017;14:613 10.3390/ijerph14060613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoek J, Thrul J, Ling P. Qualitative analysis of young adult ENDS users' expectations and experiences. BMJ Open 2017;7:e014990 10.1136/bmjopen-2016-014990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keane H, Weier M, Fraser D, et al. ‘Anytime, anywhere’: vaping as social practice. Critical Public Health 2017;27:465–76. [Google Scholar]
- 19. Kistler CE, Crutchfield TM, Sutfin EL, et al. Consumers' preferences for electronic nicotine delivery system product features: a structured content analysis. Int J Environ Res Public Health 2017;14 10.3390/ijerph14060613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mühlbacher AC, Kaczynski A, Zweifel P, et al. Experimental measurement of preferences in health and healthcare using best-worst scaling: an overview. Health Econ Rev 2016;6:2 10.1186/s13561-015-0079-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gendall P, Eckert C, Hoek J, et al. Estimating the effects of novel on-pack warnings on young adult smokers and susceptible non-smokers. Tob Control 2018;27:519–25. 10.1136/tobaccocontrol-2017-053719 [DOI] [PubMed] [Google Scholar]
- 22. Hoek J, Gendall P, Eckert C, et al. Dissuasive cigarette sticks: the next step in standardised (’plain') packaging? Tob Control 2016;25:699–705. 10.1136/tobaccocontrol-2015-052533 [DOI] [PubMed] [Google Scholar]
- 23. Hoek J, Gendall P, Eckert C, et al. Effects of brand variants on smokers' choice behaviours and risk perceptions. Tob Control 2016;25:160–5. 10.1136/tobaccocontrol-2014-052094 [DOI] [PubMed] [Google Scholar]
- 24. Orme B. The maxdiff system technical paper. Technical Paper Series 2013. https://www.sawtoothsoftware.com/download/techpap/maxdifftech.pdf (Accessed 8 Feb 2018). [Google Scholar]
- 25. Louviere JJ, Flynn TN. Using best-worst scaling choice experiments to measure public perceptions and preferences for healthcare reform in australia. Patient 2010;3:275–83. 10.2165/11539660-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 26. Orme B. Accuracy of HB Estimation in MaxDiff Experiments. Sawtooth Software: Research Paper Series. Sequim, WA: Sawtooth Software, Inc, 2005. [Google Scholar]
- 27. West R, Raw M, McNeill A, et al. Health-care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development. Addiction 2015;110:1388–403. 10.1111/add.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goniewicz ML, Gupta R, Lee YH, et al. Nicotine levels in electronic cigarette refill solutions: a comparative analysis of products from the U.S., Korea, and Poland. Int J Drug Policy 2015;26:583–8. 10.1016/j.drugpo.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cunny KA, Perri M. Single-item vs multiple-item measures of health-related quality of life. Psychol Rep 1991;69:127–30. 10.2466/pr0.1991.69.1.127 [DOI] [PubMed] [Google Scholar]
- 30. Heatherton TF, Kozlowski LT, Frecker RC, et al. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict 1989;84:791–9. [DOI] [PubMed] [Google Scholar]
- 31. Prevention CfDCa. National Adult Tobacco Survey Questionnaire, 2012–2013, 2014. [Google Scholar]
- 32. Szeinbach SL, Barnes JH, McGhan WF, et al. Using conjoint analysis to evaluate health state preferences. Drug Inf J 1999;33:849. [Google Scholar]
- 33. Flynn TN, Louviere JJ, Peters TJ, et al. Best–worst scaling: what it can do for health care research and how to do it. J Health Econ 2007;26:171–89. 10.1016/j.jhealeco.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 34. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014;23 Suppl 3:iii3–iii9. 10.1136/tobaccocontrol-2014-051670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Academies of Sciences E, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: The National Academies Press, 2018. [PubMed] [Google Scholar]
- 36. Carnevale R, Sciarretta S, Violi F, et al. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest 2016;150:606–12. 10.1016/j.chest.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 37. Yong H-H, Borland R, Cummings KM, et al. US smokers’ beliefs, experiences and perceptions of different cigarette variants before and after the FSPTCA ban on misleading descriptors such as “light,” “mild,” or “low”. Nicotine & Tobacco Research 2016;18:2115–23. 10.1093/ntr/ntw107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McNeill A, Gravely S, Hitchman SC, et al. Tobacco packaging design for reducing tobacco use. 2017. [DOI] [PMC free article] [PubMed]
- 39. Leas EC, Ayers JW, Strong DR, et al. Which cigarettes do Americans think are safer? A population-based analysis with wave 1 of the PATH study. Tob Control 2017;26:e59–e60. 10.1136/tobaccocontrol-2016-053334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quisenberry AJ, Koffarnus MN, Hatz LE, et al. The experimental tobacco marketplace i: substitutability as a function of the price of conventional cigarettes. Nicotine Tob Res 2016;18 10.1093/ntr/ntv230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grace RC, Kivell BM, Laugesen M. Estimating cross-price elasticity of e-cigarettes using a simulated demand procedure. Nicotine Tob Res 2015;17:592–8. 10.1093/ntr/ntu268 [DOI] [PubMed] [Google Scholar]
- 42. Huang J, Tauras J, Chaloupka FJ. The impact of price and tobacco control policies on the demand for electronic nicotine delivery systems. Tob Control 2014;23 Suppl 3:iii41–iii47. 10.1136/tobaccocontrol-2013-051515 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-027247supp001.pdf (54.6KB, pdf)